Summary
Creosote bush, Larrea tridentata, is known as chaparral or greasewood in the United States and as gobernadora or hediondilla in Mexico. Nordihydroguaiaretic acid (NDGA), the main metabolite of the creosote bush, has been shown to have promising applications in the treatment of multiple diseases, including cardiovascular diseases, neurological disorders and cancers. Creosote bush is a promising agent of North American herbal medicine, and it has extensive pharmacological effects and specific mechanisms of actions. This review provides an update of recent in vitro and in vivo research about NDGA and describes experimental studies using NDGA as antioxidant. Also, potential medical uses based on the effects of NDGA on the cardiovascular, immune and neurological systems; cancer; tissue engineering; as well as pharmacokinetics and toxicity are discussed.
Keywords: NDGA, antioxidants, inflammation, cardiovascular, cancer, tissue engineering
BACKGROUND
The use of plants for medicinal purposes predates written human history. Ancient Chinese, Egyptian, and Assyrian texts detail the use of herbal therapies. Over the years, the widespread use of herbal medicine has grown to the point that the World Health Organization estimated in 1985 that 65%–80% of the world’s population, or 4 billion people, rely on herbs for their medical needs [1]. Historically, thousands of herbs and their derivatives have been utilized in the treatment of numerous illnesses. They remain the mainstay of indigenous healing practices, and in many countries, they have been regulated as drugs. Among western countries, they are commonly prescribed and have been utilized as adjuncts alongside conventional medical therapies for many years.
Creosote bush, Larrea tridentata, is known as chaparral or greasewood in the United States and as gobernadora or hediondilla in Mexico. It is abundant in the desert areas of the Mexican states such as San Luis Potosi, Coahuila, Chihuahua, Durango, Sonora, Zacatecas, Baja California Norte and Sur, and in the southwest states of the United States such as Arizona, California, Nevada, Texas and New Mexico. Similar species are found in arid zones of South America, mostly in Argentina and Bolivia [2, 3]. Creosote bush is an important plant with a long history of medicinal use. Many indigenous tribes of North America like the Pima, Yaqui, Maricopa and Seri have used extracts and preparations from this plant to treat a wide variety of disorders including chicken pox, skin sores, diabetes, kidney and gallbladder stones, cancer, venereal disease, tuberculosis, colds, and rheumatism [3]. Similarly, the aqueous extracts of the creosote bush have been used by native healers of the southwest region of North America, and is commonly referred to as chaparral tea. This is traditionally implemented for the treatment of kidney and gallbladder stones; it is reported that an infusion of the leaves dissolves gallbladder and kidney stones when the tea is consumed throughout the day [3, 4].
Creosote bush is a notable source of natural products. Approximately 50% of the leaves’ dry weight is extractable matter. The leaves are shiny with a thick resinous coating, which discharges a strong odor and has a sour flavor. The resin that covers the leaves yields multiple flavonoid aglycones, essential oils, halogenic alkaloids as well as several lignans. Among the latter are included, notably, the antioxidant nordihydroguaiaretic acid (NDGA) [5]. NDGA and other phenols of the leaf surface function as antimicrobial agents and as protection against herbivores, UV radiation and water loss. As such, they are potentially important in the preservation of the species in the desert mileu.
NDGA, the main metabolite of the creosote bush, has shown to have promising applications in the treatment of multiple diseases. Several medicinal properties have been supported in cell culture and animal studies as well as historical reports. However, the safety and possible toxicity from its application must still be determined in clinical studies. Among the proposed medicinal properties of NDGA, and one of the main focuses of this review, are its antioxidant effects [6]. Over the years, this compound has been studied and has gained popularity and interest due to its antineoplastic, antiviral and anti-inflammatory characteristics. The molecular mechanisms and medical applications of NDGA have attracted much attention and hundreds of papers have been published in the last few years. This review provides an update of recent in vitro and in vivo research about NDGA and describes experimental studies using NDGA as antioxidant. Also, potential medical uses based on the effects of NDGA on the cardiovascular, immune and neurological systems; cancer; tissue engineering; as well as pharmacokinetics and toxicity are discussed.
CHEMICAL STRUCTURE AND GENERAL PROPERTIES OF NDGA
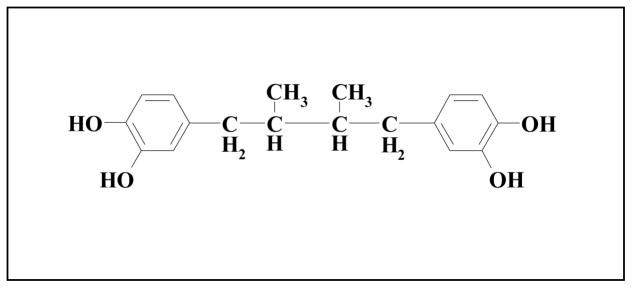
The resin that covers the leaves of creosote bush yields 19 flavonoids as well as several lignans. In terms of natural product chemistry, creosote bush is best known by the large amount of the lignan or pure compound NDGA, also known as Masoprocol. It is estimated that NDGA composes approximately 5 to 10% of the leaves’ dry weight; this corresponds to 80% of all phenolics in the resin. NDGA is a natural compound with broadspectrum of biological properties. It is a polyphenol-bearing o-dihydroxy (catechol) structure (Figure 1); it possesses four phenolic hydroxyl groups. As such, NDGA is recognized as a strong antioxidant with several beneficial health effects [7].
Figure 1.
Chemical structure of nordihydroguaiaretic acid (NDGA). Other names of NDGA are 1,4-bis(3,4-dihydroxyphenyl)-2,3-dimethylbutane, 4,4′-(2,3-dimethyltetramethylene)dipyrocatechol, and Masoprocol.
The Floriano-Sanchez group demonstrated that NDGA is a potent in vitro scavenger of reactive oxygen species (ROS) such as peroxynitrite (ONOO−), singlet oxygen (1 O2), hydroxyl radical (•OH), superoxide anion (O2−•), hydrogen peroxide (H2O2), and hypochlorous acid (HOCl); it was also able to prevent lung tyrosine nitration in vivo [7]. Excessive amounts of ROS can have deleterious effects on many molecules including protein, lipid, RNA and DNA. The unpaired electron(s), coupled with the small size of ROS, make ROS highly reactive; they can attack bases in nucleic acids, amino acid side chains in proteins and the double bonds of unsaturated fatty acids [8]. As described above, the specific scavenging properties of NDGA help to explain its recognized antioxidant properties.
In order to determine the relative efficiency of NDGA in scavenging these species, the authors compared the ability of NDGA to scavenge the above mentioned reactive species with reference compounds such as uric acid, penicillamine, glutathione, and mannitol. They concluded that NDGA is more efficient ONOO−, 1O2, •OH, O2−• scavenger than the reference compounds studied. These strong antioxidant properties may be due to the presence of four reducing equivalents from the two catechol groups in NDGA; hydrogen atoms of the four phenolic hydroxyl groups react with reactive oxygen species [7]. These antioxidant effects, meanwhile, make NDGA an agent that ameliorates the observed renal and hepatic toxicity induced by ferric-nitrilotriacetate and streptozotocin-induced diabetic nephropathy. Also, ozone-induced tyrosine nitration in the lung is prevented. As a result of the lipophilic antioxidant properties of NDGA, it has also been used as a preservative [7, 9, 10].
NDGA has been proven to selectively inhibit arachidonic acid 5-lipoxygenase activity, which reduces leukotriene and prostaglandin synthesis, thus leading to a reduction of inflammatorypathways [11, 12]. NDGA also has profound effects on the secretory pathway, reflected in its ability to block production of leukotriene B4, degranulation, phagocytosis, and the respiratory burst by exerting effects on the mitochondria and nonspecifically inhibiting NADPH oxidase and protein kinase C. Because the ROS generated by mitochrondria during respiration have been implicated in several disease processes, this ability of NDGA may contribute significantly to its therapeutic value [13–15]. NDGA has also been shown to block protein transport from the endoplasmic reticulum (ER) to the Golgi complex, induce the redistribution of Golgi proteins into the ER and affect levels of intracellular calcium [16, 17].
Morerecently, NDGA has been shown to disrupt the actincytoskeleton and exert effects on cell adhesion [18, 19] and also to directly inhibit activationof two receptor tyrosine kinases (RTKs), the Insulin-like growth factor-1 receptor and the c-erbB2/HER2/neu receptor. This results in decreased cellular proliferation [20, 21]. Some reports indicate that NDGA may exert anti-tumor effects by inducing apoptosis in a range of cell lines, including breast cancer, pancreatic carcinoma, multiple myeloma and HL-60 cells [22, 23]. Others showed NDGA’s capacity to block apoptosis either by tumor necrosis factor-α (TNF α) or CD95 ligand [24, 25].
L. tridentata leaves also contain 3-O-methyl NDGA, another lignan with one methoxyl and three hydroxyl side chains, rather than the four hydroxyl groups found on NDGA [26, 27]. 3-O-methyl NDGA, like NDGA, has been shown to exert important antiviral properties by inhibition of viral replication; it also displays anti-inflammatory activity by means of potent cyclooxygenase-2 inhibition [26, 27].
Tetra-O-methyl NDGA (M4N) is a semi-synthetic derivative of NDGA also known as terameprocol (TMP). Several studies have demonstrated the ability of M4N to inhibit the secretion of cytokines, chemokines, and inflammatory lipids from activated macrophages; these include TNF-α in vivo and chemokines monocyte chemotactic protein 1, prostaglandin E2 and other cytokines and inflammatory lipids which are linked to inflammatory diseases like psoriasis and rheumatoid arthritis. These results raise the possibility that M4N may be useful and even advantageous over other TNF-α blockers already available in the treatment for inflammatory diseases mediated by TNF-α and other key inflammatory cytokines and chemokines [25, 26, 28].
VASCULAR BIOLOGY AND CARDIOVASCULAR DISEASE
Antioxidants and antioxidant enzyme systems play an important role in the regulation of free radical levels in the vasculature which can potentially play a role in the development of disease. Numerous studies have verified the benefit that antioxidant therapy offers. Antioxidants diminish the long-term harmful effects on the vascular wall exerted by pathological entities such as hypercholesterolemia, atherosclerosis, diabetes mellitus, and hypertension; the use of antioxidants can halt disease progression. In these pathological states, increased production of ROS is observed, resulting in loss of biological activity of nitric oxide (NO) and impairment of endothelium-dependent relaxation properties.
Zhou et al. [29] have demonstrated in a variety of models that administration of antioxidant therapy can diminish endothelial dysfunction. Ramasamany et al. [30, 31] verified that antioxidants such as NDGA, N-acetylcysteine (NAC), probucol and catechol enhance the expression of endothelial nitric oxide synthase (eNOS) in cultured endothelial cells; not surprisingly, NDGA increased eNOS expression by as much as 3-fold, and its effects were augmented when combined with non-phenolic small antioxidant molecules such as ascorbic acid and NAC. Of note, increases in eNOS and NO bioavailability after treatment with NDGA and/or other antioxidants substantially reduce endothelial dysfunction; as such, the compounds are considered vasoprotective. Kumar et al. [32] observed similar results: NDGA increased eNOS protein expression by 2 to 2.5-fold in fetal pulmonary arterial endothelial cells (FPAECs). These investigators were able to identify the activation protein-1(AP-1) specific binding site as a key element in the mechanism of action of this effect.
There is substantial evidence that antioxidants may inhibit the atherosclerotic process via a variety of mechanisms. These antiatherogenic properties of antioxidants include prevention of low-density lipoprotein oxidation, inhibition of vascular adhesion molecule expression, increase of expression of eNOS and production of endothelial NO. Wedgwood et al. [33] investigated the role of AP-1 in the upregulation of eNOS expression in FPAECs treated with NDGA, and they concluded, importantly, that NDGA increases AP-1 binding, resulting in increased promoter activity. Modulation of eNOS expression by NDGA and similar compounds maybe a useful clinical target for future drug design and clinical studies.
Meanwhile, other studies indicated that NDGA, besides being a specific lipoxygenase inhibitor, also inhibits several other enzymes. For instance, NDGA has been shown to markedly inhibit the ability of TNF-α to activate the expression of vascular cell adhesion molecule 1 (VCAM-1) mRNA levels at concentrations that did not inhibit the activation of transcriptional factor NF-κB. These results suggest that activation of the lipoxygenase pathway and c-Fos activation may mediate, at least in part, the effect of TNF-α in regulating the expression of VCAM-1 in human microendothelial cells [34]. NDGA was also found to selectively inhibit platelet-derived growth factor (PDGF)-stimulated DNA synthesis in Swiss 3T3 cells, diploid murine cells and rat and human fibroblasts [35]. NDGA also selectively inhibited PDGF receptor tyrosine phosphorylation in a dose-dependent manner in intact cells. Protein tyrosine phosphorylation stimulated by epidermal growth factor or bombesin was not altered by NDGA treatment. Crucially, NDGA inhibited in vitro the tyrosine kinase activity of anti-phosphotyrosine and anti-PDGF receptor [35].
CANCER
NDGA has been identified to have a significant role in cancer therapy including that of breast, prostate, lung, esophageal and skin cancers. Models of carcinogenesis have demonstrated the capacity of NDGA to inhibit the growth of several human cancer types both in cell cultures and in animal models. These encouraging results suggest a possible therapeutic chemotherapy role for NDGA [2, 9, 36–38].
In lung cancer, there is evidence to suggest that NDGA exerts a chemo protective activity; NDGA suppresses lung cancer cell growth in cell lines exposed to NDGA [39]. Furthermore, one study demonstrated that adding 0.1% NDGA to the drinking water of athymic mice bearing non-small cell lung cancer tumors significantly inhibits tumor growth compared with control mice [38]. In addition, NDGA has not only been shown to suppress breast cancer cell growth, it has a synergistic effect with retinoic acid on the inhibition of mammary tumor cell transformation and proliferation [40]. Furthermore, NDGA has been shown to be able to induce apoptosis in several human pancreatic and cervical cancer cell lines, which is thought to be due to its activity as a lipooxygenase inhibitor [41]. Similarly, NDGA has been reported to promote cell death of trastuzumab-naive and trastuzumab-refractory human epidermal growth factor receptor 2 (HER2)-overexpressing breast cancer cells by inducing DNA fragmentation. It has also been demonstrated that combination treatment with NDGA and trastuzumab suppressed proliferation and survival of trastuzumab-refractory cells to a greater degree than either agent alone, suggesting that NDGA increases the sensitivity of refractory cells to trastuzumab, a monoclonal antibody to HER2 [42]. Due to the tendency of some breast cancers to develop resistance to trastuzumab, the possibility of developing adjunctive treatments has been explored [43], and NDGA may prove useful in this approach.
Furthermore, NDGA has been shown to significantly inhibit damaging Ultraviolet B irradiation induced signaling pathways in human keratinocytes by two suggested modes of action. First, NDGA acts as an antioxidant to prevent the harmful effect of reactive oxygen species. Secondly, NDGA affects gene expression and differentiation, which is likely through its effects on leukotriene synthesis [2]. Moreover, when topical NDGA is used in the treatment of actinic keratoses, it has been shown to be superior to 5-fluorouracil [44].
The complete mechanisms by which NDGA is anti-tumorigenic and anti-proliferative are still being elucidated. Preliminary in vivo studies have revealed that NDGA suppresses tumor growth by inhibiting metabolic enzymes as well as RTK phosphorylation, which is overexpressed in certain cancer cells [21]. Meanwhile, NDGA has been shown to modulate tumor cell sensitivity to vinblastine by a mechanism independent of interference with the multidrug resistance (MDR1) gene product. NDGA has also been shown to inhibit the incorporation of [3H]-thymidine by K562 chronic myelogenous leukemia cells in culture and reversibly inhibits DNA synthesis by human glioma cells [21, 45]. Additionally, it has been confirmed that NDGA exerts a unique and strong inhibitory effect on transforming growth factor β (TGF-β) type I receptor, a serine threonine kinase receptor, which has a unique signaling pathway involving the Smad family: NDGA treatment inhibited the translocation of Smad2 to the nucleus and strongly repressed Smad2 phosphorylation induced by TGF-β family [46]. NDGA also inhibited downstream transcriptional activation mediated by TGF-β. It is thought that NDGA inhibitory properties are strongly related to the oxidative pathways carried by TGF -β signals [46, 47].
Modification of the structure of NDGA can lead to increased potency. Not surprisingly, this finding has stimulated researchers towards the development of newer, more potent compounds. M4N has been shown to inhibit the growth of certain tumor-derived cell lines and is now in clinical trials for the treatment of human cancer [26]. Several studies have examined and demonstrated its ability to inhibit cancer cell growth in vitro as well as tumoricidal activity in vivo in human tumor cell xenografts in mice [26, 48–50]. It is postulated that M4N blocks cell cycle progression by inhibiting expression of the Sp1-dependent gene coding for cyclin-dependent kinase 1 (cdk1, also known as cdc2 and cyclin B kinase) and promotes apoptosis by inhibiting expression of the survivin gene [49–51]. Cdc2 is one of several kinases controlling mitosis and is deregulated in cancer cells. Survivin is an inhibitor of apoptosis over expressed in many cancers. Tumor cells that overexpress survivin are prevented from entering the caspase-induced cell-death pathway that would otherwise lead to their destruction [49, 51, 52]. Meanwhile, survivin is not normally expressed in non-diseased tissue [53]. Hansel et al. [54] demonstrated that esophageal adenocarcinoma cell lines treated with M4N demonstrated a dose-dependent reduction in cell proliferation, paralleling down-regulation of CDC2/CDK1 transcript and protein levels. M4N, then, may represent a novel site-specific transcription inhibitor that can potentially regulate the function of cell cycle and apoptosis genes aberrantly expressed in many types of human cancers. The safety of M4N, meanwhile, has been assessed in phase I studies using intratumoral administration in bladder, vaginal and oral squamous carcinoma in head and neck cancer patients at different doses. There was no acute or delayed toxicity. Furthermore, a phase I dose-escalation study in patients with cervical intraepithelial neoplasia revealed that intravaginal administration of M4N was well tolerated, and there were no serious or treatment-related adverse events [55, 56]. Clinical trials with M4N in the treatment of human tumors are currently underway for treatment of cervical dysplasia as well as treatment-refractory tumors and brain tumors [57]
NEUROLOGICAL DISORDERS
A range of experimental approaches have revealed a tight connection between neurodegenerative diseases and oxidative stress. Free radicals are implicated in a variety of acute and chronic neurodegenerative disorders, such as cerebral ischemia and Alzheimer’s disease; ROS are thought to be responsible for neuronal injury [58]. A study using hippocampal neurons from rats demonstrated that NDGA prevents neuronal injury and accumulation of ROS. Also, the cultured neurons were protected against the toxicity of amyloid β-peptide (Aβ); Aβ-induced accumulation of ROS and intracellular calcium were suppressed [59]. The study concluded that NDGA has a neuroprotective role which is likely mediated by its lipoxygenase inhibitor/antioxidant properties. As such, it could potentially play a key role in the disruption of neurodegenerative pathways that contribute to the pathophysiology of Alzheimer’s disease and other neurological disorders [59, 60]. Indeed, as mentioned, NDGA is a very potent inhibitor of 5-lipoxygenase in different tissues. It also has considerable inhibitory activity against cyclooxygenase, resulting in a inhibitory effect in arachidonic acid metabolism, and limited n-metyl-D-aspartate (NMDA, a subclass of glutamate receptor) induced neuronal toxicity [61].
Furthermore, the antioxidant activity of NDGA strongly diminishes cytokine secretion by dendritic cells. It significantly protects against post-ischemic cellular and functional damage in the brain by multiple mechanisms, including an α-tocopherol-like scavenging of lipid hydroperoxides [62]. NDGA has also been proven to be a potent anti-ischemia–reperfusion injury agent in vitro and in animal models through different antioxidant pathways [60]. Under this same premise, as neurodegenerative diseases are tightly connected to oxidative stress, NDGA’s antioxidant characteristics make it a potential therapeutic tool for protection against oxidative stress in cerebellar neurons by activation of the nuclear factor erythroid-2 related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) axis [63].
Another cellular mechanism recognized as being neurotoxic is glutamate homeostasis. Numerous studies suggest that glutamate, while being critical for normal synaptic neurotransmission, may be neurotoxic if not tightly regulated. Glutamate is the predominant excitatory neurotransmitter. Abnormal accumulation of glutamate in the synaptic cleft and excessive activation of glutamate receptors contribute to neuronal death, a process known as ‘excitotoxicity.’ This phenomenon is thought to contribute to neuronal loss in neurodegenerative diseases that include Amyotrophic Lateral Sclerosis, Alzheimer’s disease, Parkinson’s disease, stroke, and epilepsy [64]. NDGA has been identified as a compound capable of inducing glutamate uptake and upregulation of expression levels and activity of the glutamate transporter EAAT2 (GLT-1) in mice [65].
VIRUS INFECTION AND IMMUNE MODULATION
In addition to antioxidant, anti-inflammatory and anti-proliferative features, NDGA has also been found to be a potent antiviral and inhibitor of viruses such as human immunodeficiency virus (HIV-1), herpes simplex virus (HSV), human papilloma virus (HPV) and influenza virus [66]. NDGA has been found to suppress Sp1 regulated transcription within HIV and HSV. HPV also contains a prominent Sp1 DNA binding site that plays an active role in the virus gene expression; this is also suppressed by NDGA, leading to inhibition of gene expression from the early promoter P(97) of HPV16. Studies suggest that NDGA and its derivatives bind to DNA, which prevents Sp1 interaction and thus interferes with Sp1 protein transactivation function in gene transcription [58].
Interestingly, NDGA derivatives tetramethyl-O-NDGA and tetra-acetyl NDGA exhibited similar or even higher anti-viral activities than natural compounds. Among these, the synthetic derivative M4N showed the highest anti-HIV activity and inhibition of the replication of HSV in Vero cells and HPV [2, 66]. Moreover, Hwu et al. [67] screened their newly synthesized NDGA derivatives against HIV by using HIV long terminal repeat (LTR) promoter, SEAP, and CMV promoter driven Tat, and found several heterocycle-containing ether NDGAs were stable in aqueous medium and possessed inhibitory activity toward HIV Tat-regulated transactivation in human epithelial cells.
The pathogenesis of influenza virus infection involves not only virus-proliferation-mediated apoptosis in infected cells but also direct cellular injury to the infected as well as the non-infected cells from ROS produced by neutrophils and macrophages infiltrating the infected organs [68]. NDGA possesses antiviral, anti-apoptotic and antioxidant activities. The treatment of influenza virus-infected chorion cells with NDGA revealed not only an effective inhibition of the virus proliferation, but also showed a more potent effect over previous antioxidant tested. These findings raise the possibility of a potential treatment option for patients with influenza virus infection [68]. When cytomegalovirus and adenovirus promoters were challenged against NDGA, there was no effect, suggesting that the antiviral activity of NDGA and its derivatives is selective depending on the virus type [66].
TISSUE ENGINEERING
Tissue engineering involves combining genetic engineering of cells with chemical engineering to create artificial organs and tissues, such as skin, tendons, bones and blood vessels. In recent years, biomaterials have been used to develop tissues such as bridge gapped tendons. Implementation of appropriate tendon bioprosthetic devices requires biocompatable tissues and materials with mechanical properties sufficient to withstand the loads associated with post-surgical mobilization, as well as effective means of integrating these materials into the repair site. Biological materials intended for bioprosthetic tissue replacement are most often treated with cross-linking agents to reduce antigenicity and cell mediated degradation. Recent approaches have focused on cross-linking agents that have low cell toxicity and that can form biocompatible adducts.
NDGA is a bioactive natural product which is able to crosslink collagen. It has been proven to be effective in preparing a scaffold for tendon tissue engineering. Koob et al. [69] have developed methods for stabilizing collagen-based materials with catechol-containing monomers, which were developed in order to produce fibers with mechanical properties with respect to tension that were comparable to those of normal tendon. They found that NDGA and the products resulting from oxidative polymerization with collagen fibers are biocompatible [70]. As a result, NDGA cross-linking may provide a viable approach to stabilizing collagenous materials for use in repair of ruptured, lacerated or surgically transected tendons, as well as other biomaterial constructs for surgical repair of musculoskeletal injuries and disease.
Meanwhile, a new 3D porous and biostable collagen scaffold has been developed by Ju et al. [71, 72] to improve the biocompatibility of implantable glucose sensors by minimizing tissue reactions while stimulating angiogenesis around the sensors. This novel collagen scaffold was crosslinked using NDGA to enhance biostability. NDGA-treated collagen scaffolds have been shown to be stable without physical deformation in the subcutaneous tissue of rats for 4 weeks, an improvement over the frequently used glutaraldehyde (GA)-treated collagen scaffolds, which were extremely damaged following implantation [71]. GA is currently the most common crosslinking agent used for fixation of collagen scaffolds for tissue bioengineering. Ultimately, evaluation of these scaffolds indicates that application of NDGA-crosslinked collagen scaffolds might be a good method for enhancing the function and lifetime of implantable biosensors by minimizing the in vivo foreign body response [72].
NDGA crosslinking material is superior to those treated with GA because the chemistry of the NDGA crosslinking reaction differs from that of GA. The two aldehyde functional groups of the GA molecule react with amine groups between two neighboring polypeptide chains, lysine side chains especially. This links the two collagen chains. However, the presence of unreacted residual groups or the release of monomers and small polymers during enzymatic degradation may potentially cause cytotoxicity [72]. NDGA crosslinking, on the other hand, does not involve forming crosslinks with amino acid side chains of collagen. NDGA possesses two reactive catechols which undergo autooxidation at neutral or alkaline pH producing reactive quinones. These two quinones then couple via aryloxy free radical formation and oxidative coupling, forming bisquinone crosslinks at each end. The NDGA continues forming a large crosslinked bisquinone polymer network in which the collagen fibrils are embedded [72].
NDGA polymerized collagen fibers have also been introduced as a novel local drug delivery system. The drug loading of these biocompatible fibers has been described with the anti-inflammatory agents such as dexamethasone and dexamethasone 21-phosphate, however these fibers can be used to load other agents as well. These fibers were coated with poly(lactic-co-glycolic acid) (PLGA) to control the rate of drug release from the fiber. This novel drug delivery system has the potential broad application for the treatment of various human diseases [73].
Decellularized heart valve scaffolds, the most prevalent tool for heart valve replacement, possess many properties desirable in valvular tissue engineering. However, the non-crosslinked scaffolds have their own limitations, such as short durability, structural dysfunction and immunological incompetence. Lü et al. [74] used NDGA to crosslink decellularized heart valve scaffolds resulting in higher tensile strength, better enzymatic hydrolysis resistance and higher store stability than the non-crosslinked ones. Furthermore, its mechanical properties and cytocompability were superior to that of GA-crosslinked heart valve matrix. Their study demonstrated that NDGA-crosslinking of decellularized valvular matrix is a promising approach for preparation of heart valve tissue engineering scaffolds. Once again, NDGA holds promise as a tool in tissue engineering.
TOXICITY
Herbal medicines are considered by the public and some health care providers as gentle and safe, but in many instances there is a lack of scientific basis for that belief. Many herbal preparations, botanicals and plant extracts that are believed to be harmless and commonly used for self-medication contain chemicals that are similar to those in purified medications. As such, they have the potential to cause serious adverse effects if they are not utilized with caution and under supervision. These compounds may be found mixed within such extracts at random concentrations making it complicated to control the precise dose. Furthermore, they may generate unpredictable interactions with other medications that may be poorly studied.
As reviewed by Arteaga et al. [2], creosote bush and its main metabolite NDGA have shown to be useful in traditional medicine, industry and research, but none has been tested at the clinical level. The toxicity of creosote bush has been demonstrated through in vitro and in vivo studies. However, the reported toxic doses in humans and experimental animals always exceeded the traditional use of the plant. Traditionally, Creosote bush extracts containing NDGA used in healing practices are prepared by boiling in water and then either applied topically as a paste or consumed as a tea. This extraction process may possibly result in a significant alteration of the compound into a weaker, stronger or even a toxic derivate [75].
NDGA has been utilized in traditional healing practices for many years in a wide range of remedies, but at present, it application in clinical settings is limited due to reported toxicity in isolated cases. Its use as a food antioxidant was initially approved by the Meat Inspection Division of the US War Food Administration in 1943. It was utilized commercially in the United States as a preservative for fats and butter until it was shown to induce a cystic nephropathy in the rat; thereafter, it was removed from the FDA’s list of generally regarded as safe (GRAS) agents in 1968. However, Larrea-containing products remained on the over-the-counter, dietary-supplement market. Although consumption of low doses of such products appear to be harmless, high doses has been associated with dermatitis, nephrotoxicity, biliary toxicity, and hepatotoxicity in humans which includes fulminant liver failure, and renal cell carcinoma [76–78].
Hepatic metabolism has been described in mice. Lambert et al. [45] found the lethal dose 50% (LD50) of 75 mg/kg 5 days after a single intraperitoneal dose, and an increase in alanine amino transferase with administration of 50 mg/kg. The high clearance of NDGA, which doubles that of creatinine, suggests that its clearance is not by renal means. One possibility is that it may be extracted by the liver as O-methylation and glutathione conjugation or other metabolic derivatives [76]. Most interestingly, structural modification of NDGA changed the in vivo toxicity. For example, the LD50 of NDGA was found to be 75 mg/kg (intraperitoneal injection), whereas M4N was well-tolerated at 1000 mg/kg (intraperitoneal injection) [49]. Several other tetra-O-substituted NDGA analogs also showed decreased in vitro toxicity [79].
SUMMARY AND FUTURE DIRECTIONS
The main metabolite of Creosote bush NDGA has shown to have promising applications in the treatment of multiple diseases, such as cardiovascular diseases, neurological disorders and cancers, and in the exciting and important field of tissue engineering. Several medicinal properties have been supported by in vitro and in vivo experimental studies, as well as historical reports. This North American herb has extensive pharmacological effects and specific mechanisms of actions. NDGA is a strong antioxidant: it can scavenge ROS or inhibit ROS production, stimulate NO production, increase immune function, enhance central nervous system function, and prevent cardiovascular or other diseases. Tissue engineering studies demonstrate that NDGA-crosslinking is an effective way to improve the mechanical properties and biocompatibility of artificial tissues and organs. However, its clinical applications, safety and toxicity, are still to be determined in clinical studies. Future research on NDGA should include detailed mechanisms of action, specificity, clinically relevant pharmacokinetic and toxicity studies, and therapeutic studies in both animal models and human trials. The observation that chemical modification of this compound reduces toxicity, combined with the observed enhanced therapeutic effects, indicates that the derivatives of NDGA may become important drugs in the future.
Acknowledgments
Source of support: This work was partially supported by grants (R01HL083471, R21CA140828 and R01DE15543) from the National Institutes of Health (NIH) and by the Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, TX, U.S.A. WeakleySM was supported by a NIH grant (T32HL083774).
References
- 1.Pribitkin ED, Boger G. Herbal therapy: what every facial plastic surgeon must know. Arch Facial Plast Surg. 2001;3:127–32. doi: 10.1001/archfaci.3.2.127. [DOI] [PubMed] [Google Scholar]
- 2.Arteaga S, Andrade-Cetto A, Cardenas R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J Ethnopharmacol. 2005;98:231–9. doi: 10.1016/j.jep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Lia VV, Confalonieri VA, Comas CI, Hunziker JH. Molecular phylogeny of Larrea and its allies (Zygophyllaceae): reticulate evolution and the probable time of creosote bush arrival to North America. Mol Phylogenet Evol. 2001;21:309–20. doi: 10.1006/mpev.2001.1025. [DOI] [PubMed] [Google Scholar]
- 4.Lara F, Marquez C. Medicinal plants from Mexico: composition, uses and biological activity. Mexico: UNAM; 1996. [Google Scholar]
- 5.Konno C, Lu ZZ, Xue HZ, Erdelmeier CA, Meksuriyen D, Che CT, et al. Furanoid lignans from Larrea tridentata. J Nat Prod. 1990;53:396–406. doi: 10.1021/np50068a019. [DOI] [PubMed] [Google Scholar]
- 6.Sheikh NM, Philen RM, Love LA. Chaparral-associated hepatotoxicity. Arch Intern Med. 1997;157:913–9. [PubMed] [Google Scholar]
- 7.Floriano-Sanchez E, Villanueva C, Medina-Campos ON, Rocha D, Sanchez-Gonzalez DJ, Cardenas-Rodriguez N, et al. Nordihydroguaiaretic acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion and hypochlorous acid and prevents in vivo ozone-induced tyrosine nitration in lungs. Free Radic Res. 2006;40:523–33. doi: 10.1080/10715760500419365. [DOI] [PubMed] [Google Scholar]
- 8.Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J Cell Mol Med. 2009 Sep 14; doi: 10.1111/j.1582-4934.2009.00897.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ansar S, Iqbal M, Athar M. Nordihydroguairetic acid is a potent inhibitor of ferric-nitrilotriacetate-mediated hepatic and renal toxicity, and renal tumour promotion, in mice. Carcinogenesis. 1999;20:599–606. doi: 10.1093/carcin/20.4.599. [DOI] [PubMed] [Google Scholar]
- 10.Anjaneyulu M, Chopra K. Nordihydroguairetic acid, a lignin, prevents oxidative stress and the development of diabetic nephropathy in rats. Pharmacology. 2004;72:42–50. doi: 10.1159/000078631. [DOI] [PubMed] [Google Scholar]
- 11.Salari H, Braquet P, Borgeat P. Comparative effects of indomethacin, acetylenic acids, 15-HETE, nordihydroguaiaretic acid and BW755C on the metabolism of arachidonic acid in human leukocytes and platelets. Prostaglandins Leukot Med. 1984;13:53–60. doi: 10.1016/0262-1746(84)90102-1. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacherjee P, Boughton-Smith NK, Follenfant RL, Garland LG, Higgs GA, Hodson HF, et al. The effects of a novel series of selective inhibitors of arachidonate 5-lipoxygenase on anaphylactic and inflammatory responses. Ann N Y Acad Sci. 1988;524:307–20. doi: 10.1111/j.1749-6632.1988.tb38554.x. [DOI] [PubMed] [Google Scholar]
- 13.Bokoch GM, Reed PW. Effect of various lipoxygenase metabolites of arachidonic acid on degranulation of polymorphonuclear leukocytes. J Biol Chem. 1981;256:5317–20. [PubMed] [Google Scholar]
- 14.Wagenknecht B, Gulbins E, Lang F, Dichgans J, Weller M. Lipoxygenase inhibitors block CD95 ligand-mediated apoptosis of human malignant glioma cells. FEBS Lett. 1997;409:17–23. doi: 10.1016/s0014-5793(97)00468-7. [DOI] [PubMed] [Google Scholar]
- 15.Milagros Rocha M, Victor VM. Targeting antioxidants to mitochondria and cardiovascular diseases: the effects of mitoquinone. Med Sci Monit. 2007;13:RA132–45. [PubMed] [Google Scholar]
- 16.Pardini RS, Heidker JC, Fletcher DC. Inhibition of mitochondrial electron transport by nor-dihydroguaiaretic acid (NDGA) Biochem Pharmacol. 1970;19:2695–9. doi: 10.1016/0006-2952(70)90095-x. [DOI] [PubMed] [Google Scholar]
- 17.Biswal SS, Datta K, Shaw SD, Feng X, Robertson JD, Kehrer JP. Glutathione oxidation and mitochondrial depolarization as mechanisms of nordihydroguaiaretic acid-induced apoptosis in lipoxygenase-deficient FL5.12 cells. Toxicol Sci. 2000;53:77–83. doi: 10.1093/toxsci/53.1.77. [DOI] [PubMed] [Google Scholar]
- 18.Holland JA, Goss RA, O’Donnell RW, Chang MM, Johnson DK, Ziegler LM. Low-density lipoprotein induced actin cytoskeleton reorganization in endothelial cells: mechanisms of action. Endothelium. 2001;8:117–35. doi: 10.3109/10623320109165321. [DOI] [PubMed] [Google Scholar]
- 19.Papadogiannakis N, Barbieri B. Lipoxygenase inhibitors counteract protein kinase C mediated vents in human T lymphocyte proliferation. Int J Immunopharmacol. 1997;19:263–75. doi: 10.1016/s0192-0561(97)00068-4. [DOI] [PubMed] [Google Scholar]
- 20.Zavodovskaya M, Campbell MJ, Maddux BA, Shiry L, Allan G, Hodges L, et al. Nordihydroguaiaretic acid (NDGA), an inhibitor of the HER2 and IGF-1 receptor tyrosine kinases, blocks the growth of HER2-overexpressing human breast cancer cells. J Cell Biochem. 2008;103:624–35. doi: 10.1002/jcb.21435. [DOI] [PubMed] [Google Scholar]
- 21.Blecha JE, Anderson MO, Chow JM, Guevarra CC, Pender C, Penaranda C, et al. Inhibition of IGF-1R and lipoxygenase by nordihydroguaiaretic acid (NDGA) analogs. Bioorg Med Chem Lett. 2007;17:4026–9. doi: 10.1016/j.bmcl.2007.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S, Hahm ER, Lee DK, Yang CH. Inhibition of AP-1 transcription activator induces myc-dependent apoptosis in HL60 cells. J Cell Biochem. 2004;91:973–86. doi: 10.1002/jcb.10768. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura K, Tsumagari H, Morioka A, Yamauchi Y, Miyashita K, Lu S, et al. Regulation of apoptosis through arachidonate cascade in mammalian cells. Appl Biochem Biotechnol. 2002;102–103:239–50. doi: 10.1385/abab:102-103:1-6:239. [DOI] [PubMed] [Google Scholar]
- 24.Wagenknecht B, Schulz JB, Gulbins E, Weller M. Crm-A, bcl-2 and NDGA inhibit CD95L-induced apoptosis of malignant glioma cells at the level of caspase 8 processing. Cell Death Differ. 1998;5:894–900. doi: 10.1038/sj.cdd.4400435. [DOI] [PubMed] [Google Scholar]
- 25.Meyer AN, McAndrew CW, Donoghue DJ. Nordihydroguaiaretic acid inhibits an activated fibroblast growth factor receptor 3 mutant and blocks downstream signaling in multiple myeloma cells. Cancer Res. 2008;68:7362–70. doi: 10.1158/0008-5472.CAN-08-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eads D, Hansen R, Oyegunwa A, Cecil C, Culver C, Scholle F, et al. Terameprocol, a methylated derivative of nordihydroguaiaretic acid, inhibits production of prostaglandins and several key inflammatory cytokines and chemokines. J Inflamm (Lond) 2009;6:2. doi: 10.1186/1476-9255-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gnabre JN, Brady JN, Clanton DJ, Ito Y, Dittmer J, Bates RB, et al. Inhibition of human immunodeficiency virus type 1 transcription and replication by DNA sequence-selective plant lignans. Proc Natl Acad Sci U S A. 1995;92:11239–43. doi: 10.1073/pnas.92.24.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Yang L, Gao L, Gao TW, Li W, Liu YF. A functional promoter polymorphism in monocyte chemoattractant protein-1 is associated with psoriasis. Int J Immunogenet. 2008;35:45–9. doi: 10.1111/j.1744-313X.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhou W, Chai H, Courson A, Lin PH, Lumsden AB, Yao Q, et al. Ginkgolide A attenuates homocysteine-induced endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2006;44:853–62. doi: 10.1016/j.jvs.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Ramasamy S, Drummond GR, Ahn J, Storek M, Pohl J, Parthasarathy S, et al. Modulation of expression of endothelial nitric oxide synthase by nordihydroguaiaretic acid, a phenolic antioxidant in cultured endothelial cells. Mol Pharmacol. 1999;56:116–23. doi: 10.1124/mol.56.1.116. [DOI] [PubMed] [Google Scholar]
- 31.Bismuth J, Chai H, Lin PH, Yao Q, Chen C. Lactosylceramide causes endothelial dysfunction in porcine coronary arteries and human coronary artery endothelial cells. Med Sci Monit. 2009;15:BR270–4. [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Wedgwood S, Black SM. Nordihydroguaiaretic acid increases endothelial nitric oxide synthase expression via the transcription factor AP-1. DNA Cell Biol. 2007;26:853–62. doi: 10.1089/dna.2007.0614. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Sun X, Wedgwood S, Black SM. Hydrogen peroxide decreases endothelial nitric oxide synthase promoter activity through the inhibition of AP-1 activity. Am J Physiol Lung Cell Mol Physiol. 2008;295:L370–7. doi: 10.1152/ajplung.90205.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmad M, Theofanidis P, Medford RM. Role of activating protein-1 in the regulation of the vascular cell adhesion molecule-1 gene expression by tumor necrosis factor-alpha. J Biol Chem. 1998;273:4616–21. doi: 10.1074/jbc.273.8.4616. [DOI] [PubMed] [Google Scholar]
- 35.Domin J, Higgins T, Rozengurt E. Preferential inhibition of platelet-derived growth factor-stimulated DNA synthesis and protein tyrosine phosphorylation by nordihydroguaiaretic acid. J Biol Chem. 1994;269:8260–7. [PubMed] [Google Scholar]
- 36.Huang JK, Chen WC, Huang CJ, Hsu SS, Chen JS, Cheng HH, et al. Nordihydroguaiaretic acid-induced Ca2+ handling and cytotoxicity in human prostate cancer cells. Life Sci. 2004;75:2341–51. doi: 10.1016/j.lfs.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 37.Hofmanova J, Soucek K, Pachernik J, Kovarikova M, Hoferova Z, Minksova K, et al. Lipoxygenase inhibitors induce arrest of tumor cells in S-phase of the cell cycle. Neoplasma. 2002;49:362–7. [PubMed] [Google Scholar]
- 38.Moody TW, Leyton J, Martinez A, Hong S, Malkinson A, Mulshine JL. Lipoxygenase inhibitors prevent lung carcinogenesis and inhibit non-small cell lung cancer growth. Exp Lung Res. 1998;24:617–28. doi: 10.3109/01902149809087390. [DOI] [PubMed] [Google Scholar]
- 39.Soriano AF, Helfrich B, Chan DC, Heasley LE, Bunn PA, Jr, Chou TC. Synergistic effects of new chemopreventive agents and conventional cytotoxic agents against human lung cancer cell lines. Cancer Res. 1999;59:6178–84. [PubMed] [Google Scholar]
- 40.Kubow S, Woodward TL, Turner JD, Nicodemo A, Long E, Zhao X. Lipid peroxidation is associated with the inhibitory action of all-trans-retinoic acid on mammary cell transformation. Anticancer Res. 2000;20:843–8. [PubMed] [Google Scholar]
- 41.Seufferlein T, Seckl MJ, Schwarz E, Beil M, Wichert vG, Baust H, et al. Mechanisms of nordihydroguaiaretic acid-induced growth inhibition and apoptosis in human cancer cells. Br J Cancer. 2002;86:1188–96. doi: 10.1038/sj.bjc.6600186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowe DL, Ozbay T, Bender LM, Nahta R. Nordihydroguaiaretic acid, a cytotoxic insulin-like growth factor-I receptor/HER2 inhibitor in trastuzumab-resistant breast cancer. Mol Cancer Ther. 2008;7:1900–8. doi: 10.1158/1535-7163.MCT-08-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camirand A, Lu Y, Pollak M. Co-targeting HER2/ErbB2 and insulin-like growth factor-1 receptors causes synergistic inhibition of growth in HER2-overexpressing breast cancer cells. Med Sci Monit. 2002;8:BR521–6. [PubMed] [Google Scholar]
- 44.Olsen EA, Abernethy ML, Kulp-Shorten C, Callen JP, Glazer SD, Huntley A, et al. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J Am Acad Dermatol. 1991;24:738–43. doi: 10.1016/0190-9622(91)70113-g. [DOI] [PubMed] [Google Scholar]
- 45.Lambert JD, Meyers RO, Timmermann BN, Dorr RT. Pharmacokinetic analysis by high-performance liquid chromatography of intravenous nordihydroguaiaretic acid in the mouse. J Chromatogr B Biomed Sci Appl. 2001;754:85–90. doi: 10.1016/s0378-4347(00)00592-2. [DOI] [PubMed] [Google Scholar]
- 46.Li F, Pham JD, Anderson MO, Youngren JF. Nordihydroguaiaretic acid inhibits transforming growth factor beta type 1 receptor activity and downstream signaling. Eur J Pharmacol. 2009;616:31–7. doi: 10.1016/j.ejphar.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee CH, Jang YS, Her SJ, Moon YM, Baek SJ, Eling T. Nordihydroguaiaretic acid, an antioxidant, inhibits transforming growth factor-beta activity through the inhibition of Smad signaling pathway. Exp Cell Res. 2003;289:335–41. doi: 10.1016/s0014-4827(03)00282-9. [DOI] [PubMed] [Google Scholar]
- 48.Park R, Chang CC, Liang YC, Chung Y, Henry RA, Lin E, et al. Systemic treatment with tetra-O-methyl nordihydroguaiaretic acid suppresses the growth of human xenograft tumors. Clin Cancer Res. 2005;11:4601–9. doi: 10.1158/1078-0432.CCR-04-2188. [DOI] [PubMed] [Google Scholar]
- 49.Chang CC, Heller JD, Kuo J, Huang RC. Tetra-O-methyl nordihydroguaiaretic acid induces growth arrest and cellular apoptosis by inhibiting Cdc2 and survivin expression. Proc Natl Acad Sci U S A. 2004;101:13239–44. doi: 10.1073/pnas.0405407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heller JD, Kuo J, Wu TC, Kast WM, Huang RC. Tetra-O-methyl nordihydroguaiaretic acid induces G2 arrest in mammalian cells and exhibits tumoricidal activity in vivo. Cancer Res. 2001;61:5499–504. [PubMed] [Google Scholar]
- 51.Mak DH, Schober WD, Chen W, Heller J, Andreeff M, Carter BZ. Tetra-O-methyl nordihydroguaiaretic acid inhibits growth and induces death of leukemia cells independent of Cdc2 and survivin. Leuk Lymphoma. 2007;48:774–85. doi: 10.1080/10428190601186143. [DOI] [PubMed] [Google Scholar]
- 52.Huang RC, Chang CC, Mold D. Survivin-dependent and -independent pathways and the induction of cancer cell death by tetra-O-methyl nordihydroguaiaretic acid. Semin Oncol. 2006;33:479–85. doi: 10.1053/j.seminoncol.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 53.Chiou SK, Jones MK, Tarnawski AS. Survivin - an anti-apoptosis protein: its biological roles and implications for cancer and beyond. Med Sci Monit. 2003;9:PI25–9. [PubMed] [Google Scholar]
- 54.Hansel DE, Dhara S, Huang RC, Ashfaq R, Deasel M, Shimada Y, et al. CDC2/CDK1 expression in esophageal adenocarcinoma and precursor lesions serves as a diagnostic and cancer progression marker and potential novel drug target. Am J Surg Pathol. 2005;29:390–9. doi: 10.1097/00000478-200503000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Khanna N, Dalby R, Tan M, Arnold S, Stern J, Frazer N. Phase I/II clinical safety studies of terameprocol vaginal ointment. Gynecol Oncol. 2007;107:554–62. doi: 10.1016/j.ygyno.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 56.Khanna N, Dalby R, Connor A, Church A, Stern J, Frazer N. Phase I clinical trial of repeat dose terameprocol vaginal ointment in healthy female volunteers. Sex Transm Dis. 2008;35:577–82. doi: 10.1097/OLQ.0b013e31816766af. [DOI] [PubMed] [Google Scholar]
- 57.Smolewski P. Terameprocol, a novel site-specific transcription inhibitor with anticancer activity. IDrugs. 2008;11:204–14. [PubMed] [Google Scholar]
- 58.Noor R, Mittal S, Iqbal J. Superoxide dismutase--applications and relevance to human diseases. Med Sci Monit. 2002;8:RA210–5. [PubMed] [Google Scholar]
- 59.Goodman Y, Steiner MR, Steiner SM, Mattson MP. Nordihydroguaiaretic acid protects hippocampal neurons against amyloid beta-peptide toxicity, and attenuates free radical and calcium accumulation. Brain Res. 1994;654:171–6. doi: 10.1016/0006-8993(94)91586-5. [DOI] [PubMed] [Google Scholar]
- 60.Shishido Y, Furushiro M, Hashimoto S, Yokokura T. Effect of nordihydroguaiaretic acid on behavioral impairment and neuronal cell death after forebrain ischemia. Pharmacol Biochem Behav. 2001;69:469–74. doi: 10.1016/s0091-3057(01)00572-x. [DOI] [PubMed] [Google Scholar]
- 61.Rothman SM, Yamada KA, Lancaster N. Nordihydroguaiaretic acid attenuates NMDA neurotoxicity--action beyond the receptor. Neuropharmacology. 1993;32:1279–88. doi: 10.1016/0028-3908(93)90022-u. [DOI] [PubMed] [Google Scholar]
- 62.Aktan S, Aykut C, Yegen BC, Okar I, Ozkutlu U, Ercan S. The effect of nordihydroguaiaretic acid on leukotriene C4 and prostaglandin E2 production following different reperfusion periods in rat brain after forebrain ischemia correlated with morphological changes. Prostaglandins Leukot Essent Fatty Acids. 1993;49:633–41. doi: 10.1016/0952-3278(93)90171-r. [DOI] [PubMed] [Google Scholar]
- 63.Guzman-Beltran S, Espada S, Orozco-Ibarra M, Pedraza-Chaverri J, Cuadrado A. Nordihydroguaiaretic acid activates the antioxidant pathway Nrf2/HO-1 and protects cerebellar granule neurons against oxidative stress. Neurosci Lett. 2008;447:167–71. doi: 10.1016/j.neulet.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 64.Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–23. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 65.Boston-Howes W, Williams EO, Bogush A, Scolere M, Pasinelli P, Trotti D. Nordihydroguaiaretic acid increases glutamate uptake in vitro and in vivo: therapeutic implications for amyotrophic lateral sclerosis. Exp Neurol. 2008;213:229–37. doi: 10.1016/j.expneurol.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Craigo J, Callahan M, Huang RC, DeLucia AL. Inhibition of human papillomavirus type 16 gene expression by nordihydroguaiaretic acid plant lignan derivatives. Antiviral Res. 2000;47:19–28. doi: 10.1016/s0166-3542(00)00089-9. [DOI] [PubMed] [Google Scholar]
- 67.Hwu JR, Hsu MH, Huang RC. New nordihydroguaiaretic acid derivatives as anti-HIV agents. Bioorg Med Chem Lett. 2008;18:1884–8. doi: 10.1016/j.bmcl.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 68.Uchide N, Ohyama K, Bessho T, Toyoda H. Inhibition of influenza-virus-induced apoptosis in chorion cells of human fetal membranes by nordihydroguaiaretic Acid. Intervirology. 2005;48:336–40. doi: 10.1159/000085103. [DOI] [PubMed] [Google Scholar]
- 69.Koob TJ, Hernandez DJ. Material properties of polymerized NDGA-collagen composite fibers: development of biologically based tendon constructs. Biomaterials. 2002;23:203–12. doi: 10.1016/s0142-9612(01)00096-5. [DOI] [PubMed] [Google Scholar]
- 70.Koob TJ, Willis TA, Qiu YS, Hernandez DJ. Biocompatibility of NDGA-polymerized collagen fibers. II. Attachment, proliferation, and migration of tendon fibroblasts in vitro. J Biomed Mater Res. 2001;56:40–8. doi: 10.1002/1097-4636(200107)56:1<40::aid-jbm1066>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 71.Ju YM, Yu B, Koob TJ, Moussy Y, Moussy F. A novel porous collagen scaffold around an implantable biosensor for improving biocompatibility. I. In vitro/in vivo stability of the scaffold and in vitro sensitivity of the glucose sensor with scaffold. J Biomed Mater Res A. 2008;87:136–46. doi: 10.1002/jbm.a.31756. [DOI] [PubMed] [Google Scholar]
- 72.Ju YM, Yu B, West L, Moussy Y, Moussy F. A novel porous collagen scaffold around an implantable biosensor for improving biocompatibility. II. Long-term in vitro/in vivo sensitivity characteristics of sensors with NDGA- or GA-crosslinked collagen scaffolds. J Biomed Mater Res A. 2009 doi: 10.1002/jbm.a.32400. [DOI] [PubMed] [Google Scholar]
- 73.Moussy Y, Guegan E, Davis T, Koob TJ. Transport characteristics of a novel local drug delivery system using nordihydroguaiaretic acid (NDGA)-polymerized collagen fibers. Biotechnol Prog. 2007;23:990–4. doi: 10.1021/bp0700509. [DOI] [PubMed] [Google Scholar]
- 74.Lü X, Zhai W, Zhou Y, Zhang H, Chang J. Crosslinking effect of Nordihydroguaiaretic acid (NDGA) on decellularized heart valve scaffold for tissue engineering. J Mater Sci Mater Med. 2009 doi: 10.1007/s10856-009-3924-9. [DOI] [PubMed] [Google Scholar]
- 75.Billinsky JL, Krol ES. Nordihydroguaiaretic acid autoxidation produces a schisandrin-like dibenzocyclooctadiene lignan. J Nat Prod. 2008;71:1612–5. doi: 10.1021/np8001354. [DOI] [PubMed] [Google Scholar]
- 76.Lambert JD, Zhao D, Meyers RO, Kuester RK, Timmermann BN, Dorr RT. Nordihydroguaiaretic acid: hepatotoxicity and detoxification in the mouse. Toxicon. 2002;40:1701–8. doi: 10.1016/s0041-0101(02)00203-9. [DOI] [PubMed] [Google Scholar]
- 77.Obermeyer WR, Musser SM, Betz JM, Casey RE, Pohland AE, Page SW. Chemical studies of phytoestrogens and related compounds in dietary supplements: flax and chaparral. Proc Soc Exp Biol Med. 1995;208:6–12. doi: 10.3181/00379727-208-43824. [DOI] [PubMed] [Google Scholar]
- 78.Alderman S, Kailas S, Goldfarb S, Singaram C, Malone DG. Cholestatic hepatitis after ingestion of chaparral leaf: confirmation by endoscopic retrograde cholangiopancreatography and liver biopsy. J Clin Gastroenterol. 1994;19:242–7. doi: 10.1097/00004836-199410000-00016. [DOI] [PubMed] [Google Scholar]
- 79.Meyers RO, Lambert JD, Hajicek N, Pourpak A, Kalaitzis JA, Dorr RT. Synthesis, characterization, and anti-melanoma activity of tetra-O-substituted analogs of nordihydroguaiaretic acid. Bioorg Med Chem Lett. 2009;19:4752–5. doi: 10.1016/j.bmcl.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]