Abstract
Two small-interfering RNAs (siRNAs) targeting α-synuclein (α-syn) and three control siRNAs were cloned in an adeno-associated virus (AAV) vector and unilaterally injected into rat substantia nigra pars compacta (SNc). Reduction of α-syn resulted in a rapid (4 week) reduction in the number of tyrosine hydroxylase (TH) positive cells and striatal dopamine (DA) on the injected side. The level of neurodegeneration induced by the different siRNAs correlated with their ability to downregulate α-syn protein and mRNA in tissue culture and in vivo. Examination of various SNc neuronal markers indicated that neurodegeneration was due to cell loss and not just downregulation of DA synthesis. Reduction of α-syn also resulted in a pronounced amphetamine induced behavioral asymmetry consistent with the level of neurodegeneration. In contrast, none of the three control siRNAs, which targeted genes not normally expressed in SNc, showed evidence of neurodegeneration or behavioral asymmetry, even at longer survival times. Moreover, co-expression of both rat α-syn and α-syn siRNA partially reversed the neurodegenerative and behavioral effects of α-syn siRNA alone. Our data show that α-syn plays an important role in the rat SNc and suggest that both up- and downregulation of wild-type α-syn expression increase the risk of nigrostriatal pathology.
Introduction
α-Synuclein (α-syn) is a 140 amino acid protein that is expressed abundantly throughout the central nervous system (reviewed in ref. 1). α-Syn is present during development throughout the brain and accumulates in nerve terminals, but is also found in the perinuclear zone of the cytoplasm and in the nucleus.
α-Syn appears to play a crucial role in the pathogenesis of several neurodegenerative disorders including Parkinson's disease. The brains of Parkinson patients typically contain detergent insoluble intracellular protein inclusions called Lewy bodies. α-Syn is a major component of Lewy bodies as well as a related disease, dementia with Lewy bodies.1 It is also the nonamyloid component in amyloid plaques of Alzheimer's disease. Human α-syn locus duplication or triplication with abnormal protein overexpression, as well as point mutations in the gene, have been shown to result in Parkinson-like neurodegeneration in humans, characterized by the loss of dopamine (DA) neurons in the substantia nigra pars compacta (SNc), the loss of DA in striatal terminals and accumulation of α-syn inclusions.1
The function of α-syn remains unclear. α-Syn is associated with synaptic vesicles, and there is evidence that it regulates the size of the synaptic vesicular pool or is involved in exocytosis or endocytosis of multiple neurotransmitter release pathways.1 Paradoxically, α-syn transgenic mice, overexpressing wild-type or mutant α-syn, have often shown no signs of SNc neurodegeneration or the accumulation of α-syn aggregates.2 In contrast, several groups have demonstrated that virus vector–mediated α-syn overproduction in SNc of rodents and monkeys induces Parkinson-like neurodegeneration.3,4,5,6,7
Studies of α-syn knockout mice have revealed no overt pathogenesis, but have produced evidence for altered DA release, lower DA levels, changes in DA vesicle number, or changes in sensitivity to amphetamine or neurotoxins.8,9,10,11,12,13,14 Several studies have shown successful α-syn downregulation in both tissue culture and in animal models using antisense, ribozymes, small-interfering RNAs (siRNAs), or micro RNA15,16,17,18 without obvious pathogenesis. This has suggested that α-syn silencing might be a therapeutic approach for synucleinopathies.
In this study, we examine two α-syn siRNAs and three control siRNAs embedded as short-hairpin RNAs (shRNAs) in adeno-associated virus (AAV) vectors and delivered into rat SNc. We demonstrate that silencing of the rat α-syn gene by vector based RNA interference can induce nigrostriatal degeneration, suggesting that α-syn plays a critical role in the SNc.
Results
Screening siRNAs for efficient silencing of rat α-syn in vitro
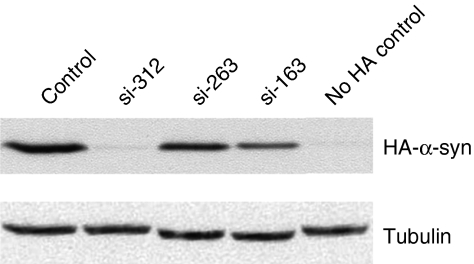
siRNA sequences were selected according to current siRNA design recommendations.19,20,21 All siRNA sequences were 19 bases long and were cloned as shRNA constructs into AAV vector plasmids under the control of H1 promoters. The siRNA vector plasmids were then co-transfected with AAV vectors expressing the targeted rat α-syn gene (2:1 ratio) or with empty vector as a control into 293 cells and knockdown of α-syn expression was determined by immunoblotting (Figure 1). Two different α-syn siRNAs were chosen to avoid the possibility that one of the siRNAs had off target effects unrelated to α-syn. si-312 and si-163, which showed ~93 and 60% knockdown efficiency, respectively, were packaged in AAV2/5 vectors for subsequent injection into rats.
Figure 1.
Identification of active rat α-synuclein (α-syn) siRNAs. Three different short-hairpin RNA expressing plasmids (si-312, si-163, si-263) were co-transfected with a plasmid expressing HA tagged rat α-syn into 293 cells. Whole cell extracts prepared 48 hours post-transfection were fractionated on a sodium dodecyl sulfate–polyacrylamide gel and immunoblotted with HA antibody. Control lane received no siRNA expression plasmid, no HA control lane received no α-syn plasmid. Tubulin was used to normalize the amount of protein loaded and α-syn expression was estimated by densitometry using Image Quant 5. HA, hemagglutinin.
Because it has become clear that expression of shRNA in brain sometimes leads to off target effects that are believed to be due to inhibition of RNA processing,20 we also chose three irrelevant shRNA vectors that were designed to reduce the expression of the rat rhodopsin, peripherin/retinal degeneration slow (rds), and myocardin genes. All of the control genes are not normally expressed in SNc at detectable levels. Rhodopsin is expressed exclusively in the eye, and the rhodopsin shRNA has been shown to be effective in reducing rhodopsin protein levels when injected into a rat eye in the context of an AAV vector.22 Similarly, myocardin controls differentiation of smooth muscle cells, and rds is a membrane protein unique to rods and cones in the retina. Both the α-syn siRNAs and the control siRNAs were under the control of the same H1 promoter in what were otherwise identical AAV constructs, all of which were packaged into AAV2/5 virus. In addition to the siRNA cassettes, all of the vectors used in this study also contained green fluorescent protein (GFP) expression cassettes for the purpose of identifying the injected brain region.
Silencing of α-syn in vivo
Animals were injected in the SNc with ~4 × 109 genome containing virus particles (1.5 µl) of one of the α-syn or control siRNA vectors. An approximately tenfold higher level of the control rhodopsin siRNA and 17-fold higher level of GFP virus were used to test for nonspecific neurodegenerative effects. Injections were done in the SNc on one side of the brain; the other side was kept as a nontransduced internal control. Brain tissue was harvested at 4, 8, or 12 weeks postinjection, and delivery of virus to the SNc was verified by detection of GFP fluorescence. As shown previously, transduction spread throughout the SNc and extended into neighboring structures,23 including neurons of the ventral tegmental area (VTA).
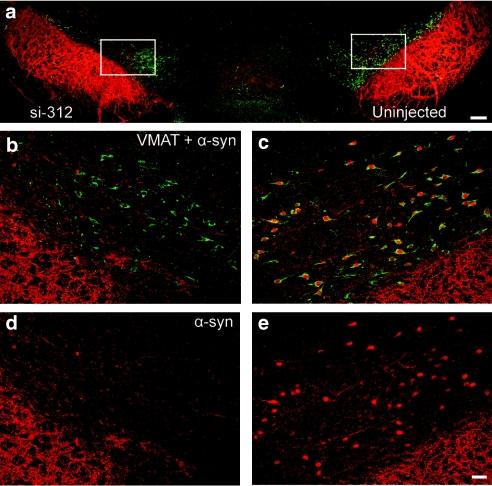
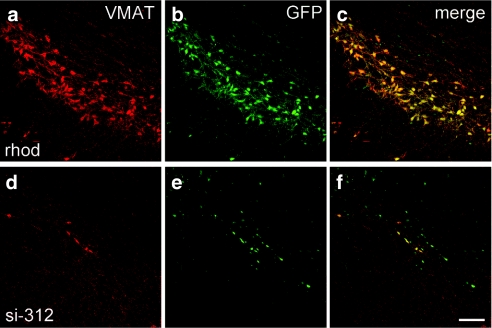
To estimate the level of reduction at the protein level, the nearby VTA region was stained for α-syn and vesicular monoamine transporter (VMAT), and the level of α-syn staining was estimated in cells that stained positively for VMAT. There was a substantial reduction of α-syn in VMAT positive cells (Figure 2). Using confocal image analysis software (Leica Applications Suite, Leica Microsystems CMS GmbH, Mannheim, Germany), it was determined that the level of α-syn staining in VMAT positive cells was ~14% of the control side in animals injected with si-312 (P < 0.0001) and 32% in animals injected with si-163 (P < 0.001); n = 4 for both groups, 200 cells/group. No significant change was seen in α-syn levels in animals injected with control siRNAs.
Figure 2.
In vivo reduction of rat α-synuclein (α-syn) protein. Confocal images show α-syn immunoreactivity (Cy3, red) and VMAT (Cy5, artificially colored green) of rat substantia nigra pars compacta at 4 weeks after injection of α-syn si-312. VMAT is used as a marker of dopamine cells in the ventral tegmental area. b and d are enlarged images of the white frame on the injected side of a; c and e are enlargements of the frame on the control side of a. d and e illustrate expression of α-syn (red) in VMAT positive neurons (green) shown in b and c, respectively. Bar = 150 µm (a) and 50 µm (b–e). VMAT, vesicular monoamine transporter.
Both of the α-syn siRNAs were also effective in reducing the steady-state level of α-syn mRNA in the SNc compared to the uninjected side as judged by reverse transcriptase-PCR of pooled SNc tissues. At 4 weeks postinjection, rat α-syn mRNA was reduced to 10% (si-163) and 5% (si-312) of the uninjected control side (n = 4 for both groups). No change in α-syn mRNA was seen in rats injected with control siRNAs or with GFP. Thus, both at the mRNA and protein levels, there was a significant decrease in α-syn expression. In these measurements and in other measurements described below, si-312 was consistently more potent than si-163. The apparently higher level of reduction at the RNA level in SNc compared to the protein levels seen in VTA may reflect the higher level of neuronal loss seen in SNc (see below).
α-Syn silencing in the SNc appears to induce the loss of TH positive neurons
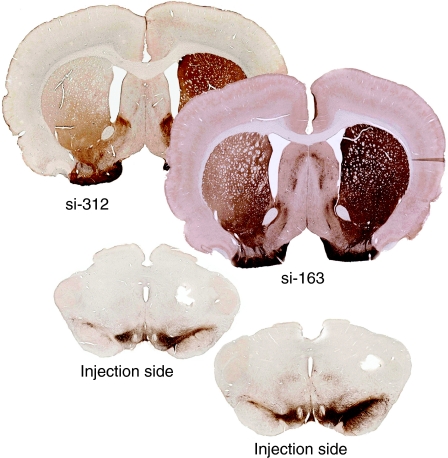
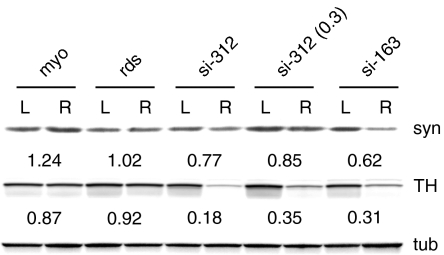
When we immunostained SNc and striatal tissues for tyrosine hydroxylase (TH), there was a significant loss of TH staining in animals injected with both si-312 and si-163 compared to the uninjected side (Figure 3). This was confirmed by immunoblotting of striatal tissue from individual animals injected with control or α-syn targeted siRNA virus (Figure 4). An si-312 injected rat retained 18% of the TH seen on the uninjected side compared to ~90% for rds and myocardin control injections at 4 weeks. Injection of a smaller dose of si-312 (0.3×) or the less potent si-163 siRNA produced more modest reductions in TH (Figure 4). In contrast, the level of α-syn in the striatum was not significantly reduced, presumably because of the contribution of uninjected striatal neurons to the total α-syn pool.
Figure 3.
Tyrosine hydroxylase (TH) immunohistochemistry of substantia nigra pars compacta (SNc) and striatum at 4 weeks postinjection of animals treated with α-synuclein (α-syn) siRNAs. TH immunostaining is significantly lower in both SNc (lower sections) and striatum (upper sections) on the injected side with both si-163 and si-312.
Figure 4.
Immunoblot of small-interfering RNA (siRNA)-treated striatal tissue. Tissue from individual animals that had received substantia nigra pars compacta injections of α-synuclein (α-syn) siRNAs (si-312, si-163, or a lower dose of si-312, 0.3× si-312) were compared with control injections of siRNAs directed against myocardin and rds for the level of tyrosine hydroxylase (TH) and α-syn on the injected (R) versus uninjected (L) sides. Fluorescent antibody signals from α-syn and TH were normalized to tubulin and the ratio of injected to uninjected side is listed beneath each blot.
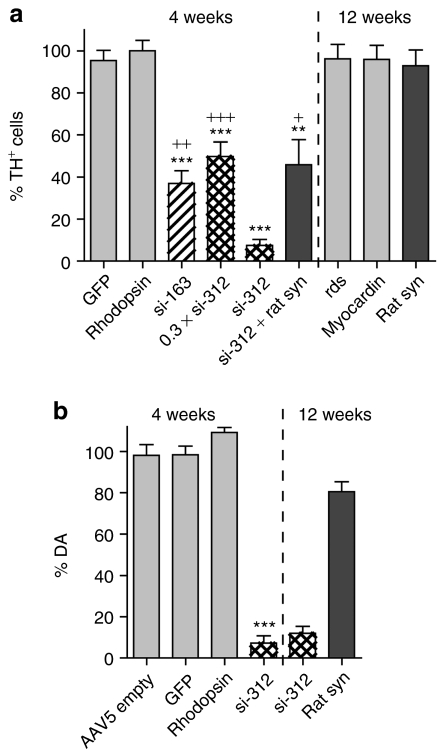
To obtain a quantitative estimate of the extent of neurodegeneration, we used unbiased stereology to count the number of TH positive cells in the SNc. We found that both α-syn shRNAs (si-312 and si-136) produced a rapid and significant SNc neurodegeneration (Figure 5). si-136 was less potent at approximately the same input genome dose than si-312, consistent with its lower activity when tested in 293 cells (Figure 1) and in vivo (Figure 4, TH). In addition, the neuron loss produced by si-312 appeared to be dose dependent; a 3.5-fold lower input dose of si-312 produced significantly less (~6.6-fold) neurodegeneration at 4 weeks (Figure 5a). No additional TH positive cells were lost in si-312 injected cells at longer time points (8 and 12 weeks) postinjection (Supplementary Figure S1, compare with Figure 5a). This may reflect that some cells are not transduced or that there are cells that are resistant to the siRNA. Finally, we also measured the loss of DA in the striatum. Striatal DA levels as determined by high-performance liquid chromatography mirrored the number of TH positive cells remaining at 4 weeks in si-312-injected animals (Figure 5b), and 3,4-dihydroxyphenylacetic acid/DA ratios, when compared to the uninjected side, were elevated in si-312 injected animals (Supplementary Figure S2) as expected.
Figure 5.
Quantitative estimation of the reduction of tyrosine hydroxylase (TH)+ cells and dopamine (DA) after injection of α-synuclein (α-syn) small-interfering RNAs (siRNAs). (a) Unbiased estimation of TH+ cells remaining in substantia nigra pars compacta (SNc) after expression of α-syn or control siRNAs in rat SNc. The mean percentage of TH+ cells remaining plus standard error at 4 or 12 weeks was calculated by comparison with the uninjected side. One-way group analysis of variance (ANOVA) for the 4 weeks time point groups was F(5,23) = 17.47, P < 0.0001, Tukey's post hoc results are indicated as *P < 0.05, **P < 0.01, and ***P < 0.001, when a group was compared to GFP or rhodopsin; +P < 0.05, ++P < 0.01, and +++P < 0.001 when a group was compared to si-312. There were no other significant differences between groups; N = 4–8 for each group. (b) Measurement of striatal DA. The amount of DA in striatal tissue was measured on the injected and uninjected sides of individual animals and is displayed as the mean percentage of DA remaining on the injected side compared with the uninjected side plus standard error. One-way group ANOVA statistics F(3,24) = 74.51, P < 0.0001, Tukey's post hoc for si-312 versus all controls at 4 weeks: ***P < 0.001. There were no other significant differences between groups; N = 4–10 for each group.
In contrast to the α-syn siRNAs, no significant loss of TH positive cells was seen in SNc of animals injected with any of the control siRNAs or with GFP-expressing vectors. The control siRNA targeting rhodopsin and the GFP-expressing vector were injected at a tenfold and 17-fold higher dose, respectively, than si-312 in an attempt to produce off target toxicity due to transcriptional repression, inhibition of mRNA transport or processing, or a viral inflammatory response; however, neither GFP nor the rhodopsin siRNA produced any significant neurodegeneration or loss of DA at 4 weeks postinjection (Figure 5a,b). Similarly, in companion experiments, injection of control siRNAs directed against myocardin and rds, which were injected at the same dose as si-312, produced no significant neuronal loss even at 12 weeks postinjection compared to uninjected controls (Figure 5a, ~96% of uninjected side), or compared to the GFP-injected control. Injection of high titer empty vector particles also did not produce any apparent toxicity at 4 weeks as judged by DA content in the striatum (Figure 5b) or by unbiased counting of TH positive cells (data not shown).
To determine whether the reduction in SNc TH positive neurons was specifically due to a loss of α-syn expression, animals were injected with a mixture containing both the siRNA-expressing virus, si-312, and a virus that expressed rat α-syn at a 1:1 genome dose. This reduced the loss of DA neurons approximately sixfold compared to si-312 injection alone, suggesting that the toxicity of si-312 was due to the loss of rat α-syn activity (Figure 5a). In contrast, injection of rat α-syn alone did not produce statistically significant loss of TH positive neurons or striatal DA at 8 or 12 weeks postinjection (Figure 5a,b).
Finally, to determine whether the loss of TH positive neurons was due simply to the loss of TH expression, we examined the effect of si-312 injection on the expression of two other markers for DA neurons. Expression of both VMAT (Figure 6) and aromatic amino acid decarboxylase (AADC) appeared to be absent to the same extent as TH following injection of si-312 but not rhodopsin siRNA. To be certain that we were observing cell loss and not changes in gene expression, we also measured the ratio of GFP positive cells to TH positive cells in the SNc of animals injected with equal amounts of GFP alone, si-312 and rhodopsin siRNA. The expression level of GFP provided an independent measure of the number of surviving cells. The ratio of GFP to TH positive cells as a function of the vector injected was: GFP = 0.92 ± 0.06 SD; rhodopsin = 0.95 ± 0.08; si-312 = 1.06 ± 0.06 (Supplementary Figure S3). Thus, the number of GFP positive cells was essentially equal to the number of TH positive cells in all groups. This implied that GFP expression and TH expression in SNc were lost to the same extent in si-312 injected animals and suggested that loss of TH expression was due to the loss of DA neurons. Had the loss of TH immunoreactivity in si-312 treated animals been due simply to lower expression of TH, then the GFP/TH ratio would have been significantly higher than one. This result also demonstrated that 92–95% of the TH positive cells in the SNc had been successfully transduced with vector. We also measured the loss of cells in SNc that stained with cresyl violet, and the loss of cells that stained with cresyl violet was similar to the loss of TH+ cells for both si-312 and si-163 (compare Supplementary Figure S4 with Figure 5a).
Figure 6.
Comparison of vesicular monoamine transporter (VMAT) staining (red) in substantia nigra pars compacta (SNc) injected with either si-312 or rhodopsin small-interfering RNA expressing virus. Both vectors also express green fluorescent protein (GFP) (green). Note the loss of VMAT staining cells in the animal injected with si-312.
Loss of α-syn expression also induces behavioral effects
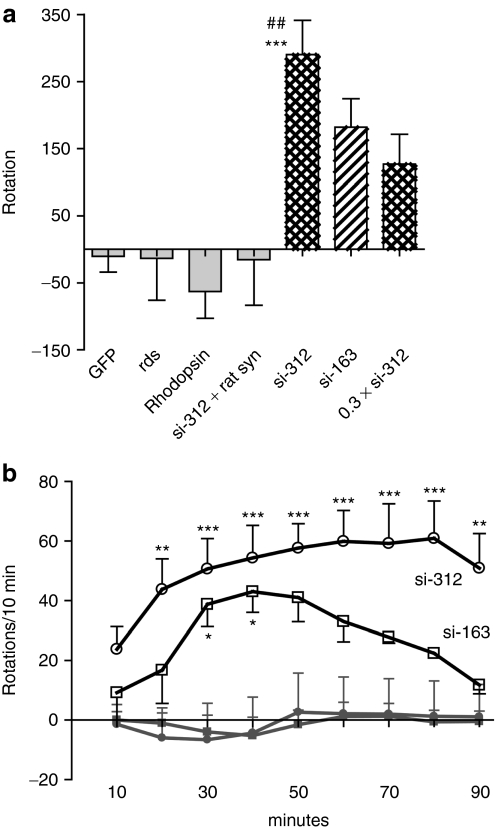
In addition to the loss of TH positive neurons in the SNc, we also observed significant amphetamine-induced rotational asymmetry at the 4-week time point in si-312-injected animals (Figure 7a). Furthermore, co-injection of the α-syn siRNA, si-312, with a vector that expressed rat α-syn protein entirely eliminated the asymmetric rotational behavior (Figure 7a). Animals injected with a lower dose of si-312 and animals injected with si-163 also displayed ipsilateral rotation but it did not reach statistical significance. This was consistent with the level of TH positive cells and DA remaining on the injected side (Figure 5). Further analysis of the amphetamine-induced rotation as a function of time revealed that si-163-injected animals also showed significant rotational asymmetry at intermediate times following amphetamine injection (Figure 7b).
Figure 7.
Amphetamine-induced rotation at 4 weeks postinjection. (a) Injection with α-synuclein (α-syn) small-interfering RNA (siRNA) induced significant rotation ipsilateral to the injected side for si-312 in a 60-minute period after amphetamine injection. Group statistics were F(6,50) = 6.038, P < 0.0001, Tukey's post hoc: ***P < 0.001 versus control siRNA (rds or rhodopsin), ##P < 0.01 versus si-312+rat syn. There were no other significant differences between groups; N = 4–12 for each group. (b) Time course of amphetamine-induced rotation at 4-week time point for green fluorescent protein (GFP) (closed grey squares), myocardin (closed grey circle), si-312 (open circles), and si-163 (open squares). Net rotation for each group is shown for each 10-minute interval following treatment with amphetamine. Positive rotation indicates rotation toward the injected side; negative rotation indicates rotation contralateral to the injected side. Two-way analysis of variance statistics: time: F (9,297) = 1.20, P = 0.298; gene: F(3,297) = 64.1, P < 0.0001; time × gene interaction: F (24,297) = 0.571, P = 0.949. Bonferroni post-tests are indicated as *P < 0.05, **P < 0.01, ***P < 0.001. Asterisks indicate that the group is being compared to the GFP or myocardin group in the same time bin; N = 5–12 for each group.
Discussion
Our results suggest that α-syn may be essential for the survival of DA neurons. Expression of two different α-syn siRNAs in the rat SNc produced a rapid loss of TH positive neurons that appeared to correlate with the potency of the siRNA. One of the siRNAs, si-312, was consistently better both in vitro and in vivo in reducing rat α-syn mRNA and protein, and was also consistently better at inducing neurodegeneration. Moreover, injection of two different doses of si-312 suggested that the effect was dose dependent. The use of multiple markers in the SNc, including AADC, VMAT and GFP, indicated that loss of TH immunoreactivity was due to neuron loss, rather than a reduction of TH enzyme expression.
It is difficult to ascribe the loss of DA neurons in the SNc following α-syn siRNA injection to nonspecific or off target effects. First, three different control siRNAs (rhodopsin, rds, and myocardin) produced little if any toxicity even at longer time points postinjection. This argues against a nonspecific effect due to overloading of the RNA processing, transport, or translational machinery. In the case of the control rhodopsin siRNA, a tenfold higher input genome dose did not produce neurodegeneration. Second, two different siRNAs targeted to α-syn produced the same effect (si-312 and si-163); both caused a loss of TH positive cells in the SNc. Third, both loss of TH positive neurons and the rotational phenotype induced by α-syn siRNA were reversed by injection of rat α-syn, suggesting that this siRNA was specific for the α-syn gene. In addition, the fact that no neurotoxicity was seen with GFP and three control siRNA viruses (myocardin, rhodopsin, and rds), as well as empty virus capsids, demonstrates that nigrostriatal degeneration was not the result of an inflammatory response due to viral injection or some other nonspecific effect. Recently, Ulusoy et al.24 demonstrated recombinant AAV vector injections using titers in excess of 3–4 × 1013 genome particles/ml produced nonspecific neurodegeneration in the SNc regardless of the expression cassette that was used. The input doses of virus used in our studies were below those that have been shown to cause neurodegeneration by this group (see Materials and Methods). Taken together, this suggests that neurotoxicity was due to a reduction of α-syn and not due to an off target effect. We note that it is still remotely possible that each of the α-syn siRNAs could have targeted alternative but equally essential genes that were unrelated to α-syn.
Two previous reports of α-syn knockdown with siRNAs or ribozymes demonstrated that viral vector–mediated reduction of α-syn could have a therapeutic effect. Hayashito-Kinoh et al.15 used AAV-mediated expression of α-syn ribozymes in rat SNc to reduce the increase in α-syn level that resulted from 1-methyl-4-phenylpyridinium+ (MPP+) treatment. This had a protective effect against MPP+-induced nigral TH+ neuron loss. Similarly, Sapru et al.18 used lentivirus-mediated expression of an siRNA directed to human α-syn to successfully silence human α-syn expression in rat SNc. This siRNA was specific for human α-syn and it was not clear whether there was any effect on endogenous α-syn expression. In addition, Lewis et al.17 infused chemically modified naked siRNAs into rat hippocampus. α-Syn was reduced for at least 1 week and recovered by week 3 with no obvious toxicity. Although this transient reduction is not directly comparable to the chronic reduction reported here, the lack of toxicity reported in hippocampus may suggest that SNc is preferentially susceptible to reduction of α-syn. Indeed, reduction of α-syn in the nearby VTA during our experiments did not appear to have as severe an effect as in SNc, although we could not be certain that this was not due to the lower dose of vector that reached the VTA. Our results also support a recent report that demonstrated an ~50% loss in cell viability when an α-syn siRNA was expressed in the dopaminergic cell line MN9D.25 The α-syn siRNA used in the MN9D study was different from the two reported here. As expected from previous work,26 the reduction in α-syn in MN9D cells had no affect on TH protein levels, but increased both the level of TH phosphorylation and enzymatic activity.
Although our results do not necessarily conflict with other α-syn knockdown experiments, they are not in agreement with a number of reports of α-syn knockout mice. Transgenic knockout of α-syn,11,12,13,14 or a combination of α-syn and β-syn,10 did not appear to induce any obvious changes in mouse brain. However, modest (20%) reductions in striatal DA10,11 or changes in DA release11,12 were noted in knockout mice. In addition, one study showed that knockout of α-syn, γ-syn, or both significantly reduced the number of TH positive neurons (20%) in SNc9 and another demonstrated that loss of striatal DA (but not SNc neurons) became more severe with age.8 This implies that knockdown of a gene in adults is not the same as congenital deletion. It is not uncommon for knockout mice to undergo compensation during development and to date a conditional knockout of α-syn in the adult mouse brain has not been constructed. In contrast, the use of viral-mediated gene expression modifies α-syn expression in adult rats and primates, and this may not allow time for adaptation. In the case of α-syn, two potentially redundant pathways are available, β-synuclein, and γ-synuclein, and both are expressed in the SNc.11 This suggestion was also made by Robertson et al.,9 who showed an increase in β-synuclein expression in adult α-syn and γ-syn knockout mice. We note that α-syn transgenic mice, in which human α-syn or α-syn mutants are overexpressed, also do not recapitulate the human phenotype.2 This is in contrast to what has been seen in both rodents and primates when α-syn is expressed via viral-mediated gene transfer.3,4,5,6,7 Finally, the absence of toxicity seen in mouse α-syn knockout models suggested the possibility that siRNA knockdown might be a viable therapeutic strategy for Parkinson Disease. Our results suggest caution in using knockdown of α-syn as a strategy for treating Parkinson disease.
The toxicity that was seen when nigral α-syn was reduced raises new questions about the role of α-syn in Parkinson disease. Much of the attention to α-syn has focused on the possibility that α-syn protein can aggregate to form soluble complexes, fibrils, or large inclusion bodies.1 One or more of these aggregated species is believed to lead to cell death by modifying DA release, proteolytic processes, endoplasmic reticulum, or vesicle formation. Cell death due to protein aggregation is consistent with the progressive nature of Parkinson disease and the progressive neurodegeneration that is seen in both rodent and primate models of viral-mediated α-syn overexpression.3,4,5,6,7 Overexpression of human α-syn in rat SNc leads to ~50% loss of DA neurons at 6 months postinjection.4 Similar losses are seen in primates 1 year after injection of the A53T mutant of α-syn.3 In contrast, the toxicity seen with α-syn knockdown is immediate and severe, suggesting an acute rather chronic defect. This suggests that α-syn is performing a critical biochemical role in DA neurons, and highlights the importance of identifying α-syn's biochemical function in the SNc. α-Syn has been shown to interact with a variety of cellular proteins and is present in membranes, nuclei, and cytoplasm.27 It is not immediately clear which of these interactions is responsible for the acute phenotype we have observed. One possible interaction that might be relevant is phospholipase D2. Elsewhere, we will present evidence that overexpression of phospholipase D2 in SNc also causes an immediate and severe toxicity with a time course similar to that seen with knockdown of α-syn, and that α-syn overexpression reduces the toxicity of phospholipase D2 (O.S. Gorbatyuk and N. Muzyczka, manuscript submitted). Thus, our results are consistent with α-syn being an in vivo inhibitor of phospholipase D2. However, a variety of other interactions could also contribute to the rapid neurodegeneration we have seen.
Finally, it is also possible that the neurodegeneration caused by α-syn overexpression and the neurodegeneration induced by α-syn downregulation are due to the same mechanism. Overexpression of α-syn or the expression of a dominant α-syn mutant may lead either to sequestration of α-syn into nonfunctional aggregates, or misdirection of α-syn to cellular compartments where it is not functional. The net effect would be the same as downregulating α-syn expression by siRNA. This theoretical possibility was also suggested by Perez et al.28
Materials and Methods
Recombinant AAV-shRNA plasmid and virus construction. siRNAs were designed based on the Genbank rat α-syn sequence NM019169. All sequences were BLAST confirmed for specificity. Synthetic DNA encoding shRNA sequences targeting rat α-syn were cloned under the control of an H1 promoter as previously described.22 shRNA vector plasmids were then co-transfected with AAV vectors expressing target genes (2:1 ratio) or with empty vector as control into 293 cells for 48 hours to determine the level of knockdown of gene expression by immunoblotting. The siRNA sequences are shown in Table 1.
Table 1. Small-interfering RNA target sequences.
All viral vectors contained AAV2 terminal repeats and were packaged in AAV5 capsids that were purified as described previously.4,23 The GFP and rhodopsin shRNA expressing viruses have been described.22,23 All shRNA vectors contained a GFP cassette in addition to the shRNA cassette driven by an H1 promoter. Empty AAV5 virus particles were obtained by fractionation on iodixanol density gradients and subsequent monoQ chromatography as described for full particles. Titers were determined by dot-blot assays and reverse transcriptase-PCR. Dot-blot assay titers were used to determine the input dose used for injection. For comparison with work reported by others, we found that the dot-blot titers are about 8–10 times higher than titers determined by reverse transcriptase-PCR. The dot-blot virus titers were 2.6 × 1012 vector genomes (vg)/ml for si-312, rds, myocardin, and rat α-syn, 9 × 1012 vg/ml for si-163, 2.6 × 1013 vg/ml for rhodopsin, and 4.5 × 1013 vg/ml for GFP. Empty AAV5 particles had a titer of 7 × 1013 particles/ml as determined by B1 AAV antibody normalized to GFP virus. Viruses were stored and diluted in Lactated Ringers solution.
Intracerebral injections. All surgeries were performed as previously described.4 The rats were placed in the stereotaxic frame and recombinant AAV vectors were injected into the SNc (anteroposterior: −5.6 mm, lateral: −2.4 mm from bregma, and dorsoventral: −7.2 mm from dura), through a glass micropipette with an inner diameter ~30–40 µm at a rate of 0.5 µl/minute. Animals were injected with a total of 1.5 µl containing ~4 × 109 vg/ml of the appropriate gene unless otherwise indicated.
Tissue preparation. Animals were deeply anesthetized by pentobarbital injection (150 mg/kg, intraperitoneally). Brains were removed and divided into two parts at approximately −3.5 mm behind bregma. The caudal part containing the SNc was fixed in the ice-cold 4% paraformaldehyde in 0.1 mol/l phosphate buffer (PB), pH 7.4. The rostral piece of brain tissue was dissected on the right and left striatum. The tissue pieces were weighed, frozen separately on dry ice, and kept at −80 °C until assayed. The fixed part of brains were stored overnight at 4 °C and then transferred into 30% sucrose in 0.1 mol/l PB for cryoprotection. Forty micrometer thick coronal sections were cut on a freezing stage sliding microtome.
For preparation of tissues that were used to measure the reduction of α-syn in VTA, an alternative method was used as previously described.29 Rats were perfused sequentially through the ascending aorta with: 20 ml of heparinized saline; 50 ml of 3.75% acrolein, and 2% paraformaldehyde in 0.1 mol/l PB, pH 7.4 (PB); and finally with 200 ml of 2% paraformaldehyde in PB. Coronal sections (40-µm thick) were cut on a Vibratome (Leica) in chilled PB. Sections were incubated for 30 minutes in 1% sodium borohydride in PB to inactivate excess aldehydes and washed in PB. After a rinse in 0.1 mol/l Tris-buffered saline, pH 7.6, sections were processed for immunocytochemical labeling. This method shows increased nuclear staining of α-syn as described previously.30
Immunohistochemistry. For bright-field microscopy analysis sections were preincubated first with 1% H2O2–10% methanol for 15 minutes and then with 5% normal goat serum for 1 hour. Sections were incubated overnight at room temperature with mouse anti-TH (at 1:2,000 dilution; Chemicon, Temecula, CA) or mouse anti- α-syn (clone 42; BD Laboratories, Franklin Lakes, NJ) antibodies. When a biotinylated secondary antibody was used, this was followed by incubation with avidin–biotin–peroxidase complex (Vector Laboratories, Burlingame, CA) and reactions were visualized using 3,3-diaminobenzidine.
For confocal microscopy, sections were incubated with the indicated primary antibodies for α-syn, TH and hemagglutinin (Abcam, Cambridge, MA), VMAT (Santa Cruz Biotechnology, Santa Cruz, CA), and a secondary antibody labeled with Cy2, Cy3, or Cy5 (Jackson Immunoresearch Laboratories, West Grove, PA). The sections were examined with a Leica laser scanning confocal microscope.
Unbiased stereology. Unbiased estimation of the total number of the TH+ neurons in SNc was performed as previously described4,23 using sections stained with TH antibody. Some measurements were also made with sections stained with cresyl violet or with antibody to AADC, VMAT, or GFP.
Immunoblotting. Tissue homogenates described above were adjusted to a final concentration of 1% NP-40, 0.1% sodium dodecyl sulfate, incubated on ice for 30 minutes and centrifuged for 15 minutes at 4 °C. Protein concentrations were determined by Bradford. 50 µg of each lysate was separated on Biorad precast 4–20% sodium dodecyl sulfate–polyacrylamide (gradient) gel electrophoresis, transferred to polyvinylidene fluoride (Millipore, Bedford, MA) membranes, and immunoblotted. Membranes were incubated with primary antibodies for 2 hours at room temperature or 4 °C overnight. Primary antibodies were diluted at 1:2,000 (α-syn antibody, BD, and TH antibody; Chemicon), or 1:5,000 (GAPDH antibody; Abcam) before incubation. After washing, membranes were incubated with peroxidase-conjugated or fluorescently labeled secondary antibodies for 1 hour. Peroxidase-conjugated hemagglutinin (Roche, Basel, Switzerland) antibody was used to detect hemagglutinin-tagged proteins and the intensity of the bands was determined by densitometry using Image Quant 5.0 software (GE Healthcare Life Sciences, Piscataway, NJ). Cy3 and Cy5 fluorescently labeled antibodies (Jackson Immunoresearch Laboratories) were used for TH and α-syn detection and the intensity of the bands was determined using a Typhoon fluorescent phosphoimager and ImageQuant TL software.
Striatal DA measurements. DA samples were thawed and the equivalent of 3 mg starting tissue was diluted into 1 ml of 0.1 N HClO4 containing dihydrobenzylamine as an internal control, and thoroughly homogenized. The sample was centrifuged and the supernatant was filtered through a 0.2-µm filter. DA and 3,4-dihydroxyphenylacetic acid levels were analyzed on a Beckman Gold System using a C18 Waters Symmetry column and an ESA Coulochem electrochemical detector equipped with a 5011A analytical cell. The mobile phase was: 8.2 mmol/l citric acid, 8.5 mmol/l sodium phosphate monobasic, 0.25 mmol/l EDTA, 0.30 mmol/l sodium octyl sulfate, and 7.0% acetonitrile, pH 3.5.
Rotational behavior. Drug-induced rotational behavior was measured following an injection of -amphetamine sulfate (2.5 mg/kg intraperitoneally; Sigma, St Louis, MO) at 4, 8, and 12 weeks after the viral injection. Rotations were measured during a 90-minute period, and full 360° turns were counted.
Statistical analysis. Data were analyzed using unpaired t-test, one-way analysis of variance with Tukey post-test or two-way analysis of variance with Bonferroni post-test using Prism 4 (GraphPad Software, La Jolla, CA).
SUPPLEMENTARY MATERIAL Figure S1. Unbiased estimation of TH+ cells remaining in SNc after expression of GFP, α-syn siRNAs or control siRNAs in rat SNc. Figure S2. Expression of α-syn siRNA increases the DOPAC to DA ratio. Figure S3. GFP and TH positive cells were lost or retained at the same rate. Figure S4. Unbiased estimation of cresyl violet (CV) positive cells remaining in SNc after expression of GFP or α-syn siRNAs in rat SNc.
Acknowledgments
We thank Isabelle Williams for technical support. This work was supported by grants from NIH (PO1 NS36302, N.M. and R.J.M.) and the ACS Edwin R. Koger endowment (N.M.). N.M. is an inventor of patents related to rAAV technology and owns equity in a gene therapy company that is commercializing AAV for gene therapy applications.
Supplementary Material
Unbiased estimation of TH+ cells remaining in SNc after expression of GFP, α-syn siRNAs or control siRNAs in rat SNc.
Expression of α-syn siRNA increases the DOPAC to DA ratio.
GFP and TH positive cells were lost or retained at the same rate.
Unbiased estimation of cresyl violet (CV) positive cells remaining in SNc after expression of GFP or α-syn siRNAs in rat SNc.
REFERENCES
- Cookson MR., and, van der Brug M. Cell systems and the toxic mechanism(s) of alpha-synuclein. Exp Neurol. 2008;209:5–11. doi: 10.1016/j.expneurol.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet MF. In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson's disease. Exp Neurol. 2008;209:22–27. doi: 10.1016/j.expneurol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslamboli A, Romero-Ramos M, Burger C, Bjorklund T, Muzyczka N, Mandel RJ, et al. Long-term consequences of human alpha-synuclein overexpression in the primate ventral midbrain. Brain. 2007;130 Pt 3:799–815. doi: 10.1093/brain/awl382. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk OS, Li S, Sullivan LF, Chen W, Kondrikova G, Manfredsson FP, et al. The phosphorylation state of Ser-129 in human alpha-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc Natl Acad Sci USA. 2008;105:763–768. doi: 10.1073/pnas.0711053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Bianco C, Ridet JL, Schneider BL, Deglon N., and, Aebischer P. alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral based model of Parkinson's disease. Proc Natl Acad Sci USA. 2002;99:10813–10818. doi: 10.1073/pnas.152339799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, et al. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002;22:2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Iwatsubo T, Mizuno Y., and, Mochizuki H. Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson's disease. J Neurochem. 2004;91:451–461. doi: 10.1111/j.1471-4159.2004.02728.x. [DOI] [PubMed] [Google Scholar]
- Al-Wandi A, Ninkina N, Millership S, Williamson SJ, Jones PA., and, Buchman VL. Absence of alpha-synuclein affects dopamine metabolism and synaptic markers in the striatum of aging mice. Neurobiol Aging. 2010;31:796–804. doi: 10.1016/j.neurobiolaging.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DC, Schmidt O, Ninkina N, Jones PA, Sharkey J., and, Buchman VL. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J Neurochem. 2004;89:1126–1136. doi: 10.1111/j.1471-4159.2004.02378.x. [DOI] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, et al. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci USA. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, et al. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci USA. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter OM, Fornai F, Alessandrí MG, Takamori S, Geppert M, Jahn R, et al. Role of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Neuroscience. 2003;118:985–1002. doi: 10.1016/s0306-4522(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Hayashita-Kinoh H, Yamada M, Yokota T, Mizuno Y., and, Mochizuki H. Down-regulation of alpha-synuclein expression can rescue dopaminergic cells from cell death in the substantia nigra of Parkinson's disease rat model. Biochem Biophys Res Commun. 2006;341:1088–1095. doi: 10.1016/j.bbrc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- Junn E, Lee KW, Jeong BS, Chan TW, Im JY., and, Mouradian MM. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci USA. 2009;106:13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Melrose H, Bumcrot D, Hope A, Zehr C, Lincoln S, et al. In vivo silencing of alpha-synuclein using naked siRNA. Mol Neurodegener. 2008;3:19. doi: 10.1186/1750-1326-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapru MK, Yates JW, Hogan S, Jiang L, Halter J., and, Bohn MC. Silencing of human alpha-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp Neurol. 2006;198:382–390. doi: 10.1016/j.expneurol.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Khvorova A, Reynolds A., and, Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115:209–216. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- McBride JL, Boudreau RL, Harper SQ, Staber PD, Monteys AM, Martins I, et al. Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: implications for the therapeutic development of RNAi. Proc Natl Acad Sci USA. 2008;105:5868–5873. doi: 10.1073/pnas.0801775105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS., and, Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk M, Justilien V, Liu J, Hauswirth WW., and, Lewin AS. Suppression of mouse rhodopsin expression in vivo by AAV mediated siRNA delivery. Vision Res. 2007;47:1202–1208. doi: 10.1016/j.visres.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Ulusoy A, Sahin G, Björklund T, Aebischer P., and, Kirik D. Dose optimization for long-term rAAV-mediated RNA interference in the nigrostriatal projection neurons. Mol Ther. 2009;17:1574–1584. doi: 10.1038/mt.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Jin L, Wang H, Zhao H, Zhao C, Duan C, et al. Silencing alpha-synuclein gene expression enhances tyrosine hydroxylase activity in MN9D cells. Neurochem Res. 2008;33:1401–1409. doi: 10.1007/s11064-008-9599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Peng XM, Tehranian R, Dietrich P, Stefanis L., and, Perez RG. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci. 2005;118 Pt 15:3523–3530. doi: 10.1242/jcs.02481. [DOI] [PubMed] [Google Scholar]
- Yu S, Uéda K., and, Chan P. Alpha-synuclein and dopamine metabolism. Mol Neurobiol. 2005;31:243–254. doi: 10.1385/MN:31:1-3:243. [DOI] [PubMed] [Google Scholar]
- Perez RG, Waymire JC, Lin E, Liu JJ, Guo F., and, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk OS, Milner TA, Wang G, Regunathan S., and, Reis DJ. Localization of agmatine in vasopressin and oxytocin neurons of the rat hypothalamic paraventricular and supraoptic nuclei. Exp Neurol. 2001;171:235–245. doi: 10.1006/exnr.2001.7746. [DOI] [PubMed] [Google Scholar]
- Yu S, Li X, Liu G, Han J, Zhang C, Li Y, et al. Extensive nuclear localization of alpha-synuclein in normal rat brain neurons revealed by a novel monoclonal antibody. Neuroscience. 2007;145:539–555. doi: 10.1016/j.neuroscience.2006.12.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Unbiased estimation of TH+ cells remaining in SNc after expression of GFP, α-syn siRNAs or control siRNAs in rat SNc.
Expression of α-syn siRNA increases the DOPAC to DA ratio.
GFP and TH positive cells were lost or retained at the same rate.
Unbiased estimation of cresyl violet (CV) positive cells remaining in SNc after expression of GFP or α-syn siRNAs in rat SNc.