Abstract
Based on the discovery of coenzyme Q (CoQ) as an obligatory cofactor for H+ transport by uncoupling protein 1 (UCP1) [Echtay, K. S., Winkler, E. & Klingenberg, M. (2000) Nature (London) 408, 609–613] we show here that UCP2 and UCP3 are also highly active H+ transporters and require CoQ and fatty acid for H+ transport, which is inhibited by low concentrations of nucleotides. CoQ is proposed to facilitate injection of H+ from fatty acid into UCP. Human UCP2 and 3 expressed in Escherichia coli inclusion bodies are solubilized, and by exchange of sarcosyl against digitonin, nucleotide binding as measured with 2′-O-[5-(dimethylamino)naphthalene-1-sulfonyl]-GTP can be restored. After reconstitution into vesicles, Cl− but no H+ are transported. The addition of CoQ initiates H+ transport in conjunction with fatty acids. This increase is fully sensitive to nucleotides. The rates are as high as with reconstituted UCP1 from mitochondria. Maximum activity is at a molar ratio of 1:300 of CoQ:phospholipid. In UCP2 as in UCP1, ATP is a stronger inhibitor than ADP, but in UCP3 ADP inhibits more strongly than ATP. Thus UCP2 and UCP3 are regulated differently by nucleotides, in line with their different physiological contexts. These results confirm the regulation of UCP2 and UCP3 by the same factors CoQ, fatty acids, and nucleotides as UCP1. They supersede reports that UCP2 and UCP3 may not be H+ transporters.
Uncoupling proteins (UCPs) are supposed to uncouple oxidative phosphorylation. They are localized in the inner mitochondrial membrane and there return H+ pumped by the respiratory chain (1–4). Whereas this function is well established for UCP1 from brown adipose tissue, uncertainty reigns in the function of the “new” uncoupling proteins: UCP2, -3, -4 and brain mitochondrial carrier protein (see reviews in refs. 5–10). An extensive literature has developed, dealing in particular with the regulation of UCP expression by a variety of factors. Besides their role in thermogenesis, other potential roles of the UCP variants are proposed, such as in obesity, diabetes, fatty acid (FA) metabolism, suppression of oxygen radicals, and, thus, aging and neurodegeneration (11–24). This abundance of cellular research contrasts with the scarcity of biochemical characterization of these UCP variants. One reason is their low rate of occurrence, which prevents their isolation as native proteins. No convincing demonstration of a regulated uncoupling (H+ transport) function of the UCP variants has been forthcoming, despite considerable efforts by several laboratories (22, 25–31). When making this judgment we assume that the characteristics of UCP1, such as H+ transport capacity, stimulation by FAs, and inhibition by nucleotides, also apply to the new UCP variants. We find that, despite only 58% (UCP2) and 55% (UCP3) similarity to UCP1, 10 or 11 of the 12 residues identified in UCP1 so far that are involved in the above-mentioned functions are conserved in UCP2 and UCP3 (32).
Because UCP2 and UCP3 occur only in low amounts in the respective tissues, functional characterization depended on overexpression in yeast or in Escherichia coli. The expression of UCP2 and UCP3 in Saccharomyces cerevisiae was based on the expectation that the previous successes with UCP1 in yeast (33–36) will also apply to these variants. However, the results were quite disparate from those for UCP1. Yeast cells with UCP2 and UCP3 seemed to be more uncoupled than with UCP1 (37–41), and uncoupling of isolated mitochondria demonstrated no clear FA dependence or inhibition by nucleotides (22, 25–28, 31). The conclusion that uncoupling by UCP2 and UCP3 is different from that by UCP1 (i.e., not regulated by FA and nucleotides) has now proved to be premature. We have shown that UCP3 expressed in yeast, which is different from UCP1, is not in a native conformation (31). Traces of UCP3 incorporated into mitochondria cause H+ leakage, but the majority of UCP3 is in an aggregated state in inclusion bodies. Thus the yeast expression system, which has been of great value for UCP1, is unsuitable for UCP3 and probably also for UCP2. In E. coli abundant expression of these UCP variants in inclusion bodies was achieved (29, 30). Disparate claims are made for the reconstituted UCP3. We reported the ability of UCP1 as well as that of UCP3 from E. coli to transport Cl− but not H+ (29). The Cl− transport was highly nucleotide sensitive. Thus we had identified for UCP3 a regulation by nucleotides. In contrast, Jaburek et al. (30) claimed that reconstituted UCP2 and UCP3 from E. coli transport H+ but required for inhibition a more than 1,000-fold higher nucleotide concentration than UCP1.
On the basis of the specific lack of H+ transport we proposed that a cofactor might be missing in UCP1 when expressed in E. coli and possibly also in UCP3 (30). Extensive experimentation with mitochondrial UCP1 showed that the cofactor is not covalently bound and is present in mitochondrial lipids (42). From the total lipid extracts a neutral lipid fraction was obtained that strongly activated H+ transport. By a variety of experiments, finally coenzyme Q (CoQ, ubiquinone) was identified as the cofactor obligatory for H+ transport by UCP1 (42). The high H+ transport rate activated with CoQ in conjunction with FA matched the maximum rates of native UCP1 and were extremely sensitive to nucleotides (KI ≈ 0.1 μM).
Here we show that UCP2 and UCP3, when renatured and reconstituted under the same conditions established for the activation of UCP1, can also exhibit high H+ transport activity in the presence of CoQ. This H+ transport is highly sensitive to nucleotides and fulfills our criteria for an UCP-linked activity. Thus H+ transport by UCP2 and UCP3 is clearly established. The important implications of these findings are discussed.
Materials and Methods
Strains and Reagents.
Restriction enzymes for subcloning were purchased from Roche Molecular Biochemicals or New England Biolabs and used as recommended by the supplier. Hamster uncoupling protein 1 (UCP1) gene was cloned from hamster brown adipose tissue as described earlier (36). Human UCP2 and UCP3 genes were obtained from J. P. Giacobino and P. Muzzin (University of Geneva Medical School, Geneva). UCP1 gene from hamster was isolated as described (36). E. coli cells BL21 (DE3) plysS were transfected with the plasmid (pET24a+) obtained from Novagen. The fluorescence dye pyranine (8-hydroxypyrene-1,3,6-trisulfonic acid, trisodium salt) was from Molecular Probes. 2′-O-[5-(Dimethylamino)naphthalene-1-sulfonyl]-GTP (dansyl-GTP) was synthesized as described by Huang and Klingenberg (43, 44). Egg yolk phospholipid was isolated from fresh eggs and purified with alumina B Supper 1 from ICN as described previously (45). The detergent n-decylpentaoxyethylene (C10E5), N-laurylsarcosine, and digitonin were obtained from Fluka. The water-soluble form of digitonin was generated by recrystallization. Dowex 1-X8 (200–400 mesh), Amberlite XAD2, and CoQ (CoQ0, CoQ1, CoQ2, CoQ6, CoQ10) were obtained from Sigma. CoQ compounds were dissolved in dichloromethane, and the concentration was estimated from UV spectra.
Bacterial Expression, Isolation, and Renaturation of Inclusion Bodies Containing UCPs.
A colony of E. coli cells that has been transfected with the plasmid containing the UCP gene was inoculated into LB medium (10 g tryptone, 5 g yeast extract, 10 g NaCl, 30 mg kanamycin in 1 liter). The culture was grown at 37°C. Expression was induced with 1 mM isopropyl β-d-thiogalactoside. Inclusion bodies (IBs) were isolated as described (9). IB-UCP was solubilized and prepared for nucleotide binding and reconstitution according to the following procedure (see also ref. 43): IB (≈5 mg) were dissolved in 400 μl of 2% (wt/vol) sarcosyl in buffer A [50 mM NH4HCO3/2 mM dithioerythritol/2 mM PMSF, pH 8.0] followed by centrifugation at 12,500 rpm for 30 min. The supernatant was diluted by buffer A to a final sarcosyl concentration of 0.5% and a protein concentration of 3 mg/ml. For renaturing the UCP sarcosyl was replaced by digitonin. The protein was diluted to a final concentration of 0.6 mg/ml by buffer B (1 mM dithioerythritol/50 μM β-mercaptoethanol/100 mM potassium phosphate/0.2% water-soluble digitonin/0.1% sarcosyl, pH 8.0), concentrated 3-fold by pressure dialysis at 4°C and diluted again to the original volume by buffer B without sarcosyl. This step was repeated four times and followed twice by buffer C (1 mM dithioerythritol/1 mM EDTA/100 mM potassium phosphate, pH 8.0). The whole process took about 10 h. In an attempt to remove potential residual sarcosyl, 1 ml of digitonin-solubilized IB protein (≈1.5 mg protein/ml) was treated with anion exchange resin 21K Dowex mesh 20–50 (800 mg Cl− form plus 20 mg normal OH− form) with shaking at 4°C overnight. The yield of the renatured protein was 80%.
Reconstitution of UCPs from Inclusion Bodies into Phospholipid Vesicles and Measurements of H+ Transport.
The solubilized digitonin-treated protein was reconstituted into liposomes similar to those described previously (29, 42). Egg yolk phospholipid was solubilized with C10E5 to a final detergent/phospholipid ratio of 1.4 by mass and a solution of 100 mM potassium phosphate, 0.2 mM EDTA, and 1 mM PMSF (pH 7.6). The addition of digitonin-treated IB at a phospholipid/protein ratio of 350 by mass resulted in a phospholipid concentration of 17 mg/ml and a protein concentration of 0.05 mg/ml. The detergent was stepwise removed with 360 mg Amberlite XAD2 in 20-min intervals with gentle shaking overnight at 4°C. For the removal of external solutes the vesicles were passed over Sephadex G-75 equilibrated with 0.5 mM Hepes, 0.2 mM EDTA (pH 7.3), and 0.28 M sucrose. H+ transport was assayed with the use of pyranine as a pH fluorescence probe at λex = 467 nm and λem = 510 nm in a medium containing 280 mM sucrose, 0.5 mM Hepes, 0.2 mM EDTA, 1 μM pyranine (pH 6.8), and laurate. Valinomycin (2.5 μM) was added to initiate the H+ uptake driven by the K+ efflux. The uncoupler carbonylcyanide m-chlorophenylhydrazone (1 μM) was added to determine the vesicle capacity for H+ uptake.
Results
Renaturation of Recombinant UCPs from Inclusion Bodies of E. coli.
Our efforts improved the procedure for renaturation of UCP from inclusion bodies (IB-UCP) over the previously described method (29). As reported, UCP1 as well as UCP3 solubilized by sarcosyl from inclusion bodies and reconstituted into phospholipid vesicles displayed a normal Cl− transport activity similar to that of the mitochondrial UCP1 but failed to yield equivalent FA-dependent H+ transport activity. The specific involvement of UCP1 and UCP3 was demonstrated by the high sensitivity to purine nucleotides. The inability to reconstitute H+ transport activity with UCP1 expressed in E. coli posed a challenge. Several explanations were considered: (i) H+ transport requires a more precise folding of the protein than Cl− transport. (ii) Traces of the solubilizing sarcosyl are still bound to UCP, which as a FA analogue may prevent FA binding. (iii) A cofactor necessary for H+ transport and present in UCP from mitochondria is missing from UCP from inclusion bodies.
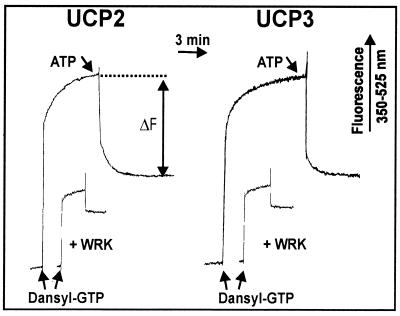
As an intermediate step toward regaining the native functions we have been able to restore nucleotide binding to the solubilized IB-UCP1 by replacing the sarcosyl stepwise with digitonin, followed by a treatment with anion exchanger in an attempt to remove residual sarcosyl (42). The same procedure was here applied to IB-UCP2 and IB-UCP3. The preparations consist approximately of 70% UCP as determined from dansyl-GTP binding, taking native UCP1 as a standard. The results presented in Fig. 1 show that, after this treatment, UCP2 and UCP3 have acquired the nucleotide binding capacity, as documented with the fluorescent derivative dansyl-GTP. On addition to UCP2 and UCP3, the fluorescence of dansyl-GTP is strongly increased. Only the fluorescence decrease observed with the subsequent addition of ATP is attributed to UCP binding. As a further control for the native conformation, the carboxyl reagent Woodward Reagent K (WRK) was used, which in UCP1 blocks specifically the carboxyl E190 at the entrance to the nucleotide binding site (36, 45) and should be expected to react with the homologues E192 (UCP2) and E195 (UCP3). As shown in Fig. 1, preincubation with low amounts of WRK largely suppresses the ATP-sensitive fluorescence in both UCP2 and UCP3. These results show that both UCP2 and UCP3 are able to bind nucleotides in a manner similar to that of UCP1. Furthermore, the sensitivity to WRK indicates that the nucleotide binding may have a similar pH dependence under the control of the homologues E192 (UCP2) and E195 (UCP3).
Figure 1.
Fluorescence response of [2′-O-(dimethylamino)-naphthaline-1-sulfonyl]-GTP (dansyl-GTP) binding to digitonin-treated IB-UCP2 and IB-UCP3. Dansyl-GTP (5 μM) was added to a solution of 45 μg protein/ml in 10 mM Mes/Hepes buffer containing 0.3% digitonin (pH 6.5) at 10°C. To differentiate the UCP-linked binding, 0.5 mM ATP was added to displace the fluorescent ligand. For a further discrimination, the binding site was inactivated by Woodward reagent K (WRK). WRK at a concentration of 10 μM was incubated with the protein for 30 min before the addition of dansyl-GTP. Fluorescence was observed at λexc = 350 nm and λem = 525 nm.
Coenzyme Q Is an Obligatory Cofactor for H+ Transport by UCP2 and UCP3.
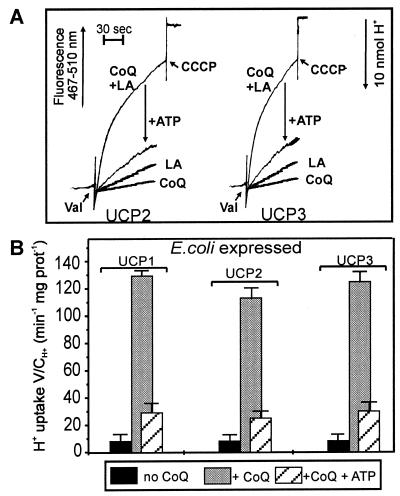
With these IB-UCP preparations, after incorporation into phospholipid vesicles, Cl− but still no H+ transport could be measured (results not shown). Thus, despite the progress of recovering the nucleotide binding to the soluble protein by the intervening digitonin treatment, there was no progress in the recovery of H+ transport. The persistence of this defect rendered it more probable that a cofactor rather than an imperfect folding was responsible. In the previous report we outlined the search in mitochondrial lipid extracts for, and the final discovery of, this cofactor, which enabled us to recover H+ transport by IB-UCP1 (42). At an earlier stage in this pursuit, we found that a total mitochondrial lipid extract and then the neutral lipid fraction of this extract (see figure 2 in ref. 42) were also able to activate H+ transport in proteoliposomes with IB-UCP2 and IB-UCP3 as well as with IB-UCP1 (data not shown). After the identification of CoQ in this extract as the activating principle for IB-UCP1, we show here that addition of CoQ10 to reconstituted IB-UCP2 and -3 also resulted in a strong stimulation of H+ transport reaching a rate of 120 μmol/min⋅mg protein (Fig. 2). This H+ transport is only activated in conjunction with FA and is inhibited by micromolar concentrations of ATP. The ATP-insensitive H+ transport is the same as when the solvent alone is added (not shown). Thus the CoQ-enhanced activity is fully sensitive to nucleotide.
Figure 2.
Activation of H+ transport in UCP2 and UCP3 by CoQ10. H+ influx into phospholipid vesicles reconstituted with digitonin-treated IB-UCP2 and IB-UCP3 was recorded. (A) Recordings of H+ uptake in reconstituted UCP liposomes in the presence of CoQ10 (2 nmol) and absence of LA, with LA (125 μM) and without CoQ10, with CoQ10 and LA, and the inhibition with 20 μM ATP. (B) Evaluated H+ transport rates. H+ influx was measured as the change in external pH monitored by pyranine fluorescence at λex = 467 nm and λem = 510 nm in the presence of 125 μM LA. In all experiments (A and B) a 50-μl portion of vesicles containing 1.3 μg protein and 420 μg phospholipid was added to 0.5 mM Hepes buffer (pH 7.3) containing 1 μM pyranine, 0.5 mM EDTA, and 280 mM sucrose to a final volume of 330 μl at pH 6.8 and 10°C. H2SO4 was added in steps of 10 nmol H+ to adjust the pH to 6.8, and valinomycin (Val) was added to a concentration of 2.5 μM. Uncoupling by 1 μM carbonylcyanide m-chlorophenylhydrazone determined the capacity of H+ uptake of the vesicles. Results are presented as the initial H+ transport rate (V: μmol/min mg protein) divided by the capacity of the vesicles (C: μmol/ml).
Dose Dependence on CoQ and FA.
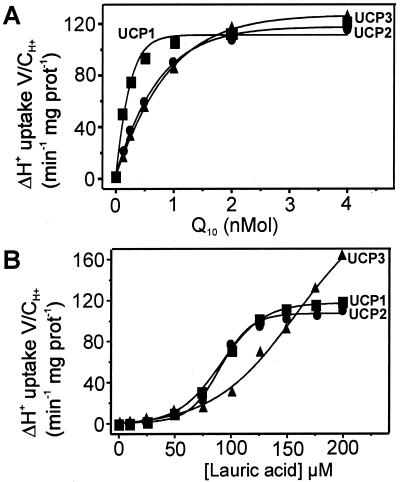
Because both CoQ and FA must be regarded as potential regulatory factors for UCP, the dependence of H+ transport on their dose is of particular interest. With the same conditions and measurements as presented in Fig. 2, the H+ transport activity was titrated with increasing amounts of CoQ at a near-saturating concentration of lauric acid (125 μM) (Fig. 3A). The uptake rate corresponds to the nucleotide sensitive portion, i.e., the difference to the rate measured with 20 μM ATP. Saturation is reached with low amounts of CoQ (between 1 and 2 nmol). The half-saturations are 0.3 nmol CoQ for UCP1 and 1.13 nmol for UCP2 and -3 (Table 1). Because most of the CoQ should be transferred from the solvent into the phospholipid, the molar ratio of CoQ to phospholipid is critical. Full activation is reached when CoQ reaches molar ratios of 1:300 to phospholipids and 80:1 to UCP dimer. Fig. 3B gives the dependence on FA concentration at a given saturating amount of CoQ (2 nmol). Again only the difference in the ATP-inhibited H+ transport rate is presented. The increase is nonlinear up to 50 μM lauric acid (LA) for all three UCP variants. This nonlinearity is unexpected, because in proteoliposomes with native UCP1 a linear dependence of FA was observed (46). Presumably low amounts of FA were sequestered by the solvent in which CoQ had to be added to the vesicles. There are notable differences between the UCP variants. With UCP1 and UCP2 the H+ transport is saturated with 150 μM lauric acid, whereas with UCP3 no saturation up to 200 μM is observed. The half-saturation is at 80 μM lauric acid with UCP1 and -2, and with UCP3 it is >130 μM.
Figure 3.
Dependence of H+ transport by UCP2 and UCP3 on the dose of CoQ and FA. H+ transport activity measured with IB-UCP1, -2, and -3. (A) Titration of activity with CoQ10 (nmol/0.4 mg phospholipid). H+ transport of IB-UCP in reconstituted vesicles is measured as shown in Fig. 2 with an increasing amount of CoQ at saturating concentration of lauric acid (125 μM). (B) Titration with lauric acid at 2 nmol CoQ. The net transport activity given was obtained by subtracting the activity in the presence of 20 μM ATP.
Table 1.
The K1 values for ATP and ADP inhibition and the Km values for CoQ10 activation of H+ transport in proteoliposomes reconstituted with E. coli expressed UCP1, UCP2, and UCP3 at pH 6.8 and 10°C
| IB-UCP |
Ki
(μM)
|
KiATP/KiADP | Km (nmol) | |
|---|---|---|---|---|
| ATP | ADP | |||
| UCP1* | 0.05 | 0.08 | 0.62 | 0.3 |
| UCP2 | 0.09 | 0.19 | 0.47 | 1.14 |
| UCP3 | 0.23 | 0.08 | 2.87 | 1.13 |
Values from ref. 42. UCP1 is from hamster, UCP2 and -3 from human.
Concentration Dependence of Inhibition by Nucleotides.
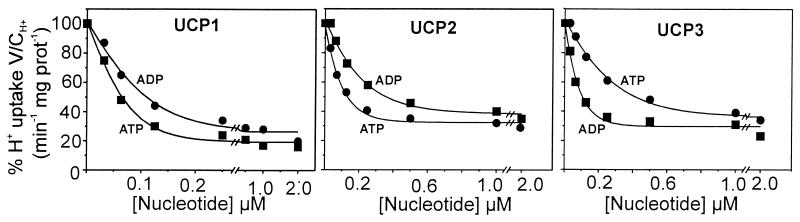
The activation of H+ transport by CoQ now makes possible the determination of the inhibition by purine nucleotides, which is a most characteristic attribute of UCP. Previously a striking difference in nucleotide specificity between IB-UCP1 and IB-UCP3 for the inhibition of the Cl− transport was found, i.e., an inversion of the KI between nucleoside di- and triphosphate (29). With this background we determined here the dependence of the inhibition on both the ADP and ATP concentrations, with saturating additions of CoQ10 (2 nmol) and LA (125 μM). Fig. 4 also includes the published (42) nucleotide concentration dependence for UCP1 to illustrate the difference between the three UCP variants. The residual activity after saturating amounts of nucleotide is still 25%, but this is largely due to the solvent-induced H+ leakage and to a lesser extent to UCP inverted in the membrane, the inhibition site of which cannot be accessed by external ADP or ATP.
Figure 4.
Inhibition of H+ transport by UCP1, -2, and -3 from E. coli by ATP and ADP after stimulation with CoQ and FA. H+ transport measurements were performed as described in the legend to Fig. 2 at fixed amounts of lauric acid (125 μM) and CoQ (2 nmol). The KI values for ATP and ADP inhibition are contained in Table 1. The values for UCP1 are from (42).
The most striking feature of H+ transport inhibition by nucleotide is the inverse sensitivity toward ADP and ATP between UCP1 and UCP2 on the one side and UCP3 on the other side. UCP2 has a higher sensitivity toward ATP than to ADP as UCP1, whereas UCP3 is more sensitive to ADP than to ATP. The KI values presented in Table 1 underline the very high sensitivity of H+ transport to nucleotide in the submicromolar range. The KI for ATP shows the strongest variation among the three UCP variants. It increases by nearly 2-fold from UCP1 to UCP2 and by nearly 5-fold from UCP1 to UCP3. The KI for ADP varies less, with a 2-fold increase for UCP2 and no change for UCP3. More significant is the ratio KIATP/KIADP, which is about 0.5 for UCP1 and UCP2 but increases to nearly 3 for UCP3. The important implications for the intracellular regulation and function of these UCP variants will be discussed below.
Structural Requirements of CoQ.
The structural requirements of CoQ and its analogues for the activation of H+ transport were studied by changing the hydrophobic side chain. It had been shown for UCP1 that the activation depended on the hydrophobic tail length of CoQ and decreased markedly with a shorter polyisoprenoid chain. It is likely that similar structural requirements for the quinone apply to UCP2 and UCP3. The results are summarized in Table 2. As in UCP1, and in UCP2 and UCP3, a similar decrease in H+ transport stimulation with a shorter isoprenoid chain was observed. Only minor activation was left when no or one isoprenoid unit was present. With CoQ2 a somewhat better activation of UCP3 than of UCP1 and UCP2 was noted.
Table 2.
The dependence of H+ transport activation of E. coli expressed UCP1, UCP2, and UCP3 on CoQ analogues
| Percentage of Δ H+
transport V/CH+,
min−1 mg⋅protein−1
|
|||
|---|---|---|---|
| UCP1 | UCP2 | UCP3 | |
| CoQ10 | 100 | 100 | 100 |
| CoQ6 | 77 | 75 | 62 |
| CoQ2 | 25 | 15 | 37 |
| CoQ1 | 10 | 7 | 20 |
| CoQ0 | 10 | 7 | 7 |
Transport activities of IB-UCPs in reconstituted phospholipid vesicles were measured as described in Fig. 2 in the presence of CoQ analogues (2 nmol). Influxes (V; μmol H+/min mg protein; C, capacity: μmol/ml) are given as net-transport activities obtained by subtracting the activity in the presence of 20 μM ATP and presented as a percentage of the activity in the presence CoQ10.
Discussion
This paper reports two major findings for the function of UCP2 and UCP3. First, the discovery that coenzyme Q is an obligatory cofactor for the H+ transport function of UCP1 (42) applies also to UCP2 and UCP3. Second, UCP2 and UCP3 are highly active H+ transporters, regulated by activation with FA and inhibition with nucleotides. The existence of UCP2 and UCP3 was reported in 1997 (37–41), but despite numerous efforts it has not been possible to convincingly delineate the functions of UCP2 and UCP3. Several authors have expressed uncertainty about whether UCP2 and UCP3 are H+ transporters at all, inasmuch as the response of expressing UCP2 and UCP3 in mammalian cells to many stimuli often seemed not to be compatible with an uncoupling function of UCP2 and UCP3 (13, 18, 23, 47). Moreover, the ablation of UCP3 in mice did not produce obesity (19, 20). However, overexpression of UCP3 in mice caused hyperphagicity (24).
A common way to screen the functions of UCP variants was the expression in S. cerevisiae (37–41). In contrast to UCP1, in yeast cells expression of UCP2 and UCP3 seemed to cause uncoupling of the mitochondria. Accordingly, in isolated yeast mitochondria the uncoupling by UCP2 and UCP3 was not inhibited by low concentration of nucleotide and barely stimulated by FA, whereas uncoupling in UCP1-containing yeast mitochondria was inhibited by nucleotides and required FA (25–28). These authors concluded that the regulation of uncoupling of UCP3 is basically different from that of UCP1. At variance, we inferred that UCP3 in yeast mitochondria, in contrast to UCP1, is in a deranged conformation allowing uncontrolled H+ leakage (31). The same conclusion can possibly be extended to UCP2, as judged from the results published with yeast mitochondria containing UCP2. Thus the yeast must be regarded as an unsuitable host for the expression of UCP variants.
With UCP2 and UCP3 expressed in E. coli and reconstituted into proteoliposomes, H+ transport has been reported (30). In view of the present finding some clarifications are required. The maximum H+ transport rates were 10–30 μmol/min⋅mg protein. Because the authors did not specify the temperature, we assume room temperature (25°C). Our measurements are performed at 10°C and give rates of 110–130 μmol/min⋅mg protein. Allowing for the 15 C° temperature difference and a concomitant 3-fold rate change, the rates in the presence of CoQ are about 20 times higher than those reported (30). Our measurement without CoQ but with LA gives 7 μmol/min⋅mg (Fig. 2), which, when converted to room temperature, would be 20 μmol/min⋅mg and thus would be similar to the transport rates reported. This H+ transport was not inhibited by 100 μM ATP, the maximum concentration that we apply, and 90% inhibition was reached at 2 mM ATP (30). The very low sensitivity to nucleotide indicates that the reported low H+ transport was not due to UCP but to an unspecific H+ leakage, e.g., to the ternary FA-valinomycin-K+ complex diffusion induced by the K+ gradient. Traces of detergent introduced with UCP to the reconstituted vesicles may enhance the ternary complex diffusion. An inhibition by millimolar ATP is unphysiological, inasmuch as in the cell there are only about 100 μM free ATP (not complexed with Mg2+). It was misleading (30) to compare UCP1 isolated from mitochondria with UCP2 and UCP3 from E. coli when it was stressed that UCP1 is much more sensitive to nucleotides than are UCP2 and UCP3. We showed that UCP1 reconstituted from E. coli has the same inability for a high H+ transport activity with the same nucleotide sensitivity in Cl− transport as UCP3 (29).
UCP2 and UCP3 are H+ Transporters Similar to UCP1.
With this background of conflicting results, the present finding that UCP2 and UCP3 are highly active H+ transporters gains enhanced significance. It vindicates our working hypothesis that UCP2 and UCP3 essentially follow the paradigm UCP1, which was based on the conservation in UCP2 and UCP3 of most residues involved in nucleotide binding and H+ transport. We can now conclude that the extension of the designation “uncoupling protein,” originating from UCP1 (46), to UCP2 and UCP3 is justified. However, it could not yet be foreseen on the basis of the amino acid sequence that UCP2 and UCP3, like UCP1, also require CoQ, whereas nucleotide binding could be predicted. This dependence underlines the fundamental role of CoQ in activating H+ transport. In the presence of CoQ, H+ transport in UCP2 and UCP3 is also dependent on FA and fully inhibited by very low nucleotide concentration. Thus FA dependency and nucleotide sensitivity remain hallmarks of UCP-catalyzed H+ transport.
The Structural Requirement for Ubiquinone.
The comparison of the activation by various CoQ homologues shows a certain structural tolerance for the hydrophobic side chain, which anchors CoQ in the membrane. Only a minimum length of 10 to 13 C atoms is required. This requirement indicates that CoQ with its quinone head group has to be deeply imbedded to interact with UCP, apparently at the interface with the hydrophobic layer. Here again UCP3 differs from UCP1 and UCP2 in that it accepts shorter chain length, i.e., C10 derivatives, which are unable to activate UCP1 and UCP2. The lower hydrophobicity may indicate that the quinone docking site in UCP3 is positioned more to the membrane surface than in UCP1 and UCP2. In this context it is interesting that UCP3 requires higher FA amounts than UCP1 and UCP2 for H+ transport activation.
The Mechanism of the CoQ Effect as a Catalyst for FA.
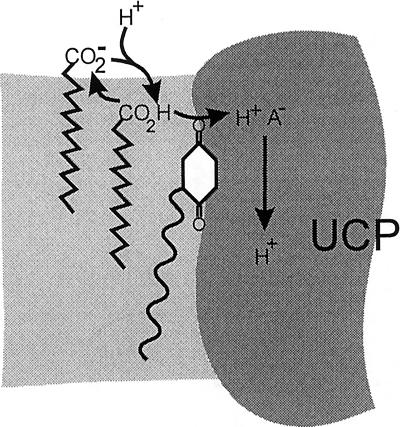
The cofactor role of CoQ for H+ transport is accompanied by the exclusive requirement for FA. An attractive model for the mechanistic function of CoQ seems to associate CoQ directly with the role of FA. Both CoQ and FA are amphiphiles and are bound to the phospholipids. Whereas the hydrophilic carboxyl group of FA is primarily anchored to the phospholipid head group region, the quinone group of CoQ can penetrate more easily into the hydrophobic layer (48). It can be visualized that by forming a hydrogen bond between the quinone oxo group and the FA carboxyl group, CoQ may facilitate the entry of undissociated FA (FAH) into the H+ translocation route. For example, in an active catalytic role, CoQ would subtract H+ from FAH+ and inject the H+ into the UCP to a H+ acceptor group (Fig. 5). The quinone oxo group could provide an energetic advantage for the H+ dissociation from FAH+ in the low dielectric environment of the lipid phase.
Figure 5.
Tentative mechanism of the cofactor role of CoQ as a catalyst of FA-mediated H+ transfer into UCP. FAH forms a H-bond with the quinone oxo group bound to UCP. The FAH-CoQ complex facilitates the delivery of the FA carboxyl to UCP, where it donates H+ to an acceptor group. The FA− returns to the surface and diffuses back to the membrane, where it collects another H+. The light shaded area corresponds to phospholipids and the dark shaded area to UCP protein.
Recently, activating and inhibiting effects of CoQ and derivatives on the mitochondrial transition pore were reported (49, 50). In contrast to UCP, the water-soluble CoQ0 was also effective as an inhibitor. In view of the possible involvement of the ADP/ATP carrier in the mitochondrial transition pore (51, 52), it is tempting to speculate that the interactions with CoQ are similar with the mitochondrial transition pore and UCP. However, the different structural requirements and the entirely different functions involved render similar mechanisms unlikely.
The Inhibition by Nucleotides and Diverse Control by the ATP/ADP Ratio Among the UCP Variants.
The inhibition by low concentration of nucleotide as formerly reported for the UCP1- and UCP3-catalyzed Cl− transport (29) is extended here to CoQ-activated H+ transport of both UCP1 and UCP3. Formerly with reconstituted native UCP1 a micromolar range was determined (29, 44, 46). The low KI not only of UCP2 and UCP3 but also of UCP1 when they are reconstituted from E. coli is surprising and seems to argue against a role for nucleotide in that regulation. Possibly the quite different mitochondrial phospholipids decrease the binding affinity of UCPs in their natural environment. This aspect is crucial for the in vivo control and has still to be explored. Another factor is the strong pH dependency known for native UCP1. Preliminary experiments show that at pH > 7, also with the reconstituted UCP2 and UCP3, the inhibition requires a higher concentration of ATP and ADP.
An interesting feature of nucleotide binding is the inverse sensitivity to ATP and ADP among the UCP variants. Noted first for Cl− transport by reconstituted UCP1 and UCP3 from E. coli (29), it is here extended to the CoQ-activated H+ transport with the three UCP variants. The different responses to nucleotides are best described by the ratio KIATP/KIADP, which is similar (0.5) for UCP1 and UCP2 but increases to nearly 3 for UCP3. This increase is mostly due to a 3- to 5-fold decrease in the affinity for ATP in UCP3. Herewith a regulatory difference between the UCP variants has been defined. The physiological implications have previously been discussed, under the premise that the differential sensitivity of Cl− transport will eventually translate to the H+ transport (29), as confirmed by the present results. The essence of the argument is as follows: UCPs would be regulated not only by the concentration of free (i.e., not Mg2+-bound) ATP and ADP and by the pH, but also by the ATP/ADP ratio. In brown adipose tissue uncoupling thermogenesis is the dominant function and is associated with a strong uncoupling and a low ATP/ADP ratio. In active skeletal muscle, the major site of UCP3, abundant heat is generated by the contraction cycles, and no additional uncoupling is required. Accordingly, UCP3 would be inhibited by the increased ADP in conjunction with the lower ATP/ADP ratio. In the resting state additional heat is required, and UCP3 would be activated by the decrease in ADP.
UCP2 is more widely distributed (7, 8), and its regulation by ATP/ADP is similar to that of UCP1, i.e., it is more active at a low ATP/ADP ratio. This observation may indicate that UCP2 has a more general role in uncoupling and metabolic stimulation. UCP2 would favor conditions where the rate of energy production is more important than the maximum energy efficiency. A partial uncoupling decreases the intramitochondrial NADH/NAD ratio, provides more NAD to the dehydrogenase, and alleviates this bottleneck of substrate oxidation. A concomitant role in lowering reactive oxygen species has been discussed for UCP2 (8).
Acknowledgments
We thank Jean-Paul Giacobino and Patrick Muzzin, University of Geneva Medical School, for supplying the cDNA of UCP2 and UCP3. The work was supported by a grant from the Deutsche Forschungsgemeinschaft.
Abbreviations
- CoQ
coenzyme Q (ubiquinone)
- IB-UCP
uncoupling protein from inclusion bodies of E. coli
- dansyl-GTP
2′-O-[5-(dimethylamino)naphthalene-1-sulfonyl]-GTP
- WRK
Woodword reagent K
- FA
fatty acid
- LA
lauric acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Nicholls D G, Locke R M. Physiol Rev. 1984;64:1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Cannon B, Nedergaard J. Essays Biochem. 1985;20:110–164. [PubMed] [Google Scholar]
- 3.Klingenberg M. Trends Biochem Sci. 1990;15:108–112. doi: 10.1016/0968-0004(90)90194-g. [DOI] [PubMed] [Google Scholar]
- 4.Klingenberg M, Huang S G. Biochim Biophys Acta. 1999;1415:269–271. doi: 10.1016/s0005-2736(98)00232-6. [DOI] [PubMed] [Google Scholar]
- 5.Muzzin P, Boss O, Giacobino J P. J Bioenerg Biomembr. 1999;31:467–473. doi: 10.1023/a:1005448423731. [DOI] [PubMed] [Google Scholar]
- 6.Boss O, Muzzin P, Giacobino J P. Eur J Endocrinol. 1998;139:1–9. doi: 10.1530/eje.0.1390001. [DOI] [PubMed] [Google Scholar]
- 7.Ricquier D, Fleury C, Larose M, Sanchis D, Pecqueur C, Raimbault S, Gelly C, Vacher D, Cassard-Doulcier A M, Levi-Meyrueis C, et al. J Intern Med. 1999;245:637–642. doi: 10.1046/j.1365-2796.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 8.Ricquier D, Bouillaud F. Biochem J. 2000;345Part 2:161–179. [PMC free article] [PubMed] [Google Scholar]
- 9.Fleury C, Sanchis D. Int J Biochem Cell Biol. 1999;31:1261–1278. doi: 10.1016/s1357-2725(99)00049-7. [DOI] [PubMed] [Google Scholar]
- 10.Boss O, Hagen T, Lowell B B. Diabetes. 2000;49:143–156. doi: 10.2337/diabetes.49.2.143. [DOI] [PubMed] [Google Scholar]
- 11.Skulachev V P. Biochim Biophys Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 12.Reitman M L, He Y, Gong D W. Int J Obes Relat Metab Disord. 1999;23,Suppl. 6:56–59. doi: 10.1038/sj.ijo.0800948. [DOI] [PubMed] [Google Scholar]
- 13.Surwit R S, Wang S, Petro A E, Sanchis D, Raimbault S, Ricquier D, Collins S. Proc Natl Acad Sci USA. 1998;95:4061–4065. doi: 10.1073/pnas.95.7.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong D W, He Y, Reitman M L. Biochem Biophys Res Commun. 1999;256:27–32. doi: 10.1006/bbrc.1999.0239. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita H, Sato Y, Mori N. FEBS Lett. 1999;458:157–161. doi: 10.1016/s0014-5793(99)01143-6. [DOI] [PubMed] [Google Scholar]
- 16.Cadenas S, Buckingham J A, Samec S, Seydoux J, Din N, Dulloo A G, Brand M D. FEBS Lett. 1999;462:257–260. doi: 10.1016/s0014-5793(99)01540-9. [DOI] [PubMed] [Google Scholar]
- 17.Van Der Lee K A, Willemsen P H, Van Der Vusse G J, Van Bilsen M. FASEB J. 2000;14:495–502. doi: 10.1096/fasebj.14.3.495. [DOI] [PubMed] [Google Scholar]
- 18.Nedergaard J, Matthias A, Golozoubova V, Jacobsson A, Cannon B. J Bioenerg Biomembr. 1999;31:475–491. doi: 10.1023/a:1005400507802. [DOI] [PubMed] [Google Scholar]
- 19.Gong D W, Monemdjou S, Gavrilova O, Leon L R, Marcus-Samuel B, Chou C J, Everett C, Kozak C, Harper M E, Reitman M L. J Biol Chem. 2000;275:16251–16257. doi: 10.1074/jbc.M910177199. [DOI] [PubMed] [Google Scholar]
- 20.Vidal-Puig A J, Grujic D, Zhang C Y, Hagen T, Boss O, Ido Y, Szczepanik A, Wade J, Mootha V, Muoio D M, Lowell B B. J Biol Chem. 2000;275:16258–16266. doi: 10.1074/jbc.M910179199. [DOI] [PubMed] [Google Scholar]
- 21.Samec S, Seydoux J, Dulloo A G. Pflugers Arch. 2000;439:723–729. doi: 10.1007/s004249900237. [DOI] [PubMed] [Google Scholar]
- 22.Hagen T, Zhang C Y, Vianna C R, Lowell B B. Biochemistry. 2000;39:5845–5851. doi: 10.1021/bi992980+. [DOI] [PubMed] [Google Scholar]
- 23.Matthias A, Ohlson K B, Fredriksson J M, Jacobsson A, Nedergaard J, Cannon B. J Biol Chem. 2000;275:25073–25081. doi: 10.1074/jbc.M000547200. [DOI] [PubMed] [Google Scholar]
- 24.Clapham J C, Arch J R, Chapman H, Haynes A, Lister C, Moore G B, Piercy V, Carter S A, Lehner Beeley L J, Godden R J, et al. Nature (London) 2000;406:415–418. doi: 10.1038/35019082. [DOI] [PubMed] [Google Scholar]
- 25.Hinz W, Faller B, Gruninger S, Gazzotti P, Chiesi M. FEBS Lett. 1999;448:57–61. doi: 10.1016/s0014-5793(99)00331-2. [DOI] [PubMed] [Google Scholar]
- 26.Hagen T, Zhang C Y, Slieker L J, Chung W K, Leibel R L, Lowell B B. FEBS Lett. 1999;454:201–206. doi: 10.1016/s0014-5793(99)00811-x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C Y, Hagen T, Mootha V K, Slieker L J, Lowell B B. FEBS Lett. 1999;449:129–134. doi: 10.1016/s0014-5793(99)00441-x. [DOI] [PubMed] [Google Scholar]
- 28.Hinz W, Gruninger S, De Pover A, Chiesi M. FEBS Lett. 1999;462:411–415. doi: 10.1016/s0014-5793(99)01568-9. [DOI] [PubMed] [Google Scholar]
- 29.Echtay K S, Liu Q, Caskey T, Winkler E, Frischmuth K, Bienengraber M, Klingenberg M. FEBS Lett. 1999;450:8–12. doi: 10.1016/s0014-5793(99)00460-3. [DOI] [PubMed] [Google Scholar]
- 30.Jaburek M, Varecha M, Gimeno R E, Dembski M, Jezek P, Zhang M, Burn P, Tartaglia L A, Garlid K D. J Biol Chem. 1999;274:26003–26007. doi: 10.1074/jbc.274.37.26003. [DOI] [PubMed] [Google Scholar]
- 31.Heidkaemper D, Winkler E, Mueller V, Frischmuth K, Liu Q, Caskey T, Klingenberg M. FEBS Lett. 2000;480:265–270. doi: 10.1016/s0014-5793(00)01949-9. [DOI] [PubMed] [Google Scholar]
- 32.Echtay K S, Winkler E, Bienengraeber M, Klingenberg M. Biochemistry. 2000;39:3311–3317. doi: 10.1021/bi992448m. [DOI] [PubMed] [Google Scholar]
- 33.Murdza-Inglis D L, Patel H V, Freeman K B, Jezek P, Orosz D E, Garlid K D. J Biol Chem. 1991;266:11871–11875. [PubMed] [Google Scholar]
- 34.Bathgate B, Freebairn E M, Greenland A J, Reid G A. Mol Microbiol. 1992;6:363–370. doi: 10.1111/j.1365-2958.1992.tb01479.x. [DOI] [PubMed] [Google Scholar]
- 35.Arechaga I, Raimbault S, Prieto S, Levi-Meyrueis C, Zaragoza P, Miroux B, Ricquier D, Bouillaud F, Rial E. Biochem J. 1993;296:693–700. doi: 10.1042/bj2960693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Echtay K S, Bienengraeber M, Klingenberg M. Biochemistry. 1997;36:8253–8260. doi: 10.1021/bi970513r. [DOI] [PubMed] [Google Scholar]
- 37.Gong D W, He Y, Karas M, Reitman M. J Biol Chem. 1997;272:24129–24132. doi: 10.1074/jbc.272.39.24129. [DOI] [PubMed] [Google Scholar]
- 38.Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin M F, Surwit R S, Ricquier D, Warden C H. Nat Genet. 1997;15:269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- 39.Boss O, Samec S, Paoloni-Giacobino A, Rossier C, Dulloo A, Seydoux J, Muzzin P, Giacobino J P. FEBS Lett. 1997;408:39–42. doi: 10.1016/s0014-5793(97)00384-0. [DOI] [PubMed] [Google Scholar]
- 40.Vidal-Puig A, Solanes G, Grujic D, Flier J S, Lowell B B. Biochem Biophys Res Commun. 1997;235:79–82. doi: 10.1006/bbrc.1997.6740. [DOI] [PubMed] [Google Scholar]
- 41.Gimeno R E, Dembski M, Weng X, Deng N, Shyjan A W, Gimeno C J, Iris F, Ellis S J, Woolf E A, Tartaglia L A. Diabetes. 1997;46:900–906. doi: 10.2337/diab.46.5.900. [DOI] [PubMed] [Google Scholar]
- 42.Echtay K S, Winkler E, Klingenberg M. Nature (London) 2000;408:609–613. doi: 10.1038/35046114. [DOI] [PubMed] [Google Scholar]
- 43.Huang S G, Klingenberg M. Biochemistry. 1995;34:349–360. doi: 10.1021/bi00001a043. [DOI] [PubMed] [Google Scholar]
- 44.Winkler E, Klingenberg M. J Biol Chem. 1994;269:2508–2515. [PubMed] [Google Scholar]
- 45.Winkler E, Wachter E, Klingenberg M. Biochemistry. 1997;36:148–145. doi: 10.1021/bi962178x. [DOI] [PubMed] [Google Scholar]
- 46.Lin C S, Klingenberg M. FEBS Lett. 1980;113:299–303. doi: 10.1016/0014-5793(80)80613-2. [DOI] [PubMed] [Google Scholar]
- 47.Ricquier D, Fleury C, Larose M, Sanchis D, Pecqueur C, Raimbault S, Gelly C, Vacher D, Cass A M, Levi-Meyrueis C, et al. J Intern Med. 1999;245:637–642. doi: 10.1046/j.1365-2796.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 48.Di Bernardo S, Fato R, Casadio R, Fariselli P, Lenaz G. FEBS Lett. 1998;426:77–80. doi: 10.1016/s0014-5793(98)00313-5. [DOI] [PubMed] [Google Scholar]
- 49.Fontaine E, Ichas F, Bernardi P. J Biol Chem. 1998;273(40):25734–25740. doi: 10.1074/jbc.273.40.25734. [DOI] [PubMed] [Google Scholar]
- 50.Walter L, Nogueira V, Leverve X, Heitz M P, Bernardi P, Fontaine E. J Biol Chem. 2000;275(38):29521–29527. doi: 10.1074/jbc.M004128200. [DOI] [PubMed] [Google Scholar]
- 51.Halestrap A P. Biochem Soc Symp. 1999;66:181–203. doi: 10.1042/bss0660181. [DOI] [PubMed] [Google Scholar]
- 52.Brustovetsky N, Klingenberg M. Biochemistry. 1996;35:8483–8488. doi: 10.1021/bi960833v. [DOI] [PubMed] [Google Scholar]