Abstract
Inflammation induced by recognition of pathogen-associated molecular patterns dramatically impacts subsequent adaptive responses. We asked if the adaptive immune system can also affect the character and magnitude of innate inflammatory responses. We find that the response of memory, but not naïve, CD4+ T cells enhances production of multiple innate inflammatory cytokines and chemokines (IIC) in the lung, and that during influenza infection, this leads to early control of virus. Memory CD4+ T cell induced IIC and viral control require cognate antigen recognition and are optimal when memory cells are either T helper type 1 (TH1)- or TH17-polarized, but are independent of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) production and do not require activation of conserved pathogen recognition pathways. This represents a novel mechanism by which memory CD4+ T cells induce an early innate response that enhances immune protection against pathogens.
Introduction
Recognition of pathogen-associated molecular patterns (PAMP) by their receptors results in the production of inflammatory mediators that act to control initial infection and mobilize elements of the innate immune system 1,2. PAMP recognition also facilitates optimal development of adaptive immune responses by activating antigen-presenting cells (APC), while ensuring that enhanced antigen-specific responses occur only when a pathogen is present 3,4. However, while the importance of innate immune recognition in shaping adaptive immune responses is established, a role for adaptive immune cells in regulation of innate inflammation is largely unexplored.
Here, we investigate the ability of memory CD4+ T cells to regulate innate inflammatory cytokine and chemokine (IIC) production following influenza (flu) infection. Memory CD4+ T cells are critical for optimal heterosubtypic immunity against flu 5, but how they contribute to protection is not well understood 6. While virus-specific T cell responses peak about one week after heterosubtypic challenge, distinguishing characteristics of memory as compared to naïve T cells including less stringent requirements for antigen density and co-stimulation, and rapid production of a broader range of cytokines, suggest that memory cells could have important functions at earlier stages of infection 7–9.
We show that memory, but not naïve, CD4+ T cells act to markedly enhance early expression of IIC and enhance viral control. Induction of IIC requires that TH1- or TH17-polarized memory cells recognize antigen presented by CD11c+ major histocompatibility complex (MHC)-II+ cells in the lung. The protective response is coincident with activation of CD11c+ cells, but independent of IFN-γ, TNF-α, and PAMP-recognition pathways. Similar IIC induction occurs when protein-specific memory cells recognize antigen in the absence of infection. These results show that memory CD4+ T cells responding at the site of infection provide enhanced protection via a novel, pathogen-independent pathway for inducing inflammatory mediators.
RESULTS
Memory CD4+ T cells enhance production of IIC
To investigate if memory CD4+ T cells impact innate inflammatory responses upon flu challenge, we measured a panel of IIC following A/PR8 challenge of naïve mice versus mice primed with a heterobsubtypic strain (A/Philippines). At 40 hours post-infection increased levels of IIC were detected in lung homogenates from primed mice (Fig 1a Primed vs Unprimed), and some were enhanced systemically in serum (unpublished observations). Most of the IIC detected remained elevated for several days in primed mice (Supp Fig 1). To determine if memory CD4+ T cells are responsible for enhanced IIC, primed mice were depleted of CD4+ or Thy1.2+ cells by antibody treatment before re-challenge (Supp Fig 2). Both treatments similarly reduced levels of most IIC in primed mice (Fig. 1a), suggesting that memory CD4+ T cells enhance a broad range of innate inflammatory responses at early time-points following flu challenge.
Figure 1. Memory CD4+ T cells induce an acute increase in IIC upon flu infection.
(a) Naïve B6 mice, or mice primed with A/Phil 60 days prior, were treated with isotype, CD4−, or Thy1.2-depleting antibody prior to A/PR8 challenge and levels of IIC in lungs assessed after 40 hrs (n = 5). (b) Bulk CD4+ T cells were isolated from naïve or A/PR8 primed mice (polyclonal memory), and equal numbers transferred to naive hosts or, alternatively, naive or in vivo- or in vitro-generated HNT memory cells were adoptively transferred to naive BALB/c hosts. All recipients were challenged with A/PR8 and lung homogenates assessed for IIC after 40 hrs (n = 5). Dotted lines in all figures represent levels of IIC in the absence of infection. Error bars indicate SD; * P < 0.05, ** P < 0.005, *** P < 0.001 (one-way ANOVA followed by Bonferroni’s post hoc test).
Since other populations are altered by priming 10,11 and since antibody may not deplete memory T cells completely 12, we next transferred bulk CD4+ T cells from flu primed mice to unimmunized hosts. Despite the small fraction of flu-specific memory cells in the bulk population, transfer of primed cells significantly enhanced IIC at 40 hours post-infection (Fig 1b Naïve vs. Memory Polyclonal) but did not result in global increases in inflammation as levels of interleukin-4 (IL-4), IL-5, IL-10, and IL-13 were unchanged (unpublished observations). To compare equal numbers of antigen-specific naïve and memory cells, and to facilitate mechanistic analysis, we utilized T cell receptor (TCR) transgenic CD4+ T cells recognizing the A/PR8 hemagglutinin protein (HNT) 13. We generated memory cells in vivo by transferring naïve HNT cells to hosts and then infected with a sublethal dose of A/PR8. We allowed virus to clear and memory cells to develop for at least 40 days before re-isolation. As flu-specific CD4+ T cell responses are largely TH1 in nature 6, we also generated memory cells in vitro by resting TH1-polarized effectors for 3 days in the absence of cytokine and antigen. We showed previously that in vitro-generated memory cells are virtually identical to long-term in vivo memory cells as assessed by broad criteria 14. We found that transfer of either in vivo- or in vitro-generated memory cells induced similar increases in IIC (Fig 1b), and levels remained elevated for several days in the lung and serum (Supp Fig 3). These results demonstrate that memory CD4+ T cells present in otherwise naïve hosts drive enhanced IIC with comparable kinetics to those observed after flu challenge of primed mice.
Memory cells enhance IIC independently of TH1 cytokines
TH1 but not other subsets of memory CD4 T cells produce abundant IFN-γ upon stimulation (Supp Fig 4) and could thus account for increased IFN-γ levels observed upon flu challenge. To determine whether memory cells stimulate IFN-γ production from other sources, we generated TH1-polarized memory cells recognizing ovalbumin (OVA) from IFN-γ-deficient TCR transgenic OT-II cells, transferred them to B6 hosts, and infected with virus expressing OVA (A/PR8-OVAII)15. Memory cells induced elevated IFN-γ on days 2 and 3 post-infection (Fig 2a), indicating IFN-γ production from sources other than donor cells. Employing IFN-γ reporter (Yeti) mice as hosts 16, increased IFN-γ (eYFP) signal was observed from natural killer (NK) cells and γδ+ T cells but not from MHC-II+, CD8+, or Gr-1+ cells, indicating contributions from multiple innate populations to the enhanced IIC response (Fig 2b). In support of this conclusion, memory cell transfer to host mice carrying the severe combined immune deficiency (SCID) mutation drove elevated IIC comparable to wild-type (WT) hosts upon flu challenge (Supp Fig 5).
Figure 2. Role of IFN-γ, TNF-α, and CCL3 in IIC upregulation by memory CD4+ T cells.
(a) Naïve or TH1-polarized memory populations generated from Ifng−/− CD4+ T cells were transferred to unprimed mice then infected with A/PR8. Fold-increase in IFN-γ detected in lung homogenate with transfer of memory versus naïve cells for days 2 and 3 post-infection is shown (n = 5). (b) Memory OT-II cells were transferred to unprimed B6 YETI hosts and lung cells stained for indicated surface markers to identify IFN-γ producing cells (eYFP+) (n = 5). Naïve or TH1-polarized memory OT-II cells were transferred to WT, Ifngr−/−, Tnfrsf1ab−/−, or Ccr5−/− hosts then infected with A/PR8. Level of IIC at 40 hours post-infection (c) and (d) viral titer on day 4 post-infection (n = 5 mice per day). Error bars indicate SD; * P < 0.05, ** P < 0.005, *** P < 0.001 (Students t-test).
Because IFN-γ is a potent regulator of inflammation 17,18, we evaluated its role in driving enhanced IIC. We transferred memory cells to IFN-γ receptor-deficient (Infgr−/−) or WT hosts and observed similar IIC with the exception of CXCL9 and CXCL10 (Fig 2c). As TH1 memory cells also produce TNF-α and CCL3 (Supp Fig 4), we employed TNF-α receptor-deficient (Tnfrsf1ab−/−) and Ccr5−/− hosts and again observed similar upregulation of IIC (Fig 2c). These results indicate that memory CD4+ T cells enhance a broad spectrum of IIC in an IFN-γ-, TNF-α-, and CCL3-independent manner, but are consistent with a requirement for IFN-γ for optimal CXCL9 and CXCL10 induction 19,20.
To investigate how enhanced IIC responses impact the course of flu infection we measured viral titers. Compared to naïve OT-II cells, memory transfer resulted in significantly lower titers in WT, Infgr−/−, Tnfrsf1ab−/−, and Ccr5−/− mice on day 3 (not shown) and 4 post-infection with A/PR8-OVAII (Fig 2d). These results demonstrate that enhanced IIC responses mediated by memory CD4+ T cells correlate with viral control following flu infection.
Lung-resident TH1 and TH17 memory cells enhance IIC and viral control
As TH1-polarized memory cells enhance IIC and viral control independently of major TH1 cytokines, we next tested the ability of other subsets of memory CD4+ T cells to mediate the same response. We transferred TH1, TH2, TH17, or unpolarized (TH0) memory HNT cells to naïve mice and infected with A/PR8. TH1 and TH17 memory cells drove similar enhanced IIC levels at 40 hours post-infection while TH2 transfer only increased IL-13 and CCL2 and TH0 transfer had little impact on most IIC measured (Fig 3a). Strikingly, TH1 and TH17, but not TH2 or TH0, transfer also reduced viral titers at day 3 and 4 post-infection (Fig. 3b). These results support the hypothesis that the IIC response induced by TH1 and TH17 memory CD4+ T is responsible for protection, and are consistent with studies demonstrating that TH1- and TH17- but not TH2-polarized HNT responses protect unprimed mice against lethal flu challenge 21, 22.
Figure 3. TH1- or TH17-polarization is required for enhanced IIC response and viral control.
Naïve, TH1-, TH17-, TH2-polarized or TH0 unpolarized memory HNT cells were transferred to BALB/c hosts then infected with A/PR8. (a) Levels of IIC in lungs 40 hrs post-infection (n = 5). (b) Pulmonary viral titers (n = 5 per day). Error bars indicate SD; * P < 0.05, ** P < 0.005, *** P < 0.001 (one-way ANOVA followed by Bonferroni’s post hoc test).
To understand the mechanisms leading to enhanced IIC responses, we examined the fate of transferred HNT cells 40 hours after flu infection. Similar numbers of naïve and memory cells were detected in all organs tested (Fig 4a), neither population had undergone division as assessed by CFSE analysis, and both had up-regulated expression of the early activation marker CD69 in draining lymph nodes but not the spleen (Fig 4b). In the lung, however, only memory cells up-regulated CD69, and did so in infected but not uninfected mice. To evaluate if enhanced IIC production required memory cell activation in the DLN before extravasating to the lung, or if activation in the lung 23 was sufficient, we employed lymphotoxin-deficient (Lta−/−) hosts that lack peripheral LN 24 with or without splenectomy. There was similar enhancement of IIC in both sham-treated and splenectomized Lta−/− mice compared to WT mice following transfer of memory CD4+ T cells (Fig 4c), suggesting that interactions in the lung are sufficient to enhance IIC and control virus (Fig 4d).
Figure 4. Recognition of antigen in the lung is sufficient for IIC upregulation.
CFSE-labeled Thy-disparate naïve or TH1-polarized memory HNT cells were transferred to separate hosts then infected with A/PR8 (n = 5 per group). (a) Numbers of donor cells in spleen, draining lymph node (dLN), and lung 40 hrs post-infection. (b) Representative CFSE and CD69 expression of donor cells 2 and 6 days post-infection. Naïve or memory OT-II cells were transferred to sham treated or splenectomized Lta−/− hosts and infected with A/PR8-OVAII. (c) Pulmonary IIC levels at 40 hrs post-infection (n = 5). (d) Pulmonary viral titers (n = 5 per day). Error bars indicate SD; * P < 0.05, ** P < 0.005, *** P < 0.001 (one-way ANOVA followed by Bonferroni’s post hoc test or t-test).
Cellular mechanism of induction of IIC by memory T cells
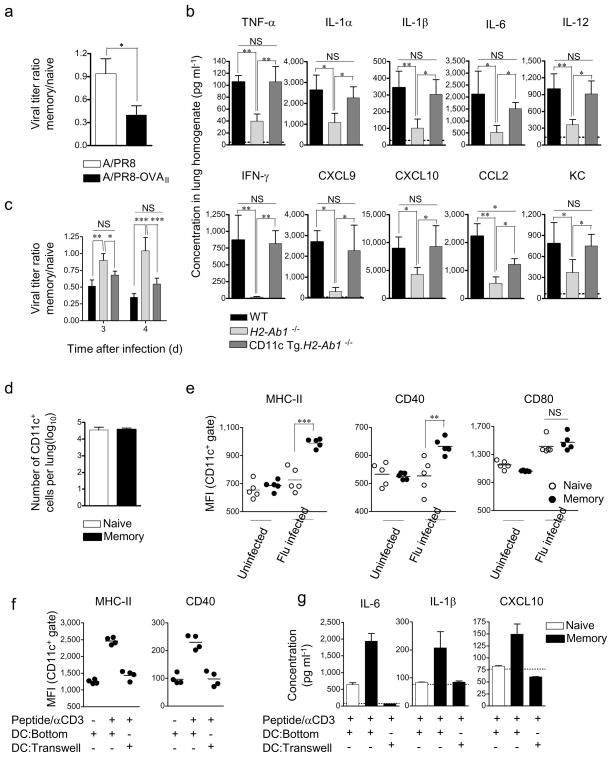
We next studied the cellular interactions required for IIC induction and viral control. To determine the need for cognate antigen recognition we transferred OT-II memory cells and infected with either PR8 or PR8-OVAII. OVA-specific cells drove increased IIC (not shown) and reduced viral titers following infection with A/PR8-OVAII, which expresses the antigen recognized by OT-II cells, but not A/PR8, which does not (Fig 5a). To test whether antigen recognition on lung epithelial cells, which up-regulate MHC-II upon infection 25, and or on CD11c+ APC is critical, we transferred memory OT-II cells to MHC-II-deficient (H2-Ab1−/−) hosts or to hosts expressing MHC-II only on CD11c+ cells (CD11c Tg.H2-Ab1−/−) 26. There was no induction of IIC or viral control in H2-Ab1−/− hosts, but both were observed in CD11c Tg.H2-Ab1−/− mice (Fig 5b and c), indicating that antigen recognition is critical, and that presentation by MHC-II+ CD11c+ cells is sufficient, for memory CD4+ T cells to enhance IIC and control virus.
Figure 5. Cognate recognition of antigen on MHC Class II expressing CD11c+ cells is sufficient to induce IIC upregulation.
(a) Naïve or TH1-polarized OT-II memory cells were transferred to naive B6 hosts then infected with A/PR8 or A/PR8-OVAII. To facilitate comparison of the different viruses, the ratio of viral titers on day 4 in mice receiving memory and naïve OT-II cells is shown (n = 5 per group per virus). (b) Levels of IIC in lung homogenates 40 hrs post A/PR8-OVAII infection in WT, H2-Ab1−/−, or CD11c Tg.H2-Ab1−/− mice receiving TH1-polarized memory OT-II cells (n = 5) and (c) pulmonary viral titers. (d) Number of CD11c+ cells in the lung 40 hrs post infection in mice receiving naïve or memory cells and (e) expression of MHC-II, CD40 and CD80 on CD11c+ cells. (f) Expression of MHC-II and CD40 on DC cultured with memory cells. (g) Cytokines detected in 48 hr supernatants of naïve or memory HNT cells cultured with DC. Error bars indicate SD; * P < 0.05, ** P < 0.005, *** P < 0.001 (one-way ANOVA followed by Bonferroni’s post hoc test or t-test).
To investigate if memory cells also regulate APC function upon flu challenge we assessed the number and activation status of CD11c+ cells in the lung. We found similar numbers of CD11c+ cells in recipients of memory or naïve CD4+ T cells following infection (Fig 5d), but memory transfer dramatically enhanced the activation of CD11c+ cells as shown by up-regulation of MHC-II and CD40 expression (Fig 5e). These were also up-regulated when memory, but not naïve, HNT cells were cultured in vitro with bone marrow-derived dendritic cells (DC) and peptide for 40 h (Fig 5f). Memory cells also induced secretion of higher levels of several IIC from DC versus culture with naïve cells (Fig 5g), and similar results were observed employing the alveolar macrophage line MH-S (unpublished observations). To evaluate if direct cell contact was required for DC activation, memory cells were separated from DC in a transwell system and stimulated with CD3-specific antibody to induce cytokine production. DC separated by the transwell did not upregulate MHC-II or CD40 (Fig 5f) and elevated levels of IIC were not seen (Fig 5g). These results show that memory CD4+ T cells deliver cell-contact-dependent signals that enhance the activation of the APC and drive their production of several IIC.
Memory CD4+ T cells amplify IIC independent of PAMPs
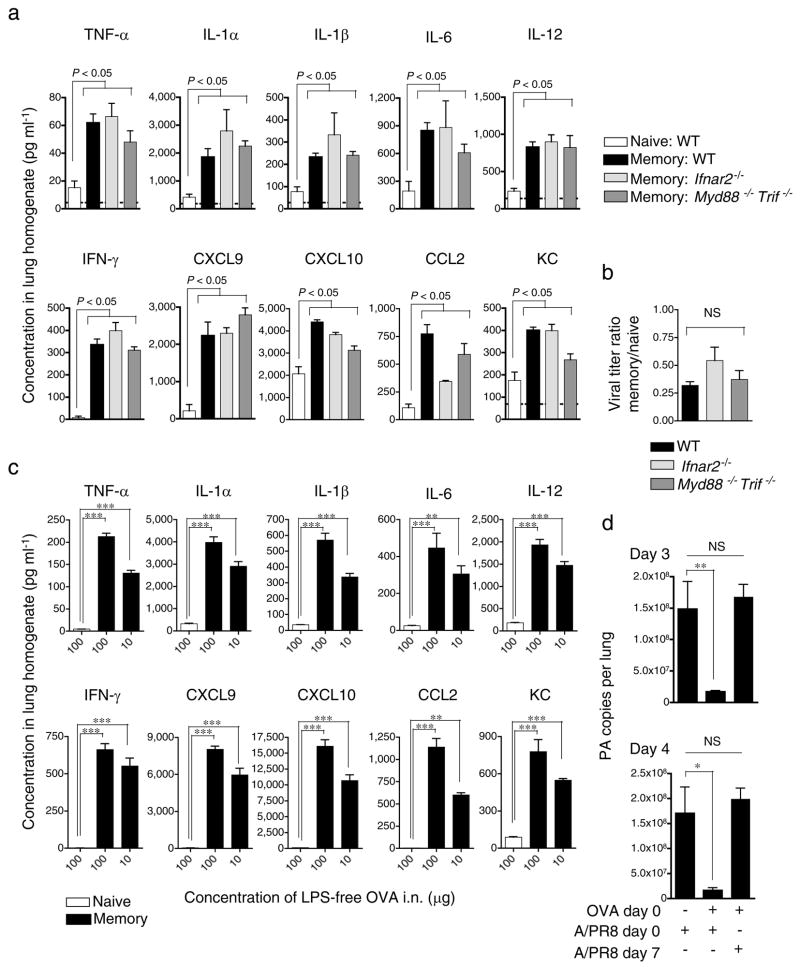
Flu viruses trigger Toll-like receptors 27,28 and drive type I IFN production through RIG-1 29. Flu-induced effects through these pathways might synergize with or facilitate those mediated by memory CD4+ T cells leading to enhanced IIC and viral control. We transferred memory OT-II cells to IFN-α/β receptor-deficient (Ifnar2−/−), MyD88 and TRIFF-deficient (MyD88−/− Trif−/−) or WT hosts and infected with A/PR8-OVAII. We found similar enhanced IIC and decreased viral titers in all hosts 40 hours post-challenge (Fig 6a and b), suggesting that these major PAMP receptor pathways are not involved.
Figure 6. Memory CD4+ T cells induce IIC responses independently of PAMP recognition.
(a) Naïve or TH1-polarized OT-II memory cells were transferred to naive WT, Ifnar2−/−, or Myd88−/− Trif−/− hosts then infected with A/PR8-OVAII. Levels of IIC detected in lungs 40 hrs post-infection (n = 5), and (b) viral titers. (c) Naïve or TH1-polarized memory OT-II cells were transferred to B6 hosts then administered 100 or 10 μg of soluble LPS-free OVA i.n. and levels of IIC assessed after 40 hrs (n = 5). (d) Memory OT-II cells were transferred to B6 hosts administered LPS-free OVA as in (c) and infected with A/PR8 on the same day or 7 days later, or only infected with A/PR8, and viral titers determined (n = 5 per group). Error bars indicate SD; * P < 0.05, ** P < 0.005, *** P < 0.001 (one-way ANOVA followed by Bonferroni’s post hoc test or t-test).
Given this independence, we asked if enhanced IIC could be induced by protein antigen in the absence of infection. We administered lipopolysaccharide (LPS)-free OVA protein intranasally and still found broad increases in IIC in the presence of memory OT-II cells (Fig 6c). Moreover, when LPS-free OVA was administered together with A/PR8, the presence of memory cells specific for OVA resulted in reduced viral titers, demonstrating that while antigen recognition is required for enhanced IIC, the impact of IIC and viral control occur by an indirect mechanism no longer requiring T cell recognition (Fig 6d). To test if viral control correlates with a transient burst of IIC or if memory cells induce a longer-lived protective state, we transferred memory cells to mice and administered LPS-free OVA on the same day (Day 0), and infected with A/PR8 7 days later. When infection was delayed, OVA-specific memory cells provided no protection (Fig 6d). These results show that memory CD4+ T cells induce IIC and that this or other aspects of their activation results in non-specific induction of a transient protective state that acts during the initial phases of infection.
DISCUSSION
Here we demonstrate a novel form of direct control by memory CD4+ T cells over innate inflammatory responses. We show that memory CD4+ T cells recognizing antigen presented by APC results in the activation of the latter and enhanced production of a broad range of IIC from multiple cellular sources independently of their own production of major TH1-associated cytokines. Most importantly, we show this response is strongly correlated with control of virus during the early phases of pathogen challenge.
How might this mechanism contribute to heterosubtypic responses against flu? Our previous studies demonstrated that while survival of flu-primed mice after heterosubtypic challenge was CD8+ T cell-dependent, CD4+ T cell depletion resulted in severely compromised protection 5. We therefore suggest that early viral control provided by memory CD4+ T cells may be mediated by enhanced IIC that could provide an important restraint on viral replication before the development of substantial effector CD8+ T cell responses that ultimately clear virus. Furthermore, since innate responses also regulate the development of adaptive immunity, we speculate that CD8+ T cell and B cell responses against flu may be significantly altered or enhanced through the action of virus-specific memory CD4+ T cells, in particular through the earlier activation of APC populations. In support of this hypothesis, we show that the protective impact of enhanced IIC itself is transitory, which may help to explain the failure of memory CD4+ T cells alone to protect against supra-lethal heterosubtypic challenge 5.
Previous studies suggested that naïve CD4+ T cells or uncharacterized effectors and memory phenotype cells obtained from unmanipulated mice dampen inflammation thereby preventing pathology 30,31. We found that only well-polarized TH1 and TH17, but not TH2 or unpolarized memory cells upregulated IIC. Our results thus highlight the ability of polarized CD4+ T cells to differentially impact IIC. We speculate that the activation status and polarization of T cells, as well as the nature of the challenge, and the context of antigen encounter may all influence regulation of IIC. In agreement with recent studies, we show that augmentation of IIC during flu infection can be a key element of a protective response 32,33, and since TH2 or TH0 cells neither protect nor induce IIC we suggest that tempering acute inflammation IIC may be deleterious.
We envisage several teleological benefits for memory CD4+ T cell induction of IIC. First, this mechanism may represent an important failsafe for eliciting optimal inflammation and rapid containment of infection in situations where pathogens evade PAMP recognition 34. Second, since memory cells can be induced by low concentrations of antigen and costimulation, they may activate innate effector mechanisms before pathogen levels are sufficient to trigger robust responses through PAMP recognition. Independent recruitment of IIC by memory cells may also counteract strategies to dampen innate responses employed by pathogens, including influenza 35. Finally, cytokines such as IL-12 and IL-6 are key in the activation of CD8+ T cells and B cells 36,37, and memory CD4+ T cell enhancement of IIC may thus facilitate optimal development of adaptive responses at later stages following infection.
The protective versus detrimental role of individual IIC during flu infection is not well understood 38. Our results suggest that IFN-γ, TNF-α, CCL3, CXCL-9, and CXCL-10 are not required for early viral control. On the other hand, protective roles for IL-1, IL-6 and IL-12 have been reported 39–41 and these factors were significantly enhanced by protective memory CD4+ T cells. Thus, we suggest that it is most likely that the protective impact of memory CD4+ T cells is not dependent on any one element of IIC alone, but a combination of many. Further studies will be required to determine the degree to which these factors, and others, alone or in combination contribute to viral control.
Our results support the hypothesis that cognate recognition of antigen on CD11c+ APC by memory CD4+ T cells is the initiating step in driving IIC production from various innate cells. Most likely, several of the IIC assayed are produced by multiple cellular populations. For example, alveolar macrophages produce robust amounts IL-1, IL-6 and TNF-α; alveolar epithelial cells can produce IL-1, TNF-α, and CCL2; NK cells produce IFN-γ, TNF-α, and CCL3; and activated DC are sources IL-6, IL-12, TNF-α, CXCL10, and type I IFN 36,42–45. Our observations of enhanced levels of many IIC from DC and alveolar macrophages stimulated in vitro combined with observations of multiple cellular sources of IFN-γ in vivo support the hypothesis that the initial activation of APC is sufficient to initiate a cytokine and chemokine cascade that ultimately results in viral control 46. Defining critical signals involved in this process will require further study, although our preliminary experiments have ruled out roles for the costimulatory molecules CD28, CD40L, OX40, and ICOS (unpublished observations).
Of particular interest is the fact that very few donor memory T cells (thousands) are seen in the lung in these studies. Yet these small numbers are sufficient to induce IIC production even when induced by low levels of non-replicating antigen. We speculate that this novel mechanism of IIC upregulation could help explain why memory CD4+ T cells have been implicated in several models of autoimmunity including diabetes, experimental autoimmune encephalomyelitis, and collagen-induced arthritis 47–49. Further studies will need to determine the full risks and benefits of IIC induced by memory CD4+ T cells in the absence of PAMP recognition.
METHODS
Mice
Male BALB/c, C57BL/6, MHC-II-deficient (H2-Ab1−/−), CD11c Tg.H2-Ab1−/−, Ltα−/−, IFNg−/−, Ifnar2−/−, Ccr5−/−, Myd88−/− Trif−/−, Bicistronic IFN-γ reporter mice (YETI), and HNT (Vα15, Vβ8.3) and OT-II (Vα2, Vβ5) TCR Tg mice were obtained from Trudeau Institute. Male SCID, Tnfrsf1ab−/−, and nude mice were purchased from Jackson Laboratories. All infected animals were at least 8 weeks old. All experimental animal procedures were conducted in accordance with the Trudeau Institute Animal Care and Use Committee guidelines.
Naïve and memory CD4 T cell isolation
Naïve CD4+ T cells were obtained from pooled spleen and lymph nodes of unimmunized mice as previously described 14. TH0, TH1, TH2, and TH17 effectors were generated from naïve TCR Tg CD4+ T cells as previously desribed14,22 and in vitro-generated memory cells generated from effectors as previously described 14. In some experiments, CD4+ T cells were CFSE labeled.
Polyclonal flu-specific memory CD4 T cells were enriched by MACS purification (Miltenyi) of bulk CD4+ T cells from mice primed 60 days previously with A/PR8. In vivo-generated memory HNT cells were obtained by first transferring naïve HNT cells to nude hosts then infected with a sublethal dose of A/PR8. Donor HNT cells were isolated after 40–60d via CD4+ MACS, after host mice had completely cleared virus. The purity of Vβ8.3+ Thy1.1+ CD4+ cells was determined by flow cytometry.
Adoptive T cell transfers and T cell depletion
Naïve or memory cells were adoptively transferred in 200 μl PBS by i.v. injection to naïve hosts. In all experiments, mice received equal numbers of naïve and memory CD4+ T cells (5 × 106 TCR Tg or 1 × 107 polyclonal cells).
In some experiments, mice were administered 500 μg of either CD4-depleting (GK1.5) or Thy1.2-depleting (30H12) antibody via i.p. injection.
Virus stocks, quantitation of viral titer, OVA protein, and infections
Influenza A/PuertoRico/8/34 (A/PR8, H1N1) virus was produced in the allantoic cavity of embryonated hen eggs from virus stocks originating at St. Jude Children’s Hospital and characterized by a core facility at the Trudeau Institute. A/Philippines (A/Phil, H3N2), obtained from S. Epstein, and engineered virus A/PR8-OVAII obtained from P. Doherty, were prepared similarly.
Mice were infected intranasally under light isoflurane anesthesia (Webster Veterinary Supply) with 50 μl of virus in PBS. In some experiments, mice first received a 500 EID50 (about 0.1 LD50) of priming virus 30–60 days previous to heterosubtypic challenge. All challenge doses of virus were 10,000 EID50 (about 2 LD50). Viral titers were determined by quantitation of viral RNA as previously described 22 and the number of copies of PA gene per lung calculated.
LPS-free whole OVA protein was a generous gift from Dr. T. Moran (Mount Sinai School of Medicine, NY, NY)
Cell Culture
Naïve or memory HNT CD4+ T cells, 5 × 104 per well, were cultured in vitro in Costar 24 well plates (Corning) in the absence or presence of HNT peptide with 1 × 105 bone marrow-derived dendritic cells (DC), prepared as previously described 50. Alternatively, naïve and memory CD4+ T cells were stimulated with CD3-specific antibody (αCD3) and were cultured with BMDC present either in the bottom of the well or within transwell inserts (0.4 μm).
Flow cytometry
Mice were euthanized followed by exsanguination achieved by perforation of the abdominal aorta and lungs perfused by injecting 10 ml of PBS in the left ventricle. Single cell suspensions were prepared from lungs, spleen, and mediastinal lymph nodes by mechanical disruption and passage through a nylon membrane.
Flow cytometry was performed as previously described14 using APC-labeled Thy1.1-specific (OX-7) Thy1.2-specific (53-2.1), PerCP-labeled CD4-specific (RM4.5), and PE-labeled Vβ8.3-specific (1B3.3) antibody to identify donor T cells. PE-labeled CD69-specific (H12F3) antibody, and isotype controls were used for phenotype analysis. Analysis was performed using Becton Dickson FACS Scan (BD Biosciences) and FlowJo (Tree Star) software.
Detection of inflammatory cytokines and chemokines
Lungs were harvested and homogenized in RPMI 1640 media supplemented with 2mM L-glutamine, 100 IU penicillin, 100 μg ml−1 streptomycin (Invitrogen), 10 mM HEPES (Research Organics), 50 μM 2-mercaptoethanol (Sigma-Aldrich) and 7.5% fetal bovine serum (Hyclone).
Levels of cytokines and chemokines in lung homogenates, serum, or culture supernatants were determined using mouse multi-plex luminex kits (Invitrogen) read on a Luminex 100 reader (Luminex Corp.) Levels of cytokines and chemokines in unprimed mice are represented by dotted lines in all figures; as we generally observed no significant differences in levels of protein in unprimed mice of different strains, WT values are depicted.
Statistical analysis
Unpaired, two-tailed, Students t-tests, ∝ = 0.05, were used to assess whether the means of two normally distributed groups differed significantly. The Welch-correction was applied when variances were found to differ. One-way ANOVA analysis with Bonferroni’s multiple comparison post-test was employed to compare multiple means. Significance is indicated as * P < 0.05, ** P < 0.005, *** P < 0.001. All error bars represent the standard deviation.
Supplementary Material
Acknowledgments
Supported by the US National Institutes of Health (P01AI04630 to and P01AI04566 to S.L.S), the Department of Defense (HR#3222), and Trudeau Institute. We thank A. Cooper, J. Kohlmeier, M. Mohrs, and D. Woodland for generously providing mice.
Footnotes
Note: Supplementary information is available at the Nature Medicine website
AUTHOR CONTRIBUTIONS
T.M.S. and K.K.M. contributed equally to the design, processing, collection, and analysis of data, and together with S.L.S wrote the paper. S.L.S. and R.W.D. contributed to study design. J.P.D., Y.K., C.W., J.D.C., and G.H. processed and collected data. All authors discussed results and commented on the manuscript.
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annual review of immunology. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature immunology. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 3.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199:227–250. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 4.Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science (New York, N Y) 2001;293:253–256. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- 5.Powell TJ, et al. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J Immunol. 2007;178:1030–1038. doi: 10.4049/jimmunol.178.2.1030. [DOI] [PubMed] [Google Scholar]
- 6.Swain SL, et al. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–2346. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 8.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164:265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 9.Bradley LM, Duncan DD, Yoshimoto K, Swain SL. Memory effectors: a potent, IL-4-secreting helper T cell population that develops in vivo after restimulation with antigen. J Immunol. 1993;150:3119–3130. [PubMed] [Google Scholar]
- 10.Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nature immunology. 2004;5:337–343. doi: 10.1038/ni1041. [DOI] [PubMed] [Google Scholar]
- 11.Didierlaurent A, et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. The Journal of experimental medicine. 2008;205:323–329. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chace JH, Cowdery JS, Field EH. Effect of anti-CD4 on CD4 subsets. I. Anti-CD4 preferentially deletes resting, naive CD4 cells and spares activated CD4 cells. J Immunol. 1994;152:405–412. [PubMed] [Google Scholar]
- 13.Scott B, et al. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994;1:73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 14.McKinstry KK, et al. Rapid default transition of CD4 T cell effectors to functional memory cells. The Journal of experimental medicine. 2007;204:2199–2211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas PG, et al. An unexpected antibody response to an engineered influenza virus modifies CD8+ T cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2764–2769. doi: 10.1073/pnas.0511185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer KD, et al. The functional heterogeneity of type 1 effector T cells in response to infection is related to the potential for IFN-gamma production. J Immunol. 2005;174:7732–7739. doi: 10.4049/jimmunol.174.12.7732. [DOI] [PubMed] [Google Scholar]
- 17.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annual review of immunology. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 18.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 19.Amichay D, et al. Genes for chemokines MuMig and Crg-2 are induced in protozoan and viral infections in response to IFN-gamma with patterns of tissue expression that suggest nonredundant roles in vivo. J Immunol. 1996;157:4511–4520. [PubMed] [Google Scholar]
- 20.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 22.McKinstry KK, et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science (New York, N Y) 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 24.De Togni P, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science (New York, NY. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 25.Debbabi H, et al. Primary type II alveolar epithelial cells present microbial antigens to antigen-specific CD4+ T cells. American journal of physiology. 2005;289:L274–279. doi: 10.1152/ajplung.00004.2005. [DOI] [PubMed] [Google Scholar]
- 26.Lemos MP, Fan L, Lo D, Laufer TM. CD8alpha+ and CD11b+ dendritic cell-restricted MHC class II controls Th1 CD4+ T cell immunity. J Immunol. 2003;171:5077–5084. doi: 10.4049/jimmunol.171.10.5077. [DOI] [PubMed] [Google Scholar]
- 27.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science (New York, N Y) 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 28.Imai Y, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pichlmair A, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science (New York, NY. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 30.Guarda G, et al. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009;460:269–273. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- 31.Kim KD, et al. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salomon R, Hoffmann E, Webster RG. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc Natl Acad Sci U S A. 2007;104:12479–12481. doi: 10.1073/pnas.0705289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuvim MJ, Evans SE, Clement CG, Dickey BF, Gilbert BE. Augmented lung inflammation protects against influenza A pneumonia. PLoS One. 2009;4:e4176. doi: 10.1371/journal.pone.0004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalinski P, Moser M. Consensual immunity: success-driven development of T-helper-1 and T-helper-2 responses. Nat Rev Immunol. 2005;5:251–260. doi: 10.1038/nri1569. [DOI] [PubMed] [Google Scholar]
- 35.Hale BG, Randall RE, Ortin J, Jackson D. The multifunctional NS1 protein of influenza A viruses. J Gen Virol. 2008;89:2359–2376. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- 36.Joffre O, Nolte MA, Sporri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 37.Dienz O, et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. The Journal of experimental medicine. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szretter KJ, et al. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol. 2007;81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SW, Youn JW, Seong BL, Sung YC. IL-6 induces long-term protective immunity against a lethal challenge of influenza virus. Vaccine. 1999;17:490–496. doi: 10.1016/s0264-410x(98)00223-0. [DOI] [PubMed] [Google Scholar]
- 41.Hama Y, et al. Interleukin 12 is a primary cytokine responding to influenza virus infection in the respiratory tract of mice. Acta Virol. 2009;53:233–240. doi: 10.4149/av_2009_04_233. [DOI] [PubMed] [Google Scholar]
- 42.GeurtsvanKessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 2008;1:442–450. doi: 10.1038/mi.2008.39. [DOI] [PubMed] [Google Scholar]
- 43.McGill J, Heusel JW, Legge KL. Innate immune control and regulation of influenza virus infections. J Leukoc Biol. 2009 doi: 10.1189/jlb.0509368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monteiro JM, Harvey C, Trinchieri G. Role of interleukin-12 in primary influenza virus infection. J Virol. 1998;72:4825–4831. doi: 10.1128/jvi.72.6.4825-4831.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao MQ, et al. Alveolar epithelial cell chemokine expression triggered by antigen-specific cytolytic CD8(+) T cell recognition. J Clin Invest. 2000;106:R49–58. doi: 10.1172/JCI9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annual review of immunology. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 47.Le Saout C, Mennechet S, Taylor N, Hernandez J. Memory-like CD8+ and CD4+ T cells cooperate to break peripheral tolerance under lymphopenic conditions. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19414–19419. doi: 10.1073/pnas.0807743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elyaman W, et al. Distinct functions of autoreactive memory and effector CD4+ T cells in experimental autoimmune encephalomyelitis. Am J Pathol. 2008;173:411–422. doi: 10.2353/ajpath.2008.080142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Latham KA, Whittington KB, Zhou R, Qian Z, Rosloniec EF. Ex vivo characterization of the autoimmune T cell response in the HLA-DR1 mouse model of collagen-induced arthritis reveals long-term activation of type II collagen-specific cells and their presence in arthritic joints. J Immunol. 2005;174:3978–3985. doi: 10.4049/jimmunol.174.7.3978. [DOI] [PubMed] [Google Scholar]
- 50.Strutt TM, Uzonna J, McKinstry KK, Bretscher PA. Activation of thymic T cells by MHC alloantigen requires syngeneic, activated CD4+ T cells and B cells as APC. Int Immunol. 2006;18:719–728. doi: 10.1093/intimm/dxl009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.