Abstract
We describe some biological and molecular characteristics of a Trypanosoma cruzi isolate derived from a Triatomine captured in Nicaragua. PCR based typification showed that this isolate, named Nicaragua, belonged to the lineage Tc I. Nicaragua infected culture cells were treated with allopurinol, showing different behaviour according to the cellular compartment, being cardiomyocyte primary cultures more resistant to this drug. The course of the infection in a mice experimental model and its susceptibility to benznidazole and allopurinol was analysed. In benznidazole treatment, mice reverted the high lethal effect of parasites during the acute infection, however, a few parasites were detected in the heart of 88 % of mice one year post-infection. Since Trypanosoma cruzi is a heterogeneous species population it is important to study and characterize different parasites actually circulating in humans in endemic areas. In this work we show that T. cruzi Nicaragua isolate, is sensitive to early benznidazole treatment.
Keywords: Trypanosoma cruzi, isolate lineage identification, allopurinol, benznidazole
1. Introduction
One of the problems of Chagas’ disease is the diversity of clinical and pathological manifestations that occur following the infection by Trypanosoma cruzi. This protozoan parasite is a heterogeneous species population circulating in human, insect vectors and animals (Tibayrenc, 2003). Different authors have reported the occurrence of genetically different parasite isolates displaying a broad range of biological behaviors, and pharmacological susceptibilities (Andrade et al., 1985; Filardi and Brener, 1987; Machado and Ayala, 2001; Murta and Romanha, 1999). It is now accepted that there are six defined groups, T. cruzi I to T. cruzi VI (Zingales et. al, 2009). In spite of the numerous studies on the molecular aspects of this parasite reported in the literature, the information relating the phylogeny of T. cruzi and the pathogenicity of Chagas disease is scanty.
Approximately 30 % of the T. cruzi infected individuals develop chronic Chagas disease, with a potentially lethal cardiopathy, megacolon or megaoesophagus. Although it is well accepted that the acute form of Chagas disease can be treated with the nitrofurane nifurtimox or the nitroimidazole benznidazole (bz), the treatment of the chronic phase with these drugs remains controversial due to the undesirable side effects of these drugs (Viotti et al., 2009). Other drugs such as allopurinol (4-hydroxypyrazol (3, 4-d) pyrymidine) (allo) are currently being evaluated for the clinical treatment of Chagas disease (Apt et al., 2005; Coronado et al., 2006; Rodriques Coura and Castro, 2002). In addition, this drug demonstrated effectivity in the inhibition of T. cruzi infection in vitro and in vivo (Berens et al., 1982; Nakajima-Shimada et al., 1996; Avila and Avila, 1981; Gobbi et al., 2007). The range of susceptibility of T. cruzi to bz and allo depends on the parasite strain, and was related to the predominant lineages in different geographical areas. T. cruzi I is prevalent in the northern endemic areas of South America and is usually resistant to both drugs (Coronado et al., 2006) while T. cruzi II isolates were partially resistant to bz (Toledo et al., 2004).
In this report we describe some molecular and biological characteristics of a T. cruzi isolate obtained from an endemic Chagas disease area of Nicaragua, the course of infection in a murine model, and compare its susceptibility to drugs being currently used in the treatment of Chagas disease.
2. Material and Methods
2.1. Chemical Compounds
Benznidazole (bz) (N-benziyl-2-nitro-1-imidazole-acetamide) was obtained from Roche, allopurinol (4-hydroxypyrazol (3, 4-d) pyrymidine) was obtained from Gador. Fetal bovine serum (FBS) (Gibco, Rockville, Md), horse serum (Internegocios SA, Córdoba Argentina), kidney epithelial cells of the African green monkey, VERO cells (ABAC, Pergamino Argentina), bovine embryo skin and muscle BESM cells (ATCC, Manassas, VA, USA), brain and heart infusion (BHT, Difco, Detroit, Mich), tryptose (Difco, Detroit, Mich), bovine hemin (Sigma, Saint Louis Mo), Hanks’ balanced salt solution (HBSS, Gibco, Rockville, Md.), Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Rockville, Md.). 24-well and 96-well culture plates (Nunc, Naperville, Ill), 12mm-coverslips (Fisherbrand Microscope Cover).
2.2. Parasites
A Trypanosoma cruzi isolate, named Nicaragua, was obtained from the intestinal content of a Triatoma dimidiata vector captured in an urban endemic area of Nicaragua (Rodriguez M. T. and Postan M. personal communication). Parasites were maintained in BHT medium containing 0.3% tryptose, 0.002% bovine hemin and 10% heat inactivated FBS in VERO and BESM cell cultures and through serial passages in Balb/c mice. T. cruzi CL Brener clone and Y, Brazil and Tulahuen 2 strains were used as standard parasites in some of the experiments.
2.3. Lineage identification
The lineage of the Nicaragua isolate was determined using PCR strategies targeted to lineage-specific genomic markers. The intergenic miniexon of the spliced leader genes was amplified with TC, TC1 or TC2 primers (Souto et al., 1996). PCR products were visualized in 3 % agarose gel. The A10 DNA fragment was amplified with primers P3 and P6 (Brisse et al., 2000) and PCR products were visualized in 3 % agarose gel. The 24S-alpha rDNA gene was amplified in a heminested PCR with primers D71, D75 and D76. The first round was performed with D75-D76 primers (Briones et al., 1999), the second round with D71-D76 primers (Burgos et al., 2005) and PCR products were visualized in 12% polyacrilamide gel. In addition, the amplification of the cytochrome c oxidase, subunit 2, was achieved by a full-nested PCR with TcMit 31 and TcMit 40 primers for the first round and TcMit 10 y TcMit 21 primers for the second round (Freitas de et al. 2006). Amplified DNA products were digested by AluI restriction enzyme and separated by 6% polyacrilamide gel.
2.4. In vitro growth rate
Epimastigotes, (1 × 106 parasites/ml), from T. cruzi Nicaragua, CL Brener clone, Tulahuen and Brazil strains were grown in BHT medium with tryptose and bovine hemin as described above with 10% FBS and the number of parasites were daily counted in a Neubauer chamber for 20 days. To characterize the population of the Nicaragua T. cruzi isolate, we calculated the coefficient of exponential growth at low population densities r0, and the maximum stationary phase concentration, K. The values of r0 and K were calculated by Verhulst differential equation through a linear regression, made with Microsof Excel (Finley and Dvorak, 1987).
2.5. Drug IC50 determination
Assays were performed in 96-well microplates with 1.5 × 105 Nicaragua or CL Brener epimastigotes, with or without drugs and cultured at 28°C. Benznidazole was resuspended in 0.2 % DMSO, and allopurinol in culture medium. Bz and allo were tested at a concentration ranging from 0.018 mM to 0.6 mM. All experiments were performed at least twice and by triplicate. Parasite numbers were counted after 72 h drug incubation with Neubauer chambers. Allo and bz 50 % inhibition concentration values (IC50) were estimated by linear and polynomial regression using Origin program version 6.
2.6. Preparation of ventricular myocytes
Ventricular myocytes were isolated from Swiss rat as described (Ellingsen et al., 1993; Fichera et al., 2004). Briefly, rat hearts were cut into small fragments and dissociated with HBSS containing 0.02% trypsin at 37°C for 15 min, a procedure repeated four times. Cells suspensions were pooled and resuspended in HBSS supplemented with 10% FBS. Remaining cells in the supernatant were resuspended in DMEM supplemented with 10% FBS and 10% horse serum and transferred to 12 mm coverslips placed in 24-well culture plates.
2.7. Allopurinol inhibitory effects on cultured derived trypomastigotes
Mammalian VERO and BESM cell lines and rat cardiac myocyte primary cell cultures were transferred to 12 mm coverslips placed in 24-wells culture plates. After 48 h cultured cells were infected with T. cruzi Nicaragua trypomastigotes (10:1 parasites: cells ratio) and incubated for 3 h at 37°C in a 5% CO2.. Then, cultures were thoroughly washed with medium and cells were incubated with complete RPMI medium with 7% FBS and 7% horse serum with or without different concentrations of allo (1, 10, 20 and 50 μM) for 72 h. Culture wells were set up in triplicate for each drug treatment and non-infected treated cultures were used as controls.
2.8. Assessment of infection rate
Number of cells containing amastigotes in the cytoplasm were evaluated. Covers with infected cells were removed from culture plates after 72 h drug treatment, gently rinsed with phosphate buffer saline, air dried and fixed in absolute methanol for 10 min and stained with Giemsa. In each experiment, a total of 300 cells (100 cells/cover) were evaluated for each treatment in randomly selected fields.
2.9. Experimental design for T. cruzi infection in vivo
Experiments were performed in groups of 15–20 C3H/HeN one month old mice, three groups were inoculated by intraperitoneal injection with 106 T. cruzi Nicaragua culture derived trypomastigotes. The experimental groups were designed as: non-infected non-treated mice, non-infected allo or bz-treated mice, T. cruzi-infected non-treated mice and T. cruzi infected allo or bz - treated mice. Drug treatment started two days post-infection. Bz treatment was performed with 100mg/kg/day drug diluted in olive oil and allo treatment was performed with 20 mg/kg/day of pulverized drug diluted in water. Depending on the treatment, 20 μl of each drugs, were daily administered by oral route with a pipette tip during 30 days. The course of infection was studied evaluating mice parasitemia, survival and histopathology. Parasitemia was scored as previously described (Brener, 1962). The area beneath parasitemia curves was determined using Graph Prism 5.0. Mice survival was daily checked. All procedures involving experimental protocols in animals were conducted in accordance with the ethical legislations and regulatory entities, established in Argentina and International Guides.
2.10. Tissue tropism and histopathological studies of infected mice
Mice inoculated with T. cruzi (n=8) were sacrificed on days 10–14 pi. Heart, spleen, skeletal muscle and digestive tract tissues were removed from non-infected and infected mice. Tissues were fixed in 10% formaldehyde solution and embedded in paraffin. Each organ was cut in 5 μm sections and stained with hematoxylin-eosin. Heart sections were thoroughly examined under a microscope. At least 50 areas were analyzed for all tissues in each mice group with a 40× objective. The infection extent was evaluated as described (Postan et al., 1987). The infected areas of the heart were scored on a scale of 0–4 according to the extension of inflammation. Briefly infected areas of the heart were scored on a scale of 0–4 according to the extension of inflammation. 1: For one focus with a minimal of 14 lymphocytes/400× optic field; 2: for multiple inflammatory foci, 3: for confluent inflammation with partial compromise of the wall; and 4: for diffuse inflammation with total wall compromise.
2.11. Statistics
T student test was used for K and r values, obtained in epimastigotes growth. Fisher’s exact test was used for comparing T. cruzi infected tissues and organs. Log-rank (Mantel-Cox) Test and Gehan-Breslow-Wilcoxon Test were applied for mice survival.
3. Results
3.1 PCR-based identification of Nicaragua phylogenetic lineage
PCR amplification of the miniexon intergenic region showed a 350 bp DNA amplicon for the Nicaragua isolate, as well as Sylvio X-10 and CAI-72 clones, which are reference parasite strains for Lineage I. T. cruzi Y strain showed a 300 bp DNA band as has been described for Lineages Tc II, V and VI (Fig. 1A). Lineages IV and III do not amplify any DNA fragment with these primers (Souto et al., 1996). Furthermore, Lineage I was confirmed for the Nicaragua isolate with other three PCR strategies. The A10 DNA marker was amplified with P3 and P6 primers (Brisse et al., 2000). No DNA amplification is usually obtained for Lineage Tc I and Tc II parasites. Control strains like Tul2 and CL Brener clone showed a 657 bp DNA fragment as has been also described for the other lineages IV, III, VI and VI (Brisse et al., 2000). We did not observe any DNA fragment amplified with P3 and P6 primers for the Nicaragua isolate nor Sylvio X-10 or Y strain (Tc II) (Fig. 1B). Another marker studied was the 24S alpha rDNA by a heminested PCR strategy with primers D71, D75 and D76. This PCR technique amplified a unique 125 bp DNA product for Nicaragua isolate and Sylvio X-10 clone, while other sizes were obtained for the rest of our control T. cruzi strains (Briones et al., 1996) (Fig. 1C). Furthermore, when the cytochrome oxidase marker was amplified (Freitas et al., 2006), the Alu I restriction DNA patterns observed in PAGE gels were identical to those obtained with Sylvio X-10 parasite (data not shown). Based on these four PCR strategies performed, we confirmed that the Nicaragua isolate belongs to Lineage I.
Fig. 1.
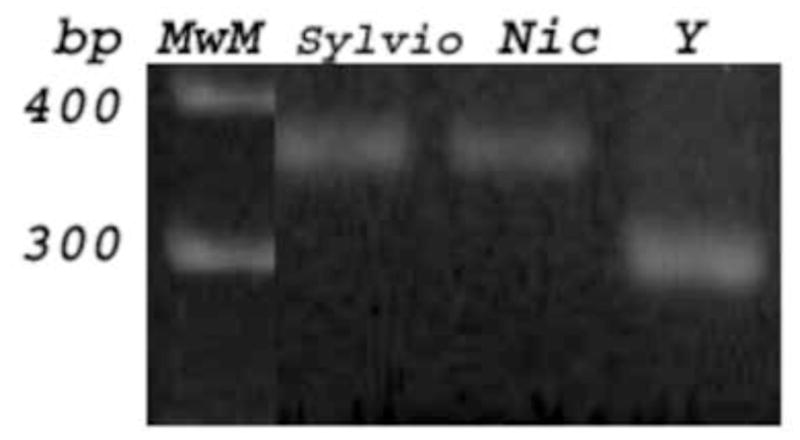

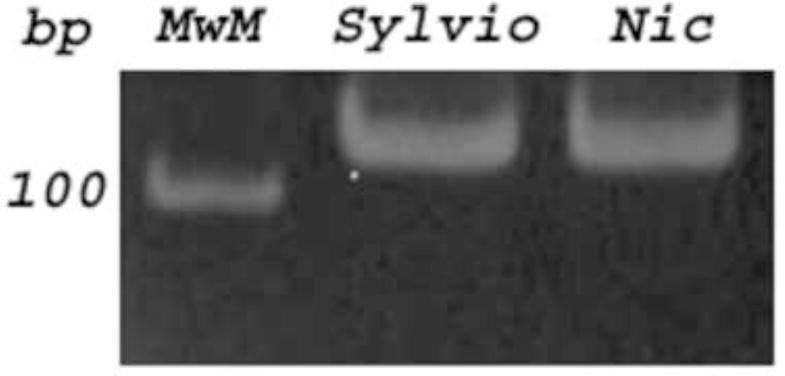
Typification was PCR based on Nicaragua isolate DNA (Nic) amplified with primers flanking several T. cruzi DNA markers. Fig 1A: The intergenic miniexon of the spliced leader genes was amplified with TC, TC1 or TC2 primers showing a 350 bp fragment for Nic and Sylvio X-10 clone (Tc I) DNA. Fig. 1B: The A10 fragment was amplified with P3 and P6 primers, and a 657 bp band was amplified for Tul2 and CL Brener DNA; no DNA band was observed for Nic, Sylvio and Y (Tc II), as was expected for lineages I and II. Fig 1C: The 24 S alpha rDNA marker was amplified with D71, D75 and D76 primers, and a 125 bp product was visualized for Nic and Sylvio.
3.2. T. cruzi Nicaragua epimastigote growth
The number of epimastigotes in axenic cultures reached its maximum at 9 days for the Nicaragua isolate and the CL Brener clone while Tulahuen and Brazil strains reached the stationary growth phase at 10 and 12 days respectively. To analyze the population of the Nicaragua isolate, we determined the values of r0 and K (the constant r defines the growth rate and K is the carrying capacity). These parameters were compared with the r0 and K of CL Brener clone, Tulahuen and Brazil strains, (Table 1). The growth rate of the Nicaragua isolate was according to other T. cruzi strains studied, with similar r0 values, what could place it as belonging to the same species (Finley and Dvorak, 1987). But a significantly lower K value (Dvorak, 1984) was observed, (Table 1), with respect to the other T. cruzi strains studied (Student’s test; p<0,025).
Table 1. Values r0 and K for T. cruzi Nicaragua isolate, Brazil and Tulahuen strains and CL Brener clone.
Growth values of r0 and K for T. cruzi Nicaragua isolate compared with Brazil and Tulahuen 2 strains and CL Brener clone. The linear regression, R >0.5, was calculated with at least five data within the exponential growth phase, and r and K values were determined. Data were from three independent experiments in duplicate.
| Nicaragua | Brazil | Tulahuen | CL Brener | |
|---|---|---|---|---|
| r0 | 0.59 ± 0.1 | 0.66 ± 0.13 | 0.53 ± 0.13 | 0.69 ± 0.15 |
| K | 23.55 ± 0.35 | 35.45 ± 6.66 | 34.01 ± 2.52 | 33.89 ± 0.71 |
3.4. Nicaragua isolate epimastigote drug susceptibility
The 50 % inhibitory concentration (IC50) of bz on T. cruzi Nicaragua isolate was 110.9 μM and 88.67 μM for CL Brener clone, being Nicaragua isolate less susceptible than the CL Brener, the reference T. cruzi clone, to bz. Regarding allopurinol, Nicaragua isolate epimastigotes showed an IC50 of 38.91 μM being more sensitive than the CL Brener clone, which showed an IC50 of 131.22 μM.
3.5. Allopurinol effect on the host cell infection
T. cruzi Nicaragua infected BESM, VERO cells and rat cardiomyocytes primary cultures were treated with 1, 10, 20 and 50 μM allo. This drug showed different behavior being dose-and cellular compartment-dependent (Fig. 2). We observed that 1 μM allo concentration was trypanocidal in VERO cells and 10 μM significantly reduced the percentage of infected cells regardless the cell line culture. In cardiomyocyte primary cultures, a 20 μM concentration was necessary to exhibit the same inhibitory effect than that observed for VERO and BESM cells with 10 μM. Viability of non-infected cells was evaluated for each drug concentration by the Blue Trypan (0.5%) exclusion test. A 50 μM allo concentration was toxic for all cell types.
Fig. 2.
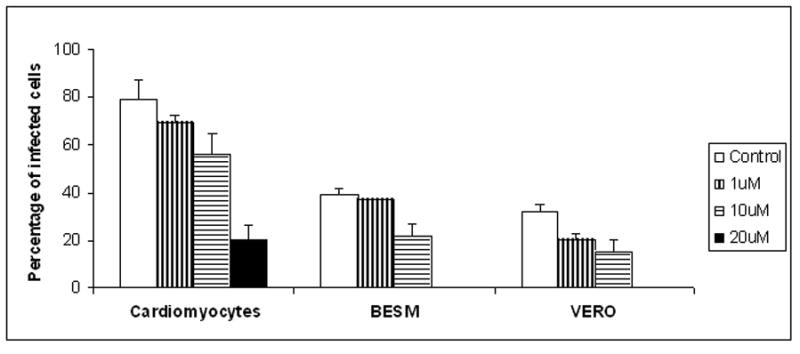
Effects of allopurinol on the amastigote rate of infection of T. cruzi Nicaragua isolate in culture cell. T. cruzi Nicaragua infected BESM, VERO cell lines and rat cardiomyocytes primary cultures treated. The cultures were incubated for 48 hs and were infected with T. cruzi Nicaragua isolate trypomastigotes and treated for 48 hs with 1, 10, 20 and 50 μM of allopurinol.
3.6. T. cruzi Nicaragua course of infection
Mice infected with 1× 106 trypomastigotes showed a parasitemia peak at 19 days post-infection (Fig. 3). The mortality of infected non-treated mice was 77 % during the acute phase. Survival rate of C3H/HeN infected mice in the different experimental groups is represented (Fig. 4). Non-treated infected mice showed a significantly higher mortality rate when compared with bz treated animals (Gehan-Breslow-Wilcoxon Test, p < 0.0001) based on the known course of infection with other T. cruzi isolates in C3H/HeN mice.
Fig. 3.
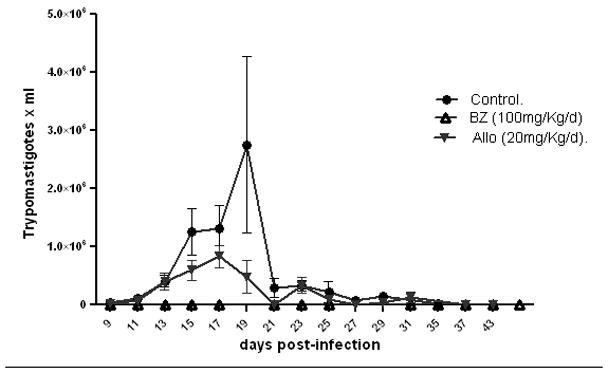
Parasitemia of mice infected with 1× 106 T. cruzi Nicaragua isolated trypomastigotes and treated with bz and allo, as described in Material and Methods. Parasitemia peak at 19 days post-infection in the acute phase is shown.
Fig. 4.
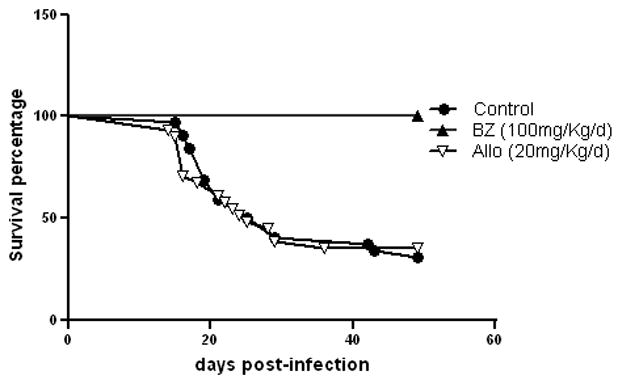
Survival rate of C3H/HeN infected mice infected with 106 T. cruzi Nicaragua isolate trypomastigotes in control and drug treated groups.
3.7. Bz and allo treatment in Nicaragua T. cruzi-infected mice
Bz treatment of mice infected with the Nicaragua isolate reverted the lethal effect of parasites observed during the acute infection. All infected mice survived with this bz treatment (Fig. 4). However, a few parasites were detected in the heart of 89 % of mice one year post-infection. The effect observed with 20 mg/kg/day of allo treatment is a parasitemia peak displacement to day 17 p.i, (Fig. 3) and although the parasitemia was lower than in allo non-treated mice, we could not see any significant difference in mice survival between both groups.
3.8. T. cruzi Nicaragua isolate tissue parasitism
The histopathological analysis of animals during the acute phase of infection with the Nicaragua isolate showed nests of parasites in all organs examined, in association with cellular necrosis and inflammatory mononuclear and polymorphonuclear cell infiltrates (Table 2). In the chronic phase of infection, intracellular parasites and interstitial mononuclear infiltrates were found predominantly in cardiac, skeletal and smooth muscles. Parasite nests were found in hearts of all mice analyzed in the chronic phase, being less frequently detected in the skeletal and smooth muscles. Although the density of parasite nests per section in the heart of mice in the chronic phase was less than in the acute phase (p < 0.015), the extension of the inflammatory infiltrate was similar in both phases. Interstitial fibrosis was detected in the myocardium of chronic infected mice. Parasites were not detected in the liver parenchyma or Kupffer cells in the chronic phase regardless of the type of treatment. However, non-specific Kupffer cell hyperplasia and mononuclear cell infiltrates were found in infected mice.
Table 2. Percentage of mice with parasite nests in different tissues.
Asterisc means the significant difference (p < 0.024) between the number of parasites in skeletal muscle during the chronic phase of infection with the bz treatment. n means number of mice infected or infected and drug treated.
| n | heart | smooth muscle | spleen | liver | kidney | ||
|---|---|---|---|---|---|---|---|
| Acute | 9 | 100% | 87.5% | 85.71% | 55.56% | 25% | 22.22% |
| Chronic | 6 | 100% | 66.67% | 33.33% | 0% | 0% | 0% |
| Chronic + bz | 9 | 88.89% | 20%* | 11. % | 0% | 0% | 0% |
| Chronic + allo | 5 | 60% | 40% | 20% | 0% | 0% | 0% |
3.9. Histopathology of drug treated infected mice
Treatment with bz significantly reduced the number of parasites in skeletal muscle during the chronic phase of T. cruzi Nicaragua infection (p < 0.024). We did not find any significant difference in the frequency of animals with parasite nests, density of nests or the extension of inflammation in the heart and smooth muscle in infected mice during the chronic phase either treated with allo or bz (Table 2).
4. Discussion
In American Trypanosomiasis the presence of the parasite and the consequent inflammation of tissues are responsible for cardiac and digestive pathogenesis. It is possible that the genetic characteristics of the parasite are involved in the development of lesions. In the present study we studied some biological and molecular aspects of a T. cruzi Nicaragua isolate and its susceptibility to currently used parasiticidal drugs in an experimental murine model.
Nicaragua isolate belongs to lineage I, TcI, in coincidence with other T. cruzi parasites isolated in Central America, Colombia and Venezuela, in which lineage I is predominant (Botero et al., 2007; Añez et al., 2006) and coexists in the domestic and sylvatic areas (Pennington et al., 2009). Although the distribution of different T. cruzi lineages in different areas is now extensively studied, the underlying causes of this distribution are still unknown. A recent study showed that the parasites circulating in domiciled triatomines and in T. cruzi mammalian reservoirs share the same lineages (Cardinal et al., 2008).
The behaviour of this T. cruzi isolate in axenic cultures showed that the maximum stationary phase concentration was lower than in other T. cruzi strains studied. The K value observed could be explained by the fact that the Nicaragua isolate might use the culture medium resources in different ways than other T. cruzi strains. Another possibility is that this parasite isolate is not adapted to the culture medium.
Allo treatment inhibited amastigote growth in BESM and VERO cell cultures, showing a dose-dependent inhibitory effect over Nicaragua T. cruzi, in agreement with allo inhibition on T. cruzi Tulahuen strain in other cell types (Nakajima-Shimada et al., 1996). To obtain the same inhibitory effect on Nicaragua isolate infected cardiomyocyte cultures it was necessary a higher drug concentration. It is possible that a potential mechanism of the xanthine oxidase could be related to its ability to reduce reactive oxygen species in this injured primary infected cardiomyocytes (Xiao et al., 2009).
The experimental infection with 106 parasites per mouse, induced high parasitemia during the acute phase of infection and 30 % of mice developed a chronic infection according to what was previously demonstrated for other T. cruzi lineage I parasites (Botero et al., 2007). The Nicaragua isolate did not show a marked cell tropism in the acute phase of infection in mice, and thus could be considered pantropic. However, parasites were repeatedly observed in heart cells and skeletal muscle during the chronic phase, in association with inflammatory cell infiltrates. The predilection of Nicaragua parasites for cardiac and skeletal muscle in the chronic infected mice is similar to what has been described for other lineage I T. cruzi parasite infections (Botero et al., 2007; Camandaroba et al., 2001). Other authors described T. cruzi lineage I nests in endomyocardial biopsies of a chronic chagasic patient with end-stage heart failure (Texeira et al., 2006) and also reported to cause mainly chagasic cardiomyopathy (Toledo et al., 2002). Studies with Nicaragua parasites in the chronic phase of infection and drug treatment sensitivity are being performed with a lower parasite inoculum.
The bz treatment of Nicaragua infected mice was effective for the clearance of parasites in the acute phase of infection. Although all treated animals survived, parasites were found in some of their tissues probably due to the high parasite inoculum used in this study. The mice treatment with bz showed in this work only reduced significantly the number of parasites in skeletal muscle during the chronic phase, compared with all other tissues analysed. Infected chronic mice treated with allo did not exhibit a significant decrease of parasite nests in the heart, although the number of nests is lower (Table 2). Our experimental mice reached the chronic phase of the infection because we had treated with bz and allo or a combination of both drugs in the acute phase.
Our results are in accordance with previous observations for the T. cruzi lineage I, in which partial susceptibility to bz was demonstrated in the acute and chronic phases of infection (Zafra et al., 2008). Recent studies suggest that the pattern of heart lesions in the chronic phase of bz-treated animals is T. cruzi - dependent but not related with drug resistance (Caldas et al., 2008). Mice treated with allo reduced the parasitemia peak with respect to non-treated control mice. Nevertheless, no significant differences in survival were observed as compared to control non-treated mice. Bz and allo treatments have demonstrated so far, different efficiency for different parasite lineages, in distinct geographic areas (Camandaroba et al., 2003).
T. cruzi Nicaragua isolate belongs to lineage I which is prevalent in many endemic areas in South America. The partial resistance to trypanocidal drugs clearly indicates the need of drug protocol optimization through the combination of most effective drug treatments. This study confirms the benefits of early bz treatment expressed as reduction of parasitemia and mortality of mice. Further studies with the Nicaragua isolate will contribute to the knowledge of some therapeutic aspects of the T. cruzi infection.
Acknowledgments
We thank the personal of the animal facility of the INP Adrian Gentile, America M. Gabi and Beatriz Asencio, for their collaboration in animal care. We thank Dr. Marta Lauricella for reference strains epimastigote cultures and Dr. Mirta Carlomagno for critical review of this manuscript. We also are indebted to Leticia Orellana for her excellent technical help. J. B., M. P. and L. E. F., are members of the Research Career of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina. This work was partially supported by the Instituto Nacional de Parasitología, ANLIS C.G. Malbrán, the CAECIS - Universidad Abierta Interamericana, Secretaría de Ciencia y Técnica (PICT 2005 38187) Buenos Aires, Argentina and from the Fogarty International Center (Grant Number D43TW007888) USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Noelia L. Grosso, Email: noelialorenag@yahoo.com.ar.
Jacqueline Bua, Email: jacbua@yahoo.com.
Alina E. Perrone, Email: alinaperrone@yahoo.com.
Mariela N. Gonzalez, Email: marnatch@yahoo.com.ar.
Patricia L. Bustos, Email: pato54mar@yahoo.com.ar.
Miriam Postan, Email: miriampostan@yahoo.com.
Laura E. Fichera, Email: lfichera@yahoo.com.
References
- Andrade V, Barral-Netto M, Andrade SG. Patterns of resistance of inbred mice to Trypanosoma cruzi are determined by parasite strain. Brazilian Journal of Medical Biological Research. 1985;18:499–506. [PubMed] [Google Scholar]
- Añez N, Crisante G, da Silva FM, Rojas A, Carrasco H, Umezawa ES, Stolf AM, Ramirez JL, Texeira MM. Predominance of lineage I among Trypanosoma cruzi isoltes from Venezuela patients with different clinical profiles of acute Chagas’ disease. Tropical Medicine & International Health. 2006;9:1319–1329. doi: 10.1111/j.1365–3156.2004.01333.x. [DOI] [PubMed] [Google Scholar]
- Avila JL, Avila A. Trypanosoma cruzi: allopurinol in the treatment of mice with experimental acute Chagas disease. Experimental Parasitology. 1981;51:204–208. doi: 10.1016/0014-4894(81)90109-0. [DOI] [PubMed] [Google Scholar]
- Apt W, Arribada A, Zulantay I, Solari A, Sánchez G, Mundaca K, Coronado X, Rodríguez J, Gil LC, Osuna A. Itraconazole or allopurinol in the treatment of chronic American trypanosomiasis: the results of clinical and parasitological examinations 11 years post-treatment. Annals of Tropical Medicine and Parasitology. 2005;99:733–741. doi: 10.1179/136485905X75403. [DOI] [PubMed] [Google Scholar]
- Berens RL, Marr JJ, Steele da Cruz F, Nelson DJ. Effect of allopurinol on Trypanosoma cruzi: metabolism and biological activity in intracellular and bloodstream forms. Antimicrobial Agents and Chemotherapy. 1982;22:657–661. doi: 10.1128/aac.22.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Revista do Instituto de Medicina Tropical de São Paulo. 1962;4:389–396. [PubMed] [Google Scholar]
- Briones MR, Souto RP, Stolf BS, Zingales B. The evolution of two Trypanosoma cruzi subgroups inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Molecular and Biochemical Parasitology. 1999;104:219–232. doi: 10.1016/s0166-6851(99)00155-3. [DOI] [PubMed] [Google Scholar]
- Brisse S, Barnabe C, Tibayrenc M. Identification of six Trypanosoma cruzi phylogenetic lineages by random amplified polymorphic DNA and multilocus enzyme electrophoresis. International Journal for Parasitology. 2000;30:35–44. doi: 10.1016/s0020-7519(99)00168-x. [DOI] [PubMed] [Google Scholar]
- Botero LA, Mejía AM, Triana O. Caracterización biológica y genética de dos clones pertenecientes a los grupos I y II de Trypanosoma cruzi de Colombia. Biomedica. 2007;27:64–74. [PubMed] [Google Scholar]
- Burgos JM, Begher SB, Freitas JM, Bisio M, Duffy T, Altcheh J, Teijeiro R, Lopez Alcoba H, Deccarlini F, Freilij H, Levin MJ, Levalle J, Macedo AM, Schijman AG. Molecular diagnosis and typing of Trypanosoma cruzi populations and linages in cerebral Chagas disease in patients with AIDS. American Journal of Tropical Medicine and Hygiene. 2005;73:1016–1018. [PubMed] [Google Scholar]
- Caldas IS, Talvani A, Caldas S, Carneiro CM, de Lana M, da Matta Guedes PM, Bahia MT. Benznidazole therapy during acute phase of Chagas disease reduces parasite load but does not prevent chronic cardiac lesions. Parasitology Research. 2008;103:413–421. doi: 10.1007/s00436-008-0992-6. [DOI] [PubMed] [Google Scholar]
- Cardinal MV, Lauricella MA, Ceballos LA, Lanati L, Marcet PL, Levin M, Kitron U, Gürtler RE, Schijman AG. Molecular epidemiology of domestic and sylvatic Trypanosoma cruzi infection in rural northwestern Argentina. International Journal for Parasitology. 2008;38:1533–1543. doi: 10.1016/j.ijpara.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camandaroba ELP, Campos RF, Magalhaes JB, Andrade SG. Clonal structure of Trypanosoma cruzi Colombian strain (Biodeme type III): biological, isoenzymatic and histopathological analysis of seven isolated clones. Revista da Sociedade Brasileira de Medicina Tropical. 2001;34:151–157. doi: 10.1590/s0037-86822001000200001. [DOI] [PubMed] [Google Scholar]
- Camandaroba ELP, Reis EAG, Gonςalves MS, Reis MG, Andrade SG. Trypanosoma cruzi: susceptibility to chemotherapy with benznidazole of clones isolated from the high resistant Colombian strain. Revista da Sociedade Brasileira de Medicina Tropical. 2003;36:201–209. doi: 10.1590/s0037-86822003000200002. [DOI] [PubMed] [Google Scholar]
- Coronado X, Zulantay I, Rozas M, Apt W, Sanchez G, Rodriguez J, Ortiz S, Solari A. Dissimilar distribution of Trypanosoma cruzi clones in human after chemotherapy with allopurinol and itraconazole. The Journal of Antimicrobial Chemotherapy. 2006;58:216–219. doi: 10.1093/jac/dkl192. [DOI] [PubMed] [Google Scholar]
- Dvorak JA. The Natural Heterogeneity of Trypanosoma cruzi: Biological and Medical implications. Journal of Cellular Biochemistry. 1984;24:357–371. doi: 10.1002/jcb.240240406. [DOI] [PubMed] [Google Scholar]
- Ellingsen O, Davidoff SK, Prasad HJ, Berger JP, Springhorn JD, Marsh JD, Kelly RA, Smith TW. Adult rat ventricular myocytes cultured in defined medium: phenotype and electromechanical function. The American Journal of Physiology. 1993;265:747–754. doi: 10.1152/ajpheart.1993.265.2.H747. [DOI] [PubMed] [Google Scholar]
- Filardi LS, Brener Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Transaction of the Royal Society of Tropical Medicine and Hygiene. 1987;81:755–759. doi: 10.1016/0035-9203(87)90020-4. [DOI] [PubMed] [Google Scholar]
- Fichera LE, Albareda MC, Laucella SA, Postan M. Intracellular growth of Trypanosoma cruzi in cardiac myocytes is inhibited by cytokine-induced nitric oxide release. Infection and Immunity. 2004;72:359–363. doi: 10.1128/IAI.72.1.359–363.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley RW, Dvorak JA. Trypanosoma cruzi: analysis of the population dynamics of heterogeneous mixtures. The Journal of Protozoology. 1987;34:409–415. doi: 10.1111/j.1550-7408.1987.tb03202.x. [DOI] [PubMed] [Google Scholar]
- Freitas de JD, Augusto-Pinto L, Pimenta JR, Bastos-Rodriguez L, Gonçalvez VF, Teixeira SMR, Chiari E, Junqueira ACV, Fernandes O, Macedo AM, Machado CR, Pena SDJ. Ancestral genomes, sex and the population structure of Trypanosoma cruzi. PLoS Pathogens. 2006;2:226–235. doi: 10.1371/journal.ppat.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi P, Lo Presti MS, Fernandez AR, Enders JE, Fretes R, Gea S, Paglini-Oliva P, Rivarola HW. Allopurinol is effective to modify the evolution of Trypanosoma cruzi infection in mice. Parasitology Research. 2007;101:1459–1462. doi: 10.1007/s00436-007-0644-2. [DOI] [PubMed] [Google Scholar]
- Machado CA, Ayala FJ. Nucleotide sequences provide evidence of genetic exchange among distantly related lineages of Trypanosoma cruzi. Proceeding of the National Academy of Sciences USA. 2001;98:7396–7401. doi: 10.1073/pnas.121187198.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murta SM, Romanha AJ. Characterization of Trypanosoma cruzi. Memorias do Instituto Oswaldo Cruz. 1999;94:177–180. doi: 10.1590/s0074-02761999000700024. [DOI] [PubMed] [Google Scholar]
- Nakajima-Shimada J, Hirota Y, Aoki T. Inhibition of Trypanosoma cruzi growth in mammalian cells by purine and pyrimidine analogs. Antimicrobial Agents and Chemotherapy. 1996;40:2455–2458. doi: 10.1128/aac.40.11.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington PM, Paiz C, Grajeda LM, Cordon-Rosales Short Report: Concurrent detection of Trypanosoma cruzi lineages I y II in domestic Triatoma dimidiata from Guatemala. American Journal of Tropical Medicine and Hygiene. 2009;80:239–241. [PubMed] [Google Scholar]
- Postan M, Bailey JJ, Dvorak JA, McDaniel JP, Pottala EW. Studies of Trypanosoma cruzi clones in inbred mice III. Histopathological and electrocardiographical responses to chronic infection. American Journal Tropical of Medicine and Hygiene. 1987;7:541–549. doi: 10.4269/ajtmh.1987.37.541. [DOI] [PubMed] [Google Scholar]
- Rodriques Coura J, de Castro SL. A critical review on Chagas disease chemotherapy. Memorias do Instituto Oswaldo Cruz. 2002;97:3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- Souto RP, Fernández O, Macedo AM, Campbell DA, Zingales B. DNA markers define two major phylogenetic linages of Trypanosoma cruzi. Molecular and Biochemical Parasitology. 1996;83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]
- Texeira MM, da Silva FM, Marcili A, Umezawa ES, Shikan ai-Yasuda MA, Cunha-Neto E, Kalil J, Stolf N, Stolf AM. Short communication: Trypanosoma cruzi lineage I in endomyocardial biopsy from a north-eastern Brasilian patient at end-stage chronic Chagasic cardiomyopathy. Tropical Medicine & International Health. 2006;11:294–298. doi: 10.1111/j.1365-3156.2006.01575.. [DOI] [PubMed] [Google Scholar]
- Tibayrenc M. Genetic subdivisions within Trypanosoma cruzi and their relevance for molecular epidemiology and experimental evolution. Kinetoplastid Biology and Disease. 2003;2:12. doi: 10.1186/1475-9292-2-12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo MJ, de Ornelas Bahia MT, Carneiro CM, Martins-Filho OA, Tibayrenc M, Barnabe C, Tafuri WL, Lana M. Chemotherapy with Benznidazole and itraconazole for mice infected with different Trypanosoma cruzi clonal genotypes. Antimicrobial Agents and Chemotherapy. 2002;47:223–230. doi: 10.1128/AAC.47.1.223-230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo MJO, Bahia MT, Veloso VM, Carneiro CM, Machado-Coelho GLL, Alves CF, Martins HR, Cruz RE, Tafuri WL, Lana M. Effects of specific treatment on parasitological and histopathological parameters in mice infected with different Trypanosoma cruzi clonal genotypes. The Journal of Antimicrobial Chemotherapy. 2004;53:1045–1053. doi: 10.1093/jac/dkh224. [DOI] [PubMed] [Google Scholar]
- Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, Bertocchi G, Armenti A. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Review of Anti- Infective Therapy. 2009;7:157–163. doi: 10.1586/14787210.7.2.157. [DOI] [PubMed] [Google Scholar]
- Xiao J, She Q, Wang Y, Luo K, Yin Y, Hu R, Huang K. Effect of allopurinol on cardiomyocyte apoptosis in rats after myocardial infarction. European Journal of Heart Failure. 2009;11:20–27. doi: 10.1093/eurjhf/hfn003. [DOI] [PubMed] [Google Scholar]
- Zafra G, Mantilla JC, Valadares HM, Macedo AM, González CI. Evidence of Trypanosoma cruzi II infection in Colombian chagasic patients. Parasitology Research. 2008;103:731–734. doi: 10.1007/s00436-008-1034-0. [DOI] [PubMed] [Google Scholar]
- Zingales B, Andrade SG, Briones MR, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Memorias do Instituto Oswaldo Cruz. 2009;104:1–4. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]


