Abstract
Previous reports have shown that B-cell-mediated gene therapy can induce tolerance in several animal models for autoimmune diseases and inhibitory antibody formation in hemophilia A mice. We know from our previous work that the induction of tolerance following B-cell therapy is dependent upon CD25+ regulatory T cells (Tregs). To extend these studies and identify the effects of this gene therapy protocol on the target CD4 T cells, we have adapted in vitro suppression assays using Tregs isolated from treated and control mice. Using carboxyfluorescein succinimidyl ester (CFSE) dilution as a measure of T-cell responsiveness to FVIII, we show that CD25+ Tregs from treated mice are more suppressive than those from control animals. To monitor the induction of antigen-specific Tregs, we repeated these studies in ovalbumin (OVA) peptide-specific DO11.10 T-cell receptor (TCR) transgenic mice. Tregs from DO11.10 mice treated with a tolerogenic OVA–Ig construct are better than polyclonal Tregs at suppressing the proliferation of responder cells stimulated with OVA peptide 323–339 (pOVA). Furthermore, we show that following B-cell therapy, there is an increase in antigen-specific FoxP3+ Tregs, and there is also a distinct decrease in antigen-specific CD4+ effector T cells. These changes in the lymphocyte population shift the balance away from effector function toward a tolerogenic phenotype.
Introduction
Induction of antigen-specific immune tolerance is a major goal to control adverse immune responses in autoimmune diseases, transplantation, gene therapy, as well as inhibitory responses to therapeutic proteins.1 As an important step to achieve tolerance induction, we developed a gene therapy protocol using a unique antigen–Ig construct that is delivered to syngeneic B cells via retroviral transduction.2,3 This strategy was based on the observations that B cells are tolerogenic antigen-presenting cells (APCs) and IgG is a known tolerogenic carrier.4,5,6,7 Adoptive transfer of these transduced B cells has been shown to induce tolerance in a number of animal models for diseases including type 1 diabetes,8,9,10 multiple sclerosis,8,11 rheumatoid arthritis,12 posterior uveitis13,14 and inhibitor formation in hemophilia A.15
Hemophilia is not an autoimmune disease per se, but shares the characteristics of a predictable and debilitating immune response to a known antigen and it represents an important clinical burden. Hemophilia A affects one in 5,000 males and up to one-third of those being treated will eventually produce blocking antibodies known as inhibitors, to therapeutic FVIII.16,17,18 Patients who form inhibitors will suffer from increased morbidity and premature mortality if they do not respond to the available tolerance induction techniques.19,20,21 In animal models for this disease, it has been reported that treatment will be effective when tolerance is induced to the immunodominant A2 and C2 domains of FVIII.22,23,24 Even though active FVIII has five domains total (A1, A2, A3, C1, C2), tolerance induction to these two sites was sufficient to reduce or completely abrogate B- and T-cell responses to FVIII in naive and even in primed mice.15 Additionally, an anti-CD25 antibody (PC61), which has been shown to deplete and/or inhibit the function of regulatory T cells25 (Tregs), was able to prevent tolerance initiation.15 These data are in agreement with recent results from several laboratories invoking a role for Tregs in tolerance to FVIII.26,27,28 Therefore, these observations led to the hypothesis that when tolerogenic B cells are introduced to a host, they drive an increased concentration of antigen-specific (Ag-specific) FoxP3+ Tregs to actively diminish the response to immunization and subsequent challenge with FVIII.
Recent advances in our understanding of Tregs in the mouse have allowed for study of this T-cell subset during tolerance induction based on their expression of the FoxP3 transcription factor.29,30,31 In particular, the creation of mice expressing a FoxP3-GFP fusion-protein reporter facilitates the study of cells expressing this important transcription factor.30 Although FoxP3+ cells represent a fraction of total T cells, the GFP reporter allows for characterization and sorting of viable cells using flow cytometry. Herein, we demonstrate that following the introduction of tolerance, Ag-specific Tregs are induced and are more suppressive than nTregs (natural Tregs). Moreover, we show that this treatment also leads to a loss of Ag-specific effector T cells.
Results
Effects of Tregs in hemophilic mice carrying the FoxP3-GFP fusion following tolerance induction
Based on the requirement for CD25+ Tregs in both the induction and maintenance of tolerance induced by B-cell gene therapy10,15,32 we proposed that our tolerance protocol would yield a significant increase in Treg levels. To facilitate analysis of Tregs in the tolerance system, we first crossed the mouse that was transgenic for the FoxP3-GFP insertion30 against the hemophilic mouse with a targeted deletion in exon 16 (ref. 33) of the FVIII gene to obtain the appropriate crossover in the X chromosome (see Methods and Supplementary Figure S1). These mice were then backcrossed to the E16 hemophilia A mouse line for 10 generations and maintained by sib-mating. In these mice, FoxP3 is still functionally active although it carries a GFP fusion.30
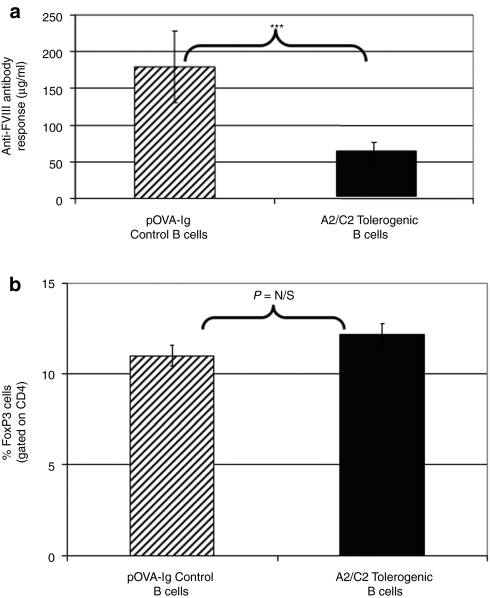
Using the newly derived mouse line, it was necessary to reproduce the established tolerance induction protocol. Following tolerogenic B-cell gene therapy with FVIII domains, reduced antibody (Figure 1a) and T-cell responses (data not shown) to FVIII were noted in syngeneic FoxP3-GFP/FVIII−/− mice consistent with previous reports in FVIII−/− mice.15 Spleen cells from treated and control mice were analyzed at numerous time points between 3 and 30 days after gene therapy. However, there was no significant increase in the percentage of Tregs in the specific treatment group compared to recipients of an unrelated antigen–Ig fusion–transduced B cells at any time point (Figure 1b). This is not surprising as the numbers of antigen-specific (in this case FVIII-domain specific) T cells in a polyclonal population is quite small and, at present, no known clonotypic anti-TCR antibody or tetramers are available to isolate and track these cells.
Figure 1.
Effect of tolerogenic B-cell gene therapy on responsiveness to FVIII and FoxP3 T-cell levels. Hemophilic mice (n = 8/group) were treated with 107 B cells that were transduced with C2–Ig and A2–Ig (tolerogenic) or with pOVA–Ig (control). After 10 days, mice received the first of 5 weekly injections of 1 µg FVIII intraperitoneally. Ten days after the last injection, mice were bled, euthanized, and their spleens harvested. (a) Serum samples were tested via a standard anti-FVIII enzyme-linked immunosorbent assay. As indicated, this treatment yields a significantly lower immune response in A2–Ig and C2–Ig B-cell-treated mice (***P value = 0.001). (b) Utilizing the GFP marker for T regulatory cells (Tregs), we were also able to track and quantify regulatory T cells in the spleen. The group that received the tolerogenic treatment had an increased percentage of Tregs on day 10, but this result was not significant. This is not surprising as the numbers of FVIII-specific T cells in a polyclonal population is quite small and without the necessary clonotypic tetramers, significant changes in their levels were not identified. pOVA, ovalbumin peptide.
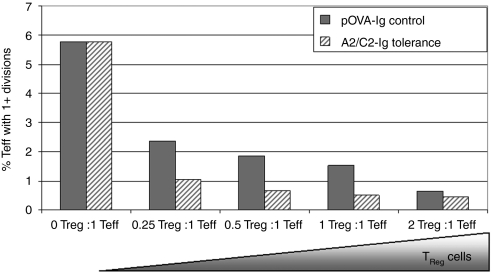
However, one can analyze the suppressive potential of Tregs through in vitro assays. Figure 2 shows data from a modified suppression assay demonstrating the ability of Tregs to suppress effector T-cell responses. In this assay, carboxyfluorescein succinimidyl ester (CFSE)–labeled effector cells from an FVIII-immunized animal were mixed with putative (CD4+CD25+) purified Tregs at different ratios, an optimal amount of FVIII (1 µg/ml) and APCs were also added. After 72 hours in culture, the cells were analyzed by flow cytometry for CFSE dilution. If functional Tregs were induced, then effector cells incubated with Tregs should divide less frequently than those incubated in the absence of Tregs. As shown in Figure 2, although the overall frequency of responder T cell is low, suppression of their proliferation was observed when the ratio of Tregs to T effector cells was as low as 1:4 (0.25:1), and suppression increased as the ratio increased. Notably, at all ratios, Tregs from mice that received tolerance induction were significantly more suppressive than Tregs from control mice.
Figure 2.
Suppression of FVIII-induced T-cell proliferation by CD25+ Tregs from FVIII-tolerized mice compared to control. Hemophilic mice (n = 8/group) received FVIII tolerogenic treatment, C2–Ig- and A2–Ig-transduced B cells, or control treatment, pOVA–Ig. Following tolerance induction, splenocytes from each group were pooled and Tregs were isolated. These cells were added at increasing ratios to effector cells stimulated with an optimal dose of FVIII in a standard suppression assay. To prepare FVIII responsive T cells (Teff), mice were immunized with FVIII/CFA. At 10–14 days following immunization, lymphocytes were harvested and stained with carboxyfluorescein succinimidyl ester (CFSE) (1 µmol/l). Cells were activated with a previously determined optimal dose of FVIII, 0.1 µg/ml, and their divisions were estimated by fluorescence-activated cell sorting analysis of CFSE dilution. A maximum of nearly 6% of T cells were FVIII-responsive and entered cycle when stimulated. As more Tregs were added, the Teff divided less frequently. In this assay, Tregs from mice treated with tolerogenic B cells were more suppressive than Tregs from control-treated mice at each ratio tested. For example, at the ratio with four times the number of effector T cells as Tregs, only 1.04% of the Teff divided at least once when mice had received tolerogenic treatment. In the control, 2.37% of the Teff divided at least once. Overall, this trend was significant with a P value of 0.05. As splenocytes were pooled for this assay, error bars are not shown. pOVA, ovalbumin peptide; Teff, T effector cells; Treg, T regulatory cells.
Kinetics of FoxP3 induction following “tolerance” induction in DO11.10 mice
To examine the effect of our gene therapy protocol further, we utilized the DO11.10 T-cell receptor (TCR) transgenic mice. DO11.10 mice express a TCR specific for the ovalbumin (OVA) peptide 323–339 (pOVA) which could be labeled with the clonotypic, anti-TCR antibody, KJ1.26. Using these mice, we were able to monitor changes in Ag-specific populations of cells. Both DO11.10 and DO11.10/Rag2−/− (which lack endogenously rearranged TCR) mice were crossed to the FoxP3-GFP line that we backcrossed to a Balb/C (H-2d) background. Supplementary Figure S2 illustrates that DO11.10/FoxP3-GFP mice have 75–80% KJ1.26+ T cells, of which about 5% are FoxP3+. Approximately 15% (2.4/16) of the KJ1.26− cells are FoxP3+, presumably representing the endogenous nTreg pool; these values are similar to those of the Balb/C. In contrast, virtually all of the T cells in the DO11.10/Rag2−/− line were KJ1.26+ and none of those were FoxP3+.
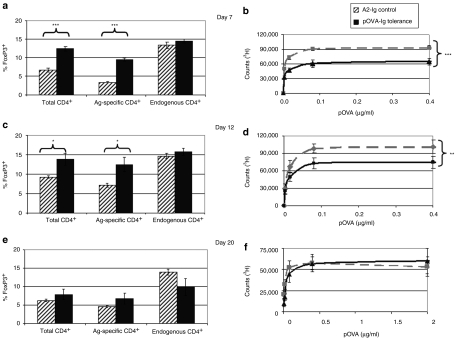
DO11.10 mice were treated with pOVA–Ig-transduced B cells (tolerogenic B cells) or with FVIII A2–Ig (control)-transduced B cells. On day 3, no changes in the numbers of FoxP3+ cells were detected between experimental and control groups (data not shown). However, as shown in Figure 3a, by day 7 there was a significant increase in the percent of CD4+ cells that are FoxP3+. At this time, the tolerogenic effect of OVA–Ig B-cell gene therapy could also be detected by T-cell proliferation (Figure 3b). The latter effect is partial but nonetheless is noteworthy considering that the frequency of antigen-specific T cells is 3–4 orders of magnitude greater than in a polyclonal T-cell population.
Figure 3.
Effect of tolerance induction to pOVA on FoxP3 levels and T-cell proliferation in DO11.10 mice. DO11.10 mice (n = 6/group) were treated with 107 pOVA–Ig-transduced tolerogenic B cells or 107 A2–Ig-transduced B cells as control. After B-cell treatment, mice were assayed at (a,b) 7 days, (c,d) 12 days (e,f) and 20 days. In this system, the effects of B-cell treatment can be monitored by the percent of cells that express FoxP3 and by in vitro T-cell responses to pOVA at numerous concentrations. In these mice, ~80% of CD4+ cells express the antigen-specific T-cell receptor and the remainder express endogenously recombined TCRs. On day 7, there was a significant change in the percent of total CD4+ cells that express FoxP3. (a,c) By comparing the antigen-specific cells to those with endogenous TCRs, it was clear that the expansion is limited to the endogenous population. (b,d) Furthermore, T-cell proliferative responses were blunted in response to treatment. This effect was also seen on day 12, but by the 20th day following treatment, the effect was lost. (*P < 0.05, **P < 0.01, ***P < 0.001). pOVA, ovalbumin peptide.
The effect of tolerogenic B cells in terms of increase in FoxP3+ cells and decreased T-cell proliferation was also be detected at 12 days following treatment, but by day 20 the effect was lost (Figure 3c–f). The time course of FoxP3 induction and return toward baseline has been reproducible in >10 experiments. Notably, in these experiments, the significant increase in CD4+FoxP3+ cells was only found in the KJ1.26+ Ag-specific group. This provides an explanation for the exquisite specificity of this mode of tolerance induction. Antigen–Ig-transduced B cells only affect antigen-specific Tregs, which are responsible for the Ag-specific tolerance induction. The difference in responses over time also demonstrates the maximal tolerance induction would occur 1–2 weeks following tolerance induction.
Kinetics of FoxP3 induction following “tolerance” induction in DO11.10/Rag2−/− mice
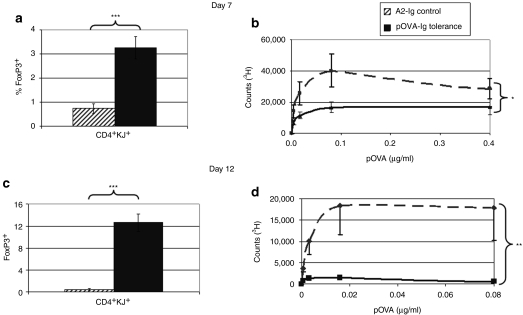
In the DO11.10 mouse, it was clear that the ratio of antigen-specific FoxP3+ to FoxP3− was increasing in response to transduced B-cell treatment. However, it was not possible to determine whether this was from an expansion of nTregs, or from the induction of FoxP3 in responder (effector) T cells. To answer this question, FoxP3-GFP/D011.10/Rag2−/− mice, which have no nTregs, were generated and treated with B cells transduced with OVA–Ig or control A2–Ig. As shown in Figure 4, 7 days following treatment, there was a significant increase in the frequency of FoxP3+KJ1.26+ T cells, and this increase was correlated with decreased T-cell proliferative responses. The peak response was seen on day 12 at which time nearly 13% of all CD4 T cells expressed FoxP3 and tolerance in terms of the T-cell proliferative response was almost complete. This represents an entirely induced (adaptive), clonotypic Treg population.
Figure 4.
Effect of tolerance induction to OVA on FoxP3 and T-cell proliferation in DO11.10/Rag2−/− mice. DO11.10/Rag2−/− mice (n = 5/group) were treated with 107 pOVA–Ig-transduced tolerogenic B cells or 107 A2–Ig-transduced B cells as control. After B-cell treatment, mice were followed for (a,b) 7 days and (c,d) 12 days. In this system, the effects of B-cell treatment can be monitored by the percent of cells that express FoxP3 and by in vitro T-cell responses to pOVA at numerous concentrations. In these mice, all of the CD4+ T cells express the T-cell receptor (TCR) transgene and none of the cells form endogenously recombined TCRs. On day 7, there was a clear induction of FoxP3 in treated mice from below baseline to >3%. Furthermore, T-cell recall responses were blunted in response to treatment. This effect is further amplified on day 12 with a completely diminished T-cell recall response to pOVA (*P < 0.05, ***P < 0.001). These data were reproducible in five experiments. pOVA, ovalbumin peptide.
Function of different Tregs following tolerogenic B-cell treatment
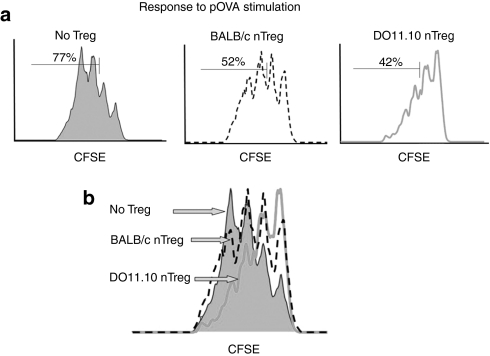
From the above experiments, it was clear that tolerogenic, antigen–Ig-transduced B cells can induce FoxP3 in antigen-specific Tregs. Also, the T-cell responses in these TCR transgenic mice were suppressed. To further demonstrate the role of Ag-specific Tregs, a series of suppression assays were performed. Initially, we asked whether nTregs from polyclonal populations of naive BALB/c mice or from naive DO11.10 mice (with primarily OVA-specific Tregs) had comparable activity. Responder (effector) CD4+FoxP3− cells were isolated from DO11.10/Rag2−/− mice and were stimulated with pOVA antigen (0.02 µg/ml). In this experiment, 77% of responders have gone into cycle in the absence of Tregs (Figure 5a, left). When Tregs have been added at a 1:1 ratio, 52% of the cells divided in the presence of polyclonal Balb/C Tregs (middle) and 42% of responders divided in the presence of TCR transgenic OVA-specific Tregs (right). Thus, both subsets of Tregs are suppressive, but the antigen-specific Tregs exert a more suppressive effect than polyclonal Tregs (Figure 5b). This is graphically represented by a curve shift to the right. This assay was repeated using T-cell activating anti-CD3/anti-CD28 beads. In this system, the specificity of Tregs should be irrelevant because the beads give the proper stimulatory signals regardless of antigen specificity. Indeed, under these conditions, nTregs from BALB/c or DO11.10 mice suppress equally (data not shown).
Figure 5.
Suppression of DO11.10 proliferation to OVA by nTregs. To determine the role of Treg antigen specificity, polyclonal BALB/c Tregs were compared to OVA-specific DO11.10 Tregs. (a) pOVA antigen (0.02 µg/ml) was used to stimulate division in CFSE-labeled DO11.10/Rag2−/− responder cells. In the absence of Tregs, 77% of the responder cells will divide two or more times in 72 hours. Polyclonal nTregs were purified from three wild-type BALB/c mice and added at a 1:1 ratio to the CFSE-labeled responder cells. The cells are suppressive and only 52% of the responder cells divide two or more times in 72 hours. OVA-specific DO11.10 Tregs were purified from three DO11.10 mice and added at a 1:1 ratio to the CFSE-labeled responder cells. These cells are more suppressive than the BALB/c Tregs and only 42% of the CFSE-labeled responder cells divide two or more times in 72 hours. (b) An overlay of the three conditions described demonstrates that the addition of Tregs to the incubation reduces the number of responder cells that respond to antigen, and antigen-specific Tregs shift the division curve further to the right. That is, antigen-specific Tregs are more suppressive than nonspecific Tregs. CFSE, carboxyfluorescein succinimidyl ester; pOVA, ovalbumin peptide; Tregs, T regulatory cells.
Finally, to examine the properties of the Tregs isolated from recipients of tolerogenic or control B cells, CD25+ cells from pOVA–Ig treatment and A2–Ig control mice were next used in a suppression assay. Tregs from the pOVA–Ig B-cell-treated DO11.10/Rag2−/− mice represent an entirely induced, antigen-specific population, whereas Tregs from A2–Ig control-treated DO11.10 mice represent a noninduced Treg population with an approximate ratio of 3:2 Ag-specific to endogenous cells. Tregs from pOVA–Ig-treated DO11.10 mice (80% KJ1.26+) should fall in between those two extremes.
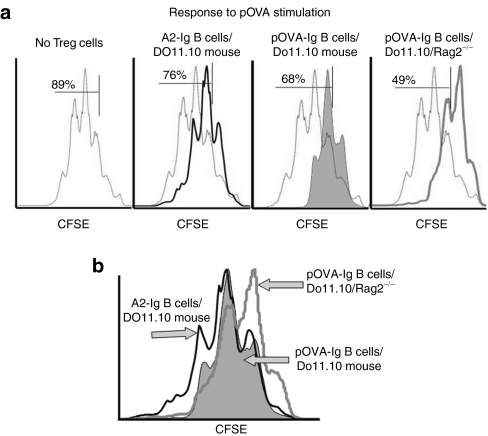
The results in Figure 6a demonstrate that although Tregs from each treatment group were suppressive, Tregs from pOVA–Ig B-cell-treated DO11.10/Rag2−/− mice (which have the highest number of Ag-specific cells) were the most suppressive. Thus, 89% of CD4+ responder cells divided at least once, whereas this was reduced to 76% in the presence of Tregs from A2–Ig-treated DO11.10 control mice. Tregs from pOVA–Ig-treated DO11.10 mice reduce proliferation to 68% whereas Tregs from pOVA–Ig-treated DO11.10/Rag2−/− mice further reduce proliferation to 49%. This is most clearly observed with the histograms overlaid (Figure 6b). There was a progressively enhanced suppression as a higher percentage of Tregs were antigen-specific. Again, this is graphically represented as a shift to the right.
Figure 6.
Suppression of DO11.10 proliferation to OVA by antigen-specific induced and noninduced Tregs. To identify differences between natural and induced antigen-specific cells, Tregs from treated and control T-cell receptor (TCR) transgenic mice were compared. (a) pOVA antigen (0.02 µg/ml) was used to stimulate division in CFSE-labeled DO11.10/Rag2−/− responder cells. When present, Tregs from were added to CFSE-labeled responder cells at a 1:1 ratio. In the absence of Tregs, 89% of responder cells had divided at least once after 72 hours of incubation. Using Tregs from DO11.10 mice treated with A2–Ig-transduced control B cells (n = 5), 76% of responder cells had divided. When Tregs from DO11.10 mice (n = 5) treated with tolerogenic pOVA–Ig-transduced B cells were used, 68% had divided. When Tregs from tolerogenic pOVA–Ig transduced B cells treated DO11.10 Rag2−/− mice (n = 6) were used, 49% of cells had divided. (b) An overlay of the histograms clearly demonstrates that the CFSE curve from the Rag2−/− Tregs is shifted more to the right. The Tregs from these mice must be induced and are entirely antigen specific. CFSE, carboxyfluorescein succinimidyl ester; pOVA, ovalbumin peptide; Tregs, T regulatory cells.
Mechanisms of Treg-dependent suppression
Using the Rag2−/− mouse, we concluded that treatment with tolerogenic B cells leads to the induction of Ag-specific adaptive Tregs. We further queried whether in addition to changes in Tregs, are there measurable changes to effector cells in the spleen? There are three scenarios that could account for an increase in the percent of Ag-specific Tregs with or without a change in the percentage of CD4+ effector T cells. If the increase of Tregs is limited to induction of FoxP3 in antigen-specific responder cells without expansion, then the total number of Ag-specific cells (FoxP3+ and FoxP3−) in the spleen would not be changed. If, however, there is an induction of FoxP3 and an expansion of either nTregs or induced Tregs, then the percent of FoxP3+ cells and the total number of Ag-specific cells in the spleen will both increase. Finally, the induction of Tregs could be seen in conjunction with a decrease in the total number of antigen-specific FoxP3− effector T cells. If this happened, the increase in percent of antigen-specific Tregs would be further amplified.
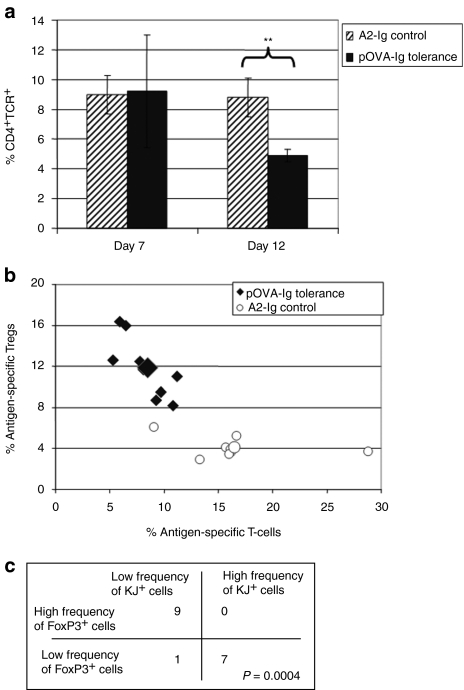
In Figure 7a, we show that when the data from Figure 4 (pOVA–Ig- versus A2–Ig-transduced B cells transferred to DO11.10/Rag2−/− mice) were reanalyzed in terms of the percent of total splenocytes that were CD4+KJ+, it became clear that effector T cells were decreasing as a percent of total splenocytes by day 12. Importantly, this treatment not only leads to an increase of these highly suppressive cells, but also leads to a decrease of OVA-specific responder cells.
Figure 7.
OVA-specific tolerance also results in a loss of effector T cells. (a) DO11.10/Rag2−/− mice (n = 6 per group) were treated with 107 pOVA–Ig-transduced B cells or 107 A2–Ig-transduced B cells as control. The percentage of total splenocytes that were CD4+KJ1.26+ were plotted for each of the groups on day 7 and 12. On day 12, there was a significant decrease in the percent of splenocytes that were antigen-specific T cells in the treatment group. The values in the control group were unchanged. (b) To confirm that OVA-specific tolerance also results in the loss of antigen-specific T cells in addition to the induction of Tregs, the experiment was repeated in a large group (n = 17) of DO11.10 mice. The data have been graphed to compare the percent of antigen-specific T cells (CD4+KJ+ gated on live cells) to the percent of antigen-specific Tregs (FoxP3+ gated on CD4+KJ+ live cells). In this graph, the values from mice that received tolerogenic B cells have moved to the upper left of this figure (the larger symbol represents the group average). This indicates that two changes occur in the spleens of treated mice: (i) an increase in the percent of CD4+KJ+ cells that express FoxP3+ (an increase on the y axis) and (ii) a decrease in the percent of splenocytes that are CD4+KJ+ (a decrease on the x axis). (c) To statistically interpret this observation, a Fisher's exact test has been applied to the data. This table identifies how many data points fell into the indicated categories for high and low frequency KJ+ cells and high and low frequency FoxP3+ cells. For example, all nine mice in the pOVA–Ig-treated group had a high frequency of FoxP3+ cells and a low frequency of KJ+ cells. Seven of the eight mice in the control group had a low frequency of FoxP3+ cells and a high frequency of KJ+ cells. The P value for this analysis is highly significant (P = 0.0004) indicating that these two variables are contingent on each other in this system, and the means are indicated by the larger symbols. pOVA, ovalbumin peptide; TCR, T-cell receptor; Tregs, T regulatory cells.
To further examine this hypothesis, a large cohort of DO11.0 recombinase-sufficient mice were treated with either pOVA–Ig tolerogenic B cells or A2–Ig control B cells to allow for statistical interpretation of the theoretical analysis described above. A plot of the experimental data (Figure 7b) clearly demonstrates that in addition to induction of FoxP3 there is an overall decrease in the percent of splenocytes that are CD4+KJ+. In this graph, the A2–Ig control group represents the baseline and is unchanged. The values for the pOVA–Ig-treated group are shifted upward and to the left. This change can be due to destruction of CD4+KJ+FoxP3− cells or effective elimination if they are somehow excluded from secondary lymphoid tissues. To calculate the statistical significance of our results, we applied a χ2 analysis (Fisher's exact test). The data points were placed into one of four categories depending on whether or not the percent of FoxP3 cells is high or low and whether or not the percent of CD4 T cells in the spleen was high or low. Figure 7c illustrates how many data points fell into each quadrant (P value = 0.0004). This indicates that the two mechanisms are highly contingent on each other. The loss of total Ag-specific effector T cells in the context of an increase in Ag-specific Tregs provides an additional mechanism to our understanding of tolerance induction using retrovirally transduced B cells.
Discussion
Treatment with antigen–Ig-transduced B cells induces tolerance to the antigen by inducing Ag-specific Tregs and depleting Ag-specific effector T cells
Gene therapy for the induction of tolerance and the treatment of hemophilia A and B offers great promise.32,34,35,36 B-cell-delivered gene therapy has been shown to induce tolerance to FVIII in both naive and primed mice for several months following treatment15 as has been observed in our previous work with autoimmune models.14 Herein, we have described two animal models that provide insight into tolerance induction by B-cell-delivered fusion Ig gene therapy and the effect it has on both regulatory and effector T cells. Initially, we sought to reproduce the initial observation that tolerance to FVIII can be induced in hemophilic mice. We treated hemophilic mice with tolerogenic B cells expressing FVIII A2 and C2 domains fused to an IgG heavy-chain scaffold and showed that treated mice have reduced antibody and T-cell responses compared to controls. Using FoxP3-GFP knock-in mice backcrossed against hemophilia A mice, we asked whether an increase in FoxP3+ cells could be detected in tolerized mice. Although there was no significant increase in FoxP3 Tregs in a polyclonal T-cell population, we nonetheless could show Treg function in CFSE dilution assays. Thus, the experiments in the hemophilia model have shown that in a physiological system the effect of tolerance can be monitored through Treg assays. These data are consistent with observations made by Herzog group showing that induced Tregs maintain tolerance following adeno-associated virus–mediated hepatic gene transfer.37
Recently, others have demonstrated the importance of Tregs in maintaining tolerance in mice with hemophilia. Miao et al. have pioneered a transgenic model of hemophilic mice with a majority of CD4+ T cells expressing FoxP3. These mice do not form inhibitory immune responses when treated with FVIII replacement gene therapy as opposed to typical hemophilic mice. They used this model to provide direct evidence that antigen-specific Tregs play an important role in modulating immune responses in vivo. They also identified the timeframe of 1–2 weeks as critical for adoptive transfer of antigen-specific Tregs for maintaining long-term tolerance.27 Two other reports have also been published recently to strengthen the hypothesis that antigen-specific Tregs are fundamental to tolerance induction in hemophilia.26,28
To overcome the low precursor frequency of antigen-specific T cells in the hemophilia A mouse and lack of tetramers for anti-FVIII T cells, we adopted the DO11.10 TCR transgenic mouse system for our gene therapy protocol. We found that in the DO11.10 mouse, on day 7 following treatment, there was an increase in Ag-specific Tregs and a significant decrease in T-cell responses. Furthermore, in the DO11.10/Rag2−/− mouse, which contains no nTregs, there were significant levels of induced Tregs found on day 12 with the corresponding decrease in T-cell responsiveness. With these mice, we clearly identified that FoxP3+ Tregs could be induced and we could identify a selective loss in antigen-specific effector cells. We believe that this effect is long-term, though the most significant changes in this subset of T-cell populations occur within 2 weeks. Whether FoxP3 expression is unstable in this population is not yet clear.
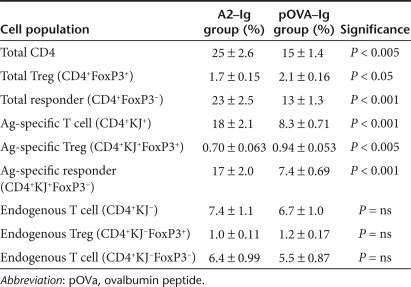
Table 1 summarizes the different T-cell populations in the DO11.10 TCR transgenic mouse and their relative changes following pOVA–Ig-transduced B-cell treatment. The significant changes are only found in the antigen-specific cells, and not in the cells with endogenously recombined TCRs. As mentioned, there is a significant increase in Ag-specific Tregs and a significant decrease in Ag-specific responders (i.e., Ag-specific FoxP3− cells). Ultimately, the immune response is dictated by the ratio of responder (effector) T cells to Tregs. These results suggest that there are at least two mechanisms that likely drive tolerance, both an increase in Ag-specific FoxP3+ Tregs and a selective loss of antigen-specific responder cells. These changes in the regulatory cell pool work synergistically to shift the lymphocyte balance toward a tolerogenic phenotype.
Table 1. Summary of the changes in different T-cell populations in tolerized DO11.10 mice 7 days following pOVA–Ig-transduced B-cell treatment.
The exact nature of the relationship between the ability of Tregs to suppress and signaling through its TCR is unclear. For example, Tregs that receive TCR stimulation without exogenous interleukin-2 are not suppressive.38 However, many have shown that if in vitro assays mimic a physiologic environment by making necessary cytokines available, then suppression is dependent on Treg activation through the TCR,38,39,40 although it has recently been suggested that TCR signaling may not be necessary for Treg function.41 Herein, we have presented data to suggest that both views may be correct. Our data demonstrate that Tregs can be suppressive if they are not Ag-specific, but they are more suppressive when they are. For example, we found that Tregs isolated from a BALB/c mouse with polyclonal repertoire can suppress OVA-specific T cells responding to pOVA. However, Tregs isolated from a DO11.10 mouse that are specific for pOVA, were more suppressive. Notably, induced Ag-specific Tregs isolated from DO11.10/Rag2−/− mice were the most suppressive T-cell subset we studied.
What is the underlying mechanism behind tolerogenic B-cell mediated suppression in this model? A recent review42 summarized four basic mechanisms that Tregs use and they include: inhibitory cytokines, cytolysis, metabolic disruption, and targeting dendritic cells. We performed assays for these methods, but we could not identify obvious differences when comparing Tregs from mice treated with tolerogenic cells and control treatment. That is, both natural and induced Tregs had the expected phenotypic markers that include: TGFβ+, CD25+, CTLA-4+, IDO+, CD44+, FR4+, Granzyme B+, interleukin-2+, CD127−. Further markers may need to be analyzed to determine the molecular basis for the differences between nTregs and induced Tregs in this system. Hopefully, the complete mechanism by which the tolerogenic B cells confer antigen-specific tolerance will become clear in the near future as we develop the molecular tools to fully characterize this phenomenon.
Materials and Methods
Mouse models. Factor VIII deficient mice33 (FVIII−/−) were used as the model for Hemophilia A. FVIII−/− mice on a BALB/c background were the generous gift of Dr Lillicrap (Queen's University). The parental strain of FoxP3-GFP30 mice was obtained from Dr Rudensky (University of Washington). These mice have a targeted insertion of GFP in the first exon of the FoxP3 gene, on the X chromosome that does not alter the function of the transcribed protein.30 The strategy for identifying FVIII−/−/FoxP3-GFP mice is shown in Supplementary Figure S1 and offspring that contained the desired crossover mutations (FVIII−/− and FoxP3-GFP). Additionally, TCR transgenic DO11.10 and DO11.10/Rag2−/− were established by crossing a FoxP3-GFP mouse against a stable parental line and then backcrossing for a total of 10 generations. Presence of the GFP mutation could be tested by genotyping PCR (using primers F: AGCCTGCCTCTGACAAGAAC; R: CAAGTACCCCACCCTGCTTA) or by flow cytometry. The presence of the TCR transgene product was identified using the clonotypic mAb, KJ1.26. All strains were backcrossed for 10 generations and then maintained by sibling mating. Animals were housed and bred in pathogen-free microisolator cages at the animal facilities operated by the University of Maryland School of Medicine.
Retroviral-mediated gene transfer. Production of retrovirus producing packaging cells has been previously described.15 Briefly, complementary DNAs encoding immunodominant domains of FVIII (C2 or A2) or the pOVA were cloned in the BSSK–IgG construct and then the antigen–Ig construct was subcloned into a bicistronic MSCV-IRES-GFP vector (Dr Bunting, Case Western Reserve University). Packaging cell lines that stably produce retrovirus were made by co-transfection of the MSCV-(C2 or A2 or pOVA)-IgG-IRES-GFP, the helper plasmids pSRα-G, and pEQPAM3e. The retroviral supernatants were then used to stably transduce the GPE-86 packaging cell line. Mouse primary B cells were stimulated with bacterial LPS at 10 µg/ml for 24 hours. Next, the activated cells were cocultured with irradiated (1,500 rad) viral packaging cells in the presence of 6 µg/ml polybrene for an additional 24 hours. The virally infected B cells were washed and 107 cells were then delivered to each syngeneic recipient. On the basis of GFP expression level in B cells, the percentages of productively transduced cells were estimated to be about 60% for C2–Ig, 30% for A2–Ig, and 40% of pOVA–Ig.
Treg purification. CD4+CD25+ cells were isolated using magnetic beads and columns (Miltenyi, Bergisch Gladbach, Germany). Anti-CD25 (PC61) antibody was produced and purified in house and conjugated with biotin using a commercial kit (AnaSpec, Fremont, CA). Lymphocytes were processed into a single-cell suspension, blocked with 24G.2 (anti-Fc), and labeled with PC61-biotin for 10 minutes at 1 µg/ml. The cells were then washed twice, resuspended in labeling buffer and incubated with streptavidin-coated magnetic beads and column purified following the vendor's protocol (Miltenyi). Typically, about 90% of purified CD4+ cells are FoxP3+.
CFSE labeling. Cell proliferation was measured by examining CFSE dilution in labeled cell populations. Vendor specifications were optimized for proper labeling (Invitrogen, Carlsbad, CA). Briefly, cells were reconstituted at 106 cells/ml in 37 °C phosphate-buffered saline+0.1%bovine serum albumin and incubated with 1 µmol/l CFSE for 10 minutes at 37 °C. They were placed on ice for 5–10 minutes with RPMI 5% and washed three times.
Treg suppression assays. Lymph nodes were collected from the FVIII/CFA treated mice or DO11.10 TCR transgenic mice and stained with CFSE. In preliminary experiments, it was observed that the optimal FVIII dose for CFSE dilution of these primed lymph node cells was 0.1–1 µg/ml and the optimal OVA dose for DO11.10 T cells was 0.01–0.1 µg/ml (data not shown). Splenocytes from mice in each experimental group were pooled and CD25+ selection beads were used to purify Tregs. Purified Tregs were mixed with effector cells in different ratios, and FVIII (or OVA) was used to stimulate cells for 72 hours before flow cytometric analysis. Suppression assays were established in round bottom 96-well plates in 300 µl/well in triplicates. Typically, 2 × 105 CFSE-labeled effector (responder) cells were added. Antigen was added at a standard concentration and dilutions of Treg cells were added.
Statistical analysis. In all experiments, the data are expressed as the mean ± SEM. To compare antibody concentrations between experimental groups an unpaired Student's t-test was used. To compare trends in T-cell proliferation analysis, a two-factor analysis of variance without replication was used. To compare categorical data for objects that can be classified in two different ways, a Fisher's exact test was used.
SUPPLEMENTARY MATERIAL Figure S1. Derivation of FVIII−/−/FoxP3-GFP mice. Figure S2. Detection of FoxP3+ T cells in transgenic and non-transgenic mice.
Acknowledgments
This study was supported by NIH grants, AI035622 and HL061883 (D.W.S.) and an AHA pre-doctoral fellowship (J.S.).
Supplementary Material
Derivation of FVIII−/−/FoxP3-GFP mice.
Detection of FoxP3+ T cells in transgenic and non-transgenic mice.
REFERENCES
- Wasserfall CH., and, Herzog RW. Gene therapy approaches to induce tolerance in autoimmunity by reshaping the immune system. Curr Opin Investig Drugs. 2009;10:1143–1150. [PubMed] [Google Scholar]
- Zambidis ET, Kurup A., and, Scott DW. Genetically transferred central and peripheral immune tolerance via retroviral-mediated expression of immunogenic epitopes in hematopoietic progenitors or peripheral B lymphocytes. Mol Med. 1997;3:212–224. [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Melo M, Deng E, Tisch R, El-Amine M., and, Scott DW. Induction of hyporesponsiveness to intact foreign protein via retroviral-mediated gene expression: the IgG scaffold is important for induction and maintenance of immune hyporesponsiveness. Proc Natl Acad Sci USA. 1999;96:8609–8614. doi: 10.1073/pnas.96.15.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eynon EE., and, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs EJ., and, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- Borel Y. Haptens bound to self IgG induce immunologic tolerance, while when coupled to syngeneic spleen cells they induce immune suppression. Immunol Rev. 1980;50:71–104. doi: 10.1111/j.1600-065x.1980.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Venkataraman M, Aldo-Benson M, Borel Y., and, Scott DW. Persistence of antigen-binding cells with surface tolerogen: isologous versus heterologous immunoglobulin carriers. J Immunol. 1977;119:1006–1009. [PubMed] [Google Scholar]
- Melo ME, Qian J, El-Amine M, Agarwal RK, Soukhareva N, Kang Y, et al. Gene transfer of Ig-fusion proteins into B cells prevents and treats autoimmune diseases. J Immunol. 2002;168:4788–4795. doi: 10.4049/jimmunol.168.9.4788. [DOI] [PubMed] [Google Scholar]
- Song L, Wang J, Wang R, Yu M, Sun Y, Han G, et al. Retroviral delivery of GAD-IgG fusion construct induces tolerance and modulates diabetes: a role for CD4+ regulatory T cells and TGF-β. Gene Ther. 2004;11:1487–1496. doi: 10.1038/sj.gt.3302327. [DOI] [PubMed] [Google Scholar]
- Soukhareva N, Jiang Y., and, Scott DW. Treatment of diabetes in NOD mice by gene transfer of Ig-fusion proteins into B cells: role of T regulatory cells. Cell Immunol. 2006;240:41–46. doi: 10.1016/j.cellimm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Xu B., and, Scott DW. A novel retroviral gene therapy approach to inhibit specific antibody production and suppress experimental autoimmune encephalomyelitis induced by MOG and MBP. Clin Immunol. 2004;111:47–52. doi: 10.1016/j.clim.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Satpute SR, Soukhareva N, Scott DW., and, Moudgil KD. Mycobacterial Hsp65-IgG-expressing tolerogenic B cells confer protection against adjuvant-induced arthritis in Lewis rats. Arthritis Rheum. 2007;56:1490–1496. doi: 10.1002/art.22566. [DOI] [PubMed] [Google Scholar]
- Liang W, Karabekian Z, Mattapallil M, Xu Q, Viley AM, Caspi R, et al. B-cell delivered gene transfer of human S-Ag-Ig fusion protein protects from experimental autoimmune uveitis. Clin Immunol. 2006;118:35–41. doi: 10.1016/j.clim.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Agarwal RK, Kang Y, Zambidis E, Scott DW, Chan CC., and, Caspi RR. Retroviral gene therapy with an immunoglobulin-antigen fusion construct protects from experimental autoimmune uveitis. J Clin Invest. 2000;106:245–252. doi: 10.1172/JCI9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei TC., and, Scott DW. Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood. 2005;105:4865–4870. doi: 10.1182/blood-2004-11-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer LW. Hemophilia A. N Engl J Med. 1994;330:38–47. doi: 10.1056/NEJM199401063300108. [DOI] [PubMed] [Google Scholar]
- Lenting PJ, van Mourik JA., and, Mertens K. The life cycle of coagulation factor VIII in view of its structure and function. Blood. 1998;92:3983–3996. [PubMed] [Google Scholar]
- Dasgupta S, Navarrete AM, Delignat S, Wootla B, Andre S, Nagaraja V, et al. Immune response against therapeutic factor VIII in hemophilia A patients–a survey of probable risk factors. Immunol Lett. 2007;110:23–28. doi: 10.1016/j.imlet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Kempton CL., and, White GC., 2nd How we treat a hemophilia A patient with a factor VIII inhibitor. Blood. 2009;113:11–17. doi: 10.1182/blood-2008-06-160432. [DOI] [PubMed] [Google Scholar]
- Waters B., and, Lillicrap D. The molecular mechanisms of immunomodulation and tolerance induction to factor VIII. J Thromb Haemost. 2009;7:1446–1456. doi: 10.1111/j.1538-7836.2009.03538.x. [DOI] [PubMed] [Google Scholar]
- DiMichele DM., and, Kroner BL. North American Immune Tolerance Study Group. The North American Immune Tolerance Registry: practices, outcomes, outcome predictors. Thromb Haemost. 2002;87:52–57. [PubMed] [Google Scholar]
- Ananyeva NM, Lacroix-Desmazes S, Hauser CA, Shima M, Ovanesov MV, Khrenov AV, et al. Inhibitors in hemophilia A: mechanisms of inhibition, management and perspectives. Blood Coagul Fibrinolysis. 2004;15:109–124. doi: 10.1097/00001721-200403000-00001. [DOI] [PubMed] [Google Scholar]
- Pratt KP, Qian J, Ellaban E, Okita DK, Diethelm-Okita BM, Conti-Fine B, et al. Immunodominant T-cell epitopes in the factor VIII C2 domain are located within an inhibitory antibody binding site. Thromb Haemost. 2004;92:522–528. doi: 10.1160/TH03-12-0755. [DOI] [PubMed] [Google Scholar]
- Jacquemin MG., and, Saint-Remy JM. Factor VIII alloantibodies in hemophilia. Curr Opin Hematol. 2004;11:146–150. doi: 10.1097/01.moh.0000130312.87668.bf. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Carpentier PA, Anger HA., and, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- Peng B, Ye P, Rawlings DJ, Ochs HD., and, Miao CH. Anti-CD3 antibodies modulate anti-factor VIII immune responses in hemophilia A mice after factor VIII plasmid-mediated gene therapy. Blood. 2009;114:4373–4382. doi: 10.1182/blood-2009-05-217315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao CH, Harmeling BR, Ziegler SF, Yen BC, Torgerson T, Chen L, et al. CD4+FOXP3+ regulatory T cells confer long-term regulation of factor VIII-specific immune responses in plasmid-mediated gene therapy-treated hemophilia mice. Blood. 2009;114:4034–4044. doi: 10.1182/blood-2009-06-228155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Shibata M, Brown B, Labelle A, Hegadorn C, Andrews C, et al. A murine model for induction of long-term immunologic tolerance to factor VIII does not require persistent detectable levels of plasma factor VIII and involves contributions from Foxp3+ T regulatory cells. Blood. 2009;114:677–685. doi: 10.1182/blood-2009-03-202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S, Nomura T., and, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG., and, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P., and, Yamaguchi T. Regulatory T cells: how do they suppress immune responses. Int Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- Skupsky J, Su Y, Lei TC., and, Scott DW. Tolerance induction by gene transfer to lymphocytes. Curr Gene Ther. 2007;7:369–380. doi: 10.2174/156652307782151443. [DOI] [PubMed] [Google Scholar]
- Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD., and, Kazazian HH., Jr Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10:119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Cao O, Hagstrom JN., and, Wang L. Gene therapy for treatment of inherited haematological disorders. Expert Opin Biol Ther. 2006;6:509–522. doi: 10.1517/14712598.6.5.509. [DOI] [PubMed] [Google Scholar]
- Cao O, Loduca PA., and, Herzog RW. Role of regulatory T cells in tolerance to coagulation factors. J Thromb Haemost. 2009;7 Suppl 1:88–91. doi: 10.1111/j.1538-7836.2009.03417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RW.ed.) Gene Therapy Immunology Wiley-Blackwell: Hoboken, New Jersey; 2009 [Google Scholar]
- Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM., and, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM., and, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- Szymczak-Workman AL, Workman CJ., and, Vignali DA. Cutting edge: regulatory T cells do not require stimulation through their TCR to suppress. J Immunol. 2009;182:5188–5192. doi: 10.4049/jimmunol.0803123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali DA, Collison LW., and, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Derivation of FVIII−/−/FoxP3-GFP mice.
Detection of FoxP3+ T cells in transgenic and non-transgenic mice.