Abstract
The ability to track moving objects, a crucial skill for mature performance on everyday spatial tasks, has been hypothesized to require a specialized mechanism that may be available in infancy (i.e., indexes). Consistent with the idea of specialization, our previous work showed that object tracking was more impaired than a matched spatial memory task in individuals with Williams syndrome (WS), a genetic disorder characterized by severe visual-spatial impairment. We now ask whether this unusual pattern of performance is a reflection of general immaturity or true abnormality, possibly reflecting the atypical brain development in WS. To examine these two possibilities, we tested typically developing 3- and 4-year-olds and people with WS on multiple object tracking (MOT) and memory for static spatial location. The maximum number of objects that could be correctly tracked or remembered (estimated from the k statistic) showed similar developmental profiles in typically developing 3- and 4-year-old children, but the WS profile was not similar to that of either age group. People with WS could track more objects than 3-year-olds, and the same number as 4-year-olds, but they could remember the locations of more static objects than both 3- and 4-year-olds. Combining these data with those from our previous studies, we found that typically developing children show increases in the number of objects they can track or remember between ages 3 and 6, and these increases grow in parallel across the two tasks. In contrast, object tracking in older children and adults with WS remains at the level of 4-year-olds, while the ability to remember multiple locations of static objects develops further. As a whole, the evidence suggests that MOT and memory for static location develop in tandem typically, but not in WS. Atypical development of the parietal lobe in people with WS could play a causal role in establishing the abnormal, uneven pattern of performance in WS. This interpretation is consistent with the idea that multiple object tracking engages different mechanisms from memory for static object location, and that the former can be particularly disrupted by atypical development.
Keywords: development, preschoolers, Williams syndrome, spatial, location memory, tracking, object, multiple object tracking, developmental disorder
Adults are skilled at tracking several objects at a time, even if the objects move or briefly disappear. This ability supports performance on a wide-range of spatial tasks, including simple tasks such as reaching and more complex tasks such as driving and interpreting events – for instance, watching sports. Pylyshyn and his colleagues have argued that object tracking is accomplished by a limited number of “mental pointers” (around 4) that “adhere to” objects on the basis of spatiotemporal continuity, allowing adults to track objects without explicitly representing their spatial locations from moment to moment. These mental pointers have been called Fingers of Instantiation (FINSTs: Pylyshyn & Storm, 1988) or indexes (Leslie, Xu, Tremoulet, & Scholl, 1998; Pylyshyn, 2000), and have been proposed to underlie object tracking from infancy onward (see Carey & Xu, 2001; Leslie et al., 1998; Pylyshyn, 2000; and related ideas in Richardson & Kirkham, 2004; Ullman, 1984; Ballard, Hayhoe, Pook, & Rao, 1997; Scholl & Leslie, 1999).1 In this paper, we ask whether the mechanism underlying multiple object tracking can be selectively impaired with a genetic deficit, and how the resulting profile for tracking compares to that of typically developing children.
The theory of indexing (Pylyshyn, 2000) proposes that the tracking of multiple objects engages a mechanism that is quite different from the one most commonly assumed to represent an object's static spatial locations. The contrast can be made by comparing the requirements for representing and storing the locations of several static objects, compared to several moving objects. The locations of a set of static objects are often assumed to be represented in terms of a reference system, with individual locations represented in terms of direction and distance from the origin (see, e.g. Landau, 2002; Carlson-Radvansky & Irwin, 1993; McCloskey et al., 2004; Regier & Carlson, 2001). An observer could represent and remember these static locations in terms of the relevant sets of x and y coordinates. In contrast, tracking multiple moving objects has been argued to require a different mechanism—one that does not depend at all on such coordinate representations (Pylyshyn, 2007). The hallmark example that is used to demonstrate this point is the multiple object tracking task (MOT), first introduced by Pylyshyn and Storm (1988). Participants are presented with eight identical objects, and a subset of these is designated as targets. The eight objects then move randomly along independent paths for several seconds and the observer's task is to indicate—after motion is completed—which of the objects were initially designated as targets. The subjective experience of tracking multiple objects is striking: Adults can correctly track up to four objects in a display of 8 to 10 objects, even though the computational task is quite demanding.
According to Pylyshyn (Pylyshyn & Storm, 1988; Pylyshyn, 2000), the ability to track objects that move along independent trajectories is unlikely to be accomplished by representing or recalling multiple sets of coordinates, as these are continually changing. Rather, he argues that the task is accomplished by attaching a pointer or index to each target object, which then travels with the object throughout its motion and adheres to it even during periods of temporary occlusion (Scholl & Pylyshyn, 1999).2
Despite the strong claim made about the different mechanisms underlying the representation of multiple moving vs. static targets, little is known about the relationship between these two tasks. However, substantial differences in performance across a static and moving task would be consistent with the idea that the two tasks utilize different mechanisms, and that the indexing mechanism may possibly be particularly useful for multiple object tracking.
In a previous study (O'Hearn et al, 2005), we offered evidence consistent with this idea. We found that people with WS-- who have visuospatial impairments -- performed significantly differently on the two tasks relative to typically developing children, with particular impairment on the MOT task relative to the task requiring memory for static location. The general spatial-cognitive profile of people with WS, along with the atypical brain function and structure found in this disorder, suggests a possible basis for this abnormal pattern of performance, inviting further study of this phenomenon.
WS is a rare genetic disorder that presents with severe visuospatial impairment (e.g., block construction, copying, visual-motor action; Brown, Johnson, Paterson, Gilmore, Longhi, & Karmiloff-Smith, 2003; Dilks, Landau, & Hoffman, 2007; Hoffman, Landau, & Pagani, 2003; Meyer-Lindenberg, Kohn, Mervis, Kippenhan, Olsen, Morris, & Berman, 2004; Nardini, Atkinson, Braddick, & Burgess, 2008; O'Hearn, Landau, & Hoffman, 2005; Paul, Stiles, Passarotti, Bavar, & Bellugi, 2002;Vicari & Carlesimo, 2006). The overall profile of spatial impairment appears to be selective, however, with relatively strong performance in tasks thought to engage the ventral stream -- such as object and face perception (Landau, Hoffman, & Kurz, 2005; Tager-Flusberg, Plesa-Skwerer, Faja, & Joseph, 2003) -- and severe impairment in tasks thought to engage the dorsal stream -- such as visual-manual action and construction tasks. Consistent with substantial abnormality in the dorsal stream, studies have reported decreased grey matter volume (Eckert et al., 2005; Meyer-Lindenberg et al., 2004; Reiss et al., 2000; Chiang et al., 2007), sulcal depth (Kippenhan et al., 2005; Van Essen et al., 2006), and functional activation (Meyer-Lindenberg et al., 2004; Mobbs et al., 2004) in the parietal and dorsal occipital regions with WS, including in children (Boddeart et al., 2005). One compelling study suggested there is a structural abnormality in the area of the intra-parietal sulcus, and that this abnormality lead to impaired function in parietal areas more generally (Meyer-Lindenberg et al., 2004). The MOT task has been found to rely on these areas in normal adults (Culham et al., 2001), raising the possibility that the brain abnormality in this region in WS could especially impair the ability to track multiple objects.
In our previous study of MOT, older children and adults with WS (10 -38 years of age, mean age 18) performed a MOT task, as well as a companion task examining memory for static location. The two tasks were identical except that the objects in the static location task did not move. People with WS performed like typically developing 4-year-olds on the MOT task, but were better than 4-year-olds-- and similar to typically developing 5- to 6-year-olds-- on the static location task. The impairment in MOT among individuals with WS is unlikely to simply reflect deficits in motion perception, because these would lead to impaired performance even at small set sizes and this was not observed. Moreover, recent studies of people with WS show little evidence for severe impairment in either motion coherence or biological motion perception (Reiss, Hoffman, & Landau, 2005), both of which require tracking many objects (dots) over time. In fact, participants with WS in these studies did not perform differently from typically developing 6 year-olds, whereas they were reliably different from 6 year-olds in the MOT task. Studies of form-from-motion do suggest some impairment in people with WS (Atkinson, King, Braddick, Nokes, Anker, & Braddick, 1997; Atkinson, Braddick, Rose, Searcy, Wattam-Bell, & Bellugi 2006; Reiss et al., 2005), but this may be due to the strong requirement for visual segmentation in this task (see Reiss et al., 2005).
In this previous study (O'Hearn et al., 2005), the profile of people with WS on the two tasks (MOT and Static Location) was not like 4-, 5-, or 6-year-olds-- they performed like 4-year-olds on MOT but like 5- to 6-year-olds on Static Location. This profile could occur for one of two reasons. First, it could be caused by general immaturity of spatial development. If this is true, then we should observe the same pattern in younger children than have been previously tested on the MOT task, i.e. among 3- to 4-year-olds. Indeed, many of the documented cases of spatial impairment turn out to have such a profile; for example, although people with WS are severely impaired in tasks requiring visual-manual action, their profile turns out to be quite similar to normally developing 3-4 year-olds (Dilks et al., 2008). A second possibility is that the unusual pattern of performance across MOT and Static Location is a true abnormality evident in people with WS. If this is true, then we should not see it in typically developing children of any age–either the younger group of 3- to 4-year-olds, or the age range more closely matched to mental age (between 5-7 years of age). In either case, the comparison of profiles for people with WS and typically developing preschoolers should shed light on the nature of the mechanism underlying MOT—whether its development is similar to that underlying memory for static location, and whether it can be selectively impaired with a genetic deficit.
To examine these issues, we tested performance on the MOT and Static Location tasks in normally developing 3- and 4-year-olds as well as people with WS. Then, by combining these data with a re-analysis of our previous data, we constructed a broader developmental profile for the two tasks across the age range of 3 to 7 years. We compared this with the WS profile. In all cases, we measured performance in terms of Hulleman's (2005) K --the average number of objects correctly remembered or tracked. Although previous developmental studies (including our own) have generally analyzed percent correct (using different levels of chance for different numbers of targets), this measure does not clearly establish the number of targets being tracked or remembered. More recent studies of tracking among adults use the K statistic to estimate the number of objects tracked (Alvarez & Franconeri, 2007; Wolfe, Place, & Horowitz, 2007). Using this measure enables us to determine whether and when there are developmental changes in the number of objects tracked/ remembered across the two tasks, from the earliest age tested thus far on MOT, to our knowledge. This allowed us to construct a substantive typical developmental profile for the tasks, to compare to the profile of individuals with WS.
Methods
Participants
We tested 16 typically developing 3-year-olds (9 M; M age 3 years, 7 months, range 3;4 - 3;10), 16 typical 4-year-olds (7 M; M age 4 years, 4 months, range 4;0 - 5;0), and 15 children and adults with WS (8 M; M age 18 years, 1 month, range 10;9 - 28;0). All participants with WS had been positively diagnosed by the FISH test (Ewart, Morris, Atkinson, Weisan, Sternes, & Spallone, 1993). Twelve of these participants had been tested in our original study between one and two years earlier (data reported in O'Hearn et al., 2005). All children were recruited through local preschools or had older siblings that had participated in our studies. WS participants were recruited through the Williams Syndrome Association.
Design, Stimuli, & Procedure
Participants viewed an LCD monitor from a distance of approximately 18 to 25 inches. The screen (resolution 1024 × 768 × 32) subtended approximately 28 × 21 degrees-of-visual-angle (dva) from a viewing distance of approximately 18 to 25 inches. Order of presentation of the Moving and Static Conditions was counterbalanced and, in each, there were at least 2 practice trials followed by 12 randomly ordered test trials, 3 each with 1, 2, 3, or 4 targets.
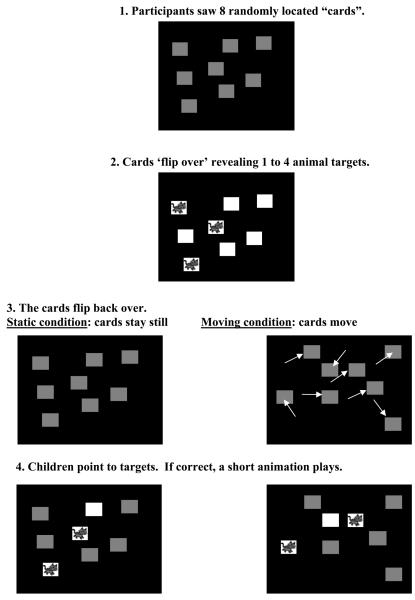
In each condition, 8 red “playing cards” (2.8 dva on a side) appeared on a black background (Figure 1). Before each trial, the cards were assigned random starting positions, with centers at least 5.7 dva apart and edges a minimum of 0.8 dva from the screen boundaries. The red cards then ‘flipped over’ one at a time to reveal between 1 and 4 targets. Within a given trial, these targets were either a cat, a dog (“Odie”), or a fish in a bowl. Participants were told to remember the target locations (Static condition), or to track the targets (Moving condition or MOT). They were given as much time as they wanted to study the initial display, and they often counted or named the targets. The experimenter then clicked the mouse and all the cards flipped back over, becoming identical again. The cards then either remained stationary (Static condition) or moved (Moving condition) for 6 seconds. Both the delay and the characteristics of the object trajectories were the same in this paradigm as in the previously used one (O'Hearn et al., 2005). Participants were encouraged throughout the delay to ‘look at the screen’ and ‘remember where the cats are hiding’ (or ‘running’). Motion paths were computed by assigning each object an initial random starting direction, which changed as a function of the object's distance from other objects or the sides. If the center of one object was within 3.78 dva of the center of another or one of the sides, it was assigned a new random direction to avoid contact. Velocity was constant at 3.6 dva per second.
Figure 1.
Illustration of task.
After the 6 seconds, a ‘splash’ sounded and the mouse pointer reappeared; in the Moving condition, the cards also stopped moving. Participants pointed to the cards they thought were targets, and the experimenter clicked the mouse on them, recording the choices. With each click, the selected card ‘flipped over’, revealing either a target or a non-target (plain white side). When a child chose a target, they saw an animation in which the target animal moved and made noises (dog and cat dancing, or a cat reaching into the fishbowl). We used a full report method, rather than the partial report used by Pylyshyn and Storm (1988), to maximize the amount of data we extracted from each trial (see also Trick, et al., 2005).
Results
We converted percent correct into a measure of capacity (k) or number of objects tracked or remembered, using the high threshold guessing model (Hulleman, 2005):
where n = total number of elements (always 8 in this study), t = number of targets to be tracked (from 1 to 4) and c = the number of targets correctly identified. Since the number of targets varied from 1 to 4, the upper limit of the k statistic also varied from 1 to 4 (black line in Figures 2 and 4). All statistical analyses were carried out on these k values.
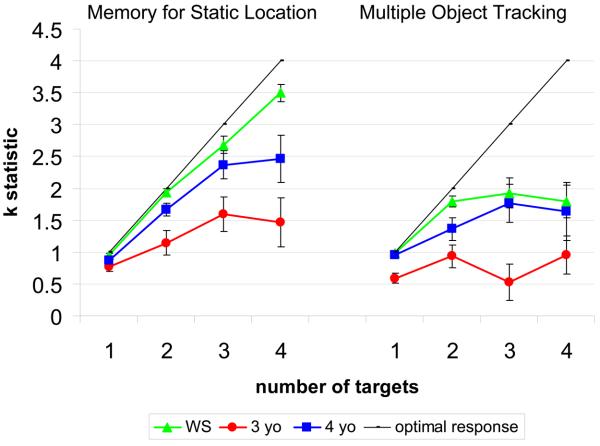
Figure 2.
The mean k statistic from the present experiment, including the WS, 3-year-old, and 4-year-old groups. The black line (optimal performance) represents perfect performance, which leads to increase k values across target number.
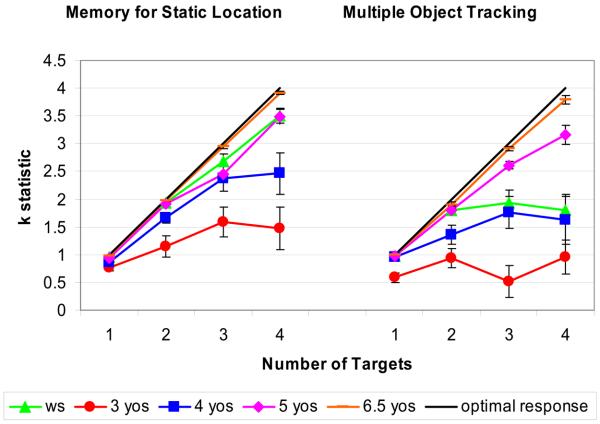
Figure 4.
The mean k statistic across condition and number of targets in each age group.
We first report the analyses on k values derived from the current study, in order to determine whether either 3- or 4-year-olds showed the same uneven profile of performance as people with WS. Next, we attempt to construct a broader developmental timeline, to understand the change in numbers of objects tracked vs. remembered between age 3 and 7, and how the WS profile of performance compares to the normal pattern of change over age. To do this, we use the data from the current study along with the data from our original study, now re-analyzed to show the average number of targets remembered or tracked, i.e. the k values. All repeated measures ANOVAs reported use the Greenhouse Geisser correction, to correct for possible violations of the sphericity assumption.3
Data from the present study
We first asked whether our WS participants would show a different performance profile from normal 4-year-olds across the MOT and Static tasks, as we had found in our earlier study. The k values from the present study, shown in Figure 2, suggest that people with WS again performed better than 4-year-olds in the Static condition with higher numbers of targets: they were also better than the 4-year-olds at tracking 2 targets in MOT. In the Static condition, people with WS were close to ceiling at remembering 1 object (.95), similar to 4-year-olds (.9); in the 2 target condition, the corresponding numbers were 1.9 and 1.7; in the 3 target condition, they were 2.7 and 2.4; most notably, in the 4 target condition, people with WS remembered an average of 3.5 objects, whereas 4-year-olds remembered only 2.5 objects. This suggests that the mean upper limit in the Static condition differs between the two groups. In contrast, neither group could track more than 2 targets in MOT. The mean number of targets tracked on 1 target trials was .98 in WS and .96 in 4-year-olds; on 2 target trials, 1.8 and 1.4; on 3 target trials, 1.9 and 1.8; on 4 targets, 1.8, and 1.6.
A 2 (group) × 2 (condition) × 4 (target number) analysis of variance on these data revealed significant main effects of condition [F(1, 29) = 23.94, p<.01], target number [F(1.64, 47.47) = 34.99, p<.001] and a marginal effect of group [F(1, 29) = 3.54, p=.07]. The effect of condition reflected the fact that both groups achieved higher k scores in the Static than the MOT condition. The effect of target number reflected higher k scores for larger target numbers. There was also a two way interaction between target number and condition [F(2.25, 65.11) = 15.67, p<.01] reflecting increasing disparity between the two conditions as the number of targets increased. The three-way interaction of group, number, and condition was not significant, [F(2.25, 65.11) = 2.37, p=.09].
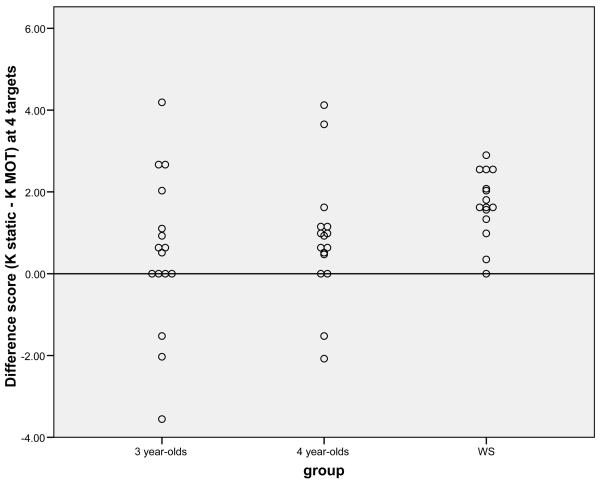
On the basis of our previous results, we looked specifically at whether there was a larger discrepancy between conditions in WS than in typical 4-year-olds. Planned comparisons were carried out on the difference scores between the two conditions (i.e., kSTATIC MEMORY – kMOT) at each target number. There was a difference between groups only for trials with 4 targets (1 target: t(25.86) = −.90, p = .38, 2 target: t(25.83) = .99, p = .33, 3 target: t(26.91) = −.33, p = .74, 4 target: t(23.10) = −2.0, p = .057). While this analysis did not quite reach significance (using a 2-tailed p value), visual inspection of the data suggested the distributions might be skewed (see Figure 3 for 4 target trials). Thus, we followed up with a nonparametric analyses which showed a significant difference between groups on the 4 target trials only (Mann-Whitney U z= −2.44, p = .02). Individuals with WS showed a larger discrepancy across the tasks with better performance at higher numbers in the Static memory task than in the MOT task relative to typical 4-year-olds. This result is similar to the original pattern we found.
Figure 3.
Individual scores in each group (3-year-olds, 4-year-olds and WS) on the difference score between the Static condition and MOT on the 4 target trials only. A score of 0 indicates no difference between conditions.
Next, we compared the WS data to those of typical 3-year-olds (also shown in Figure 2) in order to determine whether the superiority of the static task over MOT observed in WS might be a pattern observed in younger, typically developing children as well. People with WS achieved significantly higher k scores than 3-year-olds on all target numbers in both conditions. In the Static Condition, 3-year-olds remembered fewer than 1 target on the 1 target trials and never more than 1.5 targets, making the difference between groups larger at the higher target numbers (1 target: WS .95, 3-year-olds .8; 2 target: WS 1.9, 3-year-olds 1.2; 3 target: WS 2.7, 3-year-olds 1.6; 4 target: WS 3.5, 3-year-olds 1.5). In the MOT, 3-year-olds tracked at most 1 target regardless of the number of targets in the trial, while the WS group tracked almost 2 targets in trials requiring tracking of two or more (1 target: WS .98, 3-year-olds .6; 2 targets: WS 1.8, 3-year-olds .9; 3 targets: WS 1.9, 3-year-olds .5; 4 targets: WS 1.8, 3-year-olds .95).
A 2 (group) × 2 (condition) × 4 (target number) analysis of variance was performed on these data. There were main effects of group [F(1, 29) = 42.02, p < .001], condition [F(1, 29) = 25.28, p < .001] and target number [F(2.07, 60.10)= 29.24, p < .001]; these reflected overall better performance among people with WS than normal 3-year-olds, better performance of both groups in the Static condition relative to MOT, and the higher k values for larger target numbers. There was also interactions of number × group [F(2.07, 60.10)= 8.52, p < .001], and condition × number [F(1.62, 46.87)= 7.95, p < .01]; these were subsumed by a reliable interaction among group, condition and target [F(1.62,46.87)= 3.63, p = .04]. This significant 3-way interaction reflected the fact that the pattern of performance across target number and condition differed between the two groups.
Planned comparisons on the difference scores in each condition helped clarify how the WS group differed from the 3-year-old group. This score, comparing conditions (kstatic – kMOT), differed significantly between the two groups for the 4 target trials only (1 target t(19.85)=1.92, p=.07; 2 target t(18.24)=.23, p=.82; 3 target t(26.25)=.69, p=.50; 4 target t(20.62)= −2.29, p=.03), reflecting the fact that there was a larger discrepancy in performance among people with WS across the two tasks than among 3-year-olds (nonparametric analysis on 4 target trials: Mann-Whitney U z = −2.16 p = .03). Individuals with WS performed disproportionately better when remembering the static locations of four objects than when tracking four objects. Three-year-olds displayed more similar performance across tasks than did people with WS.
Having established that the pattern of performance found in WS differs from both typically developing 3- and 4-year-olds, we now examine whether there is greater developmental improvement between 3 and 4 in the Static condition relative to MOT. The data in Figure 2 suggest that performance on both tasks improves at the same rate across this age range. A 2 (group) × 2 (condition) × 4 (target number) analysis of variance showed main effects of age group [F(1, 30) = 12.29, p = .001], condition [F(1, 30) = 11.6, p = .002] and number of targets [F(1.75, 52.47)= 10.00, p < .001], but no interactions of age group with either condition or number of targets (age group × condition [F(1, 30)= .1, p = .76]: age group × target [F(1.75, 52.47)= 2.17, p = .13]: age group × condition × target [F(1.89, 56.78)= .78, p = .46]). The main effects reflected overall improvement in performance between 3 and 4, better performance in the Static task relative to the MOT, and better performance for smaller compared to larger numbers of targets. In addition, there was an interaction between condition and target [F(1.89, 56.78)= 3.31, p = .05], reflecting that the Moving condition was more difficult than the Static Condition at 3 and 4 targets (1 target, t(31) = .75, p=.46; 2 target, t(31) = 1.66, p=.11; 3 target, t(31)= −3.17, p=.003; 4 target, t(31)=−2.23, p=.03). Most importantly, however, the lack of interaction between age group and any of the other factors suggests that the effects of task and target number were the same for the 3- and 4-year-olds.
The analyses so far suggest that both the average number of items remembered and tracked grow at about the same rate between ages 3 and 4. The mean upper limit for static location memory increased from 1.6 to 2.5 targets, while the mean upper limit for MOT increased from .95 to 1.8 targets. These data also suggest that people with WS show an unusual pattern of performance in which these two tasks show different profiles: The average number of items tracked on MOT is roughly the same as normally developing 4-year-olds (WS 1.9; 4-year-olds 1.8), but the average number of static items remembered is greater than that for 4-year olds (WS 3.5; 4-year-olds 2.5).
Broader developmental trajectory
We now turn to constructing a broader developmental timeline for the two tasks, and to comparing the WS data with this timeline. To do this, we combined the data from our present experiment with our original data. The two studies were identical except for changes made to allow us to test preschoolers. Namely, the previous study included twice the number of trials in each condition (24 instead of 12), did not include an animation when a target was selected (instead subjects saw a cat and heard a ‘meow’), and had a beep instead of the splash sound when the delay was over. The combined data included the 3-year-olds, 4-year-olds, and WS group from the present study, and the 5 and 6- to 7-year-olds who participated in our original study (N = 12, mean age = 5;4: N =12, mean age = 7;0, respectively). The mean numbers of objects tracked (k values) for all groups of participants in all conditions are shown in Figure 4. Before combining these two studies, we examined whether the changes in the task modified performance across the experiments in either the 4-year-olds or individuals with WS. There were no significant differences across experiments in either group.4
Using the broader data set, we first examined performance across the two tasks in normally developing children, with a 4 (age group) × 2 (condition) × 4 (target number) repeated measures, mixed model analysis of variance on the k values. This resulted in main effects of age group [F(3,52) = 35.22, p < .001], condition [F(1,52)= 10.35, p = .002] and target number [F(1.77, 92.03)= 111.05, p < .001]. Post-hoc comparisons indicated that each age group performed significantly better overall than all the younger age groups (e.g., 6.5-year-olds performed better than 3-, 4-, and 5-year-olds). Age group interacted with target number ([F(5.31, 92.03)= 11.51, p < .001]), indicating that older children performed better than younger children with higher numbers of targets. Age group did not interact with condition ([F(3,52)= 2.08, p = .11]) or condition × target number ([F(5.79, 100.34)= 1.3, p = .28]). This indicates that there is substantial and similar developmental improvement, especially on higher numbers of targets, on both object tracking and memory for static locations.5
Separate analyses of variance were conducted on each condition (MOT and Static) to provide information on how the pattern of performance differed between people with WS and typically developing children. There were group differences at each target number in both conditions (all F′s > 2.85, p's < .03). Posthoc comparisons showed that, in the Static Condition, the WS group performed significantly better than the 3-year-olds at 1, 2, 3, and 4 targets and better than 4-year-olds with 4 targets; and they performed no differently from 5-year-olds or 6.5-year-olds on any target number (all significant differences, p < .05). Thus, memory for Static location in the WS group is similar to 5- to 6.5-year-olds. In the MOT condition, the WS group performed reliably better than 3-year-olds at 1, 2, 3, and 4 targets, better than 4-year-olds with 2 targets, and worse than 5 or 6.5-year-olds at 3 and 4 targets (all p's < .05). The combined pattern in the Static and MOT conditions shows that people with WS perform more like 4-year-olds in the number of objects they track in MOT (though they are more reliable with 2 targets), but are similar to 5 to 6.5-year-olds in the number of static object locations they can remember. The pattern replicates our original finding of a unique profile for the two tasks in people with WS.
Discussion
The present studies examined performance profiles for typically developing children and people with WS when they tracked multiple objects and when they remembered static locations. We first asked whether typically developing children – younger than those previously tested on MOT – display the uneven pattern of performance across these tasks that is evident in individuals with WS. They did not. The pattern of performance across tasks in WS--with relatively better performance when remembering the locations of multiple static objects compared to tracking multiple moving objects-- differed from that of both 3- and 4-year-olds, indicating that the pattern in WS was not merely “immature” but instead atypical. For the highest numbers of targets tested (4), people with WS could remember the static locations of more objects than 4-year-olds (3.5 vs. 2.5 objects) but could not track more moving objects than 4-year-olds (2 objects in both groups).6 Compared to 3-year-olds, people with WS performed better overall, but there was again a significantly greater discrepancy in performance between the two tasks in individuals with WS. The uneven pattern in WS argues against a single module for spatial representation.
Unlike the profile for people with WS, typically developing 3- to 4-year-olds showed general increases in the numbers of objects tracked/ remembered across the two tasks. The effect of age did not interact with task, indicating that the two tasks showed similar changes with age. Developmental improvement in the number of objects tracked/ remembered continued through the age of 6, with older children becoming more capable on trials with 3 and 4 targets. But again, developmental improvement was similar across tasks. In MOT trials with 4 possible targets, children improved from .95 targets at 3 years of age, to 1.9 targets at 4 years, to 3.2 targets at 5 years, to near ceiling performance (3.8 targets) at 6 to 7 years of age. In Static memory trials with 4 targets, children progressed from remembering 1.5 targets at 3 years of age, to 2.5 targets at 4 years, to 3.5 targets at 5 years, and to ceiling performance (3.9 targets) at 6 to 7 years of age. While performance on MOT was poorer than on spatial memory overall, the tasks did not show different developmental profiles with increasing numbers of targets between the ages of 3 and 7. This suggests that performance on the two tasks develops in parallel in typically developing preschool and school-age children-- a profile quite different from that shown by people with WS.7
Previous studies in older children suggest that this developmental improvement continues for both static memory and MOT. Studies of spatial memory (for static location) reveal a positive correlation between spatial memory span and age (although these studies are on children who are older than our sample; see Gathercole, 1999 for a review; Logie and Pearson, 1998; Farrell, Pagulayan, Busch, Medina, Bartok, & Krikorian, 2006). The growth in ability to track multiple objects over age is also consistent with previous developmental studies, which have shown developmental improvement from age 6 onward (Trick et al., 2005) and between 4 and 7 (O'Hearn et al., 2005), using a task with a longer duration than the one we used here.
While we predicted that 3-year-olds might display the same uneven pattern of performance as the WS group, the performance profile for people with WS did not resemble typical development at any age between 3 and 7 years old. This confirms and extends our earlier results of greater impairment on a MOT task than on a static memory task in WS (O'Hearn et al., 2005) to a task appropriate for preschoolers. It also suggests that the mechanism underlying the capacity to track multiple objects is specifically damaged in WS relative to the capacity to remember the locations of multiple static objects. In contrast, the functions of MOT and memory for static location appear to grow in parallel in typically developing children, suggesting that the two tasks share some cognitive component(s) that develops substantially during the preschool years.
How can the pattern found in typical development be reconciled with the pattern in people with WS? Let us first consider typical development. The literature on brain function in typical adults suggests that both tasks engage overlapping areas of the parietal and frontal lobes. For example, Culham and colleagues (1998, 2001) found that attentive tracking of moving objects activates areas in parietal (intraparietal sulcus or IPS, postcentral sulcus, superior parietal lobule or SPL, and precuneus) and frontal areas, as well as area MT which is specialized for motion. In addition, Jovicich, Peters, Koch, Braun, Chang, & Ernst (2001) reported linear increases in activation in both IPS and SPL with increases in the number of objects to be tracked. Studies of visuospatial working memory have likewise found increased brain activation in frontal and parietal areas. For example, Todd and Marois (2004) found that the number of objects retained in a working memory task leveled off at a maximum of four objects (in agreement with previous studies of the capacity of VSTM; Vogel, Woodman, & Luck, 2001), and that a single area in the intraparietal and intraoccipital sulci showed an activation pattern that was similar to the behavioral pattern. Xu and Chun (2006) also found that activation in the intraparietal sulcus (IPS) increased when the number of items stored in working memory increased, up to a limit of approximately four, regardless of object complexity.
Although we are not aware of any neuroimaging studies of developmental changes in brain areas activated during MOT, studies of visuospatial working memory point to the importance of a frontal-parietal circuit. For example, Klingberg and associates (Klingberg, 2006; Klingberg, Forssberg, & Westerberg, 2002) found that developmental increases in performance on visuospatial WM tasks between 9-18 years of age are accompanied by higher activation in superior frontal cortex and intraparietal sulcus (see also, Kwon, Reiss, & Menon, 2002). These findings are consistent with our findings that, for typically developing children, performance on MOT and spatial working memory tasks improves with age in a parallel fashion: both tasks appear to utilize a similar network of frontal and parietal brain areas that mature into adolescence.
In the case of people with WS, there are structural and functional abnormalities in the posterior parietal areas related to their atypical development (e.g., Eckert et al., 2005; Boddeart et al., 2005; Meyer-Lindenberg et al., 2004). Most relevant may be the results of Meyer-Lindenberg et al. (2004), who found abnormal brain structure in IPS in WS, and that this abnormal structure was related to decreased activation in more superior parietal areas during visuospatial tasks. These authors suggest that this might be the origin of abnormal information processing throughout the dorsal pathway. There are also behavioral abnormalities among people with WS on tasks thought to tap the frontal areas, for example, severe impairment on Stroop-type tasks requiring spatially directed responses (Atkinson et al., 2003).
The parietal lobe abnormalities found in WS are consistent with impairment on MOT and Static location (both of which are performed at the levels of typically developing 4-6 year olds) but they do not account for the unique pattern of disproportionate impairment on MOT. We speculate that the relative dissociation between the two tasks in people with WS is caused by a difference in underlying mechanisms. Although both tasks may well engage much of the same neural tissue, the more significant “hit” to MOT in people with WS suggests two different mechanisms, one of which is more vulnerable to the particular brain differences found in people with WS.
The idea of distinct mechanisms is plausible not only because of the behavioral evidence we have presented but also by the logical requirements of the two tasks. Remembering the locations of multiple static objects could be accomplished through a variety of strategies that would be precluded by the movement of objects in MOT. For example, one could remember the locations of static objects in terms of a reference system (with 2 axes) superimposed on the screen and display. This strategy would be more difficult to implement in MOT because the locations are constantly changing. People with WS and children in the age range we tested can represent object locations in terms of such reference systems (Landau & Hoffman, 2005), making it plausible that they could use such a mechanism to remember the locations of multiple static objects. Observers could also encode the locations of static objects relative to salient parts of the screen (e.g. the edge; the corner, etc), or perhaps even by using spatial language (e.g. the objects are all “in a line”). But these would be useless in the MOT task. In fact, the variety of mechanisms that can be used to remember static object locations contrasts with those available to track multiple moving objects. Indeed, part of Pylyshyn's argument for the existence of the indexing mechanism is that it can uniquely explain the ease of tracking multiple moving objects without the use of an external spatial reference system. In this view, the observation that people with WS can track a maximum of two objects at a time--at the level of a normally developing 4-year-old-- while remembering the locations of up to four static objects could be attributed to selective impairment of a mechanism such as indexing that can be reliably used for tracking moving objects.
Although our data are consistent with the idea of a separate mechanism for performing MOT, it is also possible that the unusual WS profile is due to a deficit in attention that has more impact on the MOT task than the Static Location task. Perhaps the MOT task is more attention demanding than the static version, and people with WS may be unable to meet the additional demands for attention associated with larger set sizes in the MOT task. In terms of the number of objects tracked, the moving version of the task was indeed more “difficult” than the static version, as children of all ages were able to “track” more objects in the static version than the moving one. The integration of spatial and temporal information required in MOT may require attentional resources, and the limits on the number of trackable objects may reflect the limited capacity of our attentional systems (Franconeri & Alvarez, 2007). Such attentional networks might particularly rely on parietal areas that are atypical in WS.
We conclude by pointing out that the unusual profile among people with WS provides unique insight into the development of mechanisms underlying two fundamental cognitive activities-- the capacity to track multiple objects, and the capacity to remember the locations of multiple static objects. By studying typical development, we have found that children's ability to track multiple moving objects and remember their static locations both develop with age, and that tracking multiple objects appears to show a lower “ceiling” -- that is, at all ages, children can track fewer objects than they can remember static locations. By itself, this would not suggest two different mechanisms. By studying people with WS as well, we raise the possibility that the two tasks do engage different mechanisms and that these are differentially affected in this disorder. Such side-by-side comparisons enrich our understanding of typical development, as well as the ways in which it can go awry.
Acknowledgements
This work was done at Johns Hopkins University and supported by grants from NICHD (F32 HD42346 to KO), NSF (BCS 0117744 and 9808585 to BL/JEH) and March of Dimes (12-01-0087 to BL). We thank Gitana Chunyo, Elizabeth Crowe, Eric Hsiao, and Jay Reiss for their assistance. We gratefully acknowledge our participants with Williams syndrome. Preliminary results were presented at the International Conference on Infant Studies, May 2005.
Footnotes
Whether infants can track multiple objects moving on simultaneous and independent paths, as in MOT, is unclear but they can track objects under some circumstances. Five month-old infants can represent the location of 3 occluded objects, tracking each object as they move one at a time behind the occluder (e.g., Feigensen & Carey, 2005; Spelke, 1990; Wynn, 1992; for a review, see Feigenson, Dehaene, & Spelke, 2004). In addition, 6-month-olds can track objects moving synchronously on a circular trajectory (Richardson & Kirkham, 2004).
Though it is not Pylyshyn's view, we thank an anonymous reviewer for pointing out that some sort of spatial representation may be needed to integrate the spatial location over time in order to perform MOT, and that a body-centered spatial representation may make the most sense. This sort of spatial representation is also subserved by parietal regions (Goodale & Milner, 1992; Colby, 1998).
This correction modified the degrees of freedom in some of the results. T-tests were also corrected for uneven variances between groups, which differed significantly in some comparisons.
Twelve individuals with WS participated in both studies, and we compared their performance on the two experiments using a repeated measures analysis of variance, with a 2 (experiment) × 2 (condition) × 4 (number of targets) design. There was no effect of experiment: [F(1,11) = 1.67, p = .22], nor any interactions of experiment with the other factors (all p's > .14). We also compared performance across experiments for the 4-year-olds, using a mixed model analysis of variance with the between subjects factor of experiment (2 levels) and within subjects factor of condition (2 levels) and number of targets (4 levels). Again, we found no effects of experiment (main effect [F(1, 26) = .48, p = .5]), nor interactions of this factor with the others (all p's > .30).
Since the 6.5-year-olds were approaching ceiling performance on this task, we wanted to ensure that this wasn't affecting the pattern of statistical results. Thus, the analysis was redone without the 6.5-year-old group and the pattern remained the same. There were main effects of age group [F(2,41) = 23.87, p < .001], condition [F(1,41)= 10.27, p = .003] and target number [F(1.77, 72.52)= 42.61, p < .001). Age group interacted with target number [F(3.54, 84.44)= 8, p < .001]), reflecting that older children performed better than younger children with higher numbers of targets, but it did not interact with condition or condition x target number (all p's > .22).
We do not want to imply that the number of targets represented is set in either condition. The impact of task demands on MOT performance is evident in the developmental literature; previous developmental MOT studies have shown increases in the number of targets tracked with increasing age, but findings show different upper limits at 6 years in the two studies; Trick et al. (2005) found a tracking limit of 2 targets at 6 years old (the youngest age they tested) whereas OHearn et al. (2005) found a limit of 4 targets at 6 years old (the oldest age they tested). This difference is not surprising because O'Hearn's task had fewer total objects than Trick et al's (8 vs 10) and a shorter delay (6 vs 10 s). These results are consistent with recent evidence from adults showing that tracking limits vary within an individual, depending on object speed (Alvarez & Franconeri, 2007), the path of the objects (Tripathy, Narasimhan, & Barrett, 2007), and previous experience (Green & Bavelier, 2005).
There was no significant developmental improvement with age in the WS group.
References
- Alvarez GA, Franconeri SL. How many objects can you track?: Evidence for a resource-limited attentive tracking mechanism. Journal of Vision. 2007;7(13):14.1–10. doi: 10.1167/7.13.14. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Braddick O, Rose FE, Searcy YM, Wattam-Bell J, Bellugi U. Dorsal-stream motion processing deficits persist into adulthood in Williams syndrome. Neuropsychologia. 2006;44:828–833. doi: 10.1016/j.neuropsychologia.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Atkinson J, King J, Braddick O, Nokes L, Anker S, Braddick F. A specific deficit of dorsal stream function in Williams syndrome. Neuroreport. 1997;8:1919–1922. doi: 10.1097/00001756-199705260-00025. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Braddick O, Anker S, Curran W, Andrew R, Wattam-Bell J, et al. Neurobiological models of visuospatial cognition in children with Williams syndrome: Measures of dorsal-stream and frontal function. Developmental Neuropsychology. 2003;23:139–172. doi: 10.1080/87565641.2003.9651890. [DOI] [PubMed] [Google Scholar]
- Ballard D, Hayhoe M, Pook P, Rao R. Deictic codes for the embodiment of cognition. Behavioral and Brain Sciences. 1997;20:723–767. doi: 10.1017/s0140525x97001611. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Mochel F, Meresse I, Seidenwurm D, Cachia A, Brunelle F, et al. Parieto-occipital grey matter abnormalities in children with williams syndrome. Neuroimage. 2005;30:721–725. doi: 10.1016/j.neuroimage.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Brown JH, Johnson MH, Paterson SJ, Gilmore R, Longhi E, Karmiloff-Smith A. Spatial representation and attention in toddlers with Williams syndrome and Down syndrome. Neuropsychologia. 2003;41:1037–46. doi: 10.1016/s0028-3932(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Carey S, Xu F. Infants' knowledge of objects: Beyond object files and object tracking. Cognition. 2001;80:179–213. doi: 10.1016/s0010-0277(00)00154-2. [DOI] [PubMed] [Google Scholar]
- Carlson-Radvansky LA, Irwin DE. Frames of reference in vision and language: where is above? Cognition. 1993;46:223–44. doi: 10.1016/0010-0277(93)90011-j. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Reiss AL, Lee AD, Bellugi U, Galaburda A, Korenberg JR, et al. 3D pattern of brain abnormalities in Williams syndrome visualized using tensor-based morphometry. Neuroimage. 2007;36:1096–1109. doi: 10.1016/j.neuroimage.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby C. Action-oriented spatial reference frames in cortex. Neuron. 1998;20:15–24. doi: 10.1016/s0896-6273(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. Journal of Cognitive Neuroscience. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Culham J, Brandt S, Cavanagh P, Kanwisher N, Dale A, Tootell R. Cortical fMRI activation produced by attentive tracking of moving targets. Journal of Neurophysiology. 1998;80:2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavanagh P, Kanwisher NG. Attention response functions: characterizing brain areas using fMRI activation during parametric variations of attentional load. Neuron. 2001;32:737–45. doi: 10.1016/s0896-6273(01)00499-8. [DOI] [PubMed] [Google Scholar]
- Dilks D, Landau B, Hoffman J. Vision for perception and vision for action: Normal and unusual development. Developmental Science. 2008;11:474–86. doi: 10.1111/j.1467-7687.2008.00693.x. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Hu D, Eliez S, Bellugi U, Galaburda A, Korenberg J, et al. Evidence for superior parietal impairment in Williams syndrome. Neurology. 2005;64:152–153. doi: 10.1212/01.WNL.0000148598.63153.8A. [DOI] [PubMed] [Google Scholar]
- Ewart AK, Morris CA, Atkinson D, Weisan J, Sternes K, Spallone P, et al. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nature Genetics. 1993;5:11–16. doi: 10.1038/ng0993-11. [DOI] [PubMed] [Google Scholar]
- Farrell Pagulayan K, Busch RM, Medina KL, Bartok JA, Krikorian R. Developmental normative data for the Corsi Block-tapping task. Journal of Clinical and Experimental Neuropsychology. 2006;28(6):1043–52. doi: 10.1080/13803390500350977. [DOI] [PubMed] [Google Scholar]
- Feigenson L, Carey S. On the limits of infants' quantification of small object arrays. Cognition. 2005;3:295–313. doi: 10.1016/j.cognition.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Feigenson L, Dehaene S, Spelke E. Core systems of number. Trends in Cognitive Science. 2004;8:307–14. doi: 10.1016/j.tics.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in Neurosceince. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Gathercole SE. The development of memory. Journal of Child Psychology and Psychiatry. 1998;39(1):3–27. [PubMed] [Google Scholar]
- Green CS, Bavelier D. Enumeration versus Multiple Object Tracking: The case of action video game players. Cognition. 2006;101:217–45. doi: 10.1016/j.cognition.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J, Landau B, Pagani B. Spatial breakdown in spatial construction: Evidence from eye fixations in children with Williams syndrome. Cognitive Psychology. 2003;46:260–301. doi: 10.1016/s0010-0285(02)00518-2. [DOI] [PubMed] [Google Scholar]
- Hulleman J. The mathematics of multiple object tracking: from proportions correct to number of objects tracked. Vision Research. 2005;45:2298–309. doi: 10.1016/j.visres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Jordan H, Reiss JE, Hoffman JE, Landau B. Intact perception of biological motion in the face of profound spatial deficits: Williams syndrome. Psychological Science. 2002;13(2):162–167. doi: 10.1111/1467-9280.00429. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Peters RJ, Koch C, Braun J, Chang L, Ernst T. Brain areas specific for attentional load in a motion-tracking task. Journal of Cognitive Neuroscience. 2001;13:1048–1058. doi: 10.1162/089892901753294347. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends in Cognitive Sciences. 1998;2(10):389–398. doi: 10.1016/s1364-6613(98)01230-3. [DOI] [PubMed] [Google Scholar]
- Kippenhan JS, Olsen RK, Mervis CB, Morris CA, Kohn P, Meyer-Lindenberg A, et al. Genetic contributions to human gyrification: sulcal morphometry in Williams syndrome. J Neurosci. 2005;25:7840–7846. doi: 10.1523/JNEUROSCI.1722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44(11):2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau B, Hoffman JE. Parallels between spatial cognition and spatial language: Evidence from Williams syndrome. Journal of Memory and Language. 2005;53:163–185. [Google Scholar]
- Landau B. Spatial cognition. In: Ramachandran V, editor. Encyclopedia of the human brain. Vol. 4. Academic Press; San Diego: 2002. pp. 395–418. [Google Scholar]
- Landau B, Hoffman JE. Parallels between spatial cognition and spatial language: Evidence from Williams syndrome. Journal of Memory and Language. 2005;53(2):163–185. [Google Scholar]
- Landau B, Hoffman JE, Kurz N. Object recognition with severe spatial deficits in Williams syndrome: Sparing and breakdown. Cognition. 2006;100:483–510. doi: 10.1016/j.cognition.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Leslie A, Xu F, Tremoulet P, Scholl B. Indexing and the object concept: Developing ‘what’ and ‘where’ systems. Trends in Cognitive Science. 1998;2:10–18. doi: 10.1016/s1364-6613(97)01113-3. [DOI] [PubMed] [Google Scholar]
- Logie RH, Pearson DG. The inner eye and the inner scribe of visuo-spatial working memory: Evidence from developmental fractionation. European Journal of Cognitive Psychology. 1998;9:241–257. [Google Scholar]
- McCloskey M. Spatial representations and multiple-visual-systems hypotheses: evidence from a developmental deficit in visual location and orientation processing. Cortex. 2004;40:677–94. doi: 10.1016/s0010-9452(08)70164-3. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn P, Mervis CB, Kippenhan JS, Olsen R, Morris CA, Berman KF. Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron. 2004;43:623–31. doi: 10.1016/j.neuron.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Garrett AS, Menon V, Rose FE, Bellugi U, Reiss AL. Anomalous brain activation during face and gaze processing in Williams syndrome. Neurology. 2004;62:2070–6. doi: 10.1212/01.wnl.0000129536.95274.dc. [DOI] [PubMed] [Google Scholar]
- Nardini M, Atkinson J, Braddick O, Burgess N. Developmental trajectories for spatial frames of reference in Williams syndrome. Developmental Science. 2008;11:583–95. doi: 10.1111/j.1467-7687.2007.00662.x. [DOI] [PubMed] [Google Scholar]
- O'Hearn K, Landau B, Hoffman JE. Multiple object tracking in people with Williams syndrome and in normally developing children. Psychological Science. 2005;16:905–12. doi: 10.1111/j.1467-9280.2005.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BM, Stiles J, Passarotti A, Bavar N, Bellugi U. Face and place processing in Williams syndrome: evidence for a dorsal-ventral dissociation. Neuroreport. 2002;13:1115–9. doi: 10.1097/00001756-200207020-00009. [DOI] [PubMed] [Google Scholar]
- Pylyshyn Z. Situating the world in vision. Trends in Cognitive Science. 2000;4:197–207. doi: 10.1016/s1364-6613(00)01477-7. [DOI] [PubMed] [Google Scholar]
- Pylyshyn Z. Things and Places: How the Mind Connects with the World (Jean Nicod Lectures) MIT Press; Cambridge MA: 2007. [Google Scholar]
- Pylyshyn Z, Storm R. Tracking multiple independent objects: Evidence for a parallel tracking mechanism. Spatial Vision. 1988;3:179–197. doi: 10.1163/156856888x00122. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eckert MA, Rose FE, Karchemskiy A, Kesler S, Chang M, et al. An experiment of nature: brain anatomy parallels cognition and behavior in Williams syndrome. J Neurosci. 2004;24:5009–15. doi: 10.1523/JNEUROSCI.5272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Eliez S, Schmitt JE, Straus E, Lai Z, Jones W, et al. IV. Neuroanatomy of Williams syndrome: a high-resolution MRI study. J Cogn Neurosci. 2000;12(Suppl 1):65–73. doi: 10.1162/089892900561986. [DOI] [PubMed] [Google Scholar]
- Regier T, Carlson LA. Grounding spatial language in perception: an empirical and computational investigation. J Exp Psychol Gen. 2001;130:273–98. doi: 10.1037//0096-3445.130.2.273. [DOI] [PubMed] [Google Scholar]
- Reiss JE, Hoffman JE, Landau B. Motion processing specialization in WS. Vision Research. 2005;27:483–510. doi: 10.1016/j.visres.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Richardson DC, Kirkham NZ. Multi-modal events and moving locations: Eye movements of adults and 6-month-olds reveal dynamic spatial indexing. Journal of Experimental Psychology: General. 2004;133:46–62. doi: 10.1037/0096-3445.133.1.46. [DOI] [PubMed] [Google Scholar]
- Scholl BJ. Objects and attention: the state of the art. Cognition. 2001;80:1–46. doi: 10.1016/s0010-0277(00)00152-9. [DOI] [PubMed] [Google Scholar]
- Scholl B, Leslie A. Explaining the Infant's Object Concept: Beyond the Perception/Cognition Dichotomy. In: Lepore E, Pylshyn ZW, editors. Rutgers Lectures on Cognitive Science. Oxford; Blackwell: 1999. [Google Scholar]
- Scholl B, Pylyshyn Z. Tracking multiple objects through occlusion: Clues to visual objecthood. Cognitive Psychology. 1999;38:259–290. doi: 10.1006/cogp.1998.0698. [DOI] [PubMed] [Google Scholar]
- Spelke E. Principles of object perception. Cognitive Science. 1990;14:29–56. [Google Scholar]
- Tager-Flusberg H, Plesa-Skwerer D, Faja S, Joseph RM. People with Williams syndrome process faces holistically. Cognition. 2003;89:11–24. doi: 10.1016/s0010-0277(03)00049-0. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Trick LM, Jaspers-Fayer F, Sethi N. Multiple-object tracking in children: The “Catch the Spies” task. Cognitive Development. 2005;20(3):373–387. [Google Scholar]
- Tripathy SP, Narasimhan S, Barrett BT. On the effective number of tracked trajectories in normal human vision. Journal of Vision. 2007;7:2. doi: 10.1167/7.6.2. [DOI] [PubMed] [Google Scholar]
- Ullman S. Visual routines. Cognition. 1984;18:97–159. doi: 10.1016/0010-0277(84)90023-4. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dierker D, Snyder AZ, Raichle ME, Reiss AL, Korenberg J. Symmetry of cortical folding abnormalities in Williams syndrome revealed by surface-based analyses. J Neurosci. 2006;26:5470–5483. doi: 10.1523/JNEUROSCI.4154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S, Carlesimo GA. Short-term memory deficits are not uniform in Down and Williams syndromes. Neuropsychol Rev. 2006;16(2):87–94. doi: 10.1007/s11065-006-9008-4. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Woodman GF, Luck SJ. Storage of features, conjunctions, and objects in visual working memory. Journal of Experimental Psychology-Human Perception and Performance. 2001;27(1):92–114. doi: 10.1037//0096-1523.27.1.92. [DOI] [PubMed] [Google Scholar]
- Wilson J, Scott JH, Power KG. Developmental differences in the span of visual memory for pattern. British Journal of Developmental Psychology. 1987;5:249–255. [Google Scholar]
- Wolfe JM, Place SS, Horowitz TS. Multiple object juggling: changing what is tracked during extended multiple object tracking. Psychon Bull Rev. 2007;14(2):344–9. doi: 10.3758/bf03194075. [DOI] [PubMed] [Google Scholar]
- Wynn K. Addition and subtraction by human infants. Nature. 1992;358:749–750. doi: 10.1038/358749a0. [DOI] [PubMed] [Google Scholar]
- Xu YD, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440(7080):91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]