Abstract
Irinotecan is a camptothecin analog used as an anticancer drug. Severe, potentially life-threatening toxicities can occur from irinotecan treatment. Although multiple genes may play a role in irinotecan activity, the majority of evidence to date suggests that variation in expression of UGT1A1 caused by a common promoter polymorphism (UGT1A1*28) is strongly associated with toxicity; however, this link is dose dependent. Variations in other pharmacokinetic genes, particularly the transporter ABCC2, also contribute to irinotecan toxicity. In addition, recent studies have shown that pharmacodynamic genes such as TDP1 and XRCC1 can also play a role in both toxicity and response.
Keywords: ABCC2, irinotecan, pharmacogenomics, TDP1, toxicity, UGT1A1, XRCC1
Camptothecin, a cytotoxic agent found in Camptotheca acuminata, was developed as an anticancer agent in the early 1970s [1–3]. Its mechanism of action is to bind to the DNA/topoisomerase I complex during DNA replication, preventing the resealing of single-strand breaks. Ultimately, the replication machinery collides with the camptothecin/toposiomerase I complex, shattering the DNA [4]. However, camptothecin is insoluble and attempts to address this both reduced the efficacy and increased the toxicity of the drug.
Camptothecin analogs were developed in the 1990s to circumvent the solubility problems. Irinotecan (also known as CPT-11, Camptosar®) is an analog approved for first-line therapy of advanced colorectal cancer in combination with 5-fluorouracil and/or leucovorin. In addition, irinotecan/cisplatin combination therapy is used for other cancers, for example lung and ovarian [5–6]. Recent studies have involved the combination of irinotecan with bevacizumab or cetuximab [1–2,7–8].
Diarrhea and neutropenia are major limiting factors for irinotecan, with up to 36% of patients experiencing severe, potentially life-threatening toxicities [9]. Methods such as pharmacogenomics to prospectively screen patients for DNA variations (Table 1) prior to selecting irinotecan therapy or dose would help improve patient care and reduce healthcare costs [10–12].
Table 1.
Summary of genes and variants from irinotecan pharmacogenomics studies.
| Gene | Variant | dbSNP ID | Effect | Ref. |
|---|---|---|---|---|
| ABCB1 | 1236C>T | rs1128503 | Decreased irinotecan clearance; risk of toxicity and reduced survival when in haplotype with 2677 and 3435 | [56,58,59] |
| ABCB1 | 2677G>A/T | rs2032582 | Risk of toxicity and reduced survival when in haplotype with 1236 and 3435 | [58,59] |
| ABCB1 | 3435C>T | rs1045642 | Increased toxicity; risk of toxicity and reduced survival when in haplotype with 1236 and 2677 | [57–59] |
| ABCC2 | −24C>T | rs717620 | Increased response and survival with 3972; toxicity as part of ABCC2 haplotype in patients without GT1A1*28 | [46,61–63] |
| ABCC2 | 3972T>C | rs3740066 | Increased response and survival with −24; toxicity as part of ABCC2 haplotype in patients without UGT1A1*28 | [43,46,60–63] |
| ABCG2 | 34G>A | rs2231137 | Increased toxicity; no association with toxicity and outcome | [60,61] |
| ABCG2 | 421C>A; Q141K | rs2231142 | Reduced expression; irinotecan resistance; toxicity with IVS12 +49G>T | [49,61,66,67] |
| ABCG2 | IVS12+49G>T | rs3832043 | Toxicity with 421C>A | [49,61,66,67] |
| CES1 | −816A>C | Unknown | Altered CES1 promoter activity | [22] |
| CES2 | 830C>G; −171C>G | rs11075646 | No association with expression, catalytic activity, toxicity or outcome | [20] |
| CYP3A4 | Activity | Altered irinotecan dosing requirement | [24] | |
| NR1I2 | Expression | Altered SN-38 glucuronidation | [79] | |
| TDP1 | IVS12+79T>G | rs2401863 | Response; no toxicity association at higher doses | [74,75] |
| UGT1A1 | −3156G>A | rs10929302 | Increased risk of toxicity | [32,33] |
| UGT1A1 | (TA)7 TAA, *28 | rs8175347 | Increased risk of toxicity; dose dependent | [32,33,38–41] |
| UGT1A1 | G71R, *6 | rs4148323 | Increased risk of toxicity | [44,47] |
| UGT1A7 | *2 (N129K and R131K) | rs17868323, rs17868324 | SN-38 glucuronidation; response | [47,52] |
| UGT1A7 | *3 (N129K, R131K and W208R) | rs17868323, rs17868324, rs11692021 | SN-38 glucuronidation; increased risk of toxicity; altered response | [47,52,54] |
| UGT1A9 | −118(dT) | rs3832043 | SN-30 glucuronidation; response | [44,47,52,54] |
| XRCC1 | Haplotype (−1149delGGCC, R399Q) | rs321329 rs25487 | Response | [74] |
| XRCC1 | R399Q | rs25487 | No association with toxicity; association with response | [76,77] |
dbSNP: SNP database
Irinotecan pharmacokinetics
Metabolism
Irinotecan is a prodrug, metabolized into the active form, SN-38, via human carboxylesterases CES1 and CES2. CYP3A4 converts irinotecan into the inactive metabolite, APC. The active SN-38 can be subsequently inactivated through glucuronidation via members of the UDP-glucuronosyltransferase family [12]. UGT1A enzymes are a product of alternative splicing from the UGT1A locus located on chromosome 2q37. A total of 13 UGT1A genes are encoded at this locus (including four pseudogenes). Each UGT1A enzyme has a unique promoter and a unique exon 1, while the remaining four exons are shared with all members of the UGT1A family [13].
Metabolism pharmacogenomics
Carboxylesterases
Carboxylesterase 2 is the key enzyme responsible for hydrolyzing CPT-11 to the active SN-38 form [14]. CES2 expression is highly variable among individuals [15–16], and in vitro studies suggest that increased CES2 expression leads to increased irinotecan metabolism [17]. However, extensive assessment of the CES2 gene did not identify any functional polymorphisms [18–19], and characterization of a common promoter variant in the 5′-UTR of CES2 (referred to as 830C>G; located at −171C>G) did not identify any associations with CES2 expression or catalytic activity, or irinotecan toxicity or outcome [20]. CES2 is, however, controlled by three distinct promoter regions [21], and it is possible that control of promoter choice may explain some of the individual variation in CES2 expression.
CES1 plays a minor role in irinotecan metabolism, and extensive resequencing of CES1 also did not identify any functional polymorphisms [18]. However, in a Japanese hypertensive patient population, a −816A>C variant in the CES1 promoter region has been reported to affect CES1 promoter activity [22], but this remains to be assessed in the context of irinotecan metabolism.
CYP3A4
CYP3A4 inactivates irinotecan through conversion into the metabolite APC [23]. While there is no evidence of variants in the CYP3A4 gene providing a useful screen for APC conversion, the interindividual variability in CYP3A4 activity can be exploited for irinotecan dosing [24].
UGT1A1
The most comprehensively studied genetic marker linked to toxicity from irinotecan therapy is found in the UDP-glucuronosyltransferase gene, UGT1A1. The UGT1A1 enzyme is responsible for hepatic bilirubin glucuronidation, and reduced UGT1A1 expression leads to Gilbert's syndrome [25]. Expression of UGT1A1 is, in part, controlled by a polymorphic dinucleotide repeat within the UGT1A1 promoter TATA element consisting of between five and eight copies of a TA repeat ([TA]nTAA), with the (TA)6TAA allele the most common (considered wild-type) and (TA)7TAA the most frequently recorded variant allele (usually denoted UGT1A1*28) [26]. The longer the repeat allele, the lower the corresponding UGT1A1 gene expression, with patients carrying the (TA)7TAA and (TA)8TAA alleles having significantly lower UGT1A1 expression. The frequency of the UGT1A1*28 allele has been assessed worldwide and ranges from approximately 15% in Asians to 45% in Africans. It is also found in 26–38% of Caucasians, African–Americans and Hispanics [27–29]. As increasing the number of TA repeats decreases UGT1A1 expression, the presence of more than six TA repeats in the UGT1A1 promoter region leads to reduced glucuronidation, including reduced SN-38G formation. This results in an excess build-up of SN-38, leading to toxicity [12,25,30].
Early studies confirmed the link between UGT1A1*28 and irinotecan toxicity, specifically diarrhea and neutropenia [27,31], and a retrospective analysis of DNA from 524 metastatic colorectal cancer patients on the N9741 study also associated UGT1A1*28 with the incidence of toxicities (neutropenia, febrile neutropenia and vomiting) [32]. Furthermore, a prospective study of 66 patients with advanced disease treated with irinotecan found that patients homozygous for UGT1A1*28 had a significantly greater risk of grade IV neutropenia compared with patients with at least one wild-type allele [33].
UGT1A1 in the clinic
In 2005, the US FDA approved a genetic test for UGT1A1*28 [34] and altered the irinotecan package insert to include toxicity and dosing warnings relating to the UGT1A1*28 allele [35]. This marked a significant step towards incorporating pharmacogenomics into clinical practice. However, 5 years on, concerns still remain over the specific irinotecan dose required based on genotype [36–37]. A subsequent study has identified that the relationship between UGT1A1*28 and irinotecan toxicity is dependent on the irinotecan regimen used, rendering UGT1A1*28 unsuitable as a marker for toxicity with lower doses (50–180 mg/m2). For moderate-to-high doses (200–350 mg/m2), the risk of severe hematological toxicity in patients homozygous for UGT1A1*28 is 27.8-times higher than for patients with at least one wild-type allele [38]. Furthermore, a European study confirmed that toxicity from low-dose irinotecan was not affected by the UGT1A1*28 variant [39]. Consequently, it appears necessary to further amend the irinotecan package insert to include dose/genotype guidelines. A recent prospective European study of 59 patients showed that when UGT1A1*28 homozygous patients are excluded; the standard 180 mg/m2 dose is significantly lower than the irinotecan dose that can be tolerated [40]. Dosing information from a Japanese Phase I study of 27 patients receiving irinotecan and doxifluridine has suggested a starting dose of 70 mg/m2 for patients heterozygous for UGT1A1*28. No homozygous patients were identified in this study [41].
Other UGT1A1 polymorphisms
There are other significant polymorphisms in the UGT1A1 gene. Patients with haplotypes containing both the −3156G>A variant and UGT1A1*28 experienced significantly higher incidence of severe neutropenia compared with patients with haplotypes not containing −3156G>A [33], and in the N9741 study the UGT1A1 −3156 variant was associated with a significantly increased risk of neutropenia [32]. In Caucasian populations, the *28 and −3156 alleles are in strong linkage disequilibrium [33].
In Asian populations where the frequency of UGT1A1*28 is low [42], other UGT1A1 variants can also play a role in irinotecan toxicity [12,43–50]. For example, in Korean patients with non-small-cell lung cancer treated with irinotecan-containing therapy, there were associations between the exon 1 polymorphism UGT1A1*6 (G71R), irinotecan pharmacokinetics, and toxicity from irinotecan therapy [47]. In a further study of 88 Japanese cancer patients receiving irinotecan, two haplotype groups were associated with reduced area under the curve (AUC) ratios of SN-38G to SN-38, which is predicted to have an effect on irinotecan toxicity. These haplotypes were denoted *28 (containing the UGT1A1*28 allele) and *6 (containing the exon 1 G71R polymorphism) [44]. Patients with the *6 haplotype alone did not show significant variation in their AUC ratios; however, patients with one *6 haplotype and one *28 haplotype had significantly lower AUC ratios compared with patients with homozygous wild-type UGT1A1 [44].
Other UGT1A genes
Variants in UGT1A7 and UGT1A9 are also associated with SN-38 glucuronidation [51] and irinotecan toxicities (particularly diarrhea) [47,52,53], although these studies require further exploration. UGT1A7*3 has been associated with hematologic toxicity in metastatic colorectal cancer patients treated with irinotecan [54]. Furthermore, UGT1A7*2 and *3, as well as UGT1A9 -118(dT) alleles, were associated with response to irinotecan [52].
Transport
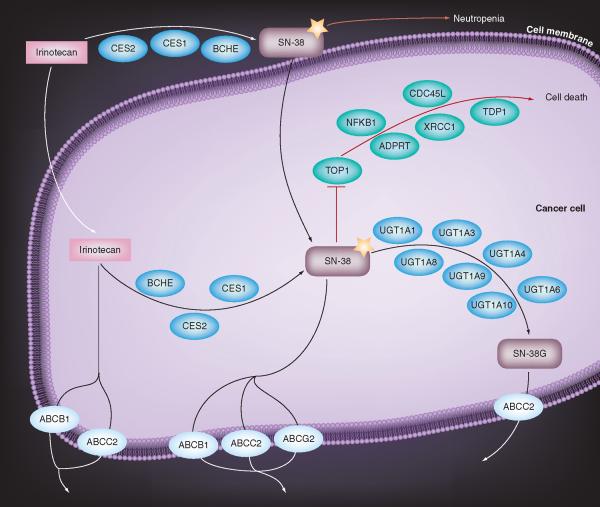
Irinotecan and SN-38 may be transported out of the cell via members of the ATP-binding cassette transporter family [55], specifically ABCB1 (MDR1; P-glycoprotein), ABCC2 (CMOAT; MRP2) and ABCG2 (BCRP). In addition, glucuronidated SN-38 can be removed from the cell by ABCC2 (Figure 1).
Figure 1. Irinotecan cancer cell pathway.
Reproduced with kind permission from PharmGKB and Stanford University [81].
Transport pharmacogenomics
ABCB1
In 65 patients treated with irinotecan, the common ABCB1 1236C>T variant caused significantly decreased clearance of irinotecan [56]. ABCB1 3435C>T was associated with diarrhea caused by irinotecan-containing therapy in a subset of 87 patients from a Phase III small-cell lung cancer trial [57]. In a further study, a haplotype containing the three most commonly studied ABCB1 polymorphisms (1236C>T, 2677G>T and 3435C>T) was associated with reduced renal clearance in 49 Asian patients receiving irinotecan [58]. The ABCB1 haplotype was also associated with response and survival in 140 colorectal cancer patients from the Nordic VI trial [59]. In the same study, ABCB1 3435C>T was also predictive of early toxic events [59].
ABCC2
In 64 patients with solid tumors treated with irinotecan, a significant correlation was observed with irinotecan and metabolite clearance, and the 3972T>C polymorphism [43], which was also associated with toxicity [60]. ABCC2 -24T homozygotes and 3972T homozygotes also experienced significantly better response rates and progression-free survival in non-small-cell lung cancer patients receiving irinotecan and cisplatin [61]. In addition, a haplotype in the multidrug transporter ABCC2 is associated with toxicity in patients lacking UGT1A1*28 [46,62–63], suggesting that this haplotype could be a secondary screen for patients who are wild-type for UGT1A1, to further reduce the risk of toxicity.
ABCG2
Cell lines overexpressing ABCG2 are resistant to several topoisomerase I inhibitors, including irinotecan [64] and SN-38 [65]. The ABCG2 variant 421C>A (Q141K) reduced ABCG2 gene expression and caused irinotecan resistance in cancer cell lines [66] and neutropenia in 55 patients receiving irinotecan monotherapy when assessed as a haplotype with ABCG2 IVS12 +49G>T [67]. Alone, the ABCG2 421C>A variant was not associated with toxicity [49,61]. A further polymorphism, ABCG2 34G>A, was significantly associated with diarrhea in 107 cancer patients [60] but was not associated with toxicity or outcome in 107 non-small-cell lung cancer patients [61].
Irinotecan pharmacodynamics
Topoisomerase I is the target for SN-38, and several downstream genes have been associated with camptothecin sensitivity, and are consequently included in the irinotecan pathway (Figure 1) including XRCC1 [68], ADPRT [69], TDP1 [70], CDC45L [71] and NF-κB1 [72–73].
Pharmacodynamics & pharmacogenomics
A retrospective analysis of 107 colorectal cancer patients identified a significant association with TDP1 IVS12 +79T>G and grade 3/4 neutropenia, and the TDP1 variant and an XRCC1 haplotype and response to irinotecan [74]. Associations with toxicity were not seen in a follow-up study of 85 cancer patients [75], although the dose of irinotecan was higher (300–350 mg/m2 compared with a median of 180 mg/m2 in [74]), and no significant association with XRCC1 R399Q and toxicity was seen in 18 colorectal cancer patients [76]. However, the variant XRCC1 R399Q was associated with overall survival in 43 Turkish metastatic colorectal cancer patients [77]. Assessment of irinotecan pharmacodynamics in the context of pharmacogenomics is in its infancy, and subsequent validation experiments are required.
Future perspective
Toxicity is a major dose-limiting, life-threatening side effect from irinotecan chemotherapy. There are comprehensive data to suggest that UGT1A1*28 may provide a genetic marker that patients can be screened for prior to irinotecan therapy and/or dose selection, and this has the potential to be a cost-effective screening approach [78]. However, a decade on from the initial association with toxicity, there are still questions remaining about how to interpret the genetic information [37]. Moreover, UGT1A1*28 does not account for all the toxicity seen from irinotecan therapy. Consequently, although screening for this allele can identify patients at risk, the lack of UGT1A1*28 does not preclude the chances of a patient experiencing severe toxicity.
Alongside variants in other UGT1A genes, transporters, and pharmacodynamic genes (Table 1), in vitro studies have shown that altered expression of PXR (encoded by the NR1I2 gene) can affect SN-38 glucuronidation [79]. Consequently, variation in the NR1I2 gene should be explored in the context of irinotecan therapy. Recent work has also suggested that epigenetic factors, such as methylation, may also play a role in altering UGT1A1 expression [80], and it is possible that screening of the tumor cells as well as germline DNA may also be needed for a comprehensive irinotecan pharmacogenomic profile.
Conclusion
Although UGT1A1*28 provides a compelling story for irinotecan toxicity, it is not the only answer. Variation in any gene involved in the irinotecan pathway (Figure 1) could play a role in either toxicity or response. As well as polymorphisms, either assessed singly or in the form of a haplotype, other genomic alterations, such as epigenetics, also need to be assessed to build a comprehensive pharmacogenomic profile. This may require assessing DNA from tumor tissue to analyze specific alterations in the tumor genome, alongside the more typical germline DNA screening. Currently, markers for irinotecan response are few, and many remain unvalidated. Further analysis, particularly of the pharmacodynamic genes, will hopefully identify the genetic basis of response to irinotecan.
Executive summary.
-
■
Irinotecan is approved, in combination, for the treatment of metastatic colorectal cancer. It is also used for treating other solid tumors such as ovarian and non-small-cell lung cancer.
-
■
Severe toxicity from irinotecan occurs in up to 36% of patients.
Irinotecan pharmacokinetics
-
■
A polymorphic dinucleotide repeat in the UGT1A1 promoter region (UGT1A1*28) is significantly associated with irinotecan toxicity.
-
■
There is now a US FDA-approved test for UGT1A1*28, and the irinotecan package insert contains warnings about UGT1A1*28 and risk of toxicity.
-
■
The UGT1A1*28 association with irinotecan toxicity is dose dependent.
-
■
Other UGT1A polymorphisms may also play a role in irinotecan toxicity, especially in populations with a low incidence of UGT1A1*28.
-
■
ABCB1 polymorphisms have been associated with both toxicity and response.
-
■
An ABCC2 haplotype may predict irinotecan toxicity in patients who are not carriers of UGT1A1*28.
Irinotecan pharmacodynamics
-
■
Initial studies have shown that XRCC1 and TDP1 are associated with toxicity and response.
Future perspective
-
■
Phase I studies aimed at determining UGT1A1*28-dependent dosing of irinotecan will help to improve the use of irinotecan pharmacogenomics in clinical practice.
-
■
Other pharmacogenomic studies, including expression panels and epigenetic markers, may prove to be useful indicators of outcome and toxicity to irinotecan.
Conclusion
-
■
Although UGT1A1*28 is a strong candidate as a pharmacogenomic marker for irinotecan toxicity, a panel of markers will be required in order to be as predictive as possible prior to irinotecan therapy selection or irinotecan dose selection.
Acknowledgments
Financial & competing interests disclosure Sharon Marsh is supported by the University of Alberta, Janelle M Hoskins is supported by the NIH Pharmacogenetics Research Network grant U01GM63340.
Footnotes
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■■ of considerable interest
- 1.Abang AM. The clinical pharmacology of topoisomerase I inhibitors. Semin. Hematol. 1998;35(3 Suppl. 4):13–21. [PubMed] [Google Scholar]
- 2.Rothenberg ML. The current status of irinotecan (CPT-11) in the United States. Ann. NY Acad. Sci. 1996;803:272–281. doi: 10.1111/j.1749-6632.1996.tb26397.x. [DOI] [PubMed] [Google Scholar]
- 3.Wall ME. Camptothecin and taxol: discovery to clinic. Med. Res. Rev. 1998;18(5):299–314. doi: 10.1002/(sici)1098-1128(199809)18:5<299::aid-med2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Lavelle F, Bissery MC, Andre S, Roquet F, Riou JF. Preclinical evaluation of CPT-11 and its active metabolite SN-38. Semin. Oncol. 1996;23(1 Suppl. 3):11–20. [PubMed] [Google Scholar]
- 5.Devore R, 3rd, Johnson D, Crawford J, Dimery I, Eckardt J, Eckhardt SG. Irinotecan plus cisplatin in patients with advanced non-small-cell lung cancer. Oncology (Williston Park) 1998;12(8 Suppl. 6):79–83. [PubMed] [Google Scholar]
- 6.Gershenson DM. Irinotecan in epithelial ovarian cancer. Oncology (Williston Park) 2002;16(5 Suppl. 5):29–31. [PubMed] [Google Scholar]
- 7.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 8.Hoff PM, Pazdur R. Progress in the development of novel treatments for colorectal cancer. Oncology. 2004;18(6):705–708. [PubMed] [Google Scholar]
- 9.Fuchs CS, Moore MR, Harker G, Villa L, Rinaldi D, Hecht JR. Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J. Clin. Oncol. 2003;21(5):807–814. doi: 10.1200/JCO.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 10.Evans WE, McLeod HL. Pharmacogenomics – drug disposition, drug targets, and side effects. N. Engl. J. Med. 2003;348(6):538–549. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- 11.Marsh S, McLeod HL. Cancer pharmacogenetics. Br. J. Cancer. 2004;90(1):8–11. doi: 10.1038/sj.bjc.6601487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsh S, McLeod HL. Pharmacogenetics of irinotecan toxicity. Pharmacogenomics. 2004;5(7):835–843. doi: 10.1517/14622416.5.7.835. [DOI] [PubMed] [Google Scholar]
- 13.Gong QH, Cho JW, Huang T, et al. Thirteen UDP glucuronosyltransferase genes are encoded at the human UGT1 gene complex locus. Pharmacogenetics. 2001;11(4):357–368. doi: 10.1097/00008571-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Humerickhouse R, Lohrbach K, Li L, Bosron WF, Dolan ME. Characterization of CPT-11 hydrolysis by human liver carboxylesterase isoforms hCE-1 and hCE-2. Cancer Res. 2000;60(5):1189–1192. [PubMed] [Google Scholar]
- 15.Xu G, Zhang W, Ma MK, McLeod HL. Human carboxylesterase 2 is commonly expressed in tumor tissue and is correlated with activation of irinotecan. Clin. Cancer Res. 2002;8(8):2605–2611. [PubMed] [Google Scholar]
- 16.Zhang W, Xu G, McLeod HL. Comprehensive evaluation of carboxylesterase-2 expression in normal human tissues using tissue array analysis. Appl. Immunohistochem. Mol. Morphol. 2002;10(4):374–380. doi: 10.1097/00129039-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Yano H, Kayukawa S, Iida S, et al. Overexpression of carboxylesterase-2 results in enhanced efficacy of topoisomerase I inhibitor, irinotecan (CPT-11), for multiple myeloma. Cancer Sci. 2008;99(11):2309–2314. doi: 10.1111/j.1349-7006.2008.00936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsh S, Xiao M, Yu J, et al. Pharmacogenomic assessment of carboxylesterases 1 and 2. Genomics. 2004;84(4):661–668. doi: 10.1016/j.ygeno.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Charasson V, Bellott R, Meynard D, Longy M, Gorry P, Robert J. Pharmacogenetics of human carboxylesterase 2, an enzyme involved in the activation of irinotecan into SN-38. Clin. Pharmacol. Ther. 2004;76(6):528–535. doi: 10.1016/j.clpt.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Bellott R, Le Morvan V, Charasson V, et al. Functional study of the 830C>G polymorphism of the human carboxylesterase 2 gene. Cancer Chemother. Pharmacol. 2008;61(3):481–488. doi: 10.1007/s00280-007-0493-9. [DOI] [PubMed] [Google Scholar]
- 21.Wu MH, Chen P, Remo BF, Cook EH, Jr, Das S, Dolan ME. Characterization of multiple promoters in the human carboxylesterase 2 gene. Pharmacogenetics. 2003;13(7):425–435. doi: 10.1097/00008571-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Geshi E, Kimura T, Yoshimura M, et al. A single nucleotide polymorphism in the carboxylesterase gene is associated with the responsiveness to imidapril medication and the promoter activity. Hypertens Res. 2005;28(9):719–725. doi: 10.1291/hypres.28.719. [DOI] [PubMed] [Google Scholar]
- 23.Haaz MC, Rivory L, Riche C, Vernillet L, Robert J. Metabolism of irinotecan (CPT-11) by human hepatic microsomes: participation of cytochrome P-450 3A and drug interactions. Cancer Res. 1998;58(3):468–472. [PubMed] [Google Scholar]
- 24.van der Bol JM, Mathijssen RH, Creemers GJ, et al. A CYP3A4 phenotype-based dosing algorithm for individualized treatment of irinotecan. Clin. Cancer Res. 2010;16(2):736–742. doi: 10.1158/1078-0432.CCR-09-1526. [DOI] [PubMed] [Google Scholar]
- 25.Innocenti F, Ratain MJ. Irinotecan treatment in cancer patients with UGT1A1 polymorphisms. Oncology (Williston Park) 2003;17(5 Suppl. 5):52–55. [PubMed] [Google Scholar]
- 26.Beutler E, Gelbart T, Demina A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proc. Natl Acad. Sci. USA. 1998;95(14):8170–8174. doi: 10.1073/pnas.95.14.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ando Y, Saka H, Ando M, et al. Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res. 2000;60(24):6921–6926. [PubMed] [Google Scholar]
- 28.Guillemette C, Millikan RC, Newman B, Housman DE. Genetic polymorphisms in uridine diphospho-glucuronosyltransferase 1A1 and association with breast cancer among African Americans. Cancer Res. 2000;60(4):950–956. [PubMed] [Google Scholar]
- 29.Hall D, Ybazeta G, Destro-Bisol G, Petzl-Erler ML, Di Rienzo A. Variability at the uridine diphosphate glucuronosyltransferase 1A1 promoter in human populations and primates. Pharmacogenetics. 1999;9(5):591–599. [PubMed] [Google Scholar]
- 30.Iyer L, Hall D, Das S, et al. Phenotype-genotype correlation of in vitro SN-38 (active metabolite of irinotecan) and bilirubin glucuronidation in human liver tissue with UGT1A1 promoter polymorphism. Clin. Pharmacol. Ther. 1999;65(5):576–582. doi: 10.1016/S0009-9236(99)70078-0. [DOI] [PubMed] [Google Scholar]
- 31.Iyer L, Das S, Janisch L, et al. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J. 2002;2(1):43–47. doi: 10.1038/sj.tpj.6500072. [DOI] [PubMed] [Google Scholar]
- 32.McLeod HL, Sargent DJ, Marsh S, et al. Pharmacogenetic predictors of adverse events and response to chemotherapy in metastatic colorectal cancer; results from Intergroup Trial N9741. J. Clin. Oncol. 2010 doi: 10.1200/JCO.2009.21.7943. DOI: 10.1200/JCO.2009.21.7943. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Innocenti F, Undevia SD, Iyer L, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J. Clin. Oncol. 2004;22(8):1382–1388. doi: 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]; ■■ Prospective study confirming the role of UGT1A1*28 in irinotecan toxicity.
- 34.Staessen JA, Kuznetsova T, Acceto R, et al. on behalf of the OASIS-HT Investigators: FDA clears Third Wave pharmacogenetic test. Pharmacogenomics. 2005;6(7):671–672. [Google Scholar]
- 35.Ratain MJ. From bedside to bench to bedside to clinical practice: an odyssey with irinotecan. Clin. Cancer Res. 2006;12(6):1658–1660. doi: 10.1158/1078-0432.CCR-06-0159. [DOI] [PubMed] [Google Scholar]
- 36.Marsh S, van Rooij T. Challenges of incorporating pharmacogenomics into clinical practice. Gastrointest. Cancer Res. 2009;3(5):206–207. [PMC free article] [PubMed] [Google Scholar]
- 37.Ratain MJ, Innocenti F. Individualizing dosing of irinotecan. Clin. Cancer Res. 2010;16(2):371–372. doi: 10.1158/1078-0432.CCR-09-2936. [DOI] [PubMed] [Google Scholar]
- 38.Hoskins JM, Goldberg RM, Qu P, Ibrahim JG, McLeod HL. UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J. Natl Cancer Inst. 2007;99(17):1290–1295. doi: 10.1093/jnci/djm115. [DOI] [PubMed] [Google Scholar]; ■■ Analysis showing that UGT1A1*28-related irinotecan toxicity is dependent on irinotecan dose.
- 39.Schulz C, Heinemann V, Schalhorn A, et al. UGT1A1 gene polymorphism: impact on toxicity and efficacy of irinotecan-based regimens in metastatic colorectal cancer. World J. Gastroenterol. 2009;15(40):5058–5066. doi: 10.3748/wjg.15.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toffoli G, Cecchin E, Gasparini G, et al. Genotype-driven Phase I study of irinotecan administered in combination with fluorouracil/leucovorin in patients with metastatic colorectal cancer. J. Clin. Oncol. 2010;28(5):866–871. doi: 10.1200/JCO.2009.23.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Identifies the optimum dose of irinotecan based on genotype.
- 41.Hazama S, Nagashima A, Kondo H, et al. Phase I study of irinotecan and doxifluridine for metastatic colorectal cancer focusing on the UGT1A1*28 polymorphism. Cancer Sci. 2010;101(3):722–727. doi: 10.1111/j.1349-7006.2009.01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Premawardhena A, Fisher CA, Liu YT, et al. The global distribution of length polymorphisms of the promoters of the glucuronosyltransferase 1 gene (UGT1A1): hematologic and evolutionary implications. Blood Cells Mol. Dis. 2003;31(1):98–101. doi: 10.1016/s1079-9796(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 43.Innocenti F, Undevia SD, Rosner GL, et al. Irinotecan (CPT-11) pharmacokinetics (PK) and neutropenia: interaction among UGT1A1 and transporter genes. Proc. Am. Soc. Clin. Oncol. 2005;23:S16. (Abstract 2006) [Google Scholar]
- 44.Sai K, Saeki M, Saito Y, et al. UGT1A1 haplotypes associated with reduced glucuronidation and increased serum bilirubin in irinotecan-administered Japanese patients with cancer. Clin. Pharmacol. Ther. 2004;75(6):501–515. doi: 10.1016/j.clpt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Hoskins JM, Mcleod HL. The move from pharmacokinetics to pharmacodynamics. Curr. Pharmacogenomics. 2006;4:39–46. [Google Scholar]
- 46.de Jong FA, Scott-Horton TJ, Kroetz DL, et al. Irinotecan-induced diarrhea: functional significance of the polymorphic ABCC2 transporter protein. Clin. Pharmacol. Ther. 2007;81(1):42–49. doi: 10.1038/sj.clpt.6100019. [DOI] [PubMed] [Google Scholar]
- 47.Han JY, Lim HS, Shin ES, et al. Comprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non-small-cell lung cancer treated with irinotecan and cisplatin. J. Clin. Oncol. 2006;24(15):2237–2244. doi: 10.1200/JCO.2005.03.0239. [DOI] [PubMed] [Google Scholar]
- 48.Takano M, Kato M, Yoshikawa T, et al. Clinical significance of UDP-glucuronosyltransferase 1A1*6 for toxicities of combination chemotherapy with irinotecan and cisplatin in gynecologic cancers: a prospective multi-institutional study. Oncology. 2009;76(5):315–321. doi: 10.1159/000209335. [DOI] [PubMed] [Google Scholar]
- 49.Jada SR, Lim R, Wong CI, et al. Role of UGT1A1*6, UGT1A1*28 and ABCG2 c.421C>A polymorphisms in irinotecan-induced neutropenia in Asian cancer patients. Cancer Sci. 2007;98(9):1461–1467. doi: 10.1111/j.1349-7006.2007.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Onoue M, Terada T, Kobayashi M, et al. UGT1A1*6 polymorphism is most predictive of severe neutropenia induced by irinotecan in Japanese cancer patients. Int. J. Clin. Oncol. 2009;14(2):136–142. doi: 10.1007/s10147-008-0821-z. [DOI] [PubMed] [Google Scholar]
- 51.Saito Y, Sai K, Maekawa K, et al. Close association of UGT1A9 IVS1+399C>T with UGT1A1*28, *6, or *60 haplotype and its apparent influence on 7-ethyl-10-hydroxycamptothecin (SN-38) glucuronidation in Japanese. Drug Metab. Dispos. 2009;37(2):272–276. doi: 10.1124/dmd.108.024208. [DOI] [PubMed] [Google Scholar]
- 52.Carlini LE, Meropol NJ, Bever J, et al. UGT1A7 and UGT1A9 polymorphisms predict response and toxicity in colorectal cancer patients treated with capecitabine/irinotecan. Clin. Cancer Res. 2005;11(3):1226–1236. [PubMed] [Google Scholar]
- 53.Hoskins JM, McLeod HL. UGT1A and irinotecan toxicity: keeping it in the family. J. Clin. Oncol. 2009;27(15):2419–2421. doi: 10.1200/JCO.2008.20.9478. [DOI] [PubMed] [Google Scholar]
- 54.Cecchin E, Innocenti F, D'Andrea M, et al. Predictive role of the UGT1A1, UGT1A7, and UGT1A9 genetic variants and their haplotypes on the outcome of metastatic colorectal cancer patients treated with fluorouracil, leucovorin, and irinotecan. J. Clin. Oncol. 2009;27(15):2457–2465. doi: 10.1200/JCO.2008.19.0314. [DOI] [PubMed] [Google Scholar]
- 55.Sparreboom A, Danesi R, Ando Y, Chan J, Figg WD. Pharmacogenomics of ABC transporters and its role in cancer chemotherapy. Drug Resist. Update. 2003;6(2):71–84. doi: 10.1016/s1368-7646(03)00005-0. [DOI] [PubMed] [Google Scholar]
- 56.Mathijssen RH, Marsh S, Karlsson MO, et al. Irinotecan pathway genotype analysis to predict pharmacokinetics. Clin. Cancer Res. 2003;9(9):3246–3253. [PubMed] [Google Scholar]
- 57.Lara PN, Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J. Clin. Oncol. 2009;27(15):2530–2535. doi: 10.1200/JCO.2008.20.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sai K, Kaniwa N, Itoda M, et al. Haplotype analysis of ABCB1/MDR1 blocks in a Japanese population reveals genotype-dependent renal clearance of irinotecan. Pharmacogenetics. 2003;13(12):741–757. doi: 10.1097/00008571-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Glimelius B, Garmo H, Berglund A, et al. Prediction of irinotecan and 5-fluorouracil toxicity and response in patients with advanced colorectal cancer. Pharmacogenomics J. 2010 doi: 10.1038/tpj.2010.10. DOI: 10.1038/tpj.2010.10. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han JY, Lim HS, Park YH, Lee SY, Lee JS. Integrated pharmacogenetic prediction of irinotecan pharmacokinetics and toxicity in patients with advanced non-small cell lung cancer. Lung Cancer. 2009;63(1):115–120. doi: 10.1016/j.lungcan.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Han JY, Lim HS, Yoo YK, et al. Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer. 2007;110(1):138–147. doi: 10.1002/cncr.22760. [DOI] [PubMed] [Google Scholar]
- 62.Fujita K, Nagashima F, Yamamoto W, et al. Association of ATP-binding cassette, sub-family C, number 2 (ABCC2) genotype with pharmacokinetics of irinotecan in Japanese patients with metastatic colorectal cancer treated with irinotecan plus infusional 5-fluorouracil/leucovorin (FOLFIRI) Biol. Pharm. Bull. 2008;31(11):2137–2142. doi: 10.1248/bpb.31.2137. [DOI] [PubMed] [Google Scholar]
- 63.Innocenti F, Kroetz DL, Schuetz E, et al. Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J. Clin. Oncol. 2009;27(16):2604–2614. doi: 10.1200/JCO.2008.20.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schellens JH, Maliepaard M, Scheper RJ, et al. Transport of topoisomerase I inhibitors by the breast cancer resistance protein. Potential clinical implications. Ann. NY Acad. Sci. 2000;922:188–194. doi: 10.1111/j.1749-6632.2000.tb07037.x. [DOI] [PubMed] [Google Scholar]
- 65.Candeil L, Gourdier I, Peyron D, et al. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int. J. Cancer. 2004;109(6):848–854. doi: 10.1002/ijc.20032. [DOI] [PubMed] [Google Scholar]
- 66.Imai Y, Nakane M, Kage K, et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol. Cancer Ther. 2002;1(8):611–616. [PubMed] [Google Scholar]
- 67.Sai K, Saito Y, Maekawa K, et al. Additive effects of drug transporter genetic polymorphisms on irinotecan pharmacokinetics/pharmacodynamics in Japanese cancer patients. Cancer Chemother. Pharmacol. 2009;66(1):95–105. doi: 10.1007/s00280-009-1138-y. [DOI] [PubMed] [Google Scholar]
- 68.Park DJ, Stoehlmacher J, Zhang W, Tsao-Wei DD, Groshen S, Lenz HJ. A Xeroderma pigmentosum group D gene polymorphism predicts clinical outcome to platinum-based chemotherapy in patients with advanced colorectal cancer. Cancer Res. 2001;61(24):8654–8658. [PubMed] [Google Scholar]
- 69.Chatterjee S, Cheng MF, Trivedi D, Petzold SJ, Berger NA. Camptothecin hypersensitivity in poly(adenosine diphosphate-ribose) polymerase-deficient cell lines. Cancer Commun. 1989;1(6):389–394. doi: 10.3727/095535489820875129. [DOI] [PubMed] [Google Scholar]
- 70.Pouliot JJ, Yao KC, Robertson CA, Nash HA. Yeast gene for a Tyr-DNA phosphodiesterase that repairs topoisomerase I complexes. Science. 1999;286(5439):552–555. doi: 10.1126/science.286.5439.552. [DOI] [PubMed] [Google Scholar]
- 71.Reid RJ, Fiorani P, Sugawara M, Bjornsti MA. CDC45 and DPB11 are required for processive DNA replication and resistance to DNA topoisomerase I-mediated DNA damage. Proc. Natl Acad. Sci. USA. 1999;96(20):11440–11445. doi: 10.1073/pnas.96.20.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cusack JC, Jr, Liu R, Houston M, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-κB inhibition. Cancer Res. 2001;61(9):3535–3540. [PubMed] [Google Scholar]
- 73.Valente P, Arzani D, Cesario A, Margaritora S, Carbone E, Russo P. TNF increases camptothecin-induced apoptosis by inhibition of NF-κB. Eur. J. Cancer. 2003;39(10):1468–1477. doi: 10.1016/s0959-8049(03)00301-0. [DOI] [PubMed] [Google Scholar]
- 74.Hoskins JM, Marcuello E, Altes A, et al. Irinotecan pharmacogenetics: influence of pharmacodynamic genes. Clin. Cancer Res. 2008;14(6):1788–1796. doi: 10.1158/1078-0432.CCR-07-1472. [DOI] [PubMed] [Google Scholar]
- 75.Hoskins JM, Rosner GL, Ratain MJ, McLeod HL, Innocenti F. Pharmacodynamic genes do not influence risk of neutropenia in cancer patients treated with moderately high-dose irinotecan. Pharmacogenomics. 2009;10(7):1139–1146. doi: 10.2217/pgs.09.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Braun MS, Richman SD, Thompson L, et al. Association of molecular markers with toxicity outcomes in a randomized trial of chemotherapy for advanced colorectal cancer: the FOCUS trial. J. Clin. Oncol. 2009;27(33):5519–5528. doi: 10.1200/JCO.2008.21.6283. [DOI] [PubMed] [Google Scholar]
- 77.Artac M, Bozcuk H, Pehlivan S, et al. The value of XPD and XRCC1 genotype polymorphisms to predict clinical outcome in metastatic colorectal carcinoma patients with irinotecan-based regimens. J. Cancer Res. Clin. Oncol. 2009;136(6):803–809. doi: 10.1007/s00432-009-0720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gold HT, Hall MJ, Blinder V, Schackman BR. Cost effectiveness of pharmacogenetic testing for uridine diphosphate glucuronosyltransferase 1A1 before irinotecan administration for metastatic colorectal cancer. Cancer. 2009;115(17):3858–3867. doi: 10.1002/cncr.24428. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Pharmacoeconomic evaluation of UGT1A1*28 genotype screening.
- 79.Raynal C, Pascussi JM, Leguelinel G, et al. Pregnane X receptor (PXR) expression in colorectal cancer cells restricts irinotecan chemosensitivity through enhanced SN-38 glucuronidation. Mol. Cancer. 2010;9:46. doi: 10.1186/1476-4598-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Belanger AS, Tojcic J, Harvey M, Guillemette C. Regulation of UGT1A1 and HNF1 transcription factor gene expression by DNA methylation in colon cancer cells. BMC Mol. Biol. 2010;11:9. doi: 10.1186/1471-2199-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klein TE, Chang JT, Cho MK, et al. Integrating genotype and phenotype information: an overview of the PharmGKB project. Pharmacogenetics Research Network and Knowledge Base. Pharmacogenomics J. 2001;1(3):167–170. doi: 10.1038/sj.tpj.6500035. [DOI] [PubMed] [Google Scholar]