Abstract
γ-hydroxybutyric acid (GHB) has been reported to disrupt spatial learning and memory in adolescent male rats. The present study was undertaken to determine the effects of GHB on the acquisition of spatial memory in adolescent female rats, and to investigate age specificity of the behavioral impairments. Adolescent female rats were subjected to repeated GHB or saline administrations, and tested in the Morris water maze. Compared to age-matched saline controls, adolescent GHB-treated rats took significantly longer and swam greater distances to find the hidden platform. In the probe trial, GHB-treated adolescent rats spent less time in the target quadrant than saline-treated controls. There was no difference in either the swim speed or in the visual task performance between GHB-treated and saline-treated rats. To test for ontogenic specificity of the behavioral responses, adult female rats were treated with GHB and tested behaviorally in two separate experiments using a 6-day learning protocol (Experiment 1) and a 16-day learning protocol (Experiment 2). In the 6-day spatial learning and memory task, adult saline-treated rats failed to learn the task, and GHB did not alter the latency to find the platform, or performance in the probe trial. In the second behavioral protocol, a modified version of the memory task was used to test adult animals. The number of test days was increased from 6 days to 16 days. Adult saline-treated females learned the task in the 16-days protocol. But unlike in adolescent female rat, GHB in adult rats had minimal effects on reference memory even when they had learned the spatial memory task. Performances in the probe trial by GHB-treated rats and saline controls were similar. Together, these data suggest that GHB impairs spatial learning specifically in adolescent female rats.
Keywords: adolescence, spatial learning and memory, Morris water maze, juvenile, cognitive dysfunction, memory deficit
Introduction
γ-hydroxybutyric acid (GHB) was developed as an anesthetic agent (Rodgers et al, 2004), and was approved by the Food and Drug Administration in the treatment of narcolepsy. But its illicit use as a dietary supplement in the early 1990s led to the amendment of the 2000 Controlled Substance Act to include it as a Schedule I agent (Barker et al, 2007; Galloway et al, 1997; Leichti et al, 2006; Nicholson et al, 2001; Sumnall et al, 2008). GHB use as a recreational drug is prevalent among young people, particularly high school and college students (Anderson et al, 2006; Camacho et al, 2005; Rodgers et al, 2004; Wong et al, 2004). According to results of the 2007 Monitoring the Future survey by the National Institute on Drug Abuse, 0.7, 0.6, 0.9 percent of students in the 8th, 10th and 12th grade, respectively, reported having used GHB during the past year.
GHB is a short-chain fatty acid found endogenously in the brain (Cash et al, 1979). It is synthesized from γ-aminobutyric acid (GABA) (Maitre, 1997). Following systemic administration, GHB rapidly crosses the blood brain barrier resulting in CNS-mediated effects. Since subcellularly GHB is located within the presynaptic terminal, it is thought to play a role as a neurotransmitter/neuromodulator (review by Snead and Gibson, 2005). Precise mechanisms underlying GHB actions have not been established, but evidence supports that GHB acts via several different receptors including the GABAB receptor (Carai et al, 2001). GHB also binds to specific brain receptors that have a distinct anatomical distribution pattern with high densities of [3H]GHB binding sites located in the hippocampus along with dentate gyrus, frontal cortex, septum, nucleus accumbens and caudate-putamen, while areas such as the cerebellum, hypothalamus, and pons-medulla are devoid of any such binding (Andriamampandry et al, 2007; Benavides et al, 1982; Crunelli et al, 2006).
In humans, GHB is known to induce short-term anterograde amnesia (Carter et al, 2006; Li et al, 1998; Schwartz et al, 2000). This amnesia-causing effect of GHB plays a prominent role in drug-facilitated sexual assault (ElSohly and Salamone, 1999; Schwartz et al, 2000; Varela et al, 2004). Although GHB-induced memory impairments have been reported in humans, relatively few studies have looked at the effects of GHB on learning and memory in animals. In monkeys, GHB failed to have any effect on the go/no-go visual discrimination task (Nakamura et al, 1987). Navarro and coworkers studied the effects of chronic low doses of GHB (5–100 mg/kg for 12 – 30 days) on the “hole-board” task performance in adult mice, and reported that working memory was significantly reduced in GHB-treated mice compared to controls (Davila et al, 2004; Garcia et al, 2006; Luna et al, 2002). In another study, GHB was reported to decrease operant behavior but not working memory in adult rats (Laraway et al, 2008). Earlier, our laboratory has shown that repeated GHB exposure in adolescent male (Sircar and Basak, 2004) and female (Sircar et al, 2008) rats significantly impaired the acquisition of spatial learning and memory. The present study was undertaken to investigate the age-specific effects of GHB on the acquisition of spatial learning and memory in female rats.
Materials and Methods
Animals and housing conditions
Adolescent (PD 30 at the beginning of the study) and adult (PD 60 – PD 90) female Sprague-Dawley rats (Taconic, Germantown, MD) were housed in institutional IACUC-approved adult rat plastic cages that can comfortably hold three adult rats, in a temperature and humidity controlled room within the animal facility. Food and water were available ad libitum, and the colony was maintained on a 12-hr light–dark cycle with the lights on at 0600 AM. All experimental protocols were approved by the institutional animal review committee, and the research was conducted in accordance with the requirements of NIH Guide for Care and Use of Laboratory Animals (1996).
Drug
GHB (γ-hydroxybutyric acid sodium salt) was purchased from Sigma-Aldrich Corp. (St. Louis, MO).
GHB treatment
Cognitive effects of GHB were measured in adolescent and adult female rats. All rats were randomly assigned to one of two groups - saline or GHB. Thirty min prior to behavioral testing each rat was administered with a single intraperitoneal (ip) injection of GHB (100 mg/kg). Adolescent animals received single daily GHB injections on 6 consecutive days. Adult rats were injected with GHB for either (i) 6 days or (ii) 16 days, the reason being that saline-treated adult female rats take longer to learn the hidden platform task in the Morris maze (Sircar et al, 2006; Sircar et al, 2009). The dosage of GHB and route of administration used were as described before (Sircar and Basak, 2004). Control rats received isovolumetric ip injections of saline on the same days. Nine to twelve rats were used in each group at each age.
Behavioral testing
Water maze apparatus
The Morris water maze (MWM) used was as previously described (Sircar and Basak, 2004; Sircar et al, 2006; Sircar et al, 2009). The maze, a circular pool (1.8 m diameter, 52 cm deep) with its interior painted white, was located in the center of a room dedicated to measuring this behavioral paradigm. The water temperature was carefully maintained between 25–27°C with the help of a submersible digital water heating system (Cleveland Process Corporation, Homestead, FL). Water was made opaque by the addition of non-toxic white paint. The pool was divided into 4 virtual quadrants and a removable 15 cm escape platform was placed in the center of one of the quadrants, one cm below the water surface. Performance was recorded and analyzed using a video tracking system (HVS Image, Hampton, UK).
One day before water maze testing, all rats were habituated to the water, and taught to escape by climbing onto the platform by placing their forepaws on the platform. Each rat was given four climb-on trials, and once on the platform it was allowed to sit there for 5 sec. The position of the platform used during habituation was not used again either for the cued visual task or during reference memory testing. No data was collected and no drug was administered before, during or after habituation.
Experimental design
Behavioral testing protocols used were as described before (Sircar and Basak, 2004; Sircar et al, 2008; Sircar et al, 2009). On the first test day, each animal was tested in the cued visual task 30 min after GHB or saline injection. This was followed by daily injections and testing in the hidden platform paradigm. A GHB or saline injection was given thirty minutes prior to the first trial of the day. On the last day of the hidden platform task, each rat was subjected to a probe trial.
Visual cued task
Thirty min after GHB or saline injection, the rat was put through the visible platform paradigm. The escape platform was made “visible” by attaching a black flag to the platform. A black curtain encircled the pool preventing the animal from using extra-maze cues to find the platform. The subject was placed in the water facing the edge of the tank in one of four pre-selected positions. The order of start locations was varied in a quasi-random fashion such that in each block of four trials the subject started from each location only one time and never started from the same place on any four consecutive trials. Rats were allowed to swim until they located the platform or 45 sec had elapsed. If the rat did not find the escape platform by the end of 45 sec, it was gently guided to the platform or was placed on the platform. The subject remained on the platform for 15 sec. Performance in the cued visual task was used to control for swimming ability, sensorimotor functions as well as motivation.
Reference memory testing in the hidden platform paradigm
One day after the visual cued task, testing for spatial memory began. Adolescent rats received GHB or saline injection on five consecutive days, and were tested in the water maze 30 min post injection. Trials were similar to those described for the visual cued task, except that the location of the escape platform was not marked, and the black curtain was removed so that the animal could see and use the visual clues provided in the room for spatial mapping. For this phase of testing, the position of the platform remained constant on all 5 days, but was different from the position used for the visual cued task.
Adult rats were treated with GHB or saline and tested behaviorally for either 5 days (Experiment 1) or 15 days (Experiment 2). This was based on our prior experience that adult female rats take longer to learn the hidden platform task (Sircar et al, 2006; Sircar et al, 2009).
Probe trial
Four hours following the last reference memory trial, each rat was subjected to a probe trial. In the probe trial, the escape platform was removed from the pool and the rat was allowed to free swim for 45 sec. The purpose of the probe trial was to provide a method for evaluating the subject's knowledge of where the platform was located by quantifying the amount of time spent in the quadrant where the platform was previously situated. During the probe trial the rat was released from a novel starting location (directly opposite to the platform location). No GHB or saline injection was given prior to the probe trial. The time spent in the target quadrant, where the platform was located prior to its removal, and other quadrants were measured.
Data analysis
In Experiment 1, where both adolescent and adult rats were put through an identical behavioral protocol, a 3-way repeated measures ANOVA was used to statistically analyze the data. In Experiment 2, behavioral data from the adolescent and adult rats were analyzed separately since adult and adolescent behavioral models were different; the number of test days for adult rats was 16 days (1 day for cued visual task and 15 days in the hidden platform task) and in adolescent rats it was 6 days (1 day for cued visual task and 5 days in the hidden platform task). Statistical analyses were performed using commercial software (SPSS for Windows 11.5.1, SPSS Inc, Chicago, IL). The general linear model (GLM) for repeated measures with Boneferroni corrections and pairwise comparisons, was used to analyze the latency, distance and swim speed data. Mixed model approach RM ANOVA was also used to analyze the probe trial quadrant data with percent time spent in each quadrant used as the repeated measure. When there were no repeated measures, either a one-way ANOVA or an unpaired, two-tailed t test was used (Prism 5.02 for Windows; GraphPad Software Inc., San Diego CA). The level of significance was set at p < 0.05.
Results
Experiment 1 Adolescent and adult rats tested in the same 5-day reference memory task
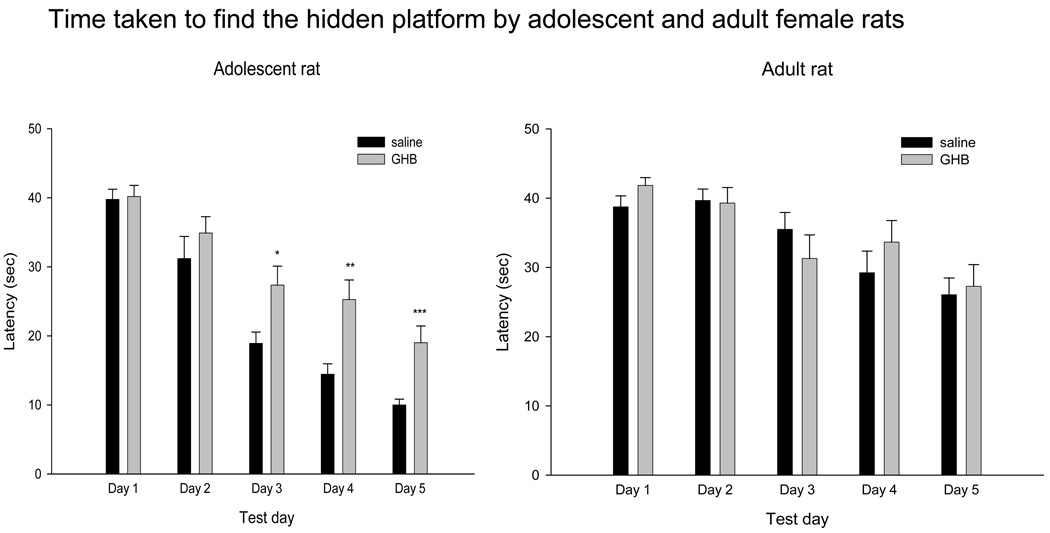
Adolescent and adult female rats were injected with a single dose of GHB (100mg/kg) or saline and tested 30 min later in the hidden platform water maze escape behavior on five consecutive days. Escape latency was subjected to RM ANOVA analyses using a mixed model approach for the longitudinal and between-drug interactions, as well as between age group patterns. The three-way interaction of age group × test day × treatment group was explored to determine if the difference between control and GHB treated animals across the 5 days was different for adolescents and adult rats. The “within subjects” factor (the repeated factor) was test day, with age group and treatment group as the “between subjects” factors. The three-way interaction of age group × test day × treatment group was not statistically significant (F(1,44) 0.919, p < 0.343). Although the 3-way interaction was not statistically significant, upon inspection treatment × test day appeared different in adolescent and adult rats (Fig 1). In addition, the adult control rats appeared not to have learned the task. Therefore, the data was then analyzed separately for each age. Compared to age-matched saline controls, adolescent female GHB-treated rats took significantly longer to find the escape platform (F (1,22) 4.22, p = 0.05). In adult females, there was no difference in latencies to find the hidden platform between GHB-treated and saline-treated rats (F (1,22) 0.101, p = 0.754). Fig 1 shows the mean latency (collapsed across four trials) to reach the hidden platform by adolescent (left panel) and adult (right panel) saline–treated and GHB-treated rats.
Figure 1.
Effect of GHB on escape latency on days 1 through 5 of hidden platform task in the Morris water maze by adolescent (left panel) and adult (right panel) rats. The latency to find the hidden platform was significantly higher in GHB exposed adolescent rats than saline-treated same-age controls. There was minimal effect of GHB on the latency to find the hidden platform in adult rats. Values indicate mean ± sem. *p<0.05, **p<0.01, ***p<0.001 compared to saline-treated same-test day, same-age rats.
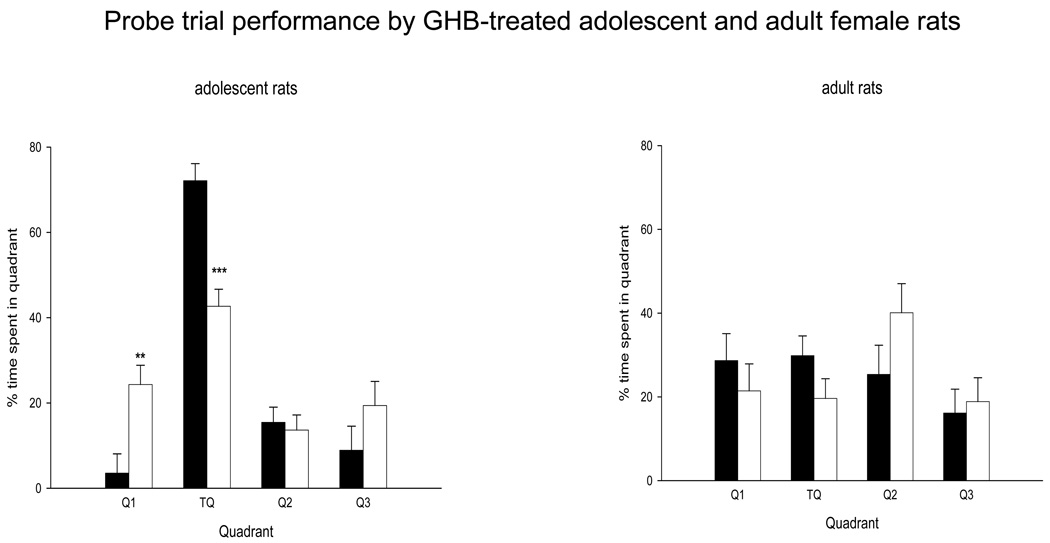
In the probe trial, there was significant treatment effect on quadrant time in adolescent rats (F (1,10) 17.202, p = 0.001). GHB-treated adolescent females spent significantly less time in the target quadrant compared to saline-treated rats. In adult female control rats, there was no difference in the time spent in the target quadrant compared to the other quadrants indicating that they had failed to learn the task. There was no treatment effect in adult female rats (F (1,10) 1.091, p = 0.407). Fig 2 shows the probe trial data from adolescent (left panel) and adult (right panel) saline-treated and GHB-treated rats.
Figure 2.
Effect of adolescent GHB on the probe trial performance in adolescent (left panel) and adult (right panel) rats following 5 days of testing in the hidden platform task. Percent time spent by GHB-treated adolescent rats in the target quadrant was significantly less than same-age saline controls. There was no difference in the % time spent in each quadrant between GHB- and saline-treated adult rats. Data shown are mean ± sem. **p<0.01, ***p<0.001 compared to percent time spent in the target quadrant by same-age rats.
Experiment 2 Effects of GHB on spatial learning and memory in adolescent and adult rats
In Experiment 1, adolescent and adult rats were treated with GHB or saline and trained in an identical 5-day Morris water maze reference memory task. By Day 5, adolescent saline-treated rats had learned the task, and compared to controls, GHB-treated rats showed significant memory deficits. Unlike adolescent rats, adult saline-treated control rats failed to learn the task, and GHB did not have any significant effect on any of the behavioral test parameters. Therefore in Experiment 2, two different training models were used to ensure that learning had taken place at both ages before drug effects were investigated.
a. Adolescent GHB on cued visual task performance
To determine whether visual and motor coordination were compromised by repeated GHB treatment, adolescent rats were tested in the cued visual task. The latency to find the platform, distance traveled and the speed with which GHB- and saline-treated rats reached the platform were measured and shown in Table I. Univariate analysis of variance was used to compare each test parameter between saline-treated and GHB-treated animals. There was no treatment effect on any of the test parameters - latency (F(1,21) = 0.327 p = 0.573), path length (F(1,21) = 0.123 p = 0.729) or swim speed (F(1,21) = 0.315 p = 0.581).
TABLE I.
Cued visual task performance by adolescent rats
| Treatment | latency (sec) | path length (cm) | swim speed (cm/sec) |
|---|---|---|---|
| saline (n=12) | 6.48 ± 0.45 | 155.52 ± 12.80 | 24.04 ± 0.81 |
| GHB (n=11) | 6.91 ± 0.61 | 149.08 ± 13.13 | 23.32 ± 0.99 |
Values are mean ± sem.
b. Adolescent GHB on the hidden platform water maze escape behavior
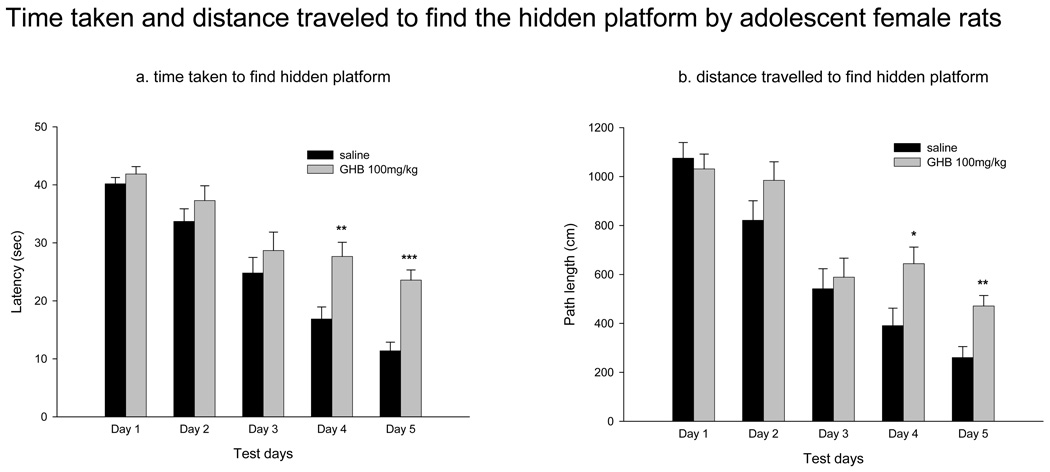
Adolescent female rats were injected daily with a single dose of GHB (100 mg/kg) for five days starting on PD 31, and tested 30 min later in the hidden platform version of the water maze task. Escape latency, path length, and swim speed data were subjected to repeated measures ANOVA analyses with treatment as the variable. Fig 3 shows the average latency (Fig 3a) and path length (Fig 3b) to reach the fixed platform by adolescent GHB-treated rats and saline controls. Repeated measures ANOVA for both latency (F(4,19) = 4.712 p = 0.008) and path length (F(4,19) = 2.996 p = 0.046) indicated that treatment was a significant variable in the regression analysis. GHB-treated rats performed significantly worse than saline-treated rats on test days 4 and 5. To test whether the deficit was not a reflection of differences in motor function, speeds at which saline-treated and GHB-treated rats swam were analyzed (Table II). There was no difference in the swim speed between experimental and control animals (F(4,19) = 0.346 p = 0.844).
Figure 3.
Deficits in performance by GHB-treated adolescent rats compared to saline-treated adolescent females in the Morris water maze hidden platform task. Starting on PD 30, each rat received a single daily injection of GHB (100 mg/kg, ip) 30 min prior to testing in the MWM. Control rats received equivalent volumes of saline. The latency (a) and path length (b) to find the hidden platform were significantly greater in GHB treated rats than saline-treated controls. Values indicate mean ± sem. *p<0.05, **p<0.005, ***p<0.001 compared to same-parameter, same-test day saline-treated controls.
TABLE II.
Adolescent swim speed (cm/min) in the hidden platform task
| Treatment | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|
| saline (n=12) | 25.88 ± 0.57 | 24.03 ± 1.07 | 23.55 ± 0.74 | 23.29 ± 0.70 | 21.68 ± .58 |
| GHB (n=11) | 26.56 ± 1.39 | 23.32 ± 2.25 | 21.03 ± 1.05 | 21.94 ± 1.10 | 20.87 ± 1.08 |
Values are mean ± sem
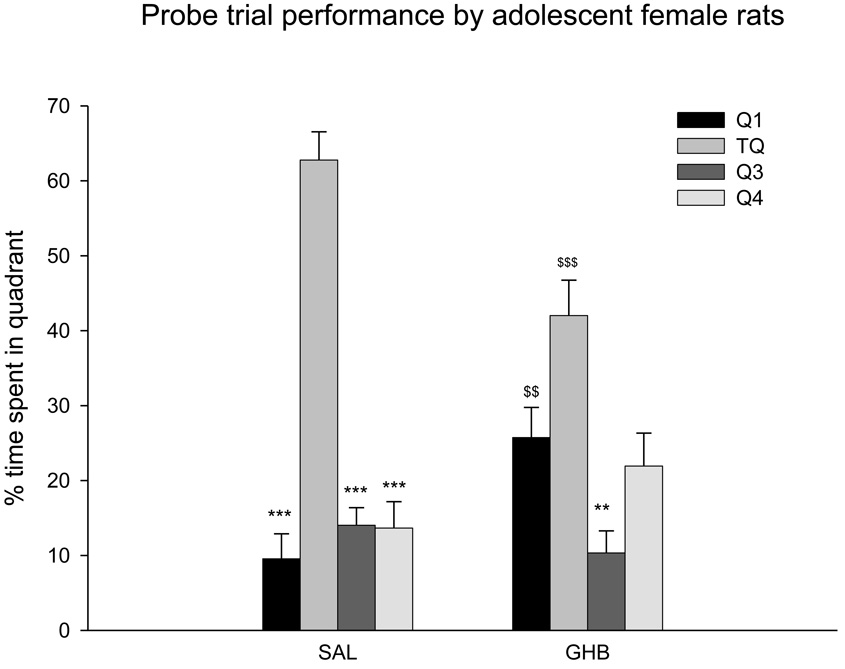
c. Adolescent GHB on probe trial performance
Repeated measures ANOVA with quadrant locations as the repeat measure, was used to analyze these data. There was significant treatment effect on the time spent in the target quadrant where the platform was located prior to its removal and other quadrants (F(3,14) = 5.195 p = 0. 013). GHB-treated rats spent less time (42.11 ± 4.72%) in the target quadrant compared to time spent by saline-treated rats (62.77 ± 3.76%). Results from the probe trial are shown in Fig 4.
Figure 4.
Effect of GHB on the probe trial performance by adolescent female rats. The platform was removed from the MWM and the percent time spent by each rat in the target quadrant (TQ), where the platform was located prior to its removal, was compared to the time spent in the other quadrants (Q1, Q2, Q3). Saline-treated adolescent rats spent significantly more time in the target quadrant than GHB-treated rats. Values indicate mean ± sem. ***p<0.001 compared to percent time spent in the target quadrant by saline-treated rats, **p<0.001 compared to percent time spent in the target quadrant by GHB-treated rats, $$$p<0.001 compared to percent time spent in the target quadrant by saline-treated rats, $$p<0.005 compared to percent time spent in the Q1 quadrant by saline-treated rats.
d. Adult GHB on cued visual task performance
As with adolescent rats, GHB in adult rats did not affect any of the test parameters in the cued visual task (Table III). There was no treatment effect on any of the parameters - latency (F(1,22) = 0.862 p = 0.363), path length (F(1,22) = 0.645 p = 0.431) or swim speed (F(1,22) = 0.221 p = 0.643).
TABLE III.
Cued visual task performance by adult rats
| Treatment | latency (sec) | path length (cm) | swim speed (cm/sec) |
|---|---|---|---|
| saline (n=9) | 13.04 ± 1.54 | 253.76 ± 37.50 | 19.54 ± 0.92 |
| GHB (n=10) | 16.07 ± 2.88 | 310.00 ± 59.16 | 20.25 ± 1.20 |
Values are mean ± sem
e. Adult GHB on the hidden platform water maze escape behavior
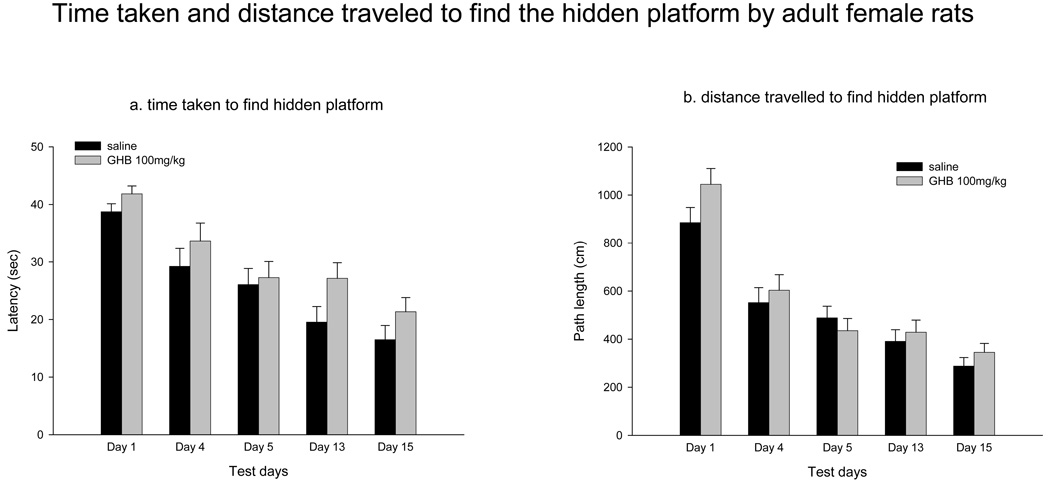
A different behavioral protocol was used to study the acquisition of spatial learning and memory in adult female rats than the one used for adolescent rats. Instead of the 5 days of behavioral testing, adult rats were tested for a longer period of time (15 days). Repeated measures ANOVA for latency (F(6,17) = 1.400 p = 0.271) and path length (F(6,17) = 1.334 p = 0.296) indicated no treatment effect, suggesting that adult GHB-treated rats did not perform any differently than saline-treated rats. The latency (Fig 5a) and path length (Fig 5b) traveled to reach the hidden platform by saline-treated and GHB-treated adults on select test days are shown in Fig 5. There was no effect of GHB on swim speed (F(6,17) = 1.387 p = 0.276); Table IV presents data from the first five test days.
Figure 5.
Performance by adult female rats in the hidden platform task (mean ± sem) following saline-treatment or GHB-treatment on select test days (Days 1, 4, 5, 13, 15). Rats were tested in the MWM 30 min after being injected (ip) with saline or 100 mg/kg GHB solution and (a) latency and (b) path length covered to reach to the hidden platform were recorded. There was no statistically significant difference between GHB-and saline-treated adult rats on any test day (see Results).
TABLE IV.
Adult swim speed (cm/min) in the hidden platform task
| Treatment | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|
| Saline (n=9) | 22.36 ± 1.17 | 19.55 ± 1.29 | 19.35 ± 0.98 | 19.84 ± 0.89 | 19.18 ± 0.60 |
| GHB (n=10) | 24.49 ± 1.10 | 23.44 ± 1.23 | 17.44 ± 1.45 | 18.29 ± 1.13 | 18.02 ± 1.11 |
Values are mean ± sem
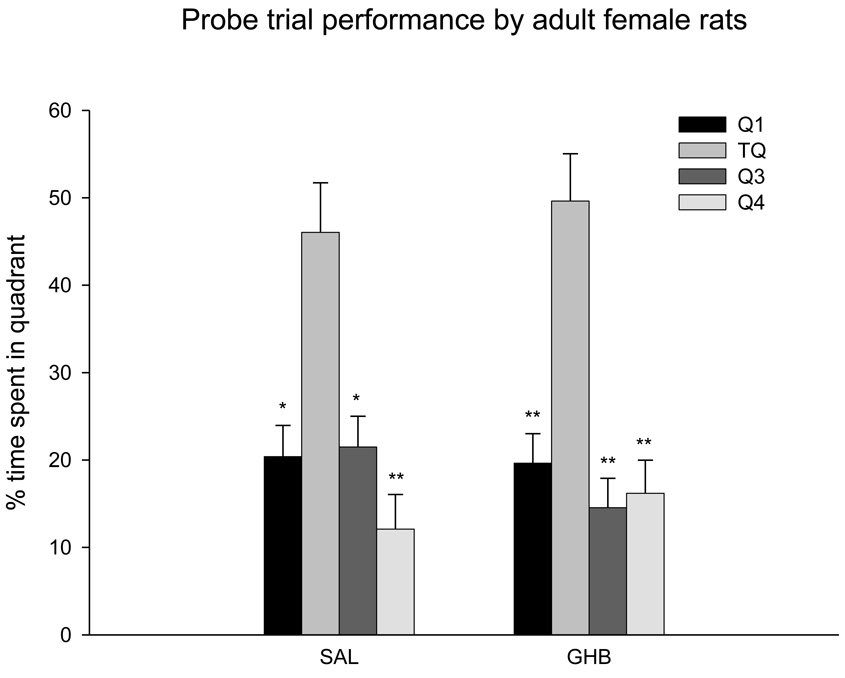
f. Adult GHB on probe trial performance
Both saline-treated and GHB-treated rats spent more time in the target quadrant compared to the other quadrants (F(3,18) = 6.60 p = 0.0003). There was no treatment effect on the time spent in each of the four quadrants between saline-treated and GHB-treated rats (F(3,18) = 2.027 p = 0.146). Probe trial data for adult animals is shown in Fig 6.
Figure 6.
Effect of GHB exposure on the probe trial performance in adult female rats. Both saline-treated and GHB-treated adult rats spent significantly more time in the target quadrant (TQ) than in the other quadrants (Q1, Q3, Q4). Time spent in the target quadrant by GHB-treated and saline-treated rats did not differ. *p<0.05, **p<0.005 compared to percent time spent in the target quadrant by same-treatment rats. Values indicate mean ± sem.
Discussion
The main finding of the study is that GHB causes age-specific disruptions in spatial learning and memory in female rats. Adolescent female rats exposed to GHB prior to acquisition displayed marked impairments in learning in a simple fixed position hidden platform task in the Morris water maze, without affecting locomotor activity or performance in the cued visual task. In the probe trial, GHB-exposed adolescent rats remained less time in the vicinity of the platform, before it was removed, than saline-treated adolescents. A second finding was that saline-treated adult female rats performed less well in the hidden platform task than saline-treated adolescent female rats. Older rats required more training days to learn the task than the younger rats. A third finding was that GHB failed to disrupt spatial learning and memory in adult females. GHB-treated adult female rats did not show any significant difference in the latency or path length to reach the hidden platform, or time spent in the target quadrant in the probe trial, than saline-treated adults. Thus in adolescent females GHB specifically disrupted acquisition of spatial learning. That was not the case with adult rats.
Repeated GHB treatment in adolescent female rats increased both time and distance traversed to find the hidden platform, indicating impairments in the acquisition of place learning i.e. performance of the reference memory (hidden platform) task. This is further supported by data obtained from the probe trial testing where the GHB-treated rats spent significantly less time in the target quadrant than the saline-treated adolescent controls. GHB did not alter any of the test parameters in the cued visual task, suggesting that impairments in the reference memory task performance were not due to compromised motor and/or visuoperceptive sensory functions. Rather it was the result of a proactive interference of spatial information processing in the performance of the task (Anisman et al, 2002). Previously, we have shown that GHB causes attenuation in the acquisition of spatial memory in adolescent male (Sircar and Basak, 2004) and female (Sircar et al, 2008) rats. Together, these data suggest that repeated GHB exposure attenuates acquisition of reference memory in adolescent rats of both sexes.
There was little impact of GHB on memory acquisition in adult female rats. Neither the time taken nor the distance traveled to reach the escape platform was affected by GHB in adult females. GHB-treated adults showed normal behavior in the probe trial, and like the saline-treated adult rats, spent greater time in the target quadrant than in the other quadrants. There was no difference in the time spent in the target quadrant by GHB-treated rats and saline-treated rats. In the cued visual task, GHB did not alter any of the test parameters (latency, pathlength or swim speed). Together, they indicate that GHB has minimal effects on the acquisition of spatial memory in adult female rats.
Acquisition of spatial memory was slower in adult rats compared to adolescent rats. The former required more training to learn the task than the latter (present study; Sircar et al, 2009). While the adolescent female rats had learned the reference memory task by the fifth test day, adult rats took several days more to learn. Since adolescent rats were peripubertal and supposedly had lower gonadal hormone levels than cycling adult females, one reason may be that gonadal hormones, particularly estrogen, may have negatively impacted the acquisition of place learning in the Morris water maze in adult female rats. This conclusion is supported by some studies (Warren and Juraska, 1997; Ziegler and Gallagher, 2005) but not others (Bucci et al, 1995; Harburger et al, 2007; Li et al, 2004). Rats in estrus, when estrogen is high, have been shown to perform better than rats in proestrus when estrogen level is low (Stackman et al, 1997; Warren and Juraska, 1997). Estrogen enhancement in spatial memory appears to be limited to working spatial memory and is not seen in the reference memory task (Luine et al, 2003; Sandstrom and Williams, 2001). Estrogen given to ovariectomized female rats failed to improve memory (Chesler and Juraska, 2000; Ziegler and Gallagher, 2005).
Intact adolescent and adult female rats were used in the present study. Effects of gonadal hormones and estrus cyclicity on GHB-induced learning in adult female rats were not investigated. Checking for estrus cyclicity would have required the examination of vaginal cytology, and that would have necessitated the use of vaginal manipulation. Female adolescent rat was not subjected to any vaginal manipulation since the vagina is not open at this age (Sircar, 1995). Since subjecting adult females to daily vaginal manipulation might have affected their behavioral performance, thereby making comparisons to the behavioral performance by adolescent female rats without vaginal stimulated difficult, adult female rats were not checked for vaginal estrous cyclicity. How gonadal (estrogen, progesterone) and stress hormones modulate GHB-induced behavioral deficits needs detailed investigation.
Earlier we have shown that GHB impairs cognitive functioning in male adolescent rats. Here we report that GHB also attenuates spatial learning and memory in adolescent female rats. We have further shown significant differences in GHB-induced effects on the acquisition of spatial memory between adolescent and adult female rats. Future studies will look at the gender-specificity of ontogenic differences in GHB-induced deficits in cognitive function.
Acknowledgments
This research project was supported by PHS Grant DA 018234 (RS). We wish to thank Ms. Barbara Napolitano, Assistant Director, Biostatistics, Feinstein Institute for Medical Research, for help with statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature references
- Anderson IB, Kim SY, Dyer JE, Burkhardt CB, Iknoian JC, Walsh MJ, Blanc PD. Trends in gamma-hydroxybutyrate (GHB) and related drug intoxication: 1999 to 2003. Annals of Emerg Med. 2006;47:177–183. doi: 10.1016/j.annemergmed.2005.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, McIntyre DC. Conceptual, spatial and cue learning in the Morris water maze in fast or slow kindling rats: Attention deficit comorbidity. J Neurosci. 2002;22:7809–7817. doi: 10.1523/JNEUROSCI.22-17-07809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriamampandry C, Taleb O, Kemmel V, Humbert JP, Aunis D, Maitre M. Cloning and functional characterization of a gamma-hydroxybutyrate receptor identified in the human brain. FASEB Journal. 2007;21:885–895. doi: 10.1096/fj.06-6509com. [DOI] [PubMed] [Google Scholar]
- Barker JC, Harris SL, Dyer JE. Experiences of gamma hydroxybutyrate (GHB) ingestion: a focus group study. J Psychoactive Drugs. 2007;39:115–129. doi: 10.1080/02791072.2007.10399870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides J, Rumigny JF, Bourguignon JJ, Wermuth CG, Mandel P, Maitre M. A high-affinity, Na+-dependent uptake system for γ-hydroxybutyrate in membrane vesicles prepared from rat brain. J. Neurochem. 1982;38:1570–1575. doi: 10.1111/j.1471-4159.1982.tb06634.x. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Chiba AA, Gallagher M. Spatial learning in male and female Long-Evans rats. Behavioral Neurosci. 1995;109:180–183. doi: 10.1037//0735-7044.109.1.180. [DOI] [PubMed] [Google Scholar]
- Camacho A, Matthews SC, Murray B, Dimsdale JE. Use of GHBB compounds among college students. Am J Drug Alcohol Abuse. 2005;31:601–607. doi: 10.1081/ada-200062545. [DOI] [PubMed] [Google Scholar]
- Carai MAM, Colombo G, Brunetti G, Melis S, Serra S, Vacca G, Mastinu S, Pistuddi M, Solinas C, Cignarella G, Minardi G, Gessa GL. Role of GABAB receptors in the sedative/hypnotic effects of γ-hydroxybutyric acid. Eur J Pharmacol. 2001;428:315–321. doi: 10.1016/s0014-2999(01)01334-6. [DOI] [PubMed] [Google Scholar]
- Cash CD, Maitre M, Mandel P. Purification from human brain and some properties of two NADPH-linked aldehyde reductase which reduce succinic semialdehyde to 4-hydroxybutyrc acid. J Neurochem. 1979;33:1169–1175. doi: 10.1111/j.1471-4159.1979.tb05261.x. [DOI] [PubMed] [Google Scholar]
- Carter LP, Richards BD, Mintzer MZ, Griffiths RG. Relative abuse liability of GHB in humans: A comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital and GHB. Neuropsychopharmacol. 2006;31:2537–2551. doi: 10.1038/sj.npp.1301146. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial Morris water maze in ovariectomized rats. Hormones Behav. 2000;38:234–242. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Emri Z, Leresche N. Unravelling the brain targets of gamma-hydroxybutyric acid. Curr Opinion Pharmacol. 2006;6:44–52. doi: 10.1016/j.coph.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila G, Garcia F, Pedraza C, Luna G, Martin M, Navarro JK. Effects of γ-hydroxybutyric acid (GHB) on memory tested in the hole-board in female mice. FENS. 2004 Abstr 2: A150.9. [Google Scholar]
- ElSohly MA, Salamone SJ. Prevalence of drugs used in cases of alleged sexual assault. J Anat Toxicol. 1999;23:141–146. doi: 10.1093/jat/23.3.141. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Frederick SL, Staggers FE, Gonzales M, Stalcup A, Smith DE. Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence. Addiction. 1997;92:89–96. [PubMed] [Google Scholar]
- Garcia FB, Pedraza C, Arias JL, Navarro JF. Effects of subchronic administration of gammahydroxybutyrate (GHB) on spatial working memory in rats. Psicothema. 2006;18:519–524. [PubMed] [Google Scholar]
- Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2007;28:602–610. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Laraway S, Snycerski S, Baker LE, Poling A. Gamma-hydroxybutyrate (GHB) reduces operant behavior without impairing working memory in rats responding under fixed-consecutive-number schedules. Pharmacol, Biochem Behav. 2008;88:205–212. doi: 10.1016/j.pbb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Leichti ME, Greminger P, Speich R, Kupferschmidt H. Clinical features of γ-hydroxybutyrate and γ-hydroxybutyrolactone toxicity and concomitant drug and alcohol use. Drug Alcohol Depend. 2006;81:323–326. doi: 10.1016/j.drugalcdep.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female rats. PNAS. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Stokes SA, Woeckener A. A tale of novel intoxication: a review of the effects of gamma-hydroxybutyric acid with recommendations for management. Ann. Emerg. Med. 1998;31:729–736. [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinol. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luna G, García F, Pedraza C, Dávila G, Martín M, Navarro JF. Effects of gammahydroxybutyric acid (GHB) on memory tested in the hole-board in male mice. Eur Neuropsychopharmacol. 2002;12 Suppl 3:389. [Google Scholar]
- Maitre M. The γ-hydroxybutyrate signaling system in brain: organization and functional implications. Prog Neurobiol. 1997;51:337–361. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Myslobodsky MS, Coppola R, Johannesen-Conway J, Mirsky AF. Effects of γ-hydroxybutyrate on the performance of go/no-go visual discrimination task. Behav Brain Res. 1987;26:19–27. doi: 10.1016/0166-4328(87)90012-x. [DOI] [PubMed] [Google Scholar]
- Nicholson KL, Balster RL. GHB: a new and novel drug of abuse. Drug Alcohol Depend. 2001;63:1–22. doi: 10.1016/s0376-8716(00)00191-5. [DOI] [PubMed] [Google Scholar]
- Rodgers J, Ashton CJ, Gilvarry E, Young AH. Liquid ecstacy: a new kid on the dance floor. Br J Psychiatry. 2004;184:104–106. doi: 10.1192/bjp.184.2.104. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CJ. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neuroscience. 2001;115:384–393. [PubMed] [Google Scholar]
- Schwartz RH, Milteer R, LeBeau MA. Drug-facilated sexual assault (’date rape’) South Med J. 2000;93:558–561. [PubMed] [Google Scholar]
- Sircar R. Chronic postnatal phencyclidine administration in female rat delays onset of puberty but has no effect on pentylenetetrazol-induced seizure-susceptibility. Brain Res. 1995;694:318–321. doi: 10.1016/0006-8993(95)00827-d. [DOI] [PubMed] [Google Scholar]
- Sircar R, Basak A. In adolescent rats GHB-induced deficits in spatial learning and memory is associated with NMDA receptor dysregulation. Pharmacol Biochem Behav. 2004;79:701–708. doi: 10.1016/j.pbb.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Sircar R, Basak AK, Sircar D. GHB alters spatial learning and memory in an age-specific manner. Annual Meeting of Soc for Neurosci. 2006 Abs 194.21. [Google Scholar]
- Sircar R, Basak AK, Sircar D. γ-hydroxybutyric acid-induced cognitive deficits in the female adolescent rat. Ann NY Acad Sci. 2008;1139:386–389. doi: 10.1196/annals.1432.044. [DOI] [PubMed] [Google Scholar]
- Sircar R, Basak AK, Sircar D. Repeated ethanol exposure affects the acquisition of spatial memory in adolescent and adult female rats. Behavioural Brain Res. 2009;202:225–231. doi: 10.1016/j.bbr.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead OC, 3rd, Gibson KM. Gamma-hydroxybutyric acid. N Engl J Med. 2005;352:2721–2732. doi: 10.1056/NEJMra044047. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Blasberg ME, Langan CJ, Clark AS. Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiol Learn Mem. 1997;67:167–171. doi: 10.1006/nlme.1996.3753. [DOI] [PubMed] [Google Scholar]
- Sumnall HR, Woolfall K, Edwards S, Cole JC, Beynon CM. Use, function, and subjective experiences of gamma-hydroxybutyrate (GHB) Drug & Alcohol Dependence. 2008;92:286–290. doi: 10.1016/j.drugalcdep.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Varela M, Nogue S, Oros M, Miro O. γ-hydroxybutyric acid use for sexual assault. Emerg Med J. 2004;21:255–256. doi: 10.1136/emj.2002.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behavioral Neurosci. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Wong CG, Gibson KM, Snead OC. From the street to the brain: neurobiology of the recreational drug γ-hydroxybutyric acid. Trends Pharmacol Sci. 2004;25:29–34. doi: 10.1016/j.tips.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Gallagher M. Spatial memory in middle-aged female rats: Assessment of estrogen replacement after ovariectomy. Brain Res. 2005;1052:163–173. doi: 10.1016/j.brainres.2005.06.006. [DOI] [PubMed] [Google Scholar]