Abstract
The outstanding migration and differentiation capacities of neural crest cells (NCCs) have fascinated scientists since Wilhelm His described this cell population in 1868. Today, after intense research using vertebrate model organisms, we have gained considerable knowledge regarding the origin, migration and differentiation of NCCs. However, our understanding of NCC development in human embryos remains largely uncharacterized, despite the role the neural crest plays in several human pathologies. Here, we report for the first time the expression of a battery of molecular markers before, during, or following NCC migration in human embryos from Carnegie Stages (CS) 12 to 18. Our work demonstrates the expression of Sox9, Sox10 and Pax3 transcription factors in premigratory NCCs, while actively migrating NCCs display the additional transcription factors Pax7 and AP-2α. Importantly, while HNK-1 labels few migrating NCCs, p75NTR labels a large proportion of this population. However, the broad expression of p75NTR – and other markers - beyond the neural crest stresses the need for the identification of additional markers to improve our capacity to investigate human NCC development, and to enable the generation of better diagnostic and therapeutic tools.
Keywords: Neural crest, vertebrate embryos, human embryogenesis, DRG, enteric nervous system, Sox9/10, Pax3/7, AP-2α, p75NTR, neural crest gene regulatory network
Introduction
Much of the fascination regarding the neural crest lies within its ability to generate a diverse array of cell types throughout the vertebrate body. These cells originate at the border of the neural and non-neural ectoderm, and later delaminate from the dorsal neural tube. In the chick, neural crest migration occurs after the neural tube has closed; however, in both the human and mouse, cranial NCCs have been shown to migrate from the unfused neural folds (Nichols 1981; O’Rahilly and Müller 2007). Once free from the neural tube, NCCs move throughout the body to generate multiple derivatives, including: the majority of the cranial connective tissue and skeletal elements, neurons and glia of the peripheral nervous system, cells contributing to the valves of the heart, secretory cells, and melanocytes.
The migration patterns of NCCs have been well characterized across model organisms. In both the mouse and chick, cranial NCCs emerge from the forebrain, midbrain and hindbrain regions of the neuroepithelium; however, in the chick, the rostral-most limit of this cell population extends only to the diencephalon (Couly and Le Douarin 1987; Serbedzija et al. 1992). More caudally, the hindbrain-derived NCCs of model organisms follow stereotypical patterns of migration, with streams of NCCs appearing adjacent to the even-numbered rhombomeres (Lumsden et al. 1991). Depending on their origin, cranial NCCs will either migrate through the facial mesenchyme and into the frontonasal process, or will populate the branchial arches (Noden 1975; Lumsden et al. 1991; Serbedzija et al. 1992). These cells go on to form the majority of the connective and skeletal tissue of the head, as well as neurons and glia of the cranial ganglia. Within the trunk, mouse NCCs first collect between the presumptive epidermis and dorsal neural tube. Cell migration then follows two main routes, with cells moving either ventro-laterally through the anterior sclerotome, or dorso-laterally between the surface ectoderm and somites (Serbedzija et al. 1990). Such migration patterns are also seen in the neural crest of the avian trunk, as evidenced by HNK-1 antibody staining (Bronner-Fraser 1986). Despite inherent differences between avian, mammalian and teleost development, zebrafish trunk NCCs undertake similar routes to those described above, with cells moving ventrally alongside the neural tube, or between the epidermis and somites (Raible et al. 1992). Collectively, these cells will form the neurons and glia of the peripheral nervous system, in addition to pigment cells.
The expression and function of several markers during neural crest development has been integrated into operational models as either a cascade, genetic network, or neural crest gene regulatory network (NC-GRN) (Mayor et al. 1999; Aybar and Mayor 2002; Meulemans and Bronner-Fraser 2004; Steventon et al. 2005; Sauka-Spengler and Bronner-Fraser 2008). These models link the expression and function of signaling molecules, transcription factors and other neural crest markers from early NCC induction events, specification, migration and eventual differentiation. According to the NC-GRN, signaling molecules (BMP, FGF, Notch, RA, and Wnt) participate in both induction and later steps of neural crest development. This induction triggers the expression of a specific set of transcription factors collectively known as border specifier genes (Msx1, Msx2, Pax3, Pax7, and Zic1), which – along with signaling molecules – direct the expression of neural crest specifiers (AP-2, FoxD3, Snail2, Sox9 and Sox10). These specifiers in turn regulate the appearance of later neural crest effector genes responsible for NCC migration and differentiation (Sox9, Sox10, Cad7, ColIIa, Ngn1, Mitf, Dct, etc.). The expression of these genes appears in a temporal fashion during embryogenesis, and marks the progression of neural crest development.
Specific roles for some of these genes in neural crest development have been illustrated through functional assays in a variety of model systems, including xenopus, zebrafish, chick and mouse. For instance, Pax3, Pax7, Sox10 and AP-2 mutant mice all demonstrate neural crest defects. These manifest as deformities of the nose and jaw in both Pax3 (Splotch) and Pax7 mutants; Pax3 mutants additionally exhibit malformations of ganglia of the peripheral nervous system (Auerbach 1954; Tremblay et al. 1995 and 1998; Mansouri et al. 1996; Conway et al. 1997). Mice carrying a mutation in Sox10 (Dom mutants) also demonstrate deformed ganglia, as well as a failure of the enteric neural crest to properly invade the gut (Lane and Liu 1984; Southard-Smith et al. 1998). Finally, AP-2 mutant mice exhibit malformed cranial nerves and ganglia, in addition to general craniofacial abnormalities (Zhang et al. 1996). As the neural crest generates the majority of the craniofacial features and connective tissue, in addition to the peripheral nervous system and ganglia therein, these observed malformations provide evidence for the role of neural plate border or neural crest specifiers within NCC development.
The migration patterns of human NCCs (hNCCs) have been described in embryos between Carnegie Stages 9–20 (O’Rahilly and Müller 2007). This study, based on careful histological analysis, suggests that hNCCs undertake similar migration routes as those observed in mouse and chick embryos. Cranial hNCCs will either move through cranial mesenchyme into the frontonasal process, or populate the branchial arches. Unlike model organisms, human forebrain NCCs appear to emerge exclusively from the optic region (O’Rahilly and Müller 2007). Within the human trunk, NCCs migrate ventrally through the somites, or laterally alongside the neural tube (O’Rahilly and Müller 2007).
Beyond cell morphology, few studies have evaluated neural crest marker expression in human embryos at a time when NCC migration is underway. To our knowledge, only Sox10 has been reported in migratory NCCs of a 4-week old human embryo (Bondurand et al. 1998); however, the RNA profiles of cell lines derived from hNCCs include Msx1, Pax3, Sox9, Sox10, and p75 transcripts, among other neural crest markers (Thomas et al. 2008). In older specimens, the in-vivo expression of Pax3, Sox9, Sox10, AP-2α and HNK-1/NC-1 has been demonstrated in neural crest derivatives such as the dorsal root ganglia (DRG) (Tucker et al. 1984; Tucker et al. 1988; Bondurand et al. 1998; Terzić and Saraga-Babić 1999; Gershon et al. 2005; Benko et al. 2009).
With the aim to expand our knowledge of human neural crest cell development, we have analyzed the expression of several molecules included in the NC-GRN, particularly during the early stages of human neural crest development. Here, we present human expression profiles of the transcription factors Msx1/2, Pax3, Pax7, Sox9, Sox10 and AP-2α, in addition to the HNK-1 monoclonal antibody and p75 neurotrophin receptor (p75NTR). We demonstrate for the first time the expression of several members of the NC-GRN within early human embryos at CS12, CS13, CS15 and CS18. We observe Pax3, Sox9 and Sox10 expression in the premigratory neural crest of a CS12 specimen. In more rostral sections at this stage, Sox9, Sox10, AP-2α, p75NTR and few HNK-1+ cells occur within the migratory neural crest directly dorsal to the neural tube, or nestled between the neural tube, surface ectoderm and somites. By CS13, Pax7, Sox9, Sox10 and p75NTR are observed in the migratory neural crest of caudal sections. At all stages examined, we note the expression of several markers within neural crest derivatives such as the facial mesenchyme, cranial and enteric ganglia, and DRGs. Comparisons are made between NCC marker staining patterns observed in human embryos and model organisms, with special focus given to the higher vertebrates. Additionally, we report interesting patterns of expression for several of these markers that have never before been seen in such young embryos.
Methods
Embryo Collection
Embryos were obtained from elective routine abortions at the Department of Gynecology, Karolinska University Hospital-Huddinge, after written and oral consent from the pregnant women. All procedures regarding the collection and use of human material were according to Swedish law and approved by the Regional Ethical Committee of Stockholm. Embryos were subsequently staged according to O’Rahilly and Müller (1987). Following their collection, CS13 and 18 embryos were fixed in 4% PF for 1–2 hours and placed in methanol. The CS12 and CS15 specimens were treated with Zinc-fixative, placed in 30% sucrose overnight and embedded in OCT.
Whole Mount Embryo Morphology
The caudal neuropores of both the CS12 and CS13 embryo appear fully closed; however, in both cases the rostral-most neural folds remain unfused, and the CS12 specimen demonstrates open portions of the neural tube more caudally (Fig. 1Q, Fig. 4H and data not shown). Regions of the frontonasal mesenchyme also appear missing in the CS12 embryo (Fig. 1Q and data not shown), likely the result of damage during the collection of this specimen. Within both the CS13 and CS15 embryos, the eye primordia and branchial arches can be seen (Fig. 4H, Fig. 5T). In the youngest three specimens, the hearts have been removed. The CS18 embryo displays normal morphology along the rostral-caudal axis (Fig. 6L and data not shown). Respectively, these embryos are equivalent to E9.5–10.25 (~CS12), E10.25–10.5 (~CS13), E11.0–11.5 (~CS15) and E12.5–13.0 (~CS18) in the mouse (for staging, see Kaufman 1992).
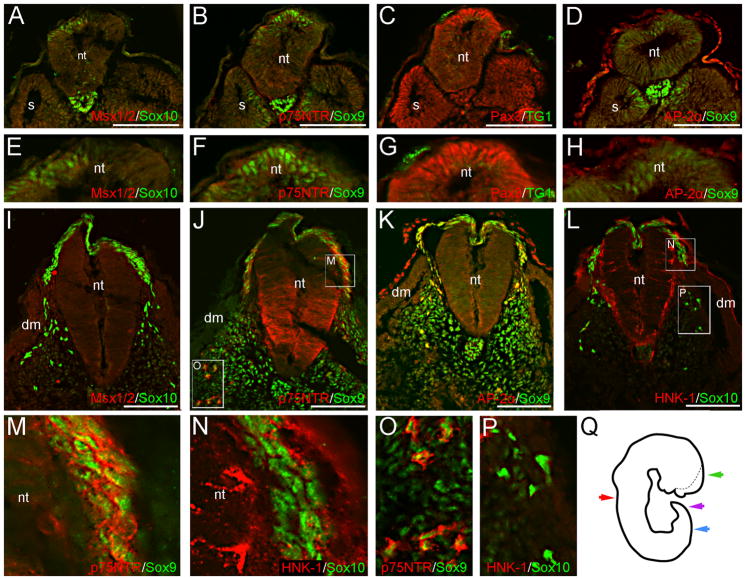
Fig. 1.
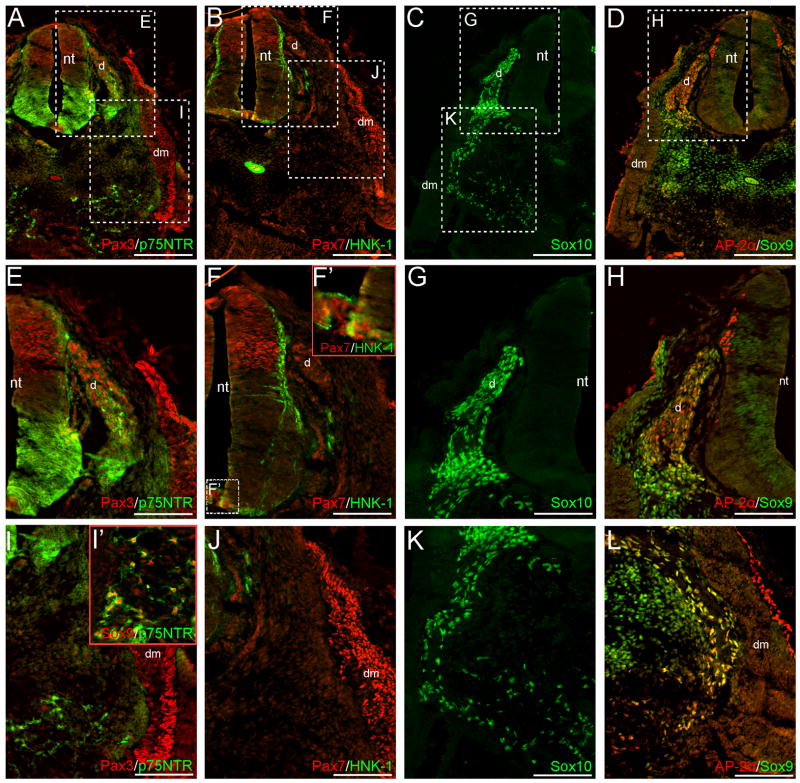
NCC markers are expressed in prospective premigratory and migratory NCCs at CS12. Sox10 (A), Sox9 (B, D) and Pax3 (C) signal occur in the dorsal neural tube of caudal trunk sections, prior to the appearance of the hindlimb. These cells likely represent premigratory NCCs. Images in (E–H) are magnifications of the dorsal neural tubes shown in (A–D). We do not observe definitive Msx1/2, p75NTR, or AP-2α signal in premigratory NCCs (A, B, D). The monoclonal antibody TG1 has previously been used to recognize other migratory cells and was thus here tried, but no signal can be associated with premigratory NCCs (C, G). In slightly rostral sections, Sox10 (I, L), Sox9 (J–K), p75NTR (J), and AP-2α (K) are expressed in migratory NCCs situated dorsal, or dorso-lateral to, the neural tube. The majority of AP-2α+ migratory NCCs appear to co-express Sox9 (K). Panels in (M–P) are magnifications of boxed regions shown in (J, L). Although robust p75NTR signal is co-expressed with Sox9 in migratory NCCs adjacent to the neural tube (M), or subadjacent to the dermomyotome (O), this cell population (when identified via Sox10 expression) does not express HNK-1 in similar sections (N, P); migratory NCCs generally appear HNK-1 negative. Image in (Q) is a tracing of the CS12 wholemount, prior to sectioning; the dotted line marks the contra-lateral side of the unfused neural tube. Color-coded arrows represent the axial levels of depicted sections as follows: purple (A–H), blue (I–P), red (Fig. 2), green (Fig. 3). Scale bars in all panels represent 100μm. Note: levels in (A–D) are modified separately from those in (I–L) to optimize signals in different embryonic regions. dm, dermomyotome; nt, neural tube; s, somite.
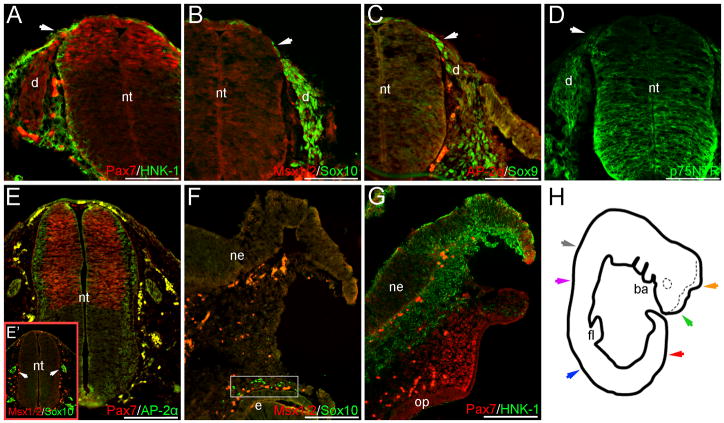
Fig. 4.
NCC markers are expressed within the caudal migratory neural crest of trunk sections, or within the cranial mesenchyme at CS13. Pax7 (A), Sox9 (C), Sox10 (B) and p75NTR (D) signal occur in migratory NCCs located directly dorsal, or dorso-lateral to (region marked by arrows), the neural tube within caudal trunk sections. Although HNK-1 is also expressed in this region, it does not appear to robustly co-localize with Pax7 (A). Pax7, Sox10, Sox9 and p75NTR are also expressed within regions of the forming DRGs (A–D). Definitive Msx1/2 or AP-2α signal are not observed in either migratory NCCs or the presumptive caudal DRGs (B, C). In cranial CS13 sections, Pax7 expression is observed in the mesenchyme adjacent to the presumptive olfactory placode, in addition to the distal-most tip of the unfused neuroepithelium (G). Punctate Sox10 expression occurs near the eye primordia of slightly caudal sections (F boxed region), and within the ganglia condensations adjacent to the neuroepithelium (E’ arrows); in similar sections, Pax7 appears in the dorsal portion of the neuroepithelium, with AP-2α signal occurring along the lateral margins (E). HNK-1 signal is also observed within the cranial mesenchyme of more rostral sections, but again appears largely independent of Pax7 expression (G). Image in (H) is a tracing of the CS13 embryo wholemount; the dotted line marks the contra-lateral side of the unfused neural tube. Color-coded arrows in (H) indicate axial levels of sections as follows: red (A–D); gray (E–E’); orange (F); green (G); blue (S1. A–C); purple (S1. D). Scale bars in (A–D) represent 100μm; those in (E–G) 200μm. Note: levels in (E’), (F) are modified separately to optimize green and red signals at different axial levels. ba, branchial arches; d, dorsal root ganglia; e, eye; fl, forelimb; ne, neuroepithelium; nt, neural tube; op, olfactory placode.
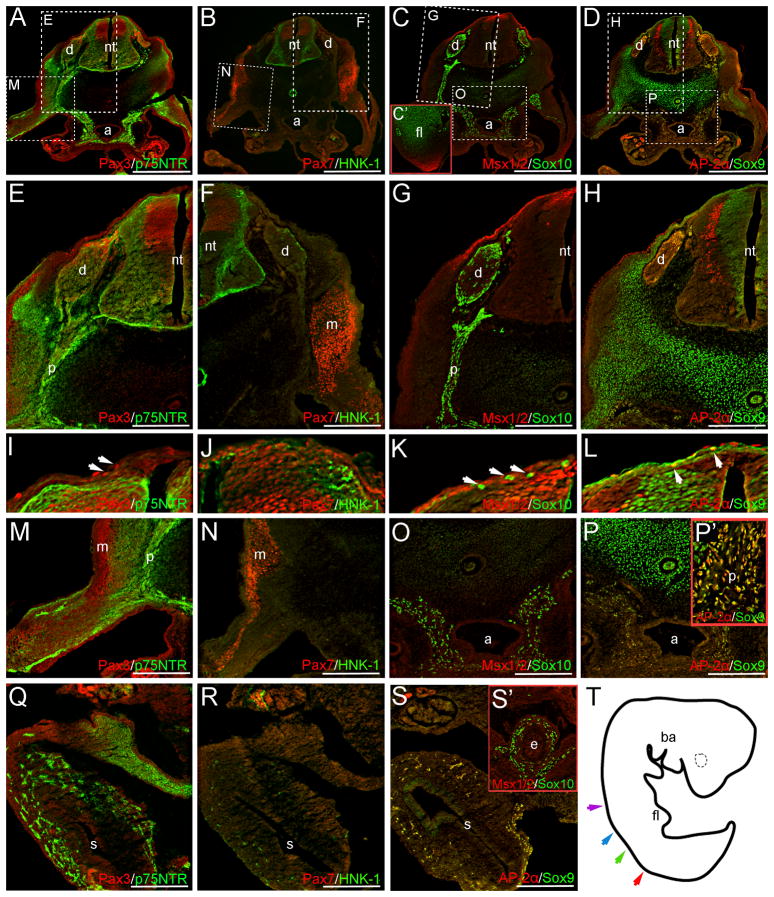
Fig. 5.
NCC markers are expressed in trunk neural crest derivatives at CS15. Panels in (E–P) are higher power images of boxed regions in (A–D). NCC-derived DRGs are positive for Pax3 (A, E), p75NTR (A, E), Sox10 (C, G), AP-2α (D, H), and Sox9 (D, H). Few HNK-1+ cells also occur in these structures (B, F). Neither Pax7 (B, F) or Msx1/2 (C, G) appear to be expressed in the DRGs at this stage. Sox10 (C, O, S’), AP-2α (D, P-P’, S), Sox9 (D, P-P’, S) and p75NTR (A, E, M, Q) staining are also observed within the mesenchyme surrounding the aorta, the presumptive peripheral nerves, and the wall of the gut; respectively, images in (C’, P’, S’) are magnifications of the forelimb bud, peripheral nerve, and esophagus in slightly rostral sections (see below). HNK-1+ cells also occur within the stomach wall at CS15 (R). Occasional Sox10+, Msx1/2+, AP-2α+ or Pax3+ cells are also observed directly dorsal to the neural tube (I, K, L arrows). Sox9 expressing cells are also suggested in similar regions (L). Outside of the neural crest or its derivatives, p75NTR (A, E, I), Pax3 (A, E, I), Pax7 (B, F, J), HNK-1 (B, F, J), Msx1/2 (C, G, K), AP-2α (D, H, L) and Sox9 (D, H, L) are all expressed in regions of the neuroepithelium. Additional Msx1/2 expression is observed in the distal forelimb mesenchyme (C’). Image in (T) depicts a tracing of the CS15 embryo wholemount prior to sectioning. Color-coded arrows indicate axial levels of sections as follows: red (A–D), green (P’, R–S), blue (Q) and purple (C’, S’). Scale bars in (A–D) represent 500μm, those in (E–S) represent 200μm. Note: levels in (C’), (E–P) and (Q–S’) are modified separately to optimize signals in different embryonic regions. a, aorta; ba, branchial arches; d, dorsal root ganglia; e, esophagus; fl, forelimb; nt, neural tube; m, presumptive musculature; p, peripheral nerve; s, stomach.
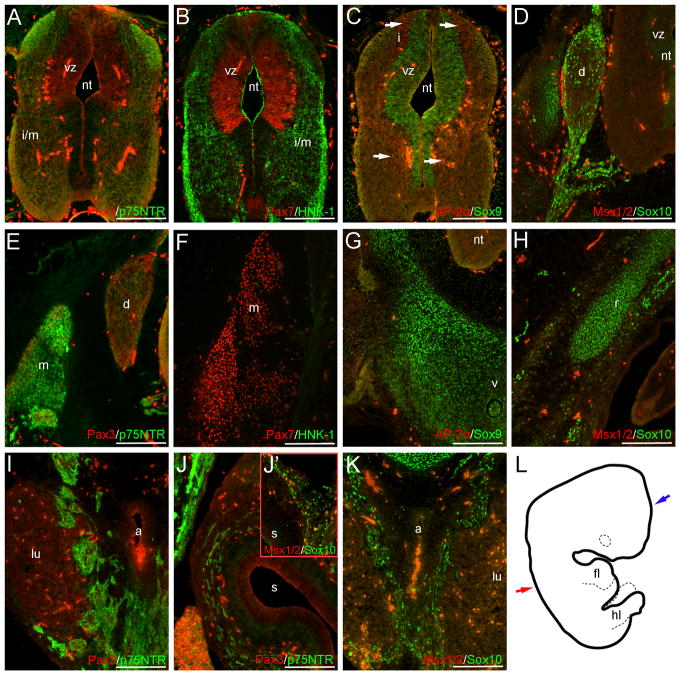
Fig. 6.
NCC markers are expressed within trunk neural crest and non-neural crest derivatives at CS18. Outside of neural crest-associated structures, Pax3 (A), Pax7 (B), Sox9 (C), AP-2α (C, arrows), Sox10 (D) and HNK-1 (B) occur in portions of the neuroepithelium. Sox10 and p75NTR signal also occur within the NCC-derived DRGs (D, E), sympathetic tissue (I, K) or presumptive enteric ganglia (J-J’); image in (J’) is a magnification of the gut wall within a similar section. Faint HNK-1 signal also occurs in the DRGs and associated nerve roots (F). Pax7 (F) and p75NTR (E) staining are additionally observed in the presumptive musculature of the back, and few Pax3+ cells occur in the dorso-medial margin of this region (E). Sox9 and Sox10 signal occur in the rib or vertebrae cartilage primordia (G, H). Image in (L) is a tracing of the CS18 embryo wholemount. Red and blue arrows represent axial levels of sections in (A–K) and Fig. 7, respectively. Scale bars in all panels indicate 200μm. Note: red levels in (B, F) are modified individually to optimize signal in the neural tube and musculature, respectively. Levels in (A, E) and (D, H) are modified separately from those in (I–K) to optimize signal in the sympathetic tissue and gut wall. a, aorta; d, dorsal root ganglia; fl, forelimb; hl, hindlimb; i, intermediate layer; i/m, intermediate/marginal layers; lu, lung; m, presumptive musculature; nt, neural tube; r, rib primordia; s, stomach; v, vertebrae primordia; vz, ventricular zone.
During human embryogenesis, the rostral neuropore generally closes at CS11, and the caudal neuropore by CS12 (Müller and O’Rahilly 1986 and 1987). The presence of rostral open neural folds in both our CS12 and CS13 specimens may be the result of damage caused by the retrieval or processing of these embryos, or may signal a developmental defect. Alternatively, this may represent inherent variability amongst normal CS12 and CS13 embryos. Given that our samples were derived from elective terminations, and that the frequency of anencephaly in the general population is low, it is unlikely that two of our four specimens demonstrate signs of cerebral dysraphia. However, previous work has indicated that even in human embryos with indications of anencephaly, neural crest development proceeds with minimal defects (Müller and O’Rahilly 1984 and 1991). Thus, for our specimens we assume that despite indications of potential abnormal neural tube fusion in cranial regions – whether caused by embryo processing or developmental abnormalities - neural crest development has proceeded normally more caudally.
Immunohistochemistry
CS13 and 18 embryos were placed in 1x PBS followed by a sucrose series (5%, 15%, 15% sucrose/7.5% gelatin), and then gelatin-embedded and sectioned at 12–14μm. The CS12 specimen was thawed in 1x DEPC PBS, and processed as above; the CS15 embryo was re-embedded in OCT and cryo-sectioned. Sections were washed in 1X PBS/ddH2O (1:1) and PT, blocked for 1hr in 10% FBS/PT, and incubated overnight at room temperature with primary antibodies. Primary antibodies were diluted in 10% FBS/PT as follows: 1:5 AP-2α (mIgG2b, AB 3B5); 1:2 Msx1/2 (mIgG1, AB 4G1); 1:25 for Pax3 and Pax7 (mIgG2a and mIgG1, respectively) (all provided by Developmental Studies Hybridoma Bank); 1:1000 p75NTR (rbIgG, Promega); 1:1000 Sox9 (gpIgG) and 1:2000 Sox10 (rbIgG) (both generous gifts from the laboratory of Vivian Lee, Medical College of Wisconsin); 1:100 HNK-1 monoclonal antibody (mIgM, Tucker et al. 1984; Bronner-Fraser 1986); 1:10 TG1 (mIGM, Beverley et al. 1980; Gomperts et al. 1994); and 1:1000 TUJ1 (mIgG2a, Covance). Sections were rinsed with PT and incubated with secondary antibodies (Alexa 488/568 at 1:2000 or Cy5 at 1:500; diluted in 10% FBS/PT) overnight at room temperature. Slides were mounted with Permafluor (Thermo Scientific) and Dapi (10μg/mL). Antibody concentrations and effectiveness were tested in mouse or chick embryos (processed in sucrose series and gelatin embedded as above; E9.5–10.0 or between Hamburger and Hamilton Stages 8–20+, respectively) (see below; S3 – S6). Images were taken with a Spot SE camera and software using a Nikon Eclipse 80i microscope, and processed with Photoshop.
Results
Carnegie Stage 12
The neural crest develops in a rostral-caudal fashion during embryogenesis; as such, caudal sections demonstrate an earlier phase of NCC formation. In caudal sections of the CS12 specimen, Pax3, Sox9 and Sox10 signal occur in the premigratory neural crest of the dorsal neural tube (Fig. 1A-H). Sox9 expression can also be seen in structures unrelated to the neural crest, including the medial portions of the developing somites and notochord (Fig. 1B, D), the latter of which also expresses Sox10 (Fig. 1A). Additionally, Pax3 expression occurs in the lateral portion of the somites, and AP-2α in the surface ectoderm (Fig. 1C-D). In more rostral sections, AP-2α, Sox9, Sox10 and p75NTR are expressed in migratory NCCs that are situated either directly above the neural tube, or wedged between the neural tube, somites and surface ectoderm (Fig. 1I-L). Staining of adjacent sections with TUJ1 confirms that the majority of cells positive for the above-described NCC markers occur outside of the neuroepithelium (data not shown). Although HNK-1 colocalizes with Sox10 in occasional migratory NCCs, the neural crest generally appears HNK-1 negative (Fig. 1L, N, P). This is especially evident when HNK-1 expression is compared to that of p75NTR in similar populations of migratory NCCs (Fig. 1J, M, O). We additionally tested the TG1 monoclonal antibody – which has previously been used to identify both migratory primordial germ cells and NCC-derived sensory neuroblasts (Sieber-Blum 1989; Gomperts et al. 1994) – in similar CS12 caudal sections. However, we do not observe definitive TG1 signal within the premigratory or migratory neural crest at this stage (Fig. 1C, G and data not shown).
In rostral CS12 trunk sections, migratory neural crest cells are not observed dorsal to the neural tube; however, NCC-derived DRGs at this axial level stain positive for Pax3, AP-2α, Sox9, Sox10 and p75NTR (Fig. 2A, C-E, G-H). HNK-1 signal occurs sparsely in the DRGs, and in the mesenchyme surrounding these structures (Fig. 2B, F, J and data not shown). Subadjacent to the dermomyotome, Sox9, p75NTR and AP-2α staining are observed (Fig. 2L and data not shown). Sox10+ cells occur in this region in similar sections (Fig 2C, K). Collectively, this staining identifies NCCs that have migrated to form a presumptive peripheral nerve. More ventrally, Sox10+ (Fig. 2C, K), p75NTR+ and p75NTR+/Sox9+ co-expressing cells (Fig. 2A, I-I’) occur in the mesenchyme surrounding the aorta/mesonephric region, indicating a population of NCCs that will later contribute to either the sympathetic tissue or enteric nervous system.
Fig. 2.
Pax3, p75NTR, Sox10, Sox9 and AP-2α are expressed in neural crest derivatives of CS12 rostral trunk sections. Images in (E–K) are magnifications of boxed regions in (A–D); image in (L) is from the contralateral side of the section shown in (D). Sox10, Sox9 and AP-2α expression appear in both the NCC-derived DRGs (C–D, G–H) and peripheral nerves (C, K, L). Pax3 and p75NTR signal additionally occur in the DRGs (A, E). In the ventral mesenchyme, NCCs that will contribute to either the sympathetic tissue or enteric nervous system are identified by their expression of p75NTR (A, I–I’), Sox10 (C, K), or Sox9 (I’). Respectively, images in (F’) and (I’) are magnifications of the boxed region in (F), and the aorta/mesonephric mesenchyme of a similar section. Axial level of sections in (A–D) is indicated by the red arrow in Fig. 1Q. Scale bars in (A–D) represent 200μm; those in (E–L) 100μm. Note: levels in (H) and (L) are modified separately to optimize green and red signals in different embryonic regions. d, dorsal root ganglia; dm, dermomyotome; nt, neural tube.
In addition to their expression in NCCs, all of the above-described markers demonstrate signal outside of the neural crest territory and associated derivatives. These patterns include: (1) Sox9 expression in the presumptive sclerotome and notochord (Fig. 1B, D, J-K; Fig. 2D, H, L); (2) AP-2α expression in the surface ectoderm and mesonephric tissue (Fig. 1D, K; Fig. 2D, H, L and data not shown); and (3) Pax7 and Pax3 expression in the dermomyotome (Fig. 2A-B, E, I-J). Several markers are also expressed in subdomains of the neural tube in rostral sections, where NCC delamination appears to have ceased. The observed patterns are as follows: (1) Pax3 and Pax7 signal in the dorsal neural tube (Fig. 2A-B, E-F); (2) AP-2α expression in the dorso-lateral margins (Fig. 2D, H); (3) Sox9 expression throughout the dorsal-ventral axis of the early ventricular zone (Fig. 2D, H); (4) HNK-1 expression along the lateral edge of the neural tube, in addition to the notochord (Fig. 2B, F); and (5) p75NTR expression primarily in the ventral half of the neural tube (Fig. 2A, E). Interestingly, we also note a small patch of Pax7+ cells in the floorplate of the CS12 neural tube (Fig. 2F-F’), which conflicts with reports that notochordal signals downregulate Pax7 expression (Goulding et al. 1994; Otto et al. 2006). To our knowledge, this represents a novel staining pattern not observed in the mouse or chick. However, faint Pax3/7 staining has been reported in the floorplate of zebrafish embryos (Hammond et al. 2007). In more caudal CS12 sections, where migratory NCCs are observed adjacent to the neural tube, NCC marker expression is generally not seen in the neuroepithelium (Fig. 1I-L). However, p75NTR signal occurs in the neural tube at this axial level, with a dorsal expression domain extended compared to the pattern observed in more rostral trunk sections (Fig. 1J; Fig. 2A, E). HNK-1 signal also occurs sparsely along the lateral edges of the neural tube in these sections (Fig. 1L).
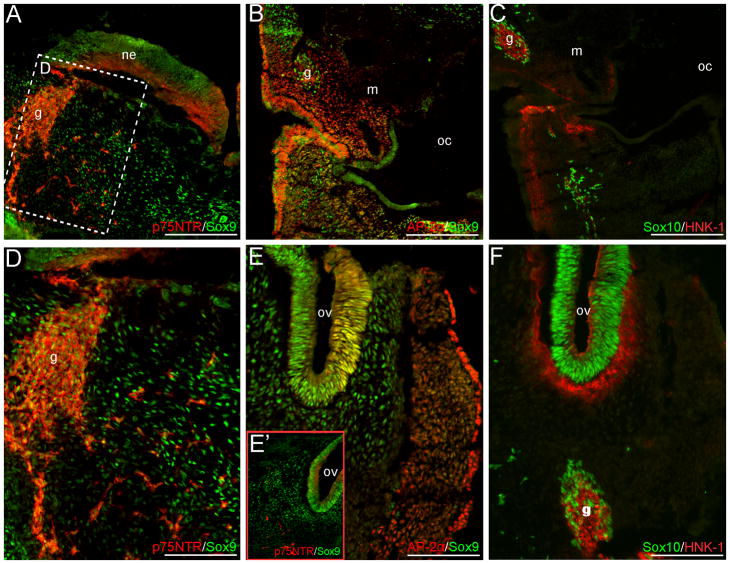
In cranial sections, several NCC markers are maintained in neural crest derivatives. Both AP-2α and Sox9 expression occur in portions of the neural crest-populated branchial arches, and the cranial mesenchyme (Fig. 3A–B, D–E’). Few Sox10+ or p75NTR+ cells can also be observed in this region (Fig. 3A, C–D, F). However, no clear Pax7 signal was detected in the cranial mesenchyme (data not shown). Cranial ganglia condensations or their associated nerves are positive for AP-2α, Sox9, Sox10, HNK-1 and p75NTR (Fig. 3A–D, F). In addition, Sox9, Sox10, AP-2α and p75NTR are expressed in the otic vesicle (Fig. 3E–F and data not shown). Strong AP-2α signal also occurs in the surface ectoderm and presumptive placodal epithelium (Fig. 3B, E), whereas HNK-1 staining can be seen in the mesenchyme subadjacent to the placodes and surrounding the otic vesicle (Fig. 3C, F). Within the cranial neuroepithelium, Pax7 and Sox9 signal can be observed in the early ventricular zone, with HNK-1 and p75NTR expression appearing in the lateral margins (Fig. 3A and data not shown).
Fig. 3.
NCC markers are expressed in the mesenchyme or neural crest derivatives of cranial CS12 sections. Sox9 and AP-2α signal occur within the NCC-populated cranial mesenchyme or branchial arches (A–B, D–E’); panel in (D) is a higher magnification of the boxed region in (A). Additional Sox10+ and p75NTR+ cells are observed within this region (A, C–D, E’–F). NCC-derived cranial ganglia condensations or associated nerves express p75NTR, Sox9, Sox10, AP-2α and HNK-1 (A–D, F). Within the otic vesicles, Sox9, AP-2α, Sox10 and p75NTR signal occur (E–F). Axial level of sections is indicated by the green arrow in Fig. 1Q. Scale bars in (A–C) represent 200μm; those in (D–F) 100μm. g, ganglia condensation or associated nerve; m, mandibular/maxillay process; ne, neuroepithelium; oc, oral-pharyngeal cavity; ov, otic vesicle.
Carnegie Stage 13
In less-developed caudal sections of the CS13 embryo, signal from Pax7, Sox9 and Sox10 are detected overlying the dorsal neural tube, suggesting the presence of few migratory NCCs (Fig. 4A–C); sparse HNK-1 signal also occurs in this region (Fig. 4A). Lateral to this, Sox9, Sox10, Pax7 and p75NTR are expressed in NCCs situated between the neural tube and surface ectoderm, migrating to populate the DRGs (Fig. 4A–D). Within regions of the forming DRGs, Pax7, Sox9, Sox10, and p75NTR signal occur (Fig. 4A–D). In both the migratory neural crest and presumptive DRGs, HNK-1 does not appear to robustly co-localize with other NCC markers (Fig. 4A). In more rostral trunk sections, Sox10 and p75NTR are maintained in the DRGs (S1. A, C, E, G), and are also seen in the mesenchyme surrounding the aorta and dorsal to the mesonephric tissue (S1. A, C, K) - suggestive of NCCs that will contribute to either the sympathetic tissue or enteric ganglia. Faint HNK-1 staining is also observed in this region (S1. B). At this same axial level, NCC marker staining within non-neural crest derivatives is largely consistent with that observed at CS12 (S1). Additionally, we note that Pax3 signal is suggested in both the dorsal neural tube and dermomyotome at CS13, although at a weaker level than that observed at CS12 (data not shown).
Despite the lack of definitive Pax7 staining in the facial mesenchyme at CS12, Pax7+ cells are observed in the mesenchyme at the level of the unfused neuroepithelium in CS13 cranial sections; signal is also observed in the dorsal tips of the open neural plate (Fig. 4G). HNK-1 staining occurs within the mesenchyme at this axial level, although its signal generally appears in close association with the ventral side of the unfused neuroepithelium (Fig. 4G). Additionally, punctate Sox10 signal is seen near the eye primordium (Fig. 4F). In slightly caudal sections, Sox10+ cells are also observed in NCC-derived ganglia condensations (Fig. 4E’ and data not shown). Within the neuroepithelium of similar sections, Pax7 expression occurs in the dorsal neural tube (Fig. 4E), with AP-2α and HNK-1 signal appearing along the lateral edges (Fig. 4E and data not shown).
Carnegie Stage 15
At CS15, Pax3, Sox10 and p75NTR are expressed in the neural crest-derived DRGs of trunk sections, with Pax3 localizing dorso-medially (Fig. 5A, C, E, G). We also note the expression of AP-2α and Sox9 in the dorso-medial or lateral margins of the DRGs at this stage (Fig. 5D, H), similar to the pattern seen for Sox10 (Fig. 5C, G). These results are consistent with previous reports of Pax3, Sox10 and AP-2α signal in the DRGs of an 8-week old embryo (Gershon et al. 2005); Sox9 expression within CS18 DRGs has also been noted (Benko et al. 2009). Few HNK-1+ cells are also observed (Fig. 5B, F). As in the CS12 sample, Sox10 and p75NTR expression appear within the presumptive peripheral nerves (Fig. 5A, C, E, G), and in more rostral sections, AP-2α+/Sox9+ cells are seen within these structures (Fig. 5P’). Ventrally, Sox10+, p75NTR+, and Sox9+/AP-2α+ cells occur within the presumptive sympathetic tissue surrounding the aorta (Fig. 5A, C, D, O, P). Within the wall of the gut, prospective enteric NCCs are positive for AP-2α, p75NTR, HNK-1, Sox9, and Sox10 (Fig. 5Q–S’). Finally, we also note the expression of Sox10, Pax3, AP-2α and Sox9 in few cells located dorsal, or dorso-lateral to, the neural tube (Fig. 5I, K–L). As roles for both Pax3 and Sox10 have been demonstrated in melanocyte development (Auerbach 1954; Lane and Liu 1984; Southard-Smith et al. 1998), it is possible that these Pax3+ or Sox10+ cells are melanoblasts. Alternatively, Pax3+ cells in this area could go on to form the presumptive musculature. The additional expression of AP-2α, and Sox9 signal in similar regions, may indicate a population of late-migrating NCCs, or may identify these cells as contributing to either the surface ectoderm or vertebrae primordia.
The expression patterns of NCC markers outside of neural crest-derivatives at CS15 is largely consistent with those observed at CS12 and CS13 (Fig. 2, S1). However, we note the ventral extension of AP-2α expression within the intermediate zone of the neuroepithelium (Fig. 5D, H). The CS15 specimen also marks the first time that definitive Msx1/2 expression is observed among any of our samples. Although suggestive Msx1/2 staining at CS12 was detected (data not shown), strong background levels limit our ability identify this as real signal. At CS15, Msx1/2 signal occurs within: (1) the dorsal-most aspect of the ventricular zone of the neural tube (Fig. 5C, G, K); (2) the surface ectoderm and/or subectodermally in regions dorso-lateral to the neural tube (Fig. 5C, G), consistent with the pattern of expression reported for Msx1 in dermal progenitors (Houzelstein et al. 2000); and (3) the distal tip of the limb mesenchyme (Fig. 5C’), supporting a role for Msx1/2 in human limb development, as has been demonstrated in mouse mutants (Lallemand et al. 2005).
Carnegie Stage 18
By CS18, migratory NCCs are no longer observed dorsal or adjacent to the neural tube; however, the expression of several markers is observed in neural crest-populated regions. In CS18 trunk sections, Sox10 and p75NTR signal are maintained in the DRGs (Fig. 6D, E). Faint HNK-1 signal can be observed in the DRGs at this axial level, and in more rostral sections (Fig. 6F and data not shown). Sox10 staining is also observed in the DRG-associated nerve roots (Fig. 6D). Ventrally, both Sox10 and p75NTR expression occur in the NCC-derived enteric ganglia of the stomach (Fig. 6J–J’), and in the sympathetic tissue surrounding the aorta (Fig. 6I, K).
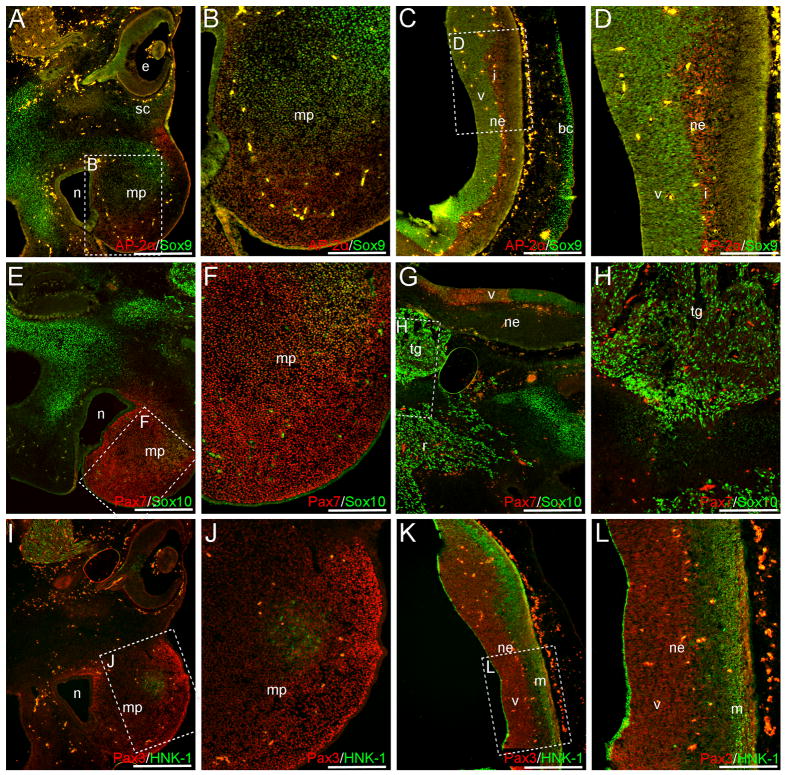
Cranially, Sox9, Sox10, AP-2α, Pax3 and Pax7 are all observed in the neural crest-rich maxillary process (Fig. 7A–B, E–F, I–J). Strong Pax7 expression is observed throughout this prominence (Fig. 7E–F), and robust AP-2α and Pax3 signal appear restricted to the lateral margin, or the mesenchyme surrounding the nasal cavity (Fig. 7A-B; I–J). In the center of the maxillary process, Pax3+ and Sox9+/AP2α+ cells are observed (Fig. 7A–B, I–J), and a circular patch of HNK-1+/Pax3+ cells occurs more laterally (Fig. 7I–J). Outside of this prominence, both Sox9 and Sox10 are expressed in the facial mesenchyme (Fig. 7A, C, E, G), with Sox9 signal also observed in the presumptive scleral cartilage (Fig. 7A). Additional NCC-derived structures - the presumptive cranial ganglia – are positive for both Sox10 and HNK-1 (Fig. 7G–I), with Sox10 signal also occurring in the associated nerve roots (Fig. 7G).
Fig. 7.
Neural crest markers are expressed in the maxillary process at CS 18. AP-2α (A, B), Sox9 (A, B), Sox10 (E, F), Pax7 (E, F), and Pax3 (I, J) signal are observed within the NCC-populated maxillary process. A circular patch of HNK-1+ cells also exists in this region (I, J). Additional Sox9 and Sox10 signal are observed throughout the facial mesenchyme or future skeletal elements of the skull (A, C, E, G). The cranial ganglia are positive for both Sox10 (G, H) and HNK-1 (I), with Sox10 signal also appearing in the associated nerve roots (G). Axial level of sections in (A, C, E, G, I, K) is indicated by blue arrow in Fig. 6L. Images in (B, D, F, H, J, L) are higher power magnifications of boxed regions in (A, C, E, G, I, K). Scale bars in (A, C, E, G, I, K) represent 500μm, those in (B, D, F, H, J, L) represent 200μm. Note: levels in (E, G), (F, H), (I, K) and (J, L) are modified individually to optimize red and green signals. bc, brain case primordia; e, eye; i, intermediate layer; m, marginal layer; mp, maxillary process; n, nasal cavity; ne, neuroepithelium; r, cranial ganglia-associated nerve root; sc, presumptive scleral cartilage; tg, trigeminal ganglia; v, ventricular zone.
Within the trunk neuroepithelium of the CS18 sample, additional marker expression patterns outside of NCC derivatives can be seen. Within the trunk neuroepithelium, these patterns include: (1) Pax3+ and Pax7+ cells in the dorsal ventricular layer, with Pax7 expression also seen in small patches of cells in both the floor- and roofplate (Fig. 6A–B); (2) Sox9 and Sox10 staining of the ventricular zone (Fig. 6C–D); (3) p75NTR expression throughout the intermediate and marginal layers, with strong signal in the dorso-lateral region (Fig. 6A); (4) few AP-2α+ cells within the dorsal and ventral intermediate zone (Fig. 6C); and (5) HNK-1 staining of the intermediate and mantle layers (Fig. 6B). In addition to the neuroepithelium, marker expression is seen in other regions, including: (1) Pax7 staining in the presumptive musculature surrounding the rib and vertebrae primordia, with few Pax3+ cells occurring in the dorso-medial tips of this region (Fig. 6E–F); (2) p75NTR expression in the trunk musculature and epidermis (Fig. 6E and data not shown); (3) Sox9 and Sox10 expression in the vertebrae and rib cartilage primordia (Fig. 6G–H); and (4) AP-2α expression in the mesenchyme of the hindlimb (S2. A–B).
The signal patterns observed within the cranial neuroepithelium include: (1) AP-2α expression in the intermediate layer (Fig. 7C–D); (2) Pax3, Pax7, Sox9 and Sox10 staining of the ventricular zone (Fig. 7C–D, G, K–L), with a boundary of Sox10 and Pax7 signal occurring ventrally (Fig. 7G); and (3) HNK-1 expression along the marginal layer (Fig. 7K–L). Additionally, Pax7+ cells occur in a small swath of epithelial cells located outside of the nasal cavity (Fig. 7E and data not shown).
Discussion
The neural crest has extensive differentiation capabilities, as evidenced by its ability to generate neurons and supportive cells of the peripheral nervous system, melanocytes, endocrine cells, and a set of derivatives collectively known as mesectoderm (which includes muscle, bone and cartilage of the head). Unfortunately, the astonishing capacity of the neural crest to generate such a broad spectrum of derivatives is linked to several human pathologies (see below). Today, after almost 150 years of intense NCC research in model organisms, we have gained much knowledge regarding the tissues and molecules involved in neural crest development. These molecules, in turn, have been integrated into a gene regulatory network responsible for the formation, migration, and differentiation of NCCs. Despite this, we remain confronted by a void of information regarding hNCC development. Specifically, we know very little about the expression – much less the possible function – of NCC-related molecules during early human embryogenesis. While the basic biology of neural crest development is very similar between model organisms, small differences exist. Effects of such differences could potentially have dramatic consequences in NCC development. It is therefore essential to establish the precise participation of specific molecules in hNCC development. This, in turn, will improve our understanding of neural crest-related pathologies, and enable the generation of better diagnostic and therapeutic tools to ameliorate their effects.
Within higher vertebrates, neural crest development was thought to start in concurrence with neurulation, and recent studies within avian embryos have demonstrated that NCC specification is underway during gastrulation (Basch et al. 2006; Patthey et al. 2008 and 2009). Equivalent stages of human embryogenesis occur during the first three weeks of gestation; however, most pregnancies are not recognized prior to four weeks, making the study of early embryonic stages particularly difficult. Here, with a small collection of human embryos, we present for the first time the expression data for several NCC markers identifying premigratory, migratory and differentiating hNCCs.
Marker Expression in the Human Neural Crest and Its Derivatives
Msx1/2, Pax3, Pax7, AP-2, Sox9, Sox10, HNK-1 and p75NTR have all been implicated in neural crest development within model organisms, based on their expression patterns and/or functional studies. These molecules appear in neural crest precursors, pre-migratory or migratory NCCs, and/or neural crest derivatives such as: the facial mesenchyme, cranial ganglia, DRGs or components of the enteric nervous system (for specific expression patterns observed in model organisms, see below) (Msx1/2: Hill et al. 1989; Robert et al. 1989; Yokouchi et al. 1991; MacKenzie et al. 1992; Foerst-Potts and Sadler 1997; Tríbulo et al. 2003; Ishii et al. 2005; Pax3 and Pax7: Goulding et al. 1991; Jostes et al. 1991; Tremblay et al. 1995; Mansouri et al. 1996; Dottori et al. 2001; Lacosta et al. 2005; Basch et al. 2006; Otto et al. 2006; Hammond et al. 2007; Minchin and Hughes 2008; Sox9 and Sox10: Ng et al. 1997; Zhao et al. 1997; Kuhlbrodt et al. 1998; Pusch et al. 1998; Southard-Smith et al. 1998; Cheng et al. 2000; Dutton et al. 2001; Li et al. 2002; Cheung and Briscoe 2003; Ishii et al. 2005; McKeown et al. 2005; Suzuki et al. 2006; AP-2: Mitchell et al. 1991; Zhang et al. 1996; Shen et al. 1997; Ishii et al. 2005; Li and Cornell 2007; HNK-1: Tucker et al. 1984; Tucker et al. 1988; Bronner-Fraser 1986; p75: Wilson et al. 2004; Anderson et al. 2006). In addition to their demonstration of in-vivo expression, few markers have been used to identify the neural crest or its precursors in different experimental paradigms. This is particularly the case for p75, which has been used to: (1) identify NCCs within avian neural fold/tube cultures (Abzhanov et al. 2003); (2) isolate neural crest precursors from rat embryonic gut and sciatic nerve preparations (Morrison et al. 1999; Bixby et al. 2002); or (3) to enrich human ES-derived crest cells (Lee et al. 2007; Jiang et al. 2009; Bajpai et al. 2010).
Despite widespread work evaluating neural crest development in model organisms, the human neural crest remains largely an enigma. Currently, only a handful of studies have evaluated neural crest marker expression within human embryos, with a focus on either the neural crest or its derivatives. These have demonstrated: (1) Pax3 expression in the neural groove or neural tube as early as CS10 and CS12, and later within the DRGs and connective tissue of the jaws (Gérard et al. 1995; Terzić and Saraga-Babić 1999; Fougerousse et al. 2002; Gershon et al. 2005); (2) Sox10 signal in the migratory neural crest of a 4-week specimen, and both Sox10 and Sox9 expression in components of the peripheral nervous system and facial mesenchyme (Bondurand et al. 1998; Gershon et al. 2005; Benko et al. 2009); (3) AP-2α staining within the DRGs of an 8-week specimen (Gershon et al. 2005); (4) NC-1 (HNK-1) expression in regions dorso-lateral to the neural tube at 5-weeks, and later signal in the DRGs (Tucker et al. 1984; Tucker et al. 1988); and (5) p75 immunoreactivity in the spinal cord at 9-weeks (Josephson 2001). Despite evidence supporting the role of Pax7 in neural crest development of the chick and mouse (Mansouri et al. 1996; Basch et al. 2006), when Pax7 expression was assessed in human embryo sections, signal was observed only in the presumptive musculature and not within the neural tube or NCC-related structures (Gershon et al. 2005). In addition to these in-vivo studies, human ES-derived NCCs or hNCC lines generated from embryonic neural tube explants have been shown to express p75, Msx1, Pax3, Sox9 and Sox10, along with other NCC markers (Pomp et al. 2005; Brokhman et al. 2008; Lee et al. 2007; Thomas et al. 2008; Jiang et al. 2009; Bajpai et al. 2010). However, the lack of information regarding in-vivo human NCC development precludes the categorical validation of the neural crest status of such cells, as the criteria used to identify them as NCCs is based in model organism - rather than human - NCC expression.
Here we present the novel expression of several neural crest markers in early human embryos, specifically revealing expression patterns in premigratory and early migratory NCCs that have never before been reported at such early stages. Our work demonstrates the expression of Pax3, Sox9 and Sox10 in premigratory NCCs at CS12 (Fig. 1A–H). In both the CS12 and CS13 specimens, we note Sox9, Sox10 and p75NTR expression in the migratory neural crest immediately dorsal or adjacent to the neural tube (Fig. 1I–L; Fig. 4B–D). AP-2α signal also occurs in this cell population at CS12 (Fig. 1K), as does Pax7 at CS13 (Fig. 4A). Few HNK-1/Sox10 or HNK-1/Pax7 co-positive cells may exist in the CS12 or CS13 migratory neural crest, respectively; however, the majority of migratory NCCs appear HNK-1 negative at both CS12 and CS13 (Fig. 1L, N, P; Fig. 4A). From CS12 to CS15, a population of NCCs is observed at the level of the aorta and in the mesenchyme dorsal to the mesonephric tissue, as evidenced by Sox10 or p75NTR staining (Fig. 2A, C, I–I’, K; S1. A, C, K; Fig. 5A, C, O). In this region, Sox9+/p75NTR+ cells are seen at CS12 (Fig. 2I’), HNK-1 expression is observed at CS13 (S1. B), and AP-2α+/Sox9+ cells occur at CS15 (Fig. 5P). Additionally, few Sox9+/AP-2α+, p75NTR+ or Sox10+ cells occur at CS12 subadjacent to the dermomyotome (Fig. 2C, K, L and data not shown). This staining likely identifies NCCs that have migrated ventrally to contribute to a peripheral nerve. The expression of all of these markers is maintained in the peripheral nerves at CS15 (Fig. 5A, C, E, G, M, P’).
Complementary to our demonstration of marker expression within the premigratory or migratory human neural crest, we also note the presence of NCC marker staining in components of the NCC-derived peripheral nervous system – specifically the dorsal root or enteric ganglia. Within all four specimens, Sox10 and p75NTR are expressed within the DRGs (Fig. 2A, C, E, G; Fig. 4B, D; Fig. 5A, C, E, G; Fig. 6D, E; S1. A, C, E, G). Furthermore, at CS12, Pax3, AP-2α and Sox9 are observed within these structures (Fig. 2A, D, E, H). By CS13, Pax7, Sox9, Sox10 and p75NTR expression occur in portions of the forming DRGs at caudal axial levels (Fig. 4A–D). Despite our failure to observe definitive AP-2α signal in the DRGs at CS13, the expression of this marker, in addition to Sox9, Pax3 and few HNK-1+ cells, are observed within the CS15 DRGs (Fig. 5A–B, D, E–F, H). We also note the expression of p75NTR, HNK-1, Sox10, Sox9 and AP-2α in the presumptive enteric neural crest populating the wall of the gut at CS15 (Fig. 5Q–S’). By CS18, Sox10 and p75NTR are maintained within the presumptive NCC-derived enteric ganglia of the stomach (Fig. 6J–J’). Previously, Sox10 signal has been reported throughout the peripheral nervous system - including the presumptive enteric ganglia - of early human embryos (Bondurand et al. 1998; Gershon et al. 2005). Sox9 has also been noted within either the DRGs or cranial ganglia of CS15 and/or CS18 specimens (Benko et al. 2009).
In more rostral CS12 sections, AP-2α, Sox9, Sox10, HNK-1 and p75NTR signal occur in the cranial ganglia condensations or associated nerves at CS12 (Fig. 3A–D, F). By CS18, only Sox10 and HNK-1 are expressed in the cranial ganglia, with Sox10 also occurring within the associated nerve roots (Fig. 7G–I). In addition to the cranial ganglia, several neural crest markers are expressed within portions of the facial mesenchyme at either CS12 or CS18 (Fig. 3; Fig. 7). In the former, AP-2α and Sox9 signal occur in either the cranial mesenchyme or branchial arches (Fig. 3A–B, D–E); occasional Sox10 or p75NTR positive cells also appear in this region (Fig. 3A, C–D, E’–F). At CS18, Pax3, Pax7, Sox9, Sox10 and AP-2α are all observed within the neural crest-populated maxillary process (Fig. 7A–B, E–F, I–J); few HNK-1+/Pax3+ cells also occur in a circular patch within this prominence (Fig. 7I–J). Sox9 and Sox10 expression appear within additional components of the facial mesenchyme at this stage (Fig. 7A, C, E, G). Although Sox10 does not persist in the cranial mesenchyme of model organisms at later stages (Kuhlbrodt et al. 1998; Cheng et al. 2000; Suzuki et al. 2006), our observation of Sox9 and Sox10 expression in both the facial mesenchyme and cranial ganglia is consistent with previous analyses of early human embryos (Bondurand et al. 1998; Benko et al. 2009).
Interspecies Comparison of Marker Expression in NCCs or Their Derivatives
Our observation of several NCC markers within migratory hNCCs from CS12–13 is largely similar to what has been reported in murine or avian model systems. Here, we discuss expression patterns for Pax3, Pax7, AP-2α, Sox9, Sox10, HNK-1 and p75NTR we have observed in either mouse E9.5–10.0 or chick HH.18 sections, with special focus on caudal axial levels. Consistent with previous reports (Goulding et al. 1991; Zhao et al. 1997; Kuhlbrodt et al. 1998; Pusch et al. 1998; Southard-Smith et al. 1998; Dottori et al. 2001), we have observed Pax3, Sox9 and Sox10 expression within either the premigratory or migratory neural crest of E9.5–10.0 mouse trunk sections; however, definitive Pax7, Msx1/2 and AP-2α signal are not seen in either of these regions (S3. A–C). Despite the lack of AP-2α expression in the neural crest of our trunk E9.5–10.0 sections, AP-2 signal has been demonstrated in potential premigratory NCCs at early stages (E8.5), and later in the DRGs and associated nerves (Mitchell et al. 1991). Furthermore, although we do not observe Pax7 or Msx1/2 within trunk mouse migratory NCCs, the expression of these markers has been reported in either migratory cranial NCCs or the craniofacial/branchial arch mesenchyme, as has Pax3, AP-2 and Sox9 (Hill et al. 1989; Robert et al. 1989; Goulding et al. 1991; Mitchell et al. 1991; MacKenzie et al. 1992; Tremblay et al. 1995; Mansouri et al. 1996; Zhang et al. 1996; Foerst-Potts and Sadler 1997; Ng et al. 1997; Zhao et al. 1997; Ishii et al. 2005). Interestingly, within our E9.5–10.0 cranial sections we observe Sox10 expression - in addition to AP-2α and Sox9 - in the branchial arch mesenchyme; Sox9 and Sox10 signal also occur in the otic vesicle (S4. A–A’, B–B’). Although HNK-1/NC-1 does not recognize the mouse neural crest (Tucker et al. 1988), p75 has been reported in murine trunk migratory NCCs, as well as the cranial neural crest and presumptive enteric tissue (Wilson et al. 2004; Anderson et al. 2006).
As in the mouse, Sox9 and Sox10 are expressed within the trunk migratory neural crest of caudal HH. St. 18 chick sections; however, Pax7, HNK-1 and AP-2α are also observed within this cell population (S5. E–H). Additionally, Pax7 and Sox9 signal occur in potential premigratory NCCs of the chick dorsal neural tube (S5. E–E’, G). In more developed (rostral) trunk sections, Pax7, HNK-1, Sox9, Sox10 and AP-2α are all observed within the forming DRGs; Sox10 and HNK-1 are also seen in the presumptive sympathetic tissue surrounding the aorta (S5. A–D and data not shown). Although few Sox9+ cells are observed abutting the aorta, it is difficult to distinguish any sympathetic tissue from the sclerotome without additional markers (S5. C). At later stages (HH. St. 20/20+), p75NTR is also apparent within the DRGs, as are Sox10 and HNK-1 (S6. A–B’). Although definitive Pax3 signal is difficult to ascertain in the neural crest/NCC-derivatives of our HH. St. 18 sections (data not shown), previous studies have demonstrated Pax3 expression in trunk migratory NCCs at similar stages (Lacosta et al. 2005). Furthermore, at HH. St. 17, Msx1 has been reported in both the trunk neural crest and craniofacial mesenchyme (Yokouchi et al. 1991).
Relevance of NCC Marker Expression to Human Neurocristopathies
The neural crest is of particular clinical significance, as several human pathologies result from aberrant NCC development. These NCC-related diseases are commonly referred to as neurocristopathies, and they encompass: (1) craniofacial malformations including cleft lip and palate; (2) tumors affecting the peripheral nervous system or melanocytes (i.e. schwannoma, neuroblastoma, and melanoma); and (3) cardiac defects generally targeting the outflow tract or arteries associated with the aortic arch (for review, see Creazzo et al. 1998; Farlie et al. 2004; Sarnat and Flores-Sarnat 2005; Etchevers et al. 2006). Specific pathologies – such as Hirschsprung disease (HSCR) and Waardenburg syndrome (WS) – are also attributed to neural crest defects; respectively, these diseases are characterized by aganglionosis of portions of the gastro-intestinal tract (implicating the enteric neural crest), or by pigmentation and hearing defects (for review, see Farlie et al. 2004; Etchevers et al. 2006; Tam and Garcia-Barceló 2009; Pingault et al. 2010).
Although the genetic causes of many neurocristopathies require clarification, several neural crest-associated genes have been implicated in these diseases. Within murine models, AP-2, Msx1/2, Pax7, Pax3, and Sox9 mutant mice demonstrate an array of craniofacial malformations ranging in severity (AP-2: Zhang et al. 1996: Msx1/2: Ishii et al. 2005; Pax7: Mansouri et al. 1996; Pax3: Tremblay et al. 1995 and 1998; Sox9: Bi et al. 2001; Mori-Akiyama et al. 2003). These include: orofacial clefts, defects in the craniofacial skeletal elements or branchial arch derivatives, and perturbations of the cranial ganglia and associated nerves. Additional pigmentation or peripheral nervous system defects – targeting either the dorsal root ganglia or enteric ganglia – are observed in Pax3 and Sox10 mutants (Auerbach 1954; Lane and Liu 1984; Tremblay et al. 1995; Conway et al. 1997; Southard-Smith et al. 1998). Finally, NCC-associated cardiac malformations have also been noted in mice carrying mutations in either Pax3 or Msx1/2 (Conway et al. 1997; Ishii et al. 2005).
Several of the above NCC-related genes have also been implicated in human neurocristopathies. For example, mutations in Msx1 and AP-2α, or the improper regulation of Sox9, can result in cleft lip or palate in humans (van den Boogaard et al. 2000; Velagaleti et al. 2005; Jakobsen et al. 2007; Milunsky et al. 2008; Benko et al. 2009). These orofacial clefts – specifically those observed in cases of AP-2α mutations or Sox9 dysregulation – are typically symptoms of a larger disorder, such as Branchio-oculo-facial syndrome or Pierre Robin syndrome (Velagaleti et al. 2005; Jakobsen et al. 2007; Milunsky et al. 2008; Benko et al. 2009). Furthermore, Pax3 and Sox10 have been shown to play a role in one or more of the variants of WS, with Sox10 mutations often resulting in Type IV WS –in which individuals exhibit both WS and HSCR symptoms (Hoth et al. 1993; Bondurand et al. 2007; Pingault et al. 2010). Finally, the expression of several NCC markers has been demonstrated in tumors affecting neural crest-derived tissues. Different combinations of Pax3, Pax7, Sox10 and AP-2α signal occur in neuroblastomas, schwannomas and additional PNS tumors (Gershon et al. 2005). Furthermore, elevated levels of Pax3 expression are also classically associated with melanoma, with Pax3 specifically facilitating cell survival in such tumors; however, Pax7, AP-2α, and Sox10 signal have all been shown in either melanoma cell lines or tumor sections (Scholl et al. 2001; Muratovska et al. 2003; Gershon et al. 2005).
As evidenced above, many of the neural crest markers we have analyzed carry profound clinical significance, due to their role in the development of neurocristopathies. Importantly, we have shown that Pax3, Sox9, Sox10, and AP-2α - all of which have been implicated in one or more human pathologies (see above)– are active in the neural crest as early as CS12 or CS13 (Fig. 1; Fig. 4B, C). Their additional expression within the DRGs at these stages, and later at either CS15 or 18, suggests a role for these markers in human PNS defects –such as those seen in the dorsal root ganglia of Pax3 or Sox10 mutant mice (Auerbach 1954; Tremblay et al. 1995; Conway et al. 1997; Southard-Smith et al. 1998). Furthermore, our observation of Sox9 and AP-2α within portions of the cranial mesenchyme or maxillary process confirms the role of these genes in human cranial NCC - and subsequent craniofacial - development (Fig. 3A–B, D–E’; 7A–C). As mutations in Pax3 can also result in craniofacial defects (i.e. dystopia canthorum) associated with either WS I and III (for review, see Pingault et al. 2010), the occurrence of Pax3 in the maxillary process at CS18 confirms a role for this marker in human craniofacial morphogenesis (Fig. 7I–J).
Interestingly, we have demonstrated the expression of Pax7 in the CS13 migratory neural crest, in addition to the maxillary process at CS18 (Fig. 4A; Fig. 7E–F). To our knowledge, Pax7 expression has only been demonstrated in PNS or melanocyte tumors (Muratovska et al. 2003; Gershon et al. 2005), and has not been implicated in any human craniofacial malformations. However, given the mild jaw and nasal defects seen in Pax7 mutant mice (Mansouri et al. 1996), in addition to our observation of Pax7 signal in the CS18 maxillary process (Fig. 7E–F), it is possible that Pax7 may be involved in the development of human orofacial clefts or additional craniofacial disturbances. In agreement with this view, a recent study suggests a strong correlation of maternally transmitted single nucleotide polymorphisms of Pax7 and Pax3 with cleft lip with or without cleft palate (Sull et al 2009). Furthermore, from CS12–18, we have shown the expression of p75NTR within the early migratory neural crest, cranial or dorsal root ganglia, sympathetic tissue, and gut wall (Fig. 1J, M, O; Fig. 2A, E, I–I’; Fig. 3A, D; Fig. 4D; Fig. 5A, E, Q; Fig. 6E, I, J). Previously, it was demonstrated that p75NTR mutant mice have perturbations of PNS development – specifically affecting Schwann cells or DRG-associated neurons (von Schack et al. 2001). Given our observed expression patterns for p75NTR within CS12–18 embryos, paired with the phenotypes seen in mutant mice, it is likely that mutations in p75NTR will affect human PNS development – targeting either the dorsal root, sympathetic or enteric ganglia. Additionally, Sox9 and AP-2α also appear to play a role in enteric nervous system development, due to their expression within the gut wall at CS15 (Fig. 5S); thus, mutations in these genes may also contribute to malformations of the human enteric ganglia.
Marker Expression in Non-NCC Derivatives of Human Embryos
In addition to their expression within the neural crest or its derivatives, all of the markers studied herein label additional cell types in non-NCC related structures; thus, no one marker is specifically restricted to NCCs. This is exemplified by AP-2, which has been shown to have a role in both facial outgrowth and limb development, in addition to neural crest development (Zhang et al. 1996; Shen et al. 1997). Consistent with reports in model organisms (Mitchell et al. 1991; Shen et al. 1997), we have observed AP-2α expression in the surface ectoderm (Fig. 1D, K; Fig. 2D, H, L; Fig. 5D, H), mesonephric tissue (Fig. 2D; Fig. 5D), dorso-lateral margins of the neuroepithelium (Fig. 2D, H; S1. D, H), and limb mesenchyme of early human embryos (S2. A–B). Below follows a discussion of additional regions - outside of the neural crest - where NCC-markers are expressed from CS12-CS15 and CS18. These include the neuroepithelium, presumptive musculature, and cartilaginous or future skeletal elements.
Neuroepithelium
All neural crest markers used within this work are expressed in some region of the neuroepithelium. Previous reports of NCC-marker expression within the neuroepithelium of human embryos have demonstrated Pax3 signal in the dorsal neural tube or ventricular layer (Gérard et al. 1995; Terzić and Saraga-Babić 1999; Gershon et al. 2005), AP-2α expression in the dorso-lateral margins (Gerhson et al. 2005), and HNK-1/NC-1 staining along the lateral edge (Tucker et al. 1988). Although Sox10 expression has been evaluated in human specimens, at no early time point has signal been demonstrated within the neural tube (Bondurand et al. 1998; Gershon et al. 2005); however, Sox9 signal has been noted within either the neuroepithelium and/or ventricular layer of embryos between CS13 and CS18 (Benko et al. 2009). These results are largely consistent with the patterns observed in model organisms (Pax3: Goulding et al. 1991; Lacosta et al. 2005; Otto et al. 2006; AP-2: Mitchell et al. 1991; Sox9: Ng et al. 1997; Zhao et al. 1997; Suzuki et al. 2006). However, within both the mouse and chick, Pax7 expression has been demonstrated in regions of the dorsal neural tube or ventricular layer (Jostes et al. 1991; Lacosta et al. 2005; Otto et al. 2006). Sox10 staining of the ventricular zone has also been reported in the avian system (Cheng et al. 2000).
In CS12 or CS13 trunk sections rostral to areas of NCC migration, we observe the following expression pattern within the neuroepithelium: (1) Pax3 or Pax7 signal within the dorsal neural tube, with Pax7 expression also observed in the floorplate (Fig. 2A–B, E–F’; S1. B, F): (2) AP-2α signal within the dorso-lateral margins (Fig. 2D, H; S1. D, H); (3) HNK-1 expression along the lateral rim (Fig. 2B, F; S1. B, F, J); and (4) p75NTR expression primarily in the ventral neuroepithelium (Fig. 2A, E; S1. A). At CS12, Sox9 staining also occurs throughout the dorsal-ventral axis of the early ventricular zone (Fig. 2D, H); however, at neither CS12 or CS13 is Sox10 expression seen within the trunk neural tube (Fig. 2C, G; S1. C, G). By CS15, we observe: (1) Pax3 and Pax7 signal within the dorsal ventricular zone, with few Pax7+ cells occurring in the floorplate (Fig. 5A–B, E–F, I–J); (2) AP-2α+ cells within the intermediate zone (Fig. 5D, H); (3) HNK-1 staining along the lateral margin of the spinal cord, extending into portions of the intermediate zone (Fig. 5B, F, J); (4) Sox9 expression throughout the dorsal-ventral axis of the ventricular zone (Fig. 5D, H, L); and (5) p75NTR staining throughout the majority of neuroepithelium, except in regions of the ventricular zone (Fig. 5A, E, I). Very faint Sox10 signal also occurs in the ventricular zone at this stage (Fig. 5C, G). Additionally, CS15 marks the first of our specimens in which definitive Msx1/2 expression is observed, with signal occurring in the dorsal-most aspect of the ventricular zone (Fig. 5C, G, K). The expression patterns of NCC markers within the neuroepithelium at CS18 are similar to those observed at CS15 (Fig. 6). Collectively, these results are largely consistent with the expression patterns observed in model organisms (see above), and studies assessing Pax3, AP-2α, Sox9 and HNK-1/NC-1 signal within human embryos (Tucker et al. 1988; Gérard et al. 1995; Terzić and Saraga-Babić 1999; Fougerousse et al. 2002; Gershon et al. 2005; Benko et al. 2009).
Presumptive Musculature
In addition to their expression within the neuroepithelium, both Pax3 and Pax7 have been implicated in muscle development (for review, see Lang et al. 2007). Within the chick and mouse, both Pax3 and Pax7 expression occur within either the somites or dermomyotome, in addition to the limb mesenchyme (Goulding et al. 1991; Goulding et al. 1994; Jostes et al. 1991; Lacosta et al. 2005; Otto et al. 2006; Galli et al. 2008). In the human, Pax3 signal has been demonstrated in the presumptive musculature of early embryos (Gérard et al. 1995; Fougerousse et al. 2002; Gershon et al. 2005). Our work provides further evidence for a role of Pax3 in human muscle development, and also implicates Pax7 in this process. Expression of both Pax3 and Pax7 appears in the dermomyotome or early musculature at CS12–CS15 (Fig. 2A–B, E, I–J; Fig. 5A–B, E–F, M–N; S1. B, J). By CS18, Pax7 staining occurs throughout the presumptive musculature surrounding the ribs and vertebrae (Fig. 6F).
Cartilage Primordia and Future Skeleton
Sox9 has also been implicated in processes outside of neural crest development, specifically chondrogenesis and sex determination (Wagner et al. 1994; Bi et al. 2001; Mori-Akiyama et al. 2003). In both the chick and mouse, Sox9+ cells appear in the sclerotomal tissue and eventual cartilaginous structures throughout the body, in addition to the male genital ridge (Kent et al. 1996; Zhao et al. 1997; Ng et al. 1997; Mori-Akiyama et al. 2003; McKeown et al. 2005; Suzuki et al. 2006). Similarly, Sox9 signal occurs within both the future skeletal elements and forming testes of human embryos (Wagner et al. 1994; Hanley et al. 2000; Benko et al. 2009). As in model systems, from CS12–CS15 we observe Sox9 expression throughout the sclerotomal tissue surrounding and ventral to the DRGs, and within the notochord itself (Fig. 1B, D, J–K; Fig. 2D, H, L; Fig. 4C; Fig. 5D, H, P; S1. D, L); faint Sox10 staining is also observed in these regions at CS15 (Fig. 5C, G, O). At CS18, both Sox9 and Sox10 signal occur in the vertebrae and rib cartilage primordia (Fig. 6G–H and data not shown). Although the role of Sox9 in chondrogenesis is well documented, Sox10 has not been reported in the trunk skeletal elements of mouse, chick or human specimens.
Conclusion
Much of the previous work evaluating hNCC migration identified these cells via their morphology, rather than specific marker expression (O’Rahilly and Müller 2007). Here, we demonstrate the novel expression of several neural crest markers in early human embryos, with specific focus on signal occurring in the premigratory or early migratory neural crest. To our knowledge, we have shown for the first time the expression of Pax3, Sox9 and Sox10 in the premigratory human neural crest (Fig. 1A–H). Furthermore, although Sox10 has been reported in the migratory neural crest of a 4-week human specimen (Bondurand et al. 1998), our study is the first to demonstrate the in-vivo expression of Pax7, Sox9, AP-2α and p75NTR in migratory hNCCs occurring either dorsal, or dorso-lateral to, the neural tube (Fig. 1I–M; Fig. 4A, C–D). At all stages examined, we have demonstrated the expression of several markers within neural crest derivatives – including the DRGs, sympathetic tissue, enteric ganglia, and cranial mesenchyme. Given our small sample size and limited access to tissue, it is difficult to determine the exact migratory routes that NCCs follow within our specimens, or to confirm those pathways previously reported (O’Rahilly and Müller 2007). This, paired with our observation of broad p75NTR expression outside of the neural crest, in addition to the limited expression of HNK-1 within migratory hNCCs, stresses the need for additional in-vivo expression analyses within early human embryos. The identification of novel markers for the human neural crest will improve our capacity to investigate hNCC development, and enable the generation of better diagnostic and therapeutic tools.
Supplementary Material
NCC markers are expressed in neural crest derivatives in rostral CS13 trunk sections. p75NTR (A, E) and Sox10 (C, G) signal are observed within the DRGs. We do not observe definitive Pax7, Msx1/2, Sox9 or AP-2α signals in the rostral DRGs (B, C, D, G, H). Additional p75NTR (A) and Sox10 (C, K) staining occurr in NCCs populating the mesenchyme surrounding the aorta/mesonephric region, which will likely contribute to either the sympathetic tissue or enteric nervous system. Faint HNK-1 signal is also observed in this region (B). Outside of the neural crest, Pax7 (B, F), p75NTR (A), HNK-1 (B, F) and AP-2α (D, H) expression are observed in portions of the neuroepithelium. The dermomyotome is positive for Pax7 (B, J), and Sox9 staining occurs within the sclerotomal tissue (D, L). Panels in (E–L) are higher power images of boxed regions in (A–D). Blue and purple arrows in Fig. 4H indicate axial levels of sections in (A–C) and (D), respectively. Scale bars (A–D) represent 200μm; those in (E–L) 100μm. a, aorta; d, dorsal root ganglia; dm, dermomyotome; nt, neural tube.
AP-2α is expressed in regions of the limb mesenchyme at CS18 (A–B). Panel in (B) is a higher power image of boxed region in (A). Additional Sox9 staining is observed in the skeletal primordia of the limbs (A–B). Scale bar in (A) represents 500μm, that in (B) 200μm. hl, hindlimb.
NCC markers are observed in the neural crest of E9.5–10.0 mouse caudal trunk sections. Pax3 (A), Sox10 (B) and Sox9 (C) are expressed in either the premigratory or migratory mouse trunk NCCs. Outside of the neural crest, Pax3 and few Pax7+ cells are observed in the forming musculature (A), Sox9 occurs in the presumptive sclerotome (C), and AP-2α signal appears in both the surface ectoderm and mesonephric tissue (C). Scale bars represent 100μm. a, aorta; dm, dermomyotome; nt, neural tube.
NCC markers are expressed in cranial neural crest derivatives at mouse E9.5–10.0. Sox10 (A–A’), AP-2α (B–B’), and Sox9 (B–B’) are all expressed within the branchial arch mesenchyme of cranial E9.5–10.0 sections. Additional Sox10 (A) and Sox9 (B) staining are observed within the otic vesicle. Sections in (A’, B’) are slightly rostral to those in (A–B). Note: levels in (A–B) and (A’–B’) are modified individually to optimize red and green signals. Scale bars represent 200μm. ba, branchial arch mesenchyme; nt, neural tube; ov, otic vesicle.
NCC markers are expressed in both prospective premigratory and migratory NCCs at HH. St. 18. In caudal trunk sections, Pax7 and Sox9 appear in both the chick dorsal neural tube, and in early migratory NCCs abutting - and lateral to - the neuroepithelium (E, G). Additional Sox10 (F), AP-2α (H) and HNK-1 (E’) expression are observed within the migratory neural crest at this axial level, or in slightly rostral sections. More rostrally, Pax7, HNK-1, Sox10, Sox9 and AP-2α are observed in either the migratory neural crest or DRG primordia (A–D). Definitive HNK-1 (A) and Sox10 (B) staining also occur in the presumptive sympathetic tissue surrounding the aorta. Outside of the neural crest, Pax7 expression is observed in the early musculature (A, E–E’), Sox9 within the sclerotomal tissue or notochord (C, G), and AP-2α within the surface ectoderm (D, H). Sections in (B) and (E’) are slightly rostral to those in (A, C–D) and (E–H), respectively. Note: levels in (A–D), (E’) and (E–H) are modified individually to optimize signal at different axial levels. All scale bars represent 200μm. d, dorsal root ganglia; dm, dermomyotome; m, early musculature/somite; nt, neural tube.
Sox10, HNK-1 and p75NTR are expressed in the DRGs or associated nerve roots at HH. St. 20/20+ (A–B’). Additional staining for these markers is observed in the presumptive sympathetic tissue near the aorta (A–B’). Scale bars represent 200μm. d, dorsal root ganglia; nt, neural tube.
Acknowledgments
The authors thank Nathan Yardley (Yale University) for his help in generating drawings of embryo samples, and Vivian Lee (Medical College of Wisconsin) for her generous gift of Sox9 and Sox10 antibodies. Additional antibodies obtained from the Developmental Studies Hybridoma Bank (Department of Biology, University of Iowa; NICHD) were generated by the following individuals: AP-2α or 3B5 - Trevor Williams (University of Colorado HSC); Msx1/2 or 4G1 – Thomas M. Jessell and Susan Brenner-Morton (Columbia University); Pax3 – Charles P. Ordahl (University of California); and Pax7 – Atsushi Kawakami (Tokyo Institute of Technology). We also thank all members of the Garcia-Castro laboratory for providing comments on this manuscript. This work was supported by by the Swedish Research Council, Stockholm’s Sjukhem Foundation, and the Knut och Alice Wallenberg Foundation (A.K. & E.S.), a fellowship from Yale University, and the Yale University Developmental Biology Training Grant (E.B.), and grants from NIH and the State of Conecticut (RO1DE017914 and 09SCAYALE45, MIGC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abzhanov A, Tzahor E, Lassar AB, Tabin CJ. Dissimilar regulation of cell differentiation in mesencephalic (cranial) and sacral (trunk) neural crest cells in vitro. Development. 2003;130:4567–79. doi: 10.1242/dev.00673. [DOI] [PubMed] [Google Scholar]
- Anderson RB, Stewart AL, Young HM. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell Tissue Res. 2006;323:11–25. doi: 10.1007/s00441-005-0047-6. [DOI] [PubMed] [Google Scholar]
- Auerbach R. Analysis of the developmental effects of a lethal mutation in the house mouse. Journal of Experimental Zoology. 1954;127:305–329. [Google Scholar]
- Aybar MJ, Mayor R. Early induction of neural crest cells: lessons learned from frog, fish and chick. Curr Opin Genet Dev. 2002;12:452–8. doi: 10.1016/s0959-437x(02)00325-8. [DOI] [PubMed] [Google Scholar]
- Bajpai R, Chen DA, Rada-Iglesias A, Zhang J, Xiong Y, Helms J, Chang CP, Zhao Y, Swigut T, Wysocka J. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 463:958–62. doi: 10.1038/nature08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch ML, Bronner-Fraser M, Garcia-Castro MI. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature. 2006;441:218–22. doi: 10.1038/nature04684. [DOI] [PubMed] [Google Scholar]
- Benko S, Fantes JA, Amiel J, Kleinjan DJ, Thomas S, Ramsay J, Jamshidi N, Essafi A, Heaney S, Gordon CT, McBride D, Golzio C, Fisher M, Perry P, Abadie V, Ayuso C, Holder-Espinasse M, Kilpatrick N, Lees MM, Picard A, Temple IK, Thomas P, Vazquez MP, Vekemans M, Roest Crollius H, Hastie ND, Munnich A, Etchevers HC, Pelet A, Farlie PG, Fitzpatrick DR, Lyonnet S. Highly conserved non-coding elements on either side of SOX9 associated with Pierre Robin sequence. Nat Genet. 2009;41:359–64. doi: 10.1038/ng.329. [DOI] [PubMed] [Google Scholar]
- Beverley PC, Linch D, Delia D. Isolation of human haematopoietic progenitor cells using monoclonal antibodies. Nature. 1980;287:332–3. doi: 10.1038/287332a0. [DOI] [PubMed] [Google Scholar]
- Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A. 2001;98:6698–703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35:643–56. doi: 10.1016/s0896-6273(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Bondurand N, Dastot-Le Moal F, Stanchina L, Collot N, Baral V, Marlin S, Attie-Bitach T, Giurgea I, Skopinski L, Reardon W, Toutain A, Sarda P, Echaieb A, Lackmy-Port-Lis M, Touraine R, Amiel J, Goossens M, Pingault V. Deletions at the SOX10 gene locus cause Waardenburg syndrome types 2 and 4. Am J Hum Genet. 2007;81:1169–85. doi: 10.1086/522090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurand N, Kobetz A, Pingault V, Lemort N, Encha-Razavi F, Couly G, Goerich DE, Wegner M, Abitbol M, Goossens M. Expression of the SOX10 gene during human development. FEBS Lett. 1998;432:168–72. doi: 10.1016/s0014-5793(98)00843-6. [DOI] [PubMed] [Google Scholar]
- Brokhman I, Gamarnik-Ziegler L, Pomp O, Aharonowiz M, Reubinoff BE, Goldstein RS. Peripheral sensory neurons differentiate from neural precursors derived from human embryonic stem cells. Differentiation. 2008;76:145–55. doi: 10.1111/j.1432-0436.2007.00196.x. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Dev Biol. 1986;115:44–55. doi: 10.1016/0012-1606(86)90226-5. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Cheung M, Abu-Elmagd MM, Orme A, Scotting PJ. Chick sox10, a transcription factor expressed in both early neural crest cells and central nervous system. Brain Res Dev Brain Res. 2000;121:233–41. doi: 10.1016/s0165-3806(00)00049-3. [DOI] [PubMed] [Google Scholar]
- Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130:5681–93. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Henderson DJ, Kirby ML, Anderson RH, Copp AJ. Development of a lethal congenital heart defect in the splotch (Pax3) mutant mouse. Cardiovasc Res. 1997;36:163–73. doi: 10.1016/s0008-6363(97)00172-7. [DOI] [PubMed] [Google Scholar]
- Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev Biol. 1987;120:198–214. doi: 10.1016/0012-1606(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Creazzo TL, Godt RE, Leatherbury L, Conway SJ, Kirby ML. Role of cardiac neural crest cells in cardiovascular development. Annu Rev Physiol. 1998;60:267–86. doi: 10.1146/annurev.physiol.60.1.267. [DOI] [PubMed] [Google Scholar]
- Dottori M, Gross MK, Labosky P, Goulding M. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development. 2001;128:4127–38. doi: 10.1242/dev.128.21.4127. [DOI] [PubMed] [Google Scholar]
- Dutton KA, Pauliny A, Lopes SS, Elworthy S, Carney TJ, Rauch J, Geisler R, Haffter P, Kelsh RN. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development. 2001;128:4113–25. doi: 10.1242/dev.128.21.4113. [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Amiel J, Lyonnet S. Molecular bases of human neurocristopathies. Adv Exp Med Biol. 2006;589:213–34. doi: 10.1007/978-0-387-46954-6_14. [DOI] [PubMed] [Google Scholar]
- Farlie PG, McKeown SJ, Newgreen DF. The neural crest: basic biology and clinical relationships in the craniofacial and enteric nervous systems. Birth Defects Res C Embryo Today. 2004;72:173–89. doi: 10.1002/bdrc.20013. [DOI] [PubMed] [Google Scholar]
- Foerst-Potts L, Sadler TW. Disruption of Msx-1 and Msx-2 reveals roles for these genes in craniofacial, eye, and axial development. Dev Dyn. 1997;209:70–84. doi: 10.1002/(SICI)1097-0177(199705)209:1<70::AID-AJA7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Fougerousse F, Durand M, Lopez S, Suel L, Demignon J, Thornton C, Ozaki H, Kawakami K, Barbet P, Beckmann JS, Maire P. Six and Eya expression during human somitogenesis and MyoD gene family activation. J Muscle Res Cell Motil. 2002;23:255–64. doi: 10.1023/a:1020990825644. [DOI] [PubMed] [Google Scholar]
- Galli LM, Knight SR, Barnes TL, Doak AK, Kadzik RS, Burrus LW. Identification and characterization of subpopulations of Pax3 and Pax7 expressing cells in developing chick somites and limb buds. Dev Dyn. 2008;237:1862–74. doi: 10.1002/dvdy.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard M, Abitbol M, Delezoide AL, Dufier JL, Mallet J, Vekemans M. PAX-genes expression during human embryonic development, a preliminary report. C R Acad Sci III. 1995;318:57–66. [PubMed] [Google Scholar]
- Gershon TR, Oppenheimer O, Chin SS, Gerald WL. Temporally regulated neural crest transcription factors distinguish neuroectodermal tumors of varying malignancy and differentiation. Neoplasia. 2005;7:575–84. doi: 10.1593/neo.04637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts M, Garcia-Castro M, Wylie C, Heasman J. Interactions between primordial germ cells play a role in their migration in mouse embryos. Development. 1994;120:135–41. doi: 10.1242/dev.120.1.135. [DOI] [PubMed] [Google Scholar]
- Goulding M, Lumsden A, Paquette AJ. Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development. 1994;120:957–71. doi: 10.1242/dev.120.4.957. [DOI] [PubMed] [Google Scholar]
- Goulding MD, Chalepakis G, Deutsch U, Erselius JR, Gruss P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 1991;10:1135–47. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond CL, Hinits Y, Osborn DP, Minchin JE, Tettamanti G, Hughes SM. Signals and myogenic regulatory factors restrict pax3 and pax7 expression to dermomyotome-like tissue in zebrafish. Dev Biol. 2007;302:504–21. doi: 10.1016/j.ydbio.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley NA, Hagan DM, Clement-Jones M, Ball SG, Strachan T, Salas-Cortes L, McElreavey K, Lindsay S, Robson S, Bullen P, Ostrer H, Wilson DI. SRY, SOX9, and DAX1 expression patterns during human sex determination and gonadal development. Mech Dev. 2000;91:403–7. doi: 10.1016/s0925-4773(99)00307-x. [DOI] [PubMed] [Google Scholar]
- Hill RE, Jones PF, Rees AR, Sime CM, Justice MJ, Copeland NG, Jenkins NA, Graham E, Davidson DR. A new family of mouse homeo box-containing genes: molecular structure, chromosomal location, and developmental expression of Hox-7.1. Genes Dev. 1989;3:26–37. doi: 10.1101/gad.3.1.26. [DOI] [PubMed] [Google Scholar]
- Hoth CF, Milunsky A, Lipsky N, Sheffer R, Clarren SK, Baldwin CT. Mutations in the paired domain of the human PAX3 gene cause Klein-Waardenburg syndrome (WS-III) as well as Waardenburg syndrome type I (WS-I) Am J Hum Genet. 1993;52:455–62. [PMC free article] [PubMed] [Google Scholar]
- Houzelstein D, Cheraud Y, Auda-Boucher G, Fontaine-Perus J, Robert B. The expression of the homeobox gene Msx1 reveals two populations of dermal progenitor cells originating from the somites. Development. 2000;127:2155–64. doi: 10.1242/dev.127.10.2155. [DOI] [PubMed] [Google Scholar]
- Ishii M, Han J, Yen HY, Sucov HM, Chai Y, Maxson RE., Jr Combined deficiencies of Msx1 and Msx2 cause impaired patterning and survival of the cranial neural crest. Development. 2005;132:4937–50. doi: 10.1242/dev.02072. [DOI] [PubMed] [Google Scholar]
- Jakobsen LP, Ullmann R, Christensen SB, Jensen KE, Molsted K, Henriksen KF, Hansen C, Knudsen MA, Larsen LA, Tommerup N, Tumer Z. Pierre Robin sequence may be caused by dysregulation of SOX9 and KCNJ2. J Med Genet. 2007;44:381–6. doi: 10.1136/jmg.2006.046177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Gwye Y, McKeown SJ, Bronner-Fraser M, Lutzko C, Lawlor ER. Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2009;18:1059–70. doi: 10.1089/scd.2008.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson A, Widenfalk J, Trifunovski A, Widmer HR, Olson L, Spenger C. GDNF and NGF family members and receptors in human fetal and adult spinal cord and dorsal root ganglia. J Comp Neurol. 2001;440:204–17. doi: 10.1002/cne.1380. [DOI] [PubMed] [Google Scholar]
- Jostes B, Walther C, Gruss P. The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech Dev. 1990;33:27–37. doi: 10.1016/0925-4773(90)90132-6. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The Atlas of Mouse Development. Academic Press; London: 1992. [Google Scholar]
- Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–22. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998;18:237–50. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacosta AM, Muniesa P, Ruberte J, Sarasa M, Dominguez L. Novel expression patterns of Pax3/Pax7 in early trunk neural crest and its melanocyte and non-melanocyte lineages in amniote embryos. Pigment Cell Res. 2005;18:243–51. doi: 10.1111/j.1600-0749.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- Lallemand Y, Nicola MA, Ramos C, Bach A, Cloment CS, Robert B. Analysis of Msx1; Msx2 double mutants reveals multiple roles for Msx genes in limb development. Development. 2005;132:3003–14. doi: 10.1242/dev.01877. [DOI] [PubMed] [Google Scholar]
- Lane PW, Liu HM. Association of megacolon with a new dominant spotting gene (Dom) in the mouse. J Hered. 1984;75:435–9. doi: 10.1093/oxfordjournals.jhered.a109980. [DOI] [PubMed] [Google Scholar]
- Lang D, Powell SK, Plummer RS, Young KP, Ruggeri BA. PAX genes: roles in development, pathophysiology, and cancer. Biochem Pharmacol. 2007;73:1–14. doi: 10.1016/j.bcp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V, Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–75. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- Li M, Zhao C, Wang Y, Zhao Z, Meng A. Zebrafish sox9b is an early neural crest marker. Dev Genes Evol. 2002;212:203–6. doi: 10.1007/s00427-002-0235-2. [DOI] [PubMed] [Google Scholar]
- Li W, Cornell RA. Redundant activities of Tfap2a and Tfap2c are required for neural crest induction and development of other non-neural ectoderm derivatives in zebrafish embryos. Dev Biol. 2007;304:338–54. doi: 10.1016/j.ydbio.2006.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden A, Sprawson N, Graham A. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development. 1991;113:1281–91. doi: 10.1242/dev.113.4.1281. [DOI] [PubMed] [Google Scholar]
- MacKenzie A, Ferguson MW, Sharpe PT. Expression patterns of the homeobox gene, Hox-8, in the mouse embryo suggest a role in specifying tooth initiation and shape. Development. 1992;115:403–20. doi: 10.1242/dev.115.2.403. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Stoykova A, Torres M, Gruss P. Dysgenesis of cephalic neural crest derivatives in Pax7−/− mutant mice. Development. 1996;122:831–8. doi: 10.1242/dev.122.3.831. [DOI] [PubMed] [Google Scholar]
- Mayor R, Young R, Vargas A. Development of neural crest in Xenopus. Curr Top Dev Biol. 1999;43:85–113. doi: 10.1016/s0070-2153(08)60379-8. [DOI] [PubMed] [Google Scholar]
- McKeown SJ, Lee VM, Bronner-Fraser M, Newgreen DF, Farlie PG. Sox10 overexpression induces neural crest-like cells from all dorsoventral levels of the neural tube but inhibits differentiation. Dev Dyn. 2005;233:430–44. doi: 10.1002/dvdy.20341. [DOI] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Dev Cell. 2004;7:291–9. doi: 10.1016/j.devcel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Milunsky JM, Maher TA, Zhao G, Roberts AE, Stalker HJ, Zori RT, Burch MN, Clemens M, Mulliken JB, Smith R, Lin AE. TFAP2A mutations result in branchio-oculo-facial syndrome. Am J Hum Genet. 2008;82:1171–7. doi: 10.1016/j.ajhg.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchin JE, Hughes SM. Sequential actions of Pax3 and Pax7 drive xanthophore development in zebrafish neural crest. Dev Biol. 2008;317:508–22. doi: 10.1016/j.ydbio.2008.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PJ, Timmons PM, Hebert JM, Rigby PW, Tjian R. Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev. 1991;5:105–19. doi: 10.1101/gad.5.1.105. [DOI] [PubMed] [Google Scholar]
- Mori-Akiyama Y, Akiyama H, Rowitch DH, de Crombrugghe B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc Natl Acad Sci U S A. 2003;100:9360–5. doi: 10.1073/pnas.1631288100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–49. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- Müller F, O'Rahilly R. Cerebral dysraphia (future anencephaly) in a human twin embryo at stage 13. Teratology. 1984;30:167–77. doi: 10.1002/tera.1420300203. [DOI] [PubMed] [Google Scholar]
- Müller F, O'Rahilly R. The development of the human brain and the closure of the rostral neuropore at stage 11. Anat Embryol (Berl) 1986;175:205–22. doi: 10.1007/BF00389597. [DOI] [PubMed] [Google Scholar]
- Müller F, O'Rahilly R. The development of the human brain, the closure of the caudal neuropore, and the beginning of secondary neurulation at stage 12. Anat Embryol (Berl) 1987;176:413–30. doi: 10.1007/BF00310083. [DOI] [PubMed] [Google Scholar]
- Müller F, O'Rahilly R. Development of anencephaly and its variants. Am J Anat. 1991;190:193–218. doi: 10.1002/aja.1001900302. [DOI] [PubMed] [Google Scholar]
- Muratovska A, Zhou C, He S, Goodyer P, Eccles MR. Paired-Box genes are frequently expressed in cancer and often required for cancer cell survival. Oncogene. 2003;22:7989–97. doi: 10.1038/sj.onc.1206766. [DOI] [PubMed] [Google Scholar]
- Ng LJ, Wheatley S, Muscat GE, Conway-Campbell J, Bowles J, Wright E, Bell DM, Tam PP, Cheah KS, Koopman P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol. 1997;183:108–21. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- Nichols DH. Neural crest formation in the head of the mouse embryo as observed using a new histological technique. J Embryol Exp Morphol. 1981;64:105–20. [PubMed] [Google Scholar]
- Noden DM. An analysis of migratory behavior of avian cephalic neural crest cells. Dev Biol. 1975;42:106–30. doi: 10.1016/0012-1606(75)90318-8. [DOI] [PubMed] [Google Scholar]
- O'Rahilly R, Müller F. The development of the neural crest in the human. J Anat. 2007;211:335–51. doi: 10.1111/j.1469-7580.2007.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rahilly RaFM. Developmental Stages in Human Embryos. Carnegie Institution of Washington; 1987. [Google Scholar]
- Otto A, Schmidt C, Patel K. Pax3 and Pax7 expression and regulation in the avian embryo. Anat Embryol (Berl) 2006;211:293–310. doi: 10.1007/s00429-006-0083-3. [DOI] [PubMed] [Google Scholar]
- Patthey C, Edlund T, Gunhaga L. Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development. 2009;136:73–83. doi: 10.1242/dev.025890. [DOI] [PubMed] [Google Scholar]
- Patthey C, Gunhaga L, Edlund T. Early development of the central and peripheral nervous systems is coordinated by Wnt and BMP signals. PLoS One. 2008;3:e1625. doi: 10.1371/journal.pone.0001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingault V, Ente D, Dastot-Le Moal F, Goossens M, Marlin S, Bondurand N. Review and update of mutations causing Waardenburg syndrome. Hum Mutat. 31:391–406. doi: 10.1002/humu.21211. [DOI] [PubMed] [Google Scholar]
- Pomp O, Brokhman I, Ben-Dor I, Reubinoff B, Goldstein RS. Generation of peripheral sensory and sympathetic neurons and neural crest cells from human embryonic stem cells. Stem Cells. 2005;23:923–30. doi: 10.1634/stemcells.2005-0038. [DOI] [PubMed] [Google Scholar]
- Pusch C, Hustert E, Pfeifer D, Sudbeck P, Kist R, Roe B, Wang Z, Balling R, Blin N, Scherer G. The SOX10/Sox10 gene from human and mouse: sequence, expression, and transactivation by the encoded HMG domain transcription factor. Hum Genet. 1998;103:115–23. doi: 10.1007/s004390050793. [DOI] [PubMed] [Google Scholar]
- Raible DW, Wood A, Hodsdon W, Henion PD, Weston JA, Eisen JS. Segregation and early dispersal of neural crest cells in the embryonic zebrafish. Dev Dyn. 1992;195:29–42. doi: 10.1002/aja.1001950104. [DOI] [PubMed] [Google Scholar]
- Robert B, Sassoon D, Jacq B, Gehring W, Buckingham M. Hox-7, a mouse homeobox gene with a novel pattern of expression during embryogenesis. EMBO J. 1989;8:91–100. doi: 10.1002/j.1460-2075.1989.tb03352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat HB, Flores-Sarnat L. Embryology of the neural crest: its inductive role in the neurocutaneous syndromes. J Child Neurol. 2005;20:637–43. doi: 10.1177/08830738050200080101. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–68. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- Scholl FA, Kamarashev J, Murmann OV, Geertsen R, Dummer R, Schafer BW. PAX3 is expressed in human melanomas and contributes to tumor cell survival. Cancer Res. 2001;61:823–6. [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116:297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Fraser SE, Bronner-Fraser M. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development. 1990;108:605–12. doi: 10.1242/dev.108.4.605. [DOI] [PubMed] [Google Scholar]
- Shen H, Wilke T, Ashique AM, Narvey M, Zerucha T, Savino E, Williams T, Richman JM. Chicken transcription factor AP-2: cloning, expression and its role in outgrowth of facial prominences and limb buds. Dev Biol. 1997;188:248–66. doi: 10.1006/dbio.1997.8617. [DOI] [PubMed] [Google Scholar]
- Sieber-Blum M. SSEA-1 is a specific marker for the spinal sensory neuron lineage in the quail embryo and in neural crest cell cultures. Dev Biol. 1989;134:362–75. doi: 10.1016/0012-1606(89)90108-5. [DOI] [PubMed] [Google Scholar]
- Southard-Smith EM, Kos L, Pavan WJ. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat Genet. 1998;18:60–4. doi: 10.1038/ng0198-60. [DOI] [PubMed] [Google Scholar]
- Steventon B, Carmona-Fontaine C, Mayor R. Genetic network during neural crest induction: from cell specification to cell survival. Semin Cell Dev Biol. 2005;16:647–54. doi: 10.1016/j.semcdb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Sull JW, Sull JW, Liang K-Y, Hetmanski JB, Fallin MD, Ingersoll RG, Park J, Wu-Chou Y-H, Chen PK, Chong SS, Cheah F, Yeow V, Park BY, Jee SH, Jabs EW, Redett R, Scott AF, Beaty TH. Maternal transmission effects of the PAX genes among cleft case-parent trios from four populations. Eur J Hum Genet. 2009;17:831–839. doi: 10.1038/ejhg.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Sakai D, Osumi N, Wada H, Wakamatsu Y. Sox genes regulate type 2 collagen expression in avian neural crest cells. Dev Growth Differ. 2006;48:477–86. doi: 10.1111/j.1440-169X.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- Tam PK, Garcia-Barceló M. Genetic basis of Hirschsprung's disease. Pediatr Surg Int. 2009;25:543–58. doi: 10.1007/s00383-009-2402-2. [DOI] [PubMed] [Google Scholar]
- Terzić J, Saraga-Babić M. Expression pattern of PAX3 and PAX6 genes during human embryogenesis. Int J Dev Biol. 1999;43:501–8. [PubMed] [Google Scholar]
- Thomas S, Thomas M, Wincker P, Babarit C, Xu P, Speer MC, Munnich A, Lyonnet S, Vekemans M, Etchevers HC. Human neural crest cells display molecular and phenotypic hallmarks of stem cells. Hum Mol Genet. 2008;17:3411–25. doi: 10.1093/hmg/ddn235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay P, Dietrich S, Mericskay M, Schubert FR, Li Z, Paulin D. A crucial role for Pax3 in the development of the hypaxial musculature and the long-range migration of muscle precursors. Dev Biol. 1998;203:49–61. doi: 10.1006/dbio.1998.9041. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Kessel M, Gruss P. A transgenic neuroanatomical marker identifies cranial neural crest deficiencies associated with the Pax3 mutant Splotch. Dev Biol. 1995;171:317–29. doi: 10.1006/dbio.1995.1284. [DOI] [PubMed] [Google Scholar]
- Tríbulo C, Aybar MJ, Nguyen VH, Mullins MC, Mayor R. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development. 2003;130:6441–52. doi: 10.1242/dev.00878. [DOI] [PubMed] [Google Scholar]
- Tucker GC, Aoyama H, Lipinski M, Tursz T, Thiery JP. Identical reactivity of monoclonal antibodies HNK-1 and NC-1: conservation in vertebrates on cells derived from the neural primordium and on some leukocytes. Cell Differ. 1984;14:223–30. doi: 10.1016/0045-6039(84)90049-6. [DOI] [PubMed] [Google Scholar]
- Tucker GC, Delarue M, Zada S, Boucaut JC, Thiery JP. Expression of the HNK-1/NC-1 epitope in early vertebrate neurogenesis. Cell Tissue Res. 1988;251:457–65. doi: 10.1007/BF00215855. [DOI] [PubMed] [Google Scholar]
- van den Boogaard MJ, Dorland M, Beemer FA, van Amstel HK. MSX1 mutation is associated with orofacial clefting and tooth agenesis in humans. Nat Genet. 2000;24:342–3. doi: 10.1038/74155. [DOI] [PubMed] [Google Scholar]
- Velagaleti GV, Bien-Willner GA, Northup JK, Lockhart LH, Hawkins JC, Jalal SM, Withers M, Lupski JR, Stankiewicz P. Position effects due to chromosome breakpoints that map approximately 900 Kb upstream and approximately 1.3 Mb downstream of SOX9 in two patients with campomelic dysplasia. Am J Hum Genet. 2005;76:652–62. doi: 10.1086/429252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schack D, Casademunt E, Schweigreiter R, Meyer M, Bibel M, Dechant G. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat Neurosci. 2001;4:977–8. doi: 10.1038/nn730. [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–20. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Wilson YM, Richards KL, Ford-Perriss ML, Panthier JJ, Murphy M. Neural crest cell lineage segregation in the mouse neural tube. Development. 2004;131:6153–62. doi: 10.1242/dev.01533. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y, Ohsugi K, Sasaki H, Kuroiwa A. Chicken homeobox gene Msx-1: structure, expression in limb buds and effect of retinoic acid. Development. 1991;113:431–44. doi: 10.1242/dev.113.2.431. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon AP, Flavell RA, Williams T. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature. 1996;381:238–41. doi: 10.1038/381238a0. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Eberspaecher H, Lefebvre V, De Crombrugghe B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209:377–86. doi: 10.1002/(SICI)1097-0177(199708)209:4<377::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NCC markers are expressed in neural crest derivatives in rostral CS13 trunk sections. p75NTR (A, E) and Sox10 (C, G) signal are observed within the DRGs. We do not observe definitive Pax7, Msx1/2, Sox9 or AP-2α signals in the rostral DRGs (B, C, D, G, H). Additional p75NTR (A) and Sox10 (C, K) staining occurr in NCCs populating the mesenchyme surrounding the aorta/mesonephric region, which will likely contribute to either the sympathetic tissue or enteric nervous system. Faint HNK-1 signal is also observed in this region (B). Outside of the neural crest, Pax7 (B, F), p75NTR (A), HNK-1 (B, F) and AP-2α (D, H) expression are observed in portions of the neuroepithelium. The dermomyotome is positive for Pax7 (B, J), and Sox9 staining occurs within the sclerotomal tissue (D, L). Panels in (E–L) are higher power images of boxed regions in (A–D). Blue and purple arrows in Fig. 4H indicate axial levels of sections in (A–C) and (D), respectively. Scale bars (A–D) represent 200μm; those in (E–L) 100μm. a, aorta; d, dorsal root ganglia; dm, dermomyotome; nt, neural tube.
AP-2α is expressed in regions of the limb mesenchyme at CS18 (A–B). Panel in (B) is a higher power image of boxed region in (A). Additional Sox9 staining is observed in the skeletal primordia of the limbs (A–B). Scale bar in (A) represents 500μm, that in (B) 200μm. hl, hindlimb.
NCC markers are observed in the neural crest of E9.5–10.0 mouse caudal trunk sections. Pax3 (A), Sox10 (B) and Sox9 (C) are expressed in either the premigratory or migratory mouse trunk NCCs. Outside of the neural crest, Pax3 and few Pax7+ cells are observed in the forming musculature (A), Sox9 occurs in the presumptive sclerotome (C), and AP-2α signal appears in both the surface ectoderm and mesonephric tissue (C). Scale bars represent 100μm. a, aorta; dm, dermomyotome; nt, neural tube.
NCC markers are expressed in cranial neural crest derivatives at mouse E9.5–10.0. Sox10 (A–A’), AP-2α (B–B’), and Sox9 (B–B’) are all expressed within the branchial arch mesenchyme of cranial E9.5–10.0 sections. Additional Sox10 (A) and Sox9 (B) staining are observed within the otic vesicle. Sections in (A’, B’) are slightly rostral to those in (A–B). Note: levels in (A–B) and (A’–B’) are modified individually to optimize red and green signals. Scale bars represent 200μm. ba, branchial arch mesenchyme; nt, neural tube; ov, otic vesicle.
NCC markers are expressed in both prospective premigratory and migratory NCCs at HH. St. 18. In caudal trunk sections, Pax7 and Sox9 appear in both the chick dorsal neural tube, and in early migratory NCCs abutting - and lateral to - the neuroepithelium (E, G). Additional Sox10 (F), AP-2α (H) and HNK-1 (E’) expression are observed within the migratory neural crest at this axial level, or in slightly rostral sections. More rostrally, Pax7, HNK-1, Sox10, Sox9 and AP-2α are observed in either the migratory neural crest or DRG primordia (A–D). Definitive HNK-1 (A) and Sox10 (B) staining also occur in the presumptive sympathetic tissue surrounding the aorta. Outside of the neural crest, Pax7 expression is observed in the early musculature (A, E–E’), Sox9 within the sclerotomal tissue or notochord (C, G), and AP-2α within the surface ectoderm (D, H). Sections in (B) and (E’) are slightly rostral to those in (A, C–D) and (E–H), respectively. Note: levels in (A–D), (E’) and (E–H) are modified individually to optimize signal at different axial levels. All scale bars represent 200μm. d, dorsal root ganglia; dm, dermomyotome; m, early musculature/somite; nt, neural tube.
Sox10, HNK-1 and p75NTR are expressed in the DRGs or associated nerve roots at HH. St. 20/20+ (A–B’). Additional staining for these markers is observed in the presumptive sympathetic tissue near the aorta (A–B’). Scale bars represent 200μm. d, dorsal root ganglia; nt, neural tube.