Abstract
Objective
To examine the rate of joint space width (JSW) loss in both knees of patients with unilateral medial joint space narrowing (JSN) at baseline.
Methods
Cases were selected from a pool of 2,678 subjects enrolled in the Osteoarthritis Initiative cohort. Inclusion criteria for the present study were unilateral medial JSN, bilateral frequent knee pain, and body mass index (BMI) ≥25 kg/m2. Baseline and 1-year fixed flexion radiographs of both knees were read (blinded to time point) using an automated algorithm for minimum JSW and JSW at 4 fixed locations in the medial compartment.
Results
Sixty-seven participants met the inclusion criteria: 43 women and 24 men, with mean ± SD age 60 ± 9 years and mean ± SD BMI 31 ± 4 kg/m2. Thirty-seven subjects (55%) had ≥1 definite tibiofemoral osteophyte. The average progression in no-JSN knees was comparable with that in JSN knees (approximately −0.2 mm/year). However, JSW change was more variable in no-JSN knees, resulting in standardized response means (SRMs; the mean/SD) of approximately −0.24 in no-JSN knees versus approximately −0.41 in JSN knees on average at the 4 fixed locations, and SRMs of −0.24 and −0.35, respectively, for minimum JSW. Young age and high BMI were associated with increased progression, especially in JSN knees.
Conclusion
JSN and no-JSN knees progressed at a comparable rate, but a wider distribution of JSW change in no-JSN knees resulted in a poorer sensitivity to change in these knees.
INTRODUCTION
Clinical development of disease-modifying osteoarthritis drugs (DMOADs) requires enrollment of a patient population with various and potentially conflicting characteristics. On the one hand, these patients should be at high risk of structural progression so that a trial with a reasonable sample size and duration would provide adequate power to detect a treatment difference between active drug and placebo. Multiple risk factors have been identified for knee osteoarthritis (OA) progression (1). Advanced OA may be one of these risk factors, being that several lines of evidence suggest that knees with baseline joint space narrowing (JSN) are at higher risk of radiographic progression than those without JSN (2-4). On the other hand, joints that already have established OA may be less responsive to pharmacologic treatment due to the overwhelming influence of biomechanical factors (altered alignment, loss of cartilage surface smoothness, ligament laxity, and meniscal damage).
The aim of this study was to assess the rate of structural progression in both knees of patients with baseline unilateral JSN as a means of evaluating the feasibility of detecting a DMOAD effect in a clinical trial enrolling this population. This specific OA population was chosen because the risk of structural progression in a given knee without baseline JSN (no-JSN knee) is higher when the contralateral knee has already lost some joint space (index knee = JSN-knee) than when the contralateral knee has not lost joint space (4). The no-JSN knee would be the target knee in a DMOAD trial, because it would be expected to have less damage than a JSN knee (and therefore less biomechanical constraints) but may have a significant progression over time due to the prevalence of a contralateral JSN, hence allowing the detection of a drug effect versus placebo.
We investigated the 1-year change in joint space width (JSW) in both knees of patients with baseline unilateral JSN who were overweight (body mass index [BMI] >25 kg/m2) and experienced frequent bilateral knee pain. High BMI increases the likelihood that the disease origin is general rather than local (5,6) and therefore increases the risk of progression in the no-JSN knee. In the same line of thought, prevalence of bilateral frequent symptoms increases the likelihood that the no-JSN knee also has OA and avoids inclusion of patients with unilateral disease who would be at low risk of progression in the no-JSN knee.
PATIENTS AND METHODS
Data for these analyses are from the public use data sets (0.2.1 and 1.2.1 clinical data sets and 0.B.1 and 1.B.1 imaging data sets) of the Osteoarthritis Initiative (OAI; online at www.oai.ucsf.edu/datarelease/DataImaging.asp), a multi-center, longitudinal cohort study designed to identify biomarkers for the development and/or progression of symptomatic knee OA.
Patient selection
The inclusion criteria for the present study were BMI >25 kg/m2, bilateral chronic frequent pain, unilateral medial tibiofemoral JSN (Osteoarthritis Research Society International [OARSI] grades 1–3), and no JSN in the lateral compartment (or less than in the medial compartment and with an OARSI JSN grade ≤1). The OAI protocol defined chronic frequent pain as “pain, aching, or stiffness on most days of at least a month during the previous year.”
Patients included in the present study were selected from the OAI cohort based on the above criteria and had baseline and 1-year knee radiographs and magnetic resonance images (MRIs) that were of adequate quality for review (only a few participants were excluded based on the absence of 1-year followup radiographs or the presence of metal artifacts on MRI analyses). The MRI analyses of this subgroup have been published elsewhere (7). Radiographs at baseline and 1 year were performed in fixed flexion position using a SynaFlexer Plexiglass positioning frame (Synarc) to achieve a reproducible foot fixation and knee flexion (8). The full OAI radiographic procedures manual can be downloaded from the OAI Web site after registration (online at www.oai.ucsf.edu/datarelease/OperationsManuals.asp ). The screening radiographs were read at each site and these readings are available in the OAI database.
Patients were selected in this study using 2 different processes (Figure 1). In the first process, after patients were selected based on the above inclusion criteria (according to the information available in the OAI database), their baseline radiographs were re-read centrally and adjudicated (in case of disagreement between site reading and central reading) for the presence and scoring of JSN and osteophytes in each compartment of both knees. Patients were included in the present study only if they fulfilled the above radiographic inclusion criteria based on central reading and, when necessary, the adjudicated reading. Of the 2,678 patients for whom radiographic data were already available in the OAI database at the time of this analysis, 48 patients were included based on this process.
Figure 1.
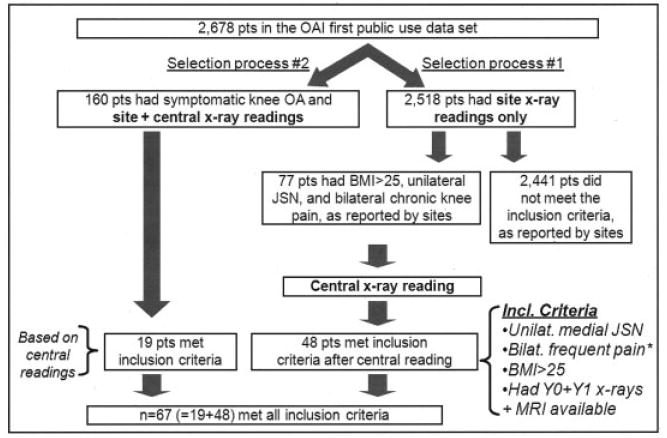
Patient selection process. pts = patients; OAI = Osteoarthritis Initiative; OA = osteoarthritis; BMI = body mass index; JSN = joint space narrowing; MRI = magnetic resonance imaging.
In the second process, files for 160 patients with symptomatic OA who had previously been selected from the OAI database for other published analyses (9-11) were screened for the current study. Radiographs for these patients had already been centrally re-read and, if needed, adjudicated for the presence of osteophytes and JSN. Of these 160 subjects, 19 fulfilled the present study inclusion criteria.
Image analysis methods
Measurement of medial JSW was facilitated by the use of automated software that delineated the femoral and tibial margins of the joint (12). JSW(x) was measured at 4 fixed locations (x = 0.2, x = 0.225, x = 0.25, and x = 0.275) (Figure 2). These measures of medial JSW(x) were defined as the distance from the tibial margin to the femur margin at each fixed location on the x-coordinate system (13). Measurement of minimum JSW was made by the software at the location of the smallest distance between the femur and tibia margins in the medial compartment. Readings of baseline and 1-year radiographs were paired but blinded to time point.
Figure 2.
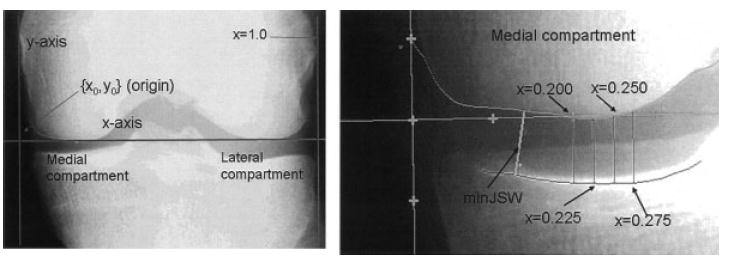
Method for measurement of joint space width (JSW). Baseline (Y0) and year 1 (Y1) fixed flexion radiographs of both knees were used. The x-axis is tangent to the femur (software placement). The y-axis and the (x = 1.0) line were placed manually, perpendicular to the x-axis. Medial JSW was measured at the location of the minimum distance between the femur and tibia margins (minimum JSW) and at 4 fixed locations (x = 0.200, 0.225, 0.250, and 0.275) in a previously described coordinate system adjusted on lower femur epiphysis width (13).
Statistical analysis
Change from baseline significance was assessed with 1-sample t-tests and P values calculated separately for JSN knees and no-JSN knees. Paired t-tests were used to compare change from baseline between JSN knees and no-JSN knees. Comparison of the variability in change from baseline in JSN and no-JSN knees was performed with an adaptation of Levene’s test for paired data, based on a paired t-test of the absolute value of the difference between each patient’s change and the mean change. Comparisons of change from baseline between subgroups (e.g., presence of osteophytes) were performed with 2-sample t-tests. Spearman’s correlations and partial correlations (adjusting for age) were used to evaluate univariate correlations of baseline characteristics with change from baseline measures. Comparisons between age groups for BMI (Wilcoxon’s rank sum test) and presence of osteophytes (chi-square test) were also performed. Multiple stepwise regression analysis was performed to assess the impact of baseline variables (age, sex, BMI, osteophytes, baseline JSW, and history of traumatic knee injury) on change in JSW from baseline to 1 year. Variables such as BMI and age were analyzed as continuous variables. Regression coefficients (β) plus or minus their SEs are presented.
The standardized response means (SRMs) comparing the baseline and 1-year radiographs were defined as the mean change divided by the SD of the change. Bootstrap resampling was performed to generate 1,000 SRMs for JSN knees and no-JSN knees, and the distribution of the difference between knees was used to compute P values.
Summary statistics for continuous variables were expressed as mean ± SDs. Two-tailed P values less than 0.05 were considered significant, and calculations were performed using SAS, version 9.1 (SAS Institute).
RESULTS
Baseline characteristics
Of 2,678 subjects for whom data were available in the OAI database, 67 were included in the present analyses (Figure 1). There were 43 women and 24 men with an average ± SD age of 60 ± 9 years (range 45–78 years). Mean ± SD BMI was 31 ± 4 kg/m2 at inclusion (range 25–42 kg/m2). A history of knee injury was reported in 27 patients (40%). All subjects had bilateral frequent chronic knee pain. The baseline radiographic characteristics of these 67 patients are presented in Table 1.
Table 1.
Radiographic characteristics at baseline*
| JSN knees (n = 67) | No-JSN knees (n = 67) | |
|---|---|---|
| JSN OARSI grade, no. (%)† | ||
| 0 | 0 | 67 (100) |
| 1 | 42 (63) | 0 |
| 2 | 18 (27) | 0 |
| 3 | 7 (10) | 0 |
| Knees with ≥1 definite tibiofemoral osteophyte, % | 52 | 19 |
| Minimal JSW, mean ± SD mm | 2.9 ± 1.2 | 4.7 ± 0.9 |
| JSW location, mean ± SD mm | ||
| x = 0.200 | 3.9 ± 1.5 | 5.7 ± 1.2 |
| x = 0.225 | 4.2 ± 1.6 | 6.0 ± 1.2 |
| x = 0.250 | 4.6 ± 1.6 | 6.4 ± 1.3 |
| x = 0.275 | 5.1 ± 1.7 | 6.9 ± 1.3 |
JSN = joint space narrowing; OARSI = Osteoarthritis Research Society International; JSW = joint space width.
Based on central reading (with or without adjudication).
Mean changes in JSW over 1 year in both JSN and no-JSN knees and distribution of change
The average progression in no-JSN knees was comparable with that in JSN knees at the 4 fixed locations, with JSW reductions of ~0.2 mm over 1 year. The change in minimum JSW was slightly less, numerically, in JSN knees than in no-JSN knees (−0.14 mm and −0.18 mm, respectively), but this difference was far from statistically significant (P = 0.62).
Variability of structural progression was higher in the no-JSN knees, as can be seen from the consistently higher SDs observed in these knees (e.g., for minimum JSW, P = 0.008) (Table 2) and the visually wider distribution of change (Figure 3). This is reflected in the P values, which were generally smaller for progression within the JSN knees compared with progression within the no-JSN knees. As a result, the sensitivity to change of JSW, as assessed by SRMs, tended to be greater in JSN knees than in no-JSN knees (Table 2), with x = 0.225 having significantly different SRMs (P = 0.04). Overall, SRMs for the change in JSW at fixed locations were approximately −0.41 in JSN knees versus −0.24 in no-JSN knees (Table 2).
Table 2.
Mean JSW change (in millimeters) over 1 year at 5 locations (x = 0.200, 0.225, 0.250, 0.275, and minimum JSW)*
| OARSI grade of JSN and location | JSN knee (n = 67) |
No-JSN knee (n = 67) |
JSN versus no-JSN knees† |
||||||
|---|---|---|---|---|---|---|---|---|---|
| JSW change, mean ± SD | SRM | P within knee | JSW change, mean ± SD | SRM | P within knee | P for mean | P for SD | P for SRM | |
| All (n = 67) | |||||||||
| x = 0.200 | −0.18 ± 0.44 | −0.42 | 0.001 | −0.20 ± 0.81 | −0.24 | 0.051 | 0.89 | 0.07 | 0.15 |
| x = 0.225 | −0.22 ± 0.48 | −0.45 | 0.0005 | −0.16 ± 0.76 | −0.21 | 0.09 | 0.53 | 0.20 | 0.04 |
| x = 0.250 | −0.21 ± 0.53 | −0.41 | 0.002 | −0.17 ± 0.75 | −0.23 | 0.07 | 0.64 | 0.38 | 0.19 |
| x = 0.275 | −0.20 ± 0.55 | −0.36 | 0.005 | −0.21 ± 0.74 | −0.29 | 0.02 | 0.86 | 0.44 | 0.56 |
| Minimum JSW | −0.14 ± 0.40 | −0.35 | 0.0006 | −0.18 ± 0.76 | −0.24 | 0.059 | 0.62 | 0.008 | 0.28 |
| Grade 1 (n = 42) | |||||||||
| x = 0.200 | −0.18 ± 0.43 | −0.42 | 0.009 | −0.11 ± 0.49 | −0.22 | 0.16 | 0.32 | 0.59 | 0.16 |
| x = 0.225 | −0.21 ± 0.48 | −0.43 | 0.007 | −0.10 ± 0.50 | −0.20 | 0.20 | 0.19 | 0.73 | 0.13 |
| x = 0.250 | −0.18 ± 0.52 | −0.34 | 0.04 | −0.09 ± 0.49 | −0.19 | 0.23 | 0.37 | 0.61 | 0.37 |
| x = 0.275 | −0.14 ± 0.49 | −0.28 | 0.08 | −0.16 ± 0.52 | −0.32 | 0.047 | 0.75 | 0.81 | 0.59 |
| Minimum JSW | −0.09 ± 0.38 | −0.25 | 0.12 | −0.10 ± 0.54 | −0.18 | 0.26 | 0.98 | 0.23 | 0.32 |
| Grade 2 (n = 18) | |||||||||
| x = 0.200 | −0.25 ± 0.48 | −0.51 | 0.046 | −0.24 ± 1.05 | −0.23 | 0.34 | 0.99 | 0.27 | 0.18 |
| x = 0.225 | −0.29 ± 0.53 | −0.55 | 0.03 | −0.18 ± 0.98 | −0.18 | 0.46 | 0.55 | 0.51 | 0.05 |
| x = 0.250 | −0.31 ± 0.51 | −0.60 | 0.02 | −0.24 ± 0.95 | −0.25 | 0.31 | 0.74 | 0.52 | 0.14 |
| x = 0.275 | −0.28 ± 0.62 | −0.45 | 0.08 | −0.20 ± 0.93 | −0.22 | 0.37 | 0.69 | 0.74 | 0.28 |
| Minimum JSW | −0.25 ± 0.46 | −0.54 | 0.03 | −0.22 ± 0.97 | −0.23 | 0.34 | 0.89 | 0.08 | 0.06 |
| Grade 3 (n = 7) | |||||||||
| x = 0.200 | −0.03 ± 0.38 | −0.08 | 0.85 | −0.61 ± 1.46 | −0.41 | 0.31 | 0.35 | 0.18 | 0.54 |
| x = 0.225 | −0.08 ± 0.41 | −0.20 | 0.62 | −0.49 ± 1.36 | −0.36 | 0.38 | 0.48 | 0.21 | 0.78 |
| x = 0.250 | −0.20 ± 0.64 | −0.31 | 0.44 | −0.44 ± 1.32 | −0.34 | 0.41 | 0.67 | 0.40 | 1.00 |
| x = 0.275 | −0.34 ± 0.69 | −0.50 | 0.24 | −0.52 ± 1.24 | −0.42 | 0.31 | 0.75 | 0.54 | 0.93 |
| Minimum JSW | −0.11 ± 0.27 | −0.41 | 0.32 | −0.56 ± 1.21 | −0.46 | 0.27 | 0.34 | 0.16 | 0.65 |
JSW = joint space width; JSN = joint space narrowing; OARSI = Osteoarthritis Research Society International; SRM = standardized response of the mean (the mean divided by the SD).
P values for within-patient comparisons of mean JSW change and SD of change.
Figure 3.
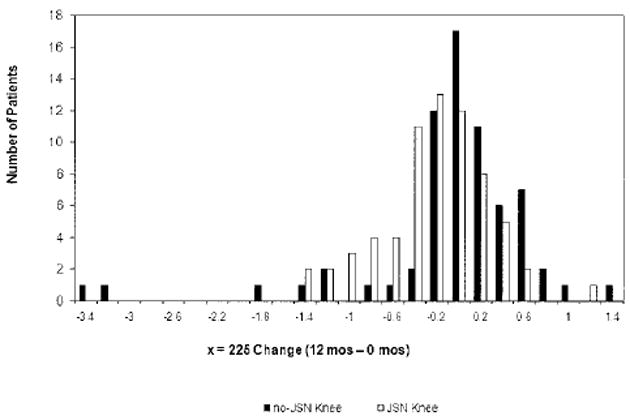
Distribution of 1-year joint space width (JSW) change (at location x = 0.225). Similar distributions of JSW change were observed at other fixed locations (x = 0.200, 0.250, and 0.275) and for minimum JSW (data not shown). Open bars = joint space narrowing (JSN) knee; solid bars = no-JSN knee.
Influence of baseline characteristics on radiographic progression
BMI had a univariate association with radiographic progression, especially in JSN knees. The average rate of progression and sensitivity to change were higher in participants with a baseline BMI ≥30 kg/m2 than in those with a BMI between 25 and 30 kg/m2, but interestingly, the differences were significant only for the JSN knees. Univariate Spearman’s correlation coefficients between baseline BMI and JSW change were significant for all 5 locations in JSN knees, but generally decreased when adjusting for age (data not shown).
Although the age cutoff of 60 years was not associated with statistically significant differences in radiographic progression (Table 3), age as a continuous variable was significantly correlated with progression for all locations in JSN knees and for 2 locations in no-JSN knees. Younger age was associated with greater JSW decreases and therefore with more progression.
Table 3.
Influence of age, sex, BMI, and definite tibiofemoral osteophyte on JSW change at 5 locations (x = 0.200, 0.225, 0.250, 0.275, and minimum JSW)*
| JSN knees |
No-JSN knees |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. patients | Mean ± SD | SRM | P† | No. patients | Mean ± SD | SRM | P† | |
| Age | ||||||||
| <60 years | 34 | 34 | ||||||
| x = 0.200 | −0.24 ± 0.42 | −0.58 | −0.36 ± 0.94 | −0.38 | ||||
| x = 0.225 | −0.31 ± 0.49 | −0.64 | −0.31 ± 0.89 | −0.35 | ||||
| x = 0.250 | −0.31 ± 0.54 | −0.57 | −0.30 ± 0.86 | −0.35 | ||||
| x = 0.275 | −0.32 ± 0.61 | −0.53 | −0.34 ± 0.86 | −0.39 | ||||
| Minimum JSW | −0.23 ± 0.41 | −0.56 | −0.29 ± 0.85 | −0.34 | ||||
| ≥60 years | 33 | 33 | ||||||
| x = 0.200 | −0.12 ± 0.45 | −0.27 | 0.26 | −0.03 ± 0.60 | −0.04 | 0.09 | ||
| x = 0.225 | −0.12 ± 0.46 | −0.26 | 0.10 | 0.00 ± 0.58 | 0.00 | 0.09 | ||
| x = 0.250 | −0.12 ± 0.51 | −0.23 | 0.14 | −0.03 ± 0.59 | −0.05 | 0.14 | ||
| x = 0.275 | −0.07 ± 0.45 | −0.15 | 0.054 | −0.08 ± 0.57 | −0.15 | 0.16 | ||
| Minimum JSW | −0.05 ± 0.37 | −0.12 | 0.06 | −0.06 ± 0.64 | −0.09 | 0.21 | ||
| Sex | ||||||||
| Male | 24 | 24 | ||||||
| x = 0.200 | −0.19 ± 0.35 | −0.53 | −0.02 ± 0.50 | −0.03 | ||||
| x = 0.225 | −0.24 ± 0.42 | −0.57 | −0.02 ± 0.53 | −0.04 | ||||
| x = 0.250 | −0.25 ± 0.46 | −0.54 | −0.02 ± 0.60 | −0.04 | ||||
| x = 0.275 | −0.18 ± 0.53 | −0.34 | −0.07 ± 0.63 | −0.12 | ||||
| Minimum JSW | −0.21 ± 0.32 | −0.66 | −0.01 ± 0.41 | −0.02 | ||||
| Female | 43 | 43 | ||||||
| x = 0.200 | −0.18 ± 0.48 | −0.37 | 0.94 | −0.30 ± 0.92 | −0.32 | 0.12 | ||
| x = 0.225 | −0.21 ± 0.52 | −0.40 | 0.81 | −0.24 ± 0.87 | −0.27 | 0.21 | ||
| x = 0.250 | −0.20 ± 0.57 | −0.34 | 0.71 | −0.25 ± 0.81 | −0.31 | 0.24 | ||
| x = 0.275 | −0.20 ± 0.56 | −0.36 | 0.87 | −0.29 ± 0.78 | −0.37 | 0.26 | ||
| Minimum JSW | −0.10 ± 0.43 | −0.22 | 0.25 | −0.27 ± 0.89 | −0.31 | 0.10 | ||
| BMI | ||||||||
| <30 kg/m2 | 23 | 23 | ||||||
| x = 0.200 | 0.08 ± 0.33 | 0.23 | −0.16 ± 0.85 | −0.19 | ||||
| x = 0.225 | 0.05 ± 0.39 | 0.13 | −0.15 ± 0.80 | −0.19 | ||||
| x = 0.250 | 0.00 ± 0.44 | −0.01 | −0.10 ± 0.76 | −0.13 | ||||
| x = 0.275 | −0.01 ± 0.41 | −0.02 | −0.15 ± 0.72 | −0.20 | ||||
| Minimum JSW | 0.02 ± 0.28 | 0.08 | −0.10 ± 0.73 | −0.13 | ||||
| ≥30 kg/m2 | 44 | 44 | ||||||
| x = 0.200 | −0.32 ± 0.43 | −0.75 | 0.0003 | −0.21 ± 0.79 | −0.27 | 0.81 | ||
| x = 0.225 | −0.36 ± 0.47 | −0.76 | 0.0007 | −0.16 ± 0.75 | −0.22 | 0.96 | ||
| x = 0.250 | −0.32 ± 0.54 | −0.60 | 0.02 | −0.21 ± 0.74 | −0.28 | 0.58 | ||
| x = 0.275 | −0.29 ± 0.59 | −0.50 | 0.04 | −0.25 ± 0.75 | −0.33 | 0.60 | ||
| Minimum JSW | −0.22 ± 0.42 | −0.52 | 0.007 | −0.22 ± 0.78 | −0.28 | 0.53 | ||
| Osteophytes‡ | ||||||||
| None | 32 | 54 | ||||||
| x = 0.200 | −0.15 ± 0.44 | −0.34 | −0.19 ± 0.71 | −0.27 | ||||
| x = 0.225 | −0.16 ± 0.49 | −0.34 | −0.16 ± 0.68 | −0.23 | ||||
| x = 0.250 | −0.16 ± 0.50 | −0.33 | −0.18 ± 0.65 | −0.27 | ||||
| x = 0.275 | −0.10 ± 0.50 | −0.21 | −0.22 ± 0.64 | −0.34 | ||||
| Minimum JSW | −0.08 ± 0.40 | −0.20 | −0.17 ± 0.66 | −0.25 | ||||
| ≥1 | 35 | 13 | ||||||
| x = 0.200 | −0.21 ± 0.44 | −0.49 | 0.55 | −0.20 ± 1.16 | −0.17 | 0.98 | ||
| x = 0.225 | −0.27 ± 0.48 | −0.55 | 0.39 | −0.18 ± 1.09 | −0.16 | 0.94 | ||
| x = 0.250 | −0.26 ± 0.56 | −0.47 | 0.45 | −0.13 ± 1.08 | −0.12 | 0.83 | ||
| x = 0.275 | −0.28 ± 0.58 | −0.48 | 0.19 | −0.19 ± 1.07 | −0.17 | 0.89 | ||
| Minimum JSW | −0.19 ± 0.40 | −0.48 | 0.26 | −0.23 ± 1.11 | −0.21 | 0.77 | ||
BMI = body mass index; JSW = joint space width; JSN = joint space narrowing; SRM = standardized response mean.
From 2-tailed 2-sample Student’s t-test comparing age <60 years versus ≥60 years, male versus female, body mass index <30 kg/m2 versus ≥30 kg/m2, and osteophyte versus no osteophyte.
Definite tibiofemoral osteophytes.
Radiographic progression, as assessed by 1-year change in JSW, tended to be faster in JSN knees with ≥1 definite osteophyte versus those without, but these differences were not statistically significant (Table 3).
Multivariate analyses were performed to determine which demographic factors were most related to JSW change. Both age (β = 0.01 ± 0.006 mm per year of age; P = 0.03) and BMI (β = −0.03 ± 0.01 mm per kg/m2; P = 0.03) as continuous variables were significantly related to the average change of the 4 fixed locations in analyses of JSN knees. Age as a continuous measure was the only variable significantly selected using stepwise regression for both minimum JSW of JSN knees (β = 0.01 ± 0.005 mm per year of age; P = 0.01) and for the average change of the 4 fixed locations (β = 0.02 ± 0.01 mm per year of age; P = 0.049) in analyses of no-JSN knees. No variables were selected at the P < 0.05 cutoff for the minimum JSW measure of the no-JSN knees; however, when the cutoff was raised to P < 0.10, age was the only selected measure (β = 0.02 ± 0.01 mm per year of age; P = 0.09).
Older patients in this population tended to have lower BMI (age ≥60 years: BMI 30.4 ± 3.9 kg/m2, age <60 years: BMI 32 ± 3.8 kg/m2; P = 0.10) and a higher prevalence of osteophytes in the JSN knee (age ≥60 years: 64% with ≥1 osteophyte, age <60 years: 41% with ≥1 osteophyte; P = 0.07), but these differences were not statistically significant.
DISCUSSION
This study is the first to our knowledge to explore the rate of progression in the population with the specified characteristics. Participants with unilateral OA have previously been studied in either an observational or a clinical trial setting (6,14). In these studies, inclusion was based on the Kellgren/Lawrence (K/L) grading system, with OA defined as a definite tibiofemoral osteophyte (with or without JSN). In the current study, we focused on JSN as an inclusion criterion rather than the presence of osteophytes. The additional inclusion criteria (bilateral frequent pain and BMI >25 kg/m2) were aimed at increasing the risk of progression in the no-JSN knee. The relatively small proportion of patients eligible for this study (67 of 2,678 subjects in this subset of the OAI database) suggests that a clinical trial aimed at enrolling this population would require significant recruitment efforts, especially if screening radiographs are not read centrally.
In our target population, no-JSN knees progressed radiographically as rapidly as JSN knees, with an annual rate of progression of ~0.2 mm. However, progression was more variable in no-JSN knees, with an SD of 0.7–0.8 mm (versus ~0.5 in JSN knees), which accounts for the relatively low SRMs observed in these knees. Two outlier knees with fast progression are shown in Figure 3. Removing them reduced 1-year progression in no-JSN knees by approximately half on average, suggesting that progression in no-JSN knees was largely driven by a few patients (although most differences between JSN and no-JSN knees remained not significant; data not shown).
Measures performed with the x-coordinate system tended to be more responsive than the measure of minimum JSW in JSN knees (nonsignificant), whereas both analysis methods were comparable in no-JSN knees. This agrees with a previously published report that used the same positioning protocol for radiographs collected at baseline and 36-month followup: the Dynamics of Health, Aging, and Body Composition study (13).
Studying JSN knees in this population provides an improved sensitivity to change versus studying no-JSN knees. Targeting no-JSN knees in a similar population, a placebo-controlled DMOAD trial would require ~800 patients per group over 1 year in order to detect a 50% difference between an active drug and placebo (80% power, 1-sided, α = 0.05, average for 4 fixed locations). The higher sensitivity to change observed in JSN knees in this population would translate into a sample size of ~270 patients per group in a DMOAD trial intended to detect a similar difference between active compound and placebo in the JSN knees. This raises questions as to whether this patient population and their no-JSN knees represent an appropriate target for a DMOAD trial. SRMs for change in cartilage thickness measured quantitatively with MRI in the same population (7) were also higher in JSN knees than in no-JSN knees, suggesting that regardless of the methodology used (radiography or MRI), a larger sample size would be required if the target knees for such a trial were the no-JSN knees.
Some of our data confirm previously published findings. Byers-Kraus et al have reported that patients recruited based on familial hand OA had a remarkably symmetric annual medial JSW change, although knee OA was not among the inclusion criteria of this study (15).
In the doxycycline trial, which included obese women with unilateral knee OA as assessed on standing anteroposterior knee radiographs, JSW reduction was 0.24 ± 0.54 mm and 0.23 ± 0.59 mm over 16 months in the index (knees with a definite tibiofemoral osteophyte) and contralateral knees, respectively (14). These figures would result in SRMs of −0.44 and −0.43, respectively, over 1 year, although only 42% of the patients included in the doxycycline trial had baseline JSN in the index knee. One of the differences between the methodology of these 2 studies lies in the knee positioning technique: the OAI study used a frame-assisted fixed flexion, whereas the doxycycline trial used a fluoroscopic-assisted alignment of radiograph beam and medial tibial plateau in a semiflexed position.
Several recent articles have suggested that the fluoroscopic-assisted positioning leads to better alignment of the tibial margin and the radiograph beam, and therefore allows less variability and more sensitivity in detecting progression than the fixed flexion (3,16-18). One of these reported the results of an observational study that compared, in the same patients (obese women with radiographic knee OA), radiographic progression over 1 year as assessed on radiographs performed in Lyon schuss and fixed flexion positions (3). Sensitivity to change of JSW showed a striking difference, with SRMs of −0.34 and −0.65 for K/L grade 2 and 3 knees, respectively, with the Lyon schuss, versus −0.01 and −0.01 for the same knees with fixed flexion.
In the present study, we did not exclude radiographs with poor or inconsistent tibial rim alignment. The software method functioned by delineating the structures of interest (femur margin and bright band associated with the tibial plateau) and made no attempt to correct for imperfect alignment. This may partially explain the relatively poor sensitivity to change of the minimum JSN measurements as compared with measurements at fixed locations in the present study.
Despite the technical limitations described above, the present study was able to detect radiographic progression over 1 year in this specific population, although sensitivity to change was modest. The relatively high variability and low SRMs observed might have improved with Lyon schuss positioning, or with the recently described modified Lyon schuss positioning (19), which also allows precise alignment of the tibial plateau without requiring fluoroscopic equipment.
Another limitation of this study is that 30 of 67 patients did not have a definite (OARSI grade ≥2) tibiofemoral osteophyte at baseline in either knee. However, all but 6 of these 30 patients had a grade 1 osteophyte in at least 1 knee. In addition, all patients had bilateral frequent chronic pain. Finally, the OAI study eligibility criteria were designed to specifically exclude patients in whom knee symptoms were likely to result from a disease process other than OA (OAI protocol, online at www.oai.ucsf.edu/datarelease/docs/StudyDesignProtocol.pdf).
The inclusion criteria used here preclude evaluation of risk factors for progression such as unilateral JSN, bilateral pain, and BMI >25 kg/m2. For instance, it is not possible to infer from our data whether JSN in 1 knee predicts future progression in the contralateral knee, or whether a BMI >25 kg/m2 predicts progression. This study was not designed to address these questions, although it does allow assessment of other potential risk factors in this population, such as age, BMI within the limits of eligibility (>25 kg/m2), and tibiofemoral osteophytes. It appeared that both age and obesity were associated with future progression and significantly improved the sensitivity to change of JSW (especially in JSN knees). Whether or not a future DMOAD trial should have inclusion criteria such as age or obesity in addition to those defining the population enrolled in the present study is debatable, being that the smaller the target population is, the more difficult it is to enroll.
In conclusion, sensitivity to change of JSW (on radiographs performed in fixed flexion) in no-JSN knees of patients with unilateral JSN is such that a placebo-controlled proof of concept DMOAD trial using the same technique would require close to 800 patients per arm over 1 year. This sample size might be decreased by the use of a positioning technique that reduces the variability of JSW change (19), although this technique adds some complexity to the procedure.
Refining inclusion criteria may also help obtain a higher sensitivity to change, especially requiring patients to be <60 years of age or to have a BMI of ≥30 kg/m2. Such highly-targeted strategies involve significant recruitment challenges and would require careful thought concerning recruitment tactics.
Acknowledgments
The authors thank Chuyun Huang for performing many of the statistical analyses and Ana Vaz for performing a thorough review of the manuscript. We would like to thank the participants, principal investigators (Michael Nevitt, Kent Kwoh, Charles B. Eaton, Rebecca Jackson, Marc Hochberg, Joan Bathon), coinvestigators, and staff of the OAI. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
ROLE OF THE STUDY SPONSOR Eli Lilly sponsored the analysis by J. Duryea of knee radiographs acquired through the Osteoarthritis Initiative (OAI). Fulltime employees of this sponsor (O. D. Benichou, D. R. Nelson, and S. L. Myers) participated in the design and performed the statistical analysis of the present image analysis study. The OAI itself is a partnership involving the National Institutes of Health as well as 4 private sponsors: Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline, and Pfizer.
The study and image acquisition were supported by the Osteoarthritis Initiative, a public-private partnership comprised of 5 contracts (N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, and N01-AR-2-2262) funded by the NIH and conducted by the Osteoarthritis Initiative Study Investigators. Image analysis was supported by Eli Lilly. Private funding partners include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmith- Kline, and Pfizer (private sector funding for the Osteoarthritis Initiative is managed by the Foundation for the NIH).
Footnotes
AUTHOR CONTRIBUTIONS All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Benichou had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Benichou, Hunter, Nelson, Guermazi, Kwoh, Myers, Duryea.
Acquisition of data. Benichou, Hunter, Guermazi, Kwoh.
Analysis and interpretation of data. Benichou, Hunter, Nelson, Guermazi, Eckstein, Wirth, Duryea.
Drs. Benichou and Myers have stock ownership or options in Eli Lilly. Dr. Guermazi has received consultancies, speaking fees, and/or honoraria from Genzyme and Facet Solutions (less than $10,000 each) and from Stryker and Merck Serono (more than $10,000 each), has stock ownership or options in Synarc, and is the president of Boston Imaging Core Lab. Dr. Eckstein has received consultancies, speaking fees, and/or honoraria from Wyeth, Genzyme, Aventis, and GlaxoSmithKline (less than $10,000 each) and from Pfizer, Novartis, and MerckSerono (more than $10,000 each), and has stock co-ownership in Chondrometrics GmbH. Dr. Kwoh has received grant funding from AstraZeneca. Dr. Duryea has received consultancies, speaking fees, and/or honoraria from Cleveland Clinic, State University of New York at Buffalo, Tufts Medical Center, and Chondrometrics GmbH (less than $10,000 each) and from Merck (more than $10,000).
References
- 1.Lohmander LS, Felson D. Can we identify a ‘high risk’ patient profile to determine who will experience rapid progression of osteoarthritis. Osteoarthritis Cartilage. 2004;12(Suppl A):S49–52. doi: 10.1016/j.joca.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Mazzuca SA, Brandt KD, Katz BP, Ding Y, Lane KA, Buckwalter KA. Risk factors for progression of tibiofemoral osteoarthritis: an analysis based on fluoroscopically standardised knee radiography. Ann Rheum Dis. 2006;65:515–9. doi: 10.1136/ard.2005.039115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Graverand MP, Vignon EP, Brandt KD, Mazzuca SA, Piperno M, Buck R, et al. Head-to-head comparison of the Lyon schuss and fixed flexion radiographic techniques: long-term reproducibility in normal knees and sensitivity to change in osteoarthritic knees. Ann Rheum Dis. 2008;67:1562–6. doi: 10.1136/ard.2007.077834. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe F, Lane NE. The long term outcome of osteoarthritis: rates and predictors of joint space narrowing in symptomatic patients with knee osteoarthritis. J Rheumatol. 2002;29:139–46. [PubMed] [Google Scholar]
- 5.Davis MA, Ettinger WH, Neuhaus JM, Cho SA, Hauck WW. The association of knee injury and obesity with unilateral and bilateral osteoarthritis of the knee. Am J Epidemiol. 1989;130:278–88. doi: 10.1093/oxfordjournals.aje.a115334. [DOI] [PubMed] [Google Scholar]
- 6.Spector TD, Hart DJ, Doyle DV. Incidence and progression of osteoarthritis in women with unilateral knee disease in the general population: the effect of obesity. Ann Rheum Dis. 1994;53:565–8. doi: 10.1136/ard.53.9.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckstein F, Benichou O, Wirth W, Nelson DR, Maschek S, Hudelmaier M, et al. for the Osteoarthritis Initiative Investigators. Magnetic resonance imaging-based cartilage loss in painful contralateral knees with and without radiographic joint space narrowing: data from the Osteoarthritis Initiative. Arthritis Rheum. 2009;61:1218–25. doi: 10.1002/art.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kothari M, Guermazi A, von Ingersleben G, Miaux Y, Sieffert M, Block JE, et al. Fixed-flexion radiography of the knee provides reproducible joint space width measurements in osteoarthritis. Eur Radiol. 2004;14:1568–73. doi: 10.1007/s00330-004-2312-6. [DOI] [PubMed] [Google Scholar]
- 9.Hunter DJ, Niu J, Zhang Y, Totterman S, Tamez J, Dabrowski C, et al. Change in cartilage morphometry: a sample of the progression cohort of the Osteoarthritis Initiative. Ann Rheum Dis. 2009;68:349–56. doi: 10.1136/ard.2007.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckstein F, Maschek S, Wirth W, Hudelmaier M, Hitzl W, Wyman B, et al. One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative Progression Subcohort: association with sex, body mass index, symptoms, and radiographic OA status. Ann Rheum Dis. 2009;68:674–9. doi: 10.1136/ard.2008.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wirth W, Hellio le Graverand MP, Wyman BT, Maschek S, Hudelmaier M, Hitzl W, et al. OAI Investigator Group. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage. 2009;17:291–7. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duryea J, Li J, Peterfy CG, Gordon C, Genant HK. Trainable rule-based algorithm for the measurement of joint space width in digital radiographic images of the knee. Med Phys. 2000;27:580–91. doi: 10.1118/1.598897. [DOI] [PubMed] [Google Scholar]
- 13.Neumann G, Hunter D, Nevitt M, Chibnik LB, Kwoh K, Chen H, et al. for the Health ABC Study. Location specific radiographic joint space width for osteoarthritis progression. Osteoarthritis Cartilage. 2009;17:761–5. doi: 10.1016/j.joca.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandt KD, Mazzuca SA, Katz BP, Lane KA, Buckwalter KA, Yocum DE, et al. Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2005;52:2015–25. doi: 10.1002/art.21122. [DOI] [PubMed] [Google Scholar]
- 15.Byers-Kraus V, Cicconetti G, Jordan JM, Renner J, Doherty M, Wilson AG, et al. Symmetry of radiographic knee osteoarthritis progression in sibling pairs with hand osteoarthritis. Osteoarthritis Cartilage. 2007;15(Suppl C):C131. [Google Scholar]
- 16.Le Graverand MP, Mazzuca S, Lassere M, Guermazi A, Pickering E, Brandt K, et al. for the Radiography Working Group of the OARSI-OMERACT Imaging Workshop. Assessment of the radioanatomic positioning of the osteoarthritic knee in serial radiographs: comparison of three acquisition techniques. Osteoarthritis Cartilage. 2006;14(Suppl A):A37–43. doi: 10.1016/j.joca.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Cline GA, Meyer JM, Stevens R, Buckland-Wright C, Peterfy C, Beary JF. Comparison of fixed flexion, fluoroscopic semiflexed and MTP radiographic methods for obtaining the minimum medial joint space width of the knee in longitudinal osteoarthritis trials. Osteoarthritis Cartilage. 2006;14(Suppl A):A32–6. doi: 10.1016/j.joca.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Brandt KD, Mazzuca SA, Conrozier T, Dacre JE, Peterfy CG, Provvedini D, et al. Which is the best radiographic protocol for a clinical trial of a structure modifying drug in patients with knee osteoarthritis? J Rheumatol. 2002;29:1308–20. review. [PubMed] [Google Scholar]
- 19.Mazzuca SA, Hellio le Graverand MP, Vignon E, Hunter DJ, Jackson CG, Kraus VB, et al. Performance of a non-fluoroscopically assisted substitute for the Lyon schuss knee radiograph: quality and reproducibility of positioning and sensitivity to joint space narrowing in osteoarthritic knees. Osteoarthritis Cartilage. 2008;16:1555–9. doi: 10.1016/j.joca.2008.04.010. [DOI] [PubMed] [Google Scholar]


