Abstract
Cisplatin is a chemotherapeutic agent that is widely used to treat a variety of malignant tumors. Serious dose-limiting side effects like ototoxicity, nephrotoxicity and neurotoxicity occur with the use of this agent. This review summarizes recent important clinical and experimental investigations of cisplatin ototoxicity. It also discusses the utility of protective agents employed in patients and in experimental animals. The future strategies for limiting cisplatin ototoxicity will need to avoid interference with the therapeutic effect of cisplatin in order to enhance the quality of life of patients receiving this important anti-tumor agent.
Keywords: ototoxicity, cisplatin, cochlea, protection, mechanisms
Cisplatin is a chemotherapeutic agent used in the treatment of solid tumors like ovarian, testicular, cervical, lung, head and neck and bladder cancers. Dose limiting side effects of cisplatin include nephrotoxicity, neurotoxicity and ototoxicity. While nephrotoxicity can to some extent be reversed by increasing saline hydration as well as mannitol diuresis, there are no known cures or preventative treatments available for ototoxicity and neurotoxicity. Elevation of hearing threshold have been reported in some audiometric studies in 75–100% of patients treated with cisplatin (McKeage 1995). Cisplatin induced hearing loss is usually bilateral and irreversible, and is particularly serious in the pediatric population (age 6 months and onwards) with cancers like neuroblastomas, CNS malignancies, head and neck cancers, where irradiation of the base of the skull or brain is also performed (Chen et al. 2006). Loss of hearing at this developmental stage hampers the speech, cognitive and social development of the child. Thus, there is an imperative need for treatments that will ameliorate ototoxicity.
Attenuation of cisplatin ototoxicity has been shown in several scientific research papers by protective agents that are predominantly anti-oxidants like N-acetyl cysteine (NAC), sodium thiosulphate, amifostine, lipoic acid etc. However, clinical trials are under way for several otoprotective agents like alpha-lipoic acid, lactated ringer’s, aspirin, sodium thiosulphate, D-methionine in chemotherapy induced ototoxicity (www.clinicaltrials.gov). This review summarizes the latest research on cisplatin induced ototoxicity and molecules that show prevention or alleviation of ototoxicity in clinical as well as experimental studies.
Clinical ototoxicity of cisplatin
Hearing loss following cisplatin chemotherapy appears to be variable. It may be related to dose, age of the patient, and other factors, such as noise exposure (Bokemeyer et al. 1998), exposure to other ototoxic drugs, depleted nutritional state, including low serum albumin and anemia (Kopelman et al. 1988), and cranial irradiation (Huang et al. 2002). Young children seem to be more susceptible than adults (Li et al. 2004). Hearing loss often is permanent and bilaterally symmetric (Bokemeyer et al. 1998). Symptoms of ototoxicity include subjective hearing loss, ear pain, or tinnitus (Reddel et al. 1982). 2% to 36% of patients treated with cisplatin complain of tinnitus (Reddel et al. 1982). High frequency audiometric thresholds often are affected first. However, hearing impairment may progress to involve the middle frequencies when doses in excess of 100 mg/m2 are used. If ultra-high-frequency audiometric testing is used, up to 100% of patients receiving high-dose cisplatin (150–225 mg/m2) may show some degree of hearing loss (Kopelman et al. 1988). Dose-related ototoxicity is illustrated by the report that 20% of patients treated with cisplatin for testicular cancer experience permanent ototoxicity, but > 50% of patients who received cisplatin in doses > 400 mg/m2 cumulative dose had permanent hearing loss (Bokemeyer et al. 1998). A good correlation between transient otoacoustic emissions and pure tone audiometric thresholds was shown in children. 90.5% of patients had a significant sensorineural hearing loss at 8 kHz. The severity of the hearing loss was correlated with the following risk factors: young age when the first dose of cisplatin was given, number of cycles and high cumulative dose (Allen et al. 1998). Cranial irradiation enhanced the probability and severity of hearing loss (Huang et al. 2002). Patients with nasopharyngeal carcinoma appear to be very susceptible to the interaction of cisplatin chemotherapy with cochlear irradiation. Radiation doses greater than 48 Gy increased the hearing loss in these patients (Chen et al. 2005). However radiation therapy for brain tumors, such as medulloblastoma, can be modified using intensity-modulated radiation therapy (IMRT) to reduce the doses of radiation to the cochlea. Only 13% of patients treated with this protocol combined with cisplatin had grade 3 or 4 hearing loss compared with 64% of the group receiving conventional radiation therapy (Huang et al. 2002). A recent study showed that patients with head and neck cancer treated with radiation therapy alone with doses of less than 40 Gy did not suffer clinically significant hearing loss. However, high frequency sensorineural hearing loss was profound in patients who received concomitant cisplatin in doses of 100 mg/m2. The predicted threshold for hearing loss in patients receiving combined radiation to the cochlea and cisplatin chemotherapy was 10 Gy (Hitchcock et al. 2009).
Although ototoxicity caused by cisplatin may occur within hours to days after drug administration, delayed ototoxicity from cisplatin may occur in children. Pediatric patients treated with cisplatin at cumulative doses approaching 400 mg/m2 showed worsening of their hearing long after treatment. Audiograms showed hearing loss in 5% of patients before the end of therapy. After more than two years of follow up, 44% had significant hearing loss (Bertolini et al. 2004). The median time for first significant decrease in hearing was 135 days in children. Additional follow-up for 6 to 44 months showed mild further progression of hearing loss of 10 to 15 dB after completion of therapy (Knight et al. 2005).
Genetic predisposition to cisplatin ototoxicity
There have been several publications suggesting a relationship between genes and susceptibility to cisplatin ototoxicity. Certain gene polymorphisms appear to play a role in increasing or decreasing the likelihood of ototoxicity in patients treated with cisplatin chemotherapy.
Functional polymorphisms in cisplatin-detoxifying enzymes have been suspected to render patients more susceptible to cisplatin-induced hearing loss. 173 cisplatin-treated survivors of testicular cancer were followed in a long-term survey that included audiometric testing and lymphocyte sampling. Hearing thresholds at 4,000 Hz were measured and compared with known functional polymorphisms in glutathione-S-transferases (GSTs). The genes encoding the enzymes GSTM1, GSTT1 and GSTP1 are polymorphic in humans. The risks of having greater hearing loss was more than four times greater in patients with 105Ile/105Ile-GSTP1 or 105Val/105Ile-GSTP1 compared with 105Val/105Val-GSTP1. Patients with the pattern of GSTT1 positive, GSTM1 positive and 105Val/105Val-GSTP1 had better hearing than those patients without this pattern (Oldenburg et al. 2007). A more recent study of 42 patients showed that pediatric patients with medulloblastoma having at least one null genotype for GSTM1 or GSTT1 had greater than 4-fold increased risk of grade 3 toxicity, including ototoxicity. They concluded that polymorphisms of GSTM1 and GSTT1 may predict adverse events (Barahmani et al. 2009).
Nucleotide excision repair genes play a key role in reversing DNA damage. Since cisplatin causes DNA cross-linking, an investigation was carried out to determine whether polymorphisms in these genes have any relationship to clinical outcome and ototoxicity in osteosarcoma patients treated with cisplatin. Eight single nucleotide polymorphisms in excision repair cross-complementing group 1, 2, 4 and 5 (ERCC1, ERCC2, ERCC4, and ERCC5) genes and xeroderma pigmentosum complementary group C and A (XPC, XPA) genes were analyzed in 91 patients with osteosarcoma treated with cisplatin. Ototoxicity was recorded in 32 patients. This adverse effect was associated with the CC genotype of XPC Lys939Gln. Tumor response was correlated with the presence of homozygosity for the T allele of ERCC2 gene in patients compared with poorer responses in patients with at least one polymorphic G allele (Caronia et al. 2009).
Megalin is a member of the low-density lipoprotein family. These genes are highly expressed in the kidney and in the stria vascularis of the cochlea. These tissues have been found to accumulate platinum-DNA adducts. A higher frequency of a particular megalin gene polymorphism, the A-allele of rs2075252, was observed in a group of cisplatin-treated patients who suffered hearing impairment compared with the group having no hearing loss after cisplatin therapy. These findings suggest that SNPs at the megalin gene may influence individual patient susceptibility to cisplatin ototoxicity (Riedemann et al. 2008).
Cisplatin-induced hearing loss has also been studied in patients with mitochondrial mutations. Five of 20 cancer survivors who suffered hearing loss were found to have a rare European J mitochondrial haplogroup that is also associated with Leber’s Hereditary Optic Atropy (Peters et al. 2003).
Experimental studies of cisplatin ototoxicity
Cisplatin undergoes hydrolysis in blood to form cisdiamineaquachloroplatinum (II), the major aquated metabolite, responsible for its cytotoxic actions (Jones et al. 1991; Huang et al. 2006). Cisplatin uptake into the cell has been linked to several different transporters like mammalian copper transporter 1 (CTR1), while ATP7A and ATP7B, the two efflux copper transporters regulate the export of cisplatin from the cells (Komatsu et al. 2000; Samimi et al. 2004; Kuo et al. 2007).
Laboratory animal as well as in vitro studies show that cisplatin interacts with cochlear tissues such as outer hair cells of the organ of Corti, stria vascularis, spiral ligament and spiral ganglionic cells to generate a robust reactive oxygen species (ROS) response (Clerici et al. 1995; Clerici and Yang 1996; Kopke et al. 1997; Dehne et al. 2001; Bánfi et al. 2004) while depleting the antioxidant enzyme system that would scavenge and neutralize this increase in superoxides (Rybak et al. 2000). Cisplatin accumulates in the cochlear tissue, integrates into the DNA, and causes inefficient and dysfunctional proteins and enzyme synthesis. The cochlea, because of its unique anatomical position and isolation is practically a closed system, and therefore is unable to flush out accumulated toxins at the rapid pace of their generation. This leads to a ROS overload combined with decreased antioxidant system leading to cell injury and apoptosis. The anti-oxidant enzymes of the cochleae include: superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) and glutathione reductase (GSH-R). Down regulation of these enzymes have been linked to increased malondialdehyde (indicator of lipid peroxidation) (Rybak et al. 2000) and in the amounts of oxidized glutathione (GSSG) (Somani et al. 2001).
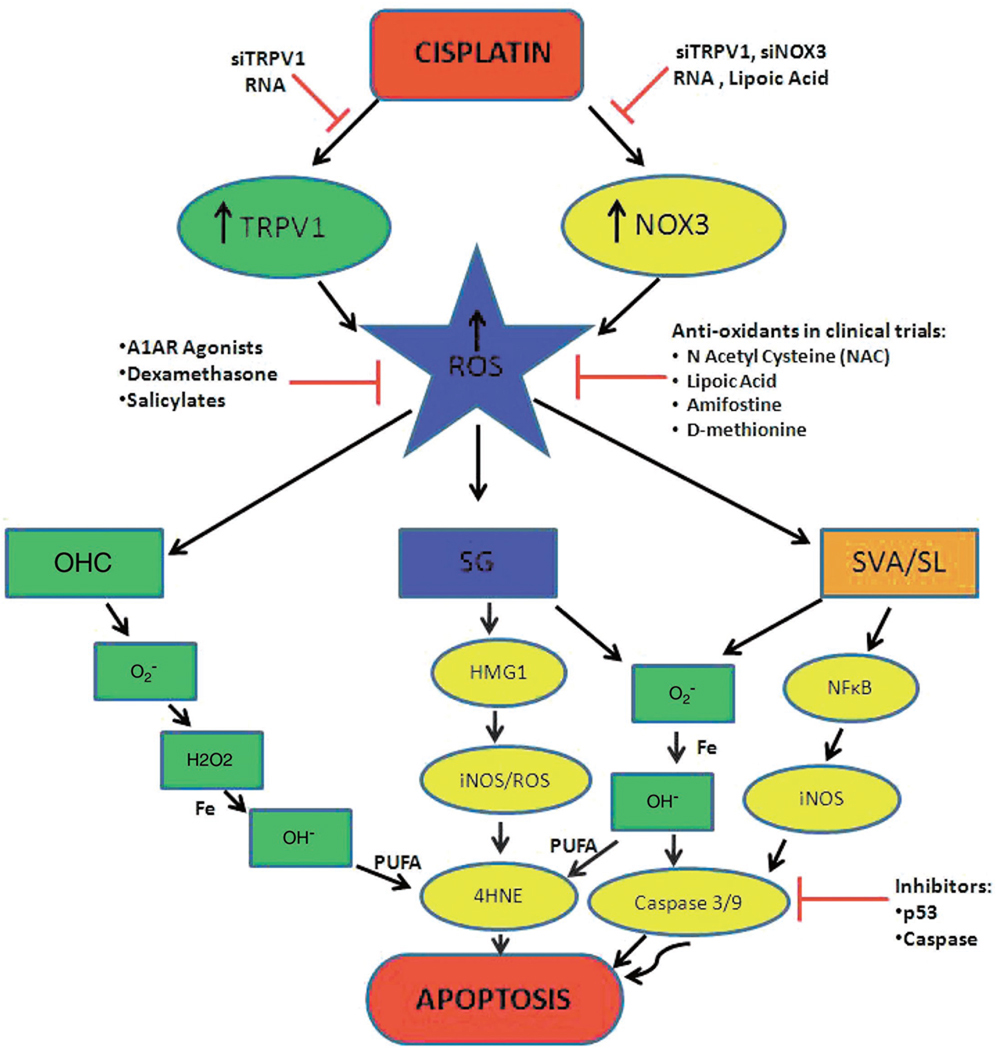
The superoxides generated by the various cochlear tissues can a) interact with nitric oxide and form peroxynitrites which nitrosylate and inactivate proteins (Lee et al. 2004a,b), b) form free hydroxyl radicals which on interaction with Iron (Fe) react with poly unsaturated fatty acids (PUFA) in the bilipid bilayer of the cell membranes to generate highly toxic aldehyde 4-hydroxynonenal (4-HNE) leading to cell death (Lee et al. 2004a,b). This increase in 4-HNE has been associated with increased Ca2+ influx into the outer hair cell and apoptosis (Ikeda et al. 1993; Clerici et al. 1995). c) inactivate antioxidant enzymes (Pigeolot 1990), and d) cause cytosolic migration of Bax, leading to release of cytochrome c from injured mitochondria which is responsible for for activation of caspase 3 and caspase 9. Caspase-activated deoxyribonuclease (CAD) is then activated, causes DNA breakdown (Watanabe et al. 2003) and cleavage of fodrin in the cuticular plates of injured hair cells (Wang et al. 2004). An overview of cisplatin induced cytotoxic mechanisms in the different areas of the cochleae and the potential inhibitors is shown in Fig. 1.
Fig. 1. An overview of cisplatin induced pathways in cochlear ototoxicity in the different areas of the cochleae and the potential inhibitors.
Cisplatin administration causes induction/activation of TRPV1 and NOX3, both of which lead to increased ROS generation. One common pathway by which increased ROS produced apoptosis in all the regions of the cochleae is by production of free superoxide radical which reacts with water to form hydrogen peroxide that will react with iron to form highly reactive hydroxyl radical. This in turn interacts with poly unsaturated fatty acids (PUFA) of the cell membrane to form 4-hydroxynonenal (4-HNE), which is a highly toxic aldehyde which leads to cell death. In addition to this pathway cisplatin has also been shown to activate NFκB which induces the formation of nitric oxide (NO) by upregulating the enzyme iNOS in the lateral wall (stria vascularis and spiral ligament, represented as SVA/SL) of the cochlea. This NO release reacts with superoxide to form peroxynitrite which will activate a caspase cascade followed by apoptosis. In the spiral ganglion (SG), cisplatin has been shown to upregulate the expression of high mobility group (HMG1) protein which upregulates iNOS and can cause cell death via either the caspase cascade or via the 4-HNE pathway in this tissue.
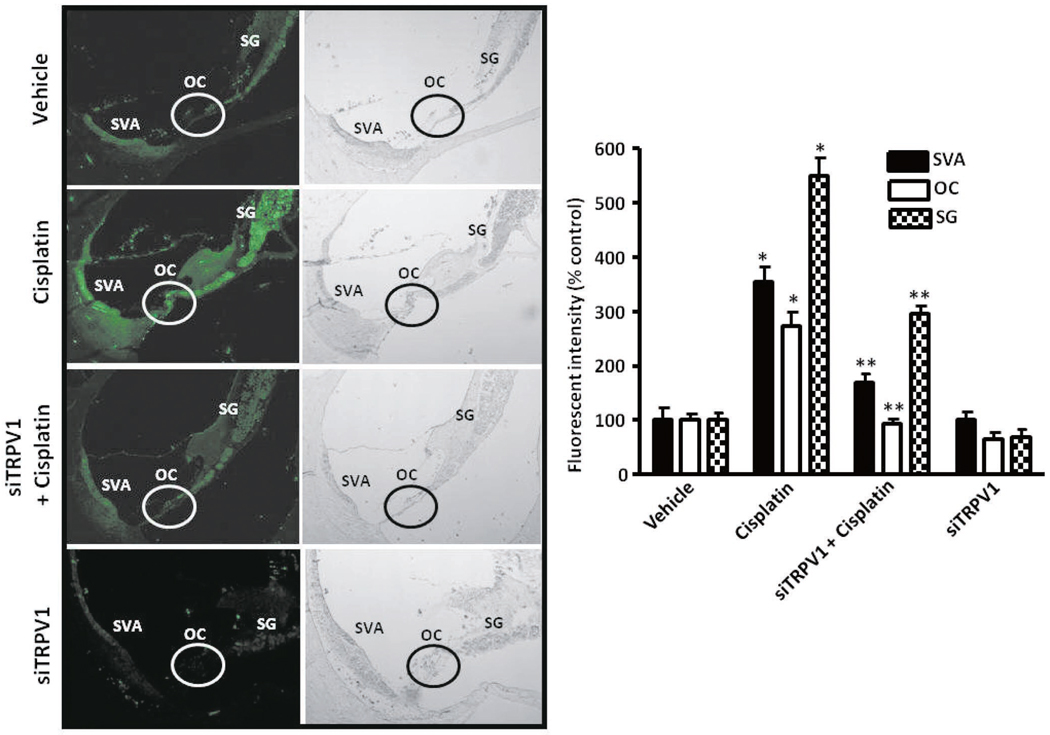
A major source of ROS which has been identified in the cochlea is an isoform of NADPH oxidase, NOX3 (Bánfi et al. 2004). This enzyme appears to be activated and induced by cisplatin in the rat cochlea (Mukherjea et al. 2006) as early as 24 hrs post cisplatin treatment, and in cochlear explants (Bánfi et al. 2004). A link between cisplatin and the activation/induction of NOX3 has not been clearly defined. Interestingly, studies from our laboratory indicate that, at least for outer hair cells, cisplatin-mediated ROS generation is dependent on induction and activation of the transient receptor potential vanilloid 1 (TRPV1) channel (Mukherjea et al. 2008). This is the first evidence of TRPV1 being used as a permeant channel by cisplatin. Thus the activation of TRPV1 brings in the added toxicity of Ca2+ influx and overload followed by activation of caspases and cell death. Therefore suppression of TRPV1 expression in UB/OC-1 cultures as well as in the rat model by short interfering (si) RNA showed suppression of cisplatin-mediated ROS generation, NOX3 expression and Ca2+ increase, thus protecting the outer hair cells from cisplatin-damage, leading to protection from cisplatin induced hearing loss (Fig. 2) (Mukherjea et al. 2008).
Fig. 2. siRNA for TRPV1 protein shows decreased immunoreactivity in the rat cochlea.
Scrambled siRNA or TRPV1 siRNA (0.9 µg) were administered to the rat cochlea by round window application, followed by vehicle or cisplatin (13 mg/kg; i.p.) treatment 48 h later. Animals were anesthetized and euthanized 72 h post cisplatin treatment, the cochleae were excised and processed for TRPV1 immunohistochemistry. Midmodiolar sections of the cochlea (magnification, 100×) are shown in the images above. Cisplatin treatment showed increased immunolabeling in the organ of Corti, spiral ganglion and stria vascularis. Administration of TRPV1 siRNA significantly decreased both basal and cisplatin-induced TRPV1 immunoreactivity in all the three regions examined. This difference in fluorescence intensity has been represented as a bar graph in the right panel. The data are presented as the mean ± SEM of 3 animals for each treatment group. Abbreviations: SVA, OC and SG represent stria vascularis, organ of Corti and stria vascularis, respectively. Asterisk (*) indicate statistically significant increase in TRPV1 immunolabeling as compared to vehicle treated controls ( p < 0.05), while (**) indicates statistically significant difference from the cisplatin group ( p < 0.05). (Mukherjea et al. 2008; This image has been republished with permission from Journal of Neuroscience).
Cisplatin also activates big conductance potassium (BK) channels in the type I spiral ligament fibrocytes of the lateral wall and disrupts the electrochemical gradient thus triggering apoptosis (Liang et al. 2005). Stria vascularis or the lateral wall maintains the ionic environment of the endolymph which has higher potassium and lower calcium concentration than the surrounding perilymph and maintains a high resting membrane potential. Cisplatin induced persistent activation of the BK channels leads to potassium efflux, thus decreasing the intracellular potassium, causing the loss of intracellular osmotic pressure and ionic concentration which in turn triggers the pro-apoptotic nucleases, cleavage of caspases leading to cell death (Hughes et al. 1997; Bortner and Cidlowski 1998; Liang et al. 2005).
Transcription factor nuclear factor kappa B (NFκB) and inducible nitric oxide synthase (iNOS) have been shown to be upregulated in the stria vascularis and spiral ligament of cisplatin treated mice in immunohistochemical preparations (Watanabe et al. 2002). However, in vitro experiments show that NFκB is essential for survival of immature auditory hair cells (Nagy et al. 2005). NFκB inhibitors have been shown to decrease cell death in HEI/OC1 cells on cisplatin teatment (Chung et al. 2008) by decreasing the caspase 3 activity. Increased NFB staining has been observed in the organ of Corti, spiral ligament, and stria vascularis in cisplatin treated rat cochlea, and NFB was shown to be activated in HEI/OC1 cells on cisplatin treatment as well (So et al. 2007). The same group has also shown that proinflammatory cytokines like TNF-alpha, IL-1beta, and IL-6 along with NFκB are upregulated by cisplatin treatment in vitro in the HEI/OC1 cells as well as in the rat cochleae (So et al. 2008) and that administration of etanercept (TNF-alpha inhibitor) as well as flunarazine (T-type calcium channel blocker) were otoprotective. This otoprotection was then shown to be via the down regulation of NFκB activation and upregulation of NRF2/HO1 activation.
Activation of transcription factor STAT1 is known to mediate cell death in exposure to ROS (Stephanou et al. 2001, 2002; Townsend et al. 2004; De Vries et al. 2004), UV radiation (Zykova 2005), tumor necrosis factor α (TNF α) and DNA damage (De Vries et al. 2004). Cisplatin induced hair cell death in utricular cultures has been shown to be mediated via STAT1 (Schmitt et al. 2009) and is attenuated by pretreatment with epigallocatechin gallate (EGCG), a green tea extract.
Cisplatin DNA adduct formation is removed by nucleotide excision repair (NER) system, which has also been linked to development of cisplatin resistance in ovarian and small cell lung cancer (Dabholkar et al. 1994; Ferry et 2000; Giaccone 2000; Selvakumaran et al. 2003). The NER identifies lesions made by two pathways: 1) The global genome-NER (GG-NER) identifies DNA damage on non-transcribed genes (de Laat et al. 1999, Costa et al. 2003), and is via activation of xeroderma pigmentosum complementation group C (XPC) and it’s conjugates (Boonstra et al. 2001, Chen et al. 2003) and 2) The transition coupled NER (TC-NER) pathway that repairs DNA damage on transcribed genes. Activation of xeroderma pigmentosum complementation group A (XPA) is essential for TC-NER and is initiated by RNA Pol II during transcription (Tornaletti et al. 2003). It has been shown that XPA activation is essential for both the NER pathways (de Laat et al. 1999). Dysregulation of XPA and XPC has been shown in cisplatin-treated rat cochleae (Guthrie et al. 2008).
Clinical studies of protection against cisplatin ototoxicity
Thiosulfate may protect against nephrotoxicity in humans (Goel et al. 1989). However, it may also reduce the anti-neoplastic activity of cisplatin (Howell and Taetle 1980; Iwamoto et al. 1985). The reductions in anti-tumor efficacy and side effects are most likely caused by a decrease in the area under the curve (AUC) of cisplatin and/or it monohydrated complex (Goel et al. 1989; Ekborn et al. 2002; Videhult et al. 2006).
Chemoradiation protocols with intra-arterial cisplatin include thiosulfate as a protective agent. However, thiosulfate given in this manner may not provide much protection. 146 patients received high-dose intra-arterial cisplatin chemotherapy (150 mg/m2, for four courses) with sodium thiosulfate rescue and concurrent radiation therapy (70 Gy) for locally advanced head and neck cancer. 23% of the ears were found to have sufficient hearing loss to require hearing aids after treatment. Cumulative dose of cisplatin and radiation and young age were correlated with increased sensorineural hearing loss during and after therapy. Furthermore, the pretreatment hearing level of the involved ear was found to be an independent predictive factor for hearing capability after therapy (Zuur et al. 2007).
Maximum threshold shifts occurred after the second cisplatin infusion, and occurred at 8 kHz. Hearing loss seemed to reach a plateau at higher levels (75–80 dB hearing loss) for frequencies above, compared with frequencies below 8 kHz (45–60 dB hearing loss) (Zuur et al. 2006).
Patients with metastatic melanoma treated with amifostine prior to cisplatin demonstrated no evidence of protection against ototoxicity (Ekborn et al. 2002). No protection was observed in children with germ cell tumors treated with amifostine in combination with cisplatin, etoposide and bleomycin (Marina et al. 2005; Sastry and Kellie 2005). Although these previous studies showed no protection from this drug, amifostine (600 mg/m2) given as an intravenous bolus immediately prior to and three hours after cisplatin and craniospinal irradiation in children with medulloblastoma, provided significant protection against hearing loss. One year after treatment initiation, 13 (37.1%) of controls compared to 9 (14.5%) of amifostine-treated patients had hearing loss sufficient to require hearing aid in at least one ear ( p = 0.005 chi square one-sided test). There was no evidence that amifostine interfered with the efficacy of the cisplatin and the side effects of amifostine therapy were mild and well tolerated (Fouladi 2008).
Strategies to prevent cisplatin ototoxicity in experimental studies
Scientific literature study suggests that cisplatin mediated hearing loss essentially involves a robust generation of reactive oxygen species (ROS) in the cochlea, outer hair cells, spiral ganglia, stria vascularis and the spiral ligament. Despite the presence of various endogenous antioxidant cytoprotective mechanisms like glutathione and other antioxidant enzymes, heat shock proteins, A1 adenosine receptors, NRF2, kidney injury molecule-1 (KIM-1), the damage seems to be inevitable as these mechanisms become overwhelmed (over time and cumulative dosage) and succumb to the lethal/cytotoxic effects of cisplatin (Rybak et al. 2007). Thus, exogenous administrations of antioxidants have been the primary focus for devising the treatment strategy against cisplatin-induced ototoxicity.
Numerous studies have reported on the protective effects of various antioxidants on cisplatin induced hearing loss. Many of the anti oxidants studied have been thiol-compounds, where the high affinity of sulfur for platinum forms the basis for their use against cisplatin toxicity. Included are some of the animal studies that formed the basis of ongoing clinical trials: a) The protective effects of N-acetyl cysteine (NAC) have been demonstrated in the rat (Dickey et al. 2004) and guinea pig (Choe et al. 2004) models of cisplatin-induced hearing loss. In guinea pigs, transtympanic administration of NAC and Ringer’s lactate resulted in the preservation of the distortion product otoacoustic emissions (DPOAE) (Choe et al. 2004), b) Administration of sodium thiosulfate (STS) along with cisplatin protected against cisplatin-induced hearing loss as assessed by auditory brainstem responses (ABR) (Otto et al. 1988). STS forms a complex with cisplatin that leads to its inactivation and excretion by kidneys, thus interfering with the tumoricidal activity of cisplatin, suggesting the use of local application of STS as an alternative and more targeted route of administration (Wimmer et al. 2004). An intracochlear perfusion of STS was shown to inhibit cisplatin-induced activation of cytochrome c, thus preventing the death of outer hair cells in guinea pigs (Wang et al. 2003), c) High doses of amifostine showed protection against cisplatin ototoxicity in hamsters, but had neurotoxicity as its own side-effect (Church et al. 2004), d) D-methionine another sulfur-containing compound has also been studied extensively in the prevention of cisplatin-induced hearing loss. Both the systemic and local administration of D-methionine effectively reduced cisplatin ototoxicity (Campbell et al. 1996; Korver et al. 2002). D-methionine also elevated the levels of antioxidant enzymes while reduced the levels of malondialdehyde (marker of lipid peroxidation) after cisplatin administration (Campbell et al. 2003). e) Other antioxidant compounds like lipoic acid, ebselen, diethyldithiocarbamate and 4-methylthiobenzoic acid also showed protection from cisplatin induced ototoxicity in rats (Rybak et al. 1999).
Endogenously expressed A1 adenosine receptors (A1ARs) have also been shown to provide cytoprotection against oxidative damage in the cochlea in chinchillas. Localized application of A1AR agonist resulted in an increase in antioxidative enzymes glutathione peroxidase and superoxide dismutase (Ford et al. 1997). Furthermore, A1AR agonists also reduced the cisplatin-mediated increase in malondialdehyde levels in the cochlea resulting in protection against cisplatin-induced hair cell damage and hearing loss (Whitworth et al. 2004).
Intra-tympanic administration of dexamethasone reduced cisplatin-induced hearing loss in a guinea pig as well as in a mouse model (Daldal et al. 2007; Hill et al. 2008). Salicylates have also been shown to be effective against cisplatin-induced outer hair cell damage. In a study by Hyppolito et al. (2006), subcutaneous administration of sodium salicylate, 90 min prior to cisplatin infusion, attenuated the loss of outer hair cells seen with cisplatin. An additional advantage of salicylate administration was lack of its effect on the antitumor activity of cisplatin (Li et al. 2002).
Neurotrophins like neurotrophin-3 (NT-3) and brain-derived nerve growth factor (BDNF) have also shown effectiveness against cisplatin ototoxicity. Mouse cochlear explants expressing NT-3 gene delivered by herpes simplex virus (HSV) amplicon vector showed protection against cisplatin-induced damage (Chen et al. 2001). BDNF protected the auditory neurons from cisplatin-mediated damage in organotypic cultures of postnatal cochlear explants without any significant protection against outer hair cells damage (Zheng and Gao 1996). However, when BDNF was administered in combination with D-methionine, it was able to protect both the auditory neurons as well as outer hair cell damage caused by cisplatin (Gabaizadeh et al. 1997). In a recent study, BDNF was also shown to be effective in the treatment of cisplatin-induced hearing loss in guinea pigs (Meen et al. 2009).
In an organ of Corti-derived cell line, HEI-OC1, cisplatin increased cell death via increase in lipid peroxidation and altered mitochondrial permeability transition, which was inhibited by a calcium-channel blocker, flunarizine (So et al. 2005). Furthermore, flunarizine mediated attenuation of cisplatin-induced cell death involved activation of a transcription factor: nuclear factor erythroid 2-related factor (Nrf2) in a PI3K/Akt-dependent pathway (So et al. 2006). Activation of this pathway by flunarizine also resulted in an increased expression of an endogenous protective molecule against oxidative stress, heme oxygenase-1 (HO-1), in both HEI-OC1 cells as well as rat primary organ of Corti explants (So et al. 2006). Additionally, flunarizine also inhibited cisplatin-induced pro-inflammatory cytokine production in an ERK1/2 MAP kinase-NF-κB- dependent pathway (So et al. 2008).
One of the mechanisms in cisplatin-induced outer hair cell damage involves activation of pro-apoptotic pathways. Use of caspase-3 and caspase-9 inhibitors prevented cisplatin-induced outer hair cell death in guinea pigs (Wang et al. 2004). However, in this study intracochlear perfusion was used as the route of administration, which is too highly invasive to be used in the clinical settings. In a different approach, a gene encoding X-linked inhibitor of apoptosis (XIAP) was delivered using adeno-associated viral (AAV) vector through round window two months before administering cisplatin for 72 h. AAV encoding XIAP prevented the cisplatin-induced shifts in ABR thresholds as well as outer hair cell loss (Cooper et al. 2006), which was lost when a mutant form of XIAP, dXIAP-t, was administered (Chan et al. 2007). Pifithrin-alpha, a p53 inhibitor, was also shown to be effective against cisplatin-induced damage to the organotypic organ of Corti cell cultures by reducing the expression of p53 as well as caspases (Zhang et al. 2003).
In a recently published study, our lab demonstrated the role of transient receptor potential vanilloid 1 (TRPV1) channels in cisplatin-mediated ototoxicity (Mukherjea et al. 2008). Using a rat model, we showed that cisplatin caused outer hair cell loss via activation of TRPV1 channels, and by using a short interfering RNA (siRNA) against TRPV1 by round window application we were able to inhibit the cisplatin response. Additionally, cisplatin increased ROS generation in the organ of Corti hair cell cultures (UB/OC-1 cells) via activation of NOX3 isoform of NADPH oxidase enzyme system, and siRNA against NOX3 blocked this high ROS generation. The NOX3-mediated ROS generation also led to an increase in TRPV1 expression as part of the positive feedback mechanism, which was suppressed by siRNA against NOX3 as well as TRPV1. The fact that the administration of naked siRNA was able to abrogate the cisplatin toxicity gives a new direction to the ongoing research looking for the treatment options against cisplatin-induced ototoxicity.
Most of the above mentioned treatment strategies for prevention of cisplatin-induced ototoxicity have been performed in vitro or have been studied in vivo by using invasive approaches for local administration. Their use in cancer patients, where they are unlikely to interfere with the cisplatin’s therapeutic effects has still to be verified.
Conclusions
A variety of otoprotective compounds have been successfully used in experimental animals. The ideal protective agent if given systemically should have three characteristics: 1) it should be non-toxic. Some of the protective agents tested in animals have significant adverse effects in patients (e.g., diethyldithiocarbamate); 2) it should achieve sufficiently high concentrations in the inner ear to protect these tissues from cisplatin injury; 3) it must not interfere with the anti-tumor effect of cisplatin. In order to achieve high concentrations of protective molecules in the inner ear, the transtympanic route of administration may be considered. The potential advantages of this route of administering protective agents are: 1) higher concentrations of protective drug in the inner ear; 2) direct treatment of the target organ for protection while avoiding systemic side effects (Light and Silverstein 2004). The disadvantages would be the need to treat each ear with a moderately invasive procedure and the lack of protection of other organs, such as the kidney. At the present time, there is no ideal protective agent in clinical use. There is a great need to find safe and effective protective agents against cisplatin ototoxicity. This would eliminate one of the dose-limiting side effects of cisplatin therapy and improve the quality of life for many patients (Van den Berg et al. 2006).
Acknowledgment
This research was supported by the National Institutes of Health (NIDCD) grant R01-DC-02396 to LPR and NIDCD F32-DC009950-01A2 to DM.
References
- Allen GC, Tiu C, Koike K, Ritchey AK, Kurs-Lasky M, Wax MK. Transient evoked otoacoustic emissions in children after cisplatin chemotherapy. Otolaryngol. Head Neck Surg. 1998;118:584–588. doi: 10.1177/019459989811800504. [DOI] [PubMed] [Google Scholar]
- Bánfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- Barahmani N, Carpentieri S, Li XN, Wang T, Cao Y, Howe L, Kilburn L, Chintagumpala M, Lau C, Okcu MF. Glutathione S-transferase M1 and T1 polymorphinsms may predict adverse effects after therapy in children with medulloblastoma. Neuro. Oncol. 2009;11:292–300. doi: 10.1215/15228517-2008-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini P, Lassalle M, Mercier G, Raquin MA, Izzi G, Corradini N, Hartmann O. Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J. Pediatr. Hematol. Oncol. 2004;26:649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Berger CC, Hartmann JT, Kollmannsberger C, Schmoll HJ, Kuczyk MA, Kanz L. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br. J. Cancer. 1998;77:1355–1362. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra A, van Oudenaren A, Baert M, van Steeg H, Leenen PJ, van der Horst GT, Hoeijmakers JH, Savelkoul HF, Garssen J. Differential ultraviolet-B-induced immunomodulation in XPA, XPC, and CSB DNA repair-deficient mice. J. Invest. Dermatol. 2001;117:141–146. doi: 10.1046/j.0022-202x.2001.01390.x. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. A necessary role for cell shrinkage in apoptosis. Biochem. Pharmacol. 1998;56:1549–1559. doi: 10.1016/s0006-2952(98)00225-1. [DOI] [PubMed] [Google Scholar]
- Campbell KC, Meech RP, Rybak LP, Hughes LF. The effect of D-methionine on cochlear oxidative state with and without cisplatin administration: mechanisms of otoprotection. J. Am. Acad. Audiol. 2003;14:144–156. [PubMed] [Google Scholar]
- Campbell KC, Rybak LP, Meech RP, Hughes L. D-methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear. Res. 1996;102:90–98. doi: 10.1016/s0378-5955(96)00152-9. [DOI] [PubMed] [Google Scholar]
- Caronia D, Patino-Garcia A, Milne RL, Zalacain-Diez M, Pita G, Alonso MR, Moreno LT, Sierrasesumaga-Ariznabarreta L, Benitez J, Gonzaler-Neira A. Common variations in ERCC2 are associated with response to cisplatin chemotherapy and clinical outcome in osteosarcoma patients. Pharmacogenomics J. 2009;9:347–353. doi: 10.1038/tpj.2009.19. [DOI] [PubMed] [Google Scholar]
- Chan DK, Lieberman DM, Musatov S, Goldfein JA, Selesnick SH, Kaplitt MG. Protection against cisplatin-induced ototoxicity by adeno-associated virus-mediated delivery of the X-linked inhibitor of apoptosis protein is not dependent on caspase inhibition. Otol. Neurotol. 2007;28:417–425. doi: 10.1097/01.mao.0000247826.28893.7a. [DOI] [PubMed] [Google Scholar]
- Chen SH, Liang DC, Lin HC, Cheng SY, Chen LJ, Liu HC. Auditory and visual toxicity during deferoxamine therapy in transfusion-dependent patients. J. Pediatr. Hematol. Oncol. 2005;27:651–653. doi: 10.1097/01.mph.0000194019.95096.b6. [DOI] [PubMed] [Google Scholar]
- Chen WC, Jackson A, Budnick AS, Pfister DG, Kraus DH, Hunt MA, Stambuk H, Levegrun S, Wolden SL. Sensorineural hearing loss in combined modality treatment of asopharyngeal carcinoma. Cancer. 2006;106:820–829. doi: 10.1002/cncr.21683. [DOI] [PubMed] [Google Scholar]
- Chen X, Frisina RD, Bowers WJ, Frisina DR, Federoff HJ. HSV ampliconmediated neurotrophin-3 expression protects murine spiral ganglion neurons from cisplatininduced damage. Mol. Ther. 2001;3:958–963. doi: 10.1006/mthe.2001.0334. [DOI] [PubMed] [Google Scholar]
- Chen Z, Xu XS, Yang J, Wang G. Defining the function of XPC protein in psoralen and cisplatin-mediated DNA repair and mutagenesis. Carcinogenesis. 2003;24:1111–1121. doi: 10.1093/carcin/bgg051. [DOI] [PubMed] [Google Scholar]
- Choe WT, Chinosornvatana N, Chang KW. Prevention of cisplatin ototoxicity using transtympanic N-acetylcysteine and lactate. Otol. Neurotol. 2004;25:910–915. doi: 10.1097/00129492-200411000-00009. [DOI] [PubMed] [Google Scholar]
- Chung WH, Boo SH, Chung MK, Lee HS, Cho YS, Hong SH. Proapoptotic effects of NF-kappaB on cisplatin-induced cell death in auditory cell line. Acta. Otolaryngol. 2008;128:1063–1070. doi: 10.1080/00016480701881811. [DOI] [PubMed] [Google Scholar]
- Church MW, Blakley BW, Burgio DL, Gupta AK. WR- 2721 (amifostine) ameliorates cisplatin-induced hearing loss but causes neurotoxicity in hamsters: dose-dependent effects. J. Assoc. Res. Otolaryngol. 2004;5:227–237. doi: 10.1007/s10162-004-4011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici WJ, DiMartino DL, Prasad MR. Direct effects of reactive oxygen species on cochlear outer hair cell shape in vitro. Hear. Res. 1995;84:30–40. doi: 10.1016/0378-5955(95)00010-2. [DOI] [PubMed] [Google Scholar]
- Clerici WJ, Yang L. Direct effects of intraperilymphatic reactive oxygen species generation on cochlear function. Hear. Res. 1996;101:14–22. doi: 10.1016/s0378-5955(96)00126-8. [DOI] [PubMed] [Google Scholar]
- Cooper LB, Chan DK, Roediger FC, Shaffer BR, Fraser JF, Musatov S, Selesnick SH, Kaplitt MG. AAV-mediated delivery of the caspase inhibitor XIAP protects against cisplatin ototoxicity. Otol. Neurotol. 2006;27:484–490. doi: 10.1097/01.mao.0000202647.19355.6a. [DOI] [PubMed] [Google Scholar]
- Costa RM, Chiganças V, Galhardo Rda S, Carvalho H, Menck CF. The eukaryotic nucleotide excision repair pathway. Biochimie. 2003;85:1083–1099. doi: 10.1016/j.biochi.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Dabholkar M, Christian M, Reed E. Cisplatin. Cancer Chemother. Biol. Response Modif. 1994;15:87–98. [PubMed] [Google Scholar]
- Daldal A, Odabasi O, Serbetcioglu B. The protective effect of intratympanic dexamethasone on cisplatin-induced ototoxicity in guinea pigs. Otolaryngol. Head Neck Surg. 2007;137:747–752. doi: 10.1016/j.otohns.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Dehne N, Lautermann J, Petrat F, Rauen U, de Groot H. Cisplatin ototoxicity: involvement of iron and enhanced formation of superoxide anion radicals. Toxicol. Appl. Pharmacol. 2001;174:27–34. doi: 10.1006/taap.2001.9171. [DOI] [PubMed] [Google Scholar]
- de Laat WL, Jaspers NG, Hoeijmakers JH. Molecular mechanism of nucleotide excision repair. Genes Dev. 1999;13:768–785. doi: 10.1101/gad.13.7.768. [DOI] [PubMed] [Google Scholar]
- DeVries TA, Kalkofen RL, Matassa AA, Reyland ME. Protein kinase C delta regulates apoptosis via activation of STAT1. J. Biol. Chem. 2004;279:45603–45612. doi: 10.1074/jbc.M407448200. [DOI] [PubMed] [Google Scholar]
- Dickey TD, Muldoon LL, Kraemer DF, Neuwelt EA. Protection against cisplatin-induced ototoxicity by N-acetylcysteine in a rat model. Hear. Res. 2004;193:25–30. doi: 10.1016/j.heares.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Ekborn A, Laurell G, Johnstrom P, Wallin I, Eksborg S, Ehrsson H. D-Methionine and cisplatin ototoxicity in the guinea pig: D-methionine influences cisplatin pharmacokinetics. Hear. Res. 2002;165:53–61. doi: 10.1016/s0378-5955(02)00277-0. [DOI] [PubMed] [Google Scholar]
- Ferry KV, Hamilton TC, Johnson SW. Increased nucleotide excision repair in cisplatin-resistant ovarian cancer cells: role of ERCC1-XPF. Biochem. Pharmacol. 2000;60:1305–1313. doi: 10.1016/s0006-2952(00)00441-x. [DOI] [PubMed] [Google Scholar]
- Ford MS, Nie Z, Whitworth C, Rybak LP, Ramkumar V. Up-regulation of adenosine receptors in the chinchilla cochlea by cisplatin. Hear. Res. 1997;111:143–152. doi: 10.1016/s0378-5955(97)00103-2. [DOI] [PubMed] [Google Scholar]
- Fouladi M, Chintagumpala M, Ashley D, Kellie S, Gururangan S, Hassall T, Gronewold L, Stewart CF, Wallace D, Broniscer A, Hale GA, Kasow KA, Merchant TE, Morris B, Krasin M, Kun LE, Boyett JM, Gajjar A. Amifostine protects against cisplatin-induced ototoxicity in children with average-risk medulloblastoma. J. Clin. Oncol. 2008;26:3749–3755. doi: 10.1200/JCO.2007.14.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaizadeh R, Staecker H, Liu W, Kopke R, Malgrange B, Lefebvre PP, Van de Water TR. Protection of both auditory hair cells and auditory neurons from cisplatin induced damage. Acta Otolaryngol. 1997;117:232–238. doi: 10.3109/00016489709117778. [DOI] [PubMed] [Google Scholar]
- Giaccone G. Clinical perspectives on platinum resistance. Drugs. 2000;59:9–17. doi: 10.2165/00003495-200059004-00002. [DOI] [PubMed] [Google Scholar]
- Goel R, Cleary SM, Horton C, Kirmani S, Abramson I, Kelly C, Howell SB. Effect of sodium thiosulfate on the pharmacokinetics and toxicity of cisplatin. J. Natl. Cancer Inst. 1989;81:1552–1560. doi: 10.1093/jnci/81.20.1552. [DOI] [PubMed] [Google Scholar]
- Guthrie OW, Li-Korotky HS, Durrant JD, Balaban C. Cisplatin induces cytoplasmic to nuclear translocation of nucleotide excision repair factors among spiral ganglion neurons. Hear. Res. 2008;239:79–91. doi: 10.1016/j.heares.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Hill GW, Morest DK, Parham K. Cisplatin-induced ototoxicity: effect of intratympanic dexamethasone injections. Otol. Neurotol. 2008;29:1005–1011. doi: 10.1097/MAO.0b013e31818599d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock YJ, Tward JD, Szabo A, Bentz BG, Shrieve DC. Relative contributions of radiation and cisplatin-based chemotherapy to sensorineural hearing loss in head-and-neck cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2009;73:779–788. doi: 10.1016/j.ijrobp.2008.05.040. [DOI] [PubMed] [Google Scholar]
- Howell SB, Taetle R. Effect of sodium thiosulfate on cisdichlorodiammineplatinum (II) toxicity and antitumor activity in L1210 leukemia. Cancer Treat. Rep. 1980;64:611–616. [PubMed] [Google Scholar]
- Huang E, The BS, Strother DR, Davis QG, Chiu JK, Lu HH, Carpenter LS, Mai WY, Chintagumpala MM, South M, Grant WH, 3rd, Butler EB, Woo SY. Intensity-modulated radiation therapy for pediatric medulloblastoma: early report on the reduction of ototoxicity. Int. J. Radiat. Oncol. Biol. Phys. 2002;52:599–605. doi: 10.1016/s0360-3016(01)02641-4. [DOI] [PubMed] [Google Scholar]
- Huang Z, Timerbaev AR, Keppler BK, Hirokawa T. Determination of cisplatin and its hydrolytic metabolite in human serum by capillary electrophoresis techniques. J. Chromatogr. A. 2006;1106:75–79. doi: 10.1016/j.chroma.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Hughes FM, Jr, Bortner CD, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J. Biol. Chem. 1997;272:30567–30576. doi: 10.1074/jbc.272.48.30567. [DOI] [PubMed] [Google Scholar]
- Hyppolito MA, de Oliveira JA, Rossato M. Cisplatin ototoxicity and otoprotection with sodium salicylate. Eur. Arch. Otorhinolaryngol. 2006;263:798–803. doi: 10.1007/s00405-006-0070-6. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Sunose H, Takasaka T. Effects of free radicals on the intracellular calcium concentration in the isolated outer hair cell of the guinea pig cochlea. Acta Otolaryngol. 1993;113:137–141. doi: 10.3109/00016489309135781. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Kawano T, Ishizawa M, Aoki K, Kuroiwa T, Baba T. Inactivation of cis-diamminedichloroplatinum (II) in blood and protection of its toxicity by sodium thiosulfate in rabbits. Cancer Chemother. Pharmacol. 1985;15:228–232. doi: 10.1007/BF00263891. [DOI] [PubMed] [Google Scholar]
- Jones MM, Basinger MA, Beaty JA, Holscher MA. The relative nephrotoxicity of cisplatin, cis-[Pt(NH3)2(guanosine)2]2+, and the hydrolysis product of cisplatin in the rat. Cancer Chemother. Pharmacol. 1991;29:29–32. doi: 10.1007/BF00686332. [DOI] [PubMed] [Google Scholar]
- Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J. Clin. Oncol. 2005;23:8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Sumizawa T, Mutoh M, Chen ZS, Terada K, Furukawa T, Yang XL, Gao H, Miura N, Sugiyama T, Akiyama S. Copper-transporting P-type adenosine triphosphatase (ATP7B) is associated with cisplatin resistance. Cancer Res. 2000;60:1312–1316. [PubMed] [Google Scholar]
- Kopelman J, Budnick AS, Sessions RB, Kramer MB, Wong GY. Ototoxicity of high-dose cisplatin by bolus administration in patients with advanced cancer and normal hearing. Laryngoscope. 1988;98:858–864. doi: 10.1288/00005537-198808000-00014. [DOI] [PubMed] [Google Scholar]
- Kopke RD, Liu W, Gabaizadeh R, Jacono A, Feghali J, Spray D, Garcia P, Steinman H, Malgrange B, Ruben RJ, Rybak L, Van de Water TR. Use of organotypic cultures of Corti’s organ to study the protective effects of antioxidant molecules on cisplatininduced damage of auditory hair cells. Am. J. Otol. 1997;18:559–571. [PubMed] [Google Scholar]
- Korver K, Rybak LP, Whitworth C, Campbell KCM. Round window application of D-methionine provides complete cisplatin otoprotection. Otolaryngol. Head Neck Surg. 2002;126:683–689. doi: 10.1067/mhn.2002.125299. [DOI] [PubMed] [Google Scholar]
- Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev. 2007;26:71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- Lee JE, Nakagawa T, Kim TS, Endo T, Shiga A, Iguchi F, Lee SH, Ito J. Role of reactive radicals in degeneration of the auditory system of mice following cisplatin treatment. Acta Otolaryngol. 2004a;124:1131–1135. doi: 10.1080/00016480410017521. [DOI] [PubMed] [Google Scholar]
- Lee JE, Nakagawa T, Kita T, Kim TS, Endo T, Shiga A, Iguchi F, Lee SH, Ito J. Mechanisms of apoptosis induced by cisplatin in marginal cells in mouse striavascularis. ORL J. Otorhinolaryngol. Relat. Spec. 2004b;66:111–118. doi: 10.1159/000079329. [DOI] [PubMed] [Google Scholar]
- Li G, Sha SH, Zotova E, Arezzo J, Van De Water T, Schacht J. Salicylate protects hearing and kidney function from cisplatin toxicity without compromising its oncolytic action. Lab. Invest. 2002;82:585–596. doi: 10.1038/labinvest.3780453. [DOI] [PubMed] [Google Scholar]
- Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: influence of age and the cumulative dose. Eur. J. Cancer. 2004;40:2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Liang F, Schulte BA, Qu C, Hu W, Shen Z. Inhibition of the calcium- and voltage-dependent big conductance potassium channel ameliorates cisplatin-induced apoptosis in spiral ligament fibrocytes of the cochlea. Neuroscience. 2005;135:263–271. doi: 10.1016/j.neuroscience.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Light JP, Silverstein H. Transtympanic perfusion: indications and limitations. Curr. Opin. Otolaryngol. Head Neck Surg. 2004;12:378–383. doi: 10.1097/01.moo.0000134438.91734.38. [DOI] [PubMed] [Google Scholar]
- Marina N, Chang KW, Malogolowkin M, London WB, Frazier AL, Womer RB, Rescorla F, Billmire DF, Davis MM, Perlman EJ, Giller R, Lauer SJ, Olson TA. Amifostine does not protect against the ototoxicity of high-dose cisplatin combined with etoposide and bleomycin in pediatric germ-cell tumors: a Children’s Oncology Group study. Cancer. 2005;104:841–847. doi: 10.1002/cncr.21218. [DOI] [PubMed] [Google Scholar]
- McKeage MJ. Comparative adverse effect profiles of platinum drugs. Drug Saf. 1995;13:228–244. doi: 10.2165/00002018-199513040-00003. [DOI] [PubMed] [Google Scholar]
- Meen E, Blakley B, Quddusi T. Brain-derived nerve growth factor in the treatment of sensorineural hearing loss. Laryngoscope. 2009;119:1590–1593. doi: 10.1002/lary.20515. [DOI] [PubMed] [Google Scholar]
- Mukherjea D, Jajoo S, Whitworth C, Bunch JR, Turner JG, Rybak LP, Ramkumar V. Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat. J. Neurosci. 2008;28:13056–13065. doi: 10.1523/JNEUROSCI.1307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjea D, Whitworth CA, Nandish S, Dunaway GA, Rybak LP, Ramkumar V. Expression of the kidney injury molecule 1 in the rat cochlea and induction by cisplatin. Neuroscience. 2006;139:733–740. doi: 10.1016/j.neuroscience.2005.12.044. [DOI] [PubMed] [Google Scholar]
- Nagy I, Monge A, Albinger-Hegyi A, Schmid S, Bodmer D. NF-kappaB is required for survival of immature auditory hair cells in vitro. J. Assoc. Res. Otolaryngol. 2005;6:260–268. doi: 10.1007/s10162-005-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg J, Kraggerud SM, Cvancarova M, Lothe RA, Fossa SD. Cisplatin-induced long-term hearing impairment is associated with specific glutathione-s-transferase genotypes in testicular cancer survivors. J. Clin. Oncol. 2007;25:708–714. doi: 10.1200/JCO.2006.08.9599. [DOI] [PubMed] [Google Scholar]
- Otto WC, Brown RD, Gage-White L, Kupetz S, Anniko M, Penny JE, Henley CM. Effects of cisplatin and thiosulfate upon auditory brainstem responses of guinea pigs. Hear. Res. 1988;35:79–85. doi: 10.1016/0378-5955(88)90042-1. [DOI] [PubMed] [Google Scholar]
- Peters U, Preisler-Adams S, Lanvers-Kaminsky C, Jürgens H, Lamprecht-Dinnesen A. Sequence variations of mitochondrial DNA and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Res. 2003;23:1249–1255. [PubMed] [Google Scholar]
- Pigeolet E, Corbisier P, Houbion A, Lambert D, Michiels C, Raes M, Zachary MD, Remacle J. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech. Ageing Dev. 1990;51:283–297. doi: 10.1016/0047-6374(90)90078-t. [DOI] [PubMed] [Google Scholar]
- Reddel RR, Kefford RF, Grant JM, Coates AS, Fox RM, Tattersall MH. Ototoxicity in patients receiving cisplatin: importance of dose and method of drug administration. Cancer Treat. Rep. 1982;66:19–23. [PubMed] [Google Scholar]
- Riedemann L, Lanvers C, Deuster D, Peters U, Boos J, Jurgens H, am Zehnhoff-Dinnesen A. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenomics J. 2008;8:23–28. doi: 10.1038/sj.tpj.6500455. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Husain K, Morris C, Whitworth C, Somani S. Effect of protective agents against cisplatin ototoxicity. Am. J. Otol. 2000;21:513–520. [PubMed] [Google Scholar]
- Rybak LP, Whitworth C, Somani S. Application of antioxidants and other agents to prevent cisplatin ototoxicity. Laryngoscope. 1999;109:1740–1744. doi: 10.1097/00005537-199911000-00003. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear. Res. 2007;226:157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Samimi G, Safaei R, Katano K, Holzer AK, Rochdi M, Tomioka M, Goodman M, Howell SB. Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin. Cancer Res. 2004;10:4661–4669. doi: 10.1158/1078-0432.CCR-04-0137. [DOI] [PubMed] [Google Scholar]
- Sastry J, Kellie SJ. Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr. Hematol. Oncol. 2005;22:441–445. doi: 10.1080/08880010590964381. [DOI] [PubMed] [Google Scholar]
- Schmitt NC, Rubel EW, Nathanson NM. Cisplatin-induced hair cell deathrequires STAT1 and is attenuated by epigallocatechin gallate. J. Neurosci. 2009;29:3843–3851. doi: 10.1523/JNEUROSCI.5842-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvakumaran M, Pisarcik DA, Bao R, Yeung AT, Hamilton TC. Enhanced cisplatin cytotoxicity by disturbing the nucleotide excision repair pathway in ovarian cancer cell lines. Cancer Res. 2003;63:1311–1316. [PubMed] [Google Scholar]
- So H, Kim H, Kim Y, Kim E, Pae HO, Chung HT, Kim HJ, Kwon KB, Lee KM, Lee HY, Moon SK, Park R. Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. J. Assoc. Res. Otolaryngol. 2008;9:290–306. doi: 10.1007/s10162-008-0126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So H, Kim H, Lee JH, Park C, Kim Y, Kim E, Kim JK, Yun KJ, Lee KM, Lee HY, Moon SK, Lim DJ, Park R. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kappaB. J. Assoc. Res. Otolaryngol. 2007;8:338–355. doi: 10.1007/s10162-007-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So HS, Kim HJ, Lee JH, Lee JH, Park SY, Park C, Kim YH, Kim JK, Lee KM, Kim KS, Chung SY, Jang WC, Moon SK, Chung HT, Park RK. Flunarizine induces Nrf2-mediated transcriptional activation of heme oxygenase-1 in protection of auditory cells from cisplatin. Cell Death Differ. 2006;13:1763–1775. doi: 10.1038/sj.cdd.4401863. [DOI] [PubMed] [Google Scholar]
- So HS, Park C, Kim HJ, Lee JH, Park SY, Lee JH, Lee ZW, Kim HM, Kalinec F, Lim D, Park R. Protective effect of T-type calcium channel blocker flunarizine on cisplatin-induced death of auditory cells. Hear. Res. 2005;204:127–139. doi: 10.1016/j.heares.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Somani SM, Husain K, Jagannathan R, Rybak LP. Amelioration of Cisplatin induced oto- and nephrotoxicity by protective agents. Ann. Neurosci. 2001;8:101–113. [Google Scholar]
- Stephanou A, Scarabelli TM, Brar BK, Nakanishi Y, Matsumura M, Knight RA, Latchman DS. Induction of apoptosis and Fas receptor/Fas ligand expression by ischemia/reperfusion in cardiac myocytes requires serine 727 of the STAT-1 transcription factor but not tyrosine 701. J. Biol. Chem. 2001;276:28340–28347. doi: 10.1074/jbc.M101177200. [DOI] [PubMed] [Google Scholar]
- Stephanou A, Scarabelli TM, Townsend PA, Bell R, Yellon D, Knight RA, Latchman DS. The carboxyl-terminal activation domain of the STAT-1 transcription factor enhances ischemia/reperfusion-induced apoptosis in cardiac myocytes. FASEB J. 2002;16:1841–1843. doi: 10.1096/fj.02-0150fje. [DOI] [PubMed] [Google Scholar]
- Tornaletti S, Patrick SM, Turchi JJ, Hanawalt PC. Behavior of T7 RNA polymerase and mammalian RNA polymerase II at site-specific cisplatin adducts in the template DNA. J. Biol. Chem. 2003;278:35791–35797. doi: 10.1074/jbc.M305394200. [DOI] [PubMed] [Google Scholar]
- Townsend PA, Scarabelli TM, Pasini E, Gitti G, Menegazzi M, Suzuki H, Knight RA, Latchman DS, Stephanou A. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J. 2004;18:1621–1623. doi: 10.1096/fj.04-1716fje. [DOI] [PubMed] [Google Scholar]
- Van den Berg JH, Beijnen JH, Balm AJM, Schellens JHM. Future opportunities in preventing cisplatin induced ototoxicity. Cancer Treat. Rev. 2006;32:390–397. doi: 10.1016/j.ctrv.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Videhult P, Laurell G, Wallin I, Ehrsson H. Kinetics of cisplatin and its monohydrated complex with sulfur-containing compounds designed for local otoprotective administration. Exp. Biol. Med. (Maywood) 2006;231:1638–1645. doi: 10.1177/153537020623101009. [DOI] [PubMed] [Google Scholar]
- Wang J, Faulconbridge RVL, Fetoni A, Guitton MJ, Pujol R, Puel JL. Local application of sodium thiosulfate prevents cisplatin-induced hearing loss in the guinea pig. Neuropharmacology. 2003;45:380–393. doi: 10.1016/s0028-3908(03)00194-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Ladrech S, Pujol R, Brabet P, Van De Water TR, Puel JL. Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment prevents cisplatininduced hearing loss. Cancer Res. 2004;64:9217–9224. doi: 10.1158/0008-5472.CAN-04-1581. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Inai S, Jinnouchi K, Bada S, Hess A, Michel O, Yagi T. Nuclear-factor kappa B (NF-kappa B)-inducible nitric oxide synthase (iNOS/NOS II) pathway damages the stria vascularis in cisplatin-treated mice. Anticancer Res. 2002;22:4081–4085. [PubMed] [Google Scholar]
- Watanabe K, Inai S, Jinnouchi K, Baba S, Yagi T. Expression of caspaseactivated deoxyribonuclease (CAD) and caspase 3 (CPP32) in the cochlea of cisplatin (CDDP)-treated guinea pigs. Auris Nasus Larynx. 2003;30:219–225. doi: 10.1016/s0385-8146(03)00049-x. [DOI] [PubMed] [Google Scholar]
- Wimmer C, Mees K, Stumpf P, Welsch U, Reichel O, Suckfull M. Round window application of D-methionine, sodium thiosulfate, brain-derived neurotrophic factor and fibroblast growth factor-2 in cisplatin-induced ototoxicity. Otol. Neurotol. 2004;25:33–40. doi: 10.1097/00129492-200401000-00007. [DOI] [PubMed] [Google Scholar]
- Whitworth CA, Ramkumar V, Jones B, Tsukasaki N, Rybak LP. Protection against cisplatin ototoxicity by adenosine agonists. Biochem. Pharmacol. 2004;67:1801–1807. doi: 10.1016/j.bcp.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu W, Ding D, Salvi R. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin induced apoptosis. Neuroscience. 2003;120:191–205. doi: 10.1016/s0306-4522(03)00286-0. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Differential damage to auditory neurons and hair cells by ototoxins and neuroprotection by specific neurotrophins in rat cochlear organotypic cultures. Eur. J. Neurosci. 1996;8:1897–1905. doi: 10.1111/j.1460-9568.1996.tb01333.x. [DOI] [PubMed] [Google Scholar]
- Zuur CL, Simis YJ, Lansdaal PE, Hart AA, Rasch CR, Schornagel JH, Dreschler WA, Balm AJ. Risk factors of ototoxicity after cisplatin-based chemo-irradiation in patients with locally advanced head-and-neck cancer: a multivariate analysis. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:1320–1325. doi: 10.1016/j.ijrobp.2007.01.042. [DOI] [PubMed] [Google Scholar]
- Zuur CL, Simis YJ, Lansdaal PE, Rasch CR, Tange RA, Balm AJ, Dreschler WA. Audiometric patterns in ototoxicity of intra-arterial Cisplatin chemoradiation in patients with locally advanced head and neck cancer. Audiol. Neurootol. 2006;11:318–330. doi: 10.1159/000095818. [DOI] [PubMed] [Google Scholar]
- Zykova TA, Zhang Y, Zhu F, Bode AM, Dong Z. The signal transduction networks required for phosphorylation of STAT1 at Ser727 in mouse epidermal JB6 cells in the UVB response and inhibitory mechanisms of tea polyphenols. Carcinogenesis. 2005;26:331–342. doi: 10.1093/carcin/bgh334. [DOI] [PubMed] [Google Scholar]