Abstract
Choclo virus (CHOV) was described in sigmodontine rodents, Oligoryzomys fulvescens, and humans during an outbreak of hantavirus cardiopulmonary syndrome (HCPS) in 1999 to 2000 in western Panama. Although HCPS is rare, hantavirus-specific serum antibody prevalence among the general population is high suggesting that CHOV may cause many mild or asymptomatic infections. The goals of this study were to confirm the role of CHOV in HCPS and in the frequently detected serum antibody and to established the phylogenetic relationship with other New World hantaviruses. CHOV was cultured to facilitate the sequencing of the small (S) and medium (M) segments and to perform CHOV-specific serum neutralization antibody assays.
Sequences of the S and M segments found a close relationship to other Oligoryzomys-borne hantaviruses in the Americas, highly conserved terminal nucleotides, and no evidence for recombination events. The maximum likelihood and maximum parsimony analyses of complete M segment nucleotide sequences indicate a close relationship to Maporal and Laguna Negra viruses, found at the base of the South American clade. In a focus neutralization assay acute and convalescent sera from 6 Panamanian HCPS patients neutralized CHOV in dilutions from 1:200 to 1:6400. In a sample of antibody-positive adults without a history of HCPS, 9 of 10 sera neutralized CHOV in dilutions ranging from 1:100 to 1:6400. Although cross-neutralization with other sympatric hantaviruses not yet associated with human disease is possible, CHOV appears to be the causal agent for most of the mild or asymptomatic hantavirus infections, as well as HCPS, in Panama.
Keywords: hantavirus, phylogeny, neutralizing antibody, Bunyaviridae
INTRODUCTION
Hantavirus cardiopulmonary syndrome (HCPS) is a severe and often fatal infection of the lung and other tissues, caused by one of at least 16 hantaviruses (Family Bunyaviridae, Genus Hantavirus) distributed throughout most of the Americas [Schmaljohn and Hjelle, 1997] [Koster and Hjelle, 2004]. Hantaviruses are negative-strand RNA viruses containing three segments, a small (S), medium (M), and large (L) segments encoding nucleocapsid (N), glycoproteins GPC, and RNA-dependent RNA polymerase, respectively. Most hantaviruses in the Americas, including the Sin Nombre virus (SNV) in North America and the Andes virus (ANDV) in South America, are associated with mortality rates of 20 to 35% and are contrasted with low levels of seroprevalence in endemic regions, usually below 2%. One exception is the Laguna Negra virus (LNV) in the Gran Chaco of western Paraguay, where indigenous peoples uncommonly experience symptomatic infection and mortality yet seroprevalence to hantavirus infection exceeds 20% in many communities [Ferrer et al., 1998; Williams et al., 1997].
The other exception is Choclo virus (CHOV), the only virus ascribed to human disease in Panama [Bayard et al., 2004; Vincent et al., 2000]. CHOV was identified by RT-PCR from samples taken from the host Oligoryzomys fulvescens, a common peridomestic sigmodontine rodent in Panama. More than 140 cases of HCPS, including 28 fatalities, have been recorded in Panama since 2000. All hospitalized patients tested by RT-PCR and amplimer sequencing were shown to have CHOV infections (Pascale et al., unpublished data). HIgh serum antibody prevalence [Armien et al., 2004] may be due to infection exclusively with CHOV or may be due in part to infections with other hantaviruses. Hantaviruses in Panama not associated with human disease include Calabazo virus (CALV) [Hjelle et al., 1995; Salazar-Bravo et al., 2004; Vincent et al., 2000] and an unnamed hantavirus in Sigmodon hirsutus [Armien et al., 2009]. Extensive cross-reactivity to nucleoproteins of the American hantaviruses prevents the use of EIA or Western blot technologies when assigning an infecting strain [Hjelle et al., 1997]. Studies utilizing neutralizing antibody specificities, on the other hand, may permit tentative assignment [Chu et al., 1994]
This study was undertaken to culture CHOV, obtain a complete sequence of the viral S and M segments, identify phylogenetic relationships, and develop a focus neutralization assay in order to implicate CHOV in the high antibody prevalence among Panamanians.
METHODS
Viral Isolation
Virus was obtained from the spleen of a rodent, O. fulvescens (specimen voucher no. NK101588, UNM MSB 96073), captured on 6 March 2000 in Las Tablas, Los Santos Province, Panama. The virus is named for a cantina ‘El Choclo’ of interesting reputation in the neighborhood Barriada 8 Noviembre near Las Tablas. One-hundred mg of tissue was homogenized by a bead beater using 2.5-mm zirconium/silica beads in phosphate buffered saline (PBS) and diluted 1:50, 1:200, and 1:1000 in 1.0 ml complete Vero media (Eagle’s minimal essential medium [EMEM] containing 10% fetal bovine serum (FBS), gentamicin (50 μg/mL), and 20 mM glutamine). Vero E6 cells (Vero C1008, ATCC CRL 1586, passage 8) were grown to confluency in 25-cm2 flasks in Vero complete media. Media was removed from the monolayer and the diluted homogenates were added, incubated on a slow plate rocker at room temperature for 2 h, then the tissue homogenate was removed. Fresh media with 2.5% FBS was added and the monolayers were incubated at 36°C in a 5% CO2 atmosphere, with media changed twice weekly. Passage of the monolayer to fresh flasks after trypsinization (0.5% trypsin/5 mM EDTA) was accomplished after 4 weeks (first passage) and thereafter every 2 to 2.5 weeks. All experiments involving infectious viruses were performed in a biosafety level 3 laboratory.
RT-PCR
A nested reverse transcriptase-polymerase chain reaction (RT-PCR) was used to detect viral RNA in culture supernatants from each passage. Typically 170-μL aliquots (approximately 2 μg total RNA) of supernatant media were extracted using the QIAampViral RNA kit (Qiagen Inc, Valencia, CA) according to the manufacturer’s directions. RT-PCR was initiated using AMVrt and Amplitaq LD with the outer antisense primer for 1 h at 42°C. Subsequent reaction conditions were 94°C for 5 min, followed by 8 cycles consisting of 10 s at 94°C, 20 s at 50°C, and 60 s at 72°C, and finally by 28 cycles with the annealing temperature of 55°C. The outer primers at the 5′ end of the segment were 5′-ACTGCACGGCAAAAGCTTAAA-3′ (58F) and 5′-GGATATAAGCACCAATTGACCT-3′ (379R) producing a 320-bp amplimer. The inner pair was 5′-GGACCCGGATGAAGTTAACAA-3′ (102F) and 5′-AATTTTTGAGCTGCCACCAA-3′ (222R) producing a 120 bp amplimer. The products were visualized on agarose gel, purified and sequenced to confirm specificity to CHOV.
Focus and Neutralization Assays
Replicating virus was titered using a focus assay as published [Bharadwaj et al., 2000]. Vero E6 cells were seeded onto 48-well plates and incubated until confluent. Ten-fold dilutions (1:10 through 1:107) of virus-containing culture supernatant were added to the monolayers in a 200-μL volume of viral culture medium (EMEM, HEPES buffer, 2.5% FBS, and 50 mg/mL gentamicin) and incubated for 2 h at 37°C. After adsorption, the supernatant was aspirated and 1 mL/well of viral overlay media (VCM and 1.2% methylcellulose) was added and incubated for 7 days. After 7 days the overlay media was removed; the monolayer was washed once with PBS. Ice cold methanol containing 0.5% H2O2 was added and incubated at room temperature for 30 min; fixative then was aspirated and PBS added for storage until immunoperoxidase assay.
For the immunoperoxidase assay, fixed cell monolayers were washed twice with PBS, and 200 μL/well of 1:1000-diluted rabbit anti-SN virus serum was incubated for 1 h at 37°C. After aspiration and washing twice with PBS, 200 μL of peroxidase-conjugated AffinPure Goat anti-rabbit IgG (H+L) (Pierce Immunochemicals, Rockford, IL) diluted 1:1000 in PBS was added and incubated for 1 h at 37°C. Following aspiration and washing, 200 μL of DAB/metal concentrate diluted with 1x peroxidase substrate buffer was added to the monolayers and incubated until brown foci appeared, usually 15 to 30 min. Foci/well were enumerated with a 20x inverted scope ocular.
Neutralizing antibody was measured in human serum using diluted test sera in the focus titration assay. Serum samples containing anti-hantavirus antibody were collected from family members and neighbors of HCPS patients in the community of San Jose, Los Santos Province from 2001 to 2003. Serum was diluted (1:20, 1:100, 1:200, 1:400, 1:800, 1:1600, 1:3200, 1:6400, and 1:12800 dilutions) was incubated for 2 h at 37°C with stock virus diluted to a concentration of 35 to 50 focus-forming units (FFU)/well prior to incubation on Vero cell monolayers. Neutralizing antibody titers were expressed as the reciprocal of the highest serum dilution that results in an 80% reduction in the number of foci compared to virus controls. Discrepant results were resolved by two additional focus neutralization assays. Stock CHOV used was E6 passage 3, with a titer of 4.3 log10 FFU/mL. Stock SNV and ANDV demonstrated approximately the same titer in the focus assay titration as was documented by the originating laboratory.
Viral Sequencing
To sequence complete S and M segments, nested primers were designed from consensus sequences of ANDV and LNV viruses in GenBank. To sequence the 5′ and 3′ termini of each segment, dephosphorylated terminal nucleotides were ligated with T4 ligase and appropriately sized amplimers were cloned into pCRII vector (Invitrogen, Carlsbad, CA). The resulting clones were sequenced by the dideoxy method. Sequences were acceptable only if forward and reverse sequencing results agreed. Multiple overlapping amplimers were sequenced to achieve an 80% duplication of the entire genome.
Phylogenetic Analysis
Sequences of the N protein gene of four strains of ANDV and one strain each of 20 other hantaviruses were compared to the sequence of the CHOV. The sequence of the GPC gene of the CHOV was compared to sequences of 4 strains of ANDV and 20 other hantaviruses (Table 1). The accession numbers of closely related viruses were Maporal virus (MAPV) (N, AY267347; GPC, AY363170) and LNV (N, AF005727; GPC, AF005728). Sequences were aligned using ClustalX [Thompson et al., 1994] followed by visual inspection using MacClade 4 [Maddison and Maddison, 2003]. The N protein and GPC gene sequences were 1302 (with stop codon) and 3465 characters in length, respectively.
Table 1.
Virus sequences used in this study. Accessions numbers are for N protein (top) and GPC gene (bottom of the pair – when present)
| Virus | Genbank No. | General locality | Host | |
|---|---|---|---|---|
| S segment | M segment | |||
| CHOV | DQ285046 | DQ285047 | Panama | Oligoryzomys fulvescens |
| ANDV | AF324902 | AF324901 | Argentina | Homo sapiens |
| ANDV | AF291702 | AF291703 | Chile | Oligoryzomys longicaudatus |
| ANDV | AF325966 | Argentina | Oligoryzomys chacoensis | |
| ANDV | AF482712 | Argentina | Homo sapiens | |
| BAYV | L36929 | L36930 | USA | Homo sapiens |
| BCCV | L39949 | L39950 | USA | Sigmodon hispidus |
| BMJV | AF482713 | Argentina | Oligoryzomys chacoensis | |
| ELMCV | U11427 | U26828 | USA | Reithrodontomys megalotis |
| HNTV | M14626 | M14627 | Korea | Apodemus agrarius |
| LECV | AF482714 | AF028022 | Argentina | Oligoryzomys flavescens |
| LNV | AF005727 | AF005728 | Paraguay | Calomys laucha |
| MACV | AF482716 | Argentina | Necromys benefactor | |
| MAPV | AY267347 | AY363179 | Venezuela | Oligoryzomys fulvescens |
| MONV | U32591 | USA | Peromyscus maniculatus | |
| MULEV | U54575 | USA | Sigmodon hispidus | |
| NYV | U09488 | U36801 | USA | Peromyscus leucopus |
| ORNV | AF482715 | AF028024 | Argentina | Oligoryzomys longicaudatus |
| PHV | M34011 | X55129 | USA | Microtus pennsylvanicus |
| PUUV | M32750 | M29979 | Russia | Clethrionomys glareolus |
| PRGV | AF482717 | Argentina | Akodon azarae | |
| RIOMV | U52136 | Bolivia | Oligoryzomys microtis | |
| RIOSV | U18100 | Costa Rica | Reithrodontomys mexicanus | |
| SEOV | M34881 | M34882 | Japan | Rattus norvegicus |
| SNV | L37904 | L37903 | USA | Peromyscus maniculatus |
Phylogenetic analyses of DNA sequences were conducted using Maximum Parsimony (MP) and Maximum Likelihood (ML) in the software package PAUP* 4.0b10 [Swofford, 2002]. Fulhorst et al. (2004) excluded third base positions from their MP and ML analyses. Third base positions were included but only for transversion changes. Transversion changes continued to increase as genetic distance increased, suggesting that a phylogenetic signal may still be present for these changes at the third position, yet transition changes plateau, suggesting saturation of substitutions has occurred for transitions at the third base position. In the MP analysis all characters were weighted based on their rescaled consistency index (RCI). A heuristic search option with 100 replications of random addition of taxa and TBR branch swapping was used to generate parsimony trees. Bootstrap support [Felsenstein, 1985] for results of MP analyses were based on 1000 repetitions of resampling data using the heuristic search option, with 10 replications of random addition of taxa.
Modeltest (v 3.06) [Posada and Crandall, 1998] was used to calculate the appropriate model for ML analyses. The GTR + G + I (0.6787 and 0.3440 for G and I, respectively) was used to construct phylogenies for the S segment and the GTR + G (0.3439) model was used to construct the M segment phylogeny based on the results of the Modeltest analysis.
RESULTS
Isolation of the Virus
RT-PCR of serial supernatants from blind passages identified the appearance of detectable viral RNA from animal #588 by the fourth blind passage in both tissue inocula diluted 1:50 and 1:1000. Virus was first detected by focus titration assay from the third passage at <100 focus forming units (FFU)/mL, and the highest level of virus was found in the sixth passage with a titer of 5 × 104 FFU/mL. Viral RNA was extracted from sixth passage supernatants, or third passage of virus-positive supernatants, for subsequent genomic sequencing.
Nucleotide Sequence Analysis
The S segment of CHOV isolate 588 (Accession No. DQ285046) was 1516 nt in length, of which 1302 bases encode a predicted nucleocapsid protein 434 amino acids in length, beginning at nt 43. The S segment also contains a potential second ORF (open reading frame) beginning at nt 122, with a predicted protein of 63 amino acids in length. Terminal nucleotides were conserved as seen in other hantaviruses [Chizhikov et al., 1995] with the 32- and 28-nt equivalent to the 5′ and 3′ ends, respectively, being identical to those of ANDV and LNV. The 20-nt differences between the CHOV and SNV in the S segment coding region included 16 nonsynonymous changes.
Comparison of the CHOV S segment sequences with those of other hantaviruses indicates the highest degree of identity with South American viruses (78.3 to 79.8% nt; 89.4 to 91.2% aa) (Table 2). Nucleotide similarity between CHOV and the North American viruses was close to that of the South American viruses and ranged from 75.9 to 79.3 % (83.2 to 89.2% aa). Comparison of deduced N protein amino acid sequences shows an 89.9% identity to MAPV, an 88.5% identity to SNV, and a 90% identity to ANDV.
Table 2.
Nucleotide differences (below diagonal) and amino acid differences (above diagonal) between pairs of hantaviruses for the S segment
| ANDV1 | ANDV2 | ANDV3 | ANDV4 | BMJV | LECV | ORNV | MACV | PRGV | MAPV | CHOV | RIOM | LNV | ELMC | RIOS | SNV | MONV | NYV | MULE | BAYV | BCCV | SEOV | HTNV | PUUV | PHV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANDV1 | 0 | 0 | 15 | 1 | 14 | 15 | 14 | 26 | 21 | 36 | 41 | 38 | 41 | 71 | 76 | 59 | 52 | 52 | 59 | 49 | 57 | 157 | 151 | 123 | 114 |
| ANDV2 | 74 | 0 | 15 | 1 | 14 | 15 | 14 | 26 | 21 | 36 | 41 | 38 | 41 | 71 | 76 | 59 | 52 | 52 | 59 | 49 | 57 | 157 | 151 | 123 | 114 |
| ANDV3 | 209 | 210 | 0 | 16 | 6 | 7 | 1 | 24 | 18 | 40 | 43 | 41 | 43 | 72 | 78 | 56 | 53 | 53 | 62 | 47 | 58 | 162 | 151 | 124 | 115 |
| AND4 | 79 | 9 | 213 | 0 | 15 | 16 | 15 | 26 | 22 | 35 | 41 | 39 | 42 | 72 | 77 | 60 | 53 | 52 | 60 | 50 | 56 | 157 | 151 | 123 | 115 |
| BMJV | 203 | 205 | 163 | 209 | 0 | 1 | 5 | 24 | 18 | 37 | 38 | 41 | 43 | 71 | 75 | 53 | 50 | 52 | 62 | 47 | 58 | 161 | 149 | 123 | 113 |
| LECV | 197 | 202 | 166 | 202 | 110 | 0 | 6 | 25 | 19 | 36 | 39 | 40 | 44 | 72 | 74 | 54 | 51 | 53 | 63 | 48 | 59 | 161 | 149 | 123 | 112 |
| ORNV | 207 | 206 | 8 | 209 | 164 | 165 | 0 | 23 | 17 | 39 | 42 | 40 | 42 | 71 | 77 | 55 | 52 | 52 | 61 | 46 | 57 | 161 | 150 | 123 | 114 |
| MACV | 241 | 241 | 227 | 240 | 231 | 228 | 227 | 0 | 17 | 42 | 46 | 45 | 50 | 70 | 77 | 62 | 57 | 54 | 60 | 52 | 57 | 159 | 155 | 124 | 115 |
| PRGV | 237 | 237 | 216 | 239 | 231 | 216 | 216 | 218 | 0 | 40 | 44 | 43 | 44 | 71 | 79 | 59 | 53 | 52 | 59 | 48 | 59 | 159 | 152 | 127 | 114 |
| MAPV | 257 | 254 | 260 | 252 | 279 | 262 | 256 | 285 | 265 | 0 | 44 | 36 | 45 | 67 | 69 | 59 | 57 | 55 | 59 | 50 | 57 | 155 | 151 | 121 | 111 |
| CHOV | 267 | 279 | 263 | 283 | 274 | 274 | 263 | 281 | 275 | 281 | 0 | 47 | 51 | 68 | 73 | 50 | 47 | 47 | 65 | 49 | 62 | 165 | 155 | 123 | 108 |
| RIOM | 258 | 245 | 244 | 246 | 259 | 251 | 244 | 276 | 264 | 234 | 280 | 0 | 29 | 72 | 77 | 65 | 58 | 56 | 58 | 50 | 56 | 161 | 156 | 124 | 116 |
| LNV | 267 | 266 | 272 | 269 | 266 | 259 | 267 | 284 | 276 | 281 | 270 | 226 | 0 | 74 | 81 | 62 | 58 | 54 | 61 | 54 | 61 | 163 | 156 | 124 | 117 |
| ELMC | 300 | 301 | 285 | 303 | 292 | 294 | 283 | 313 | 309 | 301 | 314 | 297 | 321 | 0 | 38 | 64 | 65 | 65 | 73 | 65 | 70 | 163 | 164 | 127 | 116 |
| RIOS | 328 | 326 | 322 | 328 | 321 | 320 | 317 | 329 | 323 | 308 | 320 | 324 | 316 | 268 | 0 | 70 | 73 | 71 | 80 | 69 | 76 | 167 | 164 | 123 | 119 |
| SNV | 305 | 300 | 294 | 305 | 288 | 288 | 292 | 285 | 305 | 314 | 304 | 303 | 301 | 297 | 311 | 0 | 27 | 27 | 72 | 54 | 67 | 167 | 162 | 129 | 114 |
| MONV | 276 | 281 | 283 | 282 | 289 | 291 | 279 | 307 | 291 | 294 | 294 | 295 | 315 | 298 | 324 | 217 | 0 | 16 | 68 | 48 | 58 | 166 | 159 | 128 | 109 |
| NYV | 294 | 284 | 295 | 283 | 302 | 292 | 293 | 285 | 294 | 289 | 299 | 292 | 299 | 294 | 332 | 213 | 198 | 0 | 68 | 51 | 60 | 162 | 158 | 128 | 110 |
| MULE | 293 | 307 | 301 | 307 | 299 | 291 | 303 | 307 | 310 | 296 | 310 | 290 | 305 | 312 | 351 | 314 | 314 | 304 | 0 | 30 | 41 | 168 | 160 | 124 | 115 |
| BAYV | 296 | 295 | 274 | 297 | 293 | 292 | 274 | 304 | 297 | 279 | 305 | 286 | 285 | 303 | 322 | 294 | 292 | 286 | 248 | 0 | 33 | 162 | 155 | 121 | 107 |
| BCCV | 301 | 313 | 301 | 313 | 273 | 277 | 301 | 296 | 307 | 285 | 301 | 291 | 294 | 317 | 342 | 317 | 311 | 311 | 251 | 248 | 0 | 162 | 156 | 126 | 112 |
| SEOV | 480 | 480 | 476 | 477 | 478 | 475 | 474 | 476 | 487 | 487 | 490 | 503 | 489 | 491 | 506 | 494 | 504 | 484 | 508 | 512 | 481 | 0 | 75 | 161 | 160 |
| HTNV | 469 | 475 | 473 | 475 | 479 | 481 | 472 | 468 | 476 | 465 | 484 | 478 | 476 | 486 | 509 | 484 | 491 | 497 | 482 | 461 | 474 | 343 | 0 | 166 | 163 |
| PUUV | 409 | 421 | 427 | 427 | 427 | 424 | 427 | 418 | 442 | 418 | 433 | 424 | 416 | 417 | 416 | 417 | 432 | 425 | 415 | 425 | 408 | 475 | 510 | 0 | 86 |
| PHV | 395 | 391 | 400 | 398 | 399 | 402 | 398 | 415 | 418 | 390 | 394 | 405 | 396 | 405 | 414 | 396 | 395 | 394 | 408 | 398 | 403 | 484 | 486 | 347 | 0 |
Sequence Analysis of the M Segment
The complete M segment (Accession No. DQ285047) is 3465 nt in length, encoding a glycoprotein precursor of 1155 amino acids. Again, CHOV is more similar to the South American viruses (73.4 to 74.9% and 82.6 to 83.8% nt and aa, respectively) (Table 3). CHOV and North American viruses had nucleotide similarity ranging from 70.8 to 72.3% and amino acid similarity from 76.1 to 79.6%. Comparison of amino acid sequences shows that MAPV has an 83.6% identity to, a 79.6% identity to SNV, and a 83.7% identity to ANDV.
Table 3.
Nucleotide differences (below diagonal) and amino acid differences (above diagonal) between pairs of hantaviruses for the M segment
| ANDV1 | ANDV2 | ORNV | LECV | MAPV | LNV | CHOV | SNV | NYV | BAYV | BCCV | ELMC | SEOV | HTNV | PHV | PUUV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANDV1 | 0 | 9 | 85 | 72 | 168 | 142 | 188 | 238 | 257 | 262 | 267 | 291 | 512 | 506 | 476 | 524 |
| ANDV2 | 184 | 0 | 81 | 71 | 166 | 146 | 187 | 242 | 259 | 267 | 272 | 295 | 511 | 507 | 478 | 524 |
| ORNV | 711 | 708 | 0 | 51 | 174 | 150 | 194 | 246 | 256 | 269 | 267 | 299 | 507 | 505 | 486 | 533 |
| LECV | 713 | 718 | 629 | 0 | 161 | 146 | 187 | 238 | 252 | 269 | 266 | 290 | 505 | 497 | 483 | 527 |
| MAPV | 835 | 841 | 842 | 827 | 0 | 205 | 189 | 257 | 267 | 278 | 283 | 302 | 521 | 500 | 490 | 534 |
| LNV | 820 | 832 | 807 | 812 | 876 | 0 | 201 | 257 | 267 | 269 | 268 | 288 | 512 | 508 | 485 | 535 |
| CHOV | 870 | 874 | 879 | 895 | 889 | 922 | 0 | 236 | 258 | 245 | 260 | 276 | 522 | 512 | 484 | 532 |
| SNV | 953 | 960 | 968 | 947 | 970 | 970 | 961 | 0 | 58 | 212 | 222 | 226 | 521 | 505 | 462 | 512 |
| NYV | 965 | 974 | 986 | 999 | 965 | 955 | 987 | 647 | 0 | 234 | 237 | 240 | 523 | 508 | 471 | 512 |
| BAYV | 971 | 971 | 998 | 995 | 970 | 1003 | 964 | 911 | 947 | 0 | 130 | 259 | 509 | 503 | 477 | 526 |
| BCCV | 959 | 961 | 995 | 1007 | 988 | 965 | 975 | 893 | 915 | 752 | 0 | 264 | 517 | 510 | 488 | 527 |
| ELMC | 1044 | 1050 | 1035 | 1057 | 1061 | 1019 | 1013 | 939 | 960 | 986 | 963 | 0 | 509 | 487 | 475 | 522 |
| SEOV | 1418 | 1410 | 1410 | 1413 | 1454 | 1413 | 1421 | 1414 | 1410 | 1371 | 1355 | 1358 | 0 | 258 | 594 | 621 |
| HTNV | 1400 | 1402 | 1432 | 1417 | 1392 | 1400 | 1407 | 1401 | 1376 | 1374 | 1382 | 1377 | 937 | 0 | 587 | 619 |
| PHV | 1375 | 1385 | 1397 | 1392 | 1365 | 1360 | 1396 | 1327 | 1325 | 1375 | 1378 | 1333 | 1545 | 1512 | 0 | 314 |
| PUUV | 1452 | 1471 | 1487 | 1469 | 1462 | 1454 | 1477 | 1422 | 1452 | 1463 | 1451 | 1454 | 1600 | 1590 | 1061 | 0 |
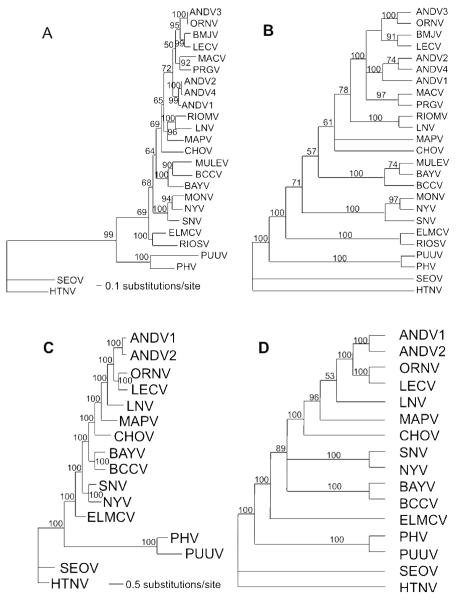
The tree topologies for the MP and ML analyses were similar. CHOV was basal to the South American viruses in the ML analyses for the S segment, whereas it formed a polytomy with MAPV and the remaining South American viruses in the MP analyses (Figure 1a and 1b). The relationship of CHOV to the South American viruses also was found in both the ML and MP analyses using the M segment (Figure 1c and 1d). However, the bootstrap support for this relationship was stronger than in the analysis of the S segment. The differences in tree topologies between this study and that of Fulhorst et al. (2004) can be explained by low bootstrap support of two nodes (compare Figure 1 to their Fig. 1 [p. 141]). Because bootstrap support is low in analyses from both studies, it is difficult to reject either hypothesis. In both studies the North American viruses are paraphyletic with respect to the South American viruses.
Figure 1.
A. Maximum likelihood analysis of 1302 nt of the S segment of 25 hantaviruses. Tree based on the GTR + G (0.6787) + I(0.3440) model of nucleotide evolution. Numbers associated with node are Bayesian posterior probabilities. Model for Bayesian analyses is the same used for Maximum Likelihood (topologies were identical). B. Single most parsimonious tree based on 675 informative of 1302 total base pairs of the N protein. Characters were weighted based on a rescaled consistency index. Third base positions were analyzed using transversions only. Tree length = 587.794; RCI = 0.5148. Numbers above branches represent percentage of 1000 bootstrap replications supporting each node. Nodes with less than 50% support are collapsed. C. Maximum likelihood analysis of 3465 nt of the M segment of 16 hantaviruses. Tree based on the GTR + G(0.3439) model of nucleotide evolution. Numbers associated with node are Bayesian posterior probabilities. Model for Bayesian analyses is the same used for Maximum Likelihood (topologies were identical). D. Single most parsimonious tree based on 2077 informative of 3465 total base pairs of the GPC gene. Tree length = 6307; CI = 0.4635. Third base positions were analyzed using transversions only.
Numbers above branches represent percentage of 1000 bootstrap replications supporting each node. Nodes with less than 50% support are collapsed.
Neutralization of Choclo Virus by Human Sera
The presence of neutralizing antibody was detected by the focus reduction assay in sera from all 6 individuals with RT-PCR-confirmed CHOV infection and typical HCPS. Three adults with 3 to 5 days of symptomatic acute disease and who required intubation had lower serum titers (1:200 to 1:1600) than the 3 individuals with milder disease who did not require intubation and who were tested 30 to 60 days after hospitalization (Table 4). Among 10 individuals with IgG antibody, as detected by EIA and strip immunoblot, and who denied previous hospitalization for respiratory infection, 9 had significant neutralizing antibody to CHOV, with titers ranging from 1:100 to 1:6400. One individual in this group did not have a detectable CHOV-neutralizing antibody, as determined in four independent assays. Seronegative controls from Panama did not have neutralizing antibody to CHOV, and no subject from Panama had neutralizing antibody to either SNV or ANDV. Two patients with mild HCPS in New Mexico had neutralizing antibody to SNV isolate 777 but not to CHOV. The hantaviruses identified by RT-PCR in Z. brevicauda (CALV) and in S. hirsutus (unnamed virus) could not be grown in Vero cell culture despite multiple blind passages in two different laboratories, and therefore neutralizing antibody to these viruses is not reported.
Table 4. Neutralization titers of Panamanian sera to CHOV isolate 588.
| Clinical Group | Pt # | Clin. Severity | Neut. CHOV | Neut. SNV | Neut. ANDV |
|---|---|---|---|---|---|
| Acute HCPS | 15355 | fatal | 1:400 | <1:20 | <1:20 |
| (day 5–8 of sxa) | 16617 | moderate | 1:200 | <1:20 | <1:20 |
| 15467 | moderate | 1:1600 | <1:20 | <1:20 | |
| Convalescent HCPS | 18483 | mild | 1:3200 | <1:20 | <1:20 |
| (day 15–20 of sxa) | 15586 | mild | 1:6400 | <1:20 | <1:20 |
| 17644 | mild | 1:3200 | <1:20 | <1:20 | |
| Seropositive/no resp hxb | 384 | No resp hx | 1:100 | <1:20 | <1:20 |
| 390 | No resp hx | 1:1600 | <1:20 | <1:20 | |
| 401 | No resp hx | 1:200 | <1:20 | <1:20 | |
| 407 | No resp hx | 1:400 | <1:20 | <1:20 | |
| 409 | No resp hx | 1:400 | <1:20 | <1:20 | |
| 412 | No resp hx | 1:1600 | <1:20 | <1:20 | |
| 703 | No resp hx | 1:6400 | <1:20 | <1:20 | |
| 705 | No resp hx | <1:20 | <1:20 | <1:20 | |
| 717 | No resp hx | 1:1600 | <1:20 | <1:20 | |
| 733 | No resp hx | 1:3200 | <1:20 | <1:20 | |
| Seronegative/neg hx | 7 sera | No resp hx | <1:20 | <1:20 | <1:20 |
| Acute HCPS, USA | NM 1 | Mild | <1:20 | 1:800 | <1:20 |
| NM 2 | mild | <1:20 | 1:1600 | <1:20 |
interval between onset of symptoms and diagnosis of HCPS.
seropositive and no history of acute respiratory infection requiring hospitalization.
DISCUSSION
The first goal of the study was to culture the virus and facilitate accurate identification of phylogenetic relationships. The complete genomic sequence of the S and M segments permitted the conclusion that CHOV is distinct from all other hantaviruses, according to established criteria [Elliott et al., 2000]. Even though CHOV and MAPV share the same host, O. fulvescens, these two strains are approximately 10% distinct at the amino acid level of the N protein. This distinction is likely due to independent evolution permitted by the extensive ecologic and physical separation between western Panama and western Venezuela. Rodents in the sigmodontine genus Oligoryzomys are natural hosts for at least 6 hantaviruses [Bharadwaj et al., 1997; Bohlman et al., 2002; Gonzalez Della Valle et al., 2002; Medina et al., 2009; Meissner et al., 2002; Powers et al., 1999]. The oryzomine rodent-borne viruses in the Argentina/Chile clade of hantaviruses show little amino acid diversity ([Bohlman et al., 2002; Medina et al., 2009], suggesting recent divergence. The greater diversity of CHOV from other oryzomine hantaviruses suggests its more remote divergence from the common ancestor.
The second goal of the study was to use the specificity of the neutralization assay to indicate whether the high serum antibody prevalence was due to previous CHOV infection. The focus neutralization assay of sera from CHOV-positive PCR-confirmed HCPS patients demonstrated the presence of neutralizing antibody titers of levels similar to other published reports [Bharadwaj et al., 2000]. As expected, there was no cross-neutralization between CHOV, ANDV, and SNV, but cross-neutralization studies with more closely related viruses, including CALV [Vincent et al., 2000] and MAPV, a hantavirus isolated from O. fulvescens in Venezuela [Fulhorst et al., 2004; Milazzo et al., 2002], is needed for definitive specificity.
In five communities in the Azuero peninsula investigators found antibody prevalence ranging from 3% to 45% [Armien et al., 2004]. Antibody was detected in samples from children as young as 4 years of age and increases steadily to highest prevalence in the 40- to 50-year-old cohort, suggesting that steady exposure and infection occurs throughout life in rural areas. From this cohort data it is estimated that among the 250,000 people of the Azuero peninsula there may be more than 10,000 individuals with serum anti-hantavirus antibody. Questionnaires indicated that none of the 275 antibody-positive individuals tested had given a history compatible with an HCPS-like illness, although histories of febrile respiratory illnesses not requiring hospitalization were common [Armien et al., 2004]. An ongoing clinical trial in four clinics during two years of observation has identified 110 individuals with fever and anti-hantavirus antibody detected by IgM-ELISA. CHOV RNA was detected in acute blood samples by RT-PCR, yet no progression to symptomatic pulmonary disease was found (B. Armien, unpublished data).
If the majority of antibody positive individuals were infected with CHOV, as suggested by neutralization assays, and if the reported 140 cases of HCPS is a good estimation of total cases, the ratio of mild or asymptomatic CHOV infection to HCPS may be as high as 50:1 in Panama. LNV may also be associated with a high ratio of mild to severe infection, although strain-specific neutralization titers have not been published [Chu et al., 2006]. In regions where multiple hantaviruses co-circulate in rodent populations, such as Panama, Paraguay [Chu et al., 2006] and Brazil [Figueiredo et al., 2009; Raboni et al., 2009], neutralization assays studies can identify the dominant hantaviruses that infect humans. Since each rodent host has habitat preferences [Armien et al., 2009; Chu et al., 2003], the identification of hantavirus species causing human disease can have useful public health implications.
ACKNOWLEDGEMENTS
We acknowledge the support of Karl M. Johnson, and sequencing support from the Center for Genomic Medicine at the University of New Mexico. We thank Brian Hjelle for providing the virus stocks of SNV and ANDV.
Funding for this work was obtained from a Gorgas Memorial Institute Research Award to R.C.; an Opportunity Pool Award and Supplements to the ICIDR Program, NIH-U19 45452; the RCE program U54 AI057156; support from the Ministry of Health, Republic of Panama (B.A. and J.M.P); and a grant from DARPA under grant no. DARPA-N00014-1-0900 (J.W.D).
BIBLIOGRAPHY
- Armien AG, Armien B, Koster FT, Pascale JM, Avila M, Gonzalez P, de la Cruz M, Zaldivar Y, Mendoza Y, Gracia F, Hjelle B, Lee S-J, Yates TL, Salazar-Bravo J. Hantavirus infection and habitat associations among rodent populations in agroecosystems of Panama: implications for human disease risk. Am J Trop Med Hyg. 2009;81(1):59–66. [PubMed] [Google Scholar]
- Armien B, Pascale JM, Bayard V, Munoz C, Mosca I, Guerrero G, Armien A, Quiroz E, Castillo Z, Zaldivar Y, Gracia F, Hjelle B, Koster F. High seroprevalence of hantavirus infection on the Azuero peninsula of Panama. Am J Trop Med Hyg. 2004;70(6):682–687. [PubMed] [Google Scholar]
- Bayard V, Kitsutani PT, Barria EO, Ruedas LA, Tinnin DS, Munoz C, de Mosca IB, Guerrero G, Kant R, Garcia A, Caceres L, Gracia F, Quiroz E, de Castillo Z, Armien B, Libel M, Mills JN, Khan AS, Nichol ST, Rollin PE, Ksiazek TG, Peters CJ. Outbreak of hantavirus pulmonary syndrome, Los Santos, Panama, 1999-2000. Emerg Infect Dis. 2004;10(9):1635–1642. doi: 10.3201/eid1009.040143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj M, Botten J, Torrez-Martinez N, Hjelle B. Rio Mamore virus: genetic characterization of a newly recognized hantavirus of the pygmy rice rat, Oligoryzomys microtis, from Bolivia. Am J Trop Med Hyg. 1997;57(3):368–374. doi: 10.4269/ajtmh.1997.57.368. [DOI] [PubMed] [Google Scholar]
- Bharadwaj M, Nofchissey R, Goade D, Koster F, Hjelle B. Humoral immune responses in the hantavirus cardiopulmonary syndrome. J Infect Dis. 2000;182(1):43–48. doi: 10.1086/315657. [DOI] [PubMed] [Google Scholar]
- Bohlman MC, Morzunov SP, Meissner J, Taylor MB, Ishibashi K, Rowe JE, Levis S, Enria D, St. Jeor SC. Analysis of hantavirus genetic diversity in Argentina: S segment-derived phylogeny. J Virol. 2002;76(8):3765–3773. doi: 10.1128/JVI.76.8.3765-3773.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov V, Spiropoulou CF, Morzunov S, Monroe M, Peters CJ, Nichol ST. Complete genetic characterization and analysis of isolation of Sin Nombre virus. J Virol. 1995;69(12):8132–8136. doi: 10.1128/jvi.69.12.8132-8136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu YK, Milligan B, Owen RD, Goodin DG, Jonsson CB. Phylogenetic And Geographical Relationships Of Hantavirus Strains In Eastern And Western Paraguay. Am J Trop Med Hyg. 2006;75(6):1127–1134. [PMC free article] [PubMed] [Google Scholar]
- Chu YK, Owen RD, Gonzalez LM, Jonsson CB. The complex ecology of hantavirus in Paraguay. Am J Trop Med Hyg. 2003;69(3):263–268. [PubMed] [Google Scholar]
- Chu YK, Rossi C, LeDuc JW, Lee HW, Schmaljohn CS, Dalrymple JM. Serologic relationships among viruses in the Hantavirus genus, family Bunyaviridae. Virol. 1994;198(1):196–204. doi: 10.1006/viro.1994.1022. [DOI] [PubMed] [Google Scholar]
- Elliott RM, Bouloy M, Calisher CH, Goldbach R, Moyer JT, Nichol ST, Pettersson RF, Plyusnin A, Schmaljohn CS. Family Bunyaviridae. In: van Regenmortel MHV, Fauquet CM, Bishop DH, Carstens EB, Estes MK, Lemon SM, Maniloff J, Mayo MA, McGeoch DJ, Pringle CR, Wickner RB, editors. Viral Taxonomy: classification and nomenclature of viruses Seventh report of the international committee on taxonomy of viruses. Academic Press; 2000. pp. 599–621. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Ferrer JF, Jonsson CB, Esteban E, Galligan D, Basombrio MA, Peralta-Ramos M, Bharadwaj M, Torrez-Martinez N, Callahan J, Segovia A, Hjelle B. High prevalence of hantavirus infection in Indian communities of the Paraguayan and Argentinean Gran Chaco. Am J Trop Med Hyg. 1998;59(3):438–444. doi: 10.4269/ajtmh.1998.59.438. [DOI] [PubMed] [Google Scholar]
- Figueiredo LTM, Moreli ML, de Sousa RLM, Borges AA, de Figueiredo GG, Machado AM, Bisordi I, Nagasse-Sugahara TK, Suzuki A, Pereira LE, de Souza RP, de Souza LTM, Braconi CT, Harsi CM, de Andreade Zanotto PM. Hantavirus pulmonary syndrome, central plateau, southeastern, and southern Brazil. Emerging Infectious Diseases. 2009;15(4):561–564. doi: 10.3201/eid1504.080289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulhorst CF, Cajimat MNB, Utrera A, Milazzo ML, Duno GM. Maporal virus, a hantavirus associated with the fulvous pygmy rice rat (Oligoryzomys fulvescens) in western Venezuela. Virus Research. 2004;104(2):139–144. doi: 10.1016/j.virusres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Gonzalez Della Valle M, Edelstein A, Miguel SD, Martinez V, Cortez J, Cacace ML, Jurgelenas G, Sosa Estani S, Padula P. Andes virus associated with hantavirus pulmonary syndrome in northern Argentina and determination of the precise site of infection. Am J Trop Med Hyg. 2002;66(6):713–720. doi: 10.4269/ajtmh.2002.66.713. [DOI] [PubMed] [Google Scholar]
- Hjelle B, Anderson B, Torrez-Martinez N, Song W, Gannon WL, Yates TL. Prevalence and geographic genetic variation of hantaviruses of New World harvest mice (Reithrodontomys): identification of a divergent genotype from a Costa Rican Reithrodontomys mexicanus. Virology. 1995;207(2):452–459. doi: 10.1006/viro.1995.1104. [DOI] [PubMed] [Google Scholar]
- Hjelle B, Jenison S, Torrez-Martinez N, Herring B, Quan S, Polito A, Pichuantes S, Yamada T, Morris C, Elgh F, Lee HW, Artsob H, Dinello R. Rapid and specific detection of Sin Nombre virus antibodies in patients with hantavirus pulmonary syndrome by a strip immunoblot assay suitable for field diagnosis. J Clin Microbiol. 1997;35(3):600–608. doi: 10.1128/jcm.35.3.600-608.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster FT, Hjelle B. Hantaviruses. In: Gorbach SL, Bartlett JG, Blacklow NR, editors. Infectious Diseases. Third ed. Lippincott Williams & Wilkins; Philadelphia: 2004. pp. 2023–2031. [Google Scholar]
- Maddison DR, Maddison WP. MacClade 4: analysis of phylogeny and character evolution. Version 4.06. Sinauer Associates; Sunderland, Massachusetts: 2003. [Google Scholar]
- Medina RA, Torres-Perez F, Galeno H, Navarrete M, Vial PA, Palma RE, Ferres M, Cook JA, Hjelle B. Ecology, genetic diversity and phylo geographic structure of Andes Virus in human and rodents in Chile. J Virol. 2009;83(6):2446–2459. doi: 10.1128/JVI.01057-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner JD, Rowe JE, Borucki MK, St. Jeor SC. Complete nucleotide sequence of a Chilean hantavirus. Virus Res. 2002;89:131–143. doi: 10.1016/s0168-1702(02)00129-6. [DOI] [PubMed] [Google Scholar]
- Milazzo ML, Eyzaguirre EJ, Molina CP, Fulhorst CF. Maporal viral infection in the Syrian Golden Hamster: A model of hantavirus pulmonary syndrome. J Infect Dis. 2002;186(10):1390–1395. doi: 10.1086/344735. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14(9):817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Powers AM, Mercer DR, Watts DM, Guzman H, Fulhorst CF, Popov VL, Tesh RB. Isolation and genetic characterization of hantavirus (Bunyaviridae: Hantavirus) from a rodent, Oligoryzomys microtis (Muridae), collected in northeastern Peru. Am J Trop Med Hyg. 1999;61:92–98. doi: 10.4269/ajtmh.1999.61.92. [DOI] [PubMed] [Google Scholar]
- Raboni SM, Hoffmann FG, Oliveira RC, Teixeira BR, Bonvicino CR, Stella V, Carstensen S, Bordignon J, D’Andrea PS, Lemos ERS, Duarte dos Santos CN. Phylogenetic characterization of hantaviruses from wild rodents and hantavirus pulmonary syndrome cases in the state of Parana (southern Brazil) J Gen Virol. 2009;90:2166–2171. doi: 10.1099/vir.0.011585-0. [DOI] [PubMed] [Google Scholar]
- Salazar-Bravo J, Armien B, Suzan G, Armien A, Ruedas LA, Avila Diaz M, Zaldivar Y, Pascale JM, Gracia F, Yates TL. Serosurvey of wild rodents for hantaviruses in Panama, 2000-2002. J Wildlife Dis. 2004 doi: 10.7589/0090-3558-40.1.103. in press. [DOI] [PubMed] [Google Scholar]
- Schmaljohn C, Hjelle B. Hantaviruses: a global disease problem. Emerg Infect Dis. 1997;3(2):95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. Phylogenetic analysis using parsimony (PAUP and other methods). Version 4.0b10. Sinauer Associates; Sunderland, Massachusetts: 2002. [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choices. Nucl Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent MJ, Quiroz E, Gracia F, Sanchez AJ, Ksiazek TG, Kitsutani PT, Ruedas LA, Tinnin DS, Caceres L, Garcia A, Rollin PE, Mills JN, Peters CJ, Nichol ST. Hantavirus pulmonary syndrome in Panama: Identification of novel hantaviruses and their likely reservoirs. Virol. 2000;277:14–19. doi: 10.1006/viro.2000.0563. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Bryan RT, Mills JN, Palma RE, Vera I, De Velasquez F, Baez E, Schmidt WE, Figueroa RE, Peters CJ, Zaki SR, Khan AS, Ksiazek TG. An outbreak of hantavirus pulmonary syndrome in western Paraguay. Am J Trop Med Hyg. 1997;57(3):274–282. doi: 10.4269/ajtmh.1997.57.274. [DOI] [PubMed] [Google Scholar]