Abstract
Adult stem cell–based gene therapy holds several unique advantages including avoidance of germline or other undesirable cell transductions. We have previously shown that liver progenitor (oval) cells can be used as a platform for liver gene delivery of human α1-antitrypsin (hAAT). However, this cell source cannot be used in humans for autologous transplantation. In the present study, we tested the feasibility of bone marrow (BM) cell–based liver gene delivery of hAAT. In vitro studies showed that BM cells can be transduced by lentiviral vector (Lenti-CB-hAAT) and recombinant adeno-associated viral vectors (rAAV1-CB-hAAT, and rAAV8-CB-hAAT). Transplantation studies showed that transplanted BM cells homed into liver, differentiated into hepatocytes and expressed hAAT in the liver. Importantly, we showed that transplantation of rAAV8-CB-hAAT vector–transduced BM cells resulted in sustained levels of hAAT in the systemic circulation of recipient mice. These results demonstrated that rAAV vector–mediated BM cell–based liver gene therapy is feasible for the treatment of AAT deficiency and implies a novel therapy for the treatment of liver diseases.
Introduction
α1-antitrypsin (AAT) deficiency is a genetic defect caused primarily by a single base substitution in the AAT gene encoding a 52 kd glycoprotein.1 This mutation results in an accumulation of polymerized mutant AAT protein in the hepatocytes where AAT is mainly synthesized and secreted into the circulation, and consequently leads to a reduced level of AAT in the serum.2 As a serine protease inhibitor, the primary function of AAT is to protect delicate tissue such as pulmonary interstitial elastin against the excessive proteolytic damage of neutrophil elastase. Deficiency of AAT in the serum can cause degradation of alveolar elastin by neutrophil elastase and leads to development of emphysema.3 Aggregated mutant AAT in the endoplasmic reticulum of hepatocytes can also result in liver diseases such as neonatal jaundice and hepatic cirrhosis.3 For AAT deficiency–associated lung disease, restoring antineutrophil elastase protection in the lung has been achieved by boosting serum AAT level via weekly intravenous infusion of human plasma AAT.4 Strategies employing overexpression of wild-type AAT gene to correct the deficiency of AAT via gene transfer to muscle are being investigated in a phase I clinical trial using recombinant adeno-associated virus (AAV) vector serotypes 1 and 2 (refs. 5,6). For AAT deficiency–associated liver disease, no effective therapy is available except for liver transplantation that is hampered by the shortage of donor organs and by immune rejection.
Adult stem cells offer a platform for ex vivo genetic manipulation followed by autologous transplantation, which may overcome many limitations, including immune rejection of allogeneic cells, ethical issues of using embryonic stem cells, and germline transduction.7,8,9,10 Nonspecific targeting is one of the major concerns of conventional gene therapy that employs direct infusion of genes into a patient. Using stem cells as a means for delivering a gene into patients could minimize unwanted cell transductions. More importantly, stem cells can self-renew and replace aged or damaged cells. Therefore, stem cell–based gene therapy may reduce or eliminate the need for repeated administration of gene therapy. Adult stem cell gene therapy, which replaces a patient's disease-causing gene with its healthy counterparts within their own stem cells, will offer a hope for those who are running out of treatment options and are tired of life-long medication regimens.11
Bone marrow (BM) is the reservoir of stem cells including two major populations, hematopoietic stem cells, and mesenchymal stem cells. The BM stem cell is one of the first stem cells successfully used in transplantation therapy for treating blood disease (e.g., leukemia). In addition, BM has been proposed to be an extrahepatic source of liver progenitor cells.12,13,14 Sex-mismatched BM transplantation in lethally irradiated dipeptidyl peptidase IV enzyme negative rats treated with 2-acetylaminofluorene and carbon tetrachloride first demonstrated cells of BM origin are capable of repopulating the injured liver.12 Both cell fusion with host hepatocytes and hepatic transdifferentiation of BM cells have been proposed as the underlying principle.15,16 Recent studies have reported that mesenchymal stem cells from BM cells can be efficiently transduced by AAV.7,11,17 In our present study, we tested the feasibility of BM cell–mediated liver gene delivery of hAAT in a mouse model.
Results
BM cell transduction
In order to test the transduction efficiency of BM cells, whole BM cells were isolated and infected with Lenti-CB-hAAT, rAAV1-CB-hAAT, and rAAV8-CB-hAAT vectors. As shown in Figure 1, all vectors can transduce BM cells, but with different levels of efficiency. Lenti-CB-hAAT infection resulted in the highest measurable levels of hAAT in culture medium. Although hAAT levels in rAAV vector–infected cells are much lower than that in lentiviral vector–infected cells, they are clearly detectable. Relatively, rAAV1-CB-hAAT mediated higher levels of hAAT than rAAV8-CB-AAT. These results suggest that both lentiviral vector and rAAV vectors can be useful in BM cell transplantation studies.
Figure 1.
Ex vivo transduction of bone marrow (BM) cells. Mouse BM cells were seeded in a 24-well plate (1 × 104 cells/well; n = 3) and infected with the Lenti-CB-hAAT vector at 100 multiplicity of infection (MOI), rAAV1-CB-hAAT, rAAV8-CB-hAAT at 104 MOI, and phosphate-buffered saline (PBS), respectively. The cumulative hAAT in the culture medium was measured by enzyme-linked immunosorbent assay. Circles, Lenti-CB-hAAT; triangles, rAAV1-CB-hAAT; squares, rAAV8-CB-hAAT; dashes, lower limit of quantification (LLOQ). hAAT level of PBS group (negative control) was below LLOQ. hAAT, human α1-antitrypsin.
Liver transplantation of ex vivo transduced BM cells
Next, we tested the feasibility of BM cell transplantation for liver gene delivery of human AAT (hAAT). As described in Figure 2, top panel, male green fluorescent protein (GFP) transgenic mice were used as donor animals and female C57BL/6 mice were used as recipients. The recipients were treated with monocrotaline (MCT) to inhibit endogenous hepatocyte proliferation. The recipients also received partial hepatectomy (PHx) before transplantation to create liver injury, thus enhancing the environment for the proliferation and differentiation of transplanted BM cells. In this experiment, freshly isolated whole BM cells (donor cells) were infected with Lenti-CB-hAAT (MOI = 100), rAAV1-CB-hAAT (MOI = 1 × 104), and rAAV8-CB-hAAT (MOI = 1 × 104) vectors, respectively. After thorough washing, 5 × 106 cells were transplanted into recipient livers through portal vein injection. All mice were killed at 14 weeks after transplantation. As shown in Figure 2, bottom panel, rAAV8-CB-hAAT vector mediated 11.93% (±10.08%) hAAT-positive cells in the recipient liver, whereas Lenti-CB-hAAT and rAAV1 vector mediated 4.67% (±2.63%) and 4.37% (±3.74%) of hAAT-positive cells, respectively (Figure 2j). To confirm that the hAAT-positive cells are derived from donors, we performed coimmunostaining experiments. As shown in Figure 3a–c, hAAT-positive cells from the rAAV8-CB-hAAT vector treatment group were also GFP+ (donor cell marker).
Figure 2.
Transplantation of BM cells into mouse liver. (a) Outline of the experiment. The recipients (female C57BL/6) were i.p. injected twice (2-week interval) with 50 mg/kg of monocrotaline (MCT) and received partial hepatectomy (PHx) to remove 70% of liver mass before transplantation. BM cells were isolated from the femurs and tibias of male C57BL/6 mice. The newly purified BM cells were infected with Lenti-CB-hAAT, rAAV1-CB-hAAT, rAAV8-CB-hAAT vector, respectively, for 2 hours, washed, and transplanted into the recipient liver by portal vein injection or intrasplenic injection. Serum hAAT levels were monitored by human AAT-specific enzyme-linked immunosorbent assay. Recipients were killed at 14 weeks after transplantation. Liver repopulation was measured by immunostaining. (b–i) Detection of expression of human α1-antitrypsin (hAAT) in recipient liver after transplantation of viral vector–infected BM cells by immunostaining. (b) Liver section from C57BL/6 mouse transplanted with Lenti-CB-hAAT-infected BM cells (brown). (c) Image b view at larger magnification. (d) Liver section from C57BL/6 mouse transplanted with rAA1-CB-hAAT-infected BM cells. (e) Image d view at larger magnification. (f) Liver section from C57BL/6 mouse transplanted with rAAV8-CB-hAAT-infected BM cells. (g) Image e view at larger magnification. (h) Human liver section served as positive control. (i) Liver section from untransplanted C57BL/6 mouse served as negative control. (j) Quantification of hAAT-positive cells in rAAV8-hAAT (n = 3), Lenti-hAAT (n = 3), and rAAV1-hAAT (n = 4) groups. BM, bone marrow.
Figure 3.
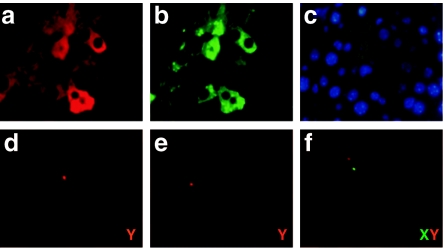
Detection of donor cells in recipient liver. (a–c) Double fluorescence immunostaining for human α1-antitypsin (hAAT) and green fluorescent protein (GFP). (a) Liver section from C57BL/6 mouse transplanted with rAAV8-CB-hAAT-infected bone marrow cells showing hAAT expression (red). (b) Liver section same as in a stained for GFP with anti-GFP antibody (green). (c) Merge image of a and b. Representative slides were viewed at ×100 magnification. (d–f) Fluorescence in situ hybridization (FISH) for Y chromosome in the recipient liver. Green signal shows X chromosome (X); red signal shows Y chromosome (Y).
BM cell transplantation resulted in sustained levels of hAAT in recipient circulation
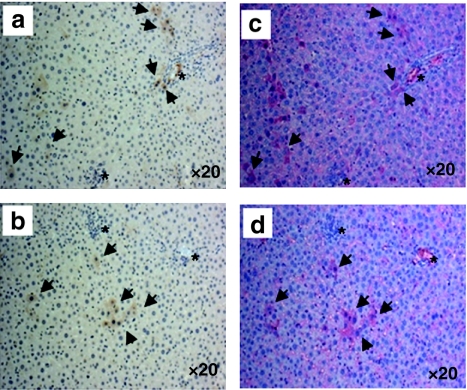
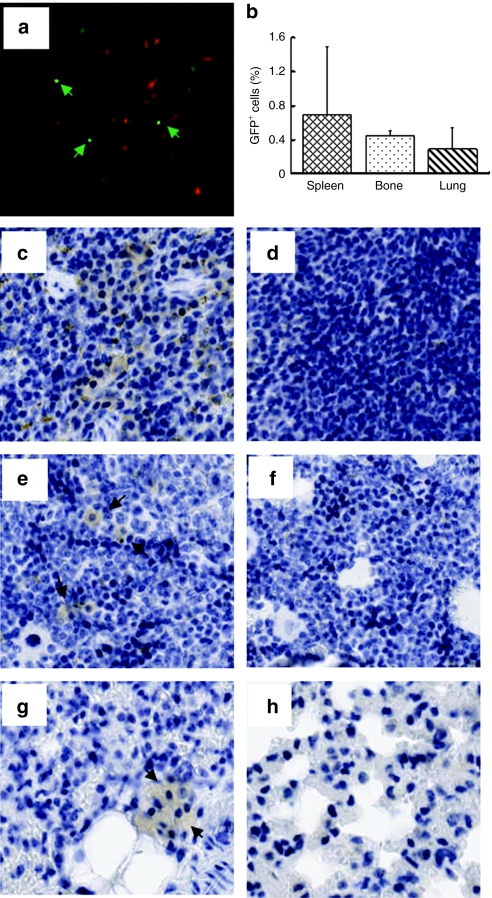
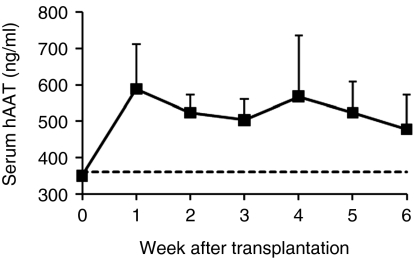
In order to further enhance transgene (hAAT) expression, we performed an additional experiment with a modified procedure. In this experiment, we transplanted 2 × 107 rAAV8-CB-hAAT-infected BM cells (from male C57BL/6) into recipients through intrasplenic injection, to avoid the possibility of portal hypertension from the increased number of cells. All mice were killed at 8 weeks after transplantation. As expected, Y-chromosome-positive cells were detected in the recipient liver demonstrating that donor cells could migrate into the recipient liver (Figure 3d–f). Immunostaining studies showed that most (>90%) of hAAT-positive donor cells were also positive for mouse albumin in the liver indicating that a majority of donor BM cells in the liver transdifferentiated or fused into hepatocytes (Figure 4, black arrow). To test the possibility that some BM cells could home to other organs and express the transgene, we have performed Y-FISH and immunostaining in spleen, BM, and lung. Both Y-FISH and GFP immunostaining showed that some donor cells were retained in the spleen (Figure 5a–c). GFP immunostaining also revealed some GFP+ cells in BM and lung indicating that intrasplenic injection of BM cells resulted in multiorgan homing of these cells (Figure 5b,e,g, black arrow). Importantly, sustained serum levels of hAAT were obtained in this experiment (Figure 6). These results imply that transplantation of rAAV8-transduced BM cells could represent a novel therapy for AAT gene deficiency.
Figure 4.
Hepatic differentiation of bone marrow (BM) cells. Coexpression of human α1-antitypsin (hAAT) and mouse albumin detected by immunostaining. (a,b) Liver section from C57BL/6 mouse transplanted with rAAV8-CB-hAAT-infected BM cells stained for hAAT (brown). (c,d) Liver section adjacent to the section in a and b, respectively, stained for albumin (red). Black arrows point to both AAT-positive and albumin-positive cells. Asterisks: location indicator. Representative slides were viewed at ×20 magnification.
Figure 5.
Multiorgan homing of transplanted bone marrow (BM) cells. (a) Spleen section subjected to fluorescence in situ hybridization for detecting Y chromosome. (b) Quantification of GFP+ cells by immunostaining. Representative images of immunostaining for GFP (brown) in spleen (c,d), in bone (e,f), and in lung (g,h). (a,c,e,g) Tissue sections were from female C57BL/6 mouse at 8 weeks after transplantation with rAAV8-CB-GFP vector–infected male BM cells. (d,f,h) Tissue sections from untransplanted C57BL/6 mouse served as negative control. Black arrowheads indicate the observed GFP staining. Representative slides were viewed at ×20 magnification. GFP, green fluorescent protein.
Figure 6.
Detection of expression of human α1-antitrypsin (hAAT) in the recipient serum. Bone marrow cells from C57BL/6 mice were infected with ssAAV8-CB-hAAT vector at 1 × 104 particles/cells for 2 hours and transplanted into the liver of partially hepatectomized C57BL/6 recipient (2 × 107 cells/mouse; n = 4) by splenic injection. The transgene expression was monitored by measuring the serum level of hAAT. Squares are the serum from the treatment group; dashes are the lower limit of quantification (LLOQ). The serum level of hAAT from untransplanted C57BL/6 mouse (negative control) was below the LLOQ.
Discussion
Adult stem cells offer a platform for ex vivo genetic manipulation followed by autologous transplantation. Adult stem cell–mediated gene delivery may overcome many limitations, including immune rejection of allogeneic cells, the ethical issue of embryonic stem cells, the shortage of donor organs, and nonspecific targeting (such as germline transduction). In addition, adult stem cell–mediated gene therapy can serve as a form of regenerative medicine to replace diseased cells with a patient's own stem cells carrying healthy gene(s). We have previously shown that liver stem (or progenitor) cell–mediated liver gene delivery of hAAT was feasible in a mouse model.18 However, liver stem cells cannot be used in humans for autologous transplantation. Considering clinical practices, we investigated the possibility of transplanting genetically modified BM cells. In the present study, we showed that rAAV8-CB-hAAT vector–transduced BM cells differentiated into hepatocytes and mediated sustained serum levels of hAAT in a mouse model. Although some donor cells might fuse to recipient hepatocytes, fused cells also contributed to the transgene expression.15,19 These results imply a novel therapy for treating AAT deficiency in humans.
In the present study, we showed that Lenti-CB-hAAT vector transduced BM cells efficiently in vitro. Interestingly, transplantation of these transduced cells resulted in undetectable levels of hAAT in the circulation, although some hAAT-positive cells were detected in the recipient mouse liver. There are several possible explanations. Studies conducted by Brown and colleagues showed that in vivo administration of lentiviral vector to mice triggered a type I interferon response.20 This innate immune response in turn triggered an adaptive response against the transgene product and also promoted vector clearance. Alternatively, transplantation of the BM cells overexpressing hAAT in immunocompetent mice might trigger an immune response eliminating most of the lentiviral vector–transduced cells. However, we did not observe this elimination in rAAV vector–transduced cells suggesting the elimination is related to the lentiviral vector. In addition, lentiviral vector integrates into the host genome randomly. It has been demonstrated that transgene expression is subjected to the negative influence of chromosomal sequences flanking the integration sites that may lead to transcriptional silencing.21,22,23 The interaction between the cis-acting elements of provirus and trans-factors of stem cells results in epigenetic modifications including DNA methylation and chromatin structure modulation, which may contribute to the lentiviral transgene silencing.21,24,25 Transcriptional silencing is most pronounced in stem cells. The trans-factors scan for foreign DNA and establish silencing in stem cells and maintain silencing in their progeny.25 Strategies for overcoming these limitations are under development including the addition of chromatin insulator elements to protect the transgene from negative positional effects or deletion/mutation of the retrovirus silencer elements.24,26,27,28
BM cells can be transduced by both rAAV1 and rAAV8 vectors in vitro. However, much higher levels of hAAT were detected in liver and serum from recipients that received rAAV8-CB-hAAT-infected BM cells than from recipients that received rAAV1-CB-hAAT-infected BM cells. It is possible that the transdifferentiation of BM cells into hepatocytes provides a favorable cellular environment for the intracellular processes of rAAV8 vector including cytoplasmic trafficking, uncoating, and nuclear entry. Recent studies have shown that mutations of capsid proteins can affect rAAV2 vector trafficking and enhance transgene expression, which supports the above hypothesis.29 Future studies will investigate the effect of stem cell transdifferentiation on rAAV vector intracellular processing.
BM cells contain hematopoietic stem cells and mesenchymal stem cells, and both have been shown to possess hepatic differentiation potential.13,30,31 Furthermore, considering the possible contribution of cellular interactions to stem cell proliferation and differentiation, the present study employed whole BM cells. After intrasplenic injection, donor cells were found not only in the liver, but also in spleen, lung, and BM. It was expected that some donor BM cells would be trapped in the injection site, spleen, whereas some cells would home back to the bone. The BM found migrating or homing to lung may be due to the pulmonary toxicity induced by MCT, a pyrrolizidine alkaloid plant toxin. MCT is bioactivated by cytochromes P450 in hepatocytes to its active compound MCT pyrrole resulting in both hepatic and pulmonary toxicity.32 It is possible that some donor cells could also migrate to other nonhepatic tissue and contribute to transgene expression.
The results of the present study suggest that the BM cell–based gene therapy approach is a promising therapy for genetic diseases. However, further improvement on transgene expression in the donor cells is required to achieve therapeutic levels (approximately >500 µg/ml). This issue could be addressed mainly from two aspects, transduction efficiency by viral vectors and the transplantation/engraftment efficiency of adult stem cells. The transduction efficiency may be improved according to recent advances in rAAV vector biology.29,33 Site-specific integration AAV vectors can establish long-term and persistent gene expression.34 Integration into the AAVS1 locus could defend against transgene silencing, a major obstacle of integration in viral vectors such as oncoretroviral vector.35 The efficiency of differentiation of BM-derived MSCs may be improved by modifying culture conditions to promote hepatic differentiation.30,31,36
In summary, we have shown that BM cells can be transduced by rAAV8 vector and that transplantation of these cells resulted in hepatic differentiation and transgene expression in the liver, and sustained levels of transgene product (hAAT protein) in the serum. Detailed studies to elucidate the mechanisms underlying interaction between viral vectors and adult stem cells, stem cell regulation on expression of a foreign gene, and migration and homing of stem cells would facilitate the application of BM cell–based gene therapy in the treatment of AAT deficiency.
Materials and Methods
Recombinant AAV vector construction and production. Recombinant single-stranded AAV vectors used in this study have been described previously.37,38 Briefly, plasmid AAV-CB-hAAT contained full-length AAV2 inverted terminal repeat sequences, hAAT cDNA flanked by two inverted terminal repeats and driven by cytomegalovirus enhancer/chicken β-actin (CB) promoter, intron, and poly (A) sequence. This vector plasmid was packaged into AAV serotype 1 and 8 capsids, respectively, as described previously.39 The physical particle titers of vector preparations are routinely assessed by quantitative dot blot analysis.
Lentiviral vector construction and production. In order to generate a lentiviral vector expressing hAAT (Lenti-CB-hAAT), hAAT cDNA fragment along with CB promoter was released from plasmid AAV-CB-hAAT and inserted into pTYF-linker vector that was derived from an LTR-modified recombinant HIV-1 plasmid.40 This lentiviral vector was packaged as previously described. Briefly, pNHP-helper (packaging helper construct), pHEF-VSV-G (envelop expression construct), and pLenti-CB-hAAT (transducing construct) were co-transfected into 293T cells using superfect reagent (Qiagen, Valencia, CA). The lentiviral vector was harvested 48 hours after transfection by collecting the cell culture medium and concentrated by centrifuging 2,500g at 4 °C for 20 minutes two times using the Amicon Ultra-15 centrifugal filter device (Millipore, Billerica, MA). The virus titer was estimated at 1 × 109 infectious units/ml.41
BM cell isolation. BM cells were isolated from the femurs and tibias of C57BL/6 male mice. The limb was sterilized by immersion in 70% ethanol, and the skin and muscles were removed. BM was exposed by cutting the ends of the bones, and extruded by inserting a 20-gauge needle attached to a 3 ml syringe and forcing 1–2 ml of DMEM (Mediatech, Manassas, VA) containing 2% fetal bovine serum (HyClone Laboratories, Logan, UT) through the bone shaft. To make a single cell suspension, BM was triturated by gently aspirating several times using the same needle and syringe and passed through a 70 µm nylon mesh strainer (Becton Dickinson Labware, Franklin Lakes, NJ). Cells were treated with red blood cell lysing buffer for 2 minutes at room temperature to deplete red blood cells and cultured for 1 hour at 37 °C to remove the macrophages that were attached to the bottom of the cell culture dish. The remaining cell suspension contained mesenchymal and hematopoietic stem cells, as well as blood progenitor cells and was ready for virus vector transduction. Transduction was performed in a 1 ml reaction volume of DMEM supplemented with 10% fetal bovine serum for 2 hours at 37 °C and 5% CO2. Cells were then washed three times with phosphate-buffered saline and resuspended in 100 µl saline for transplantation or cultured in murine myeloid long-term culture medium (MyeloCult M5300; StemCell Technologies, Vancouver, British Columbia, Canada) for in vitro transduction efficiency studies.
BM cell transplantation. Adult female C57BL/6 mice received MCT at 50 mg/kg body weight at a 2-week interval by i.p. injection for inhibiting endogenous liver cell proliferation.18 Two weeks after the second injection, the mice were partially hepatectomized (PHx) under general anesthesia to remove 70% of the liver. BM cells suspended in 100 µl saline were transplanted into the remaining liver immediately after PHx by portal vein injection as previously described.37 To transplant BM cells by intrasplenic injection, BM cells were injected into the inferior tip of the spleen of mice using a 30-gauge needle immediately after PHx. To aid in the coagulation process, the splenic injection site was ligated using sterile absorbable surgical suture (Ethicon, Somerville, NJ).
Immunohistochemistry for human AAT, GFP, and mouse albumin. Organ tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. For hAAT and GFP immunostaining, tissue sections (5 µm) were deparaffinized, rehydrated, and blocked for endogenous peroxidase with 3% hydrogen peroxide in methanol for 10 minutes. To detect hAAT expression, tissue sections were incubated with primary antibody, rabbit antihuman AAT (1:800; RDI/Fitzgerald Industries, Concord, MA), for overnight at 4 °C. Staining was detected using ABC-Rb-HRP and DAB kits (Vector Laboratories, Burlingame, CA). Antigen retrieval was performed in Digest-All (trypsin; Zymed Laboratories, Carlsbad, CA) for 5 minutes at 37 °C, followed by incubation in Trilogy (Cell Marque, Rocklin, CA) for 25 minutes at 95 °C. Immunostaining of GFP was performed using rabbit anti-GFP antibody (1: 10,000; Abcam, Cambridge, MA). Staining was detected using ABC-Rb-HRP and DAB kits (Vector Laboratories). Antigen retrieval was performed in Trilogy (Cell Marque) for 25 minutes at 95 °C. To detect albumin expression, antigen retrieval was performed using citrate retrieval for 30 minutes in a steamer. The tissues were incubated with goat anti-mouse albumin (1:5,000; Abcam) overnight at 4 °C, followed by incubation with biotinylated horse anti-goat (1:200; Vector Laboratories) for 30 minutes. Staining was developed using the Vectastain ABC-alkaline phosphatase kit (Vector Laboratories) and Vulcan Fast Red chromogen (Biocare Medical, Concord, CA). Immunofluorescence double staining for human AAT and GFP was performed as previously described with minor modification.18 Colocalization of hAAT and GFP was detected by staining sequentially with anti-GFP (1:500; Abcam) and anti-hAAT (1:100; RDI/Fitzgerald Industries). Secondary antibodies conjugated with FITC (1:1,000) or rhodamine (1:1,000) were applied. To quantify hAAT- or GFP-positive cells in the recipient livers, 10 nonoverlapping fields of view from each mouse were counted using Aperio ImageScope (Aperio Technologies, Vista, CA) and percentage of positive cells was calculated compared to total cells in the field.
Y-chromosome fluorescence in situ hybridization. Paraffin-embedded liver tissue sections (5 µm thick) were used for detecting Y chromosomes. Liver sections were deparaffinized 2 × 5 minutes in fresh xylene and rehydrated using a schedule of 2 × 2 minutes in 100% ethanol, 2 minutes in 95% ethanol, 1 minute in 70% ethanol, and 2 × 1 minutes in water. Deparaffinized liver sections were pretreated with 0.2 N HCl for 30 minutes at RT and retrieved in 1 mol/l NaSCN for 30 minutes at 85 °C. Sections were then digested in 4 mg/ml Pepsin (Sigma-Aldrich, St Louis, MO) diluted in 0.9% NaCl (pH 2.0) for 11–15 minutes at 37 °C. Digested sections were equilibrated 1 minute in 2× SSC and then dehydrated through graded alcohols. Sections were covered with Cy3- or FITC-labeled mouse Y-chromosome paint probe (Cambio, Cambridge, UK) and denatured at 65 °C for 10 minutes followed by hybridization at 37 °C overnight using a Hybrite oven (Vysis, Downers Grove, IL). FITC-labeled mouse X-chromosome paint probe was included in the hybridization solution applied to some slides. After hybridization, sections were washed three times in 50% formamide/2× SSC, followed by 2× SSC, and 4× SSC + 0.1% Igepal (NP-40) at 46 °C for 7 minutes. Slides were air-dried at RT in the dark and then mounted using glass coverslips and DAPI Vectorshield (Vector Laboratories).
Human AAT-specific ELISA. Microtiter plates were coated with 100 µl of goat anti-hAAT (1:200; Sigma Immunochemical, St Louis, MO) in Voller's buffer overnight at 4 °C. Blocking buffer, 3% bovine serum albumin, was added to saturate the remaining sites for protein binding on the microtiter plate and incubated 1 hour at 37 °C. After blocking, duplicated standard curve stocks (Sigma Immunochemical), and diluted sample serum or cell culture medium was loaded and incubated 1 hour at 37 °C. A second antibody, rabbit anti-hAAT (1:1,000; Roche Molecular Biochemicals, Indianapolis, IN), was added and reacted with captured hAAT at 37 °C for 1 hour. A third antibody, goat anti-rabbit IgG conjugated with peroxidase (1:80; Roche Molecular Biochemicals), was incubated at 37 °C for 1 hour. The plate was washed with phosphate-buffered saline-Tween 20 three times between reactions. After reacting with the substrate (o-phenylenediamine; Sigma Immunochemical), the microtiter plate was read at 490 nm on a MRX microplate reader (Dynex Technologies, Chantilly, VA).
Acknowledgments
This work was supported by grants from NIDDK (2-P01-DK05327-06), NHLBI (R21-HL079132), and Alpha-1 Foundation.
REFERENCES
- Luisetti M., and, Seersholm N. Alpha1-antitrypsin deficiency. 1: epidemiology of alpha1-antitrypsin deficiency. Thorax. 2004;59:164–169. doi: 10.1136/thorax.2003.006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMeo DL., and, Silverman EK. Alpha1-antitrypsin deficiency. 2: genetic aspects of alpha(1)-antitrypsin deficiency: phenotypes and genetic modifiers of emphysema risk. Thorax. 2004;59:259–264. doi: 10.1136/thx.2003.006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham M., and, Stockley RA. Alpha 1-antitrypsin deficiency. 3: Clinical manifestations and natural history. Thorax. 2004;59:441–445. doi: 10.1136/thx.2003.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller JK., and, Aboussouan LS. Alpha1-antitrypsin deficiency. 5: intravenous augmentation therapy: current understanding. Thorax. 2004;59:708–712. doi: 10.1136/thx.2003.006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C., and, Flotte TR. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci USA. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain JR, Schwarze U, Wang PR, Hirata RK, Hankenson KD, Pace JM, et al. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004;303:1198–1201. doi: 10.1126/science.1088757. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ., and, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- Parker AM., and, Katz AJ. Adipose-derived stem cells for the regeneration of damaged tissues. Expert Opin Biol Ther. 2006;6:567–578. doi: 10.1517/14712598.6.6.567. [DOI] [PubMed] [Google Scholar]
- Chamberlain JR, Deyle DR, Schwarze U, Wang P, Hirata RK, Li Y, et al. Gene targeting of mutant COL1A2 alleles in mesenchymal stem cells from individuals with osteogenesis imperfecta. Mol Ther. 2008;16:187–193. doi: 10.1038/sj.mt.6300339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- Oh SH, Witek RP, Bae SH, Zheng D, Jung Y, Piscaglia AC, et al. Bone marrow-derived hepatic oval cells differentiate into hepatocytes in 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Gastroenterology. 2007;132:1077–1087. doi: 10.1053/j.gastro.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Evans EP., and, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- Stender S, Murphy M, O'Brien T, Stengaard C, Ulrich-Vinther M, Søballe K, et al. 2007Adeno-associated viral vector transduction of human mesenchymal stem cells Eur Cell Mater 1393–9.discussion 99 [DOI] [PubMed] [Google Scholar]
- Song S, Witek RP, Lu Y, Choi YK, Zheng D, Jorgensen M, et al. Ex vivo transduced liver progenitor cells as a platform for gene therapy in mice. Hepatology. 2004;40:918–924. doi: 10.1002/hep.20404. [DOI] [PubMed] [Google Scholar]
- Daley GQ. Alchemy in the liver: fact or fusion. Nat Med. 2004;10:671–672. doi: 10.1038/nm0704-671. [DOI] [PubMed] [Google Scholar]
- Brown BD, Sitia G, Annoni A, Hauben E, Sergi LS, Zingale A, et al. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood. 2007;109:2797–2805. doi: 10.1182/blood-2006-10-049312. [DOI] [PubMed] [Google Scholar]
- Ramezani A, Hawley TS., and, Hawley RG. Performance- and safety-enhanced lentiviral vectors containing the human interferon-beta scaffold attachment region and the chicken beta-globin insulator. Blood. 2003;101:4717–4724. doi: 10.1182/blood-2002-09-2991. [DOI] [PubMed] [Google Scholar]
- Persons DA, Hargrove PW, Allay ER, Hanawa H., and, Nienhuis AW. The degree of phenotypic correction of murine beta -thalassemia intermedia following lentiviral-mediated transfer of a human gamma-globin gene is influenced by chromosomal position effects and vector copy number. Blood. 2003;101:2175–2183. doi: 10.1182/blood-2002-07-2211. [DOI] [PubMed] [Google Scholar]
- Pawliuk R, Westerman KA, Fabry ME, Payen E, Tighe R, Bouhassira EE, et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294:2368–2371. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- Hino S, Fan J, Taguwa S, Akasaka K., and, Matsuoka M. Sea urchin insulator protects lentiviral vector from silencing by maintaining active chromatin structure. Gene Ther. 2004;11:819–828. doi: 10.1038/sj.gt.3302227. [DOI] [PubMed] [Google Scholar]
- Pannell D., and, Ellis J. Silencing of gene expression: implications for design of retrovirus vectors. Rev Med Virol. 2001;11:205–217. doi: 10.1002/rmv.316. [DOI] [PubMed] [Google Scholar]
- Haas DL, Lutzko C, Logan AC, Cho GJ, Skelton D, Jin Yu X, et al. The Moloney murine leukemia virus repressor binding site represses expression in murine and human hematopoietic stem cells. J Virol. 2003;77:9439–9450. doi: 10.1128/JVI.77.17.9439-9450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa H, Yamamoto M, Zhao H, Shimada T., and, Persons DA. Optimized lentiviral vector design improves titer and transgene expression of vectors containing the chicken beta-globin locus HS4 insulator element. Mol Ther. 2009;17:667–674. doi: 10.1038/mt.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barklis E, Mulligan RC., and, Jaenisch R. Chromosomal position or virus mutation permits retrovirus expression in embryonal carcinoma cells. Cell. 1986;47:391–399. doi: 10.1016/0092-8674(86)90596-9. [DOI] [PubMed] [Google Scholar]
- Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurich I, Mueller LP, Aurich H, Luetzkendorf J, Tisljar K, Dollinger MM, et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007;56:405–415. doi: 10.1136/gut.2005.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- Copple BL, Banes A, Ganey PE., and, Roth RA. Endothelial cell injury and fibrin deposition in rat liver after monocrotaline exposure. Toxicol Sci. 2002;65:309–318. doi: 10.1093/toxsci/65.2.309. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma HI, Li J, Sun L, Zhang J., and, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- Zhang C, Cortez NG., and, Berns KI. Characterization of a bipartite recombinant adeno-associated viral vector for site-specific integration. Hum Gene Ther. 2007;18:787–797. doi: 10.1089/hum.2007.056. [DOI] [PubMed] [Google Scholar]
- Smith JR, Maguire S, Davis LA, Alexander M, Yang F, Chandran S, et al. Robust, persistent transgene expression in human embryonic stem cells is achieved with AAVS1-targeted integration. Stem Cells. 2008;26:496–504. doi: 10.1634/stemcells.2007-0039. [DOI] [PubMed] [Google Scholar]
- Ishii K, Yoshida Y, Akechi Y, Sakabe T, Nishio R, Ikeda R, et al. Hepatic differentiation of human bone marrow-derived mesenchymal stem cells by tetracycline-regulated hepatocyte nuclear factor 3beta. Hepatology. 2008;48:597–606. doi: 10.1002/hep.22362. [DOI] [PubMed] [Google Scholar]
- Song S, Embury J, Laipis PJ, Berns KI, Crawford JM., and, Flotte TR. Stable therapeutic serum levels of human alpha-1 antitrypsin (AAT) after portal vein injection of recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 2001;8:1299–1306. doi: 10.1038/sj.gt.3301422. [DOI] [PubMed] [Google Scholar]
- Xu L, Daly T, Gao C, Flotte TR, Song S, Byrne BJ, et al. CMV-beta-actin promoter directs higher expression from an adeno-associated viral vector in the liver than the cytomegalovirus or elongation factor 1 alpha promoter and results in therapeutic levels of human factor X in mice. Hum Gene Ther. 2001;12:563–573. doi: 10.1089/104303401300042500. [DOI] [PubMed] [Google Scholar]
- Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ., and, Walsh CE. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- Iwakuma T, Cui Y., and, Chang LJ. Self-inactivating lentiviral vectors with U3 and U5 modifications. Virology. 1999;261:120–132. doi: 10.1006/viro.1999.9850. [DOI] [PubMed] [Google Scholar]
- Lung-Ji Chang A-KZ.2002Lentiviral vectors preparation and use. In: Jeffery, RM (ed.).Gene Therapy Protocols, 2nd edn., vol. 69. Humana: Totowa, NJ. pp. 303–318 [Google Scholar]