Abstract
This study completes the longest known in vivo monitoring of adeno-associated virus (AAV)–mediated gene expression in nonhuman primate (NHP) brain. Although six of the eight parkinsonian NHP originally on study have undergone postmortem analysis, as described previously, we monitored the remaining two animals for a total of 8 years. In this study, NHP received AAV2-human -amino acid decarboxylase (hAADC) infusions into the MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-lesioned putamen. Restoration of AADC activity restored normal response to levodopa and gene expression could be quantitated repeatedly over many years by 6-[18F]fluoro-meta-tyrosine (FMT)-positron emission tomography (PET) and confirm that AADC transgene expression remained unchanged at the 8-year point. Behavioral assessments confirmed continued, normalized response to levodopa (improvement by 35% over historical controls). Postmortem analysis showed that, although only 5.6 ± 1% and 6.6 ± 1% of neurons within the transduced volumes of the striatum were transduced, this still secured robust clinical improvement. Importantly, there were no signs of neuroinflammation or reactive gliosis at the 8-year point, indicative of the safety of this treatment. The present data suggest that the improvement in the -3,4-dihydroxyphenylalanine (-Dopa) therapeutic window brought about by AADC gene therapy is pronounced and persistent for many years.
Introduction
Gene therapy for Parkinson's disease (PD) has now become the pre-eminent neurological application of this emerging medical technology in terms of patient numbers.1,2 Perhaps one of the key factors driving gene therapy for PD has been that the etiology of PD is quite well understood and this opens up opportunities to use gene therapy for symptomatic as well as disease-modifying purposes. One promising approach has been restoration of near-normal levels of aromatic -amino acid decarboxylase (AADC), the enzyme essential for conversion of -3,4-dihydroxyphenylalanine (-Dopa) to dopamine. In PD, depletion of AADC to rate-limiting levels may play a key role in progressively worsening responses to -Dopa, the mainstay symptomatic pharmacotherapy for the disease. Substantial nonclinical and clinical studies now indicate that putaminal infusion of an adeno-associated viral (AAV2) vector encoding human AADC (hAADC) confers significant benefit upon parkinsonian nonhuman primates (NHP)3,4 and a recent Phase 1 clinical study has provided indications of similar benefit in human PD patients.5,6
In this study, we extend our previous findings of long-term clinical benefit of AAV2-hAADC in parkinsonian NHP4 with a report on the stability and safety of gene transfer into the brain in the remaining two monkeys from our long-term study after 8 years. These two animals were evaluated clinically immediately before killing, and their brains were then examined for stability, extent, and efficacy of transgene expression, as well as for possible neuropathology. To our knowledge, this is the longest duration of transgene expression ever reported. This study extends and supports current and future clinical trials with this vector in advanced PD patients suffering from dose-limiting -Dopa toxicity.
Results
The study design and results from the earlier time points have already been published.4,7 Briefly, 8 hemi-parkinsonian male NHP (Macaca mulatta) were used. Two to four months after MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-lesioning, either AAV2-hAADC (n = 4) or AAV2-LacZ (nontherapeutic control gene, n = 4) was unilaterally infused into the lesioned striatum by convection-enhanced delivery in six injections of 30 µl/site of AAV2-hAADC at a concentration of 2 × 1012 vector genomes (vg)/ml into the MPTP-lesioned caudate (two sites) and putamen (four sites). Monkeys were behaviorally assessed (clinical rating scale), their brains scanned by positron emission tomography (PET) imaging for AADC gene expression and subsequently processed for immunohistochemical evaluations. Control animals were killed at 24 months. Test animals were killed at 36 (n = 2)4 and 96 months (n = 2; described in this study).
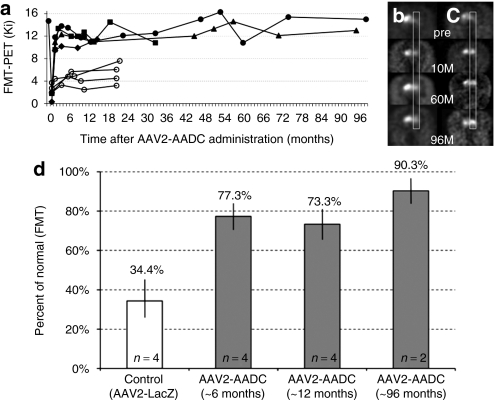
Expression of AADC can be determined in vivo by PET with the AADC-specific tracer, 6-[18F]fluoro-meta-tyrosine (FMT), an enzymatic substrate. This activity was evaluated in all monkeys at various intervals throughout the course the experiment. The remaining two monkeys received their final PET scans 96 months after the vector infusion. The PET signal on the AAV2-hAADC-infused (lesioned) side of the brain appeared unchanged between the 10, 60, and 96-month scans (Figure 1a). Figure 1 includes 10- and 60-month data from Bankiewicz et al.4 with the addition of the final PET measurements (Figure 1b,c). We also calculated the increase in FMT-PET after AAV2-AADC treatment over time (Figure 1d). After MPTP lesioning and subsequent infusion with AAV2-LacZ, the control monkeys showed a significant reduction of the signal to 34.4% of the normal, baseline value (21 months after surgery; calculated from our historical data reported in Bankiewicz et al.4). The therapeutic vector, AAV2-AADC, restored the signal to 77.3, 73.3, and 90.3% of normal PET value after 6, 12, and 96 months, respectively.
Figure 1.
PET imaging of hAADC activity in hemi-lesioned monkey striatum. Data before 72 months are taken from Bankiewicz et al.4 (a) AADC activity (measured by Ki) over time. Open circles represent four control monkeys that received AAV2-LacZ after MPTP lesioning. Solid symbols indicated AAV2-hAADC-treated NHP; two NHP, Octopus (triangle) and Max (circles), remained in life out to 96 months. (b,c) PET images from AAV2-hAADC-treated animals, (b) Octopus and (c) Max. Images indicate coronal [18F]FMT PET images at baseline (pre) and at 10, 60, and 96 months after unilateral AAV2-hAADC administration to the right side of the brain. Both monkeys showed a stable and long-term increase in AADC activity. (d) Increase in FMT-PET signal after AAV2-AADC treatment over time. This restoration of signal was expressed as a percentage of the control/baseline FMT-PET signal (FMT uptake value in normal monkeys before MPTP lesioning). As there was no significant change in FMT uptake within the control or treated monkeys over time, we grouped animals into three time points (AAV2-AADC-treated monkeys; gray columns). The white column represents the mean FMT-PET signal from control monkeys, MPTP-lesioned monkeys and animals infused with AAV2-LacZ. FMT Ki values and earlier time points for the AAV2-AADC-treated monkeys were calculated from Bankiewicz et al.4 Standard deviations are shown for each group and the difference between the control and treated animals (at each time point) is statistically significant, P < 0.005. AADC, -amino acid decarboxylase; AAV, adeno-associated virus; FMT, 6-[18F]fluoro-meta-tyrosine; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NHP, nonhuman primate; PET, positron emission tomography.
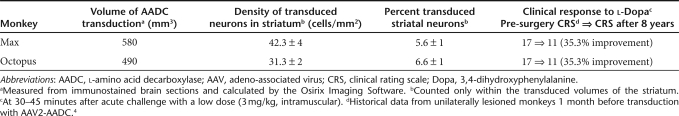
Behavioral assessments (clinical rating scale) were performed as previously described.4 Acute challenge with a normally subclinical low dose (3 mg/kg) of -Dopa given intramuscularly in both monkeys demonstrated their persistent improvement in clinical rating scale scores (35.3% change from baseline at 8 years after transduction with AAV2-AADC; Table 1) and normalized response to levodopa 96 months after gene transfer.
Table 1. Values of different parameters from two monkeys treated with AAV2-hAADC.
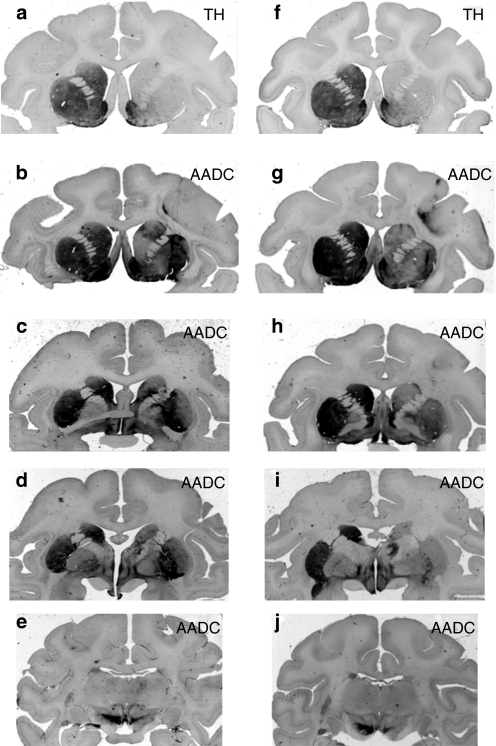
The volume of AADC gene transduction was measured from sequentially stained brain sections and calculated by OsiriX Imaging Software OsiriX Foundation, Geneva, Switzerland. The volume of the infused (Vi) vector (6 × 30 µl) resulted in a volume of expression (Ve) of 580 and 490 mm3 of the striatum of the two monkeys, Max and Octopus, respectively (Table 1), yielding Ve/Vi ratios of 3.2 and 2.7 in line with similar studies.8 Figure 2 shows the extent of AADC gene expression. Striatal transduction with AAV2-hAADC resulted in a stable, long-term restoration of AADC activity in the lesioned hemisphere. The extent of this restoration was possible to assess because of the loss of dopaminergic fibers and terminals on the MPTP-lesioned side (confirmed by tyrosine hydroxylase and vesicular monoamine transporter staining for presynaptic dopaminergic nerve terminals; Figures 2a,f and 3h). The treated side contained numerous transduced cells (AADC-positive cell bodies), whereas the contralateral side showed only a dense network of positive fibers from endogenous AADC (Figure 3a–c) The neuronal tropism of AAV2 was confirmed by dual staining with NeuN (Figure 3d–f) as shown previously.8 We also determined the density of the transduced neurons within the striatum as well as the fraction of the transduced striatal neurons (see details in Table 1).
Figure 2.
Extent of AADC transgene distribution within NHP brains. Two monkey's, Max (a–e) and Octopus (f–j) were treated long-term with AAV2-AADC. Consecutive sections (in rows) show precommissural (b,g), commissural (c,h), postcommissural (d,i), and midbrain (e,j) brain regions. The signal in the left hemisphere shows endogenous AADC expression. The right hemispheres show the extent of restoration of AADC expression in the MPTP-lesioned brains. Note the dramatic reduction of anti-TH-staining in the right striatum (a, f) compared to the intact left side, confirming profound loss of dopaminergic fibers and terminals on the lesioned side. AADC, -amino acid decarboxylase; AAV, adeno-associated virus; MPTP, 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine; TH, tyrosine hydroxylase.
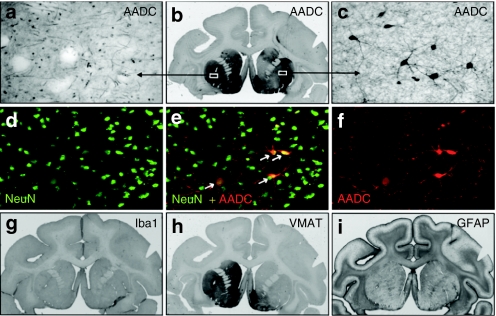
Figure 3.
Immunohistochemistry of the brain in hemi-lesioned monkeys 96 months after AAV2-AADC treatment. (a–c) AADC staining in one NHP (Max). The signal shows endogenous AADC in the unlesioned, left striatum, and almost complete restoration of the enzyme activity within the right striatum demonstrating the (b) long-term expression of AADC transgene. Endogenous AADC expression is characterized by (a) dense network of positive fibers whereas the restored lesioned side shows (c) transduced neuronal cell bodies along with the network of their processes. (d–f) Double immunofluorescent labeling of the AAV2-AADC-transduced hemisphere (Max) shows colocalization of markers AADC and NeuN (neuronal marker). The transduced neurons displayed a typical medium spinal morphology. (h) Staining against vesicular monoamine transporter (VMAT2), a marker for presynaptic neurons, shows a dramatic loss of dopaminergic terminals on the MPTP-lesioned, right hemisphere. The left side shows normal expression (compare to similar pattern of TH-staining—Figure 2a). (g) Staining for a microglia marker, Iba1, shows no inflammatory reaction on the AAV2-AADC treated side. Similarly, the lack of astrocytic activation was confirmed with (i) marker GFAP. AADC, -amino acid decarboxylase; AAV, adeno-associated virus; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NHP, nonhuman primate; TH, tyrosine hydroxylase.
The safety of long-term transduction with AAV2-hAADC was evaluated with the use of two cellular markers, Iba1 (microglia/macrophage) and GFAP (astrocytes). There were no signs of neuroinflammation or reactive gliosis in the right-hand, treated hemisphere (Figure 3g,i).
Discussion
In this report, we extend previously published data on long-term expression of AADC in parkinsonian NHP out to 96 months from a previously published 72 months. Although only two animals survived for this analysis, the unprecedented duration of in vivo clinical and PET monitoring, combined with postmortem tissue analysis, warrants publication of these limited remaining data. The data are in agreement with the concept that gene transfer into the primate brain with AAV2 is stable over many years and suggest that clinical trials underway with AAV2 for neurological indications may result in essentially permanent transduction. Accordingly, the two remaining animals in our study showed no major changes in AADC expression, as detected by FMT-PET, from the first scan at 10 months until the final scan at 96 months. In fact, this last PET scan showed almost complete restoration of the normal baseline FMT-PET (Ki): to 96.7% for Max and 83.8% for Octopus. Behavioral response to -Dopa was enhanced over the entire 8 years subsequent to AAV2-AADC transduction. Immunohistochemical analysis revealed broad, even transduction of the target putamen that covered about 40% of the striatum [41.4% (0.58 cm3—Max) and 35% (0.49 cm3—Octopus)]. It is important to note that effective coverage of the target region is a necessary precondition of therapeutic effectiveness with AAV2-hAADC in order to avoid side effects9 and, in the case of growth factor therapies, to ensure therapeutic efficacy.10,11 Although 35–40% of the striatal volume was transduced, only 5–6% of the neurons within this region expressed the AADC transgene, and only neurons were transduced by AAV2 in the brain. Although this is quite a small percentage, the wide distribution of these neurons throughout the transduced region, a significant portion of the putamen, clearly is sufficient to induce a remarkable enhancement in -Dopa response.
Safety issues in gene therapy clinical trials are of utmost importance. Immune responses can be directed to both the vector and transgene used.12 Because AAV2 transduction is neuron-specific and neurons are not antigen-presenting cells, the use of AAV2 vectors in the brain confers an added level of safety, a view born out by the remarkable safety record of AAV2 in neurological gene therapy trials.2 Attenuation of transgene expression due to pre-existing immunity to AAV does hinder the effectiveness of AAV2-AADC transduction in rats when antibody titers exceed 1:400.13 Although we did not routinely measure AAV antibody titers when this study began in 1999, we subsequently included this in later NHP studies14 and in the Phase 1 clinical trial,15 and excluded subjects with pre-existing titers exceeding 1:400. It should be noted, however, that we have no direct evidence that high pre-existing titers are problematic in primates treated for neurological disorders with gene therapy.
We have not seen any major adverse effects in our monkeys caused by the AAV2-hAADC therapy. At the end of this study, 8 years post-transduction, no signs of neuroinflammation were seen within the transduced region. Both astrocytic (GFAP) and microglia (Iba1) markers did not show any upregulation. More importantly, no attenuation of AADC expression was detected either. As mentioned above, the level of AADC activity in the lesioned striata measured by PET with [18F]FMT was maintained at the same, unchanged level until the completion of the study. Consequently, the clinical response to low doses of -Dopa gave the same positive results as with monkeys rated after earlier time points after gene transfer.
Although, the number of monkeys used in this specific component of the overall study precludes statistical analysis, the present data combined with our previous work confirms our conclusions that the improvement in the -Dopa therapeutic window brought about by AADC gene therapy is pronounced and persistent over many years.
Materials and Methods
Two monkeys (M. mulatta) from our long-term study4,7 were killed 8 years after receiving the AAV2-hAADC treatment. Relevant methods and materials (induction of parkinsonism, clinical rating scale, AAV2-hAADC vector, convection-enhanced delivery, PET, and immunohistochemistry) have been published previously.4,7 At the time of killing, NHP weighed 12 kg (Max) and 8 kg (Octopus). Additional staining for GFAP and Iba1 markers was performed as previously described.16
Volume of AADC transduction within the lesioned hemisphere. The transference of AADC immunostained areas was conducted by first scanning (Epson 1660 photo scanner at 300 dpi, Seiko Epson, Nagano, Japan) all histologically processed slides counterstained with Cresyl violet. Then sections were manually delineated based on the extent and boundary of the immunoreactive (transduced) brain area. The resulting outlines were then copied to a transparency paper, individually matched to the corresponding baseline MR images and manually drawn with the Osirix ROI tool. The software automatically calculated the volume of AADC distribution (Ve).
Cellular transduction density. Immunohistochemically stained (AADC) serial (every 0.8 mm) brain sections (40 µm) of the transduced striatum were used to count the average cellular transduction density. Six random pictures were taken from each section (~6) (objective ×20) and AADC+ cells were counted per 1 mm2 frame. The mean values were calculated from ~36 pictures from each monkey.
Fraction of the transduced striatal neurons. Fluorescence microscopy was used to calculate the number of single- and double-labeled cells in sections. DyLight 549- (red) and DyLight 488- (green) labeled secondary antibodies (Biocare Medical, Concord, CA) were used to detect AADC+ and NeuN+ cells within the transduced striatum. Approximately 36 pictures were taken (objective ×20 Imager, Z1 Zeiss microscope with ApoTome mode) from each monkey (six random frames from each six sections (separated by 0.8 mm). Counts of double-labeled cells were made on merged images from two separate channels (red and green). The mean values represent the fraction of the transduced striatal neurons.
Acknowledgments
This work was supported by a U54 grant from the NIH-NINDS (NS061802).
REFERENCES
- Edelstein ML, Abedi MR., and, Wixon J. Gene therapy clinical trials worldwide to 2007–an update. J Gene Med. 2007;9:833–842. doi: 10.1002/jgm.1100. [DOI] [PubMed] [Google Scholar]
- Fiandaca M, Forsayeth J., and, Bankiewicz K. Current status of gene therapy trials for Parkinson's disease. Exp Neurol. 2008;209:51–57. doi: 10.1016/j.expneurol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Forsayeth JR, Eberling JL, Sanftner LM, Zhen Z, Pivirotto P, Bringas J, et al. A dose-ranging study of AAV-hAADC therapy in Parkinsonian monkeys. Mol Ther. 2006;14:571–577. doi: 10.1016/j.ymthe.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70:1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson's disease. Neurology. 2009;73:1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- Fiandaca MS, Varenika V, Eberling J, McKnight T, Bringas J, Pivirotto P, et al. Neuroimage. epub ahead of print; 2008. Real-time MR imaging of adeno-associated viral vector delivery to the primate brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankiewicz KS, Daadi M, Pivirotto P, Bringas J, Sanftner L, Cunningham J, et al. Focal striatal dopamine may potentiate dyskinesias in parkinsonian monkeys. Exp Neurol. 2006;197:363–372. doi: 10.1016/j.expneurol.2005.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog CD, Brown L, Gammon D, Kruegel B, Lin R, Wilson A, et al. Expression, bioactivity, and safety 1 year after adeno-associated viral vector type 2-mediated delivery of neurturin to the monkey nigrostriatal system support cere-120 for Parkinson's disease. Neurosurgery. 2009;64:602–12; discussion 612. doi: 10.1227/01.NEU.0000340682.06068.01. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- Hadaczek P, Forsayeth J, Mirek H, Munson K, Bringas J, Pivirotto P, et al. Transduction of nonhuman primate brain with adeno-associated virus serotype 1: vector trafficking and immune response. Hum Gene Ther. 2009;20:225–237. doi: 10.1089/hum.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanftner LM, Suzuki BM, Doroudchi MM, Feng L, McClelland A, Forsayeth JR, et al. Striatal delivery of rAAV-hAADC to rats with preexisting immunity to AAV. Mol Ther. 2004;9:403–409. doi: 10.1016/j.ymthe.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Pivirotto P, Bringas J, Suzuki B, Vijay S, Sanftner L, et al. Biodistribution of adeno-associated virus type-2 in nonhuman primates after convection-enhanced delivery to brain. Mol Ther. 2008;16:1267–1275. doi: 10.1038/mt.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73:1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Kells AP, Huang EJ, Lee HS, Hadaczek P, Beyer J, et al. Safety evaluation of AAV2-GDNF gene transfer into the dopaminergic nigrostriatal pathway in aged and parkinsonian rhesus monkeys. Hum Gene Ther. 2009;20:1627–1640. doi: 10.1089/hum.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]