Abstract
Cellulose sulfate, a polyanionic compound derived from cotton, has been proposed as a topical microbicide to reduce the sexual transmission of HIV. However, a phase III clinical trial of a vaginal gel formulation of cellulose sulfate (Ushercell) had to be prematurely closed after early data indicated microbicide users had a higher rate of HIV infection than women using a placebo. The unexpected results of the cellulose sulfate trail prompted us to reexamine and attempt to replicate the available preclinical data for this compound and other polyanions. We show here that cellulose sulfate has a biphasic effect on HIV infection in vitro: at high concentrations it inhibits infection but at low concentrations it significantly and reproducibly increases HIV infection. This stimulatory effect is evident for the R5-tropic strains of virus responsible for sexual transmission, reflects the rate of infection rather than viral growth, and occurs at clinically relevant concentrations of the compound. An examination of published studies shows that the biphasic effect of cellulose sulfate was evident in previous research by independent laboratories and is also found for other polyanions such as dextrin sulfate and PRO2000. These data help in understanding the failure of the Ushercell clinical trial and indicate that cellulose sulfate is not safe for mucosal application in humans.
There is an urgent need for safe and effective topical microbicides to reduce the sexual transmission of HIV. Among the various classes of molecules proposed for this purpose, there has been particular clinical interest in sulfated polymeric anionic compounds such as cellulose sulfate (a high-molecular-weight β1,4-glucose polymer derived from cotton), carrageenan (a polygalactose + 3,6-anhydrogalactose copolymer extracted from seaweed), dextrin sulfate (a synthetic α1,4-d-glucose polymer), and PRO2000 (a synthetic naphthalene sulfonate polymer).1,2 These compounds are thought to nonspecifically inhibit HIV entry into target cells by interacting with basic regions of the viral envelope.3 Recently, however, the concept of polyanion microbicides was dealt a major setback when the Contraceptive Research and Development Program (CONRAD) announced that they had halted a phase III clinical trial of a formulated cellulose sulfate vaginal gel (Ushercell) being conducted in South Africa, Benin, Uganda, and India because the independent data monitoring committee detected increased HIV infection rates in the women who received cellulose sulfate compared to placebo gel.4,5
The premature closure of the cellulose sulfate phase III trial prompted us to reexamine and attempt to replicate the available preclinical data for this compound and other polyanions. The current work focuses on the question of how such compounds affect HIV infection in vitro, an essential prerequisite for human testing. Our main finding is that cellulose sulfate at concentrations that would be present in humans using Ushercell can increase infection by sexually transmitted R5-tropic strains of HIV.
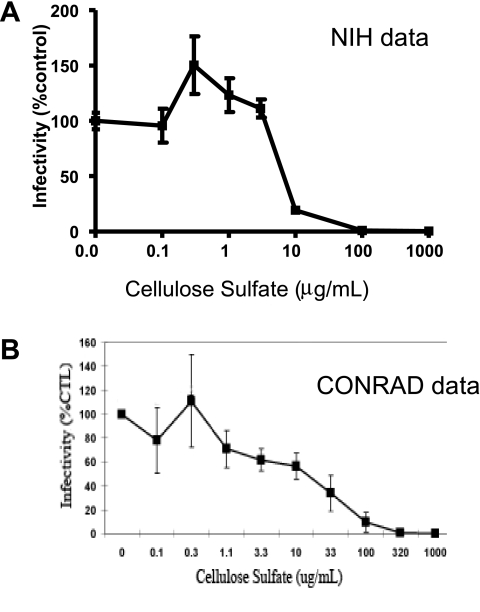
Because cellulose sulfate is thought to act by blocking viral infection rather than replication,6 we initially wished to employ an assay that would reflect the frequency of infection events rather than the ultimate viral yield. Accordingly, cellulose sulfate was titrated on the infection of human peripheral blood mononuclear cells (PBMCs) by the green fluorescent protein (GFP)-tagged R5-tropic reporter virus HIV-1JR-CSF3-GFP and infection rates were monitored by flow cytometric measurement of intracellular GFP as described.7,8 As shown in Fig. 1A, the resulting titration curve was biphasic. Concentrations of cellulose sulfate ≥10 μg/ml inhibited HIV infection. However, lower concentrations of cellulose sulfate (0.3–3 μg/ml) increased infection rates, and this was statistically significant for the data points at 0.3 μg/ml (50% increase, p = 0.007) and 1 μg/ml (23% increase, p = 0.02). The data shown in Fig. 1A represent quintuplicate replicate wells from a single experiment and were reproduced in several independent experiments using different sources of PBMCs and different viral preparations.
FIG. 1.
Biphasic effects of cellulose sulfate on HIV infection. (A) Data from the current study conducted at the National Institutes of Health. PBMC were prepared from a healthy HIV-negative donor by Ficoll-Plaque Plus density gradient centrifugation and cultured in RPMI-1640 with 10% fetal calf serum, 2 mM glutamine, 20 units/ml IL-2, and antibiotics. The cells were incubated with various concentrations of cellulose sulfate (Acros Organics) for 1 h at 37°C and then infected with the GFP-tagged R5-tropic reporter virus HIV-1JR-CSF3-GFP.7,8,25 After 72 h at 37°C, the extent of reporter virus replication was assessed by flow cytometry (Becton Dickinson FACS Calibur) by measuring the fraction of GFP+ cells, which appeared as off-the-diagonal dots in a plot of PE (background fluorescence) versus FITC (GFP signal).7,25,26 The data represent the mean ± SD for quintuplicate wells from a single experiment and were normalized to 100% for the infection level in the absence of cellulose sulfate. (B) Data from CONRAD presented at the 2007 Annual meeting of the Alliance for Microbicide Development (http://www.microbicide.org/microbicideinfo/reference/Gabelnick.AMD10.pdf). This experiment also examined infection of PBMC by an R5 virus and normalized the data to a value of 100% in the absence of compound.
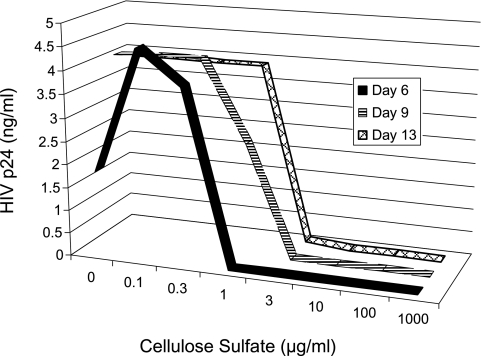
We next performed a kinetics assay in which infection was assessed by the more traditional method of measuring viral yields. PBMCs were infected with HIV-1JRFL in the presence of various concentrations of cellulose sulfate and HIV p24 (gag) protein in the cell-free supernatant was measured at different times post infection. Figure 2 shows that the titration curve measured at the earliest time point, 6 days, was clearly biphasic; concentrations of cellulose sulfate of 0.1 μg/ml and 0.3 μg/ml increased p24 levels by 150% and 110%, respectively, whereas concentrations of cellulose sulfate ≥1 μg/ml inhibited viral yields. By contrast, when supernatants from the same wells were measured at 9 or 13 days after infection, the resulting titration curves appeared to be monophasic due to the fact that the cultures with partial infection had reached the maximal viral accumulation levels that can be achieved in this system, whereas the cultures that were fully inhibited remained uninfected. This result highlights the importance of performing kinetic experiments in inhibition studies to ensure that the measured output of an assay reflects actual infection events as compared to viral yield.
FIG. 2.
Kinetics of HIV production in the presence of cellulose sulfate. PBMC were prepared as in Fig. 1A, incubated with the native R5-tropic reporter virus HIV-1JRFL for 1 h at 37°C in the presence of the indicated concentration of cellulose sulfate, then washed carefully. At 6, 9, and 13 days postinfection, aliquots of the culture fluid were centrifuged and the cell-free supernatants were assayed for HIV p24 by ELISA (Perkin Elmer Life Sciences HIV-1 p24 ELISA Kit).
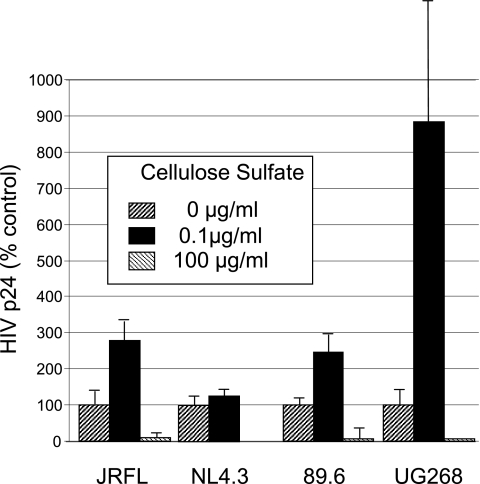
Figure 3 shows the effects of low and high concentrations of cellulose sulfate on several different HIV isolates including the laboratory-adapted X4-tropic strain HIVNL4.3, the primary X4/R5 dual-tropic strain HIV89.6, and the primary clade C strain HIVUG268 as well as the primary R5-tropic strain HIVJRFL described above. Although both the sensitivity to cellulose sulfate and the absolute infection rates of these strains varied considerably, all of them exhibited a biphasic profile, with a low concentration of cellulose sulfate increasing infection from approximately 20% for NL4.3 to more than 800% for UG268.
FIG. 3.
Effects of cellulose sulfate on various HIV isolates. PBMC were prepared and infected with the indicated virus (NIH AIDS Research and Reagent Reference Program) in the absence or presence of different concentrations of cellulose sulfate as described in Fig. 2 and the cell-free culture supernatants were assayed for HIV p24 by ELISA at 6 days postinfection. Data were normalized to a value of 100% in the absence of compound. The control p24 levels were 1.82 ng/ml for HIVJRFL, 4.6 ng/ml for HIVNL4-3, 3.14 ng/ml for HIV89.6, and 0.11 ng/ml for HIVUG268.
Our experimental observations prompted us to reexamine the previously available preclinical data for cellulose sulfate and other polyanions. The only paper from CONRAD in the peer reviewed literature is not informative because it tested only high concentrations (≥3.2 μg/ml) of cellulose sulfate on a laboratory-adapted X4-tropic virus that is not sexually transmitted (HIVIIIB).9 However, following closure of the clinical trial, CONRAD presented the results of a more relevant experiment during the 2007 Annual Meeting of the Alliance for Microbicide Development (http://www.micro-bicide.org/microbicideinfo/reference/Gabelnick.AMD10.pdf). The CONRAD data (Fig. 1B) show a biphasic pattern that is strikingly similar to that observed at the NIH (Fig. 1A). Although viral replication was inhibited at high concentrations of cellulose sulfate, there was a trend for increased replication at a concentration of 0.3 μg/ml. Importantly, the apparent spike in the CONRAD titration curve occurred at precisely the same concentration point as in the NIH titration curve; the main difference between the two experiments is that the CONRAD data do not include enough replicates to demonstrate statistical significance.
Stimulation of R5-tropic HIV infection by low concentrations of cellulose sulfate was also reported by Scordi-Bello et al.6 They found that for HIVBaL infection of primary macrophages, 1 μg/ml of cellulose increased infection by approximately 12.5% whereas even 1 mg/ml of cellulose was insufficient to completely inhibit infection. In the case of infection of primary CD4+ T cells by the primary R5-tropic isolate HIVIN/93/905, 1 μg/ml of cellulose sulfate increased infection by approximately 33%. Statistical significance could not be evaluated in these experiments because of lack of power.
The ability of other sulfated polyanions to enhance HIV replication both in vivo and in vitro is well known. In 1991, Flexner et al.10 reported that intravenous administration of dextran sulfate, a polysulfated polysaccharide, significantly increased circulating HIV p24 levels in all eight patients who received the compound for more than 3 days. Subsequently Meylan et al.11 demonstrated that this compound increased the in vitro replication of multiple primary HIV isolates in primary human macrophage.
Figure 4 shows the results of a more recent study by Fletcher et al.12 that examined the activity of the candidate polyanion microbicides PRO2000 and dextrin sulfate against an X4-tropic virus (HIVRF) and an R5-tropic virus (HIVBaL). Although the data were presented in terms of theoretical monophasic inhibition curves (black lines), inspection of the actual data reveals that the curves for the R5-tropic virus are clearly biphasic (red lines). For PRO2000 (Fig. 4A) stimulation of approximately 6–20% was apparent at concentrations of 0.6–9 μg/ml while for dextrin sulfate (Fig. 4B) stimulation of approximately 7–20% was evident at 2.4–34 μg/ml. The X4-tropic virus was more efficiently and uniformly inhibited by these compounds, but there was one spike of activation at 0.14 μg/ml of PRO2000.
FIG. 4.
Effects of two polyanionic compounds on R5 and X4 HIV infection. These data are from Fletcher et al.12 Immobilized virus was incubated with the test compound prior to addition of target PM-1 cells in the presence of compound. Cells were incubated 10 days following which viral replication was determined by reverse transcriptase measurement of culture supernatants. Black lines shows the interpretation of the data in the published paper and red lines highlight the actual data points. (A) Results for PRO2000 and (B) results for dextrin sulfate. HIVBaL (solid squares) is an R5-tropic virus and HIVRF (open squares) is an X4-tropic virus.
Enhancement of HIV infection by low concentrations of compounds that neutralize infectivity at higher concentrations is well known for other types of molecules that bind to the HIV envelope glycoprotein. Soluble CD4, which was tested as an HIV treatment in an early unsuccessful clinical trial,13 was subsequently found to strongly increase infection by certain primary isolates of HIV.14 The infectivity of primary HIV strains is also activated by a variety of monoclonal antibodies directed against multiple regions on the gp120 envelope glycoprotein including the V3 loop, CD4 binding site, and CD4-induced epitopes.14–16 In addition, naturally occurring polyanions, such as the cell surface molecule heparin sulfate, are known to facilitate HIV entry in certain cell types.3,17,18
The main clinical implication of the data reported here is that cellulose sulfate is not safe for vaginal or other topical mucosal use in humans because it can increase HIV infection rates. Because Ushercell is meant to be applied before sex rather than continuously, it is clear that if inhibitory concentrations of cellulose sulfate are ever achieved, enhancing concentrations must also occur due to natural dilution over time. The actual concentration of cellulose sulfate that occurs in the vagina in women using Ushercell is unclear since basic pharmacokinetic data have not been published and the clinical trial protocol included no biochemical measurements of compound distribution or bioavailability. It is known, however, that the modest inhibitory activity of this compound is weakened by the presence of both cervical fluids6 and seminal plasma,19 further reducing the likelihood of Ushercell's preventing HIV transmission during sexual intercourse.
Initial phase I/II clinical trials of Ushercell did not reveal any life-threatening adverse events, overt toxicity, or excessive urogenital irritation caused by cellulose sulfate;20–24 however, because none of these studies examined HIV infection rates, it was premature to conclude that cellulose sulfate is “safe” for use as a vaginal microbicide. Unfortunately, no in vivo efficacy testing in a suitable nonhuman primate model was conducted prior to the human trials.
Our experiments were conducted in a simple in vitro cell infection system in which cellulose sulfate had no obvious effects on cellular activation, apoptosis, or viability. This suggests that the stimulatory effect of the compound is due to an intrinsic property rather than a secondary effect on cellular metabolism or immune interactions. Previous preclinical safety measurement may have focused too narrowly on cellulose sulfate's inflammatory potential and not on its innate biophysical properties. The stimulatory effect of low concentrations of cellulose sulfate was in fact evident in previously available data from independent laboratories, including CONRAD, but its importance seems to have been overlooked because the studies measured viral accumulation rather than infection rates or used X4-tropic rather than R5-tropic test viruses. There are no published data on the effects of cellulose sulfate on the wide spectrum of viral clades and subtypes found in the countries in which the clinical trials were conducted.
Data from an independent laboratory indicate that the HIV stimulatory properties of cellulose sulfate are shared by two other sulfated polyanionic compounds, PRO2000 and dextrin sulfate.12 Human application of these and other sulfated polyanion products should proceed with caution. Indeed, the Population Council in South Africa recently announced the failure of an efficacy trial of carrageenan (Carraguard) and the UK Microbicide Development Program terminated the high-dose arm of their efficacy trial of PRO2000; however, the low-dose arm of the PRO2000 trial is being continued.
Our results highlight several areas for improvement of the preclinical evaluation of candidate microbicides. First, they should include a complete range of compound concentrations; the PRO2000 and dextrin sulfate clearly demonstrate that simply reporting IC50 values for HIV inhibitors is insufficient since this simplification can mask a more complex, biphasic relationship between concentration and infection rate. Second, the results should be made available both to other scientists for analysis and replication and to trial participants; relevant data for cellulose sulfate were not publicly reported until after the clinical trial had been closed. Third, candidate microbicides should not be administered to humans until they have been rigorously tested in suitable animal models. Finally, previously published research on similar compounds should not be ignored; the clinical and laboratory findings on dextran sulfate10,11 are rarely if ever cited in the literature on cellulose sulfate and other polyanion microbicide candidates.1,2
In summary, we have shown that that low concentrations of cellulose sulfate stimulate HIV infection, thereby providing a possible explanation for the results of the halted Ushercell phase III clinical trials. These results highlight the need for a more through, rigorous, and transparent preclinical evaluation procedure to develop safe and effective HIV microbicides.
Acknowledgments
We thank Jay Berzofsky, Peter Kwong, and Daniel Douek for critical reading of the manuscript and the NIH AIDS Research and Reference Program for virus samples. This research was supported by the Intramural Research Program of the National Cancer Institute Center for Cancer Research. The first two authors contributed equally to this paper.
References
- 1.Balzarini J. Van Damme L. Microbicide drug candidates to prevent HIV infection. Lancet. 2007;369:787–797. doi: 10.1016/S0140-6736(07)60202-5. [DOI] [PubMed] [Google Scholar]
- 2.McGowan I. Microbicides: A new frontier in HIV prevention. Biologicals. 2006;34:241–255. doi: 10.1016/j.biologicals.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Moulard M. Lortat-Jacob H. Mondor I, et al. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J Virol. 2000;74:1948–1960. doi: 10.1128/jvi.74.4.1948-1960.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolognesi N. AIDS gel's failure calls prevention approach into question. Nat Med. 2007;13:230. doi: 10.1038/nm0307-230b. [DOI] [PubMed] [Google Scholar]
- 5.van de Wijgert JH. Shattock RJ. Vaginal microbicides: Moving ahead after an unexpected setback. AIDS. 2007;21:2369–2376. doi: 10.1097/QAD.0b013e3282ef83fd. [DOI] [PubMed] [Google Scholar]
- 6.Scordi-Bello IA. Mosoian A. He C, et al. Candidate sulfonated and sulfated topical microbicides: Comparison of anti-human immunodeficiency virus activities and mechanisms of action. Antimicrob Agents Chemother. 2005;49:3607–3615. doi: 10.1128/AAC.49.9.3607-3615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lueders KK. De Rosa SC. Valentin A. Pavlakis GN. Roederer M. Hamer DH. A potent anti-HIV immunotoxin blocks spreading infection by primary HIV type 1 isolates in multiple cell types. AIDS Res Hum Retroviruses. 2004;20:145–150. doi: 10.1089/088922204773004851. [DOI] [PubMed] [Google Scholar]
- 8.Valentin A. Lu W. Rosati M, et al. Dual effect of interleukin 4 on HIV-1 expression: Implications for viral phenotypic switch and disease progression. Proc Natl Acad Sci USA. 1998;95:8886–8891. doi: 10.1073/pnas.95.15.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson RA. Feathergill KA. Diao XH, et al. Preclinical evaluation of sodium cellulose sulfate (Ushercell) as a contraceptive antimicrobial agent. J Androl. 2002;23:426–438. [PubMed] [Google Scholar]
- 10.Flexner C. Barditch-Crovo PA. Kornhauser DM, et al. Pharmacokinetics, toxicity, and activity of intravenous dextran sulfate in human immunodeficiency virus infection. Antimicrob Agents Chemother. 1991;35:2544–2550. doi: 10.1128/aac.35.12.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meylan PR. Kornbluth RS. Zbinden I. Richman DD. Influence of host cell type and V3 loop of the surface glycoprotein on susceptibility of human immunodeficiency virus type 1 to polyanion compounds. Antimicrob Agents Chemother. 1994;38:2910–2916. doi: 10.1128/aac.38.12.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher PS. Wallace GS. Mesquita PM. Shattock RJ. Candidate polyanion microbicides inhibit HIV-1 infection and dissemination pathways in human cervical explants. Retrovirology. 2006;3:46. doi: 10.1186/1742-4690-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daar ES. Li XL. Moudgil T. Ho DD. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc Natl Acad Sci USA. 1990;87:6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillon C. Schutten M. Boers PH. Gruters RA. Osterhaus AD. Antibody-mediated enhancement of human immunodeficiency virus type 1 infectivity is determined by the structure of gp120 and depends on modulation of the gp120-CCR5 interaction. J Virol. 2002;76:2827–2834. doi: 10.1128/JVI.76.6.2827-2834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan N. Sun Y. Binley J, et al. Determinants of human immunodeficiency virus type 1 envelope glycoprotein activation by soluble CD4 and monoclonal antibodies. J Virol. 1998;72:6332–6338. doi: 10.1128/jvi.72.8.6332-6338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan NJ. Antibody-mediated enhancement of viral disease. Curr Top Microbiol Immunol. 2001;260:145–169. doi: 10.1007/978-3-662-05783-4_8. [DOI] [PubMed] [Google Scholar]
- 17.Ugolini S. Mondor I. Sattentau QJ. HIV-1 attachment: Another look. Trends Microbiol. 1999;7:144–149. doi: 10.1016/s0966-842x(99)01474-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YJ. Hatziioannou T. Zang T, et al. Envelope-dependent, cyclophilin-independent effects of glycosaminoglycans on human immunodeficiency virus type 1 attachment and infection. J Virol. 2002;76:6332–6343. doi: 10.1128/JVI.76.12.6332-6343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neurath AR. Strick N. Li YY. Role of seminal plasma in the anti-HIV-1 activity of candidate microbicides. BMC Infect Dis. 2006;6:150. doi: 10.1186/1471-2334-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doh AS. Ngoh N. Roddy R. Lai JJ. Linton K. Mauck C. Safety and acceptability of 6% cellulose sulfate vaginal gel applied four times per day for 14 days. Contraception. 2007;76:245–249. doi: 10.1016/j.contraception.2007.05.083. [DOI] [PubMed] [Google Scholar]
- 21.El-Sadr WM. Mayer KH. Maslankowski L, et al. Safety and acceptability of cellulose sulfate as a vaginal microbicide in HIV-infected women. AIDS. 2006;20:1109–1116. doi: 10.1097/01.aids.0000226950.72223.5f. [DOI] [PubMed] [Google Scholar]
- 22.Malonza IM. Mirembe F. Nakabiito C, et al. Expanded Phase I safety and acceptability study of 6% cellulose sulfate vaginal gel. AIDS. 2005;19:2157–2163. doi: 10.1097/01.aids.0000194797.59046.8f. [DOI] [PubMed] [Google Scholar]
- 23.Mauck C. Weiner DH. Ballagh S, et al. Single and multiple exposure tolerance study of cellulose sulfate gel: A Phase I safety and colposcopy study. Contraception. 2001;64:383–391. doi: 10.1016/s0010-7824(01)00271-2. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz JL. Mauck C. Lai JJ, et al. Fourteen-day safety and acceptability study of 6% cellulose sulfate gel: A randomized double-blind Phase I safety study. Contraception. 2006;74:133–140. doi: 10.1016/j.contraception.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Valentin A. Rosati M. Patenaude DJ, et al. Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2002;99:7015–7020. doi: 10.1073/pnas.102672999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao S. Hu S. McHugh L, et al. Toward a live microbial microbicide for HIV: Commensal bacteria secreting an HIV fusion inhibitor peptide. Proc Natl Acad Sci USA. 2005;102:11993–11998. doi: 10.1073/pnas.0504881102. [DOI] [PMC free article] [PubMed] [Google Scholar]