Abstract
Multiple impairments in HIV-1-specific cytotoxic T cells (CTL) have been reported, but derangements in HIV-1-specific CD8+ T-cell chemotaxis have not been described previously. We assessed migration to SDF-1α (stromal cell-derived factor 1-alpha) and CX3CL1 in vitro and expression of cognate receptors, CXCR4 and CX3CR1, by flow cytometry in peripheral blood and lymph node CD8+ T cells from HIV-1-seropositive and -seronegative individuals. Compared with seronegative individuals, percentages of CXCR4+ CD8+ T cells were reduced (median, 26% versus 74%, p < 0.001) and percentages of CX3CR1+CD8+ T cells were increased (median, 33% versus 15%, p = 0.03) in seropositive individuals. Robust migration of peripheral blood mononuclear cell (PBMC) CD8+ T cells to SDF-1α (1 μg/ml) was observed in both HIV-1-seropositive (median chemotactic index [CI] 4.9) and -seronegative (median CI 2.8) subjects (p = 0.46). CI to SDF-1α was not significantly related to percentage of CXCR4+ CD8+ T cells or density of CXCR4, but correlated inversely with plasma HIV-1 RNA concentration (r = − 0.82, p = 0.03). Little chemotaxis was observed in response to CX3CL1 and it was unrelated to CX3CR1 expression. Lymph node CD8+ T-cell chemotaxis to SDF-1α and CX3CL1 in four subjects demonstrated the same patterns observed in PBMC. HIV-1-specific tetramer-staining CD8+ T cells exhibited chemotaxis of similar magnitude as PBMC CD8+ T cells in a subset of subjects. These data suggest that SDF-1α is a potent chemoattractant for HIV-1-specific CTL, but that impairments in migration of HIV-1-specific CTL may exist at high viral loads. Improved understanding of the determinants of CTL localization may provide insight into novel therapies to enhance delivery of CTL to sites of HIV-1 replication.
Introduction
It is widely accepted that CD8+ cytotoxic T cells (CTL) are critical to the control of HIV-1 replication. After primary infection with HIV-1, the development of HIV-1 specific CTL coincides with a decline in plasma HIV-1 RNA concentrations.1 Removal of CD8+ T cells from simian immunodeficiency virus (SIV)–infected macaques results in heightened virus replication, which declines subsequently after CD8+ T cells are replenished.2 Despite vigorous CTL responses early in disease, HIV-1 replication continues inexorably, resulting in disease progression and ultimately in death in the absence of antiretroviral therapy. Multiple impairments in HIV-1-specific CTL effector function have been described in HIV-1-seropositive individuals and hypothesized to contribute to ongoing HIV-1 replication.3–6 To date, however, derangements in HIV-1-specific CD8+ T-cell chemotaxis have not been described.
The majority of HIV-1 replication occurs in lymphoid tissues.7–9 Recruitment of lymphocytes to lymph nodes is critical to the immunopathogenesis of HIV-1, providing not only a supply of target CD4+ T lymphocytes but also potential control of HIV-1 replication by recruitment of CTL.10 Chemokines are a group of small, structurally related cytokines that act on leukocyte subsets through G-protein-coupled transmembrane receptors. These proteins are central to efficient leukocyte recirculation, appropriate structuring of lymphoid tissue, and cell recruitment to lymphoid and extralymphoid sites.11 SDF-1α (stromal cell-derived factor 1-alpha) is known to be one of the most potent chemokines for both CD4+ and CD8+ lymphocytes,12 attracting 10 times more lymphocytes than other chemokines such as monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1-alpha (MIP-1α), (IL)-8, and regulated upon activation, normal T-cell expressed and secreted (RANTES).12,13 Fractalkine (CX3CL1), a 373 amino acid protein, is known to induce chemotaxis of a variety of cell types in healthy individuals including lymphocytes,14 and it has been hypothesized that it may be a key chemoattractant for HIV-1-specific CTL.15 Both CXCR4 and CX3CR1 have been identified as HIV-1 coreceptors.13,16 Interestingly, the expression of both of these receptors is altered on CD8+ T cells in individuals with HIV-1. Decreased CXCR417,18 and increased CX3CR1 expression15 has been reported on CD8+ T cells in HIV-1-seropositive as compared to -seronegative individuals. We hypothesized that impaired migration of CD8+ T cells including HIV-1-specific CD8+ T cells might contribute to ongoing HIV-1-replication in vivo. To evaluate this, we assessed CD8+ T-cell chemotaxis to SDF-1α and CX3CL1 as well as expression of their cognate receptors in peripheral blood and lymph node of HIV-1 seropositive individuals.
Materials and Methods
Human subjects
Informed consent was obtained from all participants in accordance with the Colorado Multiple Institutional Review Board. HIV-1-infected individuals who were not receiving antiretroviral therapy and who had CD4+ T cells ≥300/mm3 were recruited to donate peripheral blood. A subset of HIV-1-infected subjects also donated inguinal lymph nodes. None of these subjects had an active opportunistic infection or malignancy or a sexually transmitted disease other than HIV-1. Inguinal lymph node excisional biopsies were performed under local anesthesia, and portions of the lymph nodes were snap frozen in OCT compound (VWR, Denver, CO) and stored at −70°C, as previously described.19 The remaining lymph node tissue was minced in phosphate-buffered saline (Life Technologies, Grand Island, NY) and either used directly in chemotaxis assays or cryopreserved immediately and stored in liquid nitrogen. Individuals who were at low risk or seronegative for HIV-1 infection were recruited to donate peripheral blood only. Peripheral blood was prepared for antibody staining by lysing the red blood cells using FACS™ lysing solution (BD Biosciences, San Jose, CA). Peripheral blood mononuclear cells (PBMC) were isolated by density centrifugation using lymphocyte separation media (Mediatech, Herndon, VA) according to the manufacturer's instructions.
Plasma virus measurements
HIV-1 RNA was measured in plasma using the HIV-1 Monitor Assay (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions.
Chemokine receptor staining of CD8+ T cells
Cells were treated with 1% normal goat serum in phosphate-buffered saline (PBS) to block nonspecific staining, then incubated with CD3-FITC (BD Biosciences, San Jose, CA), CD8-APC (BD Biosciences, San Jose, CA), and CXCR4-PE (BD Biosciences, San Jose, CA) or CX3CR1-PE (MBL International, Woburn, MA) antibodies for 30 min at room temperature. Cells were washed with 1% bovine serum albumin in PBS, fixed in 2% paraformaldehyde, and analyzed on a FACS Caliber flow cytometer using Cell Quest software (Becton Dickinson, Immunocytometry). Samples were gated on CD3+ CD8+ lymphocytes, and then histograms were generated to evaluate CX3CR1 or CXCR4 expression. Approximately 100,000 events were acquired in each analysis. For quantitative determination of the mean number of CX3CR1 or CXCR4 molecules at the surface of each CD3+ CD8+ cell, the QuantiBRITE™ system (BD Biosciences, San Diego, CA) was used. Standardized beads conjugated to four known levels of PE were used to generate a linear regression curve of the log10 of PE molecules per bead against the log10 of fluorescence, allowing for calculation of the number of fluorophores bound per cell.
Tetramer staining of PBMC
Using PBMC from subjects with known tetramer-positive CD8+ T cells, cells were incubated with PE-labeled tetramer for an A2-restricted gag epitope (SLYNTVATL) (Beckman-Coulter, Miami, FL) or APC-labeled tetramer B8-restricted nef epitope (FLKEKGGL) (provided by the NIAID MHC Tetramer Core Facility, Atlanta, Georgia) for 30 min at room temperature. Cells were washed and then stained with CD8-FITC (BD Biosciences, San Jose, CA), incubated for 30 min at room temperature, and washed again. Samples were analyzed on FACS Caliber Flow cytometer with Cell Quest software (Becton Dickinson, Immunocytometry). Between 200,000 and 300,000 events were acquired in each analysis. Results were expressed as the percentage of tetramer-staining cells in the CD8+ population.
Chemotaxis assays
Costar Transwell Plates (Corning Incorporated, Corning, NY) that had inserts composed of polycarbonate with 5-μm pore size were utilized in experiments. Serial 10-fold dilutions of SDF-1α or CX3CL1 (Peprotech Inc., Rocky Hill, NJ) were placed in the bottom well ranging from 1 pg/ml to 106 pg/ml in Hank's balanced salt solution with 0.1% bovine serum albumin, 1 mM CaCl2, and 1 mM MgCl2 (media). Inserts were placed in wells and 500,000 freshly isolated PBMC or lymph node cells in 100 μl of media were added to the upper chamber and incubated for 4 h at 37°C. All assays were performed in duplicate and included wells with media alone. After incubation, inserts were removed and the contents of the lower well were stained with CD3-FITC and CD8-PE antibodies. The number of CD8+ T cells that migrated in each well was determined by flow cytometry using a fixed unit of time (1 min), as previously described.20,21 This method of counting cells was further validated in our laboratory, and results from flow cytometry and manual counting using a hemocytometer and fluorescent microscope were highly correlated (r = 0.9847, p < 0.001). Chemotactic index (CI) was calculated by convention20,21 as the mean number of CD3+ CD8+ cells that migrated in the presence of chemokine divided by the mean number of CD3+ CD8+ cells that migrated in the absence of chemokine.
In four HIV-1-seropositive subjects, assays evaluating chemotaxis of tetramer-staining cells were performed similarly to above methods, except more wells were set up to assess migration of the lower-frequency tetramer+ CD8+ cells and wells were combined for analysis by flow cytometry. For the tetramer chemotaxis experiments, 24 wells were set up for media alone, 12 wells with SDF-1α at 105 pg/ml, and 12 wells with fractalkine at 104 pg/ml. Due to the large quantities of SDF-1α required for this experiment, the cost of SDF-1α, and the observation that majority of individuals demonstrated significant CI at SDF-1α concentration 105 pg/ml, this lower concentration of SDF-1α was utilized for these experiments.
Statistical methods
All statistical analyses assumed a two-sided significance level of 0.05. Wilcoxon two-sample t-tests were used for both within-groups and between-group comparisons. Spearman σ was used to describe correlations.
Results
Demographic and clinical characteristics of study subjects
Eighteen HIV-1-infected individuals and 10 HIV-1-seronegative individuals were recuited to this study. Clinical characteristics are shown in Table 1.22,23 Approximately half of the subjects in both the HIV-1-seropositve and -seronegative groups were women. The median age of HIV-1-seropositive subjects was 37 (range, 25–48) years and was similar to the median age of 40 (range, 25–53) years in the HIV-1-seronegative group. All of the HIV-1-infected subjects had been infected for at least 6 months, and most had been infected for more than 1 year. All but three HIV-1-infected subjects were antiretroviral naïve, and those subjects who had previously taken antiretroviral therapy had in all instances discontinued medications at least 6 months before participating in the study. Among HIV-1-seropositive subjects, the median CD4+ T-cell count was 644 (range, 305–1,103) cells/mm3, and the median plasma HIV-1 RNA concentration was 4.06 (range, 1.7–5.26) log10 copies/ml. All of the HIV-1-infected males reported a history of sex with men, and the majority of HIV-1-infected females reported heterosexual exposure (70%) as their risk factor for HIV-1 infection.
Table 1.
Demographic and Clinical Characteristics of Study Subjects
| Subject ID | Sex | Age | Race | Risk factora | CD4+T Cell count (cells/mm3) | HIV-1 Viral load (log10copies/mL) |
|---|---|---|---|---|---|---|
| HIV-1 Seropositive Subjects | ||||||
| M13 | Male | 29 | caucasian | MSM | 680 | 4.78 |
| M14 | Male | 29 | caucasian | MSM | 540 | 3.85 |
| 32 | Female | 48 | caucasian | H | 775 | 3.71 |
| 62 | Male | 32 | caucasian | MSM | 305 | 4.35 |
| 64 | Female | 25 | caucasian | Ndl stk | 467 | 3.44 |
| 67 | Female | 45 | caucasian | H | 406 | 4.37 |
| 70 | Male | 27 | caucasian | MSM | 968 | 4.17 |
| 71 | Female | 30 | hispanic | H | 447 | 3.20 |
| 73 | Female | 43 | black | H | 635 | 4.20 |
| 74 | Female | 52 | hispanic | IVDA | 895 | 1.70 |
| 77 | Male | 40 | caucasian | MSM | 913 | 5.26 |
| 78 | Male | 26 | caucasian | MSM | 1103 | 3.82 |
| 79 | Female | 42 | caucasian | IVDA | 653 | 4.74 |
| 80 | Male | 43 | caucasian | MSM | 628 | 3.94 |
| 81 | Male | 29 | hispanic | MSM | 697 | 4.23 |
| 82 | Female | 43 | black | H | 835 | 3.57 |
| 84 | Female | 39 | hispanic | H | 358 | 3.83 |
| 90 | Female | 34 | black | H | 332 | 4.86 |
| HIV-1 Seronegative Subjects | ||||||
| 72 | Female | 53 | caucasian | |||
| 99653 | Male | 45 | caucasian | |||
| 99657 | Male | 44 | black | |||
| 99665 | Female | 36 | caucasian | |||
| 99667 | Female | 46 | caucasian | |||
| 99676 | Male | 53 | caucasian | |||
| 99695 | Female | 25 | caucasian | |||
| 99697 | Female | 31 | caucasian | |||
| 99700 | Male | 25 | caucasian | |||
| 99701 | Female | 34 | caucasian | |||
*Heterosexual sex (H); male to male sex (MSM); needle stick (Ndl Stk); intravenous drug use (IVDU).
CXCR4 and CX3CR1 expression is altered on CD8+ T cells of HIV-1-infected individuals
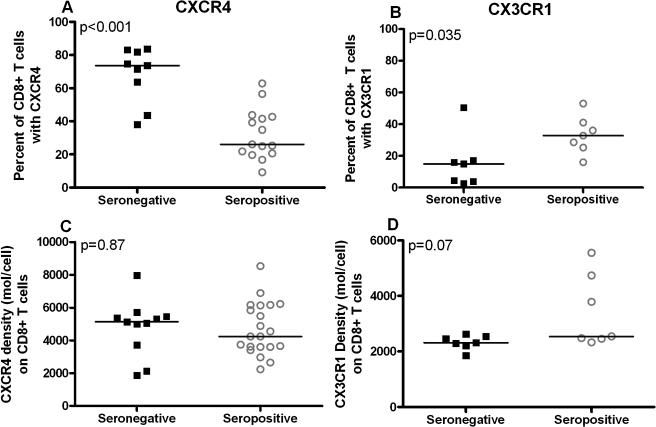
To determine whether expression of chemokine receptors on CD8+ T cells was related to HIV-1 infection, the percent of CD8+ T cells that expressed CXCR4 and CX3CR1 as well as the receptor density on CD8+ T cells was evaluated in HIV-1-seropositive and -seronegative individuals' peripheral blood (Fig. 1). The percentage of CD8+ T cells that expressed CXCR4 was substantially reduced in HIV-1-seropositive individuals (median, 26%; n = 15) compared with HIV-1-seronegative individuals (median, 74%; n = 9; p < 0.001; Fig. 1A). In contrast, the percentage of CD8+ T cells that expressed CX3CR1 was significantly increased in HIV-1-seropositive individuals (median, 33%; n = 7) compared with HIV-1 seronegative individuals (median, 15%; n = 7; p = 0.03; Fig. 1B). Interestingly, the density of CXCR4 or CX3CR1 was not significantly different between HIV-1-seropositive and -seronegative individuals (Figs. 1C and 1D). Furthermore, density of CXCR4 or CX3CR1 expression was not related to percentage of cells that expressed the respective receptor (r = 0.00, p = 1.00; r = 0.31, p = 0.27, respectively). Among HIV-1-seropositive subjects, there was no significant relationship between either CD4+ T-cell count or plasma HIV-1 RNA concentration and either the percentage of cells that expressed the chemokine receptor or the density of the chemokine receptor. Receptor expression on CD8+ T cells in peripheral blood and PBMC isolated by Ficoll-Hypaque density centrifugation was evaluated in the subset of HIV-1-seronegative and -seropositive subjects whose samples were utilized for chemotaxis assays and had available data as Ficoll-Hypaque density centrifugation has previously been demonstrated to alter expression of chemokine receptors, most notably the CCR5 receptor.15,24 On CD8+ T cells, CXCR4 density declined with Ficoll-Hypaque density centrifugation by 20% (n = 11; median = 5,462 versus 4,321 molecules/cell; p = 0.02), and CX3CR1 density remained unchanged (n = 14; median = 2,472 versus 2,486 molecules/cell; p = 0.25). The percentage of CD8+ T cells with receptor expression minimally decreased after Ficoll-Hypaque density centrifugation for both CXCR4 (median, 43–41%; p = 0.02) and CX3CR1 (median, 21–18%; p = 0.03).
FIG. 1.
Percent and density of cell surface CXCR4 and CX3CR1 on CD8+ T cells from HIV-1-seronegative (closed squares) and -seropositive (open circles) individuals. In HIV-1-seropositive subjects, the percent of CXCR4+ CD8+ T cells was significantly lower (A) and the percent of CX3CR1+ CD8+ T cells was significantly higher (B) than in HIV-1-seronegative individuals. CXCR4 (C) and CX3CR1 (D) density was similar in HIV-1-seropositive and -seronegative subjects. Horizontal lines indicate medians.
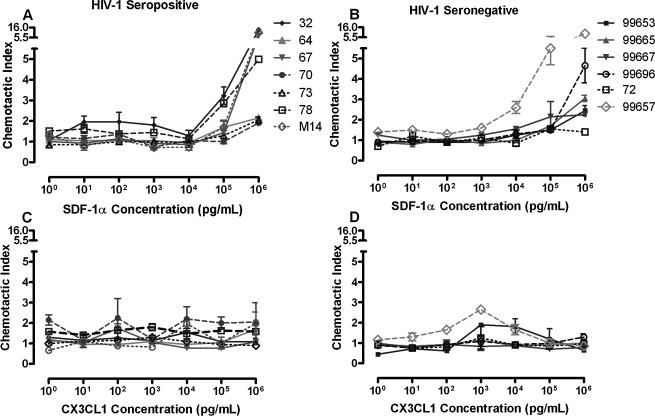
Chemotaxis of PBMC CD8+ T cells to SDF-1α and CX3CL1
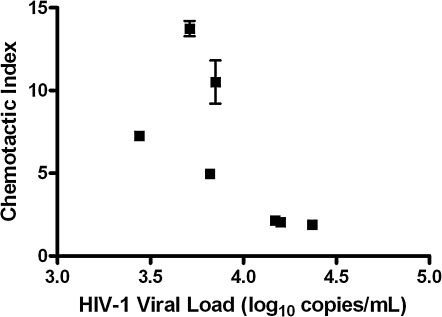
To evaluate whether migration by PBMC CD8+ T cells from HIV-1-infected individuals is impaired, chemotaxis assays were performed in parallel using serial dilutions of SDF-1α or CX3CL1 in seven HIV-1-seropositive and six HIV-1-seronegative individuals (Fig. 2). In both HIV-1-infected and -uninfected subjects, PBMC CD8+ T cells demonstrated dose-dependent migration to SDF-1α, with most manifesting a CI > 1.5 at 105 pg/ml and all at 106 pg/ml (Figs. 2A and 2B). The median CI to SDF-1α at 106 pg/ml was 4.9 in HIV-1-seropositive individuals and was not significantly different from the median CI of 2.8 in the HIV-1-seronegative subjects (p = 0.46). The CI of CD8+ T cells to SDF-1α (106 pg/ml) was not significantly related to either CXCR4 percent expression (r = 0.21, r = 0.43) or density of CXCR4 (r = − 0.03, r = − 0.03). Interestingly, HIV-1 viral load was inversely correlated with CD8+ T cell CI to SDF-1α (106 pg/ml) in HIV-1-seropositive subjects (r = − 0.82, p = 0.03; Fig. 3). In contrast, no consistent relationship was seen between any dose of fractalkine and CD8+ T-cell migration in either HIV-1-seropositive or -seronegative subjects (Figs. 2C and 2D). In addition, no relationship was seen between either the percentage or density of CX3CR1 receptor expression and CD8+ T-cell chemotaxis at any concentration of CX3CL1.
FIG. 2.
Chemotaxis of PBMC CD8+ T cells to varying concentrations of SDF-1α and CX3CL1. Dose-dependent CD8+ T-cell migration to SDF-1α was demonstrated in both HIV-1-seropositive (A) and seronegative (B) individuals. No consistent relationship between CD8+ T-cell chemotaxis and concentration of CX3CL1 was demonstrated in either serogroup (C, D).
FIG. 3.
Chemotaxis of PBMC CD8+ T cells to SDF-1α (106 pg/ml) correlated inversely with HIV-1 RNA concentration (r = − 0.82; p = 0.03). Subjects 32, 64, 67, 70, 73, 78, and M14 are included in this graph. *All data points have two replicates; some data point error bars are not visible as replicates are so close in value.
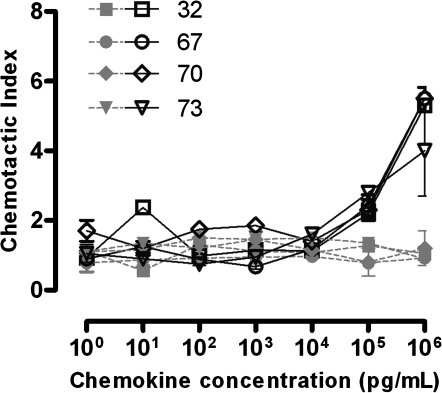
Chemotaxis of CD8+ T cells derived from lymphoid tissue
To determine whether chemotaxis in peripheral blood is reflective of that in lymphoid tissues where the majority of HIV-1 replication occurs, chemotaxis of CD8+ T cells was assessed in disaggregated lymph node cells in parallel with PBMC from four HIV-1-seropositive subjects. Lymph node CD8+ T-cell chemotaxis to SDF-1α and CX3CL1 (Fig. 4) demonstrated the same pattern as that observed in PBMC (Fig. 2), with vigorous, dose-dependent migration to SDF-1α and only weak, non-dose-dependent migration to CX3CL1. The median CIs to SDF-1α (106 pg/ml) in CD8+ T cells derived from PBMC and lymph node were 4.7 and 5.4, respectively (p = NS). The median CIs to CX3CL1 (103 pg/ml) in PBMC and lymph node were also quite similar at 1.2 and 1.3, respectively (p = NS).
FIG. 4.
Lymph node CD8+ T-cell chemotaxis to varying concentrations of SDF-1α (black lines) and CX3CL1 (gray lines) in four HIV-1-seropositive individuals. Lymph node CD8+ T-cell migration to SDF-1α was dose dependent and maximal at 106 pg/ml, although migration to CX3CL1 was relatively lower and not consistently related to any specific concentration of CX3CL1.
Expression of chemokine receptors and chemotaxis of HIV-1-specific CD8+ T cells
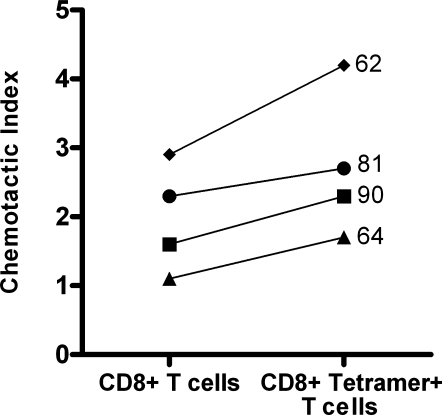
To determine whether migration of peripheral blood CD8+ T cells was reflective of migration by HIV-1-specific CD8+ T cells, chemotaxis assays were performed using PBMC from four HIV-1-infected individuals known to harbor HIV-1-specific CD8+ T cells as detected by staining with MHC-tetrameric complexes. Subjects 62 and 64 had HIV-1- specific CD8+ T cells directed against an A*0201-restricted gag epitope (SLYNTVATL) and subjects 81 and 90 had HIV-1-specific CD8+ T cells directed against a B8-restricted nef epitope (FLKEKGGL). Tetramer-positive CD8+ T cells constituted 2.0% to 5.5% of total CD8+ T cells in these subjects. A median of 22% of tetramer-staining cells expressed CXCR4, similar to the median percentage of CD8+ T cells that expressed CXCR4 in these subjects (25%). All four subjects demonstrated chemotaxis of HIV-1-specific CD8+ T cells (median CI = 2.5) to SDF-1α (105 pg/ml) with a CI that was similar if not slightly higher than that of their PBMC CD8+ T cells (median CI = 1.9, p = 0.1; Fig. 5).
FIG. 5.
Chemotaxis to SDF-1α (105 pg/ml) was similar for CD8+ T cells and HIV-1 tetramer-staining CD8+ T cells from PBMC in four HIV-1-seropositive subjects (p = 0.1). Each line represents a paired experiment from one subject with adjacent numbers indicating subject ID number.
A median of 34% of tetramer-staining cells expressed CX3CR1, similar to the median of 28% of CD8+ T cells that expressed CX3CR1 in these subjects. HIV-1-specific CD8+ T-cell migration to CX3CL1 could not be assessed in two subjects because too few cells migrated in response to CX3CL1 to evaluate for chemotaxis. In the other two (subjects 81 and 90), the CI of HIV-1-specific CD8+ T cells was 0.9 and 1.2 and was quite similar to the CIs of 1.1 and 1.3 seen in PBMC CD8+ T cells from these subjects, respectively.
Discussion
To assess the hypothesis that impaired migration of HIV-1-specific CD8+ cells might contribute to ongoing HIV-1 replication in vivo, we evaluated chemotaxis of CD8+ T cells to two known lymphocyte chemoattractants, SDF-1α and CX3CL1. To our knowledge, this is the first study to systematically examine chemotaxis of CD8+ T cells in peripheral blood and lymphoid tissues of HIV-1-infected individuals, as well as the first to evaluate chemotaxis of HIV-1-specific CD8+ T cells to these chemokines. Similar to previous studies, we observed that expression of the receptors for these chemoattractants was altered in HIV-1 infection. Specifically, CXCR4 was expressed on a lower25 and CX3CL1 was expressed on a higher15 percentage of CD8+ T cells in HIV-1-infected individuals as compared with healthy individuals at low risk for HIV-1 infection. Nevertheless, despite upregulation of CX3CR1 on CD8+ T cells from HIV-1-seropositive individuals, there was little chemotaxis of these cells in response to CX3CL1 in either HIV-1-seropositive or -seronegative individuals. Conversely, despite diminished expression of CXCR4 on peripheral blood and lymph node CD8+ T cells, as well as peripheral blood HIV-1-specific CD8+ T cells from HIV-1-seropositive subjects, robust migration of these cells in response to SDF-1α was observed. Although overall there was no significant difference between seropositive and seronegative individuals in the ability of their CD8+ T cells to migrate toward SDF-1α, plasma HIV-1 RNA concentration was inversely correlated with CI to SDF-1α, suggesting that impaired CD8+ T-cell migration is associated with higher viral loads. Migration of HIV-1-specific CD8+ T cells to SDF-1α paralleled that demonstrated by CD8+ T cells in four subjects in whom this was analyzed. These data suggest that impaired migration of HIV-1-specific CD8+ T cells exists at high viral loads and may contribute to ongoing HIV-1 replication in vivo.
Since the identification of CXCR4 as an HIV-1 coreceptor,13,26 multiple studies have evaluated its expression on peripheral blood lymphocytes.17,25 Similar to previous studies, we observed that the percentage of cells that expressed CXCR4 in HIV-1-seropositive individuals was less than half that seen in HIV-1-seronegative individuals. CXCR4 is primarily expressed on naïve and central memory CD8+ T cells, and the receptor is downregulated as cells mature to an effector memory phenotype.18 It seems likely that heightened immune activation and expansion of memory CD8+ T cells, which is seen in both PBMC and lymph node cells of HIV-1-seropositive individuals,27 is one potential cause of diminished expression of CXCR4. In addition, increased ligand-induced endocytosis28 through upregulation of SDF-1α in HIV-1 infection29 may decrease CXCR4 cell surface expression in HIV-1-infected individuals. HIV-1 Nef protein has been shown to downmodulate CXCR4 expression on the surface of lymphocytes as well.30 Thus, multiple factors could contribute to diminished expression of CXCR4 in HIV-1-infected individuals.
SDF-1α is a highly potent chemokine, attracting 80% of resting T cells as compared with other chemokines such as MCP-1, MIP-1α, IL-8, and RANTES, which only attract 1–10% of resting T cells in healthy individuals.12,13 In addition, high SDF-1α concentrations (1 μg/ml) can also cause CD8+ T-cell fugetaxis,31 or movement away from the chemokine. The present study demonstrated that SDF-1α is a potent chemoattractant for both peripheral blood and lymph node CD8+ T cells in HIV-1-infected individuals. In addition, it demonstrated for the first time that SDF-1α is a potent chemoattractant for HIV-1-specific CD8+ T cells identified by MHC class I tetrameric complexes. It is possible that this study even underestimates the magnitude of chemoattraction induced by SDF-1α in vivo, because Ficoll-Hypaque density centrifugation induced a 20% decrease in CXCR4 density on CD8+ T cells.
HIV-1 RNA concentration was inversely related to chemotaxis to SDF-1α, and impairments in chemotaxis were particularly evident at viral loads above 10,000 copies HIV-1 RNA/ml. Although overall no significant differences in CD8+ T-cell chemotaxis to SDF-1α were observed between HIV-1-seropositive and -seronegative individuals, it is conceivable that differences might be observed if larger numbers of subjects were studied. Additional studies are needed as well to determine whether impairments in migration that are seen at high viral loads occur in all CD8+ T-cell subsets, including naïve and memory subsets, as well as HIV-1-specific and other antigen-specific subsets.
No relationship was seen between the magnitude of migration of CD8+ T cells to SDF-1α and either the percentage of CD8+ T cells that expressed CXCR4 or the density of CXCR4 expression in HIV-1-seropositive or -seronegative individuals whether they were analyzed separately or collectively. These data underscore the fact that caution must be used in inferring chemotactic potential from chemokine receptor expression. These data suggest as well that the impairments of CD8+ T-cell chemotaxis seen in HIV-1-infected individuals with high viral loads are not directly mediated by downregulation of CXCR4. One possible mechanism is desensitization of CXCR4 to ligand-induced stimulation, which can occur through multiple mechanisms (reviewed in Busillo and Benovic32). Interestingly, germinal center CD4+ T cells have been reported to be refractory to SDF-1α despite high levels of CXCR4 expression, and expression of the regulator of G-protein signaling (RGS) proteins RGS13 and RGS16 is correlated with this unresponsiveness.33 It is unclear, however, whether RGS13 and RGS16 are upregulated in HIV-1 infection and whether they affect CD8+ T cells in a similar manner. T-cell receptor stimulation induces protein kinase C phosphorylation of CXCR4, resulting in impaired signaling through the receptor and diminished chemotaxis of murine CD4+ T cells as well as downregulation of CXCR4.34 In addition, HIV-1 Nef has been reported to impair downstream signaling via CXCR4 resulting in impaired chemotaxis of human CD4+ lymphocytes to SDF-1α.35 Whether these or other mechanisms explain the impaired SDF-1α responses of CD8+ T cells from individuals with high HIV-1 RNA concentrations remains to be determined.
Similar to CXCR4, CX3CR1 serves as a coreceptor for HIV-1, albeit a minor one.15,36 Polymorphisms in CX3CR1 have been reported to alter HIV-1 disease progression.37,38 although these data were not confirmed in another study39 and the putative mechanism for the influence of CX3CR1 on HIV-1 disease progression is unclear. Consistent with a previous study, we found that the percentage of CD8+ T cells that expressed CX3CR1 was significantly higher in HIV-1-seropositive compared with HIV-1-seronegative individuals.15 CX3CR1 is expressed on almost all effector CD8+ T cells in both HIV-1-seronegative40 and HIV-1-seropositive15 individuals, and the increased expression in HIV-1 infection is likely due to the expansion of effector CD8+ T cells in HIV-1-infected individuals.
The natural ligand of CX3CR1, CX3CL1, elicits very modest lymphocyte migration in healthy donors.40,41 Although Combadiere et al.15 reported that CD8+ T-cell migration to CX3CL1 was greater in PBMC from three HIV-1-seropositive (10% of CD8+ T cells seeded) as compared to two HIV-1-seronegative subjects (1% of CD8+ T cells seeded), we were unable to confirm this observation. A limitation of the present study was the lack of a strong positive control for migration to fractalkine. Nevertheless, the source of fractalkine in our study was the same as that for the study by Combadiere et al., and the manufacturer confirmed that the product elicited lymphocyte migration in vitro but acknowledged donor variability. Although our experimental conditions were identical to those in the study performed by Combadiere et al., possible reasons for the discrepancy include the way that migration was quantified, as their method did not account for potential differences in nonspecific cellular migration, or other immeasurable differences in technique. Interestingly, the magnitude of CD8+ T-cell migration to SDF-1α in their study15 at 10% of CD8+ T cells seeded was substantially lower than the average 50% migration reported in other studies.12,13 Finally, a major limitation of any in vitro study of lymphocyte migration is that it does not necessarily recapitulate the factors that may influence cell movements in vivo. Specifically, fractalkine may play a more significant role in lymphocyte migration in conjunction with other chemokines such as MIP-1β and MCP-1, as demonstrated by Nishimura et al.40 Although the present study did not confirm that soluble fractalkine is a major chemoattractant for CD8+ T cells from HIV-1-infected individuals, it does not exclude a role for this chemokine in HIV-1-specific CD8+ T-cell localization in vivo.
The reasons for the failure of virus-specific CD8+ T cells to fully suppress HIV-1 replication are unclear. Because the majority of HIV-1 replication occurs in lymphoid tissues, it is critical to understand the determinants of lymphocyte migration at those sites. In the present study we demonstrated that SDF-1α is a potent chemoattractant for CD8+ T cells from PBMC and lymph node as well as for HIV-1-specific CTL. Furthermore, the present study demonstrated that CD8+ T-cell migration to SDF-1α is impaired at high levels of plasma HIV-1 RNA concentration. Whether the impairment of CD8+ T-cell migration that exists at high viral loads is the cause or the consequence of ongoing virus replication and the mechanisms underlying this impairment merit further investigation. Studies of the impact of potent antiretroviral therapy on the ability of CD8+ T cells to migrate in response to SDF-1α would help to clarify this issue. We and others have demonstrated a high concentration of HIV-1-specific CTL within lymphoid tissues in chronic HIV-1 infection,10,19,42,43 and we recently reported that HIV-1-specific CTL do not localize at sites of HIV-1 replication in these tissues.23 A better understanding of the key determinants of CTL migration within lymphoid tissues of individuals with HIV-1 infection could provide critical insights into the immunopathogenesis of HIV-1 infection and ultimately lead to immunotherapeutic interventions to enhance delivery of CTL to sites of HIV-1 replication.
Acknowledgments
We express our gratitude to the subjects who participated in this study. We also thank Karen Whalen for nursing assistance during surgical procedures; Sonia Flores and Adela Cota-Gomez for sponsoring Kevin Lesh through the Graduate Experiences for Multicultural Students (GEMS) Program; the NIAID Tetramer Facility for provision of tetramers; the University of Colorado Center for AIDS Research Immunology Core for facilitation of the flow cytometry; and Steven Johnson, William Burman, Nancy Madinger, Patty Caraway, Beverly Putnam, Beverly Barber, Monica Carten, Pat Gourley, Ron Schimmel, and Jeff Logan for assistance in recruiting subjects to this study. This work was supported by Public Health Services Grants T35-HL080975, PO1-AI-55356, and P30-AI-054907 from the National Institutes of Health. These data were presented in part at the 12th Conference on Retro-viruses and Opportunistic Infections, Boston, MA, February 22–25, 2005 (Abstract # 465), and the 15th Conference on Retroviruses and Opportunistic Infections, Boston, MA, February 3–6, 2008 (Abstract # 434).
References
- 1.Koup RA. Safrit JT. Cao Y. Andrews CA. McLeod G. Borkowsky W, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin X. Bauer DE. Tuttleton SE. Lewin S. Gettie A. Blanchard J, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goepfert PA. Bansal A. Edwards BH. Ritter GD., Jr. Tellez I. McPherson SA, et al. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J Virol. 2000;74:10249–10255. doi: 10.1128/jvi.74.21.10249-10255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appay V. Nixon DF. Donahoe SM. Gillespie GM. Dong T. King A, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostense S. Ogg GS. Manting EH. Gillespie G. Joling J. Vandenberghe K, et al. High viral burden in the presence of major HIV-specific CD8(+) T cell expansions: evidence for impaired CTL effector function. Eur J Immunol. 2001;31:677–686. doi: 10.1002/1521-4141(200103)31:3<677::aid-immu677>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Shankar P. Russo M. Harnisch B. Patterson M. Skolnik P. Lieberman J. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood. 2000;96:3094–3101. [PubMed] [Google Scholar]
- 7.Pantaleo G. Graziosi C. Demarest JF. Butini L. Montroni M. Fox CH, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 8.Embretson J. Zupancic M. Ribas JL. Burke A. Racz P. Tenner-Racz K, et al. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 9.Tenner-Racz K. Racz P. Bofill M. Schulz-Meyer A. Dietrich M. Kern P, et al. HTLV-III/LAV viral antigens in lymph nodes of homosexual men with persistent generalized lymphadenopathy and AIDS. Am J Pathol. 1986;123:9–15. [PMC free article] [PubMed] [Google Scholar]
- 10.Tedla N. Dwyer J. Truskett P. Taub D. Wakefield D. Lloyd A. Phenotypic and functional characterization of lymphocytes derived from normal and HIV-1-infected human lymph nodes. Clin Exp Immunol. 1999;117:92–99. doi: 10.1046/j.1365-2249.1999.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein JV. Nombela-Arrieta C. Chemokine control of lymphocyte trafficking: a general overview. Immunology. 2005;116:1–12. doi: 10.1111/j.1365-2567.2005.02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bleul CC. Fuhlbrigge RC. Casasnovas JM. Aiuti A. Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleul CC. Farzan M. Choe H. Parolin C. Clark-Lewis I. Sodroski J, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 14.Foussat A. Coulomb-L'Hermine A. Gosling J. Krzysiek R. Durand-Gasselin I. Schall T, et al. Fractalkine receptor expression by T lymphocyte subpopulations and in vivo production of fractalkine in human. Eur J Immunol. 2000;30:87–97. doi: 10.1002/1521-4141(200001)30:1<87::AID-IMMU87>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Combadiere B. Faure S. Autran B. Debre P. Combadiere C. The chemokine receptor CX3CR1 controls homing and anti-viral potencies of CD8 effector-memory T lymphocytes in HIV-infected patients. AIDS. 2003;17:1279–1290. doi: 10.1097/00002030-200306130-00002. [DOI] [PubMed] [Google Scholar]
- 16.Combadiere C. Salzwedel K. Smith ED. Tiffany HL. Berger EA. Murphy PM. Identification of CX3CR1. A chemotactic receptor for the human CX3C chemokine fractalkine and a fusion coreceptor for HIV-1. J Biol Chem. 1998;273:23799–23804. doi: 10.1074/jbc.273.37.23799. [DOI] [PubMed] [Google Scholar]
- 17.Bleul CC. Wu L. Hoxie JA. Springer TA. Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi N. Takata H. Yokota S. Takiguchi M. Down-regulation of CXCR4 expression on human CD8+T cells during peripheral differentiation. Eur J Immunol. 2004;34:3370–3378. doi: 10.1002/eji.200425587. [DOI] [PubMed] [Google Scholar]
- 19.Folkvord JM. Anderson DM. Arya J. MaWhinney S. Connick E. Microanatomic relationships between CD8+cells and HIV-1-producing cells in human lymphoid tissue in vivo. J Acquir Immune Defic Syndr. 2003;32:469–476. doi: 10.1097/00126334-200304150-00001. [DOI] [PubMed] [Google Scholar]
- 20.Storie I. Sawle A. Goodfellow K. Whitby L. Granger V. Reilly JT, et al. Flow rate calibration I: a novel approach for performing absolute cell counts. Cytometry B Clin Cytom. 2003;55:1–7. doi: 10.1002/cyto.b.10051. [DOI] [PubMed] [Google Scholar]
- 21.Storie I. Sawle A. Whitby L. Goodfellow K. Granger V. Reilly JT, et al. Flow rate calibration II: a clinical evaluation study using PanLeucoGating as a single-platform protocol. Cytometry B Clin Cytom. 2003;55:8–13. doi: 10.1002/cyto.b.10050. [DOI] [PubMed] [Google Scholar]
- 22.Folkvord JM. Armon C. Connick E. Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS Res Hum Retroviruses. 2005;21:363–370. doi: 10.1089/aid.2005.21.363. [DOI] [PubMed] [Google Scholar]
- 23.Connick E. Mattila T. Folkvord JM. Schlichtemeier R. Meditz AL. Ray MG, et al. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol. 2007;178:6975–6983. doi: 10.4049/jimmunol.178.11.6975. [DOI] [PubMed] [Google Scholar]
- 24.Berhanu D. Mortari F. De Rosa SC. Roederer M. Optimized lymphocyte isolation methods for analysis of chemokine receptor expression. J Immunol Methods. 2003;279:199–207. doi: 10.1016/s0022-1759(03)00186-8. [DOI] [PubMed] [Google Scholar]
- 25.Ostrowski MA. Justement SJ. Catanzaro A. Hallahan CA. Ehler LA. Mizell SB, et al. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J Immunol. 1998;161:3195–3201. [PubMed] [Google Scholar]
- 26.Oberlin E. Amara A. Bachelerie F. Bessia C. Virelizier JL. Arenzana-Seisdedos F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 27.Yang OO. Ferbas JJ. Hausner MA. Hultin LE. Hultin PM. McFadden D, et al. Effects of HIV-1 infection on lymphocyte phenotypes in blood versus lymph nodes. J Acquir Immune Defic Syndr. 2005;39:507–518. [PubMed] [Google Scholar]
- 28.Signoret N. Oldridge J. Pelchen-Matthews A. Klasse PJ. Tran T. Brass LF, et al. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J Cell Biol. 1997;139:651–664. doi: 10.1083/jcb.139.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohashi T. Arai M. Kato H. Kubo M. Fujii M. Yamamoto N, et al. High SDF-1 expression in HIV-1 carriers does not correlate with CD8+T-cell-mediated suppression of viral replication. Virology. 1998;244:467–472. doi: 10.1006/viro.1998.9151. [DOI] [PubMed] [Google Scholar]
- 30.Venzke S. Michel N. Allespach I. Fackler OT. Keppler OT. Expression of Nef downregulates CXCR4, the major coreceptor of human immunodeficiency virus, from the surfaces of target cells and thereby enhances resistance to superinfection. J Virol. 2006;80:11141–11152. doi: 10.1128/JVI.01556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poznansky MC. Olszak IT. Foxall R. Evans RH. Luster AD. Scadden DT. Active movement of T cells away from a chemokine. Nat Med. 2000;6:543–548. doi: 10.1038/75022. [DOI] [PubMed] [Google Scholar]
- 32.Busillo JM. Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768:952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estes JD. Thacker TC. Hampton DL. Kell SA. Keele BF. Palenske EA, et al. Follicular dendritic cell regulation of CXCR4-mediated germinal center CD4 T cell migration. J Immunol. 2004;173:6169–6178. doi: 10.4049/jimmunol.173.10.6169. [DOI] [PubMed] [Google Scholar]
- 34.Peacock JW. Jirik FR. TCR activation inhibits chemotaxis toward stromal cell-derived factor-1: evidence for reciprocal regulation between CXCR4 and the TCR. J Immunol. 1999;162:215–223. [PubMed] [Google Scholar]
- 35.Choe EY. Schoenberger ES. Groopman JE. Park IW. HIV Nef inhibits T cell migration. J Biol Chem. 2002;277:46079–46084. doi: 10.1074/jbc.M204698200. [DOI] [PubMed] [Google Scholar]
- 36.Garin A. Tarantino N. Faure S. Daoudi M. Lecureuil C. Bourdais A, et al. Two novel fully functional isoforms of CX3CR1 are potent HIV coreceptors. J Immunol. 2003;171:5305–5312. doi: 10.4049/jimmunol.171.10.5305. [DOI] [PubMed] [Google Scholar]
- 37.Faure S. Meyer L. Costagliola D. Vaneensberghe C. Genin E. Autran B, et al. Rapid progression to AIDS in HIV+individuals with a structural variant of the chemokine receptor CX3CR1. Science. 2000;287:2274–2277. doi: 10.1126/science.287.5461.2274. [DOI] [PubMed] [Google Scholar]
- 38.Singh KK. Hughes MD. Chen J. Spector SA. Genetic polymorphisms in CX3CR1 predict HIV-1 disease progression in children independently of CD4+lymphocyte count and HIV-1 RNA load. J Infect Dis. 2005;191:1971–1980. doi: 10.1086/430091. [DOI] [PubMed] [Google Scholar]
- 39.McDermott DH. Colla JS. Kleeberger CA. Plankey M. Rosenberg PS. Smith ED, et al. Genetic polymorphism in CX3CR1 and risk of HIV disease. Science. 2000;290:2031. doi: 10.1126/science.290.5499.2031a. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura M. Umehara H. Nakayama T. Yoneda O. Hieshima K. Kakizaki M, et al. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol. 2002;168:6173–6180. doi: 10.4049/jimmunol.168.12.6173. [DOI] [PubMed] [Google Scholar]
- 41.Imai T. Hieshima K. Haskell C. Baba M. Nagira M. Nishimura M, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 42.Brask S. Hager H. Pallesen G. Porwit A. Biberfeld P. Gerstoft J. Quantification of CD8-positive lymphocytes in lymph node follicles from HIV-infected male homosexuals and controls. Acta Pathol Microbiol Immunol Scand [A] 1987;95:155–157. doi: 10.1111/j.1699-0463.1987.tb00024_95a.x. [DOI] [PubMed] [Google Scholar]
- 43.Wood GS. Burns BF. Dorfman RF. Warnke RA. In situ quantitation of lymph node helper, suppressor, and cytotoxic T cell subsets in AIDS. Blood. 1986;67:596–603. [PubMed] [Google Scholar]