Abstract
We tested the hypothesis that oral supplementation with the endothelial nitric oxide synthase (eNOS) cofactor tetrahydrobiopterin (BH4) improved the therapeutic efficacy of eNOS gene transfer in the ischemic rat hindlimb. BH4 or vehicle were begun 1 week before induction of hindlimb ischemia, whereas recombinant adenovirus containing bovine eNOS cDNA (AdeNOS) or vehicle [phosphate-buffered saline (PBS)] was infused intra-arterially into the ischemic hindlimb 10 days after induction of ischemia. Rats receiving co-treatment with dietary BH4 and eNOS gene transfer (the [eNOS, +BH4] group) had greater eNOS expression, phospho-eNOS expression (Ser1177), Ca2+-dependent NOS activity, and nitrite + nitrate concentrations in the ischemic gastrocnemius than did rats receiving AdeNOS alone. The [eNOS, +BH4] group demonstrated less nitrotyrosine and a higher ratio of reduced:oxidized glutathione (GSH:GSSG) in the ischemic gastrocnemius muscle than did rats receiving AdeNOS alone. The [eNOS, +BH4] group had greater flow recovery and a higher capillary:myocyte ratio in the ischemic hindlimb than did rats receiving AdeNOS alone. Finally, the [eNOS,+BH4] group had less necrosis of hindlimb muscles than rats given AdeNOS alone. We conclude that adjunctive dietary therapy with BH4 increases the beneficial effects of eNOS gene transfer within the ischemic gastrocnemius muscle, as evidenced by increased nitric oxide (NO) production, diminished oxidative stress, enhanced flow recovery, and reduced necrosis.
Introduction
Generation of novel therapeutic modalities for the clinical problem of critical limb ischemia is clearly warranted by the substantial impact this condition has on morbidity and mortality, as well as on the paucity of existing effective treatment options.1 In this context, our laboratory2,3 and others4,5 have investigated the potential utility of intra-arterial gene transfer of endothelial nitric oxide synthase (eNOS) into an ischemic limb as a therapeutic intervention designed to improve perfusion and minimize tissue loss. This treatment is based on the evidence that nitric oxide (NO) derived from eNOS is a potent vasodilator and signaling molecule that plays a essential role in vascular remodeling and perfusion recovery in response to acute hindlimb ischemia.6,7,8,9,10,11
eNOS generates NO during the oxidation of -arginine to -citrulline.12 An essential cofactor in this reaction is tetrahydrobiopterin (BH4). BH4 maintains eNOS in the dimeric configuration wherein electron transfer from the flavin of the reductase domain of one monomer to the heme group within the oxidase domain of the other monomer occurs, ultimately resulting in oxidation of -arginine. In the absence of BH4, eNOS becomes uncoupled; consequently, electron transfer between monomers fails to occur and the electron flux results in oxidation of molecular oxygen to form superoxide anion (O2−), a highly toxic free radical.13,14 Importantly, BH4 can itself undergo oxidation to form 7,8-dihydrobiopterin, which is functionally inactive. This circumstance can potentiate a vicious cycle in which uncoupled (BH4-deficient) eNOS generates O2− that in turn oxidizes available BH4, causing further uncoupling of eNOS.15 BH4 is itself a potent antioxidant and its loss might compromise redox balance by reducing the pool of antioxidants available to counteract an oxidant stress.
The capacity of eNOS to generate either NO or O2− raises an important question regarding the therapeutic efficacy of eNOS gene transfer in the setting of critical limb ischemia. It might be logically anticipated that administration of exogenous eNOS would increase production of eNOS-derived NO leading to enhancement in NO-mediated collateral artery vasodilation and remodeling. However, if eNOS is administered into an environment deficient in BH4, then it is conceivable that this exogenously delivered eNOS uncouples and therefore generates O2− in lieu of NO. To begin to address this question, this investigation tested the hypothesis that dietary supplementation with BH4 enhances the beneficial effects of intra-arterial gene transfer of eNOS in the setting of severe hindlimb ischemia in rats.
Results
Co-treatment with eNOS gene transfer and dietary BH4 significantly increased eNOS expression within the ischemic gastrocnemius muscle
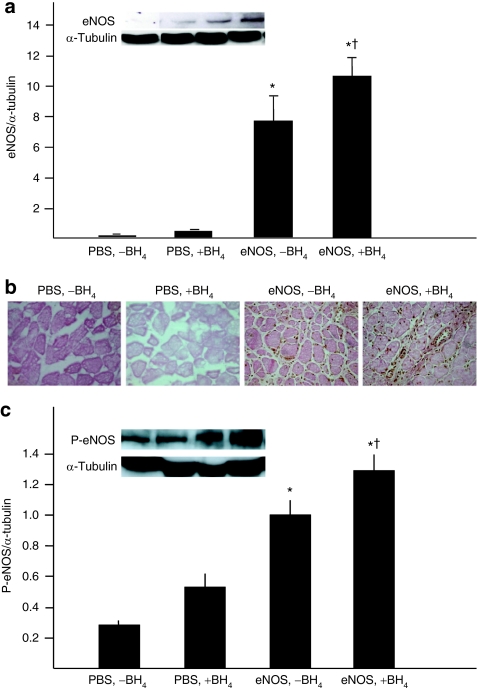
eNOS gene transfer was achieved by intra-arterial infusion of bovine eNOS cDNA linked to an adenovirus vector (AdeNOS). Rats receiving AdeNOS alone (the [eNOS, −BH4] group) demonstrated robust eNOS expression in the ischemic gastrocnemius muscle. This expression was significantly greater in rats receiving co-treatment with AdeNOS and dietary BH4 (the [eNOS, +BH4] group) (Figure 1a,b). A similar pattern was observed in the expression of phospho-eNOS in the ischemic gastrocnemius (eNOS phosphorylated at Ser1177) (Figure 1c).
Figure 1.
Co-treatment with eNOS gene transfer and dietary BH4 significantly increased eNOS expression in the ischemic gastrocnemius muscle. Studies were carried out 4 days after AdeNOS or PBS infusion (14 days after induction of ischemia). (a) Expression of bovine eNOS in the ischemic gastrocnemius muscle was greater in rats receiving AdeNOS than in rats receiving only PBS, and this expression was enhanced further by co-treatment with dietary BH4 (mean ± SD, n = 4–5, *P < 0.05 versus [PBS, −BH4], †P < 0.05 versus [eNOS, −BH4]). (b) This finding was confirmed by immunohistochemistry, which demonstrated greater evidence eNOS in rats receiving AdeNOS infusion than in rats receiving PBS infusion. Representative micrographs are shown (×400); eNOS is stained brown. (c) Expression of phospho-eNOS in the ischemic gastrocnemius muscle paralleled that of bovine eNOS; thus, expression was greater in rats receiving AdeNOS than PBS, and was greatest in rats that received both AdeNOS and dietary BH4 (mean ± SD, n = 4–5, *P < 0.05 versus [PBS, −BH4], †P < 0.05 versus [eNOS, −BH4]). BH4, tetrahydrobiopterin; eNOS, endothelial nitric oxide synthase; PBS, phosphate-buffered saline.
Co-treatment with eNOS gene transfer and dietary BH4 significantly increased eNOS activity within the ischemic gastrocnemius muscle
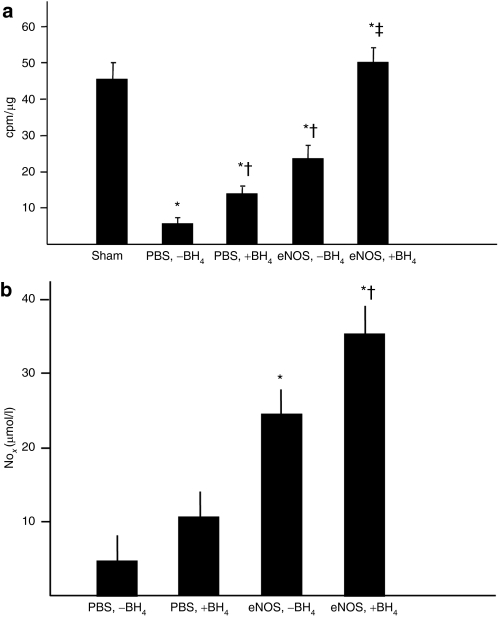
Ca2+-dependent NOS activity, i.e., the sum of endogenous eNOS, bovine AdeNOS, and endogenous nNOS (the neural is form of NOS) was significantly less in the ischemic gastrocnemius muscle of rats receiving only intra-arterial phosphate-buffered saline (PBS) (the [PBS, −BH4] group) than in sham rats, a group fed a normal diet and that underwent a sham femoral excision. Rats receiving dietary BH4 alone (the [PBS, +BH4] group) and rats receiving eNOS gene transfer alone had Ca2+-dependent NOS activity levels greater than the [PBS, −BH4] group, but these levels still remained below that noted in the sham limb. Rats in the [eNOS, +BH4] group, however, did have a NOS activity level equivalent to the sham group (Figure 2a). The Ca2+-independent NOS activity level, which reflects the inflammatory NOS isoform, was greater in the [PBS, −BH4] group than in all other groups. This level was similar among the other treatment groups (data not shown). The NOx concentration in the ischemic gastrocnemius muscle, which represents the sum of the NO metabolites NO2 and NO3, closely paralleled phospho-eNOS expression and Ca2+-dependent NOS activity levels: NOx was greater in rats receiving eNOS than PBS, was greatest in rats receiving both eNOS and BH4 (Figure 2b). Collectively, these findings indicate that co-treatment with intra-arterial AdeNOS and dietary BH4 exerted an additive effect on eNOS expression and activity in the ischemic hindlimb, leading to an increased production of NO. We have previously demonstrated that infusion of an empty adenoviral vector into the ischemic hindlimb does not affect eNOS expression or activity in the gastrocnemius muscle2 and so did not repeat these groups in the present study.
Figure 2.
Co-treatment with eNOS gene transfer and dietary BH4 significantly increased eNOS activity in the ischemic gastrocnemius muscle. Studies were carried out 4 days after AdeNOS or PBS infusion (14 days after induction of ischemia). (a) Ca2+-dependent NOS activity was measured in the gastrocnemius muscle from the four study groups as well as in rats that underwent sham femoral ligation. NOS activity was lower than the sham level in all study groups except the [eNOS,+BH4] co-treatment group, although treatment with either BH4 or AdeNOS alone increased NOS activity level above that present in rats receiving only PBS (mean ± SD, n = 5, *P < 0.05 versus sham, †P < 0.05 versus [PBS,-BH4], ‡P < 0.05 versus [eNOS, −BH4]). (b) The NOx concentration in the ischemic gastrocnemius was greater in rats receiving AdeNOS infusion than in rats receiving PBS infusion. Rats receiving co-treatment with AdeNOS and BH4 had gastrocnemius NOx levels greater than rats receiving AdeNOS alone (m ± SD, n = 4–5, *P < 0.05 versus [PBS, −BH4], †P < 0.05 versus [eNOS, −BH4]). BH4, tetrahydrobiopterin; eNOS, endothelial nitric oxide synthase; PBS, phosphate-buffered saline.
Co-treatment with eNOS gene transfer and dietary BH4 significantly reduced oxidant stress in the ischemic gastrocnemius muscle
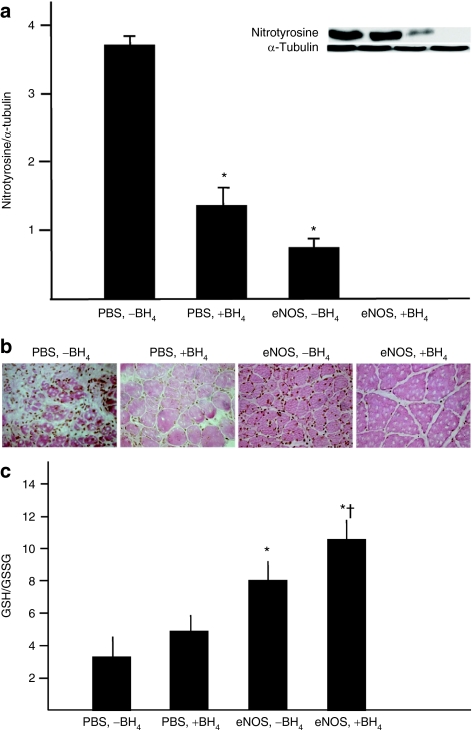
Nitrotyrosine, a marker of peroxynitrite-induced protein nitrosylation, was most abundantly present as in the ischemic gastrocnemius muscle in the [PBS, −BH4] group, as evidenced by both western blotting and immunohistochemistry (Figure 3a,b). The level of nitrotyrosine accumulation was significantly less in the [PBS, +BH4] and [eNOS, −BH4] groups than in rats receiving PBS alone, indicating that the singular additions of BH4 or AdeNOS were sufficient to reduce nitrotyrosine accumulation. Most striking, however, was the virtual absence of nitrotyrosine in the ischemic gastrocnemius muscle from the [eNOS, +BH4] group. The ratio of reduced:oxidized glutathione (GSH:GSSG) was also measured as an index of oxidant stress in the ischemic gastrocnemius muscle. Treatment with eNOS gene therapy alone was sufficient to increase the GSH:GSSG ratio, whereas the singular addition of dietary BH4 did not exert a significant effect. Rats co-treated with eNOS gene transfer and dietary BH4 had a GSH:GSSG ratio greater than rats receiving AdeNOS alone (Figure 1c). Together, these findings indicate that co-treatment with eNOS gene transfer and dietary BH4 had an additive beneficial effect in reducing oxidant stress within the ischemic gastrocnemius muscle.
Figure 3.
Co-treatment with eNOS gene transfer and dietary BH4 significantly reduced oxidative stress in the ischemic gastrocnemius muscle. These studies were carried out 4 days after AdeNOS or PBS infusion (14 days after induction of ischemia). (a) The expression of nitrotyrosine in the ischemic gastrocnemius muscle was lower in rats receiving either AdeNOS or BH4 alone than in rats receiving only PBS; moreover, nitrotyrosine was undetectable by western blotting in the ischemic gastrocnemius from rats receiving co-treatment with AdeNOS and BH4 (m ± SD, n = 4–5, *P < 0.05 versus [PBS, −BH4]). (b) These findings were confirmed by immunohistochemistry; thus, less nitrotyrosine was evident in the gastrocnemius muscles from rats receiving either AdeNOS or BH4, but virtually none was evident in rats receiving both treatments. Representative micrographs are shown (×400); nitrotyrosine is stained brown. (c) The ratio of the reduced:oxidized forms of glutathione (GSH: GSSG) in the ischemic gastrocnemius muscle was greater in rats receiving AdeNOS alone than in rats receiving PBS; in contrast, treatment with BH4 alone had no effect. Rats receiving co-treatment with AdeNOS and BH4 had a GSH:GSSG ratio greater than rats receiving AdeNOS alone (m ± SD, n = 4–5, *P < 0.05 versus [PBS, −BH4], †P < 0.05 versus [eNOS, +BH4]). BH4, tetrahydrobiopterin; eNOS, endothelial nitric oxide synthase; PBS, phosphate-buffered saline.
Co-treatment with eNOS gene transfer and dietary BH4 significantly improved blood flow and capillary density in the ischemic hindlimb
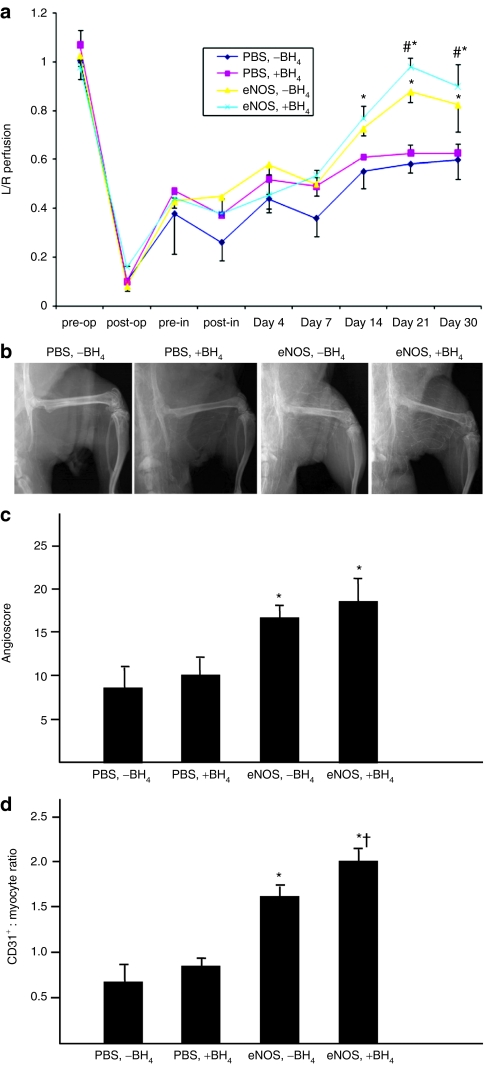
Laser Doppler perfusion imaging demonstrated that calf perfusion was similar in the four treatment groups until 14 days after induction of hindlimb ischemia (Figure 4a). Beginning at this time point, the recovery of foot perfusion was significantly greater in the two groups that received eNOS gene transfer than in the [PBS, −BH4] and [PBS, +BH4] groups. Recovery of foot perfusion was significantly greater in the [eNOS, +BH4] than in the [eNOS, −BH4] group on days 21 and 30 after induction of hindlimb ischemia. The angioscore, a measure of collateral artery enlargement, was significantly greater in the two groups that received eNOS gene transfer than in the [PBS, −BH4] or [PBS, +BH4] groups, whereas the angioscores were similar in the [eNOS, −BH4] and [eNOS, +BH4] groups (Figure 4b). Capillary density, determined as the ratio of CD31+ cells (endothelial cells):myocytes, was similar in the [PBS, −BH4] and [PBS, +BH4] groups, i.e., the ratio was not affected by the addition of dietary BH4 alone. In contrast, the [eNOS, −BH4] group had a greater capillary:myofiber ratio than groups receiving PBS, whereas the coaddition of AdeNOS and BH4 increased this ratio further (Figure 4c). We have previously demonstrated that intra-arterial infusion of an empty adenoviral vector into the ischemic hindlimb does not affect foot blood flow recovery or capillary density,2,3 and so did not repeat these groups in the present study.
Figure 4.
Co-treatment with eNOS gene transfer and dietary BH4 significantly improved blood flow and capillary density in the ischemic hindlimb. (a) LDPI data were expressed as the ratio of the ischemic:nonischemnic limb. Rats receiving AdeNOS 10 days after induction of ischemia demonstrated greater foot blood flow recovery between postischemia days 14 through 30 than rats receiving PBS infusion, and the addition of BH4 alone had no effect. Rats who received co-treatment with AdeNOS and BH4 had greater foot flow recovery between postischemia days 21 and 30 after induction of ischemia than rats receiving AdeNOS alone (m ± SD, n = 5, *P < 0.05 versus [PBS, −BH4], #P < 0.05 versus [eNOS, −BH4]). (b,c) The angioscore, determined 18 days after AdeNOS or PBS infusion (28 days after induction of ischemia), was greater in rats receiving AdeNOS than in rats receiving PBS; co-treatment with dietary BH4 and AdeNOS did not cause a further increase in the angioscore (mean ± SD, n = 5, *P < 0.05 versus [PBS, −BH4]). (d) Capillary density, expressed as the ratio of CD31+ cells (endothelial cells):myocytes, was determined in the ischemic gastrocnemius muscle 18 days after AdeNOS or PBS infusions (28 days after induction of ischemia). Rats receiving AdeNOS had higher capillary density measurements than rats receiving PBS, and this effect was increased in rats receiving co-treatment with AdeNOS and BH4 (mean ± SD, n = 5, *P < 0.05 versus [PBS, −BH4], †P < 0.05 versus [eNOS, −BH4]). BH4, tetrahydrobiopterin; eNOS, endothelial nitric oxide synthase; I/R, ischemia/reperfusion; PBS, phosphate-buffered saline.
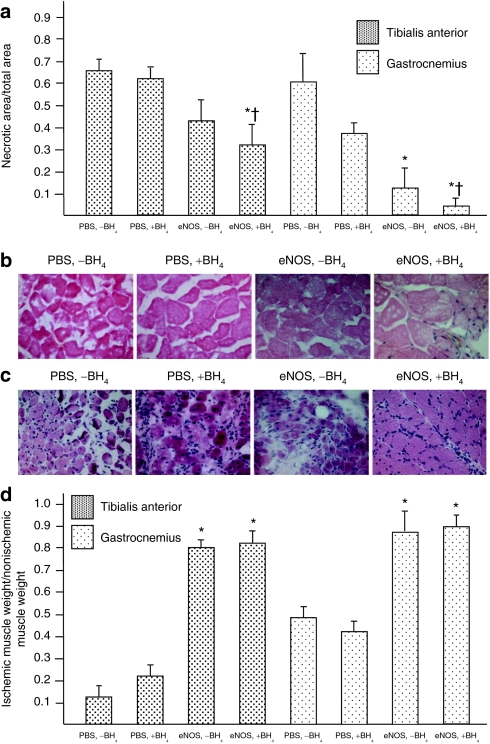
Co-treatment with eNOS gene therapy and dietary BH4 significantly reduced the extent of muscle necrosis in the ischemic hindlimb
Staining of the cut surface of tibialis anterior and gastrocnemius muscles from the ischemic hindlimb with nitroblue tetrazolium revealed substantial muscle necrosis in rats receiving only PBS. The extent of necrotic area was similar in the [PBS, −BH4] and [PBS, +BH4] groups; however, the degree of necrosis was significantly less in the [eNOS, −BH4] group than in rats receiving PBS. Rats co-treated with AdeNOS and BH4 demonstrated the least extent of muscle necrosis (Figure 5a). These differences were also evident on hematoxylin and eosin–stained sections of tibialis anterior (Figure 5b) and gastrocnemius (Figure 5c) muscles from the four study groups. Indeed, the gastrocnemius histology noted in the [eNOS, +BH4] group was remarkably free of extensive necrosis or inflammation. In contrast, muscle histology in the [PBS, −BH4] and [PBS, +BH4] groups revealed heavy nuclear staining in the intermyofibrillar connective tissue, as well as an inflammatory cell infiltrate. Finally, the weights of the ischemic tibialis anterior and gastrocnemius muscles, when expressed as a ratio to weight of the nonischemic muscles, were significantly greater in the [PBS, −BH4] and [PBS, +BH4] groups than in the [eNOS, −BH4] group. The weight ratios were similar in the [eNOS, −BH4] and [eNOS, +BH4] groups (Figure 5d). Collectively, these findings indicate that co-treatment with intra-arterial AdeNOS and dietary BH4 had an additive effect on reducing muscle necrosis in the ischemic hindlimb.
Figure 5.
Co-treatment with eNOS gene transfer and BH4 significantly reduces the extent of muscle necrosis in the ischemic hindlimb. Studies were carried out on tibialis anterior and gastrocnemius muscles taken from the thigh and calf, respectively, 7 days after AdeNOS or PBS infusion (17 days after induction of ischemia). (a) Transverse sections were made through the muscles, and the cut surfaces (≥3 per muscle) were stained with nitroblue tetrazolium to delineate between viable and necrotic tissue. The data are expressed as the ratio of necrotic surface area:total cut surface area. This ratio was less in rats receiving AdeNOS infusion than in rats receiving PBS infusion. In the gastrocnemius muscle, this ratio was reduced further by co-treatment with AdeNOS and BH4 than in rats receiving AdeNOS alone (mean ± SD, n = 5, *P < 0.05 versus [PBS, −BH4], †P < 0.05 versus [eNOS, −BH4]). Histological evaluation revealed that co-treatment with AdeNOS and BH4 significantly reduced evidence of cellular necrosis in the (b) tibialis anterior muscle and (c) gastrocnemius muscle. This difference was particularly evident in the gastrocnemius. Representative micrographs are shown (×400); sections were stained with hematoxylin and eosin. (d) The tibialias anterior and gastrocnemius muscles from the ischemic and nonischemic hindlimbs were weighed and the ratio calculated. This ratio was significantly greater in rats receiving AdeNOS infusion than PBS infusion. Co-treatment with AdeNOS and dietary BH4 did not affect the ratio further (m ± SD, n = 5, *P < 0.05 versus [PBS, −BH4]). BH4, tetrahydrobiopterin; eNOS, endothelial nitric oxide synthase; PBS, phosphate-buffered saline.
Discussion
The addition of dietary BH4 supplementation to rats receiving eNOS gene transfer had an additive effect in improving the measured outcome variables following induction of acute ischemia in the rat hindlimb and these data support the experimental hypothesis. Thus, eNOS expression and NO production, and blood flow recovery and capillary density were increased in co-treated rats, whereas evidence of oxidant stress and tissue necrosis were significantly reduced when both AdeNOS and BH4 were provided. The ultimate goal of therapy for limb ischemia is preservation of muscle and hence limb function. In this context, the present findings provide the first in vivo evidence that dietary supplementation with BH4, coupled with eNOS gene therapy, exerts a beneficial additive effect on this most clinically relevant of dependent variables, i.e., the extent of postischemic muscle necrosis.
The premise of this study was that delivery of eNOS gene therapy in the setting of critical limb ischemia will provide maximal benefit only if the substrates (-arginine, NADPH, O2) and cofactors (flavin adenine dinucleotide, flavin mononucleotide, iron protoporphyrin IX, and BH4) are present at levels sufficient to permit normal enzymatic activity.12 Of these substrates and cofactors, we focused on supplementation of BH4 for three reasons. First, BH4 is requisite to maintain eNOS in the dimeric form necessary for the transfer of electrons from NADPH to -arginine; the absence of BH4 “uncouples” eNOS monomers wherein the electron flow is diverted to O2, resulting in eNOS-derived production of O2−.13,14,15 An intracellular eNOS:BH4 molar ratio >1 is necessary to prevent eNOS “uncoupling,”16 and studies carried out in vitro16 and in vivo17 have demonstrated that BH4 supplementation enhances NO production and reduces O2− production by eNOS. Second, BH4 is readily oxidized,15 and oxidant stress is present in critical limb ischemia because of the repetitive episodes of transient reperfusion.18 It is thus possible that BH4 levels are reduced in the ischemic hindlimb and that supplementation with dietary BH4 might restore level of this cofactor within the collateral arteries and capillaries within the ischemic hindlimb, the principal sites of eNOS activity. Third, BH4 is itself a potent antioxidant that eliminates peroxynitrite 6–10 times faster than thiols (e.g., glutathione) and ascorbate.14 Peroxynitrite, formed by the combination of NO and O2−, is one of the nitrogen radicals responsible for protein nitrosylation and hence nitrotyrosine formation,15 a process established to be present in the ischemic hindlimb.2,3
The increased NOx tissue concentration, the increased expression of phospho-eNOS, the increased GSH:GSSG ratio, and virtual elimination of nitrotyrosine staining in the ischemic gastrocnemius muscle provide strong, albeit indirect evidence that dietary supplementation with BH4 reduced the extent of eNOS uncoupling in the ischemic gastrocnemius muscle of rats receiving eNOS gene transfer. NOx represents the sum of the NO metabolites NO2 and NO3, and is an accepted surrogate measurement for NO production.19 The [eNOS, +BH4] co-treatment group had an NOx concentration and a Ca2+-dependent NOS activity in the ischemic gastrocnemius that were significantly greater than rats who underwent eNOS gene transfer alone, findings that were consistent with the increased expression of phospho-eNOS in this muscle. The ratio of reduced:oxidized glutathione provides insight into the redox balance of the tissue, insofar as glutathione peroxidase is a critical oxidant detoxification pathway.20 The GSH:GSSG ratio was greater in the [eNOS, +BH4] group than in the [eNOS, −BH4] group, indicating less oxidant stress in the ischemic gastrocnemius muscle from rats receiving co-treatment. Nitrotyrosine staining reflects protein nitration by peroxynitrite, formed in the presence of excess O2−;13,14 in this context, the virtual absence of nitrotyrosine in the [eNOS, +BH4] ischemic gastrocnemius muscle indicates a marked reduction in oxidative and nitrative stress therein. We interpret these data to indicate that rats receiving co-treatment with AdeNOS and BH4 had more NO and less O2− production in the ischemic gastrocnemius, circumstances that would be anticipated in the absence of eNOS uncoupling. Demonstration of a reduced eNOS monomer:dimer ratio in the gastrocnemius of the [eNOS, +BH4] group would provide direct evidence of reduced eNOS uncoupling, but was not carried out in this study insofar as the indirect evidence was consistently supportive of the experimental hypothesis.
The present findings are entirely consistent with previous observations made in the heart and liver. Dumitrescu et al.21 correlated time-dependent reductions in eNOS activity and BH4 levels during ischemia in rat hearts, as well as a concomitant increase in NOS-derived superoxide production. Treatment with BH4 partially restored eNOS activity in ischemic hearts and improved postischemic flow recovery in a manner reminiscent to that we observed in the ischemic hindlimb. Elrod et al.22 administered AdeNOS to diabetic (db/db) mice and then induced hepatic ischemia/reperfusion; db/db mice receiving AdeNOS experienced greater hepatic ischemia/reperfusion damage than sham-treated db/db mice. However, co-treatment with AdeNOS and BH4 had the opposite effect, significantly reducing hepatic necrosis following ischemia/reperfusion. These findings are redolent to those we observed in the ischemic hindlimb, wherein co-treatment with eNOS and BH4 reduced the extent of ischemia-induced gastrocnemius muscle necrosis.
The study design, specifically the use of dietary supplementation with BH4, was based on the supposition that this cofactor would be absorbed and transported to the microvasculature within the ischemic hindlimb where it would serve to increase NO production by eNOS delivered by gene therapy. This notion was based on three facts: first, BH4 added to the chow is stable at room temperature; second, dietary BH4 is effectively absorbed;23 and third, that dietary BH4 supplementation results in an increase in tissue BH4 levels.24,25 Direct measurement of BH4 and its metabolites in the gastrocnemius would provide definitive evidence that dietary BH4 had the anticipated effect. We attempted to measure the levels of BH4 and its immediate oxidation product BH2 in homogenates of gastrocnemius muscle using established methods,15,16,17 but were encumbered by an elevated signal-to-noise ratio, even in gastrocnemius muscles from untreated sham-operated rats. This variability precluded data analysis and interpretation. We contend that the absence of tissue BH4 measurements does not abrogate the present findings. A robust literature documents the efficacy of oral BH4 supplementation on production of eNOS-derived NO and reduction of eNOS-derived O2−.13,14,15,16,17 As well, the variables measured herein consistently demonstrated that the addition of dietary BH4 to eNOS gene transfer provided an additive improvement over eNOS gene transfer alone, findings that strongly support the study hypothesis.
One of the most compelling findings was the effect of co-treatment with dietary BH4 and eNOS gene therapy on the reduction of muscle necrosis, especially in the gastrocnemius muscle that lies well downstream from the site of femoral artery excision. This reduction in muscle necrosis most likely reflects improved downstream oxygen delivery in the [eNOS, +BH4] group, which in turn reflects more effective postischemic arteriogenesis and angiogenesis in this group. In the presence of femoral excision, flow to the gastrocnemius must be reconstituted by enlargement of existing collateral arteries in a process termed arteriogenesis.26 One means to assess arteriogenesis is by measurement of blood flow to the distal-most portion of the hindlimb.24 Here, we observed that the [eNOS, +BH4] group manifest greater perfusion than other groups between postischemia days 21 and 30; interestingly, this timing is coincident with the point of maximal flow restitution in the postischemic hindlimb.27 Moreover, these perfusion data were measured in anesthetized, immobile mice wherein hindlimb muscle was in a resting state. Brevetti et al.28 reported that the flow reduction present in the resting ischemic hindlimb become more exaggerated during exercise, when the oxygen demand by contracting muscle is increased; it is thus likely that differences in flow recovery among the study groups were even more severe, making the enhanced resting flow level in the [eNOS, +BH4] group more physiologically relevant when the mice were awake and mobile. Angiogenesis is the de novo generation of capillaries within the muscle microcirculation and can be assessed by measurement of the CD31+: myofiber ratio, wherein CD31+ cells indicate the presence of endothelial cells, and hence capillaries.29 This ratio was significantly greater in rats receiving co-treatment with dietary BH4 and eNOS gene therapy than in any other group. Both arteriogenesis and angiogenesis require the mobilization of bone marrow–derived vascular progenitor cells, as well as homing of those cells to the site of injury.7,8,9,11 These processes are dependent on eNOS-derived NO;11 thus, the greater presence of arteriogenesis and angiogenesis in the [eNOS, +BH4] group is further evidence of enhanced NO production in response to co-treatment and also provide important physiological correlation to NO production.
The therapeutic goal of the medical or surgical treatment of critical limb ischemia is preservation of muscle integrity and maintenance of limb function.30 The present findings demonstrate, for the first time, that co-treatment with dietary BH4 and intra-arterial AdeNOS significantly reduce the degree of gastrocnemius muscle necrosis in the setting of acute hindlimb ischemia, a commonly used and highly relevant preclinical model of critical limb ischemia.31 It is interesting to note that previous clinical trials designed to improve vascular outcome by increasing eNOS expression or function (e.g., the VIVA trial32) have met with limited success. We propose that supplementation with BH4 might significantly improve the clinical therapeutic efficacy of treatments aimed to improve eNOS expression, particularly in circumstances wherein oxidation of endogenous BH4 might have occurred.
Materials and Methods
Experimental animals. The study was approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco, CA and the University of Massachusetts Medical School, Worcester, MA. Adult male Sprague–Dawley rats were obtained from Charles River Laboratories, Wilmington, MA. All rats underwent induction of hindlimb ischemia by excision of the left common femoral artery under 2% isoflurane.
Treatment groups. The [PBS, −BH4] group was maintained on standard rat chow and received intra-arterial PBS infusion 10 days after induction of hindlimb ischemia. The [PBS, +BH4] group was given dietary BH4 supplementation, described below, and received intra-arterial PBS infusion 10 days after induction of hindlimb ischemia. The [eNOS, −BH4] group was maintained on standard chow and received intra-arterial AdeNOS infusion, described below, 10 days after induction of hindlimb ischemia. The [eNOS, +BH4] group was given dietary BH4 supplementation and received intra-arterial AdeNOS infusion 10 days after induction of hindlimb ischemia.
Dietary BH4 supplementation. Powdered standard rat chow was obtained from Research Diets (New Brunswick, NJ). The chow was supplemented with BH4, 0.04/20 g chow (Schircks Laboratories, Jonas, Switzerland). This supplementation was designed to provide 10 mg/kg/day of BH4 based on the average amount of chow ingested per rat per day. Assigned rats were begun on the supplemented diet 1 week before induction of hindlimb ischemia and were continued on the diet until the end of study. Food supply within each cage was changed every other day.
Recombinant adenovirus preparation and in vivo gene transfer. Recombinant adenovirus containing bovine eNOS cDNA driven by the Rous sarcoma virus promoter (AdeNOS; a generous gift from Beverly Davidson, University of Iowa, Iowa City, IA) was propagated and titrated in 293 cells as described previously.3 Adenovirus stocks were stored at −80 °C, then thawed immediately before use and diluted in PBS to achieve a final concentration of 1 × 1010 plaque forming units per ml. The left common femoral vein was occluded with a microvascular clamp; thereafter, the saphenous artery was cannulated in a retrograde manner, and AdeNOS (1010 pfu/ml; 0.7 ml) or PBS (0.7 ml) was infused. The venous clamp was removed 30 minutes later. These infusions were carried out 10 days after induction of ischemia and studies of eNOS transgene expression and function, and tissue oxidant stress were carried out 4 days later. These time points were based on prior work from our lab that demonstrated optimal eNOS expression when the transgene was given 10 days after ischemia and measured 4 days thereafter.2,3,26
Laser Doppler perfusion imaging. Hindlimb blood flow was determined by means of laser Doppler imaging (Moor Instruments, Axminster, UK). Scans were obtained during inhalation of 1% isoflurane, whereas core body temperature was maintained between 36.8 and 37.2 °C. Scans were repeated three times and the average for each rat determined. Scans were obtained through day 30 postischemia as we have previously determined that maximal flow recovery occurs by this time.2,3,26
Angiograms. Barium sulfate (2.5 ml; E-Z-Paque; Merry X-Ray, South San Francisco, CA) was infused into the infrarenal aorta after ligation of the proximal aorta and inferior vena cava during inhalation of 2% isoflurane. A grid was superimposed over the film between the greater trochanter of the femur to the patella. The number of intersections between contrast-filled vessels and gridlines was determined independently by blinded observers. Angiograms were obtained on postischemia day 30 as we have previously determined that maximal flow recovery occurs by this time.2,3,26
Capillary:myofiber ratio. Cryosections were stained with 1° anti-mouse CD31 monoclonal antibody, then a biotinylated 2° antibody, and then developed with ABC (Vector Laboratories, Burlingame, CA). Slides were counterstained with eosin. Capillary density was determined by counting the number of CD31+ cells (endothelial cells) and myocytes at ×400. At least three fields were counted per muscle section and the average was taken.
Immunohistological staining. Analysis of eNOS expression by immunostaining was carried out 4 days after AdeNOS or saline administration (14 days after induction of ischemia) insofar as previous work has demonstrated maximal vascular AdeNOS expression at this time point.3 Cryosections (10 µm) of gastrocnemius and tibialis anterior muscle were stained with monoclonal antibody targeting both rat and bovine eNOS (BD Biosciences, San Diego, CA) or monoclonal antibody directed against nitrotyrosine (Cayman Chemical, Ann Arbor, MI). Negative controls were created by substituting blocking buffer for primary antibody.
Western blotting. One hundred micrograms of protein were separated on a 12% sodium dodecyl sulfate–polyacrylamide gel for nitrotyrosine or 7.5% sodium dodecyl sulfate–polyacrylamide gel for eNOS, and transferred to nitrocellulose membranes. Membranes were incubated overnight (4 °C) with monoclonal antibodies directed against eNOS, phospho-eNOS (Ser1177), or nitrotyrosine, and then horseradish peroxidase–conjugated secondary antibody was applied. Band intensity was determined by densitometry.
Ca2+-dependent NOS activity. Fifty micrograms of muscle protein homogenate were incubated in 10 mmol/l NADPH, 1 µCi/µl 14C-arginine, 6 mmol/l CaCl2, 50 mmol/l Tris-HCl (pH 7.4), 6 µmol/l BH4, 2 µmol/l FAD, and 2 µmol/l FMN for 30 minutes at 37 °C. The reaction was stopped with 400 µl of 50 mmol/l HEPES (pH 5.5) and 5 mmol/l EDTA. Identical samples were prepared without CaCl2, and all reactions were performed in duplicate. The radioactivity of the sample eluate was measured in a liquid scintillation counter. Enzyme activity was expressed as counts per million per minute per microgram protein (cpm/minute/µg). The Ca2+-NOS activity was calculated by subtracting the NOS activity measured without calcium from the total activity measured in the presence of calcium.
NOx (nitrite + nitrate) assay. Samples of gastrocnemius were homogenized and the homogenates were centrifuged; the supernatants were recovered and passed through a filter. Nitrate was reduced to nitrite via nitrate reductase and the subsequent product reacted with 2,3-diaminonaphthalene (Griess reagent) and fluorescence measured. This work was carried out with a kit from Cayman Chemical.
GSH:GSSG ratio. Samples of gastrocnemius were homogenized and the homogenates centrifuged; the supernatants were recovered and passed through a filter. The total glutathione content was measured by enzymatic reduction of all glutathione to GSH, followed by quantification with Ellman's reagent. GSSG was measured by determining GSH content in nonreduced samples. A kit from Cayman Chemical was used for this assay.
Evaluation of muscle necrosis. Muscle necrosis was determined using cut sections of tibialis anterior and gastrocnemius muscle. Muscle samples were cut transversely into three pieces (cuts made in 2 mm increment); two sections were stained with nitroblue tetrazolium. These sections were incubated in phosphate-buffered saline (pH 7.4) containing 0.033% nitroblue tetrazolium and 0.133% NADH for 10 minutes, and then fixed in 4% paraformaldehyde. The areas of viable (stained) and nonviable (unstained) tissue were quantified using Image-Pro software (Media Cybernetics, Bethesda, MD) and the average was taken for each rat. The third cut muscle section was used for hematoxylin and eosin staining.
Statistical analysis. Analysis was performed using analysis of variance. Post hoc Student–Newman–Keuls tests were carried out if the analysis of variance f-statistic was significant to determine sites of difference. Probability values less <0.05 were accepted as significant.
Acknowledgments
This work was supported by HL68042 (G.T.), HL75353 (L.M.M.), as well as grants from the Pacific Vascular Research Foundation (L.M.M.), and Wayne and Gladys Valley Foundation (L.M.M.). The authors have nothing to disclose. This work was carried out in San Francisco, CA and Worcester, MA.
REFERENCES
- Shammas NW. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Vasc Health Risk Manag. 2007;3:229–234. doi: 10.2147/vhrm.2007.3.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevetti LS, Chang DS, Tang GL, Sarkar R., and, Messina LM. Overexpression of endothelial nitric oxide synthase increases skeletal muscle blood flow and oxygenation in severe rat hind limb ischemia. J Vasc Surg. 2003;38:820–826. doi: 10.1016/s0741-5214(03)00555-x. [DOI] [PubMed] [Google Scholar]
- Yan J, Tang GL, Wang R., and, Messina LM. Optimization of adenovirus-mediated endothelial nitric oxide synthase delivery in rat hindlimb ischemia. Gene Ther. 2005;12:1640–1650. doi: 10.1038/sj.gt.3302563. [DOI] [PubMed] [Google Scholar]
- Smith RS, Jr, Lin KF, Agata J, Chao L., and, Chao J. Human endothelial nitric oxide synthase gene delivery promotes angiogenesis in a rat model of hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2002;22:1279–1285. doi: 10.1161/01.atv.0000026613.18742.67. [DOI] [PubMed] [Google Scholar]
- Tsutsui M, Chen AF, O'Brien T, Crotty TB., and, Katusic ZS. Adventitial expression of recombinant eNOS gene restores NO production in arteries without endothelium. Arterioscler Thromb Vasc Biol. 1998;18:1231–1241. doi: 10.1161/01.atv.18.8.1231. [DOI] [PubMed] [Google Scholar]
- Katusic ZS. Therapeutic angiogenesis: new indication for endothelial NO synthase gene transfer. Arterioscler Thromb Vasc Biol. 2002;22:1254–1255. doi: 10.1161/01.atv.0000026860.08501.0f. [DOI] [PubMed] [Google Scholar]
- Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter JB, Curtis VC, Valic Z, Ruble SB., and, Clifford PS. Endogenous vascular remodeling in ischemic skeletal muscle: a role for nitric oxide. J Appl Physiol. 2003;94:935–940. doi: 10.1152/japplphysiol.00378.2002. [DOI] [PubMed] [Google Scholar]
- Lloyd PG, Yang HT., and, Terjung RL. Arteriogenesis and angiogenesis in rat ischemic hindlimb: role of nitric oxide. Am J Physiol Heart Circ Physiol. 2001;281:H2528–H2538. doi: 10.1152/ajpheart.2001.281.6.H2528. [DOI] [PubMed] [Google Scholar]
- Mees B, Wagner S, Ninci E, Tribulova S, Martin S, van Haperen R, et al. Endothelial nitric oxide synthase activity is essential for vasodilation during blood flow recovery but not for arteriogenesis. Arterioscler Thromb Vasc Biol. 2007;27:1926–1933. doi: 10.1161/ATVBAHA.107.145375. [DOI] [PubMed] [Google Scholar]
- Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- Marletta MA. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993;268:12231–12234. [PubMed] [Google Scholar]
- Schmidt TS., and, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 2007;113:47–63. doi: 10.1042/CS20070108. [DOI] [PubMed] [Google Scholar]
- Vásquez-Vivar J, Kalyanaraman B, Martásek P, Hogg N, Masters BS, Karoui H, et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzkaya N, Weissmann N, Harrison DG., and, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, Hale AB, Cai S, et al. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- Bendall JK, Alp NJ, Warrick N, Cai S, Adlam D, Rockett K, et al. Stoichiometric relationships between endothelial tetrahydrobiopterin, endothelial NO synthase (eNOS) activity, and eNOS coupling in vivo: insights from transgenic mice with endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression. Circ Res. 2005;97:864–871. doi: 10.1161/01.RES.0000187447.03525.72. [DOI] [PubMed] [Google Scholar]
- Stoffels F, Lohöfener F, Beisenhirtz M, Lisdat F., and, Büttemeyer R. Concentration decrease of nitric oxide in the postischemic muscle is not only caused by the generation of O2−. Microsurgery. 2007;27:565–568. doi: 10.1002/micr.20403. [DOI] [PubMed] [Google Scholar]
- Misko TP, Schilling RJ, Salvemini D, Moore WM., and, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- Inoue M, Saito Y, Hirata E, Morino Y., and, Nagase S. Regulation of redox states of plasma proteins by metabolism and transport of glutathione and related compounds. J Protein Chem. 1987;6:207–225. [Google Scholar]
- Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, et al. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci USA. 2007;104:15081–15086. doi: 10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod JW, Duranski MR, Langston W, Greer JJ, Tao L, Dugas TR, et al. eNOS gene therapy exacerbates hepatic ischemia-reperfusion injury in diabetes: a role for eNOS uncoupling. Circ Res. 2006;99:78–85. doi: 10.1161/01.RES.0000231306.03510.77. [DOI] [PubMed] [Google Scholar]
- Fiege B, Ballhausen D, Kierat L, Leimbacher W, Goriounov D, Schircks B, et al. Plasma tetrahydrobiopterin and its pharmacokinetic following oral administration. Mol Genet Metab. 2004;81:45–51. doi: 10.1016/j.ymgme.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Sawabe K, Wakasugi KO., and, Hasegawa H. Tetrahydrobiopterin uptake in supplemental administration: elevation of tissue tetrahydrobiopterin in mice following uptake of the exogenously oxidized product 7,8-dihydrobiopterin and subsequent reduction by an anti-folate-sensitive process. J Pharmacol Sci. 2004;96:124–133. doi: 10.1254/jphs.fp0040280. [DOI] [PubMed] [Google Scholar]
- Sawabe K, Suetake Y, Nakanishi N, Wakasugi KO., and, Hasegawa H.2005Cellular accumulation of tetrahydrobiopterin following its administration is mediated by two different processes; direct uptake and indirect uptake mediated by a methotrexate-sensitive process Mol Genet Metab 86suppl. 1): S133–S138. [DOI] [PubMed] [Google Scholar]
- Scholz D, Cai WJ., and, Schaper W. Arteriogenesis, a new concept of vascular adaptation in occlusive disease. Angiogenesis. 2001;4:247–257. doi: 10.1023/a:1016094004084. [DOI] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Brevetti LS, Paek R, Brady SE, Hoffman JI, Sarkar R., and, Messina LM. Exercise-induced hyperemia unmasks regional blood flow deficit in experimental hindlimb ischemia. J Surg Res. 2001;98:21–26. doi: 10.1006/jsre.2001.6161. [DOI] [PubMed] [Google Scholar]
- Hoefer IE, van Royen N, Buschmann IR, Piek JJ., and, Schaper W. Time course of arteriogenesis following femoral artery occlusion in the rabbit. Cardiovasc Res. 2001;49:609–617. doi: 10.1016/s0008-6363(00)00243-1. [DOI] [PubMed] [Google Scholar]
- Regensteiner JG., and, Hiatt WR. Current medical therapies for patients with peripheral arterial disease: a critical review. Am J Med. 2002;112:49–57. doi: 10.1016/s0002-9343(01)01034-8. [DOI] [PubMed] [Google Scholar]
- Waters R, Terjung R, Peters K., and, Annex B. Preclinical models of human peripheral arterial occlusive disease: implications for investigating therapeutic agents. Am J Physiol. 2004;97:773–780. doi: 10.1152/japplphysiol.00107.2004. [DOI] [PubMed] [Google Scholar]
- Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, VIVA Investigators et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]