Dehydroamino acids are important moieties in biological investigations and are found in many natural products including roquefortine C and E,1 azinomycins A and B,2 AM-toxines and tentoxin.3 Dehydroamino acids introduce conformational rigidity and change the reactivities of peptides. They are also important intermediates in the biosynthesis of other non-proteinogenic amino acids and D-amino acids.
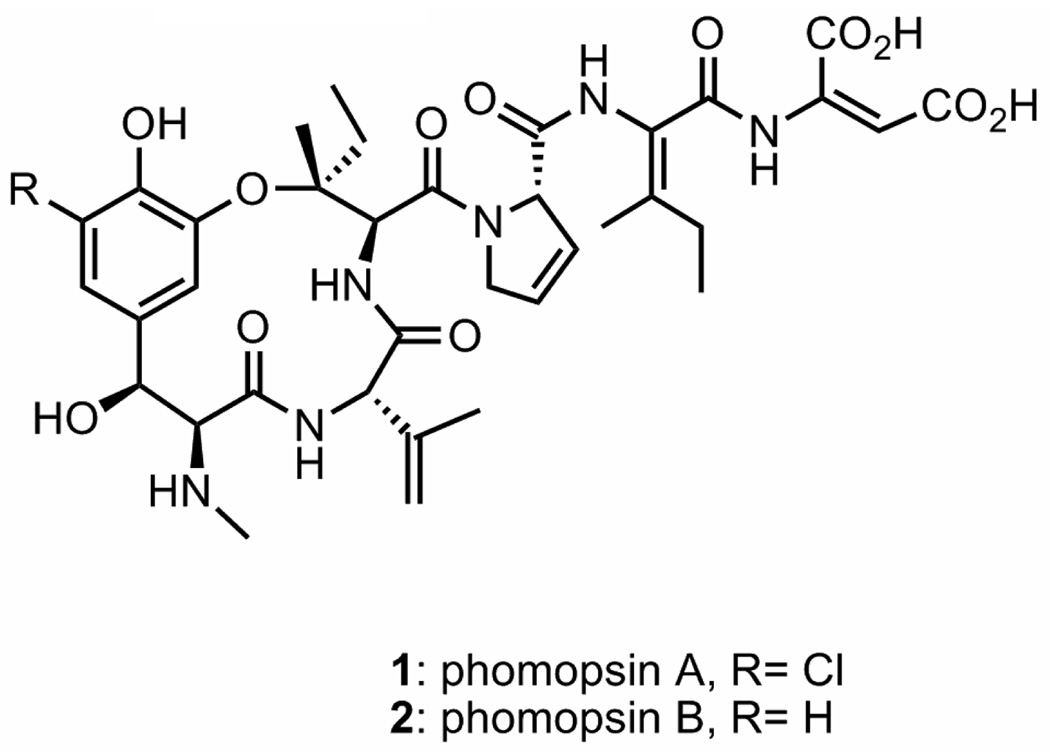
Phomopsins A (1) and B (2) are natural products isolated from the fungus species Phomopsis leptostromiformisa and potent inhibitors of microtubule polymerization (Figure 1).4 A significant structural feature of phomopsins A and B is an unsaturated tripeptide side chain, which contains dehydroisoleucine and dehydroaspartate. This side chain was reported to be important for the molecular interaction of 1 with tubulin.5 In this report, we describe an efficient and stereoselective synthesis of the phomopsin tripeptide side chain precursor.
Figure 1.
Phomopsins A and B.
Synthesis of the phomopsin side chain requires a stereoselective method to prepare (E)-dehydroisoleucine. Many methods are available for the synthesis of dehydroamino acids, and elimination of water from β-hydroxy-α-amino acids is a well established route. Activation of the hydroxyl group for elimination can be achieved by many reagents: DAST/N,N-diisopropylethyl amine (DIPEA), tosyl chloride, mesyl chloride, Martin’s sulfurane and triphenyl phospine/diethyl azodicarboxylate (DEAD).6 However, these methods are not highly stereoselective for E/Z isomers if there is no strong thermodynamic preference. Wandless et al. first reported an anti-selective two-step cyclic sulfamidate approach to the (E)-dehydroisoleucine. Based on this method they reported a total synthesis of phomopsin B in 2007.7 Ferreira et al. also reported an anti-selective Boc anhydride/DMAP elimination method. Under their conditions all amides were Boc-protected.8 In addition to elimination approaches, the Horner-Wadsworth-Emmons reaction has also been utilized to form the double bond.9 The vinyl amidation method which couples amides and vinyl halides is another useful approach to synthesize dehydroamino acids.10
We now describe a one-step copper-carbodimide elimination that provides the required (E)-dehydroisoleucine in a highly stereoselective manner. The copper-carbodimide elimination method was first reported in 1960s,11 Sai and co-workers found that this elimination method could give high syn-selective E/Z enamides.12 The utility of the copper-carbodimide method to prepare dehydroamino acids in a natural product was demonstrated in the total synthesis of roquefortine C.13
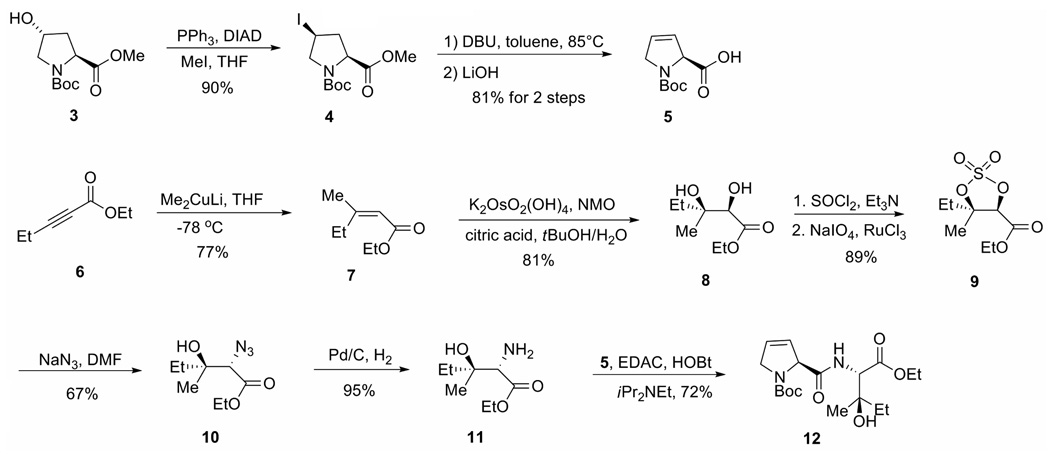
N-Boc-Dehydroproline (5) was prepared by a three-step sequence from commercially available pyrrolidine 3 (Scheme 1). Michael addition of methyl cuprate to the commercially available unsaturated ester 6, at low temperature resulted in a single anti addition product 7.14 Osmium tetroxide-mediated dihydroxylation gave syn diol 8. (Sharpless asymmetric dihydroxylation was not chosen because an enantiomeric pure compound was not needed since the syn elimination would provide a single dehydration product.) Diol 8 was converted to cyclic sulfate 9 and treated with sodium azide to provide β-hydroxy azide 10. Hydrogenation of azide 10 to amine 11 and subsequent coupling with acid 5 gave amide 12, the precursor of the dehydro amino acid moiety.
Scheme 1.
Preparation of the dehydration precursor
We screened different EDC(1-ethyl-3-(3-dimethylaminopropyl) carbodiimide)-copper mediated dehydration conditions to introduce the unsaturation and found that copper triflate in THF gave the highest yield and the single desired isomer 13 (Table 1, all the entries gave a single isomer 13). To our knowledge, this is the first example of preparing a trisubstituted enamide by using this method.
The third amino acid fragment, amine 16, was prepared by a three-step sequence from commercial available (+)-dimethyl tartrate (Scheme 2).15 The coupling between acid 17 and amine 16 resulted in the formation of an unreactive azlactone 18.7b To avoid the azlactone formation, the amide nitrogen had to be protected.
Scheme 2.
Preparation of amine 16 and azlactone formation
Thus, the ethyl ester 13 was converted to allyl ester 19 and the amide nitrogen was protected with Boc to afford compound 20 (Scheme 3). The allyl ester was cleaved under palladium catalyzed conditions to give acid 21 without reducing the double bond in the dehydroproline moiety.16 A benzyl ester was also tested but the partial reduction of the double bond occurred under hydrogenolysis conditions. Finally, acid 21 was coupled with amine 16 to give tripeptide 22 and Boc deprotection afforded 23 as the side chain precursor in the synthesis of phomopsin.17 As shown by previous workers, the dehydroaspartate unit in the phomopsin side chain isomerizes readily under basic conditions.18 Compound 23 will be coupled with the macrocycle part of phomopsins A and B directly and the β-hydroxy group will not be eliminated until the final stage of the synthesis.
Scheme 3.
Completion of the side chain
A highly stereoselective approach to make the (E)-dehydroisoleucine moiety of the phomopsin tripeptide side chain was developed to afford the material for the total syntheses of phomopsins A and B. The copper-carbodimide method provides an efficient solution to the stereoselective synthesis of dehydroamino acids. The synthesis and evaluation of the biological activities of phomopsins and their analogues will be reported in due courses.
Supplementary Material
Table 1.
Carbodimide Copper Dehydration.
 | |
|---|---|
| Conditions | Yield |
| CuCl2, 80 °C, toluene, overnight | 11% |
| CuCl2, 100 °C, toluene, 2 h | 20% |
| CuI, 100 °C, toluene, 4 h | 30% |
| No copper, toluene, 100 °C, overnight | 0 |
| Cu(OTf)2, THF, 45 °C, overnight | 42% |
| Cu(OTf)2, THF, 60 °C, 1.5 h | 73% |
Acknowledgements
Financial support for this research was provided by NIH (CA-40081) and NSF (0515443). Financial Support for the departmental instrumentation was provided by the National Institutes of Health (1S10RR23444-1). We thank Dr. George T. Furst and Dr. Rakesh Kohli of the University of Pennsylvania Spectroscopic Service Center for assistance in securing and interpreting high-field NMR spectra and mass spectra, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data associated with this article can be found in the online version.
References
- 1.(a) Ohmomo S, Sato T, Utagawa T, Abe M. Agric. Biol. Chem. 1975;39:1333. [Google Scholar]; (b) Clark B, Capon RJ, Lacey E, Tennant S, Gill JH. J. Nat. Prod. 2005;68:1661. doi: 10.1021/np0503101. [DOI] [PubMed] [Google Scholar]
- 2.Yokoi K, Nagaoka K, Nakashima T. Chem. Pharm. Bulletin. 1986;34:4554. doi: 10.1248/cpb.34.4554. [DOI] [PubMed] [Google Scholar]
- 3.(a) Okuno T, Ishita Y, Sawai K, Matsumoto T. Chem. Lett. 1974:635. [Google Scholar]; (b) Fulton ND, Bollenbacher K, Templeton GE. Phytopathology. 1965;55:49–51. [Google Scholar]
- 4.Mackay MF, Van Donkelaar A, Culvenor CC. J. Chem. Soc. Chem. Commun. 1986:1219. [Google Scholar]
- 5.Mitra A, Sept D. Biochemistry. 2004;43:13955. doi: 10.1021/bi0487387. [DOI] [PubMed] [Google Scholar]
- 6.(a) Somekh L, Shanzer A. J. Org. Chem. 1983;48:907. [Google Scholar]; (b) Pattabiraman VR, Stymiest JL, Derksen DJ, Martin N, Vederas JC. Org. Lett. 2007;9:699. doi: 10.1021/ol063133j. [DOI] [PubMed] [Google Scholar]; (c) Pravdic N, Zissis E, Pokorny M, Fletcher HtG. Carbohydr. Res. 1974;32:115. doi: 10.1016/s0008-6215(00)82921-3. [DOI] [PubMed] [Google Scholar]; (d) Yokokawa F, Shioiri T. Tetrahedron Lett. 2002;43:8679. [Google Scholar]; (e) Cherney RJ, Wang. L. J. Org. Chem. 1996;61:2544. [Google Scholar]
- 7. Stohlmeyer MM, Tanaka H, Wandless TJ. J. Am. Chem. Soc. 1999;121:6100. Stohlmeyer MM. Ph. D Thesis. CA: Stanford University; 2001. pp. 1–109. Investigating biological checkpoints through organic chemistry: synthesis of the phomopsin side-chain and ATR probes. (c) Grimley JS, Sawayama AM, Tanaka H, Stohlmeyer MM, Woiwode TF, Wandless TJ. Angew. Chem. Int. Ed. 2007;46:8157. doi: 10.1002/anie.200702537.
- 8.Ferreira PMT, Maia HLS, Monteiro LS. Tetrahedron Lett. 1998;39:9575. [Google Scholar]
- 9.Schmidt U, Griesser H, Leitenberger V, Lieberknecht A, Mangold R, Meyer R, Riedl B. Synthesis. 1992:487. [Google Scholar]
- 10.Jiang L, Job GE, Klapars A, Buchwald SL. Org. Lett. 2003;5:3667. doi: 10.1021/ol035355c. [DOI] [PubMed] [Google Scholar]
- 11.Corey EJ, Andersen NH, Carlson RM, Paust J, Vedejs E, Vlattas I, Winter REK. J. Am. Chem. Soc. 1968;90:3245. doi: 10.1021/ja01014a053. [DOI] [PubMed] [Google Scholar]
- 12.Sai H, Ogiku T, Ohmizu H. Synthesis. 2003:201. [Google Scholar]
- 13.Shangguan N, Hehre WJ, Ohlinger WS, Beavers MP, Joullié MM. J. Am. Chem. Soc. 2008;130:6281. doi: 10.1021/ja800067q. [DOI] [PubMed] [Google Scholar]
- 14.Anderson RJ, Corbin VL, Cotterrell G, Cox GR, Henrick CA, Schaub F, Siddall JB. J. Am. Chem. Soc. 1975;97:1197. doi: 10.1021/ja00838a039. [DOI] [PubMed] [Google Scholar]
- 15.Reference 6b mentioned that their starting material was (−)-dimethyl tartrate but their reported optical rotation data of intermediates agrees with the data of compounds 14–16. So we believe that (+)-dimethyl tartrate may have been used as the starting material even though the structure of the synthetic target was not affected because the dehydration reaction eliminates the stereocenters at the final stage.
- 16.Kunz H, Waldmann H. J. Chem. Soc., Chem. Commun. 1985;10:638. [Google Scholar]
- 17.Selected data: compound 13: 1H NMR (DMSO-d6, 70 °C): δ 8.91 (1 H, br), 5.97 (1 H, m), 5.72 (1 H, m), 4.89 (1 H, dd, J = 5.1, 2.6 Hz), 4.10 (2 H, m), 4.04 (2 H, q, J = 7.2 Hz), 2.37 (2 H, q, J = 7.5 Hz), 1.39 (9 H, s), 1.17 (3 H, t, J = 7.2 Hz), 1.02 (3 H, t, J =7.5 Hz); 13C NMR (DMSO-d6, 70 °C): δ 167.8, 163.8, 152.7, 144.6, 127.4, 125.7, 121.9, 78.5, 66.9, 59.2, 53.1, 27.6, 26.0, 18.0, 13.4, 12.1; HRMS (ESI) m/z calcd for C18H29N2O5 (M + H+): 353.2076, Found (M + H+): 353.2091; IR (cm−1): 3273 (w), 2977(m), 1709 (s), 1520 (w), 1401 (s), 1207 (w), 1105(w); [α]D25 = − 160 (c = 1.96, CHCl3). Compound 23: 1H NMR (CDCl3): δ 9.22 (1 H, br), 6.84 (1 H, d, J = 7.8 Hz), 5.92 (2 H, m), 5.19 (1 H, dd, J = 7.9, 2.7 Hz), 4.80 (1 H, d, J = 2.8 Hz), 4.69 (1 H, m), 4.02 (1 H, m), 3.87 (1 H, m), 3.81 (1 H, s), 3.74 (1 H, s), 2.38 (2 H, q, J = 7.6 Hz), 1.74 (3 H, s), 1.10 (3 H, t, J = 7.6 Hz); 13C NMR (CDCl3): δ 172.0, 171.7, 170.0, 165.6, 129.2, 127.8, 124.0, 123.6, 71.1, 68.9, 56.2, 54.4, 52.7, 52.5, 26.9, 17.3, 12.8; HRMS (ESI) m/z calcd for C17H26N3O7Na (M + H+): 384.1701, Found (M + Na+): 384.1765; IR (cm−1): 3288 (w, br), 2958 (w), 1748 (s), 1661 (s), 1514 (s), 1439(w), 1223 (m), 1125 (m), 1029 (w); [α]D19 = − 76 (c = 0.21, CHCl3).
- 18.Shin C, Obara T, Morita S, Yonezawa Y. Bull. Chem. Soc. Jpn. 1988;61:3265. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.