Abstract
Little is known about the relationship between CF transmembrane conductance regulator (CFTR) gene expression and the corresponding transport of Cl. The phenotypic characteristics of polarized ΔF508 homozygote CF bronchial epithelial (CFBE41o−) cells were evaluated following transfection with episomal expression vector containing either full-length (6.2kb) wild type (wt) and (4.7kb) ΔF508CFTR cDNA. Forskolin-stimulated Cl secretion in two clones expressing the full-length wild type CFTR was assessed; clone c7-6.2wt gave 13.4±2.5 µA/cm2 and clone c10-6.2wt showed 41.3±25.3 µA/cm2. Another clone (c4-4.7ΔF) complemented with the ΔF508 CFTR cDNA showed high and stable expression of vector-derived ΔF508 CFTR mRNA and a small cAMP-stimulated Cl currents (4.7±0.7 µA/cm2) indicating ΔF508CFTR trafficking to the plasma membrane at physiological temperatures. Vector-driven CFTR mRNA levels were 5-fold (c7-6.2wt), 14-fold (c10-6.2wt), and 27-fold (c7-4.7ΔF) higher than observed in normal bronchial epithelial cells (16HBE14o−) endogenously expressing wtCFTR. Assessment of CFTR mRNA levels and CFTR function showed that cAMP-stimulated CFTR Cl currents were 33%, 167% and 24%, respectively, of those in 16HBE14o− cells. The data suggest that transgene expression needs to be significantly higher than endogenously expressed CFTR to restore functional wtCFTR Cl transport to levels sufficient to reverse CF pathology.
Keywords: polarized CF bronchial epithelia, episomal expression of full-length CFTR, cell line, transfection, complementation
INTRODUCTION
Cystic fibrosis (CF) is the most common lethal, autosomal recessive disease among Caucasians and affects approximately 250,000 people worldwide. It is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene which functions as a cAMP-activated and phosphorylation-regulated Cl channel as well as a regulator of other membrane channels and/or proteins [1–4]. More than 1500 sequence variants have been detected in the CFTR gene, most of which are associated with disease pathology [5]. The predominant mutation is a trinucleotide deletion that results in the loss of a phenylalanine at amino acid 508 (ΔF508 or delF508) in the CFTR protein. This mutation accounts for approximately 66% of all CF alleles [1,5–7]. Clinically, CF is characterized by progressive deterioration of lung function that is the primary cause of morbidity and mortality [6,8]. In the airways, the CFTR protein is localized to the apical membrane of airway epithelial cells [6,9–11].
Due to the limited availability of native epithelial tissues, immortalized cell lines constitutively synthesizing the CFTR protein have been developed to analyze the biochemical and genetic mechanisms underlying CF [12–18]. A number of immortalized airway epithelial cell lines generated in the past have been critical for enhancing the understanding of the pathways responsible for CF pathology [2,19–30]. Transformed heterologous cells transfected with wt or mutant CFTR cDNA have also been widely used for biochemical studies [31–35]. These cell systems have been the models of choice when significant amounts of protein were required [36]. However, because many heterologous expression models are non-epithelial and/or are non-polarized cells, or do not normally express CFTR, they have a limited applicability for the assessment of vectorial ion transport, secretion, trafficking and other differentiated functions [37,38].
The quality of a complemented cell line for CF research is determined by both the stability and level of CFTR expression as well as its ion transport characteristics. Currently it is still unclear what level of CFTR expression is necessary for normal function of an individual cell. This is clearly a critical issue as it relates to the question of the degree of CFTR function that needs to be recovered to therapeutically reverse CF pathology. Endogenous CFTR mRNA appears to be expressed at very low levels. Apparently, 1 to 2 transcripts/cell [39,40] can result in several hundred CFTR channels/cell, thereby suggesting that low levels of wtCFTR mRNA expression may be sufficient to restore normal function. Both the lifetime of ΔF508-CFTR and its trafficking to the plasma membrane appear to be greatly reduced. However, there is evidence to indicate that, in heterologous cell systems, vector driven overexpression of ΔF508CFTR will cause some ΔF508CFTR trafficking to the plasma membrane and result in residual cAMP-dependent Cl transport [41]. Chemically-induced increases in ΔF508CFTR expression in airway epithelial cells have had equivocal results [41–45]. Even though that there may be limitations to CFTR overexpression such as mistrafficking of CFTR to the basolateral cell membrane [39], there is evidence that primary airway epithelial cells express some functional CFTR in the basolateral membrane [46], and the contribution of an overexpressed, partially functional ΔF508CFTR in the basolateral membrane may be nearer to what occurs in vivo. Furthermore, it would be useful to have an airway epithelial cell system that has endogenous CFTR to provide insight into the therapeutic potential of overexpressing ΔF508CFTR in airway epithelial cells and to quantify the relationship between ΔF508CFTR mRNA expression and CFTR function.
Currently, all wtCFTR-complemented CF cell lines in common use have been complemented with the 4.7 kb wtCFTR open reading frame (ORF) cDNA construct. Early electrophysiological studies in Xenopus oocytes used a 6.2 kb CFTR construct [47]; however, it was not used to generate stable CF cell lines that express wtCFTR. The 3’- and 5‘ untranslated regions (UTRs) of CFTR contain sequences that affect the post-transcriptional regulation and stability of CFTR mRNA and its processing. The 3'UTR appears to contain sequences that are implicated in CFTR mRNA destabilization and are controlled by the p42/p44 and p38 MAP kinase cascades [48]. The 5'UTR was shown to contain elements that modulated the translation efficiency of CFTR ORF [49]. Therefore, this study has also undertaken the task of generating a stable CF airway epithelial cell line complemented with the 6.2 kb wtCFTR cDNA construct. The parental CFBE41o− cell line is polarized and was used to derive recombinant subclones that were transfected with an episomal expression vector containing wt or ΔF508 CFTR [2,50]. Subclones were chosen based on the level of transgene-derived CFTR mRNA expression, i.e., the clones expressing the highest levels of CFTR mRNA. These isogenic lines were characterized in terms of their CFTR expression and Cl ion transport function to ascertain the degree of complementation necessary to recover CFTR-mediated Cl secretion in CF airway epithelial cells.
METHODS
Cell Culture and Cell Transformation
Experiments were performed with CF (CFBE41o−) [51] and normal (16HBE14o−) [20] human bronchial epithelial cell lines. The CFBE41o− cell line was originally derived from a bronchial tissue isolate of a CF patient homozygous for the ΔF508 CFTR mutation and immortalized with the pSVori− plasmid that contained a replication-deficient simian virus 40 (SV40) genome [22,25,52,53]. For the generation of CF cells complemented with wtCFTR and ΔF508CFTR, the parental CFBE41o− cell line was transfected by electroporation (nucleofection; Amaxa Biosystems, Germany) with an Epstein-Barr virus (EBV)-based episomal expression vector, pCEP4β (InVitrogen, Carlsbad, CA) containing either the 6.2 kb full-length wtCFTR cDNA (derived from pBQ6.2, a gift from L-C Tsui and J Rommens) [33] or the 4.7 kb ΔF508CFTR cDNA, respectively. The 4.7 kb ΔF508CFTR cDNA contained a TTT deletion at the ΔF508 locus rather than the naturally occurring CTT [54,55] thereby making it possible to differentiate between the expression of endogenous ΔF508CFTR and the plasmid derived ΔF508CFTR. Transfected CFBE41o− cells were grown in the presence of 200–500 µg/ml hygromycin B to select for clones of cells that contained the transfected plasmid. Resistant clones were isolated, expanded and characterized. PCR, reverse transcriptase PCR (RT-PCR), and quantitative PCR and RT-PCR (Q-PCR and QRT-PCR, respectively) were used to confirm the presence and amount of the CFTR transgene and its expression, respectively. Several stable clones were identified and two clones expressing the 6.2 kb wtCFTR cDNA (CFBE41o− c7-6.2wt and CFBE41o− c10-6.2wt) and one expressing the 4.7 kb ΔF508CFTR cDNA (CFBE41o− c4-4.7ΔF) were characterized further. The clones were selected based on their level of transgene derived CFTR mRNA expression. The 16HBE14o− cell line was used as a reference for the expression of endogenous wtCFTR that results in cAMP-dependent Cl transport observed in the normal airway epithelium. Cells were grown in flasks coated with an extracellular matrix cocktail comprised of human fibronectin (BD Biosciences), Vitrogen (Cohesion, Inc.), and bovine serum albumin (Biosource/Biofluids) [12,56] in MEM cell culture medium supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin sulfate under 5% CO2 at 37°C.
Immunocytochemical staining
Cells were grown on well slides (Lab-Tek) and analyzed by immunofluorescence for the presence of SV40 large tumor antigen (SV40 T-antigen), airway keratin and the presence of tight junctions. Antibodies to the SV40 large T antigen, were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The cells were fixed and stained as described previously with a FITC-labeled secondary antibody [2,19,20,22,25]. Cells were visualized by fluorescence microscopy (Olympus IM-2) at 600× magnification.
RNA extraction and genotyping
RNA was extracted from confluent cells grown on Transwell filter inserts (Costar) or on coated culture dishes using the RNeasy mini kit (Qiagen). The RNA was DNase-treated and analyzed by standard allele-specific RT-PCR. After reverse transcription, the cDNA was amplified using primers CF17 (exon 9) and CF7C or CF8C (exon 10; wt and ΔF508 mutation, respectively, Table 1) [57]. Allele-specific PCR amplification was carried out in 30 µl PCR buffer containing 1.5 mM MgCl2, 2.5 mM dNTPs, 0.031 U/µl Platinum Taq polymerase (InVitrogen), and 0.8 µM primer. The conditions for the allele-specific amplification were: 94°C for 2 min; denaturation, 94°C for 90 s; annealing, 59°C for 60 s; extension, 72°C for 30 s for 35 cycles with an 8 min extension on the final cycle. The PCR products were analyzed by 2% (w/v) agarose gel electrophoresis.
Table 1. PCR Primers.
Legend: Primers used in this study are shown with their orientation, sequence, and localization. There were 3 different allele-specific reverse primers were used to detect the mRNA specific for wt or ΔF508CFTR. The CF8C primer is specific to the endogenous CTT deletion of Δ508 CFTR, while the CF81C2 primer is specific to the TTT deletion of the recombinant ΔF508CFTR construct. r = reverse; f = forward.
| Primer | Orientation | Sequence (5'>3') | CFTR gene localization |
|---|---|---|---|
| CF7C | r | ATAGGAAACACCAAAGATGA | exon 10 |
| CF8C | r | ATAGGAAACACCAATGATAT | exon 10 |
| CF81C2 | r | ATTCATCA TAGGAAACACCGATA | exon 10 |
| CF17 | f | GAGGGATTTGGGGAATTATTTG | exon 9 |
| HQCF3 | f | GACAGTTGTTGGCGGTTGCT | exon 9 |
| HQCF4 | r | ACCCTCTGAAGGCTCCAGTTC | exon 10 |
| HGAPDH-R | r | GAAGATGGTGATGGGATTTC | |
| HGAPDH-F | f | GAAGGTGAAGGTAGGAGTC |
Real-time PCR quantification of RNA and DNA
Quantitative analysis of the DNA and RNA was performed in 25 µl with 1 µM each of primers hQCF3 and hQCF4 (Table 1), SYBR Green mix (Applied Biosystems, Foster City, CA) in a 7500 real-time PCR system using the hQCF3/hQCF4 primer pair. The ΔΔCT method was used to calculate the amount of gene expression [58]. CFTR mRNA expression was normalized to GADPH in the complemented CF cell lines and was relative to the expression of wt CFTR (normalized to GAPDH) in 16HBE14o− cells. The amount of vector per cell was quantified by real-time PCR on DNA using allele-specific primer pairs CF17/CF7C (for wtCFTR) and CF17/ CF81C2 (for vector specific ΔF508CFTR) (Table 1). The absolute amount of vector was determined using a standard curve with a known amount of vector (amount of vector/CT). Conditions of the amplification were identical to those used for the quantification of mRNA.
Measurement of transepithelial resistance (RT) and ion transport in Ussing chambers
Transepithelial short circuit current (Isc) and RT measurements were carried out by seeding the cells onto coated Snapwell (Corning Life Sciences, Acton, MD) cell culture inserts at a density of 5 ×105 cells/cm2 that were used 2 to 4 days after seeding. RT was monitored with an epithelial volt/ohm meter (World Precision Instruments, Saratoga, FL). Monolayers that exhibited a transepithelial resistance of >300 Ω·cm2 were used in Ussing chambers designed for use with the Snapwell inserts (World Precision Instruments). The serosal side of the monolayer was bathed in Krebs-Henseleit solution containing (in mM): 120 NaCl, 20 NaHCO3, 5 KHCO3, 1.2 NaH2PO4, 5.6 glucose, 2.5 CaCl2, 1.2 MgCl2. The mucosal side of the monolayer was bathed in Krebs-Henseleit solution in which all Cl salts were replaced by gluconate to increase the driving force for Cl exit across the apical membrane. Both sides were gassed with 95% air and 5% CO2 at 37°C. Transepithelial voltage was clamped to 0.0 mV using a standard four-electrode voltage clamp (Physiologic Instruments, San Diego, CA) and Isc was recorded on a computer as described previously [59]. Transepithelial voltage was clamped to 2 mV for 1 s in 50 second intervals to monitor RT. CFTR-mediated Cl transport was determined by adding forskolin (20 µM) to activate and GlyH101 or glibenclamide (20 µM) to inhibit CFTR [60].
Chemical Compounds
The adenylate cyclase activator forskolin (Calbiochem, La Jolla, CA) was prepared in DMSO (dimethyl sulfoxide) as a 20 mM stock and was added to the serosal side at a final concentration of 20 µM; GlyH101 (kindly provided by Dr. Alan Verkman and glibenclamide (Sigma, St Louis, MO) were used to block transepithelial Cl currents [60,61]. Glibenclamide was prepared as a 300 mM stock in DMSO and added to the mucosal solution at a final concentration of 500 µM. GlyH101 was prepared as a 20 mM stock in DMSO and added to the mucosal solution at a final concentration of 20 µM.
Statistical analysis
Data are presented as original values or as the mean ± SE (SEM); n refers to the number of cultures investigated. The effects of the treatment were tested using one-sample t tests. Comparisons between cell lines were carried out sequentially using the ANOVA and Bonferroni-corrected t tests. Statistical testing used StatView (version 4.57, Abacus Concepts, Berkeley, CA) or SigmaStat (version 3.5, Systat, Inc, Richmond, CA). The resulting p values are given with p < 0.05 considered significant.
Linear regression was performed from two average data sets with multiple independent measurements. Average vector copy number was determined from 2 measurements over 5 passages. The average CFTR mRNA expression was determined from 8 measurements over 8 passages.
RESULTS
The goal of this study was to develop and characterize isogenic CF airway epithelial cells lines that stably express wtCFTR or ΔF508CFTR cDNA and maintain differentiated features characteristic of the airway epithelium. Immortalized CF airway epithelial cells (CFBE41o−) were transfected with episomal expression vectors containing wtCFTR or ΔF508CFTR cDNA and a hygromycin B resistance (HygBR) gene. CFBE41o− cells transfected with a vector containing the full-length 6.2 kb wtCFTR resulted in numerous HygBR clones, two of which were selected for further characterization. The two clones expressing wtCFTR were designated as c7-6.2wt and c10-6.2wt. Since the parental CFBE41o− expresses low levels of endogenous ΔF508CFTR mRNA [51,62], a CF airway epithelial cell line with high ΔF508CFTR expression was generated following transfection with a plasmid containing 4.7 kb ΔF508CFTR cDNA. One stable subclone (c4-4.7ΔF) was selected for further characterization.
Characterization of epithelial phenotype by immunostaining
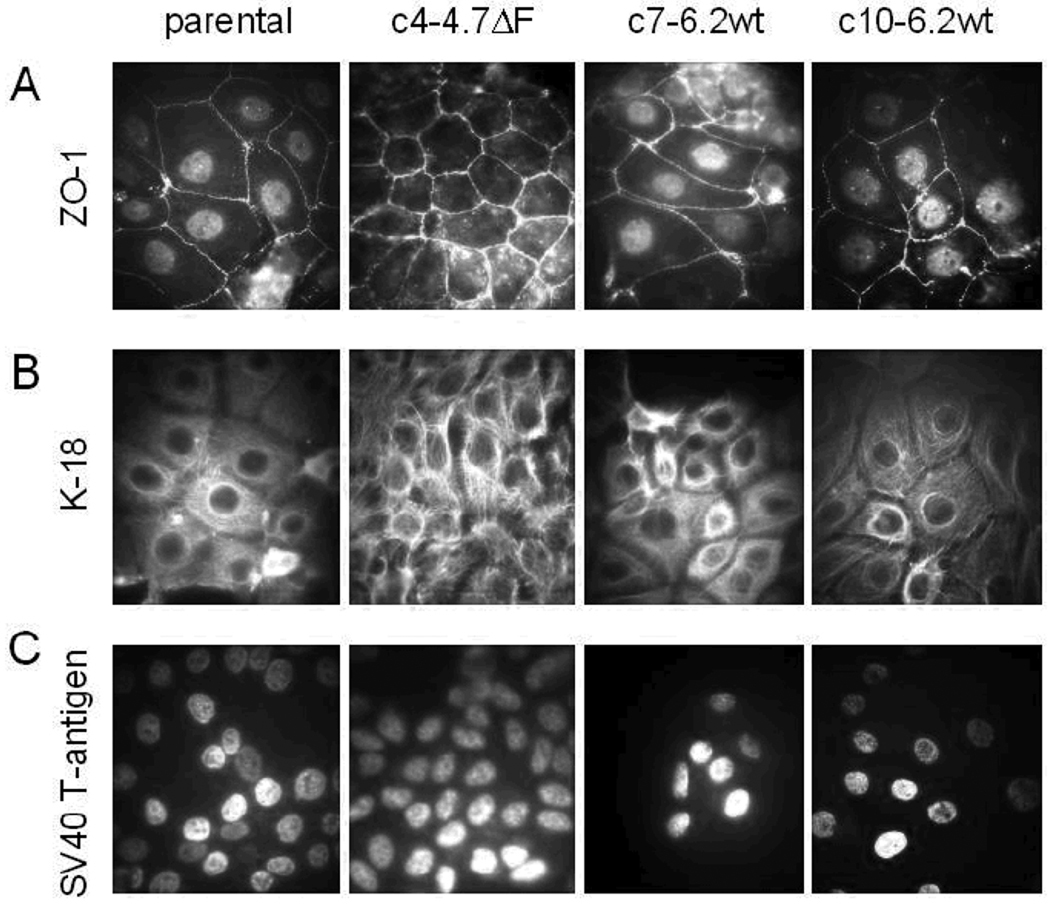
All cell lines (parental and CFTR transfected) maintained epithelial morphology and a characteristic "cobblestone" appearance. The retention of epithelial characteristics was further confirmed by immunocytochemical staining with antibodies against the epithelial cell-specific markers, ZO-1 and K-18. ZO-1 staining showed well-defined signals at the cell periphery in all clones (Figure 1A). The presence and localization of the ZO-1 is indicative of an intact junctional complex that is characteristic of the cell-cell contacts associated with tight junctions in epithelial cells. Cytokeratin staining shows well-organized cytokeratin filaments (Figure 1B) in all cell clones after staining with the airway epithelial cytokeratin, K-18 antibody. In addition, nuclei of all CFBE41o− cell clones stained positive with an antibody for the SV40 large T antigen (Figure 1C) as would be expected for cells transformed by the pSVori plasmid [13,22].
Figure 1. Immunohistochemical analysis of parental and complemented CFBE41o− cells.
Parental CFBE41o− cells and three clonal isolates expressing either the ΔF508CFTR construct (clone c4-4.7ΔF) or the 6.2kb wtCFTR construct (clones c7-6.2wt and c10-6.2wt). Cells were stained with FITC-tagged primary antibodies against ZO-1, K-18, and the SV40 large T antigen. A. Localization of ZO-1 to the plasma membrane at points of cell-cell contacts is consistent with the formation of tight junctions and maintenance of cell polarity. B. Staining for K-18 indicates a well-organized keratin filament structure in all cell lines. C. All cell clones stained positive for the SV40 large T antigen; original magnification 600×.
Expression of cDNA-derived CFTR
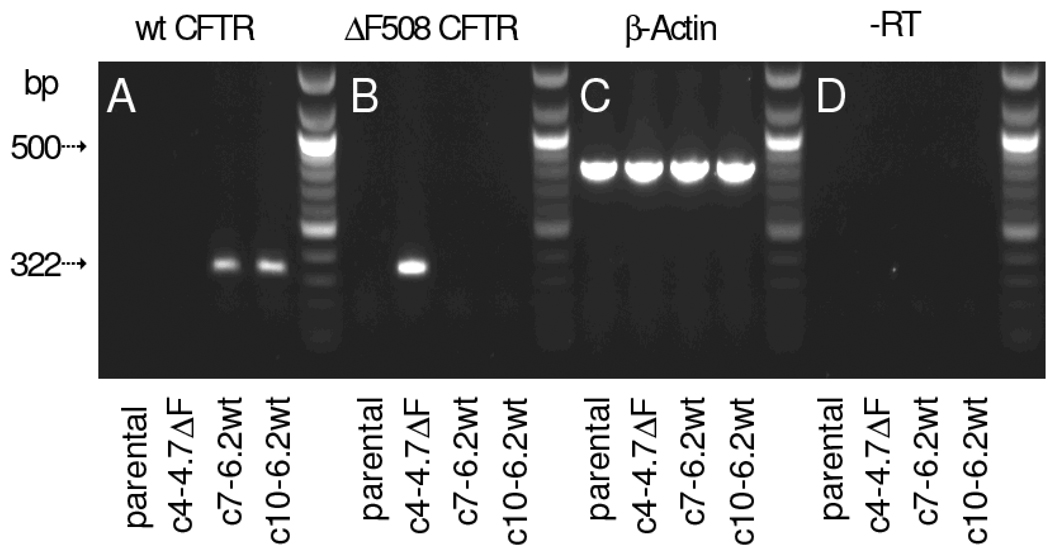
Expression of CFTR mRNA in the parental, uncomplemented and the complemented CFBE41o− cell lines was analyzed by allele-specific RT-PCR. In the amplification of the mRNA-derived CFTR cDNA, a common primer in exon 9 (CF17) was paired with allele-specific exon 10 primers to detect recombinant ΔF508CFTR (primer CF81C2) or wtCFTR (primer CF7C) (Table 1). Clones expressing wtCFTR (c7-6.2wt and c10-6.2wt) yielded a 340-bp amplicon, while no product was found in the parental or ΔF508CFTR transfected cell lines (Fig. 2A). The primer CF81C2 differentiates between the vector-derived ΔF508CFTR with its TTT deletion and the endogenous ΔF508CFTR with a CTT deletion. A 334-bp product was only detected in clone c4-4.7ΔF (Fig. 2B). Expression of β-actin (Fig. 2C) and sample processing in absence of reverse transcriptase (Fig. 2D) are shown as positive and negative controls, respectively.
Figure 2. RT-PCR analysis of recombinant CFTR expression in the complemented CFBE41o− clones.
A. Expression of wtCFTR was prominent in the two stable cell clones c7-6.2wt and c10-6.2wt. B. Using allele-specific primer to detect the expression of the recombinant ΔF508 construct showed prominent expression in clone c4-4.7ΔF, but not in the other clones. C&D. Positive and negative controls are the expression of β-actin and processing the sample without reverse transcriptase (−RT), respectively. The primer pair for wtCFTR amplification was CF7C/CF17; expression of recombinant ΔF508CFTR was detected by primer pair CF81C2/CF17 specific for the TTT deletion in the construct (see Table 1 for sequences).
Vector copy number, CFTR expression, and Cl channel function in subclones c7-6.2wt and c10-6.2wt
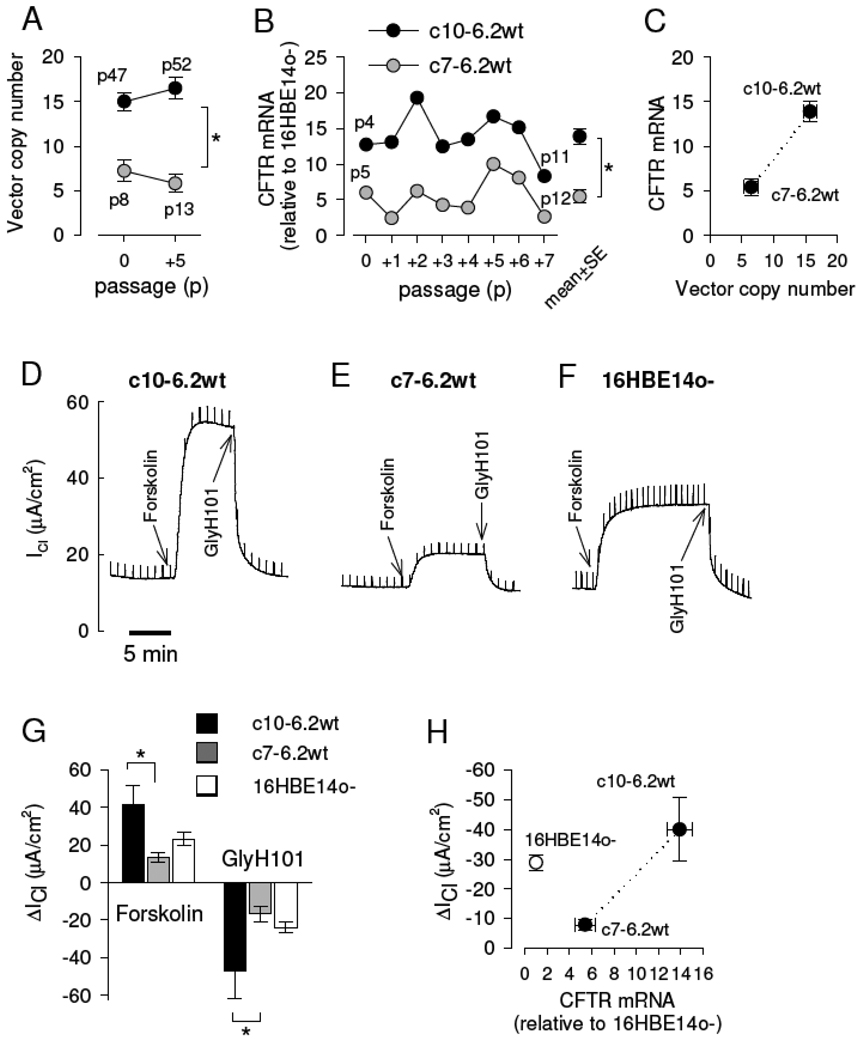
Clones c7-6.2wt and c10-6.2wt were assayed by PCR for the stability of recombinant CFTR expression and in Ussing chambers for CFTR functional activity. Quantitative PCR was used to determine the plasmid copy number relative to a known standard and to monitor for effects of subculturing on the expression of vector. Measurement of the number of vector copies in wtCFTR transfected CFBE41o− cells was determined in both clonal isolates (Fig. 3A). The vector copy number in either cell clone did not change significantly over 5 passages. However, c10-6.2wt had 2.4-times more copies (15.8±0.8 vectors per cell) when compared to c7-6.2wt (6.5±0.7 vectors per cell, p<0.001).
Figure 3. Quantitative assessment of CFTR mRNA expression and cAMP-dependent Cl transport in wtCFTR complemented CFBE41o− clones.
A. The mean number of plasmids per cell (n=3 per bar). Copy number was determined from cells that were 5 passages apart, passage numbers are given next to each symbol. The absolute number of passages (P) as denoted by “(passages after primary isolation).(passages after immortalization).(passages after CFTR transfection)” were p4.77.47 to p4.77.52 for c10-6.2wt, and p4.77.8 to p4.77.13 for c7-6.2wt. Subculturing did not affect the number of vectors per cell; c10-6.2wt expressed significantly higher levels of vector per cell than c7-6.2wt (ANOVA, p=0.005). B. CFTR expression by real-time PCR relative to that measured in the 16HBE14o− cells, relative passage numbers are as indicated. There was no change of expression with passage number (as determined by QRT-PCR analysis of expression level over passage number; c10-6.2wt, p=0.55; c7-6.2wt p=0.68); c10-6.2wt expressed significantly higher levels of mRNA compared to c7-6.2wt (p<0.001, paired t tests). The number of subcultures (P) was p4.77.4 to p4.77.11 for c10-6.2wt and p4.77.5 to p4.77.12 for c7-6.2wt. C. Vector number and CFTR mRNA levels correlated closely (0.9±0.1 mRNA increase per vector). D–F. Transepithelial recordings in the presence of a serosal-to-mucosal Cl gradient. Chloride currents (ICl) were activated by forskolin (20 µM) and inhibited by GlyH101 (20 µM) in c10-6.2wt (D), c7-6.2wt (E), and, for comparison, in 16HBE14o− (F). G. A summary of forskolin-stimulated and GlyH101-blocked chloride currents (ΔICl), n=4–10 experiments per bar; * denotes significant difference (ANOVA). H. The relationship between CFTR expression and function. The correlation between the GlyH101-blocked current and the relative CFTR mRNA expression resulted in a slope of 3.8±0.7 µA/cm2 per unit increase in CFTR mRNA levels, where one unit corresponds to the level of CFTR mRNA in 16HBE14o− cells.
CFTR mRNA expression levels in the different clones of the CFBE41o− cell line were determined by real-time PCR (using the hQCF3/hQCF4 primer pair, Table 1) and were normalized to the relative amount of wtCFTR mRNA that was expressed in the 16HBE14o− cell line (Fig. 3B). CFTR mRNA levels were monitored over 8 consecutive passages, i.e., over a period of approximately 4 weeks in culture. Although the levels of CFTR mRNA varied somewhat over time, there was no apparent trend or loss of expression (as determined by QRT-PCR analysis of CFTR mRNA levels as a function of passage number). Vector-driven wtCFTR mRNA levels were substantially higher in the complemented CFBE41o− clones compared to native CFTR mRNA in the 16HBE14o− cells, i.e., the CFTR mRNA in c7-6.2wt was 5.4±0.9-fold higher and that in c10-6.2wt was 14±1.2-fold higher than that observed in the 16HBE14o− cells (Fig. 3B, p<0.001, one-sample t tests). Average CFTR mRNA levels over the 8 passages in c10-6.2wt were 2.6-fold higher than those in c7-6.2wt (p<0.001). Fig. 3C shows a direct relationship between the average vector copy number per cell (over 5 passages) and the relative average (over 8 passages) CFTR mRNA levels in the wtCFTR-transfected CFBE41o− clones. Linear regression (dashed line, Fig. 3C) resulted in a slope of 0.9±0.1 fold CFTR mRNA increase per vector per cell based on comparing the averages of these two independent pools of measurements.
In parallel experiments, transepithelial Cl secretion was measured in both clonal isolates of the wtCFTR complemented CFBE41o− cell grown as monolayers with Ussing chambers. The parental CFBE41o− and the 16HBE14o− cell lines were used as negative and positive controls, respectively. Both c7-6.2wt and c10-6.2wt expressed moderately tight transepithelial resistances similar to that of the parental CFBE41o− (Table 2). The parental CFBE41o− did not respond to forskolin or GlyH101 (Table 2), while the wtCFTR-complemented clones showed a significant increase in cAMP-dependent Cl current after forskolin stimulation and a GlyH101-specific block of these currents (Figure 3D–3F). The CFTR-mediated chloride currents in c10-6.2wt were 3.1-fold higher than those observed in c7-6.2wt (p=0.005, Table 2) and 1.9-fold higher than in the 16HBE14o− cells (Figure 3H).
Table 2. Transepithelial electrical parameters of the CFBE41o− cell lines.
Legend: All measurements are in the presence of a serosal-to-mucosal Cl gradients. Chloride currents (ICl in µA/cm2) and transepithelial resistances (Rt in Ω·cm2) before (unstimulated), after addition forskolin, and after the addition of the CFTR inhibitor Gly101. ΔICl = ICl (after) − ICl (before) treatment.
| Unstimulated | Forskolin | GlyH101 | Forskolin | GlyH101 | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | Isc | Rt | Isc | Rt | Isc | Rt | ΔIsc | ΔIsc | |
| Parental | 7 | 25.3±7.1 | 310±53 | 24.1±6.4 | 302±68 | 25.9±6.6 | 355±83 | 0.7±0.3 | −1.1±0.9 |
| c7-6.2wt | 10 | 16.1±3.2 | 319±40 | 29.6±4.4 | 259±37 | 20.2±3.8 | 349±54 | 13.4±2.4# | −15.3±2.5# |
| c10-6.2wt | 6 | 20.3±5.3 | 313±34 | 64.1±17.1 | 243±48 | 16.7±1.6 | 332±34 | 41.3±10*# | −46.8±11*# |
| c4-4.7ΔF | 7 | 11.2±2.0 | 339±58 | 15.9±2.2 | 334±57 | 13.2±2.2 | 300±27 | 4.7±0.7# | −4.6±1.3# |
| ANOVA p | 0.20 | 0.98 | 0.002 | 0.60 | 0.29 | 0.91 | <0.001 | <0.001 | |
measurements significantly different from the parental line by ANOVA;
significant effect of treatment (one-sample t test).
Values are the mean ± SE, n= number of experiments.
The levels of CFTR mRNA and the CFTR-associated chloride currents observed in clones c7-6.2wt and c10-6.2wt were used to help define the relationship between the expression of recombinant CFTR and the cAMP-dependent CFTR Cl transport in these CF bronchial epithelial cells. GlyH101 blocked currents were used to indicate that the transepithelial chloride current was carried by CFTR [60]. The levels of wtCFTR mRNA in the wtCFBE41o− clones were normalized to the CFTR mRNA levels in 16HBE14o− cells and the corresponding magnitudes of the Cl currents blocked by GlyH-101 are plotted in Fig 3H. Accordingly, there was a positive relationship between the level of CFTR mRNA levels and the magnitude of the functional CFTR-mediated Cl currents. Linear regression (dashed line, Fig. 3H) resulted in a slope of 3.8±0.7 µA/cm2 per relative unit increase in CFTR mRNA expression (based on the average CFTR mRNA levels over 8 passages).
CFTR mRNA levels in the wtCFTR-CFBE41o− clones were significantly higher than endogenous CFTR mRNA levels in the 16HBE14o− cells. The GlyH101-blockable chloride currents were smaller despite a 5-times higher and 14-times higher CFTR mRNA expression in clones c7-6.2wt and c10-6.2wt, respectively, compared to 16HBE14o−. This suggests that expression from the CFTR transgene is not as effective at generating cAMP-dependent Cl current as the endogenously expressed CFTR. From the data plotted in Fig. 3H, it can be estimated that the expression of CFTR in CF bronchial epithelial cells requires approximately 10-fold higher levels of CFTR mRNA than that found in the endogenously expressed CFTR in 16HBE14o− cells to generate functional CFTR Cl currents (ICl) of similar magnitude.
ΔF508 CFTR mRNA expression and ISC
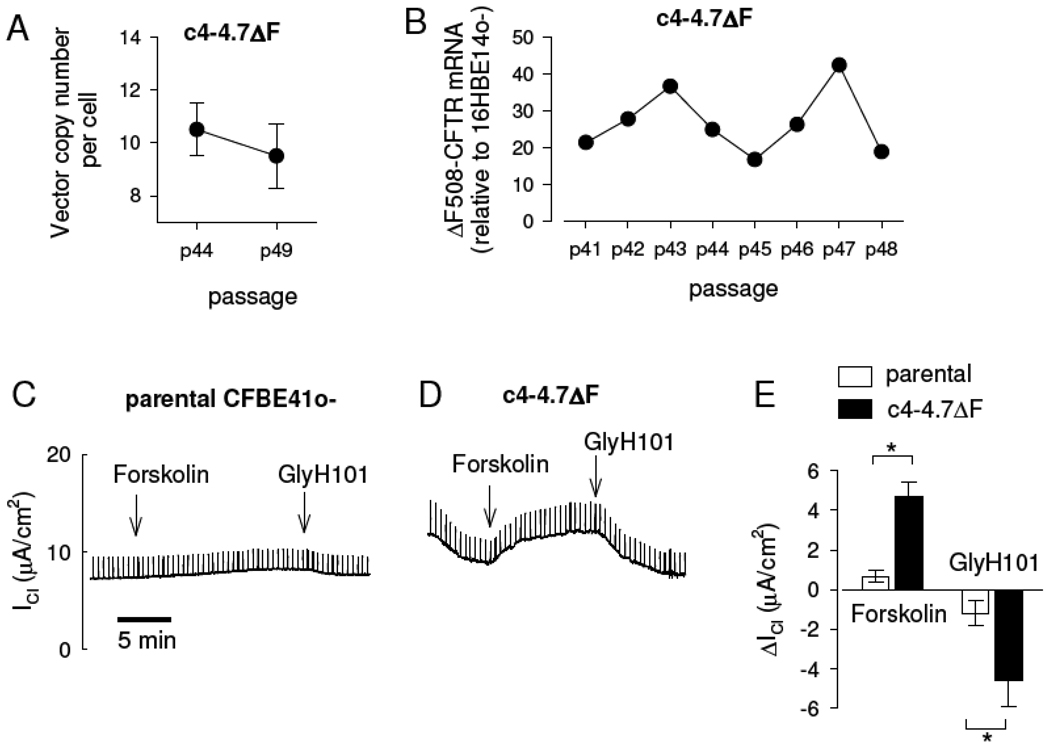
Using a similar PCR strategy as above, the maintenance of the episomal plasmid and its expression over multiple subcultures was determined for the ΔF508CFTR complemented cell line, c4-4.7ΔF (Figure 4A). The number of vectors in these cells over 5 passages (using the hQCF3/hQCF4 primer pair) was relatively high (on average, 10±1.2 vectors per cell) and did not significantly change over five passages. The relative ΔF508CFTR mRNA levels (determined relative to 16HBE14o− CFTR mRNA being 1.0) for 8 consecutive passages (using the CF17/CF81C2 primer pair) were, on average, 27±3.1-fold higher than the levels found in 16HBE14o− (Figure 4B). Despite some fluctuations over time in culture, CFTR mRNA levels over the 8 subcultures remained high.
Figure 4. Expression of ΔF508CFTR mRNA and cAMP-dependent Cl transport in ΔF508CFTR complemented CFBE41o− cells.
A. The mean number of ΔF508CFTR plasmids per cell in CFBE41o− c4-4.7ΔF. Copy number was determined over 5 consecutive passages. The absolute passage numbers were p4.72.44 to p4.72.49. Subculturing did not affect the number of vectors per cell (p=0.56). B. The expression of ΔF508CFTR mRNA in clone c4-4.7ΔF relative to the level of wtCFTR expression in 16HBE14o− cells over 8 consecutive passages. The absolute cell culture passage numbers for c4-4.7ΔF were p4.72.41 to p4.72.48. There was no significant trend between CFTR mRNA expression and passage number (p=0.9). C. No significant forskolin-stimulated or GlyH101-sensitive chloride currents (ICl) were detected in the parental CFBE41o−. D. The c4-4.7ΔF clone consistently expressed small CFTR-mediated currents. E. A A summary of forskolin-stimulated and GlyH101-blocked Cl currents (ΔICl) in parental CFBE41o− (open bars) and c4-4.7ΔF (filled bars). Small but significant CFTR-mediated currents were induced by expression of recombinant ΔF508CFTR, p<0.001 for forskolin-activated currents, p=0.043 for GlyH101-blocked currents, n=7. The responses to these compounds in the parental CFBE41o− were not significantly different from 0 (n=7, one-sample t tests).
Measurement of transepithelial Cl current in Ussing chambers for both the parental CFBE41o− and c4-4.7ΔF showed similar, moderately “tight” transepithelial resistance (Table 2). As above, the parental CFBE41o− showed no significant forskolin-stimulated or GlyH101-blockable Cl currents (Figure 4C, Table 2). On the other hand, the overexpression of ΔF508CFTR mRNA in clone c4-4.7ΔF reproducibly showed a small, but measurable forskolin-stimulated and GlyH101-blocked Cl current typical of CFTR (Figure 4D). The detection of the forskolin-activated and GlyH101-blocked Cl currents in c4-4.7ΔF (Figure 4E, Table 2) suggested that overexpression of ΔF508CFTR could result in functional cAMP-dependent Cl transport in the CFBE41o− cell line.
DISCUSSION
The major objective in generating a cell culture system for CF research is to provide in vitro models that resemble as closely as possible the properties of the native tissue from which they were derived. A number of immortalized airway epithelial cell lines generated in the past have been critical for enhancing understanding of the pathways responsible for CF pathology (reviewed in [14]). Currently, all available cell models lack one or more of the following characteristics critical for a CF-relevant airway epithelial cell model: 1) Epithelial polarization and tight junction formation, 2) isogenic cell lines expressing wt and ΔF508CFTR, 3) high levels of ΔF508CFTR expression in CF cell lines, and 4) stable expression of CFTR constructs. Thus, a prudent approach is to select a clonal cell line from the pool of available lines and select and optimize according to these criteria. Currently, a cell line that meets the above criteria is not available. The CFBE41o− cell line and the complemented CFBE41o− subclones introduced in this study do meet the above criteria. However, one notable limitation is the lack of an airway-typical ENaC-mediated Na absorption in both non-complemented and complemented CFBE41o− cells (data not shown). This characteristic is difficult to maintain under simple culture conditions and is generally lost in most human cell culture systems, whether primary or transformed.
Stable CF airway epithelial cell lines have been critical for both academic and commercial CF research. Basic mechanistic studies as well as screening drugs for their therapeutic potential have benefited from the availability of these human cell lines. Although a number of matched CF and nonCF cell lines have been developed over the years, CFTR expression is often variable, airway epithelial-specific phenotypic characteristics are lacking, or they have been derived from different individuals and thereby have different genetic backgrounds. Correction of the ΔF508CFTR trafficking defect in human airway epithelial cell lines turned out to be difficult. As a result many drug studies testing small molecules that correct this defect have used heterologous and/or non-epithelial cell systems, such as Fisher rat thyroid cells [63], MDCK canine kidney epithelial cells [64,65], LLC-PK1 porcine kidney epithelial cells [66], HEK293 human embryonic kidney cells [67], HT500 kidney cells [68], [69,70], CHO Chinese hamster ovary cells [71], C127i murine mammary carcinoma cells [72], and 3T3 fibroblasts [67,73]. To overcome potential limitations of these heterologous cell systems that can lead to a misinterpretation of results, this study strived to generate stable and effectively isogenic CF airway cell lines that have electrophysiological characteristics that reflect both the wt and ΔF508CFTR and account for the affect of overexpressing CFTR.
Both the stability and the level of CFTR expression determine the value of a complemented cell line for CF research. Currently it is not clear what level of CFTR expression is required for normal function. This also relates to the question of how much CFTR function needs to be recovered for CF treatment to normalize defective Cl secretion. This study quantifies the relative CFTR mRNA levels and the resulting CFTR-mediated currents and indicates that there are 3.8 µA/cm2 of CFTR current per unit increase in CFTR mRNA levels (Fig.3H), where one unit is defined as the amount of endogenous wtCFTR mRNA in 16HBE14o− cells. It is estimated that there are ~43 active apical wtCFTR channels per cell per fold increase in the amount of CFTR mRNA generated by the 6.2 kb wtCFTR constructs in the CFBE41o− clones assuming ~106 cells per cm2, an apical driving force for Cl of −22 mV [74], and a single channel conductance of CFTR of 8 pS with an open probability of 0.5 [75]. By comparison, the efficiency of generating a functional CFTR must be considerably higher in 16HBE14o− cells given that CFTR mRNA levels in these cells were significantly lower than those detected in the complemented cell clones. Chloride currents were about 1/3 at 5-fold higher mRNA expression levels (c7-6.2wt) and 1.6 times more at 14-fold higher mRNA expression levels (c10-6.2wt) (Fig. 3H). Using a similar calculation as above, there are ~330 active CFTR channels per cell in 16HBE14o−, i.e., the natively expressed mRNA in 16HBE14o− was more efficient for the overall chloride secretory response and might be due to a substantially more efficient expression and/or processing of CFTR protein.
Since both the life-time of ΔF508CFTR is reduced and normal trafficking to the membrane of ΔF508CFTR is largely inhibited compared to wtCFTR, increasing the levels of ΔF508CFTR expression appears as a prudent strategy for testing whether overexpressed ΔF508CFTR has a functional role in CF airway epithelial cells. Although the parental CFBE41o− is homozygous for ΔF508CFTR, native expression levels are low [51,62] and no significant CFTR-mediated currents can be detected. Clone c4-4.7ΔF showed a small, but consistent, forskolin-stimulated and GlyH101-blocked current at high levels of recombinant ΔF508CFTR mRNA suggesting that there is some CFTR-dependent function in these cells. Using the same values for driving forces and channel conductance as above, but with an open probability of 0.1 for ΔF508 CFTR [72], there would be ~9 active apical ΔF508CFTR channels per cell per unit increase in the amount of ΔF508CFTR mRNA. This estimation suggests that enhanced expression of ΔF508CFTR increases the presence of ΔF508CFTR in the apical membrane of CF bronchial epithelial cells. However, the number of active channels associated with the ΔF508CFTR is lower than the number of active channels in the clonal isolates expressing recombinant wtCFTR. Since a 4.7kb ΔF508CFTR cDNA construct was used for the expression of recombinant ΔF508CFTR mRNA and a full-length 6.2kB wtCFTR cDNA construct was used for the wtCFTR complemented cell clones, it is possible that the 4.7kB construct was not ideal for optimal expression of functional ΔF508CFTR.
The correlation between CFTR mRNA levels and Cl transport represents an important consideration for designing CF therapies that rely on modulating the levels of CFTR mRNA whether through genetic means or through pharmacological enhancement. While others have carried out studies in heterologous systems, the paucity of data in cells that are polarized and normally express CFTR is noteworthy. The studies described here suggest a direct relationship between the amount of CFTR mRNA and the number of active CFTR channels in the apical membrane of polarized airway epithelial cells. The efficacy of mRNA generated from recombinant transgene appears to be significantly diminished when compared to CFTR mRNA expressed from the endogenous gene in terms of the ability to generate CFTR-associated cAMP-dependent Cl conductances. Furthermore, while the increased expression and ΔF508CFTR-associated function in this episomal vector complementation system indicates that the c4-4.7ΔF clone has a potentially useful role in the development of pharmacological agents that augment ΔF508CFTR expression, additional studies will be needed to evaluate the potential advantages of using a full-length 6.2kB vs a 4.7kB ΔF508CFTR construct to optimize the efficacy of ΔF508CFTR in CF bronchial epithelial cells.
ACKNOWLEDGEMENTS
We would like to thank Dr Kaarin Goncz for construction of the episomal CFTR cDNA expression vectors, and Judy Cheung for her expert technical assistance. This study was supported by grants from the CF Foundation (FISCHE07G0 to HF, GRUENE05I0 to DCG, Illek08G0), the Pennsylvania CF Inc., and the California Pacific Medical Center Research Foundation (to DCG, RM), the Children’s Hospital Oakland Commercial Endowment (to BI), and the NIH (AT002620, HL071829). LW was a recipient of a summer student stipend from the Elizabeth Nash Foundation.
ABBREVIATIONS
- cAMP
3’-5’-cyclic adenosine monophosphate
- CF
cystic fibrosis
- CFTR
CF transmembrane conductance regulator
- DMSO
dimethyl sulfoxide
- HygBR
hygromycin B resistance
- ORF
open reading frame
- ICl
chloride currents
- ISC
transepithelial short circuit current
- PCR
polymerase chain reaction
- RT-PCR
reverse transcriptase PCR
- SEM
standard error of the mean
- SV40
simian virus 40
- TR
transepithelial resistance
- UTR
untranslated region
- wt
wild type
REFERENCES
- 1.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 2.Kunzelmann K, Lei DC, Eng K, Escobar LC, Koslowsky T, Gruenert DC. Epithelial cell specific properties and genetic complementation in a delta F508 cystic fibrosis nasal polyp cell line. In Vitro Cell Dev Biol Anim. 1995;31:617–624. doi: 10.1007/BF02634315. [DOI] [PubMed] [Google Scholar]
- 3.McNicholas CM, Guggino WB, Schwiebert EM, Hebert SC, Giebisch G, Egan ME. Sensitivity of a renal K+ channel (ROMK2) to the inhibitory sulfonylurea compound glibenclamide is enhanced by coexpression with the ATP-binding cassette transporter cystic fibrosis transmembrane regulator. Proc Natl Acad Sci U S A. 1996;93:8083–8088. doi: 10.1073/pnas.93.15.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riordan JR, Rommens JM, Kerem B-S, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J-L, Drumm ML, Iannuzzi MC, Collins FS, Tsui L-C. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 5.Consortium TCFGA. Cystic Fibrosis Mutation Database. 2007. In. [Google Scholar]
- 6.Collins FS. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992;256:774–783. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- 7.Tsui L-C. The spectrum of cystic fibrosis mutations. Trends in Genet. 1992;8:392–398. doi: 10.1016/0168-9525(92)90301-j. [DOI] [PubMed] [Google Scholar]
- 8.Aitken ML, Fiel SB. Cystic fibrosis. J Clin Invest. 1993;91:225–234. [Google Scholar]
- 9.Denning GM, Ostedgaard LS, Welsh MJ. Abnormal localization of cystic fibrosis transmembrane conductance regulator in primary cultures of cystic fibrosis airway epithelia. J Cell Biol. 1992;118:551–559. doi: 10.1083/jcb.118.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR, Boucher RC. Characterization of wild-type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell. 2005;16:2154–2167. doi: 10.1091/mbc.E04-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Widdicombe JH, Welsh MJ, Finkbeiner WE. Cystic fibrosis decreases the apical membrane chloride permeability of monolayers cultured from cells of tracheal epithelium. Proc Natl Acad Sci USA. 1985;82:6167–6171. doi: 10.1073/pnas.82.18.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruenert DC, Basbaum CB, Widdicombe JH. Long-term culture of normal and cystic fibrosis epithelial cells grown under serum-free conditions. In Vitro Cell Dev Biol. 1990;26:411–418. doi: 10.1007/BF02623833. [DOI] [PubMed] [Google Scholar]
- 13.Gruenert DC, Finkbeiner WE, Widdicombe JH. Culture and transformation of human airway epithelial cells. Am J Physiol. 1995;268:L347–L360. doi: 10.1152/ajplung.1995.268.3.L347. [DOI] [PubMed] [Google Scholar]
- 14.Gruenert DC, Willems M, Cassiman JJ, Frizzell RA. Established cell lines used in cystic fibrosis research. J Cyst Fibros. 2004;3 Suppl 2:191–196. doi: 10.1016/j.jcf.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 15.Kalin N, Claass A, Sommer M, Puchelle E, Tummler B. DeltaF508 CFTR protein expression in tissues from patients with cystic fibrosis. J Clin Invest. 1999;103:1379–1389. doi: 10.1172/JCI5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widdicombe JH, Coleman DL, Finkbeiner WE, Tuet I. Electrical properties of monolayers cultured from cells of human tracheal mucosa. J Appl Physiol. 1985;58:1729–1735. doi: 10.1152/jappl.1985.58.5.1729. [DOI] [PubMed] [Google Scholar]
- 17.Yankaskas JR, Cotton CU, Knowles MR, Gatzy JT, Boucher RC. Culture of human nasal epithelial cells on collagen matrix supports. Am Rev Respir Dis. 1985;132:1281–1287. doi: 10.1164/arrd.1985.132.6.1281. [DOI] [PubMed] [Google Scholar]
- 18.Yankaskas JR, Knowles MR, Gatzy JT, Boucher RC. Persistence of abnormal chloride ion permeability in cystic fibrosis nasal epithelial cells in heterologous culture. Lancet. 1985;1:954–956. doi: 10.1016/s0140-6736(85)91728-3. [DOI] [PubMed] [Google Scholar]
- 19.Cozens AL, Yezzi MJ, Chin L, Simon EM, Finkbeiner WE, Wagner JA, Gruenert DC. Characterization of immortal cystic fibrosis tracheobronchial gland epithelial cells. Proc Natl Acad Sci U S A. 1992;89:5171–5175. doi: 10.1073/pnas.89.11.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 21.Cozens AL, Yezzi MJ, Yamaya M, Steiger D, Wagner JA, Garber SS, Chin L, Simon EM, Cutting GR, Gardner P, et al. A transformed human epithelial cell line that retains tight junctions post crisis. In Vitro Cell Dev Biol. 1992;28A:735–744. doi: 10.1007/BF02631062. [DOI] [PubMed] [Google Scholar]
- 22.Gruenert DC, Basbaum CB, Welsh MJ, Li M, Finkbeiner WE, Nadel JA. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc Natl Acad Sci U S A. 1988;85:5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jefferson DM, Valentich JD, Marini FC, Grubman SA, Iannuzzi MC, Dorkin HL, Li M, Klinger KW, Welsh MJ. Expression of normal and cystic fibrosis phenotypes by continuous airway epithelial cell lines. Am J Physiol. 1990;259:L496–L505. doi: 10.1152/ajplung.1990.259.6.L496. [DOI] [PubMed] [Google Scholar]
- 24.Jetten AM, Yankaskas JR, Stutts MJ, Willumsen NJ, Boucher RC. Persistence of abnormal chloride conductance regulation in transformed cystic fibrosis epithelia. Science. 1989;244:1472–1475. doi: 10.1126/science.2472008. [DOI] [PubMed] [Google Scholar]
- 25.Kunzelmann K, Schwiebert EM, Zeitlin PL, Kuo WL, Stanton BA, Gruenert DC. An immortalized cystic fibrosis tracheal epithelial cell line homozygous for the delta F508 CFTR mutation. Am J Respir Cell Mol Biol. 1993;8:522–529. doi: 10.1165/ajrcmb/8.5.522. [DOI] [PubMed] [Google Scholar]
- 26.Lundberg AS, Randell SH, Stewart SA, Elenbaas B, Hartwell KA, Brooks MW, Fleming MD, Olsen JC, Miller SW, Weinberg RA, Hahn WC. Immortalization and transformation of primary human airway epithelial cells by gene transfer. Oncogene. 2002;21:4577–4586. doi: 10.1038/sj.onc.1205550. [DOI] [PubMed] [Google Scholar]
- 27.Scholte BJ, Kansen M, Hoogeveen AT, Willemse R, Rhim JS, van der Kamp AW, Bijman J. Immortalization of nasal polyp epithelial cells from cystic fibrosis patients. Exp Cell Res. 1989;182:559–571. doi: 10.1016/0014-4827(89)90259-0. [DOI] [PubMed] [Google Scholar]
- 28.Vaughan MB, Ramirez RD, Wright WE, Minna JD, Shay JW. A three-dimensional model of differentiation of immortalized human bronchial epithelial cells. Differentiation. 2006;74:141–148. doi: 10.1111/j.1432-0436.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 29.Wei X, Eisman R, Xu J, Harsch AD, Mulberg AE, Bevins CL, Glick MC, Scanlin TF. Turnover of the cystic fibrosis transmembrane conductance regulator (CFTR): slow degradation of wild-type and delta F508 CFTR in surface membrane preparations of immortalized airway epithelial cells. J Cell Physiol. 1996;168:373–384. doi: 10.1002/(SICI)1097-4652(199608)168:2<373::AID-JCP16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Zeitlin PL, Lu L, Rhim J, Cutting G, Stetten G, Kieffer KA, Craig R, Guggino WB. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am J Respir Cell Mol Biol. 1991;4:313–319. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]
- 31.Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O'Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 32.Kartner N, Hanrahan JW, Jensen TJ, Naismith AL, Sun SZ, Ackerley CA, Reyes EF, Tsui LC, Rommens JM, Bear CE, et al. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell. 1991;64:681–691. doi: 10.1016/0092-8674(91)90498-n. [DOI] [PubMed] [Google Scholar]
- 33.Rommens JM, Dho S, Bear CE, Kartner N, Kennedy D, Riordan JR, Tsui LC, Foskett JK. cAMP-inducible chloride conductance in mouse fibroblast lines stably expressing the human cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1991;88:7500–7504. doi: 10.1073/pnas.88.17.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiavi SC, Abdelkader N, Reber S, Pennington S, Narayana R, McPherson JM, Smith AE, Hoppe Ht, Cheng SH. Biosynthetic and growth abnormalities are associated with high-level expression of CFTR in heterologous cells. Am J Physiol. 1996;270:C341–C351. doi: 10.1152/ajpcell.1996.270.1.C341. [DOI] [PubMed] [Google Scholar]
- 35.Stutts MJ, Gabriel SE, Olsen JC, Gatzy JT, O'Connell TL, Price EM, Boucher RC. Functional consequences of heterologous expression of the cystic fibrosis transmembrane conductance regulator in fibroblasts. J Biol Chem. 1993;268:20653–20658. [PubMed] [Google Scholar]
- 36.Farinha CM, Penque D, Roxo-Rosa M, Lukacs G, Dormer R, McPherson M, Pereira M, Bot AG, Jorna H, Willemsen R, Dejonge H, Heda GD, Marino CR, Fanen P, Hinzpeter A, Lipecka J, Fritsch J, Gentzsch M, Edelman A, Amaral MD. Biochemical methods to assess CFTR expression and membrane localization. J Cyst Fibros. 2004;3 Suppl 2:73–77. doi: 10.1016/j.jcf.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Brezillon S, Hamm H, Heilmann M, Schafers HJ, Hinnrasky J, Wagner TO, Puchelle E, Tummler B. Decreased expression of the cystic fibrosis transmembrane conductance regulator protein in remodeled airway epithelium from lung transplanted patients. Hum Pathol. 1997;28:944–952. doi: 10.1016/s0046-8177(97)90010-1. [DOI] [PubMed] [Google Scholar]
- 38.Jacquot J, Puchelle E, Hinnrasky J, Fuchey C, Bettinger C, Spilmont C, Bonnet N, Dieterle A, Dreyer D, Pavirani A, et al. Localization of the cystic fibrosis transmembrane conductance regulator in airway secretory glands. Eur Respir J. 1993;6:169–176. [PubMed] [Google Scholar]
- 39.Farmen SL, Karp PH, Ng P, Palmer DJ, Koehler DR, Hu J, Beaudet AL, Zabner J, Welsh MJ. Gene transfer of CFTR to airway epithelia: low levels of expression are sufficient to correct Cl- transport and overexpression can generate basolateral CFTR. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1123–L1130. doi: 10.1152/ajplung.00049.2005. [DOI] [PubMed] [Google Scholar]
- 40.Trapnell BC, Chu CS, Paakko PK, Banks TC, Yoshimura K, Ferrans VJ, Chernick MS, Crystal RG. Expression of the cystic fibrosis transmembrane conductance regulator gene in the respiratory tract of normal individuals and individuals with cystic fibrosis. Proc Natl Acad Sci U S A. 1991;88:6565–6569. doi: 10.1073/pnas.88.15.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng SH, Fang SL, Zabner J, Marshall J, Piraino S, Schiavi SC, Jefferson DM, Welsh MJ, Smith AE. Functional activation of the cystic fibrosis trafficking mutant delta F508-CFTR by overexpression. Am J Physiol. 1995;268:L615–L624. doi: 10.1152/ajplung.1995.268.4.L615. [DOI] [PubMed] [Google Scholar]
- 42.Dragomir A, Bjorstad J, Hjelte L, Roomans GM. Curcumin does not stimulate cAMP-mediated chloride transport in cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun. 2004;322:447–451. doi: 10.1016/j.bbrc.2004.07.146. [DOI] [PubMed] [Google Scholar]
- 43.Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Glockner-Pagel J, Canny S, Du K, Lukacs GL, Caplan MJ. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304:600–602. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- 44.Lipecka J, Norez C, Bensalem N, Baudouin-Legros M, Planelles G, Becq F, Edelman A, Davezac N. Rescue of DeltaF508-CFTR (cystic fibrosis transmembrane conductance regulator) by curcumin: involvement of the keratin 18 network. J Pharmacol Exp Ther. 2006;317:500–505. doi: 10.1124/jpet.105.097667. [DOI] [PubMed] [Google Scholar]
- 45.Mall M, Kunzelmann K. Correction of the CF defect by curcumin: hypes and disappointments. Bioessays. 2005;27:9–13. doi: 10.1002/bies.20168. [DOI] [PubMed] [Google Scholar]
- 46.Fischer H, Illek B, Finkbeiner WE, Widdicombe JH. Basolateral Cl channels in primary airway epithelial cultures. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1432–L1443. doi: 10.1152/ajplung.00032.2007. [DOI] [PubMed] [Google Scholar]
- 47.Cunningham SA, Worrell RT, Benos DJ, Frizzell RA. cAMP-stimulated ion currents in Xenopus oocytes expressing CFTR cRNA. Am J Physiol. 1992;262:C783–C788. doi: 10.1152/ajpcell.1992.262.3.C783. [DOI] [PubMed] [Google Scholar]
- 48.Baudouin-Legros M, Hinzpeter A, Jaulmes A, Brouillard F, Costes B, Fanen P, Edelman A. Cell-specific posttranscriptional regulation of CFTR gene expression via influence of MAPK cascades on 3'UTR part of transcripts. Am J Physiol Cell Physiol. 2005;289:C1240–C1250. doi: 10.1152/ajpcell.00595.2004. [DOI] [PubMed] [Google Scholar]
- 49.Davies WL, Vandenberg JI, Sayeed RA, Trezise AE. Cardiac expression of the cystic fibrosis transmembrane conductance regulator involves novel exon 1 usage to produce a unique amino-terminal protein. J Biol Chem. 2004;279:15877–15887. doi: 10.1074/jbc.M313628200. [DOI] [PubMed] [Google Scholar]
- 50.Lei DC, Kunzelmann K, Koslowsky T, Yezzi MJ, Escobar LC, Xu Z, Ellison AR, Rommens JM, Tsui LC, Tykocinski M, Gruenert DC. Episomal expression of wild-type CFTR corrects cAMP-dependent chloride transport in respiratory epithelial cells. Gene Ther. 1996;3:427–436. [PubMed] [Google Scholar]
- 51.Bruscia E, Sangiuolo F, Sinibaldi P, Goncz KK, Novelli G, Gruenert DC. Isolation of CF cell lines corrected at DeltaF508-CFTR locus by SFHR-mediated targeting. Gene Ther. 2002;9:683–685. doi: 10.1038/sj.gt.3301741. [DOI] [PubMed] [Google Scholar]
- 52.Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 53.Small MB, Gluzman Y, Ozer HL. Enhanced transformation of human fibroblasts by origin-defective SV40. Nature. 1982;296:671–675. doi: 10.1038/296671a0. [DOI] [PubMed] [Google Scholar]
- 54.Hipper A, Mall M, Greger R, Kunzelmann K. Mutations in the putative pore-forming domain of CFTR do not change anion selectivity of the cAMP activated Cl-conductance. FEBS Lett. 1995;374:312–316. doi: 10.1016/0014-5793(95)01132-x. [DOI] [PubMed] [Google Scholar]
- 55.Mall M, Kunzelmann K, Hipper A, Busch AE, Greger R. cAMP stimulation of CFTR-expressing Xenopus oocytes activates a chromanol-inhibitable K+ conductance. Pflugers Arch. 1996;432:516–522. doi: 10.1007/s004240050164. [DOI] [PubMed] [Google Scholar]
- 56.Lechner JF, LaVeck MA. A serum-free method for culturing normal human bronchial epithelial cells at clonal density. J Tissue Culture Methods. 1985;9:43–48. [Google Scholar]
- 57.Goncz KK, Kunzelmann K, Xu Z, Gruenert DC. Targeted replacement of normal and mutant CFTR sequences in human airway epithelial cells using DNA fragments. Hum Mol Genet. 1998;7:1913–1919. doi: 10.1093/hmg/7.12.1913. [DOI] [PubMed] [Google Scholar]
- 58.Freeman WM, Walker SJ, Vrana KE. Quantitative RT-PCR: pitfalls and potential. Biotechniques. 1999;26:112–122. doi: 10.2144/99261rv01. 124-115. [DOI] [PubMed] [Google Scholar]
- 59.Illek B, Fischer H. Flavonoids stimulate Cl conductance of human airway epithelium in vitro and in vivo. Am J Physiol. 1998;275:L902–L910. doi: 10.1152/ajplung.1998.275.5.L902. [DOI] [PubMed] [Google Scholar]
- 60.Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structureactivity analysis, and in vivo efficacy. J Gen Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheppard DN, Welsh MJ. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ehrhardt C, Collnot EM, Baldes C, Becker U, Laue M, Kim KJ, Lehr CM. Towards an in vitro model of cystic fibrosis small airway epithelium: characterisation of the human bronchial epithelial cell line CFBE41o. Cell Tissue Res. 2006;323:405–415. doi: 10.1007/s00441-005-0062-7. [DOI] [PubMed] [Google Scholar]
- 63.Ma T, Vetrivel L, Yang H, Pedemonte N, Zegarra-Moran O, Galietta LJ, Verkman AS. High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J Biol Chem. 2002;277:37235–37241. doi: 10.1074/jbc.M205932200. [DOI] [PubMed] [Google Scholar]
- 64.Loffing-Cueni D, Loffing J, Shaw C, Taplin AM, Govindan M, Stanton CR, Stanton BA. Trafficking of GFP-tagged DeltaF508-CFTR to the plasma membrane in a polarized epithelial cell line. Am J Physiol Cell Physiol. 2001;281:C1889–C1897. doi: 10.1152/ajpcell.2001.281.6.C1889. [DOI] [PubMed] [Google Scholar]
- 65.Maitra R, Shaw CM, Stanton BA, Hamilton JW. Increased functional cell surface expression of CFTR and DeltaF508-CFTR by the anthracycline doxorubicin. Am J Physiol Cell Physiol. 2001;280:C1031–C1037. doi: 10.1152/ajpcell.2001.280.5.C1031. [DOI] [PubMed] [Google Scholar]
- 66.Bebok Z, Venglarik CJ, Panczel Z, Jilling T, Kirk KL, Sorscher EJ. Activation of DeltaF508 CFTR in an epithelial monolayer. Am J Physiol. 1998;275:C599–C607. doi: 10.1152/ajpcell.1998.275.2.C599. [DOI] [PubMed] [Google Scholar]
- 67.Van Goor F, Straley KS, Cao D, Gonzalez J, Hadida S, Hazlewood A, Joubran J, Knapp T, Makings LR, Miller M, Neuberger T, Olson E, Panchenko V, Rader J, Singh A, Stack JH, Tung R, Grootenhuis PD, Negulescu P. Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1117–L1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 68.Howard M, Fischer H, Roux J, Santos BC, Gullans SR, Yancey PH, Welch WJ. Mammalian osmolytes and S-nitrosoglutathione promote Delta F508 cystic fibrosis transmembrane conductance regulator (CFTR) protein maturation and function. J Biol Chem. 2003;278:35159–35167. doi: 10.1074/jbc.M301924200. [DOI] [PubMed] [Google Scholar]
- 69.Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJ, Verkman AS. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005;115:2564–2571. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sousa M, Ousingsawat J, Seitz R, Puntheeranurak S, Regalado A, Schmidt A, Grego T, Jansakul C, Amaral MD, Schreiber R, Kunzelmann K. An extract from the medicinal plant Phyllanthus acidus and its isolated compounds induce airway chloride secretion: A potential treatment for cystic fibrosis. Mol Pharmacol. 2007;71:366–376. doi: 10.1124/mol.106.025262. [DOI] [PubMed] [Google Scholar]
- 71.Galietta LV, Jayaraman S, Verkman AS. Cell-based assay for high-throughput quantitative screening of CFTR chloride transport agonists. Am J Physiol Cell Physiol. 2001;281:C1734–C1742. doi: 10.1152/ajpcell.2001.281.5.C1734. [DOI] [PubMed] [Google Scholar]
- 72.Haws CM, Nepomuceno IB, Krouse ME, Wakelee H, Law T, Xia Y, Nguyen H, Wine JJ. Delta F508-CFTR channels: kinetics, activation by forskolin, and potentiation by xanthines. Am J Physiol. 1996;270:C1544–C1555. doi: 10.1152/ajpcell.1996.270.5.C1544. [DOI] [PubMed] [Google Scholar]
- 73.Pedemonte N, Diena T, Caci E, Nieddu E, Mazzei M, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Antihypertensive 1,4-dihydropyridines as correctors of the cystic fibrosis transmembrane conductance regulator channel gating defect caused by cystic fibrosis mutations. Mol Pharmacol. 2005;68:1736–1746. doi: 10.1124/mol.105.015149. [DOI] [PubMed] [Google Scholar]
- 74.Illek B, Zhang L, Lewis NC, Moss RB, Dong JY, Fischer H. Defective function of the cystic fibrosis-causing missense mutation G551D is recovered by genistein. Am J Physiol. 1999;277:C833–C839. doi: 10.1152/ajpcell.1999.277.4.C833. [DOI] [PubMed] [Google Scholar]
- 75.Tabcharani JA, Low W, Elie D, Hanrahan JW. Low-conductance chloride channel activated by cAMP in the epithelial cell line T84. FEBS Lett. 1990;270:157–164. doi: 10.1016/0014-5793(90)81257-o. [DOI] [PubMed] [Google Scholar]