Abstract
Free radicals derived from oxygen, nitrogen and sulphur molecules in the biological system are highly active to react with other molecules due to their unpaired electrons. These radicals are important part of groups of molecules called reactive oxygen/nitrogen species (ROS/RNS), which are produced during cellular metabolism and functional activities and have important roles in cell signalling, apoptosis, gene expression and ion transportation. However, excessive ROS attack bases in nucleic acids, amino acid side chains in proteins and double bonds in unsaturated fatty acids, and cause oxidative stress, which can damage DNA, RNA, proteins and lipids resulting in an increased risk for cardiovascular disease, cancer, autism and other diseases. Intracellular antioxidant enzymes and intake of dietary antioxidants may help to maintain an adequate antioxidant status in the body. In the past decades, new molecular techniques, cell cultures and animal models have been established to study the effects and mechanisms of antioxidants on ROS. The chemical and molecular approaches have been used to study the mechanism and kinetics of antioxidants and to identify new potent antioxidants. Antioxidants can decrease the oxidative damage directly via reacting with free radicals or indirectly by inhibiting the activity or expression of free radical generating enzymes or enhancing the activity or expression of intracellular antioxidant enzymes. The new chemical and cell-free biological system has been applied in dissecting the molecular action of antioxidants. This review focuses on the research approaches that have been used to study oxidative stress and antioxidants in lipid peroxidation, DNA damage, protein modification as well as enzyme activity, with emphasis on the chemical and cell-free biological system.
Keywords: ROS, antioxidants, cell-free systems, radical scavenging, antioxidant enzymes, lipid peroxidation, DNA damage, protein modification
Introduction
Free radicals are atoms, molecules or ions with unpaired electrons, which are highly active to chemical reactions with other molecules. In the biology system, the free radicals are often derived from oxygen, nitrogen and sulphur molecules. These free radicals are parts of groups of molecules called reactive oxygen species (ROS), reactive nitrogen species (RNS) and reactive sulphur species (RSS). For example, ROS includes free radicals such as superoxide anion (O2−•), perhydroxyl radical (HO2•), hydroxyl radical (▪OH), nitric oxide and other species such as hydrogen peroxide (H2O2), singlet oxygen (1O2), hypochlorous acid (HOCl) and peroxynitrite (ONOO−) [1]. RNS are derived from nitric oxide through the reaction with O2−• to form ONOO−. RSS are easily formed from thiols by reaction with ROS [2]. ROS are produced during cellular metabolism and functional activities, and have important roles in cell signalling, apoptosis, gene expression and ion transportation (Fig. 1) [1]. However, excessive amounts of ROS can have deleterious effects on many molecules including protein, lipid, RNA and DNA since they are very small and highly reactive. ROS can attack bases in nucleic acids, amino acid side chains in proteins and double bonds in unsaturated fatty acids, in which ▪OH is the strongest oxidant. ROS attacking macromolecules is often termed oxidative stress. Cells are normally able to defend themselves against ROS damage through the use of intracellular enzymes to keep the homeostasis of ROS at a low level. However, during times of environmental stress and cell dysfunction, ROS levels can increase dramatically, and cause significant cellular damage in the body. Thus, oxidative stress significantly contributes to the pathogenesis of inflammatory disease, cardiovascular disease, cancer, diabetes, Alzheimer’s disease, cataracts, autism and aging [2–6]. In order to prevent or reduce the ROS-induced oxidative damage, the human body and other organisms have developed an antioxidant defence system that includes enzymatic, metal chelating and free radical scavenging activities to neutralize these radicals after they have formed. In addition, intake of dietary antioxidants may help to maintain an adequate antioxidant status in the body.
Fig 1.
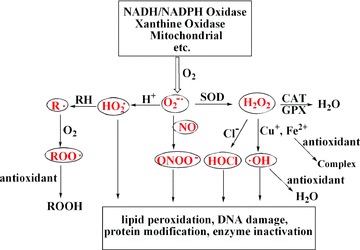
Summary of ROS types and sources, and action point of antioxidants. O2−•, superoxide anion; HO2▪, perhydroxyl radical; ▪OH, hydroxyl radical; H2O2, hydrogen peroxide; HOCl, hypochlorous acid; ONOO−, peroxynitrite; R▪, lipid alkyl radical; RH, lipid; ROO▪, lipid peroxyl radical; ROOH, lipid hydroperoxide; SOD, superoxide dismutase; CAT, catalase and GPX, glutathione peroxidase.
Antioxidants may be molecules that can neutralize free radicals by accepting or donating electron(s) to eliminate the unpaired condition of the radical. The antioxidant molecules may directly react with the reactive radicals and destroy them, while they may become new free radicals which are less active, longer-lived and less dangerous than those radicals they have neutralized. They may be neutralized by other antioxidants or other mechanisms to terminate their radical status. For example, many antioxidants have aromatic ring structures and are able to delocalize the unpaired electron (Fig. 1). Vitamin C (AscH−) in the aqueous phase and vitamin E (TOH) in the lipid phase will directly react with or neutralize hydroxyl, alkoxyl and lipid peroxyl (ROO▪) radicals and form H2O, alcohol and lipid hydroperoxides, respectively. Vitamin E itself becomes a phenyl radical and vitamin C turns to a very stable radical (Asc−•), due to its delocalized structure (Fig. 2A and B). Furthermore, vitamin C can also neutralize the radical form of other antioxidants such as glutathione radical and vitamin E radical, and regenerate these antioxidants (Fig. 2C). Vitamin C itself is readily regenerated from Asc−• with NADH or NADPH-dependent reductases [7]. Many antioxidants may directly react with ROS and/or free radical intermediates induced by ROS and terminate the chain reaction, thereby stopping the ROS-induced damage [8].
Fig 2.
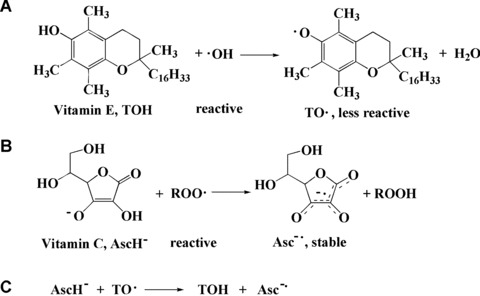
Direct reactions of vitamin E (TOH) with ▪OH (A) and vitamin C (AscH−) with ROO▪ (B) and regeneration of vitamin E from vitamin C (C).
Small molecules such as vitamin C, vitamin E, uric acid and glutathione play important roles as cellular antioxidants. Synthetic antioxidants such as tert-butylhydroxyl-toluene, tert-butylhydroxyanisole and tert-butylhydroquinone have been widely used in the food industry to retard lipid oxidation. However, such synthetic antioxidants are not preferred for pharmacologic use due to toxicological concerns. Thus, more and more interests have focused on identifying plant extracts to use as dietary antioxidant supplements [9]. Most of these natural antioxidants come from fruits, vegetables, spices, grains and herbs such as ginseng, curcuma, ginkgo, rosemary, green tea, grape, ginger and garlic. They contain a wide variety of antioxidant compounds, such as phenolics (phenol and polyphenols), flavonoids, carotenoids, steroids and thiol compounds [10]. These antioxidants may help to protect cellular damages from oxidative stress and also lower the risk of chronic diseases. For example, ginseng contains steroid-like compounds, ginsenosides, which show antioxidant activities against free radical damage on the vascular endothelium [11]. Ginkgo has been reported to have strong antioxidant activities due to flavone glycosides that scavenge free radicals [8]. Flavonoids such as catechin and epicatechin in green tea and grape seed extracts could be responsible for their potent antioxidant activities [10, 12].
Another important function of antioxidants is to regulate ROS-related enzymes. Antioxidants may decrease the cellular level of free radicals either by inhibiting the activities or expressions of free radical generating enzymes such as NAD(P)H oxidase and xanthine oxidase (XO) or by enhancing the activities and expressions of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX) [13, 14]. These antioxidant enzymes produced in the body provide an important defence against free radicals. Animal cells containing the enzyme SOD can convert two O2−• into a H2O2 and an oxygen. Also, organisms use CAT and/or GPX to eliminate H2O2 before the Fenton Reaction can create a ▪OH. These observations of inhibition or enhancement of enzyme activities by antioxidants have opened a new door for us to further understand the molecular mechanisms of action of antioxidants.
Since the 1990s, antioxidant research has expanded dramatically due to its potential benefit in disease prevention and health promotion. The antioxidant activity of pure compounds, foods and dietary supplements has been extensively studied in biological systems such as cell cultures, animal models and clinical trials [10, 12, 15, 16]. Many research models have been established in chemical and/or biological systems for the studies of mechanisms of action of antioxidants as well as identification of new antioxidants especially from natural substances. Generally, the antioxidant ability was measured and presented as total antioxidant activity [17, 18], total antioxidant capacity [19, 20], total antioxidant potentials [21], Trolox equivalent antioxidant capacity [22], total radical absorption potentials [23], ferric reducing/antioxidant power [24] and oxygen radical absorption capacity [25]. Mechanistically, these methods are based on either a single-electron transfer reaction or a hydrogen atom transfer reaction from an antioxidant or oxidant to a free radical. The change of optical absorbance of either antioxidant or oxidant is measured for the quantitation for antioxidant capability. The chemical approaches are simple and easy to study the total antioxidant activity, which includes the radical scavenging ability and reductive activity [18]. Radicals are commonly used to measure the radical scavenging ability of antioxidant. This article describes the scavenging of active radicals such as ▪OH and O2−•, stable radicals such as 1,1-diphenyl-2-picryl-hydrazyl free radical (DPPH), 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) cation radical (ABTS+) and other species such as HOCl, H2O2, ONOO−. The scavenging of ROS in the biology system is the most important.
Further studies in cell cultures and animal models give critical information on the bioavailability, metabolism and toxicity issues of antioxidants, implicating potential clinical applications of these substances. However, animal models and human studies are expensive and not suitable for the initial antioxidant screening of foods and dietary supplements. Thus cell culture models have been used for initial antioxidant screening and antioxidant research prior to animal studies and human clinical trials [15]. However, cell systems are very complicated and too many factors may affect the results compared with cell-free systems. Many chemical and cell-free systems offer unique advantages to delineate the chemical and molecular mechanisms of action of new antioxidants. It is possible that some antioxidants can work chemically, while they may not work cellularly and physiologically. The aim of this review is to update the current approaches to study properties and mechanisms of action of antioxidants with emphasis on the chemical and cell-free biological systems. This review may greatly benefit researchers and scientists who are interested in the study of detail chemical and molecular mechanisms of antioxidants. It is also helpful for physicians who are interested in antioxidant therapy or prevention for certain oxidative stress-related diseases or inflammation conditions.
Free radical scavenging
Searching and identifying natural and safe antioxidants, especially of plant origin, have been notably increased in recent years [26, 27]. Assays based on the use of O2−• and ▪OH, DPPH, ABTS+, and N,N-dimethyl-p-phenylenediamine dihydrochloride cation radical (DMPD+) are among the most popular spectrophotometric methods for determination of the antioxidant capacity of foods, beverages and vegetable extracts [26–32]. DPPH and ABTS+ scavenging methods have been the most commonly used to evaluate the antioxidant activity of compounds due to their simple, rapid, sensitive and reproducible procedures. Thus, the radical scavenging assays in the cell-free systems for antioxidant studies are often considered by researchers before further studies in cellular lines and/or animal models.
Scavenging superoxide and other ROS
Superoxide (O2−•), a predominant cellular free radical, is involved in a large number of deleterious changes often associated with an increase in peroxidative processes and linked to a low antioxidant concentration. Although O2−• itself is not so reactive to biomolecules, it helps in generation of more powerful ▪OH and ONOO−[33]. In phagocytes, O2−• is produced in large quantities by the enzyme NADPH oxidase for killing pathogens. O2−• is also a by-product of mitochondrial respiration, as well as several other enzymes such as NADH oxidase, XO, monooxygenases and cyclooxygenases [34].
Direct scavenging of O2−• has been a model for determining the antioxidant activities [28]. In the chemical systems, O2−• can be generated enzymatically or non-enzymatically from quinone derivatives, such as 6-anilino-5, 8-quinolinequinone (LY83583), 1,4-benzoquinone, 1,4-naphthaquinone, 2-methyl-1,4-naphthaquinone, riboflavin, etc. (Fig. 3) [35]. In the presence of enzymes such as NADPH-cytochrome P450 reductase and mitochondrial NADH-ubiquinone oxidoreductase or thiol compounds such as glutathione and L-cysteine, LY83583 undergoes a one-electron reduction due to low redox potential (-0.3 V versus SCE), followed by formation of LY83583 semiquinone anion radical. Under an aerobic condition, this species interacts with molecular oxygen to form O2−• and original quinones (Fig. 3C) [35]. O2−• is also generated in riboflavin/methionine /illuminate and assayed by the reduction of Nitro blue tetrazolium (NBT) to form blue formazan [28, 36, 37]. Briefly, the reaction mixture is illuminated at 25°C for 40 min. and O2−• generated from the photochemically reduced riboflavin can reduce NBT to form blue formazan which has absorbance at 560 nm (Fig. 2C). This system can be used to determine the radical scavenging activity of antioxidants. Antioxidants can be added to the reaction mixture to scavenge O2−•, thereby inhibiting the NBT reduction. Decreased absorbance of the reaction mixture indicates increased O2−• scavenging activity. The percentage of O2−• scavenged is calculated by the absorption change. NBT salt and other tetrazolium salts are chromogenic probes useful for O2−• determination. These probes are also widely used for detecting redox potential of cells for viability, proliferation and cytotoxicity assays [38]. In the cell culture system, O2−• can be increased by treating cells with a mitochondrial respiratory complex III inhibitor, antimycin A [39].
Fig 3.
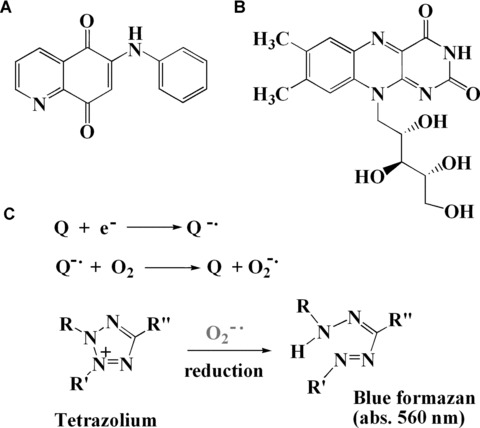
Structure of quinones (Q): LY83583 (A), riboflavin (B) and the formation of superoxide and its reaction with NBT (C), where R, R′ and R″ represent p-nitrophenyl, o-methoxyphenyl and phenyl groups, respectively.
Assessment of low-level O2−• in non-phagocytic cells is crucial for assessing redox-dependent signalling pathways and the role of enzymes such as the NADPH oxidase complex. Many probes and methods, such as enzymatic (cytochrome c, aconitase), spectrophotometric (NBT), chemiluminescent (lucigenin [Luc], coelenterazine, etc), fluorescent [dihydroethidium (DHE) and MitoSOX], as well as electron paramagnetic resonance (EPR) spin trapping, have been used to detect the production of O2−• (see review [40]). Among these probes, Luc luminescence is a more specific measure of O2−•. It involves several steps such as single-electron reduction of Luc2+ to Luc+•, coupling of Luc+• with O2−• yielding a dioxetane, decomposition of the dioxetane into two molecules of the N-methyl acridone, one of which is in the electronically excited state, and finally emission of a photon from the excited state acridone as it returns to the ground state (Fig. 4) [41]. Because Luc+• is readily autoxidized to Luc2+ and reduces O2 to O2−•, Luc2+ cannot precisely determine the cellular levels of O2−•[40, 41]. Nevertheless, Luc luminescence is still widely used in control conditions due to the convenience and sensitivity of luminescence methods.
Fig 4.
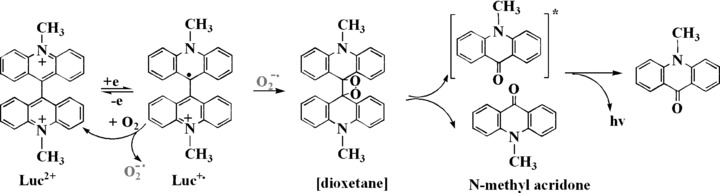
Process of Luc luminescence.
DHE is a useful fluorogenic probe for the detection of ROS including O2−•. DHE has been used increasingly as a probe for O2−• in biological systems because DHE is a hydrophobic, uncharged compound that is able to cross extra- and intracellular membranes. It undergoes significant oxidation in resting leukocytes, possibly through the uncoupling of mitochondrial oxidative phosphorylation. Cytosolic DHE displays blue fluorescence, whereas after oxidation of by oxidants such as O2−• and H2O2, it becomes 2-hydroxyethidium (2-EOH) and ethidium, which intercalates cellular DNA, staining the nucleus with a bright red fluorescence (Fig. 5). Its oxidation by different oxidizing systems has been used increasingly for fluorescent analysis of ROS output in cells and tissues. Determination of total DHE fluorescence in cells has been performed extensively in the literature for assessment of ROS and, more specifically, of O2−•. Its main drawback is that the total fluorescence of DHE is a sum of the composite spectra of all different products and thus likely reflects preferentially a measure of total cell redox state rather than production of a specific intermediate. Both 2-EOH and ethidium are fluorescent products that are difficult to discriminate them by conventional fluorescence microscopy or fluorometry. Thus, high-performance liquid chromatography (HPLC) analysis of DHE-derived fluorescent compounds (2-EOH and ethidium) has been developed in order to achieve separation and individual analysis of such products [42, 43]. This technique provides a significant increase in the accuracy of ROS output determinations and is a meaningful advance towards the precise quantification of this species in cells and tissues. Recent studies of HPLC separation and analysis of those two main products indicated that 2-EOH is generated specifically by O2−• oxidation of DHE, whereas ethidium is associated mainly with pathways involving H2O2 and metal-based oxidizing systems, including heme proteins and peroxidases [42]. More information about the DHE fluorescent probe is available in a recent review by Laurindo et al.[40]. MitoSOX™ Red mitochondrial superoxide indicator [44], a modified DHE, is a novel fluorogenic dye for highly selective detection of superoxide in the mitochondria of live cells. It is readily oxidized by superoxide inside the mitochondrion but not by other ROS- or RNS-generating systems, and oxidation of the probe is prevented by SOD. The oxidation product becomes highly fluorescent upon binding to nucleic acids [39].
Fig 5.
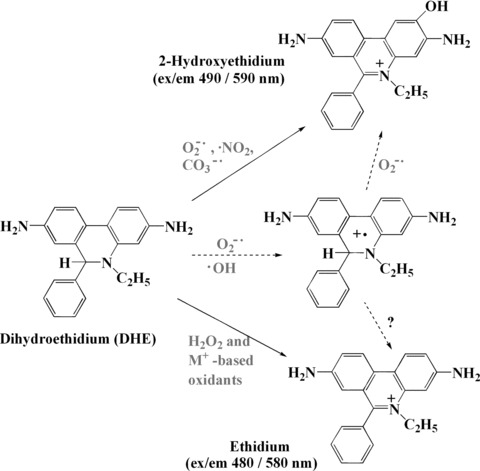
Structures of DHE, EOH and ethidium and hypothesized reaction pathways to account for the formation of DHE-derived red fluorescence. EOH is basically formed from superoxide and possibly involving ONOO−/CO2 while ethidium is mainly formed from H2O2 pathways involving metal proteins. Dotted arrows indicate possible intermediate pathways involved in the formation of these products.
Several other fluorescent reagents such as fluorescein and rhodamine are also used to detect ROS including O2−•, although they are not specific to O2−•. Non-fluorescent 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) is a cell-permeable indicator for ROS, and it may be extremely useful for assessing cellular oxidative stress. After the acetate groups of H2DCFDA are removed by intracellular esterases, the non-fluorescent H2DCFDA is oxidized to the highly fluorescent 2′,7′-dichlorofluorescein (DCF) (Fig. 6), which can be monitored by a fluorometer using excitation sources and filters appropriate for the fluorescein. Fluorescein has an absorption maximum at 494 nm and emission maximum of 521 nm [45]. H2DCF has been shown to be oxidized to DCF in human neutrophils by H2O2 and nitric oxide and FeSO4; in the cell-free system by ONOO− and horseradish peroxidase (HRP) alone; HRP in combination with H2O2; FeSO4 alone; and a mixture of FeSO4 and H2O2. The oxidation by Fe2+ in the presence of H2O2 was reduced by the ▪OH radical scavenger formation and the iron chelator deferoxamine [46]. 2′,7′-dichlorodihydrofluorescein (DCFH) was insensitive to nitric oxide and H2O2 in the cell-free system. Thus Myhre et al. suggest that the DCF assay is only suitable for measurements of ONOO− and H2O2 in combination with cellular peroxidases, peroxidases alone and ▪OH, while it is not suitable for measurement of nitric oxide, HOCl or O2−• in biological systems [46]. However, in neutrophils, H2DCFDA has proven useful for flow cytometric analysis of nitric oxide, forming a product that has spectral properties identical to those produced when it reacts with H2O2[47]. Other studies reported that oxidation of H2DCFDA was not directly sensitive to singlet oxygen, but singlet oxygen can indirectly contribute to the formation of DCF through its reaction with cellular substrates that yield peroxyl products and peroxyl radicals [48, 49]. Importantly, DCF itself can also act as a photosensitizer for H2DCFDA oxidation, both priming and accelerating the formation of DCF; thus care must be taken when using DCFH to measure oxidative stress in cells as a result of both visible and UV light exposure [49].
Fig 6.
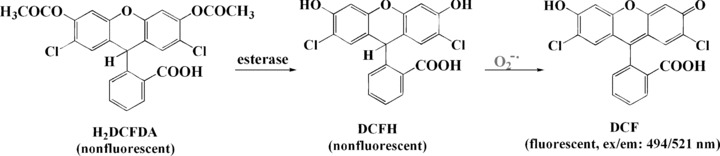
The structures of H2DCFDA, DCFH and DCF; and the reaction pathway of superoxide detection. DCF has an absorption maximum at 494 nm and emission maximum of 521 nm.
For the measurement of O2−• scavenging activity by H2DCFDA in a cell-free system, briefly, H2DCFDA mixed with esterase at pH 7.4 is incubated at 37°C for 20 min. and placed on ice in the dark until immediately prior to the study. H2DCFDA is deacetylated to non-fluorescent DCFH by esterase and subsequently oxidized to highly fluorescent DCF by O2−•. The extent of conversion of DCFH into DCF is stoichiometrically related to the amount of O2−•. The fluorescence intensity of oxidized DCFH is measured by using the fluorescence reader at excitation and emission wavelengths of 485 and 530 nm, respectively, for 1 hr with or without the addition of 2-methyl-1,4-naphthaquinone (50 mM) as an O2−• source [50].
Dihydrorhodamine 123 (DHR123) is another commonly used fluorescent mitochondrial dye. DHR123 itself is non-fluorescent, but it readily enters most of the cells and is oxidized by oxidative species or by cellular redox systems to the fluorescent rhodamine 123 that accumulates in mitochondrial membranes (Fig. 7) [51]. DHR123 is useful for detecting ROS including O2−• (in the presence of peroxidase or cytochrome c) and ONOO−[50].
Fig 7.
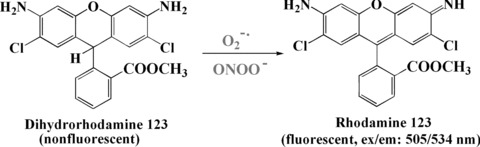
Detection of ONOO− and superoxide by DHR123.
Scavenging hydroxyl radical and other ROS
Hydroxyl radical (▪OH) is extremely reactive, more toxic than other radical species and can attack biologic molecules such as DNA, proteins and lipids. ▪OH is widely believed to be generated from the Fe2+ (or Cu+) / H2O2 Fenton reaction system, by simply incubating FeSO4 and H2O2 in aqueous solution. Thus, ▪OH scavenging activity of antioxidants can be accomplished through direct scavenging or preventing of ▪OH formation through the chelation of free metal ions or converting H2O2 to other harmless compounds. The scavenging ability of antioxidants can be determined by Gutteridge method, which is monitored in the Fe3+–EDTA–H2O2–deoxyribose system [36, 37, 52]. The extent of deoxyribose degradation by the ▪OH formed can be measured directly in the aqueous phase by thiobarbituric acid reactive species (TBARS) assay at 532 nm (Fig. 8). This method is based on the fact that the degradation of deoxyribose by ▪OH forms a reactive species malondialdihyde, which forms an adduct with thiobarbituric acid (TBA). The adduct, MDA–TBA, has an absorption at 532 nm that can be assayed spectrophotometrically. By this assay, the ability of several antioxidants to scavenge ▪OH has been studied and compared with that of DMTU, uric acid, trolox and mannitol [36, 37, 52].
Fig 8.
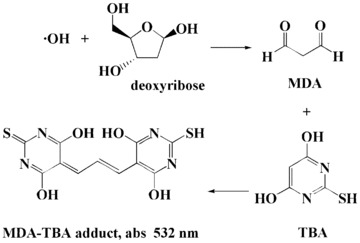
Reaction pathway of TBARS assay of ▪OH.
Another method uses a spin-trapping agent 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) to trap the generated ▪OH radical [53]. DMPO reacts with ▪OH to form a DMPO-OH radical, which can be monitored by EPR spectrum. Comparison of the EPR intensities of DMPO-OH radical in the absence and presence of antioxidants can measure the radical scavenging ability of the antioxidants. For example, Kang et al. studied the ▪OH scavenging activity of ginsenosides [53].
Stable radical scavenging
The interaction between free radicals (such as O2−• and ▪OH) and antioxidants can show direct evidence for antioxidants to scavenge free radicals. It has been widely used to evaluate the radical scavenging ability of antioxidants. For models, radical scavenging research tends to use more stable radicals as probes. Radicals such as DPPH, galvinoxyl, ABTS+ and DMPD+ are stable and coloured (Fig. 9). DPPH and gavinoxyl radicals are commercially available, and ABTS+ and DMPD+ radicals can be generated freshly before the assay by oxidizing the neutral molecules with potassium persulphate and FeCl3, respectively [28, 32]. These radicals have strong absorption in the visible region, while their absorption decreases proportionally upon receiving an electron or hydrogen from the antioxidants. Thus, the radical scavenging capacity of the antioxidants can be obtained based on the absorption change. The detailed method description for this free radical scavenging test is available from a previous report [28], where the antioxidant and radical scavenging properties of curcumin are studied.
Fig 9.
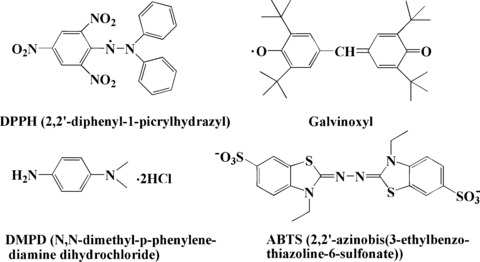
Structures of DPPH, galvinoxyl, ABTS and DMPD.
DPPH has been widely used to evaluate the free radical scavenging effectiveness of various antioxidant substances [27, 28, 30]. In the DPPH assay, the antioxidants are able to donate a hydrogen to reduce the stable radical DPPH to the yellow-coloured non-radical diphenyl-picrylhydrazine (DPPH-H). DPPH is usually used as a reagent to evaluate free radical scavenging activity of antioxidants based on the absorption change of DPPH at 517 nm measured spectrophotometrically. Similar to DPPH, galvinoxyl is a stable phenoxy radical that exhibits characteristic UV absorption at 429 nm in ethanol solution. ABTS+ radicals are more reactive than DPPH radicals, and the reactions with ABTS+ radicals involve a single-electron transfer process. Bleaching of a preformed solution of the blue-green radical cation ABTS+, which has an absorption at 734 nm, has been extensively used to evaluate the antioxidant capacity of complex mixtures and individual compounds. The reaction of the preformed radicals with free radical scavengers can be easily monitored by following the decrease of the sample absorbance at 734 nm. The principle of the DMPD+ assay is very similar to that of ABTS+. The UV–visible spectrum of DMPD+ shows a maximum absorbance at 505 nm [54].
These free radical scavenging reactions need the antioxidant to donate an electron or an active hydrogen atom such as one in reactive hydroxyl group. Generally, antioxidants that are molecules bearing active hydroxyl groups, such as vitamins E and C, polyphenol and flavonol compounds, are potent radical scavengers. Interestingly, Liu and colleagues found that ecdysteroids, which do not have active hydroxyl groups, are also active antioxidants and free-radical scavengers [55]. The most active hydrogen of the ecdysteroid may be H-9, which is an allylic hydrogen, and furthermore, conjugation with the 6-carbonyl group further weakens the C–H-9 bond. It is well known that allylic hydrogens are very active and easily abstracted by free radicals.
In the current review, we focused mainly on the scavenging of reactive species centred on oxygen, nitrogen and chlorine. These assays were commonly used to search and identify natural and safe antioxidants. Other species such as RSS have been considered as a separate class of oxidative stressors, which may provide new antioxidant drug targets [56, 57]. RSS are formed in vivo under conditions of oxidative stress. RSS are likely to include disulphide-S-oxides, sulfenic acids and thiyl radicals, and are predicted to modulate the redox status of biological thiols and disulphides.
Metal ion (Fe2+, Fe3+, Cu2+ and Cu+) chelating
Studies demonstrate that antioxidants and metal chelators may be of beneficial use in the treatment of neurodegenerative diseases, such as Alzheimer’s disease [30, 58, 59]. Transition metals such as copper and iron are known to aggravate oxidative stress. These metals (Fe2+ and Cu+) react with H2O2, which is a product formed by the dismutation of the O2−• by SOD, to produce the highly reactive ▪OH (Eq. 1). Unlike iron, copper generates more singlet oxygen than ▪OH upon its reaction with H2O2. Fe2+ and Cu+ are oxidized to Fe3+ and Cu2+, respectively. In the presence of reducing agents such as ascorbic acid (AscH−), cellular reductant such as NADH and oxidized metal ions Fe3+ and Cu2+ are reduced and allow the recycling to react with another molecule of H2O2 to generate ▪OH radical (Eq. 2). Hydroxyl radical is highly reactive and can react directly with proteins and other macromolecules to produce carbonyls (aldehydes and ketones), cross-linking and lipid peroxidation. Chelating metal ions can decrease their activity thereby reducing the formation of ROS.
| 1 |
| 2 |
Diram et al.[30] and Ak et al.[28] studied the antioxidant and iron-binding properties of curcumin, capsaicin and S-allylcysteine. These compounds show significant DPPH and O2−• scavenging ability. Chelating of ferrous ion by curcumin is demonstrated by the absorbance measurement of the ferrous iron–ferrozine complex at 562 nm [60]. Specifically, curcumin is added to a solution of FeCl2. The reaction is initiated by the addition of 5 mM ferrozine. Then, the mixture is shaken vigorously and left at room temperature for 10 min. Absorbance of the solution is then measured spectrophotometrically at 562 nm. The percentage of inhibition of ferrozine–Fe2+ complex formation is calculated [28]. For the ion-chelating ability in the tissue [30], the lipid peroxidation is initiated by incubating the tissue homogenate with FeSO4 or FeCl2 and H2O2 in either the absence or presence of antioxidants at 37°C for 1 hr. The product of the lipid peroxidation, protein-bound malonedialdehyde (MDA), is extracted from the solution after the addition of TBA in the form of MDA–TBA adduct, whose concentration is determined by its absorption at 532 nm (Fig. 8). The Fe2+ and Fe3+ binding activity of antioxidants such as curcumin, capsaicin and S-allylcysteine can be measured electrochemically. Curcumin and capsaicin compounds can effectively bind to Fe2+ and Fe3+ and prevent the redox cycling of iron, suggesting that these agents may reduce Fe2+-induced lipid peroxidation.
Cu+ generates damaging ▪OH radical 60 times faster than Fe2+[61]. It has found that selenium antioxidant or resveratrol can chelate Cu+ (generated in situ with Cu2+/ascorbic acid) very efficiently and inhibit the DNA damage from ▪OH radical (generated by Cu+/H2O2) [62, 63]. Battin et al. observed that inhibition of oxidative DNA damage requires selenium-metal coordination. This mechanism is validated by using Cu(bipy)22+ (bipy = 2,2′-bipyridine) as the copper source. Cu(bipy)22+ is reduced by ascorbic acid to Cu(bipy)2+ which causes DNA damage in the presence of H2O2. Addition of the selenlium antioxidant does not inhibit the damage, indicating that the antioxidant does not coordinate to Cu(bipy)2+ since the bipy ligands completely coordinate the Cu+ ion. The direct evidence of antioxidant-metal coordination has been observed by the large absorption bands of the complex in the UV-vis range [62, 63].
Importantly, the ▪OH radical scavenging activity by several ginsenosides was thought to be related to the Fe2+ chelating activity of their aglycone instead of direct reaction with ▪OH, studied by EPR spectroscopy [53, 64, 65]. The ion-chelating ability of ginsenosides was thought to be influenced by their type of hydrophilic sugar moieties. Furthermore, polyphenols also show iron binding activity to prevent ROS-induced DNA damage, although direct scavenging of ROS by polyphenols is the generally accepted mechanism of their antioxidant activity [66–69]. The iron binding to polyphenol groups is related to their pKa values. Polyphenol compounds with lower pKa values should bind iron more readily under physiologically relevant conditions. This predicted iron-coordination mechanism for polyphenol antioxidant activity is very useful for the design of more-potent antioxidants to treat and prevent diseases caused by oxidative stress [66].
Inhibition of free radical generating enzymes
NADPH oxidases are a group of plasma membrane associated enzymes, which transfer one electron from the cytosolic donor NADPH to a molecule of extracellular oxygen, producing O2−•[70]. XO is an enzyme involved in the formation of uric acid in the body, which catalyses the oxidation of hypoxanthine and xanthine to uric acid yielding O2−• and H2O2 and raises the oxidative level in an organism (Fig. 10) [71].
Fig 10.
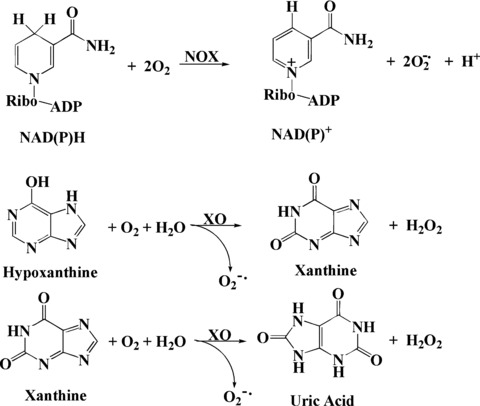
Superoxide and hydroperoxide generation from NAD(P)H, oxidase (NOX) and XO.
These enzymes are the main sources of free radicals and have been reported in various physiological and pathological conditions. O2−• is also a by-product of mitochondrial respiration, as well as several other enzymes such as NADH oxidase, monooxygenases and cyclooxygenases. It is biologically quite toxic and is deployed by the immune system to kill invading microorganisms. In phagocytes, O2−• is produced in large quantities by the enzyme NADPH oxidase for use in oxygen-dependent killing mechanisms for invading pathogens. The controlled production of reactive oxygen derivatives during the respiratory burst is essential for the defense of an organism against invading microorganisms without causing a significant loss of tissue functions [34]. However, excessive ROS promote oxidative stress such as low-density lipoprotein (LDL) oxidation. Recently, a direct association between increased phagocytic NADPH oxidase activity and elevated circulating oxidized LDL has been found in patients with metabolic syndrome. Consequently, both modulation of NADPH oxidase to prevent overproduction of ROS [7] and supplementation with antioxidants have been proposed as effective strategies to prevent the deleterious effects of oxidative stress in haemodialysis patients [72].
Physiopathological importance of ROS generating enzymes has led many researchers to develop new strategies to control the functions of these enzymes in a wide number of in vivo and in vitro experiments. A survey of the scientific literature shows that the use of inhibitors such as diphenylene iodonium and apocynin in the study of NADPH oxidase’s role has increased exponentially in the last years. A number of inhibitors of the O2−• generating NADPH oxidase have been described [73]. Treatment with an XO inhibitor largely prevents the development of endothelial dysfunction and atherosclerosis in mice [74, 75]. Searching for new inhibitors has not stopped and many natural antioxidants have shown great potential to inhibit these enzymes [34, 74, 76–80]. These investigations have clinical significance in understanding the structure and functions of NADPH oxidases and in development of new therapeutic agents for oxidative stress-related diseases.
In a clinical trial, Castilla et al.[72] compared the effects of dietary supplementation with concentrated red grape juice (RGJ), a source of polyphenols and vitamin E, on neutrophil NADPH oxidase activity and other cardiovascular risk factors in haemodialysis patients. They found that both RGJ and vitamin E reduced plasma concentrations of oxidized LDL and ex vivo neutrophil NADPH oxidase activity. These effects were intensified when the supplements were used in combination. This effect of RGJ consumption may favour a reduction in cardiovascular risk. The result indicates natural antioxidants are potential inhibitors of NADPH oxidase. However, the detail mechanism in this important aspect is largely unknown.
The NADPH oxidase activity can be determined by the absorption at 340 nm (a molar extinction coefficient of 6200/M/cm) of oxidation of NADPH by NADPH oxidase in a Tris/EDTA buffer. The production of O2−• is determined by addition of cytochrome c to the reaction mixture, and the amount of O2−• production is calculated using a molar extinction of 21,000/M/cm at 550 nm due to the reduction of cytochrome c[34, 78]. Laurindo et al. also described procedures to assess NADPH oxidase activity in vascular smooth muscle cell membrane fractions using DHE-derived fluorescence oxidation products detected by either HPLC or fluorometry [40].
Diatchuk et al.[76] assayed the lithium dodecyl sulphate (LiDS)- and NADPH-dependent oxygen consumption by cell-free mixtures, consisting of membrane and recombinant cytosolic components. They examined the inhibition of NADPH oxidase by 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF) and related compounds and found that AEBSF prevents the activation of the O2−• generating NADPH oxidase in both intact stimulated macrophages and in cell-free systems consisting of solubilized membrane or purified cytochrome b559 combined with total cytosol. AEBSF did not scavenge oxygen radicals and did not affect assay reagents, while it did have a direct effect on the NADPH oxidase. Thus, NADPH oxidase can be activated in a cell-free system by certain anionic amphiphiles. The effect of AEBSF on O2−• production can be examined in several forms of cell-free activation systems. The simplest one consists of solubilized macrophage membranes and unfractionated cytosol, both derived from unstimulated cells. AEBSF is found to exhibit a concentration-related, inhibitory influence on LiDS-induced O2−• production. In addition, the effect of AEBSF on O2−• production can be studied in a semi-recombinant cell-free system, which consists of either solubilized macrophage membrane or purified cytochrome b559 incorporated in phospholipid liposomes, combined with recombinant p47phox, p67phox and Rac1-GTPgS. AEBSF is able to act as an inhibitor of O2−• production in this system.
Inhibition of XO enzyme activity has also been studied in both cell-free and cell systems. Allopurinol, a potent inhibitor of XO, is clinically used for the treatment for gout by preventing urate from accumulating in joints. Natural polyphenols coumarin (known as 1,2-benzopyrone), consisting of fused benzene and pyrone rings, is an important group of low-molecular weight phenolics and has been widely used for the prevention and treatment of venous thromboembolism, myocardial infarction and strokes [81]. Coumarins acting to inhibit XO enzyme activity and scavenge free radical has been reported [74]. The XO enzyme activity can be measured spectrophotometrically by continuously measuring uric acid formation at 295 nm with xanthine as the substrate. The assay is initiated by adding the enzyme to the reaction mixture containing xanthine, EDTA and 3-(cyclohexylamino)-1-propanesulfonic acid, without or with inhibitors at 37°C. The extent of inhibition is expressed as the chemical concentration required to inhibit 50% of the enzyme activity (IC50). The XO enzyme activity is also assayed with XO assay kit (Cayman Chemical Co., Ann Arbor, MI, USA), based on multistep enzymatic reactions in which XO first produces H2O2 during oxidation of hypoxanthine. In the presence of horseradish peroxidase, the H2O2 reacts with ADHP (10-acetyl-3,7-dihydroxyphenoxazine) to produce the highly fluorescent compound resorufin which can be easily analysed at 563 nm (excitation) / 587 nm (emission) (Fig. 11). ADHP, market name Amplex Red™ reagent, is regarded as the best fluorogenic substrate for peroxidase because it is highly specific and stable. The substrate itself is nearly colourless and non-fluorescent until it is oxidized by H2O2 in the presence of HRP to become the highly red fluorescent resorufin (Biotium, Inc., Hayward, CA, USA). Importantly, combined ROS-scavenging and XO-inhibition activities are measured using the DMPO spin adduct generated in the hypoxanthine (HPX) and XO reaction system by the spin trapping method. The structure–activity relationships (SARs) of coumarins interacting with this enzyme suggest that the number of hydroxyl groups on the ring structure of coumarins is correlated with the effects of ROS suppression. This structure-based molecular modelling revealed interactions between coumarins and the active site, the molybdopterin region of XO. The carbonyl of coumarins points towards and closely interacts with the guanidinium group of the Arg880. The O atom of the pyrone ring forms hydrogen bonds with the hydroxyl side chain of Thr1010. One of coumarin derivative, Esculetin, which bears two hydroxyl moieties on its benzene rings, had the highest affinity towards the binding site of XO, and this was mainly due to the interaction of 6-hydroxyl with the E802 residue of XO [74].
Fig 11.
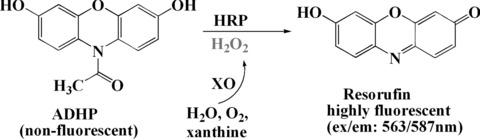
10-acetyl-3,7-dihydroxyphenoxazine (ADHP) fluorescent assay of XO activity.
Activation of internal antioxidant enzymes
During metabolism, ROS such as H2O2, O2−• and ▪OH, etc, are produced irreversibly [82]. Thus, methods to reduce the damage induced by oxidative stress are extensively investigated. Intracellular antioxidant enzymes are an important protective mechanism against ROS. These enzymes are produced in the cell and provide an important defence against free radicals. SOD, CAT, GPX, glutathione reductase (GRd), glutathione S-transferase (GST), thioredoxin reductase (TrxR), heme oxygenase and biliverdin reductase are the most important antioxidant enzymes. The enzyme SOD converts two O2−• into a H2O2 and an oxygen molecule (Eqs 3 and 4).
| 3 |
| 4 |
where M = Cu (n = 1), Mn (n = 2), Fe (n = 2) and Ni (n = 2). In this reaction, the oxidation state of the metal ion oscillates between n and n+1. SOD is an important antioxidant defense in nearly all cells exposed to oxygen. Meanwhile CAT and/or GPX can eliminate H2O2 before the Fenton Reaction can create ▪OH (Eqs 5 and 6).
| 5 |
| 6 |
where GSH represents reduced glutathione, and GS–SG represents glutathione disulphide. GRd then reduces the oxidized glutathione (GS-SG) to complete the cycle (Eq. 7).
| 7 |
Both GST and GPX are involved in eliminating peroxides that are formed during metabolism. GRd regulates the equivalent of GSH and oxidized glutathione (GSSG), and the ratio of GSH/GSSG is a well known index of oxidative stress [83]. The activation of GRd plays an important role in elevating the concentration of GSH, which maintains the oxido-redox status in the organism [14]. The brain, which is very vulnerable to free radical damage, has seven times more GPX activity than CAT activity [84]. Accordingly, increasing evidence suggests a role of oxidative stress in the development and clinical manifestation of autism. Recently, researchers have demonstrated in a comparative cellular system between individuals diagnosed with autism and controls that in a concentration and time-dependent fashion exposure to oxidative stress via the sulfhydryl reagent thimerosal resulted in a greater decrease in the GSH/GSSG ratio and increase in free radical generation in autism compared to control cells [83]. Moreover, GPX is found throughout the cell, whereas CAT is often restricted to peroxisomes. In human beings, the highest levels of CAT are found in liver, kidney and erythrocytes, where it decomposes the majority of H2O2. Nonetheless, the lifespan of transgenic mice has been extended about 20% by overexpression of human CAT targeted to mitochondria [85].
As we have addressed above, many natural substances reduces oxidative stress by radical scavenging, metal ion chelating and inhibiting the activity of radical generating enzymes. It also has been well defined that chemopreventive reagents, such as phenolic antioxidants, dithiolethiones, isothiocyanates, etc., selectively induce the activation of phase II detoxifying and antioxidant enzymes through the Keap1–Nrf2 pathway [10]. Furthermore, cell line models have been used to study the effect of antioxidants such as anthocyanins, resverratrol and curcumin on the activation of these internal antioxidant enzymes. It has been found that natural anthocyanins act as chemopreventive phytochemicals and could stimulate the intracellular antioxidant system to resist oxidant-induced injury [14]. Resveratrol, however, either has no effect on, or reduces the activities of GPX, CAT and CuZnSOD, while it dramatically and progressively induces mitochondrial MnSOD expression and activity [86]. Exposure of human cells to resveratrol for two weeks increased MnSOD protein level 6-fold and activity 14-fold. Thus, long-term exposure of human cells to resveratrol results in a highly specific up-regulation of MnSOD, and this may be an important mechanism by which it elicits its antioxidant effects in human cells. Selenomethionine (SeMet), one of major dietary selenium compounts, can be converted to hydrogen selenide and selenocystein, which are ready for incorporation into proteins at specific UGA codons to form selenoproteins such as active GPX. Thus, SeMet plays a critical role to maintain enzymatic activities of GPX for antioxidation [87–89]. In addition, SeMet is able to directly interact with some oxidant molecules or oxidant generating ions [63, 90, 91].
The GPX and GRd activity can be determined spectrophotometrically according to the method described by Shih et al.[14]. The mixture of homogenate, EDTA, NaN3, NADPH, GSH reductase and GSH in PBS buffer is pre-incubated for 5 min. at 37°C. Thereafter, the overall reaction is initiated by adding H2O2. Enzyme activity is calculated by the change of the absorbance value at 340 nm for 5 min. The GPX activity could be expressed as nanomoles of NADPH per minute per milligram of protein. The GRd assay also monitors the oxidation of NADPH consumed in the reduction of GSSG by the change in absorbance at 340 nm. The following solutions are pipetted into a 1 cm spectrophotometric cuvette: homogenate, MgCl2▪6H2O, GSSG and NADPH in PBS buffer. This mixture is pre-incubated for 5 min. at 37°C. GRd activity is calculated by the change in the absorbance value at 340 nm for 5 min. and is expressed as nanomoles of NADPH per minute per milligram of protein.
TrxR is a key enzyme for DNA synthesis by directly serving as an electron donor to ribonucleotide reductase [19]. It catalyses NADPH-dependent reduction of the redox-active disulphide in Trx, and it plays essential roles in substrate reductions, defence against oxidative stress and redox regulation by thiol redox control. TrxR has been found to be overexpressed by a number of human tumors. Fang et al. studied the inhibitory effect of curcumin on TrxR [92]. TrxR is first reduced with NADPH in a Tris-Cl/EDTA (TE) buffer, and incubated with curcumin at room temperature. The enzyme activity assay is based on the reduction of DTNB (5,5′-dithiobis[2-nitrobenzoic acid]) with NADPH to 5-thio-2-nitrobenzoic acid (TNB) which produces a yellow product that is measured at 405-414 nm with spectrophotometer [79, 92]. Curcumin is found to inhibit TrxR irreversibly, and the curcumin-modified TrxR enzyme showed a strong induction of NADPH oxidase activity to produce O2−•. The curcumin modified sites and peptides of TrxR are also identified. Modification of TrxR by curcumin provides a possible mechanistic explanation for its cancer preventive activity, shifting the enzyme from an antioxidant to a prooxidant.
Native SOD has been used as a therapeutic agent to attenuate ROS-induced injuries. However, applications of exogenous SOD still have many problems including short clearance, brain inaccessibility, chemical instability, low oral bio-availability, specific tissue targeting and immunogenicity [82, 93]. An increasing number of low molecular-weight SOD mimics have been developed to overcome these limitations. Most SOD catalytic mimics are designed with a redox active metal centre, similar to the active site metals of the native SODs, i.e. Cu, Fe or Mn. Among them, MnSOD is thought being safe and has been well developed [94, 95]. MnTBAP, Manganese (III) 5,10,15,20-tetrakis(4-benzoic acid) porphyrin (MnTBAP), is one of the most intensely explored ‘SOD mimics’ in biology and medicine [96, 97]. MnTBAP is an active, stable, non-toxic and cell permeable SOD mimetic. Most importantly, it possesses both SOD and CAT activity [98]. MnTBAP scavenges O2−•, H2O2, ONOO− and ROO▪ radicals, but does not scavenge nitric oxide [99, 100]. It is a potent inhibitor of lipid peroxidation [98, 101].
However, the molecular relationship between consumption of antioxidants and elevation of antioxidant ability is not completely clear. Many studies have been focused on the structure modification of antioxidants to improve the antioxidant property and to enhance SOD activity [1, 94, 95, 102, 103]. For example, Liu et al. modified the structure of ginsenoside Rg3 to a ocotillol type saponin and found that the lipid peroxidation (LPO) activity decreased and SOD activity increased 120 min. after administration of ginsenoside Rg3 to dogs. However, the underlying mechanisms for these effects are not clear [102]. Barik et al. have found that cupper complexes of curcumin analogues had SOD activity [95]. They have established chemical models which can be used to study the SOD activity of the metal-curcumin SOD mimics. Briefly, the SOD assay is carried in a mixture containing xanthine, NBT2+ (NBT = nitroblue tetrazolium), XO and metal-curcumin complex in PBS buffer. After incubation at room temperature for 10 min., the NBT+ formed can be measured spectrophotometrically at 560 nm. SOD activity is calculated based on the rate of absorbance change per minute (DA/min) and expressed in terms of the IC50 of NBT reduction obtained from a linear regression plot of percent SOD activity versus test compound concentrations. However, the cell-free systems for a direct interaction of antioxidants with SOD enzyme have not been established. Cell-free systems for assaying the activities of these intracellular antioxidant enzymes are necessary to elucidate the mechanisms of action of certain antioxidants.
Many diseases such as Alzheimer’s disease, audism, cardiovascular risk and cancer can be correlated with increased activity of free radial generating enzymes and decreased activity of antioxidant enzymes. One study showed that NADPH oxidase is activated in the brains of patients with Alzheimer’s disease by demonstrating the marked translocation of the cytosolic factors p47-phox and p67-phox to the membrane in Alzheimer’s disease [104]. NADPH oxidase is a highly regulated membrane-bound enzyme complex that is composed of a number of cytosolic and membrane-bound proteins. p47-phox and p67-phox, two of the components of NADPH oxidase, are normally located in the cytosol in resting cells and their translocation from the cytosol to the membrane is required for O2−• generation in vitro and in vivo. Activation of NADPH oxidase results in production of O2−•. This is then rapidly converted to secondary toxic ROS or RNS such as ONOO−, which can efficiently kill microorganisms. Melatonin shows significant antioxidative effects in Alzheimer’s disease models in vitro and in vivo. Pre-treatment of microglia with melatonin dose-dependently prevents the activation of NADPH oxidase and decreases the production of ROS. Melatonin inhibits the phosphorylation of the p47phox subunit of NADPH oxidase via a PI3K/Akt-dependent signalling pathway, blocks the translocation of p47phox and p67phox subunit to the membrane, down-regulates the binding of p47phox to gp91phox, and impairs the assembly of NADPH oxidase [105].
Autism is a severe developmental disorder with poorly understood aetiology. Oxidative stress in autism has been studied in cell culture. A mechanism has been proposed that links oxidative stress with membrane lipid abnormalities, inflammation, aberrant immune response, impaired energy metabolism and excitotoxicity, leading to clinical symptoms and pathogenesis of autism [5, 6, 83, 106]. As reviewed by Chauhan [106], several studies have observed patients with autism showed decreased activity of GPX in plasma and in erythrocytes, reduced levels of total glutathione and lower redox ratio of GSH to GSSG in plasma, and decreased CAT and SOD activity in erythrocytes.
Natural substances may directly influence the activities of these enzymes [107]. For example, a clinical trial reported that both red grape juice and vitamin E reduced plasma concentrations of oxidized LDL and ex vivo neutrophil NADPH oxidase activity, indicating that natural antioxidants are potential inhibitors of NADPH oxidase. This may favour a reduction in cardiovascular risk [72]. However, detail mechanisms in this important aspect are largely unknown.
Prevention of lipid peroxidation
Lipid peroxidation refers to the oxidative deterioration of lipids containing any number of carbon-carbon double bonds, such as unsaturated fatty acids, phospholipids, glycolipids, cholesterol esters and cholesterol itself. ROS attack the unsaturated fatty acids which contain multiple double bonds and the methylene -CH2- groups with especially reactive hydrogen atoms, and initiate the radical peroxidation chain reactions (Fig. 12) [108]. Radical scavengers can directly react and quench peroxide radicals to terminate the chain reaction. Lipid peroxidation and DNA damage are associated with a variety of chronic health problems, such as cancer, ageing and atherosclerosis [109–111].
Fig 12.
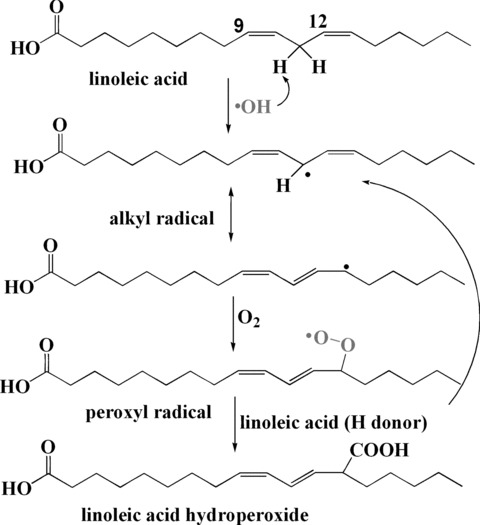
Mechanism of linoleic acid peroxidation initiated by ▪OH radical.
Antioxidant compounds may scavenge ROS and peroxide radicals, thereby preventing or treating certain pathogenic conditions. Lipid peroxidation has been extensively used as a research model for identifying natural antioxidants as well as the studies of their mechanisms of action. Studies on antioxidants such as vitamins, polyphenols (green tea), flavones and ginsenosides against free radical-induced lipid peroxidation have been undertaken in several systems such as lipid, human red cells, human LDL and rat liver microsomes in homogeneous solution or micelles. The antioxidant activity of these polyphenols depends significantly on the structure of the molecules, the initiation conditions and the microenvironment of the reaction medium. In vitro lipid peroxidation such as linoleic acid can be either initiated thermally by using a water soluble azo initiator 2, 2′-azobis(2-amidinopropane) hydrochloride (AAPH) [112], or initiated by metal ions Fe2+ or Cu+ with H2O2 (Fenton reaction) [18, 62, 113]. AAPH decomposes at physiological temperature (37°C) in aqueous solutions to generate alkyl radical (R▪), which in the presence of oxygen is converted to the corresponding peroxyl radicals (ROO▪). Because AAPH is water soluble, the rate of free-radical generation from AAPH can be easily controlled and measured. It has been extensively used as a free-radical initiator for biological and related studies and the haemolysis induced by AAPH provides a good approach for studying membrane damage induced by free radicals [55]. Fe2+ or Cu+ react with H2O2 and generate highly reactive ▪OH. These radicals (▪OH, R▪ and ROO▪) abstract an active methylene hydrogen from linoleic acid to form lipid radical and lipid peroxide in the presence of O2, and start the chain reaction. Briefly, linoleic acid substrate (or its analogues) can be incubated with initiator (either AAPH [114] or Fe2+/H2O2[18]) in the absence or presence of antioxidants in homogenous solution or SDS micelle. Peroxidation of linoleic acid gives different hydroperoxides depending on the reaction conditions. Hydroperoxide substitution at the C-9 or C-13 positions produces either trans, trans or cis, trans conjugated dienes (Fig. 12). These are the major products in the absence of antioxidants and show characteristic ultraviolet absorption at 235 nm that can be used to monitor the formation of the total hydroperoxides during the peroxidation after separation of the reaction mixture by HPLC [114]. In some studies [18], a colour indicator benzoyl leucomethylene blue can be used to reduce the linoleic acid hydroperoxide to linoleic acid hydroxide, while itself is oxidized to methylene blue which shows a absorption at 666 nm. In the presence of antioxidants, the antioxidant first inhibits the formation of linoleic acid hydroperoxides by scavenging peroxide and ▪OH radicals and also inhibits the formation of the colour indicator methylene blue by reduction of the linoleic acid hydroperoxide to the linoleic acid hydroxide. By comparing the formation kinetics of hydroperoxide in the presence and absence of antioxidants, the inhibitory ability of antioxidants can be evaluated and expressed as the total antioxidant activity. This test has been used to identify the key antioxidant in bread crust [115]. By using synthesized methyl esters of linoleic acid hydroperoxide as the substrate, the reductive activity of antioxidants can be measured. The difference between the total antioxidant activity and the reductive activity is the radical scavenging activity [18].
Other models of lipid peroxidation include the lipid peroxidation of LDL, human red cell and pospholipid microsomes. These models are very similar to those of linoleic acid peroxidation. Instead of pure linoleic acid, human red cells, LDL or microsomes are used as the lipid substrate. Erythrocyte membranes are rich in polyunsaturated fatty acids, which are very susceptible to oxidative stress mediated by free radicals. In the human red cell system, briefly, AAPH–PBS solution has been to a suspension of erythrocytes in PBS to which an antioxidant is added in advance to a certain concentration, and the suspension is incubated at 37°C. Samples are taken from the above incubation mixture and centrifuged, and the supernatant is analysed for haemoglobin (haemolysis) at 540 nm [116]. Peroxidation of polyunsaturated lipids in red cell membranes causes a quick damage and the membrane losses its integrity, leading to the release of haemoglobin (haemolysis) and intracellular K+ ions. When antioxidants such as curcumin are present the system, peroxyl radicals can be converted to non-reactive species, and thereby the radical-induced lipid peroxidation and haemolysis can be inhibited.
LDL has a highly-hydrophobic core consisting of polyunsaturated fatty acid linoleate and about 1500 esterified cholesterol molecules [117]. The peroxidation of LDL can be initiated either thermally by a water-soluble azo initiator, AAPH, or photochemically by a triplet sensitizer benzophenone. The peroxidation of LDL can be measured by the formation of conjugated dienes (absorbance at 235 nm) as above or the rate of oxygen intake [118]. A new method is to quantify the decomposition product of hydroperoxide of LDL oxidation, hexanal, by headspace GC analysis [119]. Hexanal production correlates well with the oxidation of polyunsaturated fatty acid in LDL and reflects the degree of LDL oxidation in vitro. The addition of antioxidants can inhibit the formation of lipid dienes or the rate of oxygen intake. The method has been used to study the antioxidant activities of flavonols from apple peels and green tea leaves, vitamins, and resveratrol and its analogues [118–121]. Peroxyl radical-initiated LDL oxidation in these studies is similar to the LDL oxidation under physiological conditions in human beings compared with the Cu2+-induced LDL oxidation model [119].
Similar to erythrocyte membranes, microsomes (especially smooth endoplasmic reticulum) are particularly susceptible to oxidative stress because of their high polyunsaturated fatty-acid content [122]. Iron (Fe2+ combined with a reducing reagent) is usually used for generating ▪OH radicals to induce microsome peroxidation, which can be measured by the TBA method [123]. Antioxidants may inhibit the formation of TBA reactive species. Thus, the antioxidation activity of the antioxidant can be determined [124, 125].
Lipid peroxidation processes involve radical formations including initiator radicals (R▪, ▪OH), and ROO▪ radicals. The antioxidant may directly react with initiator radicals or lipid peroxides, and it may also inhibit the formation of active radicals. These mechanisms of action of any antioxidant are critical and warrant for further investigations with radical scavenging assays and ion chelating tests.
Prevention of DNA damage
The ▪OH and ONOO− radicals generated from nitric oxide and O2−• can react directly with plasmid DNA macromolecules in vivo to cleave (or ‘nick’) one DNA strand, causing oxidative DNA damage. Cell death and mutation caused by this DNA damage are implicated in neurodegenerative and cardiovascular diseases, cancer and aging. Treating these conditions with antioxidants is of growing interest. At the same time, the DNA or plasmid damage has been used as models for the study and identification of antioxidants [27, 32, 62, 66, 91, 126]. A typical research model of DNA damage caused by Cu+ induced ▪OH has been developed [62, 63]. Briefly, metal-free plasmid DNA is combined with Cu2+, ascorbic acid and H2O2 at pH 7. Cu2+ is reduced to Cu+ in situ with ascorbic acid. The ▪OH radical generated by Cu+/H2O2 cleaves one DNA strand, causing the normally supercoiled plasmid DNA to unwind. The degree of DNA damage is assessed using electrophoresis to separate the damaged and undamaged forms. Adding antioxidants such as selenium can inhibit ▪OH-induced DNA damage. Thus, the antioxidant potential of certain compounds can be quantified and directly compared. DNA damage also produces carbonyls (aldehydes and ketones), which react with TBA to form TBARS which can be measured directly in the aqueous phase by TBARS assay at 532 nm [32].
Prevention of protein modification
Besides lipid peroxidation and DNA damage, ROS also cause protein modification by nitration or chloration of amino acids. Peroxynitrite, O = N-O-O−, formed in vivo by the reaction of O2−• with free radical nitric oxide by a diffusion-controlled reaction, is an powerful oxidant and nitrating agent [127]. ONOO− is a much more powerful oxidant than O2−• and can damage a wide variety of molecules including DNA and proteins in cells. ONOO− and its protonated form peroxynitrous acid (ONOOH) can exert direct oxidative modifications through one- or two-electron oxidation processes [128]. ONOO− reacts nucleophilically with CO2in vivo to form nitrosoperoxycarbonate, which is the predominant pathway for ONOO−. ONOOCO2− homolyses to form carbonate radical (CO3−▪) and nitrogen dioxide radical (▪NO2). ▪NO2 is also a RNS which in turn can nitrate tyrosine to nitrotyrosine. These radicals are believed to cause ONOO−-related cellular damage (Fig. 13). ONOO− itself is also a strong oxidant and can react directly with electron-rich groups, such as sulfhydryls, iron-sulphur centres, zinc-thiolates and the active site sulfhydryl in tyrosine phosphatases [127]. Another pathway utilizes heme protein myeloperoxidase (MPO)-generated HOCl [129], which reacts with NO2− to form nitryl chloride (NO2Cl), which may spontaneously decompose to ▪NO2 and Cl▪. These products may be responsible for the chlorinating and nitrating behaviour of Cl-NO2[130]. HOCl reacts with a wide variety of biomolecules including DNA, RNA, fatty acid groups, cholesterol and proteins. The presence of nitrotyrosine or chlorotyrosine has been as biomarker of damage by RNS in vivo[131].
Fig 13.
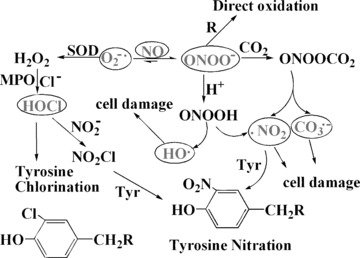
Potential pathways of nitrotyrosine and chlortyrosine formation. ONOO− , peroxynitrite; HOCl, hypochlorous acid; NO2Cl, nitryl chloride; Cl−, chloride; H2O2, hydrogen peroxide; CO3−▪, carbonate radical; ▪NO2, nitrogen dioxide radical; ONOOH) NO2−, peroxynitrous acid; NO2Cl, nitryl chloride; MPO, myeloperoxidase; Tyr, tyrosine.
These modifications often result in the alteration of protein function or structure and, usually, the inhibition of enzyme activities. Proteins containing nitrotyrosine residues have been detected in different pathologic conditions, including diabetes, hypertension and atherosclerosis, all associated with enhanced oxidative stress, including increased production of ONOO−[132]. In order to attenuate the protein modification caused by ONOO− and HOCl, antioxidants and antioxidant enzyme are used. Antioxidants or enzyme like CAT which can eliminate H2O2 should also inhibit the formation of HOCl; likewise, SOD or antioxidant such as curcumin and polyphenols may scavenge O2−•, and inhibit the formation of ONOO−. However, the formation of ONOO− from nitric oxide and O2−• is three times faster than the scavenging of O2−• by SOD. Therefore, the scavenging of ONOO− directly by using natural safe ingredients from the medicinal herbs may be a rational alternative for preventive and therapeutic interventions in diseases [133]. Indeed, reactions of ONOO− with phenoic compounds are widely reported in the literature [134]. Scavenging of ONOO− by antioxidants based on assays involving tyrosine nitration is suggested as a useful research tool which may provide additional valuable information on the antioxidant profile of the biomolecules [135, 136]. Generation of ONOO−in vivo has been implicated in a wide range of human diseases. Agents that are able to protect against ONOO− dependent damage may be therapeutically useful.
Rosmarinic acid has been well recognized especially, in regard to its antioxidant and anti-inflammatory activities [137]. Rosmarinic acid displays a strong scavenger activity for ONOO− and other free radicals. ONOO− is responsible for a wide spread biological damage in the brains of patients with Alzheimer’s disease. Amyloid β protein (Aβ), the principal component of the senile plaques, is the main cause of increased ONOO− in the brain of patients with Alzheimer’s disease. In cell culture studies, rosmarinic acid protects against the ROS induced by Aβ, and in in vivo studies, rosmarinic acid has shown a protective effect on the memory impairment in a mouse mode induced by acute intracerebroventricular injection of Aβ25-35 into the brains of mice. It is found that memory protective effects of rosmarinic acid in the neurotoxicity of Aβ25–35 is due to its scavenging of ONOO−, and that daily consumption of rosmarinic acid may protect against memory impairments observed in Alzheimer’s disease [133]. Natural product ergothioneine is also a powerful scavenger of ONOO− and is able to protect α1-antiproteinase against inactivation and tyrosine against nitration [138].
Choi et al. characterized ONOO− scavenging constituents from twenty-eight herbs with the use of a fluorometric method in the cell-free system [139]. They found the potency of scavenging activity to be as follows: witch hazel bark > rosemary > jasmine tea > sage > slippery elm > black walnut leaf > Queen Anne’s lace > Linden flower. The extracts exhibited dose-dependent ONOO− scavenging activities. Thus they found that witch hazel bark (Hamamelis virginiana L.) showed the strongest effect for scavenging ONOO− of the 28 herbs. Hamamelitannin, the major active component of witch hazel bark, was shown to have a strong ability to scavenge ONOO−. The ONOO− scavenging activity can be measured by monitoring the oxidation of DHR 123 by ONOO− with or without an antioxidant present (Fig. 7) [50]. The ONOO− scavenging ability is determined at room temperature by a microplate fluorescence spectrophotometer with excitation and emission wavelengths of 485 and 530 nm, respectively. ONOO− can be purchased from Cayman Chemical Co. or derived by 3-morpholinosydnonimine (SIN-1). SIN-1 liberates nitric oxide spontaneously when in solution and nitrifies protein. SIN-1 is also a vasodilator and inhibits platelet aggregation. It can be used both in vivo and in vitro. Using molecular oxygen, it generates both O2−• and nitric oxide that spontaneously form ONOO−.
HOCl is a powerful oxidant which plays an important role in bactericidal function [140]. However, excess HOCl is also highly reactive towards a range of biological substrates and may cause tissue injury. One of the major targets of HOCl in vivo is α1-antiproteinase (α1-AP), the most important inhibitor of the proteolytic enzymes of polymorphonuclear granulocytes, proteolytic enzymes especially elastase, which degrades elastin [141]. It has been reported that HOCl preferentially attacks one methionine (Met) residue of α1-AP, Met 358, resulting in a methionine sulfoxide and the immediate loss of inhibitory function. Several sulphur-contain compounds have been demonstrated to efficiently preventing HOCl-induced deleterious effects in vitro, including reduced glutathione, oxidized glutathione, and methylglutahione, as well as lipoic acid [142, 143], N-acetylcysteine [144], cefalosporins [145] and histamine H2 antagonists [146]. Many antioxidants usually can scavenge several types of ROS. For example, S-allylcysteine, a garlic-derived compound, has in vivo and in vitro antioxidant properties. It can scavenge ROS such as O2−•, H2O2, ▪OH and ONOO−, as reviewed by Medina-Campos et al. S-allylcysteine can also scavenge both HOCl and 1O2[147]. Sulindac and sulindac sulfone can scavenge O2−•, ▪OH, nitric oxide and ONOO−, while sulindac sulphide scavenges HOCl, O2−•, ▪OH, nitric oxide and ONOO−[148]. HOCl scavenging assay is based on the inhibition of thio-2-nitrobenzoic acid oxidation to DTNB induced by HOCl. TNB has a chromophore that has maximal absorbance at 412 nm while DTNB is colourless (Fig. 14). By following the decrease of absorbance at 412 nm, the HOCl scavenging activity is measured [37, 148].
Fig 14.
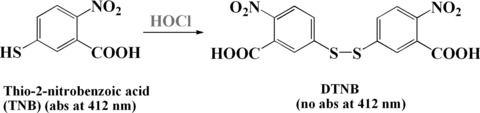
Detection of HOCl by DTNB.
Conclusions
Oxidative stress caused by ROS results in an increased risk for many diseases such as inflammatory disease, cardiovascular disease, cancer, diabetes, Alzheimer’s disease, cataracts, autism and aging. Antioxidants may directly react with the reactive radicals to destroy them by accepting or donating electron(s) to eliminate the unpaired condition of the radical, or they may indirectly decrease the formation of free radicals by inhibiting the activities or expressions of free radical generating enzymes or by enhancing the activities and expressions of other antioxidant enzymes. Many research models have been established in chemical and/or biological systems for studying the mechanisms of action of antioxidants and for identifying new antioxidants, especially from natural substances. We have reviewed the antioxidant mechanisms and experimental approaches such as direct radical scavenging, ion chelating and enzyme activities in cell-free chemical systems. The preventive and inhibitory effects of antioxidants on lipid peroxidation, DNA damage and protein modification caused by ROS are also discussed. The chemical approaches are simple and facilitate the study of the total antioxidant activity of antioxidants and the precise mechanisms of action of antioxidants. However, cell-free systems do not take bioavailability and metabolic factors into consideration, and thereby the data generated from these systems require the confirmation from cell-based systems or in vivo studies. Recently, clinical data has shown a correlation between various types of oxidative stress measurements and clinical findings in actual individuals with diseases. For example, a significant correlation was found between the degree of mercury intoxication and oxidative stress biomarkers present in autism patients [6, 149]. Also, the correlation has been observed between tissue culture models of oxidative stress and the actual pathologies observed in clinical diseases. For example, researchers have identified very similar oxidative stress pathology in human tissue culture model systems as observed in the brains of patients with autism, particularly following exposure to low-dose mercury from Thimerosal [150].
Acknowledgments
This work is partially supported by research grants from the National Institutes of Health (Q.Y.: DE15543 and AT003094, and C.C.: HL72716, EB-002436 and HL083471) and by the Michael E. DeBakey Department of Surgery, Baylor College of Medicine and Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas. The authors have declared that no competing interests exist. The authors greatly acknowledge the reviewers for their constructive comments and suggestions.
References
- 1.Vajragupta O, Boonchoong P, Berliner LJ. Manganese complexes of curcumin analogues: evaluation of hydroxyl radical scavenging ability, superoxide dismutase activity and stability towards hydrolysis. Free Radic Res. 2004;38:303–14. doi: 10.1080/10715760310001643339. [DOI] [PubMed] [Google Scholar]
- 2.Giles GI, Jacob C. Reactive sulfur species: an emerging concept in oxidative stress. Biol. Chem. 2002;383:375–88. doi: 10.1515/BC.2002.042. [DOI] [PubMed] [Google Scholar]
- 3.Ames BN, Shigenaga MK, Gold LS. DNA lesions, inducible DNA repair, and cell division: three key factors in mutagenesis and carcinogenesis. Environ Health Perspect. 1993;101:35–44. doi: 10.1289/ehp.93101s535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu RH, Hotchkiss JH. Potential genotoxicity of chronically elevated nitric oxide: a review. Mutat Res. 1995;339:73–89. doi: 10.1016/0165-1110(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 5.Geier DA, Kern JK, Garver CR, et al. A prospective study of transsulfuration biomarkers in autistic disorders. Neurochem Res. 2009;34:386–93. doi: 10.1007/s11064-008-9782-x. [DOI] [PubMed] [Google Scholar]
- 6.Geier DA, Kern JK, Garver CR, et al. Biomarkers of environmental toxicity and susceptibility in autism. J Neurol Sci. 2009;280:101–8. doi: 10.1016/j.jns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Hossain MA, Asada K. Monodehydroascorbate reductase from cucumber is a flavin adenine dinucleotide enzyme. J Biol Chem. 1985;260:12920–6. [PubMed] [Google Scholar]
- 8.DeFeudis FV, Papadopoulos V, Drieu K. Ginkgo biloba extracts and cancer: a research area in its infancy. Fundam Clin Pharmacol. 2003;17:405–17. doi: 10.1046/j.1472-8206.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 9.Rababah TM, Hettiarachchy NS, Horax R. Total phenolics and antioxidant activities of fenugreek, green tea, black tea, grape seed, ginger, rosemary, gotu kola, and ginkgo extracts, vitamin E, and tert-butylhydroquinone. J Agric Food Chem. 2004;52:5183–6. doi: 10.1021/jf049645z. [DOI] [PubMed] [Google Scholar]
- 10.Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic Biol Med. 2006;41:1727–46. doi: 10.1016/j.freeradbiomed.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 11.Lü J-M, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr. 2005;81:243S–55S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 13.Panchatcharam M, Miriyala S, Gayathri VS, et al. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 2006;290:87–96. doi: 10.1007/s11010-006-9170-2. [DOI] [PubMed] [Google Scholar]
- 14.Shih PH, Yeh CT, Yen GC. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J Agric Food Chem. 2007;55:9427–35. doi: 10.1021/jf071933i. [DOI] [PubMed] [Google Scholar]
- 15.Liu RH, Finley J. Potential cell culture models for antioxidant research. J Agric Food Chem. 2005;53:4311–4. doi: 10.1021/jf058070i. [DOI] [PubMed] [Google Scholar]
- 16.Manach C, Williamson G, Morand C, et al. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–42S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 17.Rice-Evans CA. Measurement of total antioxidant activity as a marker of antioxidant status in vivo: procedures and limitations. Free Radic Res. 2000;33:S59–66. [PubMed] [Google Scholar]
- 18.Lindenmeier M, Burkon A, Somoza V. A novel method to measure both the reductive and the radical scavenging activity in a linoleic acid model system. Mol Nutr Food Res. 2007;51:1441–6. doi: 10.1002/mnfr.200700210. [DOI] [PubMed] [Google Scholar]
- 19.Young IS. Measurement of total antioxidant capacity. J Clin Pathol. 2001;54:339. doi: 10.1136/jcp.54.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirschbaum B. Total urine antioxidant capacity. Clin Chim Acta. 2001;305:167–73. doi: 10.1016/s0009-8981(01)00381-3. [DOI] [PubMed] [Google Scholar]
- 21.Lissi E, Salim-Hanna M, Pascual C, et al. Evaluation of total antioxidant potential (TRAP) and total antioxidant reactivity from luminol-enhanced chemiluminescence measurements. Free Radic Biol Med. 1995;18:153–8. doi: 10.1016/0891-5849(94)00117-3. [DOI] [PubMed] [Google Scholar]
- 22.Van Den Berg R, Haenen GRMM, Van Den Berg H, et al. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999;66:511–7. [Google Scholar]
- 23.Evelson P, Travacio M, Repetto M, et al. Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys. 2001;388:261–6. doi: 10.1006/abbi.2001.2292. [DOI] [PubMed] [Google Scholar]
- 24.Benzie IF, Strain JJ. Ferric reducing/ antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 25.Cao G, Prior RL. Measurement of oxygen radical absorbance capacity in biological samples. Methods Enzymol. 1999;299:50–62. doi: 10.1016/s0076-6879(99)99008-0. [DOI] [PubMed] [Google Scholar]
- 26.Heo SJ, Kim JP, Jung WK, et al. Identification of chemical structure and free radical scavenging activity of diphlorethohydroxycarmalol isolated from a brown alga, Ishige okamurae. J Microbiol Biotechnol. 2008;18:676–81. [PubMed] [Google Scholar]
- 27.Kang HS, Kim KR, Jun EM, et al. Cyathuscavins A, B, and, new free radical scavengers with DNA protection activity from the Basidiomycete Cyathus stercoreus. Bioorg Med Chem Lett. 2008;18:4047–50. doi: 10.1016/j.bmcl.2008.05.110. [DOI] [PubMed] [Google Scholar]
- 28.Ak T, Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee A, Kunwar A, Mishra B, et al. Concentration dependent antioxidant/pro-oxidant activity of curcumin Studies from AAPH induced hemolysis of RBCs. Chem Biol Interact. 2008;174:134–9. doi: 10.1016/j.cbi.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Dairam A, Fogel R, Daya S, et al. Antioxidant and iron-binding properties of curcumin, capsaicin, and S-allylcysteine reduce oxidative stress in rat brain homogenate. J Agric Food Chem. 2008;56:3350–6. doi: 10.1021/jf0734931. [DOI] [PubMed] [Google Scholar]
- 31.Mantle D, Wilkins RM, Gok MA. Comparison of antioxidant activity in commercial Ginkgo biloba preparations. J Altern Complement Med. 2003;9:625–9. doi: 10.1089/107555303322524472. [DOI] [PubMed] [Google Scholar]
- 32.Zhao F, Liu ZQ, Wu D. Antioxidative effect of melatonin on DNA and erythrocytes against free-radical-induced oxidation. Chem Phys Lipids. 2008;151:77–84. doi: 10.1016/j.chemphyslip.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–57. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- 34.Steinbeck MJ, Hegg GG, Karnovsky MJ. Arachidonate activation of the neutrophil NADPH-oxidase. Synergistic effects of protein phosphatase inhibitors compared with protein kinase activators. J Biol Chem. 1991;266:16336–42. [PubMed] [Google Scholar]
- 35.Hasegawa T, Bando A, Tsuchiya K, et al. Enzymatic and nonenzymatic formation of reactive oxygen species from 6-anilino-5,8-quinolinequinone. Biochim Biophys Acta. 2004;1670:19–27. doi: 10.1016/j.bbagen.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Floriano-Sanchez E, Villanueva C, Medina-Campos ON, et al. Nordihydroguaiaretic acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion and hypochlorous acid and prevents in vivo ozone-induced tyrosine nitration in lungs. Free Radic Res. 2006;40:523–33. doi: 10.1080/10715760500419365. [DOI] [PubMed] [Google Scholar]
- 37.Haces ML, Hernandez-Fonseca K, Medina-Campos ON, et al. Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp Neurol. 2008;211:85–96. doi: 10.1016/j.expneurol.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 38.Scudiero DA, Shoemaker RH, Paull KD, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–33. [PubMed] [Google Scholar]
- 39.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–94. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 40.Laurindo FR, Fernandes DC, Santos CX. Assessment of superoxide production and NADPH oxidase activity by HPLC analysis of dihydroethidium oxidation products. Methods Enzymol. 2008;441:237–60. doi: 10.1016/S0076-6879(08)01213-5. [DOI] [PubMed] [Google Scholar]
- 41.Spasojevic I, Liochev SI, Fridovich I. Lucigenin: redox potential in aqueous media and redox cycling with O-(2) production. Arch Biochem Biophys. 2000;373:447–50. doi: 10.1006/abbi.1999.1579. [DOI] [PubMed] [Google Scholar]
- 42.Fink B, Laude K, McCann L, et al. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 43.Zhao H, Joseph J, Fales HM, et al. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci USA. 2005;102:5727–32. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MitoSOX. http://www.invitrogen.com/site/us/en/home/support/Product-Technical-Resources/Product-Structures.-36008.html. . Accessed: 15 March 2009.
- 45.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 46.Myhre O, Andersen JM, Aarnes H, et al. Evaluation of the probes 2’,7’-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol. 2003;65:1575–82. doi: 10.1016/s0006-2952(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 47.Rao KM, Padmanabhan J, Kilby DL, et al. Flow cytometric analysis of nitric oxide production in human neutrophils using dichlorofluorescein diacetate in the presence of a calmodulin inhibitor. J Leukoc Biol. 1992;51:496–500. doi: 10.1002/jlb.51.5.496. [DOI] [PubMed] [Google Scholar]
- 48.Inbaraj JJ, Bilski P, Chignell CF. Photophysical and photochemical studies of 2-phenylbenzimidazole and UVB sunscreen 2-phenylbenzimidazole-5-sulfonic acid. Photochem Photobiol. 2002;75:107–16. doi: 10.1562/0031-8655(2002)075<0107:papsop>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 49.Bilski P, Belanger AG, Chignell CF. Photosensitized oxidation of 2′,7′-dichlorofluorescin: singlet oxygen does not contribute to the formation of fluorescent oxidation product 2′,7′-dichlorofluorescein. Free Radic Biol Med. 2002;33:938–46. doi: 10.1016/s0891-5849(02)00982-6. [DOI] [PubMed] [Google Scholar]
- 50.Zou Y, Kim AR, Kim JE, et al. Peroxynitrite scavenging activity of sinapic acid (3,5-dimethoxy-4-hydroxycinnamic acid) isolated from Brassica juncea. J Agric Food Chem. 2002;50:5884–90. doi: 10.1021/jf020496z. [DOI] [PubMed] [Google Scholar]
- 51.Vowells SJ, Sekhsaria S, Malech HL, et al. Flow cytometric analysis of the granulocyte respiratory burst: a comparison study of fluorescent probes. J Immunol Methods. 1995;178:89–97. doi: 10.1016/0022-1759(94)00247-t. [DOI] [PubMed] [Google Scholar]
- 52.Gutteridge JM. Ferrous-salt-promoted damage to deoxyribose and benzoate. The increased effectiveness of hydroxyl-radical scavengers in the presence of EDTA. Biochem J. 1987;243:709–14. doi: 10.1042/bj2430709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang KS, Yokozawa T, Yamabe N, et al. ESR study on the structure and hydroxyl radical-scavenging activity relationships of ginsenosides isolated from Panax ginseng C A Meyer. Biol Pharm Bull. 2007;30:917–21. doi: 10.1248/bpb.30.917. [DOI] [PubMed] [Google Scholar]
- 54.Fogliano V, Verde V, Randazzo G, et al. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J Agric Food Chem. 1999;47:1035–40. doi: 10.1021/jf980496s. [DOI] [PubMed] [Google Scholar]
- 55.Cai YJ, Dai JQ, Fang JG, et al. Antioxidative and free radical scavenging effects of ecdysteroids from Serratula strangulata. Can J Physiol Pharmacol. 2002;80:1187–94. doi: 10.1139/y02-152. [DOI] [PubMed] [Google Scholar]
- 56.Giles GI, Tasker KM, Jacob C. Hypothesis: the role of reactive sulfur species in oxidative stress. Free Radic Biol Med. 2001;31:1279–83. doi: 10.1016/s0891-5849(01)00710-9. [DOI] [PubMed] [Google Scholar]
- 57.Giles GI, Jacob C. Reactive sulfur species: an emerging concept in oxidative stress. Biol Chem. 2002;383:375–88. doi: 10.1515/BC.2002.042. [DOI] [PubMed] [Google Scholar]
- 58.Delanty N, Dichter MA. Antioxidant therapy in neurologic disease. Arch Neurol. 2000;57:1265–70. doi: 10.1001/archneur.57.9.1265. [DOI] [PubMed] [Google Scholar]
- 59.Cuajungco MP, Faget KY, Huang X, et al. Metal chelation as a potential therapy for Alzheimer’s disease. Ann N Y Acad Sci. 2000;920:292–304. doi: 10.1111/j.1749-6632.2000.tb06938.x. [DOI] [PubMed] [Google Scholar]
- 60.Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–9. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 61.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 62.Zheng LF, Wei QY, Cai YJ, et al. DNA damage induced by resveratrol and its synthetic analogues in the presence of Cu (II) ions: mechanism and structure-activity relationship. Free Radic Biol Med. 2006;41:1807–16. doi: 10.1016/j.freeradbiomed.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 63.Battin EE, Perron NR, Brumaghim JL. The central role of metal coordination in selenium antioxidant activity. Inorg Chem. 2006;45:499–501. doi: 10.1021/ic051594f. [DOI] [PubMed] [Google Scholar]
- 64.Kang KS, Kim HY, Baek SH, et al. Study on the hydroxyl radical scavenging activity changes of ginseng and ginsenoside-Rb2 by heat processing. Biol Pharm Bull. 2007;30:724–8. doi: 10.1248/bpb.30.724. [DOI] [PubMed] [Google Scholar]
- 65.Kang KS, Kim HY, Pyo JS, et al. Increase in the free radical scavenging activity of ginseng by heat-processing. Biol Pharm Bull. 2006;29:750–4. doi: 10.1248/bpb.29.750. [DOI] [PubMed] [Google Scholar]
- 66.Perron NR, Hodges JN, Jenkins M, et al. Predicting how polyphenol antioxidants prevent DNA damage by binding to iron. Inorg Chem. 2008;47:6153–61. doi: 10.1021/ic7022727. [DOI] [PubMed] [Google Scholar]
- 67.Lopes GK, Schulman HM, Hermes-Lima M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim Biophys Acta. 1999;1472:142–52. doi: 10.1016/s0304-4165(99)00117-8. [DOI] [PubMed] [Google Scholar]
- 68.Sestili P, Diamantini G, Bedini A, et al. Plant-derived phenolic compounds prevent the DNA single-strand breakage and cytotoxicity induced by tert-butylhydroperoxide via an iron-chelating mechanism. Biochem J. 2002;364:121–8. doi: 10.1042/bj3640121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Melidou M, Riganakos K, Galaris D. Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: the role of iron chelation. Free Radic Biol Med. 2005;39:1591–600. doi: 10.1016/j.freeradbiomed.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 70.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–76. [PubMed] [Google Scholar]
- 71.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castilla P, Davalos A, Teruel JL, et al. Comparative effects of dietary supplementation with red grape juice and vitamin E on production of superoxide by circulating neutrophil NADPH oxidase in hemodialysis patients. Am J Clin Nutr. 2008;87:1053–61. doi: 10.1093/ajcn/87.4.1053. [DOI] [PubMed] [Google Scholar]
- 73.Cross AR. Inhibitors of the leukocyte superoxide generating oxidase: mechanisms of action and methods for their elucidation. Free Radic Biol Med. 1990;8:71–93. doi: 10.1016/0891-5849(90)90147-b. [DOI] [PubMed] [Google Scholar]
- 74.Lin HC, Tsai SH, Chen CS, et al. Structure-activity relationship of coumarin derivatives on xanthine oxidase-inhibiting and free radical-scavenging activities. Biochem Pharmacol. 2008;75:1416–25. doi: 10.1016/j.bcp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 75.Schroder K, Vecchione C, Jung O, et al. Xanthine oxidase inhibitor tungsten prevents the development of atherosclerosis in ApoE knockout mice fed a Western-type diet. Free Radic Biol Med. 2006;41:1353–60. doi: 10.1016/j.freeradbiomed.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 76.Diatchuk V, Lotan O, Koshkin V, et al. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem. 1997;272:13292–301. doi: 10.1074/jbc.272.20.13292. [DOI] [PubMed] [Google Scholar]
- 77.Riganti C, Costamagna C, Bosia A, et al. The NADPH oxidase inhibitor apocynin (acetovanillone) induces oxidative stress. Toxicol Appl Pharmacol. 2006;212:179–87. doi: 10.1016/j.taap.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 78.Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem. 2005;280:25284–90. doi: 10.1074/jbc.M414645200. [DOI] [PubMed] [Google Scholar]
- 79.Zhong L, Arner ES, Holmgren A. Structure and mechanism of mammalian thioredoxin reductase: the active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine-selenocysteine sequence. Proc Natl Acad Sci USA. 2000;97:5854–9. doi: 10.1073/pnas.100114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brumaghim JL, Li Y, Henle E, et al. Effects of hydrogen peroxide upon nicotinamide nucleotide metabolism in Escherichia coli: changes in enzyme levels and nicotinamide nucleotide pools and studies of the oxidation of NAD(P)H by Fe(III) J Biol Chem. 2003;278:42495–504. doi: 10.1074/jbc.M306251200. [DOI] [PubMed] [Google Scholar]
- 81.Voora D, McLeod HL, Eby C, et al. The pharmacogenetics of coumarin therapy. Pharmacogenomics. 2005;6:503–13. doi: 10.2217/14622416.6.5.503. [DOI] [PubMed] [Google Scholar]
- 82.Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005;38:995–1014. doi: 10.1590/s0100-879x2005000700003. [DOI] [PubMed] [Google Scholar]
- 83.James SJ, Rose S, Melnyk S, et al. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 2009;23:2374–83. doi: 10.1096/fj.08-128926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marklund SL, Westman NG, Lundgren E, et al. Copper- and zinc-containing superoxide dismutase, manganese-containing superoxide dismutase, catalase, and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer Res. 1982;42:1955–61. [PubMed] [Google Scholar]
- 85.Schriner SE, Linford NJ, Martin GM, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–11. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 86.Robb EL, Page MM, Wiens BE, et al. Molecular mechanisms of oxidative stress resistance induced by resveratrol: specific and progressive induction of MnSOD. Biochem Biophys Res Commun. 2008;367:406–12. doi: 10.1016/j.bbrc.2007.12.138. [DOI] [PubMed] [Google Scholar]
- 87.Pedrero Z, Madrid Y. Novel approaches for selenium speciation in foodstuffs and biological specimens: a review. Analytica Chimica Acta. 2009;634:135–52. doi: 10.1016/j.aca.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 88.Hinojosa L, Ruiz J, Marchante JM, et al. Selenium bioaccessibility assessment in selenized yeast alter ‘in vitro’ gastrointestinal digestion using two-dimensional chromatography and mass spectrometry. J Chromatogr A. 2006;1110:108–16. doi: 10.1016/j.chroma.2006.01.088. [DOI] [PubMed] [Google Scholar]
- 89.Alexander J. Selenium. Novartis Found Symp. 2007;282:143–9. [PubMed] [Google Scholar]
- 90.Assmann A, Briviba K, Sies H. Reduction of selenomethionine selenoxide to selenomethionine by glutathione. Arch Biochem Biophys. 1998;340:201–3. doi: 10.1006/abbi.1997.0462. [DOI] [PubMed] [Google Scholar]
- 91.Ramoutar RR, Brumaghim JL. Effects of inorganic selenium compounds on oxidative DNA damage. J Inorg Biochem. 2007;101:1028–35. doi: 10.1016/j.jinorgbio.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 92.Zhong L, Holmgren A. Essential role of selenium in the catalytic activities of mammalian thioredoxin reductase revealed by characterization of recombinant enzymes with selenocysteine mutations. J Biol Chem. 2000;275:18121–8. doi: 10.1074/jbc.M000690200. [DOI] [PubMed] [Google Scholar]
- 93.Czapski G, Goldstein S. Requirements for SOD mimics operating in vitro to work also in vivo. Free Radic Res Commun. 1991(Pt. 1):12–13. doi: 10.3109/10715769109145782. [DOI] [PubMed] [Google Scholar]
- 94.Barik A, Mishra B, Kunwar A, et al. Comparative study of copper(II)-curcumin complexes as superoxide dismutase mimics and free radical scavengers. Eur J Med Chem. 2007;42:431–9. doi: 10.1016/j.ejmech.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 95.Barik A, Mishra B, Shen L, et al. Evaluation of a new copper(II)-curcumin complex as superoxide dismutase mimic and its free radical reactions. Free Radic Biol Med. 2005;39:811–22. doi: 10.1016/j.freeradbiomed.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 96.Faulkner KM, Liochev SI, Fridovich I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J Biol Chem. 1994;269:23471–6. [PubMed] [Google Scholar]
- 97.Faulkner KM, Stevens RD, Fridovich I. Characterization of Mn(III) complexes of linear and cyclic desferrioxamines as mimics of superoxide dismutase activity. Arch Biochem Biophys. 1994;310:341–6. doi: 10.1006/abbi.1994.1176. [DOI] [PubMed] [Google Scholar]
- 98.Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch Biochem Biophys. 1997;347:256–62. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- 99.Zahmatkesh M, Kadkhodaee M, Moosavi SM, et al. Beneficial effects of MnTBAP, a broad-spectrum reactive species scavenger, in rat renal ischemia/reperfusion injury. Clin Exp Nephrol. 2005;9:212–8. doi: 10.1007/s10157-005-0359-6. [DOI] [PubMed] [Google Scholar]
- 100.Zingarelli B, Day BJ, Crapo JD, et al. The potential role of peroxynitrite in the vascular contractile and cellular energetic failure in endotoxic shock. Br J Pharmacol. 1997;120:259–67. doi: 10.1038/sj.bjp.0700872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bao F, Liu D. Peroxynitrite generated in the rat spinal cord induces apoptotic cell death and activates caspase-3. Neuroscience. 2003;116:59–70. doi: 10.1016/s0306-4522(02)00571-7. [DOI] [PubMed] [Google Scholar]
- 102.Liu JP, Lu D, Zhao Y, et al. A new semisynthetic ocotillol-type saponin and resuscitation of haemorrhagic shock. J Asian Nat Prod Res. 2007;9:103–13. doi: 10.1080/10286020500251741. [DOI] [PubMed] [Google Scholar]
- 103.Dutta S, Murugkar A, Gandhe N, et al. Enhanced antioxidant activities of metal conjugates of curcumin derivatives. Met Based Drugs. 2001;8:183–8. doi: 10.1155/MBD.2001.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shimohama S, Tanino H, Kawakami N, et al. Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem Biophys Res Commun. 2000;273:5–9. doi: 10.1006/bbrc.2000.2897. [DOI] [PubMed] [Google Scholar]
- 105.Zhou J, Zhang S, Zhao X, et al. Melatonin impairs NADPH oxidase assembly and decreases superoxide anion production in microglia exposed to amyloid-beta1–42. J Pineal Res. 2008;45:157–65. doi: 10.1111/j.1600-079X.2008.00570.x. [DOI] [PubMed] [Google Scholar]
- 106.Chauhan A, Chauhan V. Oxidative stress in autism. Pathophysiology. 2006;13:171–81. doi: 10.1016/j.pathophys.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 107.Tonissen KF, Di Trapani G. Thioredoxin system inhibitors as mediators of apoptosis for cancer therapy. Mol Nutr Food Res. 2009;53:87–103. doi: 10.1002/mnfr.200700492. [DOI] [PubMed] [Google Scholar]
- 108.Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–90. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 109.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–70. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 110.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91:10771–8. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bland JS. Oxidants and antioxidants in clinical medicine: past, present and future potential. J Nutr Environ Med. 1995;5:255–80. [Google Scholar]
- 112.Dai F, Chen WF, Zhou B. Antioxidant synergism of green tea polyphenols with alpha-tocopherol and l-ascorbic acid in SDS micelles. Biochimie. 2008;90:1499–505. doi: 10.1016/j.biochi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 113.Gal S, Lichtenberg D, Bor A, et al. Copper-induced peroxidation of phosphatidylserine-containing liposomes is inhibited by nanomolar concentrations of specific antioxidants. Chem Phys Lipids. 2007;150:186–203. doi: 10.1016/j.chemphyslip.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 114.Fang JG, Lu M, Chen ZH, et al. Antioxidant effects of resveratrol and its analogues against the free-radical-induced peroxidation of linoleic acid in micelles. Chemistry. 2002;8:4191–8. doi: 10.1002/1521-3765(20020916)8:18<4191::AID-CHEM4191>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 115.Lindenmeier M, Faist V, Hofmann T. Structural and functional characterization of pronyl-lysine, a novel protein modification in bread crust melanoidins showing in vitro antioxidative and phase I/II enzyme modulating activity. J Agric Food Chem. 2002;50:6997–7006. doi: 10.1021/jf020618n. [DOI] [PubMed] [Google Scholar]
- 116.Li GX, Liu ZQ. The protective effects of ginsenosides on human erythrocytes against hemin-induced hemolysis. Food Chem Toxicol. 2008;46:886–92. doi: 10.1016/j.fct.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 117.Segrest JP, Jones MK, De Loof H, et al. Structure of apolipoprotein B-100 in low density lipoproteins. J Lipid Res. 2001;42:1346–67. [PubMed] [Google Scholar]
- 118.Liu ZQ, Ma LP, Liu ZL. Making vitamin C lipophilic enhances its protective effect against free radical induced peroxidation of low density lipoprotein. Chem Phys Lipids. 1998;95:49–57. doi: 10.1016/s0009-3084(98)00064-4. [DOI] [PubMed] [Google Scholar]
- 119.Chu YF, Liu RH. Novel low-density lipoprotein (LDL) oxidation model: antioxidant capacity for the inhibition of LDL oxidation. J Agric Food Chem. 2004;52:6818–23. doi: 10.1021/jf040099j. [DOI] [PubMed] [Google Scholar]
- 120.Liu ZQ, Yu W, Liu ZL. Antioxidative and prooxidative effects of coumarin derivatives on free radical initiated and photosensitized peroxidation of human low-density lipoprotein. Chem Phys Lipids. 1999;103:125–35. doi: 10.1016/s0009-3084(99)00101-2. [DOI] [PubMed] [Google Scholar]
- 121.Cheng JC, Fang JG, Chen WF, et al. Structure-activity relationship studies of resveratrol and its analogues by the reaction kinetics of low density lipoprotein peroxidation. Bioorg Chem. 2006;34:142–57. doi: 10.1016/j.bioorg.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 122.Liebler DC, Stratton SP, Kaysen KL. Antioxidant actions of beta-carotene in liposomal and microsomal membranes: role of carotenoid-membrane incorporation and alpha-tocopherol. Arch Biochem Biophys. 1997;338:244–50. doi: 10.1006/abbi.1996.9822. [DOI] [PubMed] [Google Scholar]
- 123.Chattopadhyay A, Choudhury TD, Bandyopadhyay D, et al. Protective effect of erythropoietin on the oxidative damage of erythrocyte membrane by hydroxyl radical. Biochem Pharmacol. 2000;59:419–25. doi: 10.1016/s0006-2952(99)00277-4. [DOI] [PubMed] [Google Scholar]
- 124.Cai YJ, Fang JG, Ma LP, et al. Inhibition of free radical-induced peroxidation of rat liver microsomes by resveratrol and its analogues. Biochim Biophys Acta. 2003;1637:31–8. doi: 10.1016/s0925-4439(02)00174-6. [DOI] [PubMed] [Google Scholar]
- 125.Cai YJ, Ma LP, Hou LF, et al. Antioxidant effects of green tea polyphenols on free radical initiated peroxidation of rat liver microsomes. Chem Phys Lipids. 2002;120:109–17. doi: 10.1016/s0009-3084(02)00110-x. [DOI] [PubMed] [Google Scholar]
- 126.Zhao F, Tang YZ, Liu ZQ. Protective effect of icariin on DNA against radical-induced oxidative damage. J Pharm Pharmacol. 2007;59:1729–32. doi: 10.1211/jpp.59.12.0016. [DOI] [PubMed] [Google Scholar]
- 127.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 129.Winterbourn CC. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta. 1985;840:204–10. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- 130.Eiserich JP, Cross CE, Jones AD, et al. Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid. A novel mechanism for nitric oxide-mediated protein modification. J Biol Chem. 1996;271:19199–208. doi: 10.1074/jbc.271.32.19199. [DOI] [PubMed] [Google Scholar]
- 131.Mohiuddin I, Chai H, Lin PH, et al. Nitrotyrosine and chlorotyrosine: clinical significance and biological functions in the vascular system. J Surg Res. 2006;133:143–9. doi: 10.1016/j.jss.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 132.Viappiani S, Schulz R. Detection of specific nitrotyrosine-modified proteins as a marker of oxidative stress in cardiovascular disease. Am J Physiol Heart Circ Physiol. 2006;290:H2167–8. doi: 10.1152/ajpheart.00128.2006. [DOI] [PubMed] [Google Scholar]
- 133.Alkam T, Nitta A, Mizoguchi H, et al. A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by Abeta(25–35) Behav Brain Res. 2007;180:139–45. doi: 10.1016/j.bbr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 134.Ramezanian MS, Padmaja S, Koppenol WH. Nitration and hydroxylation of phenolic compounds by peroxynitrite. Chem Res Toxicol. 1996;9:232–40. doi: 10.1021/tx950135w. [DOI] [PubMed] [Google Scholar]
- 135.Whiteman M, Halliwell B. Protection against peroxynitrite-dependent tyrosine nitration and alpha 1-antiproteinase inactivation by ascorbic acid. A comparison with other biological antioxidants. Free Radic Res. 1996;25:275–83. doi: 10.3109/10715769609149052. [DOI] [PubMed] [Google Scholar]
- 136.Whiteman M, Tritschler H, Halliwell B. Protection against peroxynitrite-dependent tyrosine nitration and alpha 1-antiproteinase inactivation by oxidized and reduced lipoic acid. FEBS Lett. 1996;379:74–6. doi: 10.1016/0014-5793(95)01489-6. [DOI] [PubMed] [Google Scholar]
- 137.Gao LP, Wei HL, Zhao HS, et al. Antiapoptotic and antioxidant effects of rosmarinic acid in astrocytes. Pharmazie. 2005;60:62–5. [PubMed] [Google Scholar]
- 138.Aruoma OI, Whiteman M, England TG, et al. Antioxidant action of ergothioneine: assessment of its ability to scavenge peroxynitrite. Biochem Biophys Res Commun. 1997;231:389–91. doi: 10.1006/bbrc.1997.6109. [DOI] [PubMed] [Google Scholar]
- 139.Choi HR, Choi JS, Han YN, et al. Peroxynitrite scavenging activity of herb extracts. Phytother Res. 2002;16:364–7. doi: 10.1002/ptr.904. [DOI] [PubMed] [Google Scholar]
- 140.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–17. [PubMed] [Google Scholar]
- 141.Maier KL, Matejkova E, Hinze H, et al. Different selectivities of oxidants during oxidation of methionine residues in the alpha-1-proteinase inhibitor. FEBS Lett. 1989;250:221–6. doi: 10.1016/0014-5793(89)80725-2. [DOI] [PubMed] [Google Scholar]
- 142.Den Hartog GJ, Haenen GR, Vegt E, et al. Efficacy of HOCl scavenging by sulfur-containing compounds: antioxidant activity of glutathione disulfide? Biol Chem. 2002;383:709–13. doi: 10.1515/BC.2002.073. [DOI] [PubMed] [Google Scholar]
- 143.Haenen GR, Bast A. Scavenging of hypochlorous acid by lipoic acid. Biochem Pharmacol. 1991;42:2244–6. doi: 10.1016/0006-2952(91)90363-a. [DOI] [PubMed] [Google Scholar]
- 144.Aruoma OI, Halliwell B, Hoey BM, et al. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–7. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 145.Lapenna D, Cellini L, De Gioia S, et al. Cephalosporins are scavengers of hypochlorous acid. Biochem Pharmacol. 1995;49:1249–54. doi: 10.1016/0006-2952(95)00044-z. [DOI] [PubMed] [Google Scholar]
- 146.Ching TL, De Jong J, Bast A. Structural characteristics of histamine H2 receptor antagonists that scavenge hypochlorous acid. Eur J Pharmacol. 1994;268:89–93. doi: 10.1016/0922-4106(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 147.Medina-Campos ON, Barrera D, Segoviano-Murillo S, et al. S-allylcysteine scavenges singlet oxygen and hypochlorous acid and protects LLC-PK(1) cells of potassium dichromate-induced toxicity. Food Chem Toxicol. 2007;45:2030–9. doi: 10.1016/j.fct.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 148.Fernandes E, Toste SA, Lima JL, et al. The metabolism of sulindac enhances its scavenging activity against reactive oxygen and nitrogen species. Free Radic Biol Med. 2003;35:1008–17. doi: 10.1016/s0891-5849(03)00437-4. [DOI] [PubMed] [Google Scholar]
- 149.Sajdel-Sulkowska EM, Lipinski B, Windom H, et al. oxidative stress in autism: elevated cerebellar 3-nitrotyrosine levels. Am J Biochem Biotech. 2008;4:73–84. [Google Scholar]
- 150.Geier DA, King PG, Geier MR. Mitochondrial dysfunction, impaired oxidative-reduction activity, degeneration, and death in human neuronal and fetal cells induced by low-level exposure to thimerosal and other metal compounds. Toxicol Environ Chem. 2009;91:735–49. doi: 10.1080/02772240802246458. [DOI] [PMC free article] [PubMed] [Google Scholar]


