Abstract
In an effort to optimize DNA immunization-elicited antibody production responses against Aβ1-42 (Aβ42) as a therapy for Alzheimer’s disease (AD), comparisons were made between three distinct plasmid systems using gene-gun delivery. Plasmids encoding Aβ42 monomer and a novel Aβ42 trimeric fusion protein were evaluated in conjunction with CMV or Gal4/UAS promoter elements. It was found that vaccination Aβ42 trimer under the Gal4/UAS promoter elicited high levels of anti-Aβ42 antibody production. Serum antibody levels from Gal4/UAS Aβ42 trimer-immunized mice were found to be 16.6 ± 5.5 μg/ml compared to 6.5 ± 2.5 μg/ml with Gal4/UAS Aβ42 monomer or even less with CMV Aβ42 trimer. As compared to monomeric Aβ42 or Aβ42 trimer expressed under the CMV promoter, injection of the Gal4/UAS Aβ42 trimer induced high levels of Aβ42 antigen expression in tissue suggesting a mechanism for the increase in anti-Aβ42 antibody. Antibodies were found to be primarily IgG1 suggesting a predominant Th2 response (IgG1/IgG2a ratio of 9). Serum from Aβ42 trimer-vaccinated mice was also found to identify amyloid plaques in the brains of APP/PS1 transgenic mice. These results demonstrate the potential therapeutic use of Gal4/UAS DNA Aβ42 trimer immunization in preventing Alzheimer’s disease.
Introduction
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder characterized by progressive memory loss and neuronal degeneration in cerebral cortex, hippocampus, and other subcortical structures. The pathological changes in AD brain include extracellular neuritic plaques and intracellular neurofibrillary tangles [1–3]. Amyloid beta 42 peptide (Aβ42) is the primary component of the neuritic plaques [4–6] and is considered to play an essential role in initiation of the pathogenesis of AD. Active immunization with full-length Aβ42 peptide in APP transgenic mice resulted in a substantial reduction of plaque burden with significant improvement in neuritic dystrophy and gliosis [7]. The clearance of Aβ42 following immunization also protected APP-Tg mice from developing memory deficits [8, 9]. The clinical trial with Aβ42 peptide immunization in AD patients demonstrated that only 20% of vaccinated patients developed a significant antibody response. The clinical trial was stopped because of the occurrence of meningoencephalitis in 6% of patients due to infiltration of autoreactive T cells in brain [10–13]. Based on pre-clinical and clinical studies, we have proposed using the gene-gun method for DNA Aβ42 immunizations as an alternative therapy for preventing AD [14].
We have previously reported that gene gun mediated DNA Aβ42 immunization elicited a Th2 biased immune response in mice [14]. In APPswe/PS1deltaE9 double transgenic AD mice, Aβ42 DNA immunization significantly prevented brain Aβ42 plaque formation [15, 16]. Antibody expression levels have been improved using Aβ42 DNA immunization with a cascade binary plasmid system [17, 18]. We demonstrate here that the binary vaccine system using yeast transcription factor Gal4 as a transactivator elicited increased levels of antibody production as compared to a CMV plasmid system. In addition, vaccination with a plasmid encoding a trimeric fusion protein of Aβ1-42 together with the Gal4 responsive promoter elements further improved antigen expression and production of anti-Aβ42 antibodies.
2. Materials and Methods
2.1. Mice
Four to 7-wk-old female BALB/c mice were purchased from the The Jackson Laboratory (Bar Harbor, Maine) and housed in a temperature and light-cycle controlled facility. APPswe/PS1deltaE9 transgenic mice (stock number 004462) were also purchased from The Jackson Laboratory. Animal use protocols were approved by the UT Southwestern Medical Center Animal Care and Use Committee (Dallas, TX).
2.2. Construction of plasmids
The Aβ42 monomer and trimer genes were chemically synthesized and cloned into the immunization vector system [14–16]. A set of complementary oligonucleotides of the Aβ42 DNA sequence were designed using the DNA builder program and custom synthesized (Sigma, St. Louis, MO). These oligonucleotides were designed after the respective Aβ42 amino acid sequence using multiple codons for a particular amino acid allowing a more flexible design of the nucleotide sequence to avoid hairpins, primer dimer structures and other inappropriate matches among the sequences which can hinder gene synthesis by Polymerase chain reaction (PCR). A total of 32 oligonucleotides (end concentration 250 μM) were mixed for the first PCR reaction to assemble them and built the designed gene sequence (30 cycles: 94°C for 15 s, 55°C for 30 s and 72°C for 45 s; Platinum®Taq DNA Polymerase, Invitrogen, Carlsbad, CA). A second PCR was used to amplify the full-length product using a forward and a reverse primer (30 cycles: 94°C for 15 s, 55°C for 30 s and 72°C for 45 s). PCR products from this second run were purified by gel electrophoresis, digested with restriction enzymes (Promega, Madison, WI ) and cloned into the polycloning site of the plasmid vector (EcoRI/XbaI digestion). Bacteria were transformed with the ligated plasmids and clones were identified by sequence analysis (Applied Biosystem, CA, Sequencing core of UTSW). An adenovirus E3 gene leader sequence [19] and an endosomal targeting sequence [20] were cloned up and down stream of the Aβ42 gene, respectively. For the control immunizations corresponding plasmids were constructed. Plasmid pGal4/UAS-Luc consists of the same binary plasmid system as pGal4/UAS-Aβ42 trimer or monomer but without the E3 leader and endosomal targeting sequence, in which the transcription of the Luc gene is driven by binding of the Gal4 transcription factor. In pCMV-Luc, transcription is driven by a CMV promoter [21, 22].
2.3. Transfection of cells, ear skin and detection of expressed proteins by Western blot and ELISA
HEK293 cells (8 × 105) were transiently transfected with 2 μg plasmid DNA by Fugene 6 Reagent (Roche, Indianapolis, IN) or by gene gun bombardment at 200 psi (3 shots with 1 μg plasmid). After 24 h of growth (37 °C, 10% CO2) in Dulbecco’s Modified Eagles Medium supplemented with 10% heat inactivated Fetal Bovine Serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine (Invitrogen), the cells were washed with PBS, proteins were extracted with reporter lysis buffer (Promega), and protein concentrations were determined using a NanoDrop® Spectrophotometer. Cell lysates were resolved in 4–20% SDS-PAGE followed by Western Blot analysis to detect expressed Aβ trimer or monomer. An anti-Aβ1–16 monoclonal antibody (6E10, Signet, MA) was used for detection of the expression of Aβ42 trimer or Aβ42 monomer, and an anti-Gal4 polyclonal antibody (SC-729, Santa Cruz, CA) was used for the detection of Gal4 expression. Cell lysates transfected with control vector pUAS-Luc or CMV-Luc were used as the negative controls. For in-vivo expression of Aβ42, the mouse ear was transfected with Luc or Aβ42 constructs by gene gun (300 psi, 1 μg in each side of the ear). Ears were collected 24 hours after transfection and homogenized with a polytron device in 500 μl reporter lysis buffer (Promega). After measurement of the protein concentrations, extracted proteins were loaded on 4–20% SDS-PAGE followed by Western Blot analysis to detect expressed Aβ42. Here we used a polyclonal antibody Aβ42 antibody, SC5399 (Santa Cruz Biotechnology, Santa Cruz, CA), because the secondary anti-rabbit IgG antibody did not show cross-reactivity with other mouse proteins in these particular lysates. An ear lysate transfected with control vector pUAS-Luc was used as the negative control. Antibody binding was visualized with enhanced chemiluminescence detection using Luminol reagent as recommended by manufacturer (Thermo Scientific, Waltham, MA). Pixel densities of the bands were analyzed using ImageJ software (rsb.info.nih.gov/ij) and normalized against APP expression level in the same lane. In-vivo expression of Aβ42 in ear skin in the same lysates was also confirmed by ELISA. The ELISA plates were coated with the ear lysates (1 mg protein/ml coating buffer) and incubated overnight at 4°C. The plates were blocked with blocking buffer (PBS/1.5% BSA) for 30 min and incubated with anti-Aβ monoclonal antibodies, 6E10 and 4G8 (1ng/ml, Covance, Madison, WI), for 1 hour at room temperature. After washing the plates were incubated with a goat-anti-mouse IgG/HRP labeled secondary antibody for another hour. Antibody binding was detected with ABTS substrate and the plates were read at 405 nm. The concentration of Aβ42 was calculated with a standard curve for Aβ42 peptide and non-specific binding was subtracted from second antibody control and pUAS-Luc transfected lysate control.
2.4. Immunization of the mice using the Gene Gun method
All plasmid DNAs were purified using a commercial plasmid maxi kit (Qiagen, Valencia, CA). The purity and concentration of DNA were measured by optical density reading at 260/280 nm and gel electrophoresis. Immunizations were performed as described before [14 –18]: DNA coated gold particles were bombarded to both sides of the mouse ears with a helium gas pressure of 300 psi (Helios gene gun, Bio-Rad, Hercules, CA). Each vaccination consisted of 1 μg DNA into the skin of both side of the ears for a total of 4 μg DNA for each vaccination point. Mice were immunized weekly for the first 4 times and then biweekly for a total of 6–11 immunizations. Three independent experiments for each immunization vector (Gal4/UAS-Luc, Gal4/UAS-Aβ42 monomer, Gal4/UAS-Aβ42 trimer, CMV-Luc, CMV-Aβ42 trimer) were performed with 4 mice in each group.
2.5. Use of the vaccine generated immune sera for detection of Aβ42 protein in Western blots
Full length human Aβ42 gene was inserted into pMAL vector downstream of the malE gene (encoding gene of maltose-binding protein, MBP) (New England Biolabs, Ipswich, MA) and MBP-Aβ42 was expressed in E. coli BL21. The bacterial pellet was extracted with 1 x SDS loading buffer and 2 μl of supernatant (10 μg/total protein/lane) were applied to a 4–20% SDS PAGE and transferred to a PVDF membrane (Immulon-P, Millipore, Billerica, MA). The membranes were blocked with blocking buffer (TBST containing 3% milk, 1% BSA, 0.05% tween 20), and probed with 1:2000 diluted sera obtained from mice vaccinated with Aβ42 constructs or Luc control constructs as negative control. Lower dilutions of the antisera resulted in high background and could therefore not be used. HRP-conjugated goat anti-mouse IgG (1:5,000 dilution, Bio-Rad) was used as secondary antibody and antibody binding onto the membranes were detected using an enhanced chemiluminescence assay (PerkinElmer Life Sciences, Waltham, Massachusetts).
2.6. ELISAs for detection of anti-Aβ antibody, isotyping and epitope mapping
Blood was collected from mouse tail vein and the serum was analyzed for Aβ-specific antibodies by ELISA. The assay was performed as described earlier (14). Briefly, the plate was coated with 50 μl Aβ42 peptide (2 ug/ml)(BioBasic Inc., Ontario, Canada) in coating buffer and serum was added into the wells in various concentrations in blocking buffer (1% milk, 1% BSA, 0.05% Tween 20 in PBS) and incubated overnight at 4°C. Antibody binding was detected with a secondary HRP conjugated rabbit anti-mouse IgG antibody. The reaction was visualized by adding 3,3′,5,5′tetramethylbenzidine (TMB) (Pierce, Rockford, IL) substrate solution and was read after 15 min of substrate reaction in a spectrophotometer at 405 nm. Further reaction was stopped at 20 min by adding 50 μl 0.5N HCL and read again at 450 nm. Antibody concentrations were calculated using a calibration curve generated with known concentrations of an anti-Aβ42 monoclonal antibody, 4G8 (Covance). The isotypes of anti-Aβ antibodies were detected using rabbit anti-mouse IgG1, IgG2a, IgG2b and IgM as secondary antibodies (Zymed, San Francisco, CA) and an HRP conjugated anti-rabbit Ig antibody as tertiary antibody as previously described (15). Epitope mapping of the immune sera was performed by analysis of the antibody binding to various Aβ42 peptides including Aβ1-28, 1-15, 1-9, 5-14, 9-18, 16-38, and 31-42 following the ELISA procedure described above.
2.7. Detection of senile plaques with vaccine generated immune sera
Brain sections from APPswe/PS1deltaE9 transgenic mice were used to test whether sera of vaccinated animals contain antibodies that bind to senile plaques. Sera from mice vaccinated with DNA Aβ42 trimer and control plasmid (end concentration 1 μg/ml) were added to 5 μm-thick brain sections of formalin-fixed paraffin embedded APPswe/PS1deltaE9 mice. Sections were deparaffinized and pretreated with 90% formic acid, and exogenous peroxidase was quenched. Controls used were: Staining with an antibody specific for Aβ42 (A1976, Sigma) as a positive control; staining of brain sections of a wild-type mouse (C57BL/6) as negative control (no plaques); staining with pre-absorbed immune serum (1 h at 37 °C with Aβ42 peptide, 1 μM final concentration) as a specificity control. Binding of antibodies to brain sections was detected by HRP labeled secondary antibody followed by 5–10 min development with metal-enhanced diaminobenzidine substrate (Pierce). A digital camera (Olympus, Japan) was used to capture images of the plaques at 10× magnification.
2.8. Statistical analyses
Data are expressed as means and standard deviations and a standard Student T-test was used. P values <0.05 were the criterion for statistical significance.
3. Results
3.1. Construction of the binary Gal4/UAS Aβ42 trimer vaccine system
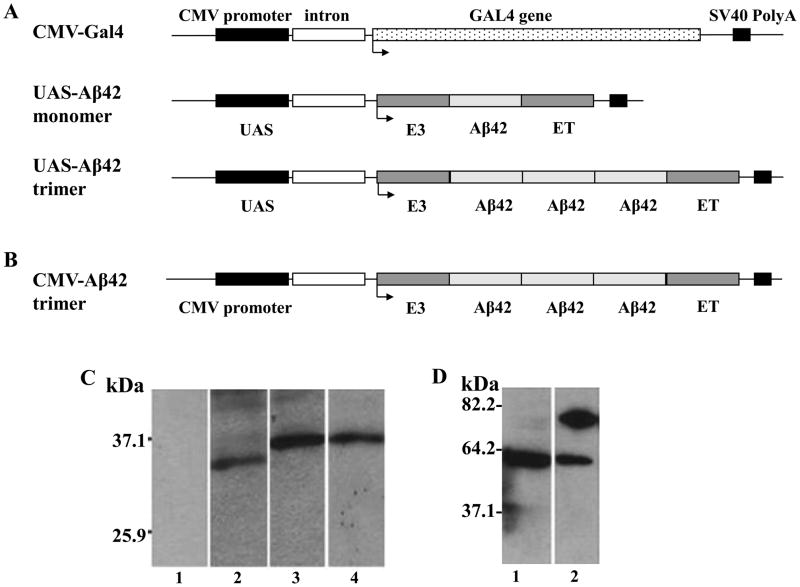
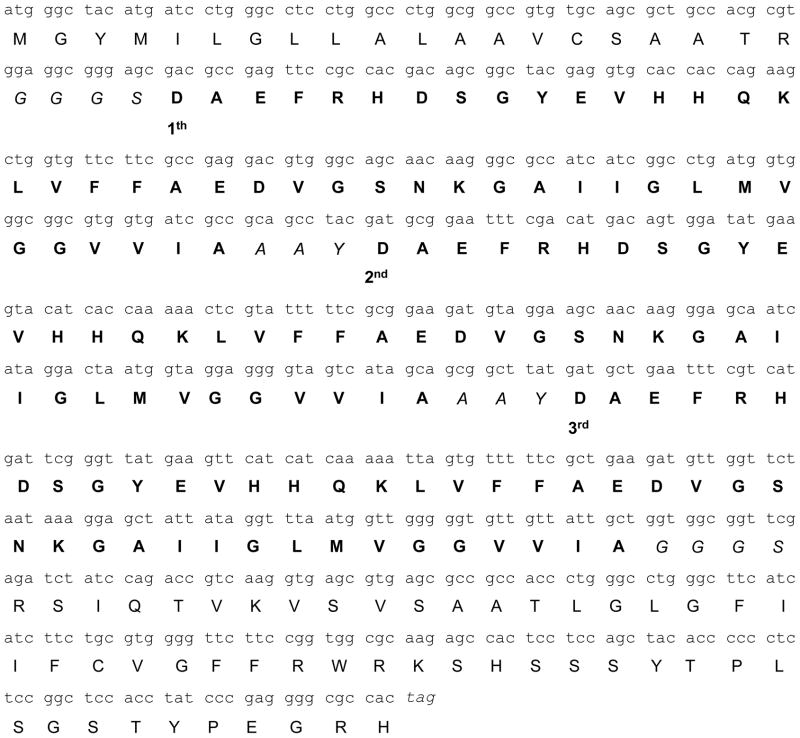
The binary Gal4/UAS Aβ42 trimer vaccine system consists of two plasmids which were co-injected into the skin: An activator plasmid encoding the yeast transcription factor Gal4 and a responder plasmid containing UAS segments to which Gal4 binds and thereby drives the transcription of the respective gene from the responder plasmid, which is Aβ42 in the system used here. We compared three different vector systems; Gal4/UAS-Aβ42 monomer, Gal4/UAS-Aβ42 trimer, and CMV-Aβ42 trimer to test the potency of the Gal4/UAS binary system in eliciting an immune response (Fig. 1). The nucleotide sequence of the Aβ42 trimer, its linkers, the leader sequence and endosomal targeting sequence as well as the respective amino acid sequences are shown in Fig. 2. Note that the respective nucleotide sequences for the three Aβ42 copies differ from each other and from human Aβ42 (72%, 79%, and 80% homology to the human Aβ42 germline sequence respectively) which are due to the development of this construct by PCR, but the amino acid sequences are identical. In-vivo expression of the plasmids was demonstrated in HEK293 cells after transient Fugene 6 transfection with Aβ42 monomer or Aβ42 trimer (Gal4/UAS and CMV) or after gene gun transfection for pCMV-Gal4 alone. Western blot analysis showed positive bands detected by an anti-Aβ monoclonal antibody, 6E10, with a molecular weight of about 30 kDa in cells transfected with Gal4/UASAβ42 monomer (Fig. 1C2). Cells transfected with Gal4/UAS-Aβ42 trimer and CMV- Aβ42 trimer showed bands of 40 kDa (Fig. 1C3 and C4) which are considered to be multimers of the Aβ42 trimer proteins (the trimer protein has an expected MW of 20 kDa, for further analyses see also Figure 7). No protein band was detected in cells transfected with control vector (pUAS-Luc, Fig. 1C1). Gal4 protein expression in HEK293 cells transfected with CMV-Gal4 was shown by a positive band of about 80 kDa using an anti-Gal4 polyclonal antibody (Fig. 1D2). No protein band of this size was detected in the cells transfected with control vector analyzed in parallel (Fig. 1D1).
Figure 1. Schematic representation of the plasmids used in this study and in-vitro expression of these plasmids in HEK293 cells.
A The binary plasmid system contains two plasmid vectors. CMV-Gal4 is the activator plasmid, in which, Gal4 was cloned into a pCMVi mammalian expression vector containing CMV promoter for initiation and SV40 polyadenylation site for termination of transcription. The effector plasmids include UAS-Aβ42 monomer or Aβ42 trimer, which contain four UAS sequences for Gal4 binding and transcription initiation and the SV40 polyadenylation sequence for termination. Aβ42 were cloned between adenovirus E3 leader signal and endosomal targeting (ET) sequence. B For comparison with the binary plasmid system, Aβ42 trimer was cloned directly downstream of a CMV promoter. All plasmids contain an intron sequence to further increase transcription. C Western Blot for detection of Aβ42 using the monoclonal antibody 6E10. No band is detected in cells transfected with a control plasmid (Lane C1). After transfection with the respective plasmids in HEK293 cells positive bands of about 30 kDa for pUAS-Aβ42 monomer (Lane C2), 40 kDa for pUAS-Aβ42 trimer (Lane C3) and 40 kDa for CMVi-Aβ42 trimer (Lane C4) confirmed expression of the constructs. D Gal4 expression in CMV-Gal4 transfected HEK293 cells was demonstrated by Gal4 antibody binding to a band of 80 kDa (Lane D2). This band was not detected in cells transfected with control plasmid (Lane D1).
Figure 2.
Nucleotide and predicted amino acid sequence (single letter code) of DNA Aβ42 trimer immunization construct including the E3 leader and endosomal targeting sequence. The short linkers are italics. Three repeats of the Aβ42 sequence are in bold letters and indicated as 1st, 2nd, and 3rd.
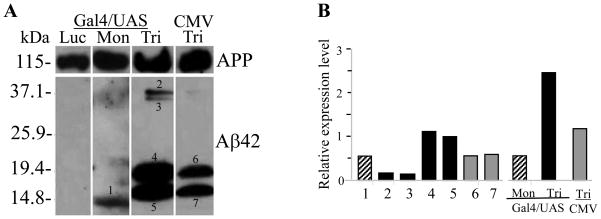
Figure 7. Analysis of Aβ42 expression in mouse ear after gene gun transfection.
A Protein lysate from mouse ear was run on a 4–20 % SDS PAGE. UAS-monomer transfection in mouse ear resulted in a unique 12 kDa band recognized by the anti-Aβ42 antibody (Mon). In ear lysate from mice immunized with Gal4/UAS Aβ42 trimer, protein bands of about 16 and 20 kDa, and dimerized bands of about 32 and 40 kDa, respectively were detected by the Aβ42 antibody (Tri). CMV-Aβ42 trimer transfection in mouse ear produced the same sized bands as Gal4/UAS-trimer but the amount was significantly reduced and dimerized products were not detected (CMV-Tri). The ear lysate transfected with control DNA (Gal4/UAS-Luc) showed no protein bands detectable by Aβ42 antibody (Luc). B Image J analyses of the bands in A which had been normalized to the level of APP protein in the same lane. The first seven bars show the intensities for the individual bands corresponding to this numbers; in the last three bars the intensities are summarized for each vaccination construct used. Black bars show values for Gal4/UAS-Aβ42 trimer, hatched bars show values for Gal4/UAS-Aβ42 monomer, and grey bars show pixel densities for CMV-Aβ42 trimer
3.2. Gal4/UAS-Aβ42 trimer gene vaccine induced high antibody levels against Aβ42 peptide in BALB/cJ wild type mice
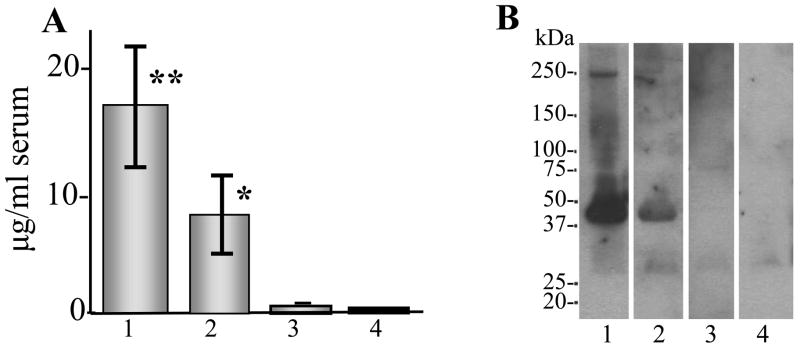
The immunization efficacy of Gal4/UAS Aβ42 trimer gene vaccine was tested in BALB/cJ mice and directly compared with the other two plasmid constructs. ELISA results showed that Gal4/UAS Aβ42 trimer elicited the highest antibody production with a mean value of 16.6 μg/ml serum after 5 vaccinations. (Fig. 3A1). Sera from mice immunized with Gal4/UAS Aβ42 monomer vaccine had significantly lower levels of anti-Aβ42 antibodies with a mean value of 6.5 μg/ml serum (Fig. 3A2). Mice immunized with CMV-Aβ42 trimer vaccine produced even lower antibody titers with a mean value of less than 1 μg/ml serum (Fig 3A3). The control plasmids (encoding luciferase) elicited no measurable anti-Aβ42 antibodies (Fig. 3A4). Consistent with the ELISA result, Western blot assay using the immune sera from vaccinated mice with binary system could detect MBP (New England Biolabs) fused Aβ42 protein with a molecular weight of 46 kDa (Fig. 3B1 for trimer and B2 for monomer vaccine), while the sera from mice vaccinated with CMV-trimer could not detect MBP fused Aβ42 proteins due to the low antibody concentration (Fig. 3B3). Control sera from mice immunized with Gal4/UAS-Luc construct showed also no binding to MBP fused Aβ42 protein (Fig. 3B4).
Figure 3. Anti-Aβ42 antibody production in BALB/cJ mice immunized with gene gun Aβ42 gene vaccine.
A ELISA results showing anti-Aβ42 antibodies of the immune sera. The highest level of antibody production were found in mice vaccinated with Gal4/UAS Aβ42 trimer gene vaccine (mean ± SD) 16.6 ± 5.5 μg/ml serum, ** P < 0.01 compared with control (A1). In mice vaccinated with Gal4/UAS Aβ42 monomer antibody levels with a mean ± SD of 6.5 ± 2.5 μg/ml serum were found, * P < 0.05 compared with control (A2). In CMV-Aβ42 trimer immunized mice Aβ42 antibody were barely detectable (A3). Control mice were immunized with a UAS-Luc plasmid and served as the negative control (A4). The graph shows means and SD of four mice in each group after 5 gene gun vaccinations and is representative of 3 experiments. B Western blot with same sera used in ELISA (1:2000) shows that MBP-fused Aβ42 can be detected with the expected band of MW 46 kDa with the immune sera of mice immunized with Gal4/UAS-Aβ42 trimer (B1) and Gal4/UAS-Aβ42 monomer (B2). The sera from mice vaccinated with CMV-Aβ42 trimer showed no specific binding to MBP-Aβ42 (B3) and the immune sera from mice vaccinated with UAS-Luc served as a negative control (B4).
3.3. Gal4/UAS-Aβ42 monomer and Gal4/UAS-Aβ42 trimer induced Th2 type anti-Aβ42 antibodies
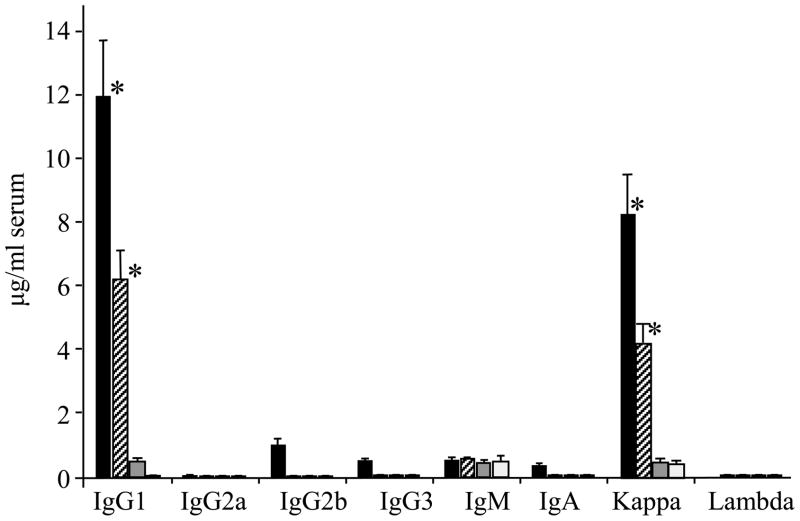
Isotype analysis of the anti-Aβ42 antibodies in the immune sera showed that the main antibody isotype was IgG1 (Th2 type) in both mice vaccinated with Gal4/UAS-Aβ42 trimer (11.9 μg/ml, 71% of total IgG) and Gal4/UAS-Aβ42 monomer (6.2 μg/ml, 95% of total IgG). Both groups had an IgG1/IgG2a ratio of 9 as calculated by the respective OD405 values, indicating a polarized Th2 immune response. Antibodies with an IgG2b isotype (also Th2 type) were 0.98 μg/ml and 0.04 μg/ml for Gal4/UAS-Aβ42 trimer and Gal4/UAS-Aβ42 monomer respectively. No significant IgG2a antibody (Th1) was found (values less than 0.1 μg/ml). IgM anti-Aβ42 antibodies were shown to be present in all groups of mice including the control group. Low levels of Aβ42 specific IgA antibodies (values less than 0.5 μg/ml) were found in immune sera vaccinated with Gal4/UAS-Aβ42 trimer as compared with the control sera. Similarly low levels of IgG3 were present in the sera of UAS-trimer group mice (0.8 μg/ml) (Fig. 4). For the light chain usage only Aβ antibodies with k chains were found.
Figure 4.
Isotype analysis of anti-Aβ42 antibodies from BALB/cJ mice immunized with Gal4/UAS-Aβ42 trimer, monomer, and CMV-Aβ42 trimer gene vaccine by ELISA. Shown are means and SD of four mice in each group. All sera were diluted 1:400 after five vaccinations. Black bars show isotypes from Gal4/UAS-Aβ42 trimer generated antisera, hatched bars show anti-Aβ42 isotypes from Gal4/UAS-Aβ42 monomer generated antisera. Grey bars show isotypes found in antisera generated after immunization with CMV-Aβ42 trimer. White bars show the reactivity of control serum. * P < 0.01 compared with control.
3.4. Aβ42 epitope mapping of the immune sera
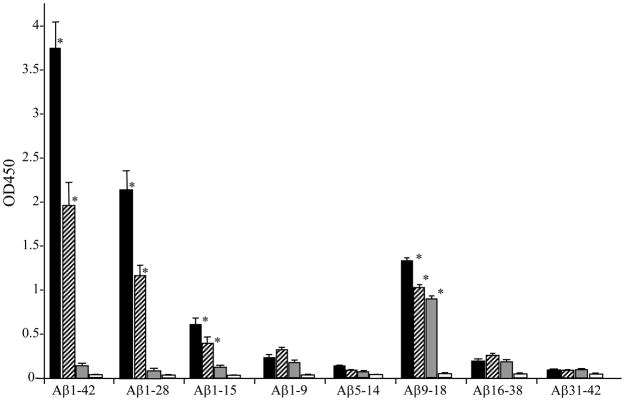
The immune sera obtained were further analyzed for their ability to bind to linear Aβ42 peptide epitopes. The sera from mice vaccinated with Gal4/UAS-Aβ42 trimer and Gal4/UAS-Aβ42 monomer showed high binding to full length Aβ1-42 and Aβ1-28. A lower binding to Aβ1-15 was found which might be due to the mouse strain used, BALB/cJ, as the B cell epitope reported for this strain is Aβ6-20 (30). For all sera tested we found high binding to Aβ9-18. It should be noted that this peptide is the only peptide for which we found significant binding of immune sera of mice vaccinated with CMV-Aβ42 trimer (Fig. 5).
Figure 5.
Epitope binding to Aβ42 peptide by the immune sera of BALB/cJ mice vaccinated with Aβ42 gene vaccine measured by ELISA showing that immune sera (1:400) mainly binds to Aβ1-42, Ab1-28 and Aβ9-18. The immune sera also show binding to Aβ1-9 and Aβ16-38 with a low titer. Shown are means and SD of 4 mice in each group after 7 vaccinations and the experiment was repeated three times. Black bars show Aβ peptides bound by antibodies in sera from Gal4/UAS-Aβ42 trimer immunized mice, hatched bars show antibody binding by antibodies from sera of Gal4/UAS-Aβ42 monomer immunized mice. Grey bars show antibody binding by antisera derived from CMV-Aβ42 trimer immunization. White bars show the reactivity of control serum. * P < 0.05 compared with control.
3.5. Anti-Aβ42 antibodies from immune sera bind senile plaques in brain of APP tg mice
Brain sections from APPswe/PS1deltaE9 transgenic mice were used to test whether antibodies from sera of vaccinated animals bind to plaques. As positive control for staining of the plaques we used a commercial anti-Aβ42 antibody (A1976, Sigma) (Fig. 6A). Sera from mice immunized with Gal4/UAS-Aβ42 trimer showed a similar binding pattern to Aβ senile plaques in brain sections of the transgenic mice as the anti-Aβ42 antibody, A1976 (Fig. 6B, C, D, E). The specificity of antibodies was confirmed by incubation of immune sera with Aβ42 peptide prior to staining of the sections (F), which completely eliminated plaque binding in transgenic mice brain tissues. Sera from control vector (Gal4/UAS-luc) vaccine mice did not show any specific binding (G). As a negative control we used C57BL/6 wild-type mouse brain sections and found no labeling with the immune sera (H).
Figure 6.
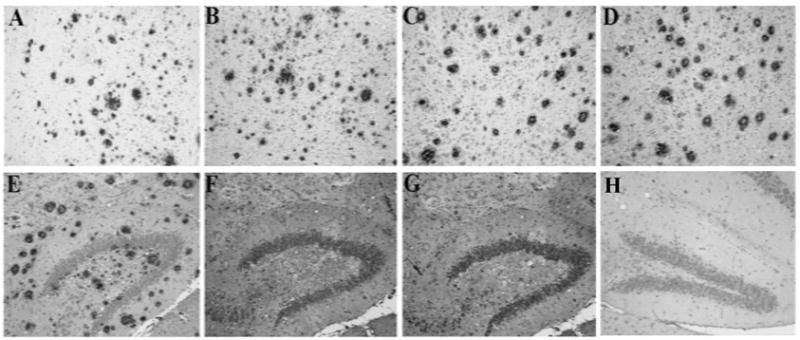
Immunostaining for senile plaques in APPswe/PS1deltaE9 Tg mouse brain section with immune sera (1 ug/ml) generated with gene gun mediated Gal4/UAS Aβ42 trimer gene vaccination in BALB/cJ mice. A Positive control: Cortex of a Tg mouse brain was stained with a commercially available Aβ42 antibody to show amyloid plaques (A1976, Sigma). B, C, D Immunosera of three mice vaccinated with Gal4/UAS Aβ42 trimer (11 immunizations) showed the same staining of senile plaques in cortex of Tg mouse brain. E Immune serum from DNA Aβ42 trimer immunized mice stained plaques in hippocampus of Tg mouse brain. F Preabsorbtion of immune serum with Aβ1-42 peptide blocks binding to plaques confirming the antigen specificity. G Staining of Tg mouse brain with immune sera from mice vaccinated with control DNA as negative control. H Immune sera from DNA Aβ42 trimer immunized mice showed no staining of brain from wild-type mice.
3.6. In-vivo demonstration of expression of the respective plasmids in mouse ear
To explain why Gal4-UAS-Aβ42 trimer is a better system for vaccination, we performed gene gun transfection of Gal4/UAS-Aβ42 trimer, Gal4/UAS-Aβ42 monomer, and CMV-trimer and compared directly the expression of Aβ42 in the skin (Fig 7). Western blots using a polyclonal anti-Aβ antibody (SC5399, Santa Cruz Biotechnology) revealed a single protein band with a MW of 12 kDa (Fig. 7A, Lane Mon-1) for Aβ42 monomer transfected ear lysates. The skin cells transfected with Gal4/UAS trimer exhibited reactive bands in the size of 20 kDa (Fig. 7A, Lane Tri-4), which is expected to be the full-length Aβ42 trimer protein (putative MW 19.8 kDa; containing three copies of Aβ42, linkers and endosomal targeting peptide, 186 amino acids). Clearly visible protein bands with lower MW were likely due to different conformations of the Aβ42 trimer in solution or due to differently cleaved products with or without endosomal targeting peptide (Fig. 7A Lane Tri-5). The bands with a higher MW (Fig. 7A, Lane Tri-2, 3) were believed to be dimerized products of these single trimeric forms as Aβ42 peptide is known as a very “sticky” peptide [23, 24]. CMV-trimer drives significant less Aβ42 trimer expression than the Gal4/UAS system (Fig. 7A, Lane CMV-Tri-6, 7) and no dimerized bands of higher MW were found. Ear lysate transfected with control plasmid (encoding luciferase) showed no Aβ42 positive bands. Analysis of the pixel densities of the bands and normalization against APP expression level in the same lane shows that the total Aβ42 trimer proteins produced by the transfection of the ear skin with Gal4/UAS Aβ42 trimer was 2.5 times more than APP expression, while ear skin transfected with CMV-Aβ42 trimer produced about 1.2 fold the amount of APP expression. Taken together, in-vivo Aβ42 trimer production after Gal4/UAS-Aβ42 trimer immunization was more than 2 fold higher than after CMV-Aβ42 trimer immunization using the same amount of DNA. This conclusion was further confirmed by ELISA using 4G8 and 6E10 antibodies to detect expressed Aβ42 in the mouse ear lysates (Figure 8). The monoclonal antibodies 4G8 (Aβ17-24) and 6E10 (Aβ1-16) are specific for different parts of the Aβ42 peptide and the very similar reactivity pattern showed that these different areas were equally accessible to antibody binding. Aβ42 quantification showed about 0.1 μg Aβ42 peptide per mg protein in ear lysates after Gal4/UAS-Aβ42 monomer and CMV-Aβ42 trimer immunization and 0.3 μg Aβ42 peptide per mg protein in ear lysates after Gal4/UAS-Aβ42 trimer immunization. These results showed again a major improvement by the use of the binary plasmid system. The high level of reactivity in the Gal4/UAS-Aβ42 trimer may result from the trimeric structure of the antigen and binding of more antibodies to an antigen.
Figure 8. ELISA showing the expression of Aβ42 peptides in ear lysates.
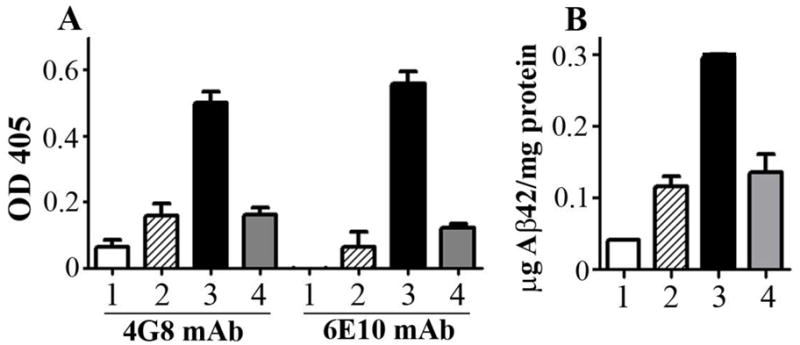
A Two Aβ42 monoclonal antibodies, 4G8 and 6E10, were used. Samples were analyzed in triplicates and shown are mean OD and SD. B To calculate the amount of peptide expression in the ears, an Aβ42 titration was used in the same ELISA. A threefold higher peptide concentration was demonstrated in ears transfected with Gal4/UAS-Aβ42 trimer compared to ears transfected with Gal4/UAS-Aβ42 monomer and CMV-Aβ42 trimer. White bars show results for ear lysates transfected with Gal4/UAS-Luc as negative control (1). Hatched and black bars show values for Gal4/UAS-Aβ42 monomer (2) and Gal4/UAS-Aβ42 trimer (3). Grey bars show expression levels for CMV-Aβ42 trimer (4).
Discussion
We have previously reported that gene gun mediated DNA Aβ42 immunization with a constitutive promoter induced a good antibody response against Aβ42 peptide in BALB/cJ mice [14]. We have further reported that prophylactic DNA Aβ42 immunization in APPswe/PS1ΔE9 transgenic mice reduced the brain Aβ42 plaque load by 42% [15, 16]. Recently we have compared the immune response of Gal4/UAS-Aβ42 trimer immunization and Aβ42 peptide immunization in detail [18]. In the present study, comparisons of three distinct plasmid systems showed that the binary Gal4/UAS system in combination with a novel Aβ42 trimer construct elicited significantly higher levels of antibody production in wild type BALB/cJ mice. Trimeric Aβ42 improved immunogenicity as compared to native monomeric forms. By comparing the Gal4/UAS Aβ42 trimer and the CMV Aβ42 trimer we have been able to determine the influence of the promoter used for a genetic immunization protocol.
The yeast Gal4 transcription factor has been shown to function as a transactivator and promote transcription of Aβ42 in the second plasmid [25]. Further processing of Aβ42 was enhanced by the cloning of an adenovirus E3 leader peptide upstream and an endosomal targeting peptide downstream of the Aβ42 sequence. Both have been considered as good combination for improved antigen presentation and boosting immunity post genetic vaccination [15, 16, 19, 20]. We found higher antibody production in the mice immunized with the Gal4/UAS Aβ42 monomer compared to the CMV-Aβ42 monomer or trimer. In wild-type mice the Gal4/UAS-Aβ42 trimer vaccine elicited a 2.5 fold higher antibody production (about 10 to 20 μg/ml serum) than the Gal4/UAS-Aβ42 monomer (6 μg/ml serum) with a highly polarized Th2 type immune response. Possible therapeutic potency of anti-Aβ antibodies from this immunization protocol was further demonstrated by binding of the respective antibodies to Aβ plaques in brain tissues from a mouse model of AD, APP/PS1deltaE9. We are now testing the preventive and therapeutic potency of the binary trimer vaccine construct in APP transgenic mice.
The Gal4/UAS system has been widely used in studies of gene regulation and protein interaction [25–29]. To our knowledge, this is the first report in which this system has been used for genetic immunization achieving high levels of antibody production. One of the mechanisms might be an improved antigen expression and presentation with the Gal4/UAS system. Indeed, we observed more than 2 fold higher Aβ42 protein expressions in cells of the ear transfected with Gal4/UAS-Aβ42 trimer than CMV-Aβ42 trimer construct as shown by Western blot and ELISA (Figure 7 and 8).
Multimeric Aβ42 peptide epitopes are likely able to elicit B cell responses more potently than single epitopes. The concept of using multimeric constructs and fusion proteins in vaccination vectors for Aβ42 has also been used by other laboratories and it appears that this approach is effective. Different from our immunization protocol, in these studies only a part of the Aβ42 sequence, 3x Aβ1-11 or even 11 tandem repeats of Aβ1-6, were included in the plasmids particularly to avoid amino acid sequences which might cause T cell activation [29–34]. In a recent study from DaSilva, full-length Aβ42 was also used carrying the Arctic mutation (E33G) combined with an attenuated Caspase gene, leading in theory to an improved antigen presentation by local antigen presenting cells (APCs). In this model, effective levels of antibodies were produced and overall plaque load and concentration of Aβ42 oligomers were reduced in the brains of immunized TgCRND8 mice [35].
DNA immunization using the gene gun method delivers the respective antigen encoding gene into dermal dendritic cells which migrate rapidly to the draining lymph nodes to function as APC. Both local and distant expression of antigens influence the kinetics and magnitude of the immune responses and antibody production is increased in a dose-dependent manner [36–38]. In general, lower levels of antibody production have been observed in DNA immunizations compared with protein immunization, especially in large animals and humans [39–41]. Methods have been used to increase Aβ42 antibody production by fusion of the antigen to endosomal targeting proteins [15,16], fusion with cytokines or other immune response-stimulating factors [42, 43]. These methods improve the Aβ42 antibody production in mice but the effectiveness in human remains to be tested. It has been shown that higher titer antibody is correlated with a better reduction in Aβ plaque pathology both in AD mouse and in patients. [44–47]. An effective and safe genetic vaccination method for clinical application must aim at two goals: maximizing the antibody production while preventing the generation of a pathogenic T cell response [18]. We conclude that the binary Gal4/UAS DNA Aβ42 trimer system presented here could be a potential candidate for a clinical use in AD patients as it meets both, higher antibody production and a low level of T cell activation.
Acknowledgments
We thank Marilyn Howell for expert assistance in preparation of the figures. Supported by the UTSouthwestern Alzheimer’s Disease Center, NIH/NIA Grant P30AG12300-16, The Rudman Foundation and an Alzheimer’s Association Research Grant IIRG-06-24428
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg RN. Translational research on the way to effective therapy for Alzheimer disease. Arch Gen Psychiatry. 2005;62(11):1186–1192. doi: 10.1001/archpsyc.62.11.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yates D, McLoughlin DM. The molecular pathology of Alzheimer’s disease. Psychiatry. 2008;7(1):1–5. [Google Scholar]
- 4.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 5.Iwatsubo T, Mann DM, Odaka A, Suzuki N, Ihara Y. Amyloid beta protein (Aβ) deposition: Aβ42(43) precedes Aβ40 in Down syndrome. Ann Neurol. 1995;37:294–299. doi: 10.1002/ana.410370305. [DOI] [PubMed] [Google Scholar]
- 6.Hardy J. Alzheimer’s disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis. 2006;9(suppl):151–153. doi: 10.3233/jad-2006-9s317. [DOI] [PubMed] [Google Scholar]
- 7.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 8.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, et al. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 9.Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- 10.Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, et al. Effects of Aβ immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 11.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg RN. Immunization with β-Amyloid (1–42) protein for Alzheimer disease: genomics predicts the response. JAMA. 2005;294:2352–2355. [Google Scholar]
- 13.Bombois S, Maurage CA, Gompel M, Deramecourt V, Mackowiak-Cordoliani MA, Black RS, et al. Absence of beta-amyloid deposits after immunization in Alzheimer disease with Lewy body dementia. Arch Neurol. 2007;64(4):583–587. doi: 10.1001/archneur.64.4.583. [DOI] [PubMed] [Google Scholar]
- 14.Qu BX, Rosenberg RN, Li L, Boyer PJ, Johnston SA. Gene vaccination to bias the immune response to amyloid-beta peptide as therapy for Alzheimer disease. Arch Neurol. 2004;61:1859–1864. doi: 10.1001/archneur.61.12.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qu BX, Boyer PJ, Johnston SA, Hynan LS, Rosenberg RN. Aβ42 gene vaccination reduces brain amyloid plaque burden in transgenic mice. J Neurol Sci. 2006;244:151–158. doi: 10.1016/j.jns.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qu BX, Xiang Q, Li L, Johnston SA, Hynan LS, Rosenberg RN. Aβ42 gene vaccine prevents Aβ42 depostion in bran of double transgenic mice. J Neurol Sci. 2007;260:204–208. doi: 10.1016/j.jns.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu BX, Fu M, Johnston SA, Rosenberg RN. Trimer Aβ42 gene vaccine elicites a high level of antibody production when delivered with the Gal4/UAS system and the gene gun. Alzheimer Dement. 2008;4(4 Suppl 1):T477. [Google Scholar]
- 18.Lambracht-Washington D, Qu B, Fu M, Eagar TN, Stüve O, Rosenberg RN. DNA β-Amyloid1-42 Trimer Immunization for Alzheimer Disease in a Wild-Type Mouse Model. JAMA. 2009;302(16):1796–1802. doi: 10.1001/jama.2009.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciernik IF, Berzofsky JA, Carbone DP. Induction of cytotoxic T lymphocytes and antitumor immunity with DNA vaccines expressing single T cell epitopes. J Immunol. 1996;156:2369–2375. [PubMed] [Google Scholar]
- 20.Marques ET, Jr, Chikhlikar P, de Arruda LB, Leao IC, Lu R, Wong J, et al. HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 as a DNA plasmid vaccine chimera is highly expressed, traffics to the major histocompatibility class II compartment, and elicits enhanced immune responses. J Biol Chem. 2003;278:37926–37936. doi: 10.1074/jbc.M303336200. [DOI] [PubMed] [Google Scholar]
- 21.Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 22.Chamber RS, Johnston SA. High-level generation of polyclonal antibodies by genetic immunization. Nature Biotechnol. 2003;21:1088–1092. doi: 10.1038/nbt858. [DOI] [PubMed] [Google Scholar]
- 23.Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, et al. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int Immunol. 2003;4:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garzon-Rodriguez W, Sepulveda-Becerra M, Milton S, Glabe CG. Soluble amyloid Aβ1-40 exists as a stable dimer at low concentration. J Biol Chem. 1997;272:21037–21044. doi: 10.1074/jbc.272.34.21037. [DOI] [PubMed] [Google Scholar]
- 25.Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid β-protein (Aβ) assembly: Aβ40 and Aβ42 oligomerize through distinct pathways. PNAS USA. 2003;100:330–335. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ornitz MD, Moreadith WR, Leder P. Binary system for regulating transgene expression in mice: Targeting int-2 gene expression with yeast GAL4/UAS control elements. PNAS USA. 1991;88:698–702. doi: 10.1073/pnas.88.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartley KO, Nutt SL, Amaya E. Targeted gene expression in transgenic Xenopus using the binary Gal4-UAS system. PNAS USA. 2002;99(3):1377–1382. doi: 10.1073/pnas.022646899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar PR, Yu Y, Sternglanz R, Johnston SA, Joshua-Tor L. NADP regulates the yeast GAL induction system. Science. 2008;319(5866):1090–1092. doi: 10.1126/science.1151903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elliott DA, Brand AH. The GAL4 system: a versatile system for the expression of genes. Methods Mol Biol. 2008;420:79–95. doi: 10.1007/978-1-59745-583-1_5. [DOI] [PubMed] [Google Scholar]
- 30.Kim HD, Tahara K, Maxwell JA, Lalonde R, Fukuiwa T, Fujihashi K, et al. Nasal inoculation of an adenovirus vector encoding 11 tandem repeats of Aβ1-6 upregulates IL-10 expression and reduces amyloid load in a Mo/Hu APPswe PS1dE9 mouse model of Alzheimer’s disease. J Gene Med. 2007;9:88–98. doi: 10.1002/jgm.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, et al. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine - a novel immunotherapeutic strategy. PLoS ONE. 2008;3 (5):e2124. doi: 10.1371/journal.pone.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou J, Yao Z, Zhang G, Wang H, Xu J, Yew DT, Forster EL. Vaccination of Alzheimer’s model mice with adenovirus vector containing quadrivalent foldable Aβ1-15 reduces Aβ burden and behavioral impairment without Aβ-specific T cell response. J Neurol Sci. 2008;272:87–98. doi: 10.1016/j.jns.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Davtyan H, Mkrtichyan M, Movsesyan N, Petrushina I, Mamikonyan G, Cribbs DH, et al. DNA prime-protein boost increased the titer, avidity and persistence of anti-Abeta antibodies in wild-type mice. Gene Ther. 2009:1–11. doi: 10.1038/gt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HD, Jin JJ, Maxwell JA, Fukuchi K. Enhancing Th2 immune responses against amyloid protein by a DNA prime-adenovirus boost regimen for Alzheimer’s disease. Immunol Lett. 2007;15:30–38. doi: 10.1016/j.imlet.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, Janus C, Lemere CA. Short amyloid-beta (Abeta) immunogens reduce cerebral Abeta load and learning deficits in an Alzheimer’s disease mouse model in the absence of an Abeta-specific cellular immune response. J Neurosci. 2006;26:4717–4728. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DaSilva KA, Brown ME, McLaurin J. Reduced oligomeric and vascular amyloid beta following immunization of TgCRND8 mice with an Alzheimer’s DNA vaccine. Vaccine. 2009;27(9):1365–1376. doi: 10.1016/j.vaccine.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 37.Deck RR, DeWitt CM, Donnelly JJ, Liu MA, Ulmer JB. Characterization of humoral immune responses induced by an influenza hemagglutinin DNA vaccine. Vaccine. 1997;15:71–78. doi: 10.1016/s0264-410x(96)00101-6. [DOI] [PubMed] [Google Scholar]
- 38.Robinson HL, Boyle CA, Feltquate DM, Morin MJ, Santoro JC, Webster RG. DNA immunization for influenza virus: Studies using hemagglutinin- and nucleoprotein-expressing DNAs. J Infect Dis. 1997;176(Suppl):S50–55. doi: 10.1086/514176. [DOI] [PubMed] [Google Scholar]
- 39.Gurunathan S, Klinman DM, Seder RA. DNA VACCINES: Immunology, Application, and Optimization. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 40.Kang Y, Calvo PA, Daly TM, Long CA. Comparison of humoral immune responses elicited by DNA and protein vaccines based on merozoite surface protein-1 from Plasmodium yoelii, a rodent malaria parasite. J Immunol. 1998;161:4211–4219. [PubMed] [Google Scholar]
- 41.Babiuk LA, Pontarollo R, Babiuk S, Loehr B, van Drunen Littel-van den Hurk S. Induction of immune responses by DNA vaccines in large animals. Vaccine. 2003;21(7–8):649–658. doi: 10.1016/s0264-410x(02)00574-1. [DOI] [PubMed] [Google Scholar]
- 42.Ghochikyan A, Vasilevko V, Petrushina I, Movsesyan N, Babikyan D, Wenqiang Tian WQ, et al. Generation and characterization of the humoral immune response to DNA immunization with a chimeric β-amyloid-interleukin-4 minigene. Eur J Immunol. 2003;33(12):3232–3241. doi: 10.1002/eji.200324000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Movsesyan N, Mkrtichyana M, Petrushin I, Ross TM, Cribbs DH, Agadjanyan MG, et al. DNA epitope vaccine containing complement component C3d enhances anti-amyloid-β antibody production and polarizes the immune response towards a Th2 phenotype. J Neuroimmunol. 2008;205:57–63. doi: 10.1016/j.jneuroim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen G, Chen KS, Kobayashi D, Barbour R, Motter R, Games D, et al. Active beta-amyloid immunization restores spatial learning in PDAPP mice displaying very low levels of beta-amyloid. J Neurosci. 2007;27:2654–2662. doi: 10.1523/JNEUROSCI.3710-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrushina I, Ghochikyan A, Mktrichyan M, Mamikonyan G, Movsesyan N, Davtyan H, et al. Alzheimer’s disease peptide epitope vaccine reduces insoluble but not soluble/oligomeric A beta species in amyloid precursor protein transgenic mice. J Neurosci. 2007;27:12721–12731. doi: 10.1523/JNEUROSCI.3201-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 47.Patton RL, Kalback WM, Esh CL, Kokjohn TA, Van Vickle GD, Luehrs DC, et al. Amyloid-beta peptide remnants in AN-1792-immunized Alzheimer’s disease patients: a biochemical analysis. Am J Pathol. 2006;169:1048–1063. doi: 10.2353/ajpath.2006.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]