Abstract
In this paper, we report a novel method for fabricating ion-selective membranes in poly(dimethylsiloxane) (PDMS)/glass-based microfluidic preconcentrators. Based on the concept of capillary valves, this fabrication method involves filling a lithographically patterned junction between two microchannels with an ion-selective material such as Nafion resin; subsequent curing results in a high aspect-ratio membrane for use in electrokinetic sample preconcentration. To demonstrate the concentration performance of this high-aspect-ratio, ion-selective membrane, we integrated the preconcentrator with a surface-based immunoassay for R-Phycoerythrin (RPE). Using a 1×PBS buffer system, the preconcentrator-enhanced immunoassay showed an approximately 100× improvement in sensitivity within 30 minutes. This is the first time that an electrokinetic microfluidic preconcentrator based on ion concentration polarization (ICP) has been used in high ionic strength buffer solutions to enhance the sensitivity of surface-based immunoassay.
Introduction
Blood samples contain a large diversity of proteins that are relevant to disease conditions. However, the concentration of these proteins varies widely (ranging from pg/mL to mg/mL), making them difficult to detect and quantify.1 Detecting low abundance protein molecules has been a continuing challenge, and a number of technologies have been developed for the detection of analytes present at low concentrations. More sensitive sensor technologies, such as surface plasmon resonance (SPR), laser induced fluorescence (LIF), and nanowire sensors offer increased sensitivity for detecting biomolecules2, but these sensor systems generally require high-quality antibodies. For a given target protein molecule, obtaining high quality, low-KD antibodies is not at all straightforward. One possible solution is to increase the pre-binding concentration by preconcentrating the analyte molecules to increase the occurrence of binding events and thus, decrease reliance on the KD value of capture antibodies.3
Our group has implemented electrokinetic preconcentration in different microfluidic chip formats. A silicon/glass device with two microchannels interconnected by nanochannels was used to electrokinetically trap molecules as the nanochannels act effectively as an ion-selective membrane.4 However, these devices required extensive micro fabrication steps and the permselectivity of nanochannels become progressively weaker with increasing ionic buffer strength. A number of poly(dimethylsiloxane) (PDMS) microfluidic chips have been developed to solve these problems.5–8 Nafion, a highly porous ion-selective material, has been used in place of nanochannels to provide high permselectivity. Lee et. al. has previously demonstrated a surface-patterned Nafion membrane, where a submicron thin layer of permselective Nafion resin was printed on a glass substrate and enclosed with a PDMS cover by plasma bonding.6 An alternative method was demonstrated by Kim et. al., where a mechanical incision was made into the PDMS chip with a blade and Nafion resin was infiltrated into the cut after opening it through bending.8 Nafion membrane made with this incision method offered high-aspect-ratio and increased sample throughput; however, this cutting method is difficult to automate and prone to process variability during fabrication. One critical issue of the self-sealing method is the uncontrolled amount of Nafion resin introduced into the junctions. This could lead to variations in operation parameters and performance of preconcentration devices. To solve this problem, we present a new fabrication method based on capillary burst valves that produce high-aspect-ratio Nafion membranes with tightly controlled location, width and length. The main advantages of this technique over existing methods are; 1) precise, repeatable positioning of the membrane junction that can be filled with controllable amount of permselective resin, and 2) applicability to more durable plastic chip materials such as poly(methyl methacrylate) (PMMA) and COC (cyclic olefin copolymer).
The key idea behind this technique is controlling liquid (Nafion) flow using one or more capillary valves, followed by curing of the liquid resin into solid. Capillary valves belong to a class of microvalves known as passive valves9, valves that don’t require active actuation. In a capillary burst valve, a sudden geometrical expansion of the microchannel causes an increase in surface tension and traps the meniscus at the expansion. In our systems, this expansion is designed in a way to confine Nafion resin to an interconnecting funnel- or rectangular-type junction between two microchannels. Briefly, a filling front advancing in a straight channel with an angle α = 90 − θc (θc: contact angle) between the meniscus and the channel sidewall would stop at a junction with abrupt opening with an angle β, if β ≥ α (see S-1 in supplementary information). In this case, the change in angle is too large for the meniscus to overcome.10 This mechanism was used in our new fabrication method for building the electrokinetic concentrator and its efficacy was shown in an immunoassay experiment.
Experimental
Design and fabrication of ion-selective membrane junctions
Devices are made in PDMS using methods described elsewhere.11 Nafion (Sigma Aldrich, Co) is a charge-selective, porous polymer developed by DuPont. This material has highly charged sulfonate groups and a porous structure, allowing for excellent charge selectivity at various ionic strengths. We tested 5 wt%, 12.5% and 20 wt% Nafion resin. The contact angle measurement showed ~ 0° of 5 wt% resin on a glass substrate, while the contact angle increased to 48.9° on a PDMS substrate. In the case of 20 wt% Nafion resin, the contact angle increased to 35.7° on a glass substrate and 52.5° on a PDMS surface (see S-2 in supplementary information for contact angle measurement of Nafion resin on different substrates). In accordance with the results of contact angle measurements, we could easily fill a PDMS/glass microchannel with up to 12.5 wt% Nafion resin, while higher wt% Nafion showed low flowability inside the microchannel. Oxygen plasma treatment of the microchannel prior to bonding helps to increase its flowability.
The critical factor here is the balance between viscosity and structural integrity of the formed Nafion junction. A higher weight percent solution results in higher viscosity, which helps the capillary valves confine the Nafion resin to the junctions and also results in junctions with higher structural integrity that can withstand higher fluidic pressure. However, the downside to a high viscosity (high weight percent solution) is that the Nafion resin shows low flowability and can dry before it reaches the junctions in the channel. Lower Nafion content means high flowability in the channel, as implied by the contact angle measurements. However, shrinkage of the resin during the curing process can cause a leakage between sample and buffer channels. From our experiment, a 12.5 wt% Nafion resin solution has resulted in the best combination of yield and performance.
The devices discussed in this paper have a single and dual junction design. As past studies have shown, a dual-gated design provides a more stable and consistent performance in preconcentration.4 In dual-junction concentrator designs, the sample channel in the center is connected to the buffer channels via a funnel- or rectangular-type junction on each side. The funnel-type junction is 10 – 20 µm wide in the narrow opening and 50 µm in the wide opening at a length of 50 µm. The rectangular-type junctions are 10 µm wide and 50 µm long.
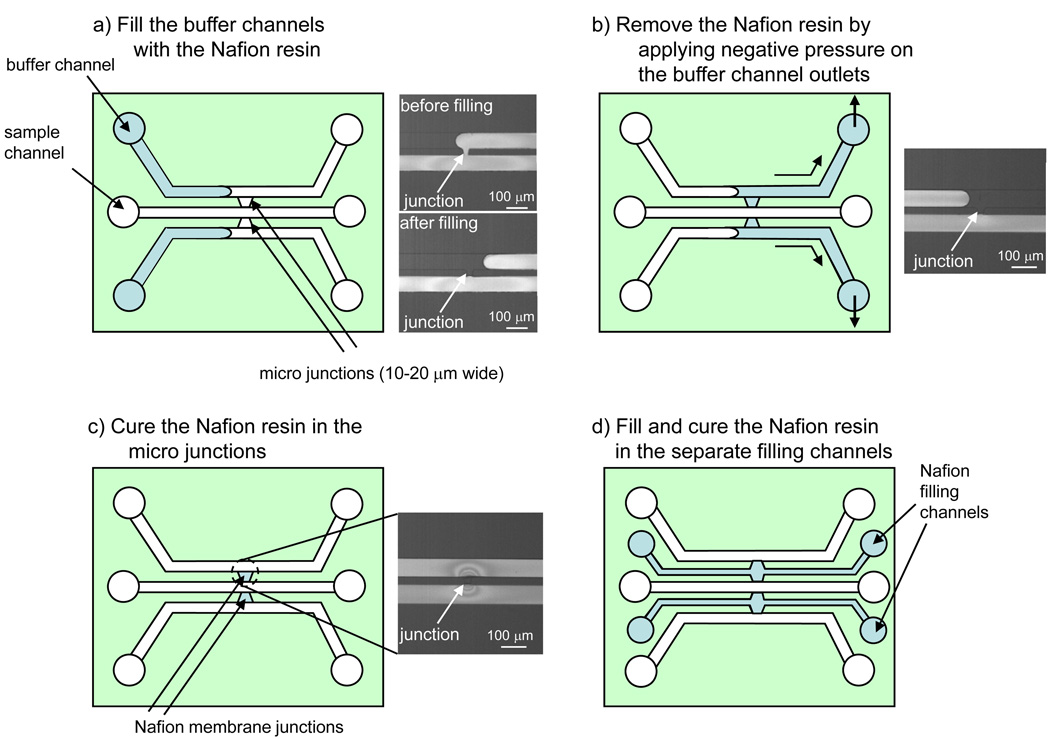
This capillary valve based fabrication method is schematically shown in Figure 1. Starting with a new device, 1 µL of Nafion resin was pipetted into each of the buffer channels and allowed to flow in via capillary force. The junctions should fill completely with capillary action (Figure 1a). At the narrow opening side of the junction, the sudden expansion to the large channel creates an angle large sufficiently high enough to stop the advancement of the Nafion meniscus (see supplemental video of the filling process). Then, a gentle negative pressure was applied on the channel outlets to remove excessive resin from the buffer channels (Figure 1b, see supplemental video of the removing process in the ESI). Nafion resin that has entered the junctions was cured rapidly due to the small openings to air-filled sample channel and therefore not withdrawn by negative pressure. In addition, the removal process left an additional, thickened, Nafion layer adjacent to the already cured membrane when the drying front passed the junction on its way out to the reservoir. After the Nafion resin was completely taken out of the channel, the device was baked on a hot plate at 70 °C for 10 min. A major drawback of this method is the added step to remove Nafion from the buffer channels which caused a variation in membrane thickness and, therefore, in amount of the electrical current with a coefficient of variance (CV) of ~20% (see S-3 in supplementary information for detailed characterization).
Fig. 1.
Schematic of the capillary-valve-based fabrication method: a) Filling the buffer channels with Nafion resin, b) removing excessive resin from the buffer channels by applying gentle negative pressure on the channel outlet, c) curing the trapped Nafion resin in the junctions on a hot plate. A leakage test with a FITC dye solution in the sample channel showed that the junctions were tightly sealed with the Nafion membrane. d) alternative fabrication method by using separate filling channels for the Nafion resin, step b) above can be eliminated as the resin can remain in the filling channels.
To alleviate this problem, we included an additional set of microchannels (Nafion filling channels) dedicated for the delivery of Nafion resin into the junctions (Figure 1d). Instead of being withdrawn, the excessive Nafion resin was simply left inside the Nafion filling channels and cured in the junctions. Since these channels were sandwiched between the sample and buffer channels, their width was limited to the spacing between those channels. In our current design, a width of 40 µm was used for these filling channels. These channels narrowed down to 10 µm near the junctions so that sufficient capillary pressure could be generated to fill the junctions properly. While these designs were more complicated, the filling operation was simpler – no withdrawal of Nafion was required after the junctions were filled. For this reason, we used this method to fabricate devices for immunoassay.
Depending on the performance requirements, we can pattern several junctions next to each other. Tradeoffs were examined in this study: the ability of a capillary valve to properly confine liquid is inversely proportional to the cross sectional area of the junctions; at the same time, junctions with larger cross sectional area result in lower electrical resistance and allows for lower voltages to be used in the devices. In our experiments, maximum repeatability was achieved when the width of each individual junction channel was limited to 10 µm, and numerically limited to three parallel channels separated by 10 µm. The total width of the Nafion junctions was described by summing the widths of all the individual junctions. Accordingly, the rectangular –shaped Nafion junctions used in this paper were 50 µm long and 30 µm wide each. Nafion resin residing in each junction can be re-wetted rapidly after injecting the sample and buffer solution into the sample and grounding channels. The trapping of air bubbles was not observed when the channels were rewetted.
Surface patterning of capture antibody and preconcentration operation
To demonstrate the performance of our preconcentrator, we integrated the concentrator with a surface immunoassay chip. Antibodies were immobilized using a simple physiosorption method12 in our device. This method was chosen because of its lack of interaction with electric fields and flow under experimental conditions. A layer of physiosorbed Neutravidin (Invitrogen, CA) was applied as an anchor for the subsequent layer of biotinylated biomolecules. In addition to the flexibility of working with any biotinylated molecules, the Neutravidin layer absorbed onto the hydrophobic surface of the PDMS channels protected the subsequent layer of molecules from denaturing and allowed for higher capture efficiency of antigen molecules.
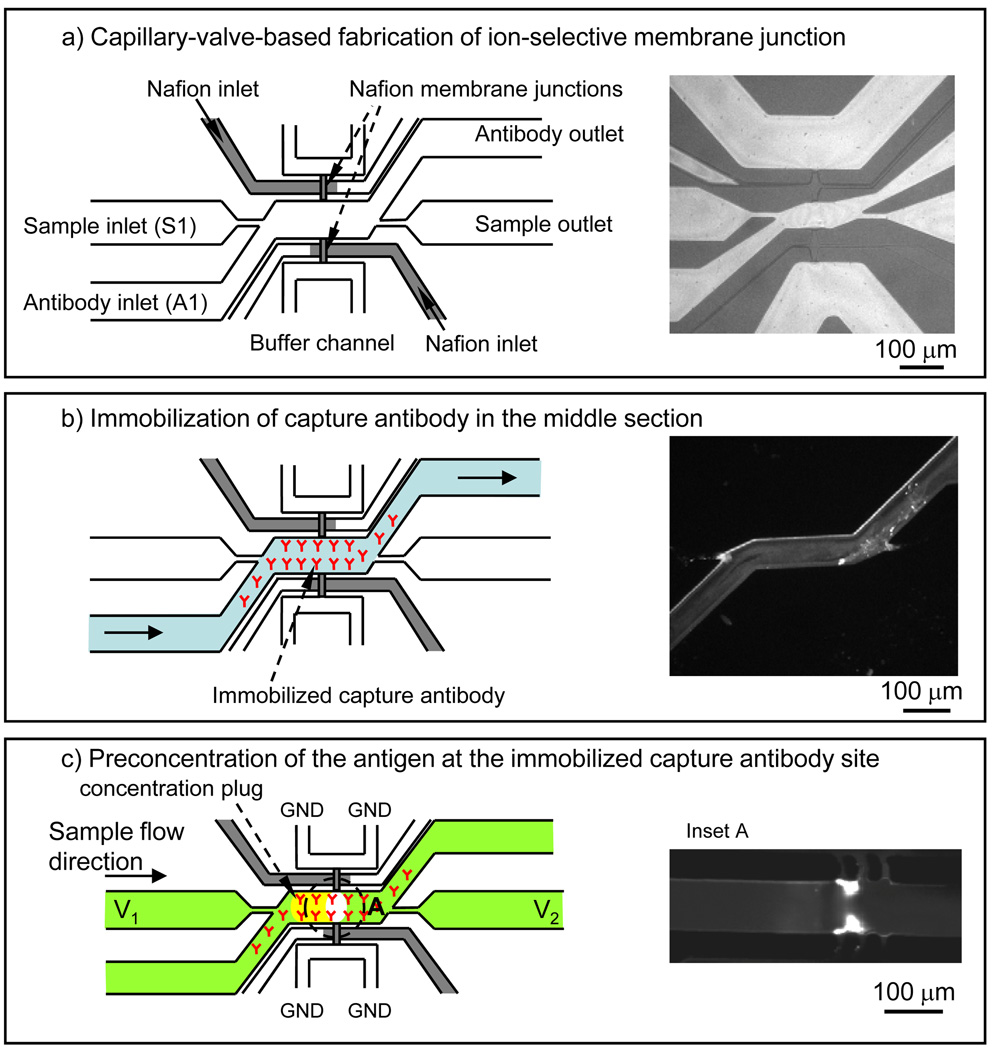
A critical design criterion for the surface immunoassay chip is prevention of sample depletion. Since the preconcentrator works by trapping molecules into a small volume, molecules that are captured elsewhere do not contribute to the added signal at the detection stage and the performance is affected. For this reason, capture antibodies need to be patterned in such a way that they only capture antigen molecules where the preconcentrator operates. To confine the antibody solution to a 200 µm × 50 µm section of the microchannel in proximity to the concentrator, we utilized the ability of hydrophobic microvalves for patterning.13 Hydrophobic microvalves rely on differential fluidic resistance in connected channels to direct flow. When liquid encounters a splitting channel, most or all of the liquid would enter the path of least resistance. Thus, we can create a valve by increasing the local fluidic resistance of one of the channels. We used channels of different widths to create differential resistance paths due to easier fabrication steps. In our devices, the sample channel was comprised of two intersecting channels, as shown in Figure 2a. The wider channel (A1) was used to functionalize the surface with an antibody and the other one (S1) to deliver sample to the concentrator. 30 µm wide, 50 µm long constrictions were placed on either sides of the channel S1; this allowed us to confine the antibody solution to A1 channel and selectively immobilize the capture antibody in the concentration zone of the sample channel S1.
Fig. 2.
Schematic and fabrication of an integrated preconcentrator and surface-based immunoassay in microfluidic format: a) Filling of the Nafion resin into the junctions and subsequent curing, b) the side channel on the bottom (A1) was used to coat the surface of the sample channel with antibodies. After immobilization of capture antibody, sample was injected through the top side channel (S1). Since this channel was not surface-functionalized with antibody except for the area around the concentrator, sample depletion was minimized, as evidenced by the lack of binding in those regions. The insert shows a fluorescence image of R-Phycoerythrin molecules binding to only the coated region of the channel, c) preconcentration of RPE on the capture antibody site.
To immobilize the capture antibody, 0.1 mg/mL solution of Neutravidin was flown into the channel A1 under a constant negative pressure and incubated for 10 min. in the sample channel. 0.01 mg/mL solution of biotinylated monoclonal anti-R-Phycoerythrin (BD Biosciences, CA) was then flown into the same channel (A1) and incubated for 10 minutes. A 1× phosphate buffered saline (PBS) was used to remove unbound molecules in between the incubation of each type of molecules. Finally, the surfaces of all channels were treated with 1% bovine serum albumin (BSA) for 5 min. to minimize non-specific binding. The devices were kept in 1× PBS buffer solution until use. As mentioned above, immunoassay samples were introduced through the non-functionalized channel (S1) to further minimize sample depletion.
Immunoassay experiments were carried out with a model protein, R-Phycoerythrin (R-PE), in 1× PBS buffer solution. 1 × PBS buffer solution was selected to model physiological ionic strength and used as a substitute for purified cell culture media or serum. Samples with different R-PE concentrations, varying from 0.01 ng/mL to 10 µg/mL, were prepared and incubated inside the channel for 30 min. to generate a calibration curve. Incubation for immunoassay without preconcentration was done in a constant flow condition at a flow rate of 1 µL/min using a syringe pump. We believe this flow rate was high enough such that binding would not be limited by axial flow, but rather be limited by diffusion at low concentrations and by reaction at high concentrations. Pressure-driven flow for preconcentration experiments was achieved with level difference of the sample solution in reservoirs across the sample channel. Typically, the difference in liquid level was 10mm, which generated a flow rate of ~50 pL/min inside the microchannel (see the calculation in S-4 of supplementary information). A voltage between V1 = V2 = 10 and 15 V was applied across the sample and buffer channels with a power supply (Keithley, OH). The application of voltage across Nafion junctions created a depletion zone, and pressure-driven flow in the sample channel moved analytes to the depletion zone, where the molecules were electrokinetically trapped and accumulated at the stacking boundary. Figure 2c illustrates this particular configuration for preconcentrator operations.
Results and Discussion
Preconcentrator performance
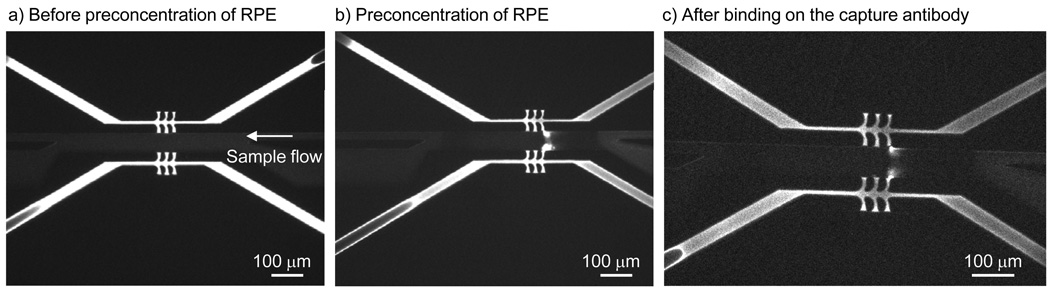
The factors that determine preconcentrator performance are flow speed and voltage applied. The flow speed determines how fast molecules are brought to the depletion zone, and the voltage applied across the sample/grounding channels determines the depletion force. Compared to the previously published electrokinetic concentrators, our preconcentrator with high-aspect-ratio permselective junctions presented here allowed for the use of pressure-driven flow instead of electroosmotic-driven flow. Several advantages can be gained with the use of pressure-driven flow. Firstly, the use of pressure-driven flow allows for a higher flow rate, which increases accumulation rate. Secondly, it allows preconcentration of protein samples in high ionic buffer solutions such as 1× PBS. Lastly, a high voltage at around 50–100 V is no longer required to drive liquid flow; this allows us to use a lower voltage to generate the depletion zone and at the same time to reduce the instabilities/vortexes generated near the preconcentration zone.14 The reduction in voltage, along with the use of pressure-driven flow, also decouples the electric field applied across the Nafion junctions and the electric field applied across the sample channel. This reduces the need for voltage adjustments and increases robustness in preconcentrator operation. In our experiments, flow rates of up to 10 nL/min were applied with the use of a syringe pump, and a stable stacking boundary could be maintained for ~30 minutes by applying just 15 V across the sample and ground channels. Preconcentration of R-Phycoerythrin is shown in Figure 3. In concentration devices with multiple nanochannels or Nafion junctions (which act effectively as nanochannels), the ion depletion region extends to the outermost nanochannel/Nafion junction and the stacking boundary for accumulating molecules would therefore only form at the outermost junction. In Figure 3c, the sample is flowing from the right outlet to the left outlet channel and therefore, the concentration region is forming at the third membrane junction.
Fig. 3.
Operation of the preconcentrator with pressure-driven flow in 1× PBS solution: a) initial condition of the channel, with uniform sample distribution. b) Depletion zone is generated with voltage application of 15 V across the Nafion junctions. c) Sample accumulation at the boundary of the stacking zone.
The shape of the preconcentration zone in the new devices was different from those shown in previously published results.3–6 Instead of a “plug-shaped” preconcentration zone, the concentrated plug was focused to areas around the Nafion junctions in “dot-shaped” regions similar to the result of Kim et al.15 (see Figure 3c). There are a number of possible explanations for the differences. First, the aspect ratio of the Nafion junctions presented in this system is different from those previously published. Due to this high aspect ratio, different amounts of depletion forces are likely produced by the new Nafion junctions and result in a different stacking pattern. Another possible reason is that the ionic buffer strength is 10 times higher than in previous experiments and this may result in a different concentration pattern. Further experiments would enable us to explore the effects of channel geometry and the behavior of electrokinetic preconcentration systems in high ionic strength buffer solutions. For additional characterization of the preconcentrator performance, we investigated the influence of flow speed and voltages by varying the voltage difference across the sample channel from Vdiff = V1 – V2 = 5 V to 15 V with an incremental step of 2.5V in 10mM phosphate buffer solution. This parameter study showed that a balance between flow speed and depletion force was well controlled between Vdiff = 7.5V and 10V (see S-5 in supplemental information).
Immunoassay results
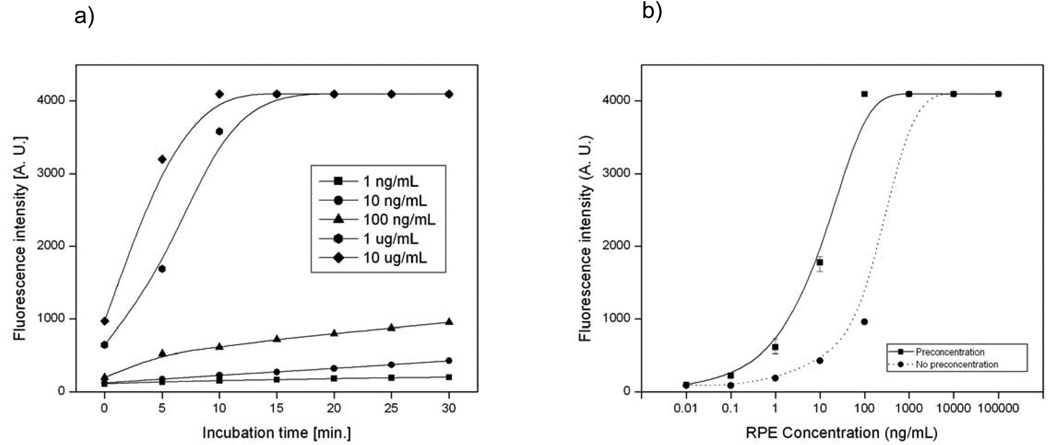
The advantage of a pre-binding amplification method (preconcentration) over a post-binding amplification method (e.g. ELISA) is that it improves both the sensitivity and the binding kinetics of the immunoassay. In order to quantify the performance of the preconcentrator, an immunoassay using the surface functionalization method described in the previous section was performed using R-Phycoerythrin as a model antigen. The intensity of R-PE captured on the surface was initially measured for 5 different concentrations (1 ng/mL, 10 ng/mL, 100 ng/mL, 1 µg/mL, 10 µg/mL) to generate a calibration curve. The samples showed negligible non-specific absorption when BSA was coated on areas not covered by antibodies, and a relatively uniform distribution on the surface was seen. This allowed for an averaged value on the surface to be taken anywhere along the channel. Figure 4a) shows the fluorescence intensity of R-PE as a function of incubation time. We can see that at low concentrations (< 1 µg/mL), binding of R-PE to capture agents is limited by kinetics and saturates at a very low rate.
Fig. 4.
a) time-lapse plots of R-PE at different concentrations., b) Immunobinding of R-PE compared with the one enhanced by preconcentration.
To quantify the performance of our dual-gated preconcentrator, we measured the increase in R-PE binding after 30 minutes of preconcentration. Figure 4b) shows a comparison between the performance of the immunoassay with and without the use of the preconcentrator. At initial concentrations of 100 ng/mL or more, the intensity saturated to a maximum within 10 minutes. In the case of 10ng/mL and 1ng/mL, the intensity improved by a factor of ~100 after 30 minutes of preconcentration. Two concentrations that were previously undetectable, 0.1ng/mL and 0.01ng/mL, became detectable after the sample was preconcentrated for a 30 minute period. With the preconcentrator, we demonstrated an increase of two orders of magnitude in intensity, and R-PE concentrations down to 10pg/mL became detectable. We used ImageJ to quantify the resulting fluorescence intensity of the concentrator-enhanced binding zone (see S-6 in supplementary information for detection protocol). In our experiments, we found that a voltage of 15 V, combined with a flow rate of ~50 pL/min resulted in the best conditions for preconcentration.
The improved detection sensitivity reported here was lower than some of the previously reported results (~350× – 1000×), including both the silicon devices and PDMS concentrators. This was likely due to the higher ionic strength buffer solution used in the experiments in this report. While low ionic strength buffers, such as 10mM phosphate buffers, were used in previous reports, we used 1× PBS solution in this experiment. Higher ionic strength buffers result in lower ion selectivity, which probably leads to a decreased sample trapping efficiency. To compare our device fabricated with the filling and curing method (see Fig. 1c) with other previously published ones, we measured the preconcentration factor of B-Phycoerythrin (B-PE) in 10mM phosphate buffer solution. We found out that we can achieve a concentration factor of ~1000× within 5 min. at a CV below 10% (see S-7 in supplementary information) which is comparable to that of the surface-patterned Nafion membrane.6 Since 1×PBS has the same ionic strength and pH as physiological fluids, it is a better media for simulating the conditions encountered with real biological samples such as serum. If we assume that we are given samples of the same ionic strength, a dilution would be required to get to a low ionic strength buffer such as those used in previous reports. When this dilution factor (~15×) is taken into account, we found that the preconcentration factors between the systems are actually similar. A lower voltage was also used in this report – we found that this led to greater preconcentration stability and thus made the system more amenable to automation, at the expense of decreased preconcentration efficiency.
Conclusions
A simple and highly efficient sample preconcentrator has been presented. Using the capillary-valve-based fabrication method, high-aspect-ratio membranes with controllable location, width and length can be integrated in junctions between microchannels and act as ion-permselective membranes. The major advantages of this new device are the ability to operate in high ionic strength buffers, such as 1× PBS buffer, and with pressure-driven flow in addition to its ease of fabrication. The use of pressure-driven flow allows the use of high ionic strength buffers, which more closely resemble physiological fluids and brings the preconcentration system closer to actual application in enhancing assay sensitivity with samples such as serum.
Supplementary Material
Acknowledgments
This work was mainly supported by NIH (CA119402, EB005743).
Footnotes
Electronic Supplementary Information (ESI) available: [Contact angle measurement of Nafion resin on different substrates, video clips showing Nafion filling and removing process, characterization of Nafion junctions]. See DOI: 10.1039/b000000x/
Notes and references
- 1.Anderson NL, Anderson NG. Mol. Cell. Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 2.Wild D. The Immunoassay Handbook. 3rd Edition. Elsevier; 2005. [Google Scholar]
- 3.Wang Y-C, Han J. Lab Chip. 2008;8:392–394. doi: 10.1039/b717220f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y-C, Stevens AL, Han J. Anal. Chem. 2005;77:4293–4299. doi: 10.1021/ac050321z. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Chung S, Kim SJ, Han J. Anal. Chem. 2007;79:6868–6873. doi: 10.1021/ac071162h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JH, Song Y-A, Han J. Lab Chip. 2008;8:596–601. doi: 10.1039/b717900f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JH, Song Y-A, Tannenbaum SR, Han J. Anal. Chem. 2008;80:3198–3204. doi: 10.1021/ac800362e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SJ, Han J. Anal. Chem. 2008;80:3507–3511. doi: 10.1021/ac800157q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh KW, Ahn CH. Journal of Micromechanics and Microengineering. 2006;16:R13–R39. [Google Scholar]
- 10.Zimmermann M, Hunziker P, Delamarche E. Microfluidics and Nanofluidics. 2008;5:395–402. [Google Scholar]
- 11.Mcdonald JC, Duffy DC, Anderson JR, Chiu DT, Wu H, Schueller OJA, Whitesides GM. Eletrophoresis. 2000;21:27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Rusmini F, Zhong Z, Feijen J. Biomacromolecules. 2007;8:1775–1789. doi: 10.1021/bm061197b. [DOI] [PubMed] [Google Scholar]
- 13.Ahn CH, Choi JW, Beaucage G, Nevin JH, Lee J-B, Puntambekar A, Lee JY. Proceedings of the IEEE. 2004;Vol 92(No.1):154–173. [Google Scholar]
- 14.Kim SJ, Wang Y-C, Lee JH, Jang H, Han J. Phys. Rev. Lett. 2007;99 doi: 10.1103/PhysRevLett.99.044501. 044501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SM, Burns MA, Hasselbrink EF. Anal. Chem. 2006;78:4779–4785. doi: 10.1021/ac060031y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.