Abstract
Oncolytic viruses (OVs) are highly immunogenic and this limits their use in immune-competent hosts. Although immunosuppression may improve viral oncolysis, this gain is likely achieved at the cost of antitumoral immunity. We have developed a strategy wherein the immune response against the OV leads to enhanced therapeutic outcomes. We demonstrate that immunization with an adenoviral (Ad) vaccine before treatment with an oncolytic vesicular stomatitis virus (VSV) expressing the same tumor antigen (Ag) leads to significantly enhanced antitumoral immunity. Intratumoral replication of VSV was minimally attenuated in Ad-immunized hosts but extending the interval between treatments reduced the attenuating effect and further increased antitumoral immunity. More importantly, our combination approach shifted the immune response from viral Ags to tumor Ags and further reduced OV replication in normal tissues, leading to enhancements in both efficacy and safety. These studies also highlight the benefits of using a replicating, OV to boost a pre-existing antitumoral immune response as this approach generated larger responses versus tumor Ag in tumor-bearing hosts than could be achieved in tumor-free hosts. This strategy should be applicable to other vector combinations, tumor Ags, and tumor targets.
Introduction
Oncolytic viruses (OVs) cure cancer in animal models if they infect tumors and replicate extensively to mediate complete destruction.1,2,3,4,5,6 However, broad clinical application requires treating immunocompetent hosts bearing malignancies that may have partially intact antiviral mechanisms. An active host immune response against the virus that rapidly eliminates viral replication, leading to incomplete or transient tumor destruction represents an important barrier to success.7 It has been shown in naive animals that the development of an acquired immune response usually takes less than a week, leaving a small window of opportunity for oncolytic vectors to function.8,9 To maximize viral replication or redeliver the same virus, a variety of approaches have been tested ranging from outright immunosuppression,10,11,12,13 to the use of carrier cells (so-called “Trojan horses”),14,15,16,17 or viral cloaking.18,19,20,21
If, however, we accept that the ensuing immune response dictates that viral oncolysis will inevitably be transient in nature then could we design the anti-OV immune response to be a useful one that enhances the therapeutic impact of the vector? We hypothesize that by designing the OV to express a tumor-associated antigen (Ag) (TAA) and then using this virus in a host that has been previously vaccinated against this same TAA one could achieve this effect. In such a vaccinated host, the boosted secondary response against the tumor-Ag transgene would dominate the primary response against viral Ags leading to a robust antitumoral immune response. If the tumor Ag in the OV is a nonstructural transgene, any antibody response against this Ag induced by preimmunization would not impede viral delivery to tumors in vivo. The T-cell response against this Ag might impair viral replication within the tumor; however, this would only occur when tumor-specific effector T cells (rather than solely viral-specific T cells) are recruited into the tumor resulting in the killing of infected tumor cells. Therefore, we predict that any reduction in viral oncolysis in such a vaccinated host would be more than compensated for by the very response that clears the oncolytic vaccine vector as this would represent a boosted antitumoral response functioning within the tumor to destroy malignant cells. This may allow us to obtain both a transient oncolytic effect and an enhanced antitumoral immune response that is long lasting in nature.
To evaluate our hypothesis, we used a recombinant adenovirus (Ad) as a vaccine vector and a complementary wild-type vesicular stomatitis virus (VSV) as an oncolytic vaccine virus in mice that had established tumors in the brain or lungs.4,22 We have previously utilized these vectors as vaccine vectors and demonstrated that they work well together to prime and boost an Ag-specific immune response.23,24 We now demonstrate that sequential treatment of a tumor-bearing host with Ad and oncolytic VSV both expressing a defined TAA induced a massive antitumoral immune response. Importantly, preimmunization with Ad did not prevent acute VSV infection within the tumor allowing significant viral replication. This combined approach leads to increased tumor infiltrating lymphocytes (TILs), epitope spreading, and superior survival benefits over that seen with either the use of viral oncolysis or tumor vaccination alone. These studies demonstrate the utility of OVs as boosters of antitumoral immunity as larger responses are achieved in tumor-bearing hosts due to amplification of the boosting vector in the tumor bed. Our results suggest that preimmunization against a tumor Ag encoded by an oncolytic vaccine virus can rig the immune response such that the response against the tumor dominates over the immune response against viral Ags thus allowing for transient viral oncolysis while leading to robust and durable antitumoral immunity and enhanced safety.
Results
VSV oncolysis is insufficient to effect cures in B16-F10 model
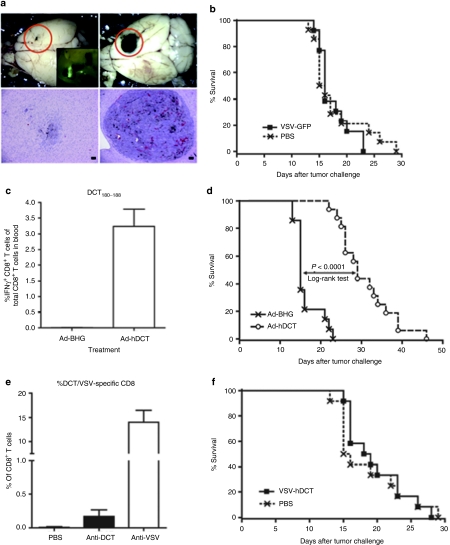
We adopted an aggressive intracranial (i.c.) B16 melanoma model to rigorously test our therapeutic strategy in an immunocompetent host using melanoma-associated Ags. In this model, C57BL/6 mice were engrafted with B16-F10 cells through i.c. injection and the median survival time following tumor delivery was 15 days. To evaluate the efficacy of VSV treatment, mice carrying 7-day-old B16 tumors were treated with a single intravenous (i.v.) dose of VSV-GFP. Figure 1a shows that the tumor was infected resulting in a clear reduction in tumor volumes as expected. However, this effect was transient and failed to translate into a survival benefit (Figure 1b). A similar phenomenon has been observed in other therapies where incomplete and partially destroyed tumors often regrow more rapidly than untreated ones due to “Gompertzian” growth kinetics.25
Figure 1.
Impact of oncolysis and tumor vaccination in naive hosts. C57BL/6 mice received intracranial injections of B16-F10 cells. (a) One week later mice were treated with intravenous injections of VSV-GFP. Fluorescent microscopy revealed that brains harvested 3 days after VSV treatment had evidence of intratumoral GFP expression (inset: upper left panel). Macroscopic examination of brains harvested at day 4 postinfection revealed a large reduction in tumor burden in VSV-GFP-treated brains (upper panels) confirmed by hematoxylin and eosin–stained sections (lower panels). (b) Survival studies failed to detect prolonged survival following oncolytic VSV-GFP treatment (PBS n = 14, VSV-GFP n = 13, pooled data from three experiments). (c) Alternatively, on day 7 postengraftment mice were treated with a single intramuscular dose of Ad-hDCT or Ad-BHG. Immunological analysis of blood was performed on day 14 postvaccination. The percentage of DCT-specific CD8+ T cells are indicated. (d) A significant extension of survival in Ad-hDCT-vaccinated mice was achieved (median survival: Ad-hDCT = 29 days, n = 16 and Ad-BHG = 15 days, n = 14; P < 0.0001, pooled data from three experiments). (e) On day 7 postengraftment, mice bearing intracranial B16 tumors were treated with a single intravenous dose of VSV-hDCT. Immunological analysis of blood was performed 14 days later. The percentage of DCT- and VSV nucleocapsid-specific CD8+ T cells are indicated. (f) Survival studies failed to detect prolonged survival following oncolytic VSV-hDCT treatment (pooled data from three experiments, n = 12 for each treatment). Ad, adenovirus; DCT, dopachrome tautomerase; hDCT, human DCT; PBS, phosphate-buffered saline; VSV, vesicular stomatitis virus.
Ad-hDCT vaccination prolonged survival
Another approach we explored in the treatment of i.c. B16 melanoma was tumor vaccination. Our recent studies have shown that immunization with a recombinant Ad expressing human dopachrome tautomerase (hDCT) could generate a response against murine DCT.22,26,27 This vaccine vector could provide prophylactic protection against an i.c. challenge with B16 cells in a CD8-dependent manner (B.W. Bridle and Y. Wan, manuscript submitted). To test the therapeutic utility of this vaccine, we engrafted C57BL/6 mice with an i.c. dose of B16 cells and then treated them intramuscularly with Ad-hDCT 7 days postengraftment. CD8+ T-cell responses against an immunodominant epitope DCT180–188 (identical between human and mouse) were evident in blood 1-week postvaccination and peaked at day 12–14 (~3.2% of CD8+ T cells, Figure 1c).22,26 In contrast to VSV treatment, Ad-hDCT vaccination significantly extended survival (median survival 29 days; P < 0.0001) (Figure 1d) but was unable to cure any of the mice.
VSV-hDCT treatment failed to prolong survival
Having determined the transient nature of VSV-mediated oncolysis but the potency of antitumor vaccination, we reasoned that engineering VSV to express a TAA might achieve both effects simultaneously. As such, an antitumoral immune response induced by an oncolytic vaccine vector would continue to have an impact on the tumor after the host cleared the virus. To this end, we engineered VSV to express hDCT (VSV-hDCT) and treated mice with i.c. B16-F10 tumors. This vector induced a small anti-DCT CD8+ T-cell response (0.26%, Figure 1e), which was 12 times smaller than that elicited by Ad-hDCT (3.2%, Figure 1c). However, a high level of CD8+ T cells against an epitope from the nucleoprotein of VSV was detected following VSV-hDCT treatment (14.0%, Figure 1e) suggesting that the antiviral response dominated the immunological outcome. Similar to the observation with VSV-GFP (Figure 1b), treatment with VSV-hDCT did not provide any survival benefit (Figure 1f). Thus, the potent antiviral immune response elicited by our OV not only causes the oncolytic impact of the vector to be transient, but also dominates attempts to directly induce immune responses against the TAA transgene.
Turning the immune response against the OV into a beneficial one
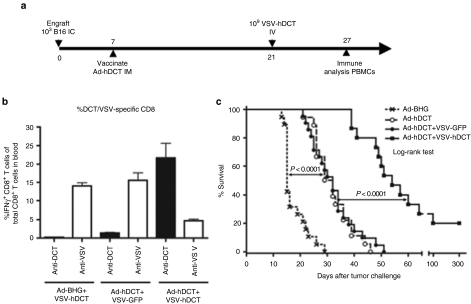
Given that our OV is going to be cleared by the immune system, we reasoned that we may be able to tailor this response in our favor. We hypothesized that by priming an immune response against a defined tumor Ag and then treating with an OV expressing that same Ag we would generate an immune response against the tumor Ag that dominated over the response against viral Ags. To test the potential utility of this combined approach, C57BL/6 mice bearing i.c. B16 tumors were treated with either Ad-BHG or Ad-hDCT. After 14 days, mice were given a single i.v. dose of VSV-GFP or VSV-hDCT (Figure 2a). As summarized in Figure 2b, Ad-hDCT immunization followed by VSV-hDCT in tumor-bearing mice resulted in 21.7% of blood-derived CD8+ T cells being DCT-specific; sevenfold (compared to Figure 1c) or 85-fold (compared to Figure 1e) higher than either vector treatment alone. Furthermore, not only did this combination significantly enhance the immune response to the TAA, it actually reduced the magnitude of the anti-VSV CD8+ T-cell response as compared to that observed following exposure of a naive mouse to this OV (from 14 to 4.7% of blood-derived CD8+ T cells; P < 0.0001; Figure 1e versus Figure 2b), demonstrating an inversion of the immune response against the oncolytic vaccine virus where the antitumoral response now dominated over antiviral immunity. Most importantly, the combination therapy led to a further extension in median survival (15 days Ad-BHG alone; 30 days Ad-hDCT alone; 54 days combo; P < 0.0001) and 20% of mice treated in this fashion displayed a long-term, durable cure (Figure 2c). It should be noted that VSV lacking the TAA transgene (VSV-GFP) failed to boost the hDCT response in Ad-hDCT-primed mice (Figure 2b) and did not extend survival (Figure 2c) indicating that the OV used must express the same TAA transgene to achieve this effect. As well, treating with the oncolytic vaccine vector VSV-hDCT first failed to slow tumor progression precluding subsequent treatment with the Ad-hDCT vector as a booster (data not shown). Thus, the optimal use of such an oncolytic vaccine vector is in the presence of a pre-existing response versus the tumor-Ag transgene.
Figure 2.
Turning the immune response against the oncolytic virus into a beneficial one. (a) Timeline for combination treatment with Ad-hDCT and VSV-hDCT. (b) Blood was collected 6 days after VSV treatment and intracellular staining for IFN-γ in response to the dominant epitopes for DCT and the VSV nucleocapsid was performed. (c) Pooled survival data from three independent experiments. Mice were treated with empty Ad vector (Ad-BHG), Ad-hDCT alone, Ad-hDCT followed by VSV-GFP or Ad-hDCT followed by VSV-hDCT (n = 19, n = 18, n = 21, and n = 15, respectively). Median survival: 15, 30, 32, and 54 days, respectively. Ad, adenovirus; DCT, dopachrome tautomerase; hDCT, human DCT; PBS, phosphate-buffered saline; i.c., intracranial; IFN, interferon; i.v., intravenous; VSV, vesicular stomatitis virus.
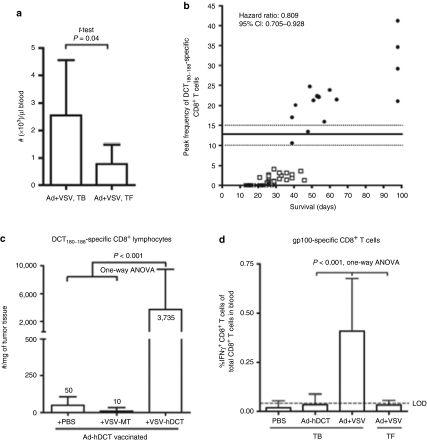
Remarkably the magnitude of the anti-DCT T-cell response was greater in tumor-bearing animals than in tumor-free animals demonstrating the advantage of using a replicating OV to deliver the transgene in the presence of a tumor (Figure 3a). Furthermore, survival was directly correlated with the level of DCT-specific CD8+ T cells (a unit increase in CD8 response resulted in a 29.1% reduction in hazard of death; 95% CI 7.2–29.5%) where the greatest extension to survival was only achieved when the magnitude of this immune response exceeded that seen in tumor-free hosts (Figure 3b). Thus, maximal therapeutic effect was mediated through replication of the boosting oncolytic vector within the tumor. As well, the frequency of CD8+ TILs specific for DCT was 100-fold higher in VSV-hDCT-treated animals as compared to those treated with the VSV-MT control virus, indicating that treatment with VSV-hDCT not only resulted in an increase in Ag-specific CD8+ T cells in the periphery but also enhanced their recruitment into the tumor (Figure 3c). Interestingly, we also detected a CD8+ T-cell response against GP100 (ref. 28), another TAA for which we did not vaccinate the mice, providing evidence of epitope spreading likely resulting from enhanced tumor destruction by both anti-DCT CTL and viral oncolysis because an anti-GP100 response was not measured with either treatment alone or in mice treated with Ad-hDCT+control VSV (Figure 3d).
Figure 3.
Immunological features of oncolytic virus immune boosting. (a) Comparison of the numbers of DCT-specific, IFN-γ+ CD8+ T cells in the blood of tumor-bearing (TB, n = 7) and tumor-free (TF, n = 5) C57BL/6 mice at the peak of the response after VSV treatment. (b) Pooled data demonstrating the correlation between the magnitude of the anti-DCT response in the blood and survival. Data includes mice that were mock vaccinated (Ad-BHG, cross), Ad-hDCT vaccinated (open squares) or treated with the Ad-hDCT+VSV-hDCT combination (closed circles). After accounting for group, a unit increase in CD8 resulted in a 29.1% reduction in hazard of death (95% CI 7.2–29.5%), P = 0.0024, hazard ratio 0.809, 95% CI 0.705–0.928. Those three mice having the highest responses actually survived much longer than 100 days. The horizontal lines indicate the mean response achieved in tumor-free mice ± SEM (dashed lines). (c) Ad-hDCT vaccinated C57BL/6 mice bearing intracranial B16-F10 tumors were subsequently treated with PBS, VSV-GFP, or VSV-hDCT. Seven days later, tumors were collected and IFN-γ+ tumor-infiltrating lymphocytes responsive to DCT180–188 peptide were enumerated. (d) gp100-responsive CD8+ T cells were enumerated by intracellular cytokine staining (ICS) for IFN-γ demonstrating that combination therapy of TB animals induces epitope spreading. Limit of detection (LOD) is indicated. Ad, adenovirus; CI, confidence interval; DCT, dopachrome tautomerase; hDCT, human DCT; PBS, phosphate-buffered saline; IFN, interferon; VSV, vesicular stomatitis virus.
Vaccination against OV transgene did not prevent oncolysis
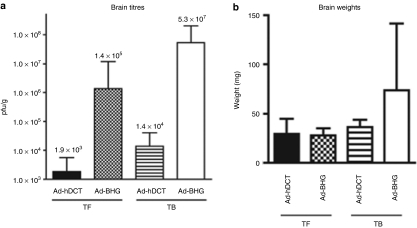
Although the observations described above suggest that VSV-hDCT remains oncolytic in the presence of an immune response against the vector transgene, the impact of such pre-existing immunity on VSV replication needed to be evaluated. To first examine this, we treated tumor-free mice and mice bearing i.c. B16-F10 tumors with Ad-hDCT or Ad-BHG. After 14 days, mice were treated with VSV-hDCT. Brains were harvested and viral titres were determined. In mock-vaccinated mice both tumor-free and tumor-bearing brains displayed abundant VSV-hDCT replication (Figure 4a) demonstrating the neurovirulence of the wild-type VSV used in these studies. As mock vaccination did not impede tumor growth, these mice had a large tumor burden (Figure 4b) and were very near end point at the time of killing. In the Ad-hDCT vaccinated mice, the VSV titres were much lower; however, the tumor-bearing brains still exhibited a higher VSV-hDCT titer (Figure 4a) even though these brains had minimal tumor burden at this time point (Figure 4b). Thus, VSV was still able to infect and replicate in this residual tumor despite pre-existing immunity to the vector transgene. As well, BrdU-labeling experiments demonstrated that there was a 3- to 4-day lag before T-cell expansion in response to VSV-hDCT (Supplementary Figure S1) providing a window of opportunity for viral oncolysis.
Figure 4.
Impact of vaccination on OV replication. Tumor-free (TF) or B16-F10 tumor-bearing (TB) C57BL/6 mice were immunized i.m. with Ad vectors as indicated. Fourteen days after Ad treatment, mice were given VSV-hDCT via i.v. injection and the brains were weighed and homogenized 3 days later. (a) Viral titers were quantified by plaque assay and are expressed as pfu/g of brain tissue. Brain weights are summarized in b. Data were pooled from two experiments with 5 mice/group. hDCT, human dopachrome tautomerase; i.m., intramuscularly; i.v., intravenously; VSV, vesicular stomatitis virus.
Vaccination against a virally encoded TAA modulates OV activity
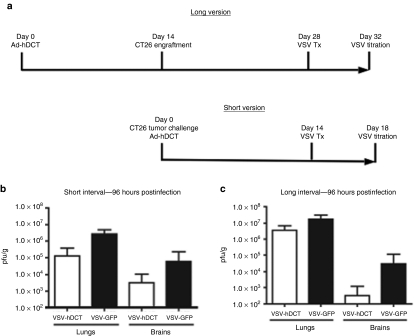
As Ad-hDCT treated tumors were smaller, it was difficult to quantitatively analyze the impact of a pre-existing immune response against a transgene on viral replication in the B16 model. Therefore, we decided to measure this effect in a different tumor model where DCT was not a tumor Ag. This allowed a comparison where mice had similar tumor burdens regardless of vaccination and also allowed for flexibility with regards to the interval between vaccination and viral oncolysis. To this end, we selected a CT26 colon carcinoma model where DCT was irrelevant. Mice were inoculated i.v. with CT26 cells and vaccinated with Ad-hDCT. After 14 days, mice received either VSV-hDCT or VSV-GFP. To measure viral replication, lungs and brains were collected for determination of VSV titers (Figure 5a). An ~1.5 log reduction of viral titers was observed in both the lungs and brains of VSV-hDCT-treated animals, as compared to VSV-GFP controls (Figure 5b). Interestingly, however, there was a smaller reduction in VSV-hDCT lung titers if mice were immunized 14 days before CT26 engraftment (28 days before VSV-hDCT treatment) (Figure 5c), suggesting an increased interval between these two treatments can increase oncolysis. Note that there was no detectable VSV titer in tumor-free lungs at this time point following identical infections.29 We also observed a large reduction in VSV-DCT brain titers (Figure 5c), demonstrating that prior vaccination against a nonstructural transgene encoded by an OV could enhance the safety profile of that virus.
Figure 5.
Impact of vaccination on oncolysis in a lung metastatic model. (a) BALB/c mice were immunized i.m. with Ad-hDCT 14 days before (long interval) or on the same day (short interval) of CT26 tumor engraftment (2 × 105 cells, i.v. injection). Fourteen days after tumor inoculation, mice were treated with 2 × 108 pfu of VSV-hDCT or VSV-GFP via tail-vein injection. (b,c) The whole lungs and brains were harvested and homogenized 4 days after VSV treatment. Viral titers were quantified by plaque assay and expressed as pfu/g of tissue. Data were pooled from two experiments with 5 mice/group. Ad, adenovirus; hDCT, human dopachrome tautomerase; i.m., intramuscularly; i.v., intravenously; VSV, vesicular stomatitis virus.
Increasing the interval between prime and boost enhances responses
To further determine whether extending the interval between vaccination and viral oncolysis can enhance Ag-specific CD8+ T-cell response, we carried out an experiment in tumor-free animals. C57BL/6 mice were boosted with VSV-hDCT 14 or 100 days after Ad-hDCT immunization. The mean frequency of CD8+ T cells specific for the immunodominant epitope of DCT reached 37% if VSV-hDCT was administered 100 days after Ad-hDCT, a level significantly higher than that achieved when boosting 14 days postprime (16.3%, P < 0.0001; Supplementary Figure S2). Although extending this interval could not be tested in tumor-bearing mice due to rapid tumor progression even in Ad-vaccinated hosts, such an increased interval may be available in the clinic.
Discussion
Strategies to escape or suppress immune responses have been proposed to prolong the duration of viral replication for oncolytic therapy.11,12,13,14,15,16,17,19,21 However, there is good evidence that oncolytic viral therapy can indirectly induce antitumoral immunity in some cases, which aids therapy.3,30,31,32 Thus, alternative approaches have also been investigated where OVs are engineered to express immunostimulatory transgenes aimed at increasing the antitumoral immune response.33,34,35,36,37,38,39,40 In this study, we propose a treatment strategy that benefits from the immune response against the OV leading to an enhanced therapeutic outcome. We demonstrate that preimmunization with a genetic vaccine against a tumor Ag allows a rapid and potent boost of the CTL response by an oncolytic VSV expressing the same Ag. Although some attenuation of intratumoral viral replication was observed in preimmunized hosts, extending the interval between the two treatments reduced the attenuating effect and further increased antitumoral immunity. This reduction of viral replication is likely due to killing of infected tumor cells by DCT-specific T cells, indicative of intratumoral recruitment of effector T cells as a beneficial trade-off. More importantly, this combination approach shifted the immune response from viral Ag to tumor Ags and reduced viral replication outside the tumor, enhancing both efficacy and safety. Overall, this work demonstrates the several notable advantages of boosting a tumor vaccine with a replicating oncolytic vaccine vector. These advantages include tumor debulking and a massive increase in tumor-specific T cells, particularly in tumor-bearing hosts, accompanied by significantly greater numbers of Ag-specific TILs.
Although others have reported the use of OVs expressing model (foreign) tumor Ags,41,42,43 which were either not expressed by the tumor cells at all41 or were artificially expressed through stable transfection before engraftment,42,43 our data suggest that engineering OVs to express natural tumor Ags may induce a weak T-cell response that is largely overshadowed by the immune response against viral Ags. This is not surprising as these viral Ags are entirely foreign and highly immunogenic.7,44 These observations suggest that immunosuppressive or tolerogenic mechanisms associated with an established tumor must be overcome and immune responses must be redirected toward the tumor in order for oncolytic vaccines to be effective.45 Our data indicate that this can be well achieved by preimmunization with a genetic vaccine against a tumor that is also expressed by an OV. Although prior immunization against an oncolytic vector transgene is counter-intuitive as it may impair viral delivery or replication, our results demonstrated that our oncolytic vaccine became more potent under these circumstances as it dramatically amplified the pre-existing antitumoral immunity while retaining oncolytic activity, leading to significantly improved clinical outcomes.
Although intratumoral replication of the OV was reduced in preimmunized mice the vastly improved efficacy indicates that this is a reasonable trade-off. Moreover, this reduction in oncolysis could be further minimized by increasing the interval between vaccination and OV administration, as the frequency of Ag-specific effectors subsides over time.46 In fact, in addition to the reduction of the initial impact of prior vaccination on viral oncolysis, increasing this interval leads to a further enhanced boosting effect as we have seen in aged tumor-free mice. Although the Ag-specific response boosted by an oncolytic vaccine may further reduce replication of the OV, our data from BrdU-labeled mice indicated there is at least a 3-day window of opportunity for viral oncolysis. In fact, recruitment of CTL into the tumor at that point is desirable and should enhance clearance of both the virus and the tumor.
An additional benefit of this approach is the enhanced safety profile exhibited by the oncolytic vaccine vector. Previous studies have shown that innate immunity can limit VSV replication in peripheral tissues but the murine brain is highly permissive for wild-type VSV infection.5 Our data indeed demonstrate i.c. infection by VSV following i.v. delivery; however, viral titers were lower in the brains of immunized animals and most strikingly, there was no hindlimb paralysis in any of the mice that have been vaccinated against the viral transgene even though wild-type VSV was used (data not shown). We have also tested this combination therapy with an interferon-inducing mutant of VSV5 expressing hDCT and have seen comparable survival in the i.c. B16 model (not shown) indicating that this approach can be successfully combined with other means of viral targeting and attenuation.
We and others have previously shown that VSV can be an effective priming or boosting vaccine vector in the prophylactic setting to elicit Ag-specific immunity against pathogen or tumor challenge.23,24,47 However, this study demonstrates that oncolytic VSV should be better used as a boosting vector in tumor-bearing hosts as a larger boosted response was seen in tumor-bearing than tumor-free animals. This surprising effect is likely the result of VSV replication within the tumor that increases and prolongs Ag presentation. Furthermore, virus-mediated oncolysis recruits more tumor-specific effector T cells into the tumor resulting in further killing of tumor cells. A CD8+ T-cell response against GP100, an indication of epitope spreading, confirms enhanced tumor destruction by both anti-DCT CTL and viral oncolysis because an anti-GP100 response was not measured with either treatment alone or in mice treated with Ad-hDCT+control VSV. Thus, some reduction in oncolysis resulting from our sequential treatment strategy is well compensated for by enhanced antitumoral immunity and local immunomodulatory effects within the tumor.
Although our studies were restricted to one vaccine platform and one OV, we believe this work provides proof-of-concept and that the strategy could be applied to other tumor vaccine platforms using various tumor Ags, in combination with other OVs to which the recipient is naive. In theory, any pre-existing anti-TAA response could be used to enhance oncolytic viral therapy in this manner potentially including instances where patients have an identified pre-existing immune response thereby precluding the necessity of administering a priming vaccine. As long as the tumor Ag itself is not incorporated into the viral particle, the pre-existing antibodies will not prevent delivery of the oncolytic vaccine vector to the tumor. The virus will replicate inside the tumor until the antitumoral immune response is boosted and a new wave of TAA-specific effector T cells traffic into the tumor. Notably, this strategy simultaneously improves the safety profile of oncolytic virotherapy while enhancing its therapeutic potency.
Materials and Methods
Mice. Age-matched (8–10 weeks old at study initiation) female C57BL/6 (H-2b) and BALB/c (H-2d) mice (Charles River Laboratories, Wilmington, MA) were housed in a specific pathogen-free facility. Animal studies complied with Canadian Council on Animal Care guidelines and were approved by McMaster University's Animal Research Ethics Board.
Viruses. Ad-hDCT is an E1/E3-deleted human type 5 Ad that expresses the full-length hDCT gene and Ad-BHG is an E1/E3-deleted virus that contains no transgene.22,48 Recombinant VSV of the Indiana serotype was engineered to express the hDCT by subcloning the transgene into both wild-type and ΔM51 mutant genome plasmids as described previously.5 Recombinant genomes were rescued using standard techniques49 to generate replication-competent VSV-hDCT. VSV-MT is a recombinant virus lacking a transgene; VSV-GFP has been described elsewhere.5 Viral titer was determined by plaque assay on Vero cells.
In vivo tumor models. To establish brain tumors, C57BL/6 mice received sterotactic i.c. injections of 1 × 103 B16-F10 cells in 2 µl of phosphate-buffered saline (PBS). BALB/c mice were inoculated with 2 × 105 CT26 cells in 200 µl of PBS via tail-vein injection. Anesthetized mice were immunized by intramuscular injection of 1 × 108 plaque-forming units (pfu) of Ad vector in 100 µl of PBS (50 µl/hamstring) or i.v. injection of 2–10 × 108 pfu of VSV in 200 µl of PBS. We have previously determined these doses to be optimal for vaccination and/or viral oncolysis (data not shown).
Peptides. The immunodominant peptide from DCT that binds to H-2Kb (DCT180–188, SVYDFFVWL; shared by human and murine DCT) was synthesized by PepScan Systems (Lelystad, The Netherlands). The H-2Kb-restricted epitope from the N protein of VSV (RGYVYQGL) and a Db-binding murine gp100 peptide (mgp10025–33; EGSRNQDWL) were purchased from Biomer Technologies (Hayward, CA).
Stereotactic surgery. To establish brain tumors, mice received i.c. injections of 1 × 103 B16-F10 cells in 2 µl of PBS. Mice were placed in a stereotaxis (Xymotech Biosystems, Cote Saint-Lu, Quebec, Canada) and an incision made in the scalp to expose the skull under anesthesia. A needle mounted on a 10-µl Hamilton syringe (Hamilton, Reno, NV) was positioned over the right hemisphere of the brain, 2.25 mm lateral to bregma. A small burr hole was drilled through the skull and the bevel of the needle inserted into the brain parenchyma to a depth of 3 mm. Cells were injected over a period of 1 minute. The needle was left in place for 2 minutes before withdrawal to minimize reflux along the injection tract. The scalp incision was closed with stainless steel clips that were removed 7–10 days later.
Lung metastatic tumors in BALB/c mice. BALB/c mice were inoculated with 2 × 105 CT26 cells in 200 µl of PBS via tail-vein injection. All untreated mice reached the end point within 24 days.
Vaccination protocol. Anesthetized mice were immunized by intramuscular injection of 1 × 108 pfu of Ad vector in 100 µl of PBS (50 µl/hamstring) or i.v. injection of 2–10 × 108 pfu of VSV in 200 µl of PBS. We have previously determined these doses to be optimal for vaccination and/or viral oncolysis (data not shown).
Viral titering in tissue homogenates. To measure intratumoral virus replication, brains or lungs were collected 3 days after i.v. inoculation of VSV vectors, weighed, and homogenized before titering. Viral titers were quantified by plaque assay on Vero monolayers and are expressed as pfu/g of tissue.
Antibodies. The following monoclonal Abs were used in flow cytometry assays: anti-CD16/CD32 (clone 2.4G2) to block Fc receptors, anti-CD3 (clone 145-2C11), anti-CD8 (clone 53-6.7) for detecting cell surface markers and anti-IFN-γ (clone XMG1.2) for intracellular staining (all reagents from BD Pharmingen).
T-cell preparation and intracellular staining. For peripheral blood mononuclear cell collection, blood was collected from the periorbital sinus and red blood cells lysed. For TIL isolation, central nervous system tumors were perfused with PBS, dissected from the brains, weighed, minced, and subsequently incubated at 37 °C for 45 minutes in Hank's buffered saline containing 0.1% collagenase type I (Invitrogen Life Technologies, Carlsbad, CA) and DNase (0.1 mg/ml; Sigma-Aldrich, St Louis, MO). Following the digestion, released cells were filtered through a 70-µmol/l strainer and TILs were purified using EasySep CD90.2-PE system (Stemcell Technologies, Vancouver, British Columbia, Canada). Mononuclear cells from blood and TILs from the brain tumors were stimulated with peptides (1 µg/ml) in the presence of brefeldin A (GolgiPlug; BD Pharmingen, 1 µg/ml added after 1 hour of incubation). After 5 hours of total incubation time, cells were treated with anti-CD16/CD32 and surface markers fluorescently labeled by addition of Abs. Cells were then permeabilized and fixed with Cytofix/Cytoperm (BD Pharmingen) and stained for intracellular cytokines. Data were acquired using a FACSCanto flow cytometer with FACSDiva 5.0.2 software (BD Pharmingen) and analyzed with FlowJo Mac, version 6.3.4 software (Treestar, Ashland, OR).
Tetramer staining and BrdU incorporation assay. Immunized mice received i.p. injections of 1 mg BrdU 24 hours before harvest and given BrdU in drinking water (0.8 mg/ml) thereafter. Lymphocytes from different organs were first stained with allophycocyanin-conjugated tetramer H-2Kb/SVYDFFVWL and then stained for BrdU using the BrdU staining kit (BD Pharmingen) according to the manufacturer's instructions.
Tissue staining. For histological analysis of brains, tissue was fixed for 3 days in 10% formalin, transferred to 70% ethanol, paraffin-embedded, sectioned at a thickness of 10 µm and stained with hematoxylin and eosin (Sigma).
Statistical analyses. GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA) or R (www.r-project.com) were used for all graphing and statistical analyses. If required, data were normalized by log transformation. T-cell responses were analyzed by Student's two-tailed t-test or one- or two-way analysis of variance. All reported P values were two-sided and were considered significant at P ≤ 0.05. Error bars indicate 95% confidence intervals throughout. Survival curves were estimated by the Kaplan–Meier method, and differences between groups were investigated using the log-rank test. The association between immune response and survival time was evaluated by regressing survival time onto immune response, with separate baseline hazards for each group, using Cox proportional hazards regression. Proportionality of the hazards corresponding to CD8+ T-cell immune response was tested by considering departures from proportionality in which the log hazard ratio was to be a linear function of the Kaplan–Meier function, then using a χ2-test based upon the scaled Schoenfeld residuals.50 This was not significant (P = 0.787) suggesting no important departure from proportionality.
SUPPLEMENTARY MATERIAL Figure S1. Kinetics of T cell replication following VSV-hDCT treatment. Figure S2. Increased interval between prime and boost enhances magnitude of response.
Acknowledgments
This work was supported by grants to Y.W. from the Canadian Institutes of Health Research (MOP-67066) and the Ontario Cancer Research Network.
Supplementary Material
Kinetics of T cell replication following VSV-hDCT treatment.
Increased interval between prime and boost enhances magnitude of response.
REFERENCES
- Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- Grote D, Russell SJ, Cornu TI, Cattaneo R, Vile R, Poland GA, et al. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97:3746–3754. doi: 10.1182/blood.v97.12.3746. [DOI] [PubMed] [Google Scholar]
- Hummel JL, Safroneeva E., and, Mossman KL. The role of ICP0-Null HSV-1 and interferon signaling defects in the effective treatment of breast adenocarcinoma. Mol Ther. 2005;12:1101–1110. doi: 10.1016/j.ymthe.2005.07.533. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Wilcox ME, Yang W, Senger D, Rewcastle NB, Morris DG, Brasher PM, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst. 2001;93:903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- Cattaneo R, Miest T, Shashkova EV., and, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva LR, Binny C, Ferreira SC., Jr, and, Martins ML. A multiscale mathematical model for oncolytic virotherapy. Cancer Res. 2009;69:1205–1211. doi: 10.1158/0008-5472.CAN-08-2173. [DOI] [PubMed] [Google Scholar]
- Russell SJ., and, Peng KW. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28:326–333. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomonte J, Wu L, Chen L, Meseck M, Ebert O, García-Sastre A, et al. Exponential enhancement of oncolytic vesicular stomatitis virus potency by vector-mediated suppression of inflammatory responses in vivo. Mol Ther. 2008;16:146–153. doi: 10.1038/sj.mt.6300343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomonte J, Wu L, Meseck M, Chen L, Ebert O, Garcia-Sastre A, et al. Enhanced oncolytic potency of vesicular stomatitis virus through vector-mediated inhibition of NK and NKT cells. Cancer Gene Ther. 2009;16:266–278. doi: 10.1038/cgt.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamfers ML, Fulci G, Gianni D, Tang Y, Kurozumi K, Kaur B, et al. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Mol Ther. 2006;14:779–788. doi: 10.1016/j.ymthe.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zeng Z, Fu X., and, Zhang X. Coadministration of a herpes simplex virus-2 based oncolytic virus and cyclophosphamide produces a synergistic antitumor effect and enhances tumor-specific immune responses. Cancer Res. 2007;67:7850–7855. doi: 10.1158/0008-5472.CAN-07-1087. [DOI] [PubMed] [Google Scholar]
- García-Castro J, Martínez-Palacio J, Lillo R, García-Sánchez F, Alemany R, Madero L, et al. Tumor cells as cellular vehicles to deliver gene therapies to metastatic tumors. Cancer Gene Ther. 2005;12:341–349. doi: 10.1038/sj.cgt.7700801. [DOI] [PubMed] [Google Scholar]
- Iankov ID, Blechacz B, Liu C, Schmeckpeper JD, Tarara JE, Federspiel MJ, et al. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol Ther. 2007;15:114–122. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
- Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT., and, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- Power AT, Wang J, Falls TJ, Paterson JM, Parato KA, Lichty BD, et al. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther. 2007;15:123–130. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Chirmule N, Zhang Y., and, Wilson JM. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J Virol. 2001;75:4792–4801. doi: 10.1128/JVI.75.10.4792-4801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto Y, Gao JQ, Sekiguchi F, Kurachi S, Katayama K, Maeda M, et al. PEGylated adenovirus vectors containing RGD peptides on the tip of PEG show high transduction efficiency and antibody evasion ability. J Gene Med. 2005;7:604–612. doi: 10.1002/jgm.699. [DOI] [PubMed] [Google Scholar]
- Fisher KD, Stallwood Y, Green NK, Ulbrich K, Mautner V., and, Seymour LW. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001;8:341–348. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- Sailaja G, HogenEsch H, North A, Hays J., and, Mittal SK. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002;9:1722–1729. doi: 10.1038/sj.gt.3301858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C, Leitch J, Tan X, Hadjati J, Bramson JL., and, Wan Y. Vaccination-induced autoimmune vitiligo is a consequence of secondary trauma to the skin. Cancer Res. 2004;64:1509–1514. doi: 10.1158/0008-5472.can-03-3227. [DOI] [PubMed] [Google Scholar]
- Bridle BW, Boudreau JE, Lichty BD, Brunellière J, Stephenson K, Koshy S, et al. Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus. Mol Ther. 2009;17:1814–1821. doi: 10.1038/mt.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger EK, Kugathasan K, Zhang X, Lichty BD., and, Xing Z. Heterologous boosting of recombinant adenoviral prime immunization with a novel vesicular stomatitis virus-vectored tuberculosis vaccine. Mol Ther. 2008;16:1161–1169. doi: 10.1038/mt.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AK. Dynamics of tumor growth. Br J Cancer. 1964;13:490–502. doi: 10.1038/bjc.1964.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kianizad K, Marshall LA, Grinshtein N, Bernard D, Margl R, Cheng S, et al. Elevated frequencies of self-reactive CD8+ T cells following immunization with a xenoantigen are due to the presence of a heteroclitic CD4+ T-cell helper epitope. Cancer Res. 2007;67:6459–6467. doi: 10.1158/0008-5472.CAN-06-4336. [DOI] [PubMed] [Google Scholar]
- Grinshtein N, Ventresca M, Margl R, Bernard D, Yang TC, Millar JB, et al. High-dose chemotherapy augments the efficacy of recombinant adenovirus vaccines and improves the therapeutic outcome. Cancer Gene Ther. 2009;16:338–350. doi: 10.1038/cgt.2008.89. [DOI] [PubMed] [Google Scholar]
- Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CW, Stephenson KB, Hanson S, Kucharczyk M, Duncan R, Bell JC, et al. The p14 FAST protein of reptilian reovirus increases vesicular stomatitis virus neuropathogenesis. J Virol. 2009;83:552–561. doi: 10.1128/JVI.01921-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Toda M, Watanabe M, Iizuka Y, Kubota T, Kitajima M, et al. In situ cancer vaccination with a replication-conditional HSV for the treatment of liver metastasis of colon cancer. Cancer Gene Ther. 2002;9:142–148. doi: 10.1038/sj.cgt.7700407. [DOI] [PubMed] [Google Scholar]
- Li H, Dutuor A, Fu X., and, Zhang X. Induction of strong antitumor immunity by an HSV-2-based oncolytic virus in a murine mammary tumor model. J Gene Med. 2007;9:161–169. doi: 10.1002/jgm.1005. [DOI] [PubMed] [Google Scholar]
- Toda M, Rabkin SD, Kojima H., and, Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- Bertrand P, Côté M, Zheng YM, Albritton LM., and, Liu SL. Jaagsiekte sheep retrovirus utilizes a pH-dependent endocytosis pathway for entry. J Virol. 2008;82:2555–2559. doi: 10.1128/JVI.01853-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KJ, Kim JH, Lee YS, Kim J, Suh BS, Kim H, et al. Concurrent delivery of GM-CSF and B7-1 using an oncolytic adenovirus elicits potent antitumor effect. Gene Ther. 2006;13:1010–1020. doi: 10.1038/sj.gt.3302759. [DOI] [PubMed] [Google Scholar]
- Fukuhara H, Ino Y, Kuroda T, Martuza RL., and, Todo T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system. Cancer Res. 2005;65:10663–10668. doi: 10.1158/0008-5472.CAN-05-2534. [DOI] [PubMed] [Google Scholar]
- Shin EJ, Wanna GB, Choi B, Aguila D, 3rd, Ebert O, Genden EM, et al. Interleukin-12 expression enhances vesicular stomatitis virus oncolytic therapy in murine squamous cell carcinoma. Laryngoscope. 2007;117:210–214. doi: 10.1097/01.mlg.0000246194.66295.d8. [DOI] [PubMed] [Google Scholar]
- Su C, Peng L, Sham J, Wang X, Zhang Q, Chua D, et al. Immune gene-viral therapy with triplex efficacy mediated by oncolytic adenovirus carrying an interferon-γ gene yields efficient antitumor activity in immunodeficient and immunocompetent mice. Mol Ther. 2006;13:918–927. doi: 10.1016/j.ymthe.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Varghese S, Rabkin SD, Liu R, Nielsen PG, Ipe T., and, Martuza RL. Enhanced therapeutic efficacy of IL-12, but not GM-CSF, expressing oncolytic herpes simplex virus for transgenic mouse derived prostate cancers. Cancer Gene Ther. 2006;13:253–265. doi: 10.1038/sj.cgt.7700900. [DOI] [PubMed] [Google Scholar]
- Vigil A, Park MS, Martinez O, Chua MA, Xiao S, Cros JF, et al. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 2007;67:8285–8292. doi: 10.1158/0008-5472.CAN-07-1025. [DOI] [PubMed] [Google Scholar]
- Zhao H, Janke M, Fournier P., and, Schirrmacher V. Recombinant Newcastle disease virus expressing human interleukin-2 serves as a potential candidate for tumor therapy. Virus Res. 2008;136:75–80. doi: 10.1016/j.virusres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Chuang CM, Monie A, Wu A, Pai SI., and, Hung CF. Combination of viral oncolysis and tumor-specific immunity to control established tumors. Clin Cancer Res. 2009;15:4581–4588. doi: 10.1158/1078-0432.CCR-08-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- Vigil A, Martinez O, Chua MA., and, García-Sastre A. Recombinant Newcastle disease virus as a vaccine vector for cancer therapy. Mol Ther. 2008;16:1883–1890. doi: 10.1038/mt.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vile R, Ando D., and, Kirn D. The oncolytic virotherapy treatment platform for cancer: unique biological and biosafety points to consider. Cancer Gene Ther. 2002;9:1062–1067. doi: 10.1038/sj.cgt.7700548. [DOI] [PubMed] [Google Scholar]
- Stewart TJ., and, Abrams SI. How tumours escape mass destruction. Oncogene. 2008;27:5894–5903. doi: 10.1038/onc.2008.268. [DOI] [PubMed] [Google Scholar]
- Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2004;25:98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Van Rompay KK, Abel K, Earl P, Kozlowski PA, Easlick J, Moore J, et al. Immunogenicity of viral vector, prime-boost SIV vaccine regimens in infant rhesus macaques: attenuated vesicular stomatitis virus (VSV) and modified vaccinia Ankara (MVA) recombinant SIV vaccines compared to live-attenuated SIV. Vaccine. 2010;28:1481–1492. doi: 10.1016/j.vaccine.2009.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P, Beauchamp C, Evelegh C, Parks R., and, Graham FL. Development of a FLP/frt system for generating helper-dependent adenoviral vectors. Mol Ther. 2001;3:809–815. doi: 10.1006/mthe.2001.0323. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Stillman EA, Whitt MA., and, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grambsch P., and, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kinetics of T cell replication following VSV-hDCT treatment.
Increased interval between prime and boost enhances magnitude of response.