Abstract
Human lysosomal β-hexosaminidase A is a heterodimer composed of α- and β-subunits encoded by HEXA and HEXB, respectively. We genetically introduced an additional N-glycosylation sequon into HEXA, which caused amino acid substitutions (S51 to N and A53 to T) at homologous positions to N84 and T86 in the β-subunit. The mutant HexA (NgHexA) obtained from a Chinese hamster ovary (CHO) cell line co-expressing the mutated HEXA and wild-type HEXB complementary DNAs was demonstrated to contain an additional mannose-6-phosphate (M6P)-type-N-glycan. NgHexA was more efficiently taken up than the wild-type HexA and delivered to lysosomes, where it degraded accumulated substrates including GM2 ganglioside (GM2) when administered to cultured fibroblasts derived from a Sandhoff disease (SD) patient. On intracerebroventricular (i.c.v.) administration of NgHexA to SD model mice, NgHexA more efficiently restored the HexA activity and reduced the GM2 and GA2 (asialoGM2) accumulated in neural cells of the brain parenchyma than the wild-type HexA. These findings indicate that i.c.v. administration of the modified human HexA with an additional M6P-type N-glycan is applicable for enzyme replacement therapy (ERT) involving an M6P-receptor as a molecular target for HexA deficiencies including Tay–Sachs disease and SD.
Introduction
Enzyme replacement therapies (ERTs) involving recombinant human lysosomal enzymes have been clinically established to treat some lysosomal storage diseases (LSDs), such as Gaucher disease,1 Fabry disease,2 and Pompe disease.3 For these ERTs for LSDs, glycan receptors including mannose receptor and cation-independent mannose-6-phosphate receptor (CI-M6PR) are usually utilized as molecular targets. In particular, the latter provides a common delivery system for the uptake of exogenous lysosomal enzymes that is expected to be applicable for the treatment of other LSDs. However, the presently used ERTs have several disadvantages. For these therapies, very large amounts of recombinant enzymes produced by mammalian cells including Chinese hamster ovary (CHO) and human fibroblastic ones are required, and their repetitive administration is necessary to maintain the therapeutic effectiveness, and therefore the costs are very high. Because intravenously administered recombinant enzymes are not delivered to the brain parenchyma across the blood–brain barrier, it is difficult to improve the neurological manifestations in LSD patients exhibiting abnormalities of the central nervous system. Therefore, the development of techniques and methods is required to decrease the frequency of the use and effective doses of enzymes as well as to deliver them into the brains of LSD patients with neurological symptoms.
Human β-hexosaminidase (Hex, EC 3.2.1.52) is a lysosomal enzyme composed of two subunits, α and β, which exhibit about 56% similarity in amino acid sequence.4 The major Hex isozymes are HexA (αβ heterodimer) and HexB (ββ homodimer),5 and only HexA can catalyze the cleavage of the β-N-acetylgalactosamine residue of GM2 ganglioside (GM2) through cooperation with a substrate specific protein cofactor, GM2-activator protein.6,7 Tay–Sachs disease and Sandhoff disease (SD) are HexA deficiencies caused by mutations of HEXA and HEXB encoding the Hex α- and β-subunits, respectively. These diseases are classified as GM2 gangliosidoses associated with lysosomal accumulation of GM2 mainly, in neural tissues, and development of neurological symptoms.8,9,10
In a previous study, Weitz and Proia demonstrated that three N-glycans are attached to the N115, N157, and N295 residues of the human Hex α-subunit.11 Among them, only the N-glycan attached to N295 is recognized by N-acetylglucosamine-1-phosphotransferase, resulting in the addition of M6P residues as a lysosomal transport signal.12,13 In contrast, two M6P-type N-glycans are attached to the N84 and N327 residues among the four N-glycosylation sites of the β-subunit.14
In this study, we succeeded in the production of a mutant HexA (NgHexA) with an additional M6P-type N-glycan by means of a CHO cell line co-expressing the wild-type HEXB and a genetically altered HEXA due to introduction of an additional N-glycosylation consensus sequon. We demonstrated that NgHexA had a superior replacement effect compared to the wild-type HexA (WTHexA) on cultured dermal fibroblasts derived from an SD patient and the brains of SD mice on intracerebroventricular (i.c.v.) administration due to enhanced uptake via CI-M6PR.
Results
Structural modeling of human wild-type Hex α- and β-subunits and comparison of N-glycosylation sites
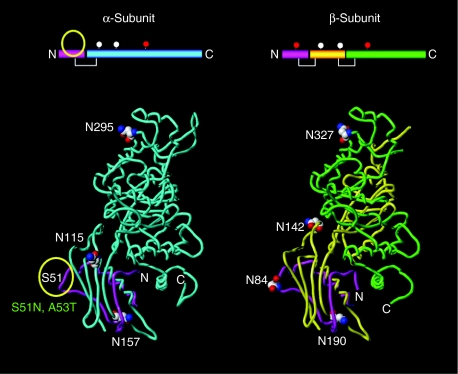
The human Hex α- and β-subunits, exhibiting about 56% amino acid sequence homology,4,5 have 3 and 4 N-glycans, respectively. Among them, the N-glycan attached to N295 in the α-subunit, and those to N84 and N327 in the β-subunit carry M6P residues.11 A structural model of the human Hex α-subunit, composed of the 502 amino acids comprising residues 23–524, was built based on the crystallographic data for the human Hex β-subunit and sequence alignment of the two subunits. We selected S51 in the α-subunit, which corresponds to N84 in the β-subunit, to introduce an additional N-glycosylation consensus sequence, and constructed a modified α-subunit model (Figure 1). Little structural change was predicted between the loop structure around the substituted N51 in the α-subunit and that around N84 in the β-subunit (data not shown).
Figure 1.
Comparison of the N-glycosylation sites between the Hex α- and β-subunits by in silico modeling. Three-dimensional structures of the human β-hexosaminidase A are presented. The location of the glycosylation sites in the α- (N115, N157, and N295) and β-subunits (N84, N142, N190, and N324) are presented as space-filling models. The N-glycan carrying M6P residues are attached to N295 in the α-subunit, and to N84 as well as N327 in the β-subunit. Amino acid substitutions (S51N and A53T) in the α-subunit are predicted to cause the additional N-glycan carrying M6P residues, and correspond to those (N84N and T86) in the β-subunit. The yellow circle indicates a novel glycosylation site in the α-subunit.
Expression of NgHexA with an additional N-linked glycan containing M6P residues on the α-subunit
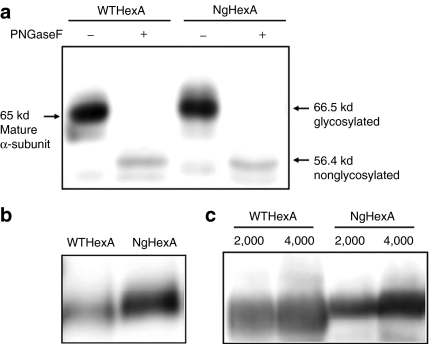
WTHEXA and the mutated complementary DNA encoding the S51N/A53T substitutions in the α-subunit were each introduced into the CHO/HEXB cell line stably expressing the wild-type β-subunit complementary DNA. The expressed HexAs (WT- and NgHexA) were isolated from cell extracts by means of a Q-type spin column, and then analyzed by immunoblotting with anti-α-subunit antibodies. As shown in Figure 2a, the expressed α-subunit of NgHexA migrated to the position of 66.5 kd, whereas that of WTHexA migrated to the 65 kd position. After digestion with PNGase F, WT- and NgHexA showed the same Rf value, corresponding to a 56.4 kd band. The results indicate that the difference in molecular mass between the α-subunits of WT- and NgHexA is due to the addition of an N-glycan attached to NgHexA.
Figure 2.
Glycosylation analysis of NgHexA with an additional N-linked glycan containing mannose-6-phosphate residues in the α-subunit. Glycosylation of the α-subunit and the M6P content of HexA were examined by western blotting. (a) HexA fractions separated from CHO/HEXA/HEXB and CHO/NgHEXA/HEXB cell extracts obtained with the Vivapure spin column were treated with (+) or without (−) PNGase F. The α-subunit molecular masses were determined by western blotting using antihuman Hex α-subunit peptide antibodies. (b) HexA fractions separated from supernatants of the CHO/HEXA/HEXB and CHO/NgHEXA/HEXB cell lines with a DEAE column were analyzed by native-polyacrylamide gel electrophoresis (PAGE) and western blotting using concanavalin A. (c) The M6P contents of N-glycans of WT- and NgHexA, each exhibiting 4-methylumbelliferyl N-acetyl-β--glucosaminide-degrading activity (2,000 and 4,000 nmol/hour/lane), were determined by native-PAGE and lectin blotting using Dom9His.
Figure 2b shows the results obtained on native-polyacrylamide gel electrophoresis (PAGE) and ConA lectin blotting. The reactivity of NgHexA to ConA lectin more remarkably increased than that of WTHexA, indicating that a high mannose-type N-glycan was attached to NgHexA. We next examined, by means of native-PAGE and M6P receptor-blotting with CI-M6PR-binding domain 9 (Dom9His), whether the additional N-glycan attached to NgHexA carries M6P residues or not. As shown in Figure 2c, the reactivity of NgHexA to Dom9His was markedly enhanced in comparison to that of WTHexA. These results demonstrate that the newly attached high mannose-type N-glycan carries M6P residues.
Enhanced uptake of NgHexA by SD fibroblasts
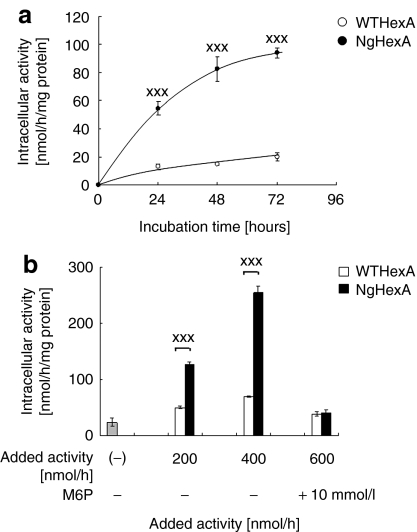
Next, the effects of the addition of an M6P-type N-glycan to HexA on uptake by fibroblasts derived from an SD patient and restoration of the Hex activity was assessed. As shown in Figure 3a, efficient uptake was observed by SD fibroblasts on treatment with NgHexA in a time-dependent manner. The significant restoration of Hex activity (P < 0.005) was revealed to be due to enhanced uptake of NgHexA compared with WTHexA on treatment with the same level of activity of recombinant HexA (Figure 3b). The uptake of NgHexA was inhibited in the presence of 10 mmol/l M6P. These results indicate that the addition of an M6P-type N-glycan caused efficient uptake of NgHexA via CI-M6PR.
Figure 3.
Effective uptake of NgHexA by Sandhoff disease (SD) fibroblasts. The uptake by WT- and NgHexA into fibroblasts derived from an SD patient was analyzed as described under “Materials and Methods”. WTHexA and NgHexA were administered to SD fibroblasts. (a) The time-course was measured at 24-hour intervals for 72 hours [4-methylumbelliferyl N-acetyl-β--glucosaminide (4-MUG) activity, 200 nmol/hour]. (b) Dose dependency was analyzed for 72 hours after administration (4-MUG activity, 200, 400, or 600 nmol/hour). In another experiment, HexA (600 nmol/hour) was administered after treatment with 10 mmol/l M6P for 30 minutes. Error bars show ±SEM, ***P < 0.005 (Tukey's post hoc test).
Enzyme replacement effect of NgHexA on reduction of intracellular GM2 ganglioside accumulated in SD fibroblasts
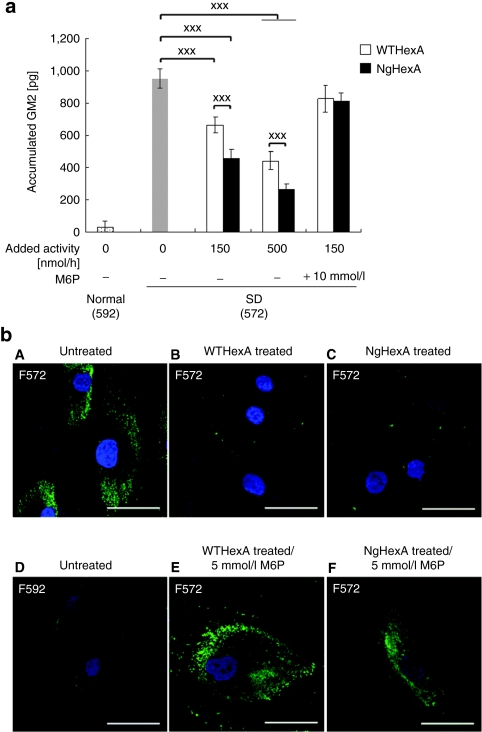
We examined the reduction of GM2 accumulated in SD fibroblasts by means of enzyme-linked immunosorbent assay and immunocytochemistry with anti-GM2 antibodies after treatment with WT- and NgHexA. Remarkable accumulation of GM2 due to the HexA deficiency was detected in the SD fibroblasts (F572) (Figure 4a,b). Both WT- and NgHexA significantly reduced the accumulated GM2 in a dose-dependent manner (Figure 4a, P < 0.005). The reduction of GM2 was also inhibited in presence of 10 mmol/l M6P, indicating that the enhanced elimination of intracellular GM2 on NgHexA administration is due to efficient uptake of NgHexA carrying an additional M6P-type N-glycan via CI-M6PR (Figure 4a,b).
Figure 4.
Enzyme replacement effect of NgHexA on reduction of intracellular GM2 gangliosides. Reduction of accumulated GM2 was quantified by means of Cell ELISA and immunofluoroscense with anti-GM2 antibodies. (a) Quantitative analysis of accumulated GM2. WT- or NgHexA was administered to SD fibroblasts, followed by incubation for 3 days, and then the GM2 level was determined by Cell ELISA. In another experiment, HexA [4-methylumbelliferyl N-acetyl-β--glucosaminide (4-MUG) activity, 150 nmol/hour] was administered after treatment with 10 mmol/l M6P for 30 minutes. Each error bar represents the average of three or four experiments, and shows ±SEM, ***P < 0.005 (Tukey's post hoc test). (b) Immunofluorescense analysis. WT- or NgHexA (4-MUG activity, 500 nmol/hour) was administered to SD fibroblasts, followed by incubation for 3 days, accumulated GM2 being visualized as immunofluororescense with anti-GM2. (A) Untreated; (B) treated with WTHexA (4-MUG activity, 800 nmol/hour/well); (C) treated with NgHexA (800 nmol/hour/well); (D) untreated WT fibroblasts; (E) treated with 5 mmol/l M6P/WTHexA (800 nmol/hour/well) after treatment with 5 mmol/l M6P; and (F) treated with NgHexA (800 nmol/hour/well) after treatment with 5 mmol/l M6P. Bar = 50 µm.
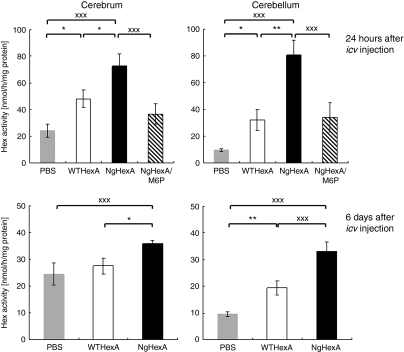
Recovery of Hex activity and reduction of GSL storage in brains of SD mice on i.c.v. treatment with NgHexA
The replacement effects of WT- and NgHexA on i.c.v. injection into the lateral cerebral ventricles of 12-week-old SD mice were examined. Twenty-four hours after injection, the brains were dissected out and divided into two parts (cerebra and cerebella). After preparation of tissue extracts, HexA activity in the extracts was measured. The HexA activity levels in the cerebra and cerebella after NgHexA injection were 1.5- and 2.5-fold, respectively, higher than those in the case of WTHexA. On co-injection of M6P (0.25 µmol) with NgHexA, the restoration of the HexA activity was decreased by 65–74% of that in the absence of M6P (Figure 5, upper panels). Six days after treatment with NgHexA, significant increases in residual HexA activity in all regions were observed in comparison with those in WTHexA-treated mice (Figure 5, lower panels; cerebrum, P < 0.05; cerebellum, P < 0.005).
Figure 5.
Significant restoration of Hex activity in SD mouse brains after NgHexA injection. Hex activity in brain extracts was measured at 24 hours (upper) and 6 days (lower) after WTHexA (n = 5) or NgHexA (n = 4) injection. NgHexA/M6P indicates the data obtained on co-injection of NgHexA and M6P (0.25 µmol). Error bars show ±SEM, *P < 0.05, **P < 0.01, ***P < 0.005 (Tukey's post hoc test).
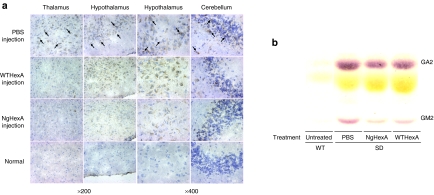
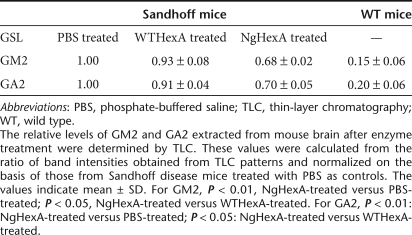
The effects of i.c.v. administration of WT- and NgHexA on reduction of the accumulated GM2 were examined by immunohistochemistry 6 days after injection (Figure 6a). In the brains of SD mice treated with phosphate-buffered saline (PBS) as controls, GM2-immunoreactivity (GM2-IR) was observed throughout the brain, especially, in the thalamus, hypothalamus, and cerebellum. On treatment with WTHexA, remarkable reduction of GM2-IR was detected in the thalamus and cerebellum, whereas a decrease of GM2-IR was hardly observed in the hypothalamus. In contrast, marked reduction of GM2-IR was seen throughout the brain, even in the hypothalamus, of NgHexA-treated mice. The content of GM2 as well as that of GA2 in brain extracts from NgHexA-treated mice were also calculated from the areas of the corresponding bands on thin-layer chromatography (Figure 6b), which were decreased, to 68 and 70%, compared to in enzyme-untreated controls after single i.c.v. injection, respectively. Table 1 also indicated that the degrees of GM2 and GA2 reduction on treatment with NgHexA carrying an additional M6P-type N-glycan were significantly greater than that with WTHexA (for GM2, P < 0.05, NgHexA versus WTHexA; for GA2, P < 0.05, NgHexA versus WTHexA).
Figure 6.
The effects of intracerebroventricular (i.c.v) administration on reduction of accumulated GSLs in the Sandhoff disease (SD) mice brain. (a) Decrease of GM2-immunoreactivity in the brains of (SD) mice after i.c.v. administration of WT- and NgHexA. WT- and NgHexA were administered to SD mice at the age of 12 weeks. Brain sections (10 µm) were prepared from SD mice 6 days after injection, and then counterstained with hematoxylin. Accumulated GM2 were detected by enzyme-linked immunostaining with anti-GM2 antibodies. Arrows indicates specific GM2-IR (brown). (b) Thin-layer chromatography patterns of glycosphingolipids (GSLs) extracted from the brains of phosphate-buffered saline–treated controls (PBS), WTHexA-injected SD mice (WTHexA), NgHexA-injected SD mice (NgHexA), and untreated WT mice (Untreated).
Table 1. Comparison of the relative ratio of glycosphingolipid (GSL) present in mouse brain.
Discussion
In recent years, intravenous ERT has become the principal treatment for several LSDs with peripheral and visceral manifestations,1,2,3 in which recombinant human lysosomal enzymes derived from mammalian cell lines as glycoprotein drugs and CI-M6PR as a molecular target are utilized.2,3 Although intravenous ERT had been believed to be ineffective with LSDs involving neurological symptoms because of the presence of the blood–brain barrier, preclinical studies on the development of ERTs for LSDs involving brain dysfunction have been performed in recent years. In these experiments, direct injection of therapeutic enzymes into the brains of LSD model animals was performed by means of intrathecal administration because such methods are also clinically available for other neurological diseases such as hydroencephalomegaly, the spinal symptoms in multiple sclerosis and chronic pain.15,16 These methods involving cerebrospinal fluid exhibit potential as expansion of the distribution and subsequent uptake of such enzymes into the brain parenchyma.17,18 Intracerebrospinal fluid administration of recombinant enzymes to animal models of other LSDs has been reported to cause a reduction in the accumulated substrate and histological improvement in the brain.19,20,21 As CI-M6PR is widely expressed in neural cells of the central nervous system,22 it should be essential for the uptake of exogenous enzymes and delivery to the late endosomal/lysosomal compartments of target neural cells.
In this study, we designed, on the basis of homology modeling between the human Hex α- and β-subunit, a novel HexA with an additional N-glycan with terminal M6P residues as a therapeutic enzyme for an i.c.v. ERT for Tay–Sachs disease and SD. We examined the effect of genetic introduction of an N-glycosylation sequon into HEXA causing amino acid substitutions (S51 to N and A53 to T) at homologous positions to N84 and T86 of the β-subunit, because the N-glycans attached at specific positions of lysosomal enzyme molecules can be recognized by GlcNAc-1-phosphotransferases and modified so as to contain M6P residues.12,13 Actually, we succeeded in the production of a recombinant HexA (NgHexA) comprising the wild-type β-subunit and a modified α-subunit with an additional N-glycan carrying terminal M6P residues. We demonstrated here that NgHexA was more efficiently taken up via CI-M6PR than WTHexA by cultured fibroblasts from an SD patient and neural cells in the brain parenchyma of SD model mice, and that NgHexA was superior to WTHexA in restoring the HexA activity and degrading GM2 accumulated in the cells by immunocytochemical analysis. Recently, Akeboshi et al. indicated that a higher number of M6P residues on N-glycans of a methylotrophic yeast-derived human HexA causes more efficient uptake of the enzyme into Tay–Sachs fibroblasts and cultured neural cells from SD mice.23 We also reported here that NgHexA exhibits superior abilities as to uptake by neural cells, maintenance of the enzyme activity and reduction of the accumulated substrate in the brains of SD mice on single i.c.v. administration, although it has been generally considered difficult to deliver recombinant enzymes into neural cells of the brain because of the relatively low expression of CI-M6PR in the central nervous system. Therefore, M6P-modification of N-glycans attached to lysosomal enzymes is also favorable for i.c.v. ERTs utilizing CI-M6PR as a target receptor. Thus, genetic engineering to introduce additional M6P-type N-glycans onto recombinant lysosomal enzymes is expected to be applicable not only to ERTs but also to other therapeutic strategies including gene and cell therapies for LSDs.
Materials and Methods
Materials. 4-Methylumbelliferone, 4-methylumbelliferyl N-acetyl-β--glucosaminide (4-MUG), 4-methylumbelliferyl β--galactoside, M6P, nutrient mixture Ham's F-10, dimethyl sulfoxide, RNaseA, a 5-bromo-4-chloro-3-indoyl phosphate/nitro blue tetrazolium liquid substrate system and bovine serum albumin were purchased from Sigma (St Louis, MO). The GenomeLab DCIT-Quick Start Kit, CEQ Separation Gel-LPAI, and CEQ separation buffer were from Beckman Coulter (North Harbor, CA). The Wizard plus midipreps DNA purification kit, dNTPs, and hygromycin were from Promega (Madison, WI). Pepstatin A and leupeptin were from Peptide Institute (Osaka, Japan). The Quick gel extraction kit and purification kit were from Qiagen (Germantown, MA). 4-Methylumbellifery-6-sulfo-β--glucosaminide potassium salt was purchased from Calbiochem (San Diego, CA). Fetal bovine serum was from Biological Industries (Tokyo, Japan). KOD plus polymerase was from Toyobo (Osaka. Japan). The DNA ligation kit ver.1 was purchased from TaKaRa (Otsu, Japan). G418 was from Wako (Osaka, Japan). Unifecter was from B-Bridge International (Mountain View, CA). The DC protein assay kit was from Bio-Rad (Hercules, CA). The Vivapure mini spin column Q type was from Viva Science (Stonehouse, UK). Amicon ultra was from Millipore (Carrigtwohill, Ireland).
Construction of human Hex α- and β-subunits. Structural data for human HexB and the β-subunit were obtained from the Protein Data Bank. To construct a structural model of the human Hex α-subunit, the structure of the β-subunit was used as a template. The models were built using molecular modeling software, SYBYL/BIOPOLYMER (Tpipos, St Louis, MO).
Construction of a vector plasmid and establishment of CHO/NgHEXA/HEXB cell lines. The pIRES-HEXA vector, previously constructed in our laboratory,24 was used as a template for PCR. The open reading frame of HEXA was amplified with a primer set (primers 1 and 2, Table 2) to create terminal XhoI and BglII sites. The digested DNA fragment (fragment 1) was subcloned into the XhoI and BglII sites of mammalian expression vector pCX-Hygro.24 The resultant vector plasmid was designated as pCX-HEXA Hygro. Fragments 2 and 3 were amplified using pCX-HEXA Hygro, as a template, and primer sets, under the PCR conditions indicated in Table 2. After purification, the combined fragments were subjected to two-step PCR. The resultant fragments were digested with XhoI and PvuII, and then ligated into the pCX-HEXA Hygro vector digested with the same endonucleases. The resultant vector plasmid was designated as pCX-NgHEXA Hygro. The expression vector was introduced by lipofection into CHO/HEXB cells stably expressing the HEXB gene as previously described.24 Hygromycin-resistant cell lines were selected by the limited dilution method, and designated as CHO/NgHEXA/HEXB cell lines co-expressing NgHEXA and the wild-type HEXB.
Table 2. Primer sets and PCR conditions for DNA fragments used in this study.
Characterization of recombinant Hex isozymes. The intracellular and extracellular Hex isozymes expressed in the transformed CHO/NgHEXA/HEXB cell lines were analyzed by means of native-PAGE and immunoblotting. To prepare cell extracts, cultured cells seeded on φ 60-mm dishes were grown to confluency, washed with PBS, harvested, and then suspended in 10 mmol/l sodium phosphate buffer (pH 6.0) (NaPB) containing protease inhibitors (0.01 mmol/l pepstatin A, 0.02 mmol/l leupeptin, 1 mmol/l EDTA, and 2 mmol/l phenylmethylsulfonyl fluoride). The cell suspension was sonicated and then centrifuged at 13,200 g for 15 minutes at 4 °C. Aliquots of supernatants (cell extracts) were subjected to native-PAGE on 7.5% acrylamide gels. The proteins were transferred to polyvinylidine difluoride membranes. On immunoblotting, each membrane was incubated with 50% (vol/vol) Blocking one (Nacalai Tesque, Kyoto, Japan) in Tris-buffered saline (TBS; pH 7.4) overnight at 4 °C. The membrane was treated with antihuman HexA polyclonal antibodies diluted with Blocking one/TBS (1:1,000 dilution) at room temperature (RT). After washing with 0.1% Tween-20/TBS, the membrane was treated with biotin conjugated anti rabbit IgG antibodies (1:1,000 dilution) at RT for 1 hour. After washing, the membrane was treated with alkaline phosphatase–conjugated streptavidin (1:1,000 dilution) (Roche Diagnosis, Indianapolis, IN) for 1 hour. After washing, the protein bands were visualized with the 5-bromo-4-chloro-3-indoyl phosphate/nitro blue tetrazolium liquid substrate system.
Enzyme assays. The Hex isozyme activity in cell extracts and conditioned media was measured using 4-MUG and 4-Methylumbellifery-6-sulfo-β--glucosaminide potassium salt as substrates in 0.1 mol/l sodium citrate buffer (pH 4.5 and 4.2, respectively). Protein levels were determined with the DC protein assay kit with bovine serum albumin as a standard.
Glycosylation analysis of recombinant Hex isozymes. The presence of N-glycans attached to the recombinant HexA was assessed by native-PAGE and lectin blotting. WT and NgHexA fractions were prepared from conditioned media as previously described,25 and then subjected to native-PAGE as described above. After the proteins had been transferred to polyvinylidine difluoride membranes, the membranes were subjected to lectin blotting to detect high mannose-type N-glycans and ones carrying terminal M6P residues. One membrane was incubated with a biotin-conjugated concanavalin A (ConA) solution diluted with Blocking one/TBS (1:1,000 dilution) at 4 °C overnight, and another one was incubated with a recombinant CI-M6PR domain 9 (Dom9His)26 solution diluted with blocking reagent (Qiagen, Tokyo, Japan) to a final concentration 3 µg/ml at RT for 1 hour. After washing with 0.1% Tween-20/TBS (pH 7.0), the membranes were incubated with horseradish peroxidase–conjugated anti-biotin antibodies diluted with Blocking one/TBS (1:1,000 dilution) or penta His horseradish peroxidase–conjugate diluted with the blocking reagent (1:1,000 dilution) at RT for 1 hour. The protein bands were visualized with a LAS 3000 (Fuji Film, Tokyo, Japan).
Immunoblotting for HexA. HexA fractions were prepared from cell extracts with the Vivapure spin column. Briefly, cell extracts were applied to the spin column pre-equilibrated with 10 mmol/l NaPB, followed by centrifugation at 2,000 g for 15 minutes at 4 °C. After washing with the same buffer, the proteins were eluted stepwise with 10 mmol/l NaPB containing 0.05, 0.1, 0.2, 0.3, 0.4, 0.6, and 1 mol/l NaCl, respectively. The 0.2 mol/l NaCl eluate containing HexA activity was concentrated and desalted. After protease inhibitors had been added, the eluate was stored at 4 °C as the HexA fraction. The HexA fraction was treated overnight with or without PNGase F according to the manufacturer's protocol, and then subjected to sodium dodecyl sulfate-PAGE on a 10% gel. After the proteins had been transferred to a polyvinylidine difluoride membrane, the membrane was incubated with antihuman Hex α-subunit peptide antibodies diluted with Blocking one/TBS (1:500 dilution) at RT for 1 hour. After washing, the membrane was incubated with biotin-conjugated anti-rabbit IgG antibodies (1:1,000 dilution) at RT for 1 hour. After washing, the membrane was treated with horseradish peroxidase–conjugated anti-biotin antibodies (1:1,000 dilution) at RT for 1 hour. The protein bands were visualized with the LAS 3000.
Assaying of uptake of recombinant HexA by SD fibroblasts. To compare the dose-dependent uptake of NgHexA by a fibroblastic cell strain (F572) from an SD patient with that of WTHexA, cells were seeded onto a type I collagen–coated 96-well plate (3 × 103 cells/well), and then the isolated WT- and NgHexA fractions (each containing 200, 400, or 600 nmol/hour/well, respectively) were added, followed by incubation for 3 days. For time-dependent uptake, HexA fractions were added (200 nmol/hour/well), followed by incubation for 24, 48, and 72 hours, respectively. In some experiments, M6P was added to a final concentration of 10 mmol/l, followed by incubation for 1 hour before the addition of each HexA fraction. After incubation, the fibroblasts were washed with PBS twice, and then treated with 10 mmol/l NaPB containing 1% NP-40/protease inhibitors. The microplate was vibrated with a EM-36 Micro Mixer (Taitec, Tokyo, Japan) for 1 hour at 4 °C, followed by pipetting 20 times in each well, and then the HexA activity in each suspension was assayed as described above.
Assaying of reduction of GM2 ganglioside accumulated in SD fibroblasts after administration of HexA fractions. To examine the effects of recombinant WT- and NgHexA replacement on the reduction of intracellularly accumulated GM2, F572 cells were seeded onto type I collagen–coated 96-well plates (3 × 103 cells/well), and then treated with the isolated WT- and NgHexA fractions for 3 days. For quantification of GM2, an enzyme-linked immunosorbent assay for immobilized cells (Cell ELISA) was performed according to the method described previously.27
Glycolipid extraction and thin-layer chromatography analysis. The extraction of glycolipids from mouse brains was performed as previously described.28 Insoluble fractions of brain tissues were homogenized in distilled water, and glycolipid fractions were extracted using 1.5 ml of chloroform, methanol, and water (4:8:3) by sonication. Glycolipid fractions were dried under vacuum, and then 3 ml of chloroform and methanol (2:1), and a 1/6 volume of water were added. Folch's upper phase was isolated and further extracted with chloroform, methanol, and 0.1% NaCl (1:10:10) three times. The total glycosphingolipid (GSL) fractions were then dried under vacuum. A 1.5 ml aliquot of 0.1 N NaOH/MeOH was added to the dried samples, which were then incubated for 2 hours at 40 °C. Samples were neutralized by the addition of 150 µl of CH3COOH/MeOH, and then dried again. GSLs were purified using a C18 Sep-Pak cartridge, and the GSL solutions were dried under vacuum. For determination of the relative GM2 and GA2 levels, GSL solutions were analyzed by means of thin-layer chromatography as described previously.29
Immunofluorescence of GM2 accumulated in Sandhoff fibroblasts. F572 cells were seeded (1 × 104 cells/well) onto 8-well Lab-Tek chamber slides (NUNC, New York, NY). After administration of the WT- and NgHexA fractions, the fibroblasts were incubated for 3 days, and then fixed with cold 4% paraformaldehyde in PBS overnight at 4 °C. After washing with PBS, the cells were treated at 4 °C with appropriately diluted primary antibodies, anti-GM2 mouse monoclonal antibodies (GMB28; IgM).30 After washing with PBS containing 0.05% Tween-20, the cells were treated with fluorescein isothiocyanate–conjugated goat anti-mouse IgM (Biosource International, Camarillo, CA). Specimens were viewed under a confocal fluorescent microscope (LSM510; Zeiss, Oberkochen, Germany).
I.c.v. administration of recombinant HexA and analysis of the enzyme replacement effect. SD mice at the age of 13 weeks were anesthetized with sodium 5-ethyl-5-(1-methylbuthyl) barbiturate (32.4 mg/kg body weight; Schering-Plough Animal Health, Japan). WT- and NgHexA (4-MUG-degrading activity, 10,000 nmol/hour/25 µl) as well as PBS, as an enzyme-untreated control, in a total volume of 25 µl were injected at a certain position (0.5 mm caudally from the bregma, 1.0 mm laterally from the central line, and 2.0 mm from the top of the skull) into each ventricle with a two-step needle attached to a glass syringe. After the injection, the needle was held at the site for 1 minute to prevent reverse flow. Twenty-four hours and 6 days after injection, SD mice were killed, and their brains were obtained after PBS perfusion. The brains were cut into two sections (cerebrum and cerebellum, respectively). These sections were weighed and homogenized by sonication in 10 mmol/l NaPB containing protease inhibitors. After centrifugation at 13,200g, the supernatants were collected as tissue extracts, and then the Hex activity in the extracts was measured. For immunohistochemical analysis, each brain hemisphere was embedded in optimum cutting temperature compound (Miles, Elkhart, IN) and frozen at −30 °C. Slices (10 µm thick) were prepared with a cryostat (Micro-Edge Instrument, Osaka, Japan) and placed on silane-coated glass slides, which were then air-dried for 2 hours. The brain slices were fixed with 4% paraformaldehyde/PBS for 2 days at 4 °C. After washing with PBS, specimens were then treated with 0.03% H2O2/PBS and washed with PBS. They were then treated with anti-GM2 mouse monoclonal antibodies (GMB28) diluted with 5% goat serum/1% bovine serum albumin/PBS (1:20 dilution) overnight at 4 °C. After washing with 0.05% Tween-20/PBS, the brain slices were treated with anti-mouse IgM antibodies diluted with 5% goat serum/1% bovine serum albumin/PBS (1:1,000 dilution) for 1 hour at RT After washing with 0.05% Tween-20/PBS, the brain slices were treated with horseradish peroxidase–conjugated streptavidin (Nichirei, Tokyo, Japan) for 30 minutes at RT After washing with 0.05% Tween-20/PBS, the immunopositive cells were visualized with diaminobenzidine (0.012% H2O2/0.0133% diaminobenzidine/50 mmol/l Tris–HCl, pH 8.0). After washing with PBS, the specimens were counterstained with hematoxylin (Muto Pure Chemicals, Tokyo, Japan).
Statistical analysis was performed using Turkey's post hoc test.
Acknowledgments
We thank R.L. Proia (NIDDKD, NIH, Bethesda, MD) for providing the SD mice. We also thank T. Tai (Department of Tumor Immunology, The Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan) for providing the mouse monoclonal antibodies, GMB28. This work was supported by JST CREST, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We also thank E. Endo for helping with the preparation of this manuscript.
REFERENCES
- Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, et al. Replacement therapy for inherited enzyme deficiency–macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, International Collaborative Fabry Disease Study Group et al. Safety and efficacy of recombinant human alpha-galactosidase A–replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- Klinge L, Straub V, Neudorf U., and, Voit T. Enzyme replacement therapy in classical infantile pompe disease: results of a ten-month follow-up study. Neuropediatrics. 2005;36:6–11. doi: 10.1055/s-2005-837543. [DOI] [PubMed] [Google Scholar]
- Proia RL. Gene encoding the human beta-hexosaminidase beta chain: extensive homology of intron placement in the alpha- and beta-chain genes. Proc Natl Acad Sci USA. 1988;85:1883–1887. doi: 10.1073/pnas.85.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneluk RG, Mahuran DJ, Neote K, Klavins MH, O'Dowd BF, Tropak M, et al. Isolation of cDNA clones coding for the alpha-subunit of human beta-hexosaminidase. Extensive homology between the alpha- and beta-subunits and studies on Tay-Sachs disease. J Biol Chem. 1986;261:8407–8413. [PubMed] [Google Scholar]
- Wu YY, Lockyer JM, Sugiyama E, Pavlova NV, Li YT., and, Li SC. Expression and specificity of human GM2 activator protein. J Biol Chem. 1994;269:16276–16283. [PubMed] [Google Scholar]
- Zarghooni M, Bukovac S, Tropak M, Callahan J., and, Mahuran D. An alpha-subunit loop structure is required for GM2 activator protein binding by beta-hexosaminidase A. Biochem Biophys Res Commun. 2004;324:1048–1052. doi: 10.1016/j.bbrc.2004.09.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel A, Kaback M, Proia RL, Sandhoff K., and, Suzuki K.2001The GM2 gangliosidoses. In: Scriver, CR, Beaudet, AL, Sly, WS and Valle, D (eds). The Metabolic and Molecular Bases of Inherited Diseases, 8th edn, Vol III. McGraw-Hill: New York. pp 3827–3877 [Google Scholar]
- Conzelmann E., and, Sandhoff K. AB variant of infantile GM2 gangliosidosis: deficiency of a factor necessary for stimulation of hexosaminidase A-catalyzed degradation of ganglioside GM2 and glycolipid GA2. Proc Natl Acad Sci USA. 1978;75:3979–3983. doi: 10.1073/pnas.75.8.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CA, Neote K, Leung A, Gravel RA., and, Mahuran DJ. Introduction of the alpha subunit mutation associated with the B1 variant of Tay-Sachs disease into the beta subunit produces a beta-hexosaminidase B without catalytic activity. J Biol Chem. 1989;264:21705–21710. [PubMed] [Google Scholar]
- Weitz G., and, Proia RL. Analysis of the glycosylation and phosphorylation of the alpha-subunit of the lysosomal enzyme, beta-hexosaminidase A, by site-directed mutagenesis. J Biol Chem. 1992;267:10039–10044. [PubMed] [Google Scholar]
- Kornfeld S., and, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Dahms NM, Lobel P., and, Kornfeld S. Mannose 6-phosphate receptors and lysosomal enzyme targeting. J Biol Chem. 1989;264:12115–12118. [PubMed] [Google Scholar]
- Sonderfeld-Fresko S., and, Proia RL. Analysis of the glycosylation and phosphorylation of the lysosomal enzyme, beta-hexosaminidase B, by site-directed mutagenesis. J Biol Chem. 1989;264:7692–7697. [PubMed] [Google Scholar]
- Boviatsis EJ, Kouyialis AT, Korfias S., and, Sakas DE. Functional outcome of intrathecal baclofen administration for severe spasticity. Clin Neurol Neurosurg. 2005;107:289–295. doi: 10.1016/j.clineuro.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Rudich Z, Peng P, Dunn E., and, McCartney C. Stability of clonidine in clonidine-hydromorphone mixture from implanted intrathecal infusion pumps in chronic pain patients. J Pain Symptom Manage. 2004;28:599–602. doi: 10.1016/j.jpainsymman.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Chang M, Cooper JD, Sleat DE, Cheng SH, Dodge JC, Passini MA, et al. Intraventricular enzyme replacement improves disease phenotypes in a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol Ther. 2008;16:649–656. doi: 10.1038/mt.2008.9. [DOI] [PubMed] [Google Scholar]
- Dickson P, McEntee M, Vogler C, Le S, Levy B, Peinovich M, et al. Intrathecal enzyme replacement therapy: successful treatment of brain disease via the cerebrospinal fluid. Mol Genet Metab. 2007;91:61–68. doi: 10.1016/j.ymgme.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge JC, Clarke J, Treleaven CM, Taksir TV, Griffiths DA, Yang W, et al. Intracerebroventricular infusion of acid sphingomyelinase corrects CNS manifestations in a mouse model of Niemann-Pick A disease. Exp Neurol. 2009;215:349–357. doi: 10.1016/j.expneurol.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Kakkis E, McEntee M, Vogler C, Le S, Levy B, Belichenko P, et al. Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I. Mol Genet Metab. 2004;83:163–174. doi: 10.1016/j.ymgme.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Hemsley KM, King B., and, Hopwood JJ. Injection of recombinant human sulfamidase into the CSF via the cerebellomedullary cistern in MPS IIIA mice. Mol Genet Metab. 2007;90:313–328. doi: 10.1016/j.ymgme.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Cheryl H., and, Satybarata K. Insulin-like growth factor-II/mannose-6-phosphate receptor:widespread distribution in neurons of the central nervous system including those expressing cholinergic phenotype. J Comp Neurol. 2003;458:113–127. doi: 10.1002/cne.10578. [DOI] [PubMed] [Google Scholar]
- Akeboshi H, Kasahara Y, Tsuji D, Itoh K, Sakuraba H, Chiba Y, et al. Production of human beta-hexosaminidase A with highly phosphorylated N-glycans by the overexpression of the Ogataea minuta MNN4 gene. Glycobiology. 2009;19:1002–1009. doi: 10.1093/glycob/cwp080. [DOI] [PubMed] [Google Scholar]
- Itakura T, Kuroki A, Ishibashi Y, Tsuji D, Kawashita E, Higashine Y, et al. Inefficiency in GM2 ganglioside elimination by human lysosomal beta-hexosaminidase beta-subunit gene transfer to fibroblastic cell line derived from Sandhoff disease model mice. Biol Pharm Bull. 2006;29:1564–1569. doi: 10.1248/bpb.29.1564. [DOI] [PubMed] [Google Scholar]
- Tsuji D, Kuroki A, Ishibashi Y, Itakura T., and, Itoh K. Metabolic correction in microglia derived from Sandhoff disease model mice. J Neurochem. 2005;94:1631–1638. doi: 10.1111/j.1471-4159.2005.03317.x. [DOI] [PubMed] [Google Scholar]
- Akeboshi H, Chiba Y, Kasahara Y, Takashiba M, Takaoka Y, Ohsawa M, et al. Production of recombinant beta-hexosaminidase A, a potential enzyme for replacement therapy for Tay-Sachs and Sandhoff diseases, in the methylotrophic yeast Ogataea minuta. Appl Environ Microbiol. 2007;73:4805–4812. doi: 10.1128/AEM.00463-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji D, Higashine Y, Matsuoka K, Sakuraba H., and, Itoh K. Therapeutic evaluation of GM2 gangliosidoses by ELISA using anti-GM2 ganglioside antibodies. Clin Chim Acta. 2007;378:38–41. doi: 10.1016/j.cca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Sphingolipid Biochemistry. Plenum Press: New York.; 1983. [Google Scholar]
- Sango K, McDonald MP, Crawley JN, Mack ML, Tifft CJ, Skop E, et al. Mice lacking both subunits of lysosomal beta-hexosaminidase display gangliosidosis and mucopolysaccharidosis. Nat Genet. 1996;14:348–352. doi: 10.1038/ng1196-348. [DOI] [PubMed] [Google Scholar]
- Kotani M, Kawashima I, Ozawa H, Ogura K, Ishizuka I, Terashima T, et al. Immunohistochemical localization of minor gangliosides in the rat central nervous system. Glycobiology. 1994;4:855–865. doi: 10.1093/glycob/4.6.855. [DOI] [PubMed] [Google Scholar]