Abstract
Canine cancers occur with an incidence similar to that of humans and share many features with human malignancies including histological appearance, tumor genetics, biological behavior, and response to conventional therapies. As observed in humans, the telomerase reverse transcriptase (TERT) activity is largely confined to tumor tissues and absent in the majority of normal dog tissues. Therefore, dog TERT (dTERT) can constitute a valid target for translational cancer immunotherapy. We have evaluated the ability of adenovirus serotype 6 (Ad6) and DNA electroporation (DNA-EP) to induce immune responses against dTERT in dogs affected by malignant lymphoma (ML). The vaccine was combined with standard chemotherapy regimen [cyclophosphamide, vincristine, prednisone (COP)]. dTERT-specific immune response was induced in 13 out of 14 treated animals (93%) and remained detectable and long-lasting with the absence of autoimmunity or other side effects. Most interestingly, the survival time of vaccine/Chemo-treated dogs was significantly increased over historic controls of Chemo-treated animals (>97.8 versus 37 weeks, respectively, P = 0.001). Our results show that Ad6/DNA-EP-based cancer vaccine against dTERT overcomes host immune tolerance, should be combined with chemotherapy, induces long-lasting immune responses, and significantly prolongs the survival of ML canine patients. These data support further evaluation of this approach in human clinical trials.
Introduction
Telomerase is a ribonucleoprotein comprising an RNA component and a catalytic protein component [telomerase reverse transcriptase (TERT)].1,2 As telomerase confers immortality to cells, telomerase activity has been detected in cancerous cell lines and in a diverse range of tumor types.3 Conversely, telomerase is inactive or only transiently expressed at low levels in normal human tissues and normal somatic cell cultures. The combination of telomerase overexpression in most tumor types as well as low or absent expression in normal cells makes TERT a tumor-associated antigen and a suitable target for cancer immunotherapy. In these conditions, TERT is processed and presented in the context of class I major histocompatibility complex molecules, and tumors are recognized by T lymphocytes specific against telomerase.4,5 These findings have justified vaccination trials in cancer patients based either on autologous dendritic cells transfected or loaded with human TERT–derived peptides.6,7
Among genetic vaccines, in vivo electroporation of plasmid DNA (DNA-EP) and replication-defective recombinant adenoviruses (Ads) have proven to be very efficacious in inducing strong antibody and cellular antigen-specific immune responses against a variety of antigens in several species.8,9,10,11,12 Combinations of heterologous modalities of immunization induce superior immune reactions as compared to single modality vaccines.13,14
However, the translational relevance of cancer immunotherapy is strictly dependent on the preclinical models employed (mostly rodents, to date) and suffers from the lack of a suitable therapeutic large animal model. In light of this, dogs represent a good research model due to their large size, spontaneous tumor occurrence, a gene expression pattern similar with human tumors15 and comparable influence to environmental factors.16 Cancers in pet dogs are characterized by tumor growth over long periods of time in the setting of an intact immune system, interindividual and intratumoral heterogeneity, the development of recurrent or resistant disease, and metastasis to relevant distant sites. Thanks to their large population size, cancer rate in pets is sufficient to power clinical trials, including assessment of new drugs. However, to date, the application of cancer vaccines in dogs has not been widely explored, with the exception of a xenogeneic DNA-based vaccine for melanoma17,18 that led to the successful approval and the commercial launch of a veterinary biological (Merial US, Duluth, GA).
Telomerase activity has been reported in the majority (>90%) of canine tumors.19,20,21,22,23 Pairwise alignments of TERT protein sequences revealed that human protein shares greater homology with Canis familiaris TERT (dTERT)24 than with mouse TERT25 and, as observed in human tumors, dTERT activity contributes to maintenance of telomere length.21 In contrast, telomere shortening and telomerase activation have only a minor effect on murine tumorigenesis. In addition, the observation that human telomeres (10–15 kb long) are more similar in size to the canine telomeres (3–23 kb) rather than the mouse telomeres (25–100 kb) suggests that the canine cancer is a better experimental model for telomerase studies and for the evaluation of therapeutic agents than those that are based on murine tumors.26,27
Malignant lymphoma (ML) is the most common hematopoietic malignancy in dogs, caused by clonal proliferation of lymphocytes in solid tissue with distinctive morphologic and immunophenotypic features. The median age of occurrence is about 7 years,28,29 and the aetiology is multifactorial (viral, genetic predisposition, etc.) with a positive association with exposure to herbicide, chemicals, and with living in highly polluted areas.16,30 Several clinical symptoms can be distinguished on the basis of anatomical location: multicentric, alimentary, mediastinal, cutaneous, and extranodal. The most common is the multicentric or generalized lymphadenopathy.31 Historically, the World Health Organization (WHO) has proposed a classification based on clinical stage: I, involvement limited to a single lymph node or lymphoid tissue in a single organ; II, involvement of many lymph nodes in a regional area; III, generalized lymph node involvement; IV, liver and/or spleen involvement; V, blood or bone marrow involvement.32 Presently, cytological evaluation of fine needle aspirates is used to diagnose canine ML and the most common is high-grade malignant polymorphic centroblastic type based on updated Kiel classification.29,33,34 In general, T-cell lymphomas are associated with the worst prognosis.35
Several multidrug chemotherapy protocols have been proposed for management of canine ML. Treatment protocols mainly consist of a high-dose chemotherapy (induction phase) and a prolonged low-dose chemotherapy (maintenance phase). Complete remission in dogs with immunophenotype B and T treated with multidrug protocol consisting of ℓ-asparaginase, vincristine, cyclophosphamide, doxorubicin, methotrexate, and prednisolone has been reported (73%).28 However, tumor relapse and resistance invariably occur, and the median survival time for dogs is 35 weeks (range 0–238).28 Similarly, a chemotherapy regimen composed of vincristine, cyclophosphamide, and prednisolone (COP) for 6–8 weeks followed by a maintenance phase with prednisone and cyclophosphamide or chlorambucil and melphalan has shown a 68.7% with a complete remission median time of 10 weeks and survival median time of 27 weeks (range 7–75).29
In this study, we show that a genetic vaccine targeting dTERT can induce strong immune response to the antigen in dogs affected by B-cell ML and that standard chemotherapy regimen does not interfere with the effects of the immunotherapy. Most importantly, survival of ML dogs is significantly augmented in comparison to historical controls of chemotherapy-treated subjects. Our data support the evaluation of Ad6/DNA-EP-based cancer vaccine in a phase III canine trial as well as in human lymphoma patients.
Results
dTERT is expressed in canine B lymphoma
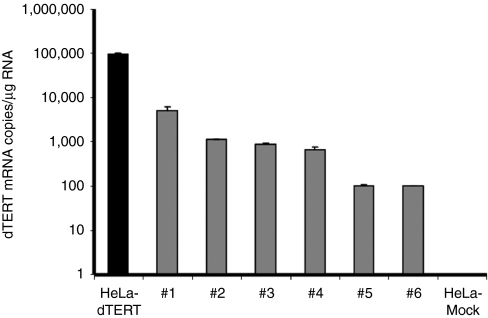
dTERT expression has been reported in the majority of canine tumors, including hematologic malignancies.36 To verify the expression of dTERT in canine B-cell lymphoma, six samples from lymph node fine needle aspirates from ML dogs were obtained and RNA was extracted. Cells transfected with a dTERT expression vector or mock-transfected were used as positive and negative controls, respectively. dTERT mRNA was detected by real-time PCR at different levels in all analyzed lymphoma samples (Figure 1). These data confirm that dTERT is a suitable tumor-associated antigen candidate for B-cell ML immunotherapy.
Figure 1.
dTERT expression in canine B-cell malignant lymphoma (ML). RNA was extracted from fine needle aspirates of lymph nodes from B-cell ML dogs. dTERT mRNA was quantified by TaqMan real-time PCR. HeLa transfected with pV1J-dTERT plasmid or mock-treated were used as positive and negative control of the assay, respectively. The amplicon resides in the exon 3–4 boundary. The results were confirmed with an independent set of primers/probe, amplifying a region in exon 2–3 boundary.
Study design
We have recently observed that genetic vectors expressing dTERT are capable of inducing an immune response against the antigen in healthy dogs and that the treatment is safe with no side effects.37 dTERT vaccine consisted of a DNA plasmid encoding a canine codon-optimized, catalytically inactive dTERT protein fused to a TPA (human tissue plasminogen activator) leader sequence at N-term and fused itself to the β-subunit of Escherichia coli heat-labile enterotoxin (LTB) at C-term (TPA-dTERT-LTBopt) and an Ad6 vector expressing dTERTopt. The vaccine regimen consisted of 5 DNA-EP (5 mg/injection) every 2 weeks in the tibial muscle and one injection of Ad6 (1011 vp) in the femoral biceps.
To evaluate the immunological response and the clinical efficacy of dTERT vaccine in the treatment of canine ML, dogs affected by WHO clinical stage III–IV of B-cell ML were enrolled in the study. Initially, the study was designed to vaccinate canine patients by DNA-EP/Ad6 regimen described above, 2 weeks after complete remission following standard COP induction without any maintenance chemotherapy. This protocol design was adopted because standard dose induction and maintenance chemotherapy can lead to transient immune suppression. However, due to recurrence of the disease soon after interrupting the standard induction chemotherapy (see Pu and Ba in Table 1), we realized that a washout period after COP before starting the dTERT vaccine was not doable. Therefore, we decided to vaccinate canine patients after complete remission concurrently with maintenance chemotherapy. Moreover, to obtain a prompt induction of the immune response, two injections of Ad6 at 2-week interval were performed at the beginning of the treatment, then followed by a cycle of five DNA-EP at 2-week intervals (see scheme in Figure 2).
Table 1. Characteristics and treatment of ML canine patients.
Figure 2.
Induction of cell-mediated immunity to dTERT in malignant lymphoma (ML) dogs by Ad and DNA-EP regimen. Top: schematic representation of the vaccination and chemotherapy combination schedule. Vaccination (Vax) indicates Ad6 injections (triangles) and DNA-EP (arrows). Dark and light gray indicate the induction and maintenance phases of chemotherapy. ELISPOT assay performed on PBMCs from dTERT immunized ML dogs 2 weeks after the second Ad6 injection. Four pools of 15 mer peptides covering dTERT (pool A, B, C, and D) were utilized. Reactivity and distribution of the immune response in (a) La, (b) Su, and (c) Ca ML patients is reported. The assay was run in duplicate, and the average value from the replicates is shown. DMSO, dimethyl sulfoxide; PBMC, peripheral blood mononuclear cell; SFC, spot-forming cell.
dTERT vaccine breaks immune tolerance in B-cell ML dogs
Table 1 shows subject age (median age is 8 years old), breed, gender, immunophenotype and cytological type, chemotherapy protocol, scheme of immunotherapy adopted, and responsiveness to the vaccination. A total of 14 dogs with B-type ML were enrolled in the study. The induced immune response was monitored by enzyme-linked immunosorbent spot (ELISPOT) using peptide pools covering dTERT sequence. No T-cell reactivity was observed in untreated animals (data not shown) or against dimethyl sulfoxide. Responses were scored positive when they were at least four times higher than background reactivity (dimethyl sulfoxide). In 13 out of 14 patients, the analysis after the first two Ad6 injections showed a detectable [ranging from 50 to 250 spot-forming cell/million peripheral blood mononuclear cells (PBMCs)] and multiepitope response (Figure 2). In fact, a significant reactivity was measured against most of dTERT peptide pools and, as expected from subject heterogeneity, it turned out to be different from patient to patient.
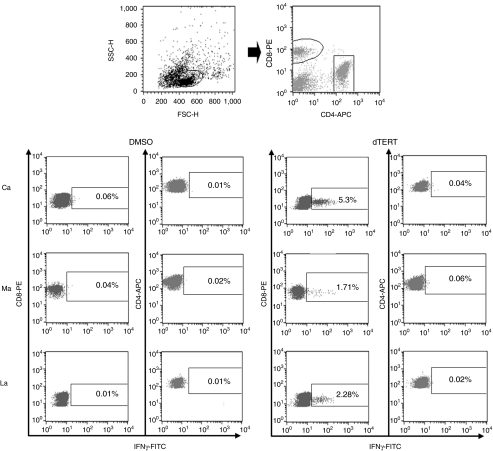
To characterize the quality of the immune response, an intracellular staining for IFNγ was performed during the vaccination protocol, 2 weeks after the second Ad6 injection. The reactivity measured in three dogs (Ca, Ma, and La, see Table 1) was exclusively due to CD8+ T cells (Figure 3).
Figure 3.
The immune response is exclusively CD8+. Dog peripheral blood mononuclear cells were analyzed by intracellular staining for IFN-γ. The gating strategy for the identification of T-cell sub-population is shown on the top: left, lymphogate; right, CD8 and CD4 surface staining. The response of Ca, Ma, and La ML canine patients following two Ad6 injections against three different pools of peptides (A, B, and C) is shown. DMSO, dimethyl sulfoxide.
The kinetics of the immune response was followed over time. Two dogs received Ad6 injections only and did not complete the vaccination protocol because one had a severe disease relapse (Gr, see Table 1) and the other one withdrew on the owner's decision (Pi dog, Table 1), however doing very well for a long period. As frequently observed with self-antigens,8,38 the response against dTERT was not long-lasting but could be fully restored and maintained by DNA-EP treatment (Figure 4a). Notably, three cycles of DNA-EP allowed measuring dTERT-specific cell-mediated response in one dog (Ca dog, Table 1) for a time frame of almost 2 years (Figure 4b). This subject, as well as others still alive, is currently being monitored for dTERT-specific immune response.
Figure 4.
Kinetics of the dTERT-specific T-cell response in malignant lymphoma (ML) dogs. ML dogs were treated as described in the text and indicated in the top scheme in Figure 2. PBMCs were purified at the indicated time points, and the response was measured by ELISPOT with dTERT peptide pools. The total reactivity for dTERT peptide pools is calculated as follows: total reactivity = σ (dTertA + dTertB + dTertC + dTertD pools) − 4 (DMSO). (a) Kinetics of the response during Ad6/DNA-EP regimen in four selected subjects: Pi, Bo, Ce, and Ma dogs. Pi received only two Ad6 injections due to owner's lack of consent. (b) An ML dog (Ca) was treated as shown in top scheme. Ad6 injections were performed 2 weeks after the induction phase and during maintenance chemotherapy at weeks 0 and 2. Three cycles of DNA-EP were executed at weeks 6, 26, and 74. At every cycle, the T-cell response was restored. Triangles indicate Ad6 injections; arrows represent vaccinations by DNA-EP. PBMC, peripheral blood mononuclear cell; SFC, spot-forming cell.
It is important to point out that the vaccination regimen does not seem to elicit any adverse effects. In fact, no changes of body weight, blood parameters, or the occurrence of clinical signs were observed during the entire course of the study (data not shown). Thus, the data indicate that dTERT vaccination is immunogenic and safe in canine ML patients during standard COP treatment and further confirm the power of the heterologous prime-boost modality.
dTERT vaccine prolongs survival of COP-treated B-cell ML dogs
Vaccine (Vax)/COP-treated ML dogs are presently being monitored over time for relapse of the disease and survival. Figure 5 compares the time to first relapse and overall survival (OS) of the 14 patients treated in this study with a historical cohort of eight ML patients at the same WHO stage III–IV, originating from the same geographic area (Tuscany, Italy) and treated with standard chemotherapy treatment in the same veterinary center.29 The characteristics of this patient population and the clinical behavior of each subject are also summarized in Table 1. Figure 5a shows the time to first relapse from the start of treatment. Most disease relapses during maintenance chemotherapy occurred within the first 3–5 months. A significant increase in this parameter (P = 0.014; exact log-rank test) is observed between the two cohorts (median, Vax/Chemo and Chemo, 26 and 14 weeks, respectively). The OS is shown in Figure 5b. A significant increase in OS is observed for the Vax/Chemo over the Chemo group (P = 0.0004, exact log-rank test). There have been six deaths in the vaccinated group (Pu, Ba, Bo, Pi, Ba, and Gr), all attributable to ML relapse. Because median survival cannot be established for the Vax/Chemo group at the moment, the 95% lower confidence interval for OS of the Vax/Chemo group is 57.6 weeks versus 26 weeks for Chemo group. There was no observable difference in survival both by gender and age. The evaluations included all Vax/Chemo dogs initiating treatment, irrespective of reason for not completing the study. These results seem to indicate that Ad6/DNA-EP dTERT vaccine added on top of standard chemotherapy has a beneficial effect on ML symptoms and OS.
Figure 5.
dTERT vaccine prolongs time to first relapse and survival in malignant lymphoma (ML) dogs. (a) Kaplan–Meier plot of time to first relapse. Vaccinated dogs (Vax/Chemo) (dashed lines; n = 14) show a significantly longer time to first relapse than dogs on chemotherapy alone (Chemo) (dashed lines; n = 8) (P = 0.014; exact log-rank test). (b) Kaplan–Meier plot of overall survival. Vax/Chemo dogs (dashed lines; n = 14) show a significantly longer overall survival than dogs on Chemo alone (dashed lines; n = 8) (P = 0.0004; exact log-rank test). Both in (a) and (b), the curves start from the diagnosis of ML, almost coincident with the beginning of the chemotherapy induction phase.
Discussion
Human B-cell ML has been considered for decades and still represents an ideal tumor type for cancer immunotherapy. In fact, the clonal immunoglobulin idiotype (Id) displayed on the surface of most malignant B cells represents a patient- and tumor-specific antigen that can be used for therapeutic vaccination. Lymphoma Id vaccines have been largely explored in clinical trials and were the first cancer vaccines to ever show biological and clinical efficacy, as well as clinical benefit, in humans.39 However, recent randomized clinical trials have failed to achieve their main end points (reviewed in ref. 40) for a variety of reasons, among them the intrinsic variability of the source of the Id vaccine, the heterogeneous response of the host to the Id as an antigen and the possible emergence of variant immunoglobulin over the course of the disease. All these issues are virtually unpredictable in rodent preclinical models in which the same Id is used to immunize and cure all animals. Conversely, the use of a tumor-associated antigen (such as TERT) instead of a customized tumor-specific antigen (such as the Id) coupled with an efficient vaccination platform could be instrumental to develop an efficacious immunotherapy readily applicable to all patients and devoid of unpredictable variability.
We have previously shown that DNA-EP/Ad vaccine regimen can elicit significant immune responses against mouse TERT and therapeutic effects in two different models of spontaneous carcinogenesis41 as well as strong immune responses in large animals such as nonhuman primates38 and healthy dogs.37 In this study, we have evaluated the canine ML as a therapeutic model of immunotherapy for TERT vaccine. ML is the most common hematopoietic tumor in dogs and about 80% are B types34 and its properties and cytological changes are comparable to human disease.42 It is very aggressive, and no cure is available apart from chemotherapy. Dogs that undergo standard COP usually survive for 8 months by average even if they often relapse.29 This makes the vaccination in dogs even more interesting because clinical effects can be observed in a short time and because ML dogs are treated with chemotherapy protocols similar to those used for human patients.
To assess the feasibility of an immunotherapy approach against dTERT, antigen expression was verified by real-time PCR. dTERT mRNA was expressed at different levels in all lymph node biopsies from dogs affected by B-cell ML (Figure 1). The aggressiveness of the disease did not allow the enrollment of patients in remission after the induction phase chemotherapy without any maintenance therapy. However, the notion that some classes of chemotherapy drugs could act as immunomodulators prompted us to combine the vaccination with maintenance therapy. In fact, chemotherapy combined to immunotherapy have been described to affect antigen crosspresentation,43 induce a cytokine storm,44 reduce the number of regulatory T cells,45 and activate homeostatic lymphoid proliferation46 that could help inducing the immune response against self-antigens.47 In fact, a strong T-cell response against dTERT in ML canine patients with Ad6/DNA-EP technology platform was measured during maintenance chemotherapy. This observation is of crucial importance because immune tolerance and cancer immune suppression are expected to play major roles in impairing vaccination efficacy. In any case, we cannot predict the biological efficacy (i.e., the extent of the immune response) of dTERT immunotherapy in the absence of concomitant chemotherapy in this particular disease setting. Two Ad6 injections appeared to be sufficient to break immune tolerance (Figures 2 and 3) in 13 out of 14 dogs (93%). The response was polyspecific, and T-cell epitopes were distributed throughout the entire antigen (Figure 2). In general, the T-cell reactivity fell down 6–14 weeks after first vaccination (Figure 4). We reasoned that maintaining dTERT immune response could contribute to the therapeutic effect48 and therefore decided to treat dogs with DNA-EP for at least two cycles. One cycle (five or three DNA-EP) treatment restored the T-cell reactivity to almost that obtained after the second Ad6 injection in most of the dogs. This observation opened up the possibility of a vaccination regimen with multiple DNA-EP injections at appropriate intervals to sustain the dTERT-specific T-cell immune response.38 This was further supported by the data obtained in all treated dogs (Figure 4a) and in one subject that received three cycles of DNA-EP and reached >98 weeks of follow-up (Figure 4b), suggesting that the 2× Ad6 followed by DNA-EP vaccination regimen was a useful strategy to stimulate enduring immune responses.
In addition to the immune response, the end point of the study was to assess the efficacy of dTERT vaccination on biological behavior, progression, and evolution of canine ML in remission treated with standard COP chemotherapy. Significant differences on time to first relapse of the disease were noted in dogs treated with the combination (Figure 5a). Most importantly, a therapeutic impact on survival time was observed in canine patients that received the combination therapy over a comparable cohort of eight dogs that received only standard therapy (i.e., induction and maintenance chemotherapy, Figure 5b). Importantly, no adverse effects were observed in any dog patient, such as changes in clinical and hematological parameters, local/systemic toxicity or organic dysfunction, and fever that could be attributed to treatment, as also reported by the owners. Nonetheless, a potential limitation of our study could be the relatively low number of canine patients and the comparison carried out with historical control cases, although managed by the same clinicians within the same veterinary center and treated with a comparable chemotherapy regimen.
Of note, the translational relevance of our study must take into account important considerations with respect to human immunotherapy trials conducted so far, making dog ML a even more interesting proof-of-concept model. For instance, human Id vaccines have been utilized only in indolent lymphoma, whereas dTERT vaccine has been tested in aggressive dog ML. Assessing this immunotherapy in aggressive ML would be ethically difficult in humans, who may well benefit from other already established treatments ranging from antiCD20 antibodies49 (that would interfere with active vaccination by depleting B cells) and/or stem cell transplant (which might or not be followed by vaccination), rather than the immunotherapy. However, our findings may open up new therapeutic combination avenues to be explored in the clinical studies.
In summary, our data indicate for the first time that dTERT Ad6/DNA-EP vaccine can break immune tolerance to this antigen in lymphoma canine patients and achieve a durable immune response. We show that dTERT vaccine can be successfully combined with COP maintenance chemotherapy, and most importantly, the combination has a significant impact on canine patients' survival. Finally, our data support the clinical use of TERT cancer vaccines in combination with chemotherapy in human lymphoma patients.
Materials and Methods
Real-time quantitative PCR. Fine needle biopsies were obtained from B-lymphoma diagnosed canine patients and stored in RNA later (Qiagen, St Louis, MO). Total RNA was isolated with the Qiagen RNeasy Mini Reagent Set (Qiagen), according to the manufacturer's instructions. All preparation and handling steps of RNA took place in a laminar flow hood under RNase-free conditions. The isolated RNA was dissolved in DEPC water and stored at −70 °C until used. RNA concentration was determined in the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). One microgram of total RNA was used to perform reverse transcription of RNA and real-time PCR (TaqMan) assay using two primer/probe sets (Cf02671898_g1 and Cf02671897_m1) that recognized canine TERT (Applied Biosystems, Carlsbad, CA) according to manufacturer's instructions. The reactions were performed using a TaqMan Gold reverse transcription (RT)-PCR kit (PE Applied Biosystems) and included a 30-minute reverse transcription step at 50 °C followed by 10 minutes at 95 °C and 40 cycles of amplification using the universal TaqMan standardized conditions (15 seconds, 95 °C denaturation step followed by a 1-minute, 60 °C annealing/extension step). RNA transcribed from a plasmid containing dTERT37 was used as a standard to establish genome equivalents. All reactions were run in duplicate by using the ABI Prism 7900 sequence detection system (PE Applied Biosystems).
Dog study design. From May 2007 to September 2009, 45 client-owned dogs with ML lymphoma were evaluated and 14 of them were enrolled in the study, approved by Animal Ethical Committee of the University of Pisa and Ministry of Health. For each dog, the veterinary staff of the Clinical Department performed a full initial clinical examination, filled up the clinical record, and carried out the scheduled investigations to reach the diagnosis and clinical staging of ML. Particularly, at the first visit for the candidate dogs, peripheral blood, lymph node, and bone marrow samples were collected, and thoracic and abdomen X-rays and abdomen ultrasound were performed. From the peripheral blood collected, we carried out complete blood count, serum biochemical profile of at least 12 analytes, coagulation profile, serum protein electrophoresis, urinalysis, serological titers for Leishmania i., Ehrlichia c., and Rickettsia r. From the fine needle aspiration of lymph nodes, both cytological smears and immunophenotyping were performed to classify the ML. From the bone marrow aspirate, cytological smears were performed to stage the ML.
Only 14 dogs stage III–IV possessed the necessary requirements for clinical trial and were enrolled. All subjects were affected by high-grade type ML, B immunophenotype and with the cytological appearance according to the Kiel updated classification were grouped as follows: centroblastic polymorphic (eight cases), centroimmunoblastic (three cases), immunoblastic (two cases), and lymphoblastic (one case). Selected animals did not show any additional significant disease. All the dogs included in the study received a standard chemotherapy protocol with vincristine (0.75 mg/m2 i.v. once a week), cyclophosphamide (50 mg/m2 p.o. 3–4 days a week), and prednisone (40 mg/m2 p.o. daily for the first week, then 20 mg/m2 p.o. daily for the further weeks) for 6/8 weeks. Then when a complete remission (CR) was reached, dogs received a maintenance therapy with low dose of prednisone (5–10 mg/m2 p.o.) and cyclophosphamide (50 mg/m2 p.o. 1–2 days a week) or chlorambucil (2 mg/m2 p.o. 2–3 days a week) and melphalan (2 mg/m2 p.o. 2–3 days a week). Similar chemotherapy protocols were applied for control patients.29 Where indicated, dogs were treated with doxorubicin (30 mg/m2, every 3 weeks). The weight of enrolled and control dogs ranged from 9 to 66 kg.
Dog immunization. Both pV1J-dTERT.LTBopt and Ad6-dTERTopt have been described elsewhere.37 The DNA injection consisted of a 1 ml solution (split over two injection sites with 0.5 ml/site) containing 5 mg pV1J-dTERT.LTBopt in dogs' tibial muscles. Electrical conditions for electroporation consisted of eight trains of 100 square bipolar pulses (1-second each) delivered every other second for a total treatment time of 10 seconds. The pulse length was 2 ms/phase with a frequency and amplitude of 100 Hz and 100 mA, respectively (constant current mode). For Ad vaccination, dogs were injected in femoral biceps muscle with a dose of 1011 Ad viral particles (vp). Blood was collected at the indicated time points, and PBMCs were isolated by density gradient centrifugation columns (Accuspin; Sigma, St Louis, MO). Fresh or frozen PBMC samples were analyzed for immunologic assays. Dogs with tumors enrolled in the vaccination protocol received two Ad-dTERTopt injections followed by DNA-EP as described above. Dogs were anesthetized to carry out the electroporation with medetomidine at about 10 µg/kg (Domitor) i.v. and with propofol at about 5 mg/kg (Rapinovet) i.v. To reverse the anesthesia, atipamezole at about 10 µg/kg (Antisedan) i.m. was used.
Peptides. Lyophilized dTERT peptides were purchased from JPT Peptide Technologies (Berlin, Germany) and resuspended in dimethyl sulfoxide at 40 mg/ml. Pools of 15 amino acid peptides overlapping by 11 residues were assembled as described previously for other genes.50 Four pools of 70 peptides, respectively, were formed for the whole length of TERT protein. The final concentration of each peptide in the pools was 0.57 mg/ml.
Interferon-γ ELISPOT. The ELISPOT assay was performed using a standard kit for the detection of canine IFNγ (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Purified dog PBMCs (Accuspin system) were plated in duplicate in 96-well MAIP plates (Millipore, Billerica, MA) coated O/N with an anti-dog IFNγ antibody, at a density of 4 × 105 and 2 × 105 cells/well. Cells were incubated for 20 hours at 37 °C with 2 µg/ml suspension of each peptide. Concanavalin A was used as positive internal control at 10 µg/ml. Plates were washed and incubated for 12 hours at 4 °C with biotin-conjugated anti-dog IFNγ (50 µg/well). Spots were detected after incubation with streptavidin-AP followed by NBT/BCIP substrate (50 µl/well; Pierce Biotechnology, Rockford, IL). Plates were washed and air dried, and spots were counted with an automated ELISPOT reader (AID ELISPOT v3.0; AID, Strassberg, Germany).
Flow cytometry analysis. IFN-γ production by stimulated T cells was measured as follow: 2–3 × 106 dog PBMCs were isolated from blood as described above and incubated overnight with 5–6 µg/ml of dog TERT peptide pools and Brefeldin A (1 µg/ml; BD Biosciences, Pharmingen, San Jose, CA) at 37 °C. Cells were washed, stained with surface antibodies, rat anti-dog CD4: Alexa Fluor 647 and anti-dog CD8-PE (AbD Serotec). Cells were then permeabilized, and incubated with mouse antibovine fluorescein isothiocyanate–conjugated IFN-γ antibody that crossreacts with dog (AbD Serotec, Raleigh, NC). Cells were analyzed on a FACSCanto flow cytometer, using Diva software (BD Biosciences Immunocytometry Systems). At least 15,000 gated events were acquired to analyze the immune response.
Statistical analysis. Differences in time to first relapse and OS were evaluated using the exact log-rank test. All reported P values are two-sided. Analyses were performed in R v. 2.8.1.
Acknowledgments
We thank the owners of the dogs for their collaboration and patience as well as the reference practitioners to send their cases to our attention. There are no competing interests.
REFERENCES
- Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, et al. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Vonderheide RH. Universal tumor antigens for cancer vaccination: targeting telomerase for immunoprevention. Discov Med. 2007;7:103–108. [PubMed] [Google Scholar]
- Chen DY, Vance BA, Thompson LB, Domchek SM., and, Vonderheide RH. Differential lysis of tumors by polyclonal T cell lines and T cell clones specific for hTERT. Cancer Biol Ther. 2007;6:1991–1996. doi: 10.4161/cbt.6.12.5078. [DOI] [PubMed] [Google Scholar]
- Su Z, Dannull J, Yang BK, Dahm P, Coleman D, Yancey D, et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- Domchek SM, Recio A, Mick R, Clark CE, Carpenter EL, Fox KR, et al. Telomerase-specific T-cell immunity in breast cancer: effect of vaccination on tumor immunosurveillance. Cancer Res. 2007;67:10546–10555. doi: 10.1158/0008-5472.CAN-07-2765. [DOI] [PubMed] [Google Scholar]
- Aurisicchio L, Mennuni C, Giannetti P, Calvaruso F, Nuzzo M, Cipriani B, et al. Immunogenicity and safety of a DNA prime/adenovirus boost vaccine against rhesus CEA in nonhuman primates. Int J Cancer. 2007;120:2290–2300. doi: 10.1002/ijc.22555. [DOI] [PubMed] [Google Scholar]
- Babiuk S, Baca-Estrada ME, Foldvari M, Storms M, Rabussay D, Widera G, et al. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine. 2002;20:3399–3408. doi: 10.1016/s0264-410x(02)00269-4. [DOI] [PubMed] [Google Scholar]
- Scheerlinck JP, Karlis J, Tjelle TE, Presidente PJ, Mathiesen I., and, Newton SE. In vivo electroporation improves immune responses to DNA vaccination in sheep. Vaccine. 2004;22:1820–1825. doi: 10.1016/j.vaccine.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Zucchelli S, Capone S, Fattori E, Folgori A, Di Marco A, Casimiro D, et al. Enhancing B- and T-cell immune response to a hepatitis C virus E2 DNA vaccine by intramuscular electrical gene transfer. J Virol. 2000;74:11598–11607. doi: 10.1128/jvi.74.24.11598-11607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- Casimiro DR, Bett AJ, Fu TM, Davies ME, Tang A, Wilson KA, et al. Heterologous human immunodeficiency virus type 1 priming-boosting immunization strategies involving replication-defective adenovirus and poxvirus vaccine vectors. J Virol. 2004;78:11434–11438. doi: 10.1128/JVI.78.20.11434-11438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZY, Wyatt LS, Kong WP, Moodie Z, Moss B., and, Nabel GJ. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J Virol. 2003;77:799–803. doi: 10.1128/JVI.77.1.799-803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uva P, Aurisicchio L, Watters J, Loboda A, Kulkarni A, Castle J, et al. Comparative expression pathway analysis of human and canine mammary tumors. BMC Genomics. 2009;10:135. doi: 10.1186/1471-2164-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazza A, Presciuttini S, Barale R, Lubas G., and, Gugliucci B. Association between canine malignant lymphoma, living in industrial areas, and use of chemicals by dog owners. J Vet Intern Med. 2001;15:190–195. [PubMed] [Google Scholar]
- Bergman PJ, McKnight J, Novosad A, Charney S, Farrelly J, Craft D, et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res. 2003;9:1284–1290. [PubMed] [Google Scholar]
- Bergman PJ, Camps-Palau MA, McKnight JA, Leibman NF, Craft DM, Leung C, et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine. 2006;24:4582–4585. doi: 10.1016/j.vaccine.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Argyle DJ., and, Nasir L. Telomerase: a potential diagnostic and therapeutic tool in canine oncology. Vet Pathol. 2003;40:1–7. doi: 10.1354/vp.40-1-1. [DOI] [PubMed] [Google Scholar]
- Nasir L. Telomeres and telomerase: biological and clinical importance in dogs. Vet J. 2008;175:155–163. doi: 10.1016/j.tvjl.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Nasir L, Devlin P, Mckevitt T, Rutteman G., and, Argyle DJ. Telomere lengths and telomerase activity in dog tissues: a potential model system to study human telomere and telomerase biology. Neoplasia. 2001;3:351–359. doi: 10.1038/sj.neo.7900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa M, Okuda M, Setoguchi A, Iwabuchi S, Nishimura R, Sasaki N, et al. Telomere length and telomerase activity in canine mammary gland tumors. Am J Vet Res. 2001;62:1539–1543. doi: 10.2460/ajvr.2001.62.1539. [DOI] [PubMed] [Google Scholar]
- Yazawa M, Okuda M, Setoguchi A, Nishimura R, Sasaki N, Hasegawa A, et al. Measurement of telomerase activity in dog tumors. J Vet Med Sci. 1999;61:1125–1129. doi: 10.1292/jvms.61.1125. [DOI] [PubMed] [Google Scholar]
- Angelopoulou K, Zavlaris M, Papaioannou N., and, Vlemmas I. Canis familiaris telomerase reverse transcriptase undergoes alternative splicing. Mamm Genome. 2008;19:647–653. doi: 10.1007/s00335-008-9144-7. [DOI] [PubMed] [Google Scholar]
- Nasir L, Gault E, Campbell S, Veeramalai M, Gilbert D, McFarlane R, et al. Isolation and expression of the reverse transcriptase component of the Canis familiaris telomerase ribonucleoprotein (dogTERT) Gene. 2004;336:105–113. doi: 10.1016/j.gene.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Lund JR, Paoloni M, Kurzman I, Padilla M., and, Argyle DJ. Inhibition of canine telomerase in vitro and in vivo using RNAi: further development of a natural canine model for telomerase-based cancer therapies. Vet J. 2008;177:192–197. doi: 10.1016/j.tvjl.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Pang LY., and, Argyle DJ. Using naturally occurring tumours in dogs and cats to study telomerase and cancer stem cell biology. Biochim Biophys Acta. 2009;1792:380–391. doi: 10.1016/j.bbadis.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Simon D, Moreno SN, Hirschberger J, Moritz A, Kohn B, Neumann S, et al. Efficacy of a continuous, multiagent chemotherapeutic protocol versus a short-term single-agent protocol in dogs with lymphoma. J Am Vet Med Assoc. 2008;232:879–885. doi: 10.2460/javma.232.6.879. [DOI] [PubMed] [Google Scholar]
- Gavazza A, Sacchini F, Lubas G, Gugliucci B., and, Valori E. Clinical, laboratory, diagnostic and prognostic aspects of canine lymphoma: a retrospective study. Comp Clin Path. 2009;18:291–299. [Google Scholar]
- Hayes HM, Tarone RE, Cantor KP, Jessen CR, McCurnin DM., and, Richardson RC. Case-control study of canine malignant lymphoma: positive association with dog owner's use of 2,4-dichlorophenoxyacetic acid herbicides. J Natl Cancer Inst. 1991;83:1226–1231. doi: 10.1093/jnci/83.17.1226. [DOI] [PubMed] [Google Scholar]
- Vail DM., and, Young KM.2007Hematopoietic tumorsIn: Withrow, SJ and MacEven, EG (eds). Small Animal Clinical Oncology 4th edn. Elsevier: St Louis, MO [Google Scholar]
- WHO 1980TNM Classification of Tumors in Domestic AnimalsWHO Proceedings, Geneva. pp. 46–54.
- Fournel-Fleury C, Magnol JP, Bricaire P, Marchal T, Chabanne L, Delverdier A, et al. Cytohistological and immunological classification of canine malignant lymphomas: comparison with human non-Hodgkin's lymphomas. J Comp Pathol. 1997;117:35–59. doi: 10.1016/s0021-9975(97)80065-5. [DOI] [PubMed] [Google Scholar]
- Willmann M, Müllauer L, Guija de Arespacochaga A, Reifinger M, Mosberger I., and, Thalhammer JG. Pax5 immunostaining in paraffin-embedded sections of canine non-Hodgkin lymphoma: a novel canine pan pre-B- and B-cell marker. Vet Immunol Immunopathol. 2009;128:359–365. doi: 10.1016/j.vetimm.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Ponce F, Magnol JP, Ledieu D, Marchal T, Turinelli V, Chalvet-Monfray K, et al. Prognostic significance of morphological subtypes in canine malignant lymphomas during chemotherapy. Vet J. 2004;167:158–166. doi: 10.1016/j.tvjl.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Yazawa M, Okuda M, Kanaya N, Hong SH, Takahashi T, Ohashi E, et al. Molecular cloning of the canine telomerase reverse transcriptase gene and its expression in neoplastic and non-neoplastic cells. Am J Vet Res. 2003;64:1395–1400. doi: 10.2460/ajvr.2003.64.1395. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Mesiti G, Ciliberto G, La Monica N., and, Aurisicchio L. Telomerase and HER-2/neu as targets of genetic cancer vaccines in dogs. Vaccine. 2010;28:1201–1208. doi: 10.1016/j.vaccine.2009.11.031. [DOI] [PubMed] [Google Scholar]
- Dharmapuri S, Peruzzi D, Mennuni C, Calvaruso F, Giampaoli S, Barbato G, et al. Coadministration of telomerase genetic vaccine and a novel TLR9 agonist in nonhuman primates. Mol Ther. 2009;17:1804–1813. doi: 10.1038/mt.2009.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inogès S, Rodrìguez-Calvillo M, Zabalegui N, Lòpez-Dìaz de Cerio A, Villanueva H, Soria E, et al. Grupo Español de Linfomas/Trasplante Autologo de Medula Oseo study group; Programa para el Estudio y Tratamiento de Hemopatias Malignas study group; 2006Clinical benefit associated with idiotypic vaccination in patients with follicular lymphoma J Natl Cancer Inst 981292–1301. [DOI] [PubMed] [Google Scholar]
- Bendandi M. Idiotype vaccines for lymphoma: proof-of-principles and clinical trial failures. Nat Rev Cancer. 2009;9:675–681. doi: 10.1038/nrc2717. [DOI] [PubMed] [Google Scholar]
- Mennuni C, Ugel S, Mori F, Cipriani B, Iezzi M, Pannellini T, et al. Preventive vaccination with telomerase controls tumor growth in genetically engineered and carcinogen-induced mouse models of cancer. Cancer Res. 2008;68:9865–9874. doi: 10.1158/0008-5472.CAN-08-1603. [DOI] [PubMed] [Google Scholar]
- Breen M., and, Modiano JF. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans–man and his best friend share more than companionship. Chromosome Res. 2008;16:145–154. doi: 10.1007/s10577-007-1212-4. [DOI] [PubMed] [Google Scholar]
- van der Most RG, Currie A, Robinson BW., and, Lake RA. Cranking the immunologic engine with chemotherapy: using context to drive tumor antigen cross-presentation towards useful antitumor immunity. Cancer Res. 2006;66:601–604. doi: 10.1158/0008-5472.CAN-05-2967. [DOI] [PubMed] [Google Scholar]
- Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13 2 Pt 1:644–653. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisticò P, Capone I, Palermo B, Del Bello D, Ferraresi V, Moschella F, et al. Chemotherapy enhances vaccine-induced antitumor immunity in melanoma patients. Int J Cancer. 2009;124:130–139. doi: 10.1002/ijc.23886. [DOI] [PubMed] [Google Scholar]
- Kamstock D, Elmslie R, Thamm D., and, Dow S. Evaluation of a xenogeneic VEGF vaccine in dogs with soft tissue sarcoma. Cancer Immunol Immunother. 2007;56:1299–1309. doi: 10.1007/s00262-007-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meerten T., and, Hagenbeek A. CD20-targeted therapy: a breakthrough in the treatment of non-Hodgkin's lymphoma. Neth J Med. 2009;67:251–259. [PubMed] [Google Scholar]
- Facciabene A, Aurisicchio L., and, La Monica N. Baculovirus vectors elicit antigen-specific immune responses in mice. J Virol. 2004;78:8663–8672. doi: 10.1128/JVI.78.16.8663-8672.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]