To the Editor
Some 2009–2010 influenza vaccine package inserts indicate that each dose may contain up to 1 μg ovalbumin; others do not provide information on ovalbumin content. The ovalbumin content of influenza vaccines is important if these vaccines are administered to patients with egg allergy. In a previous publication (1) we (MAR and JTL) proposed a protocol for administration of influenza vaccine to patients with egg allergy when the ovalbumin content of the vaccine is unknown.
Previous study of seasonal influenza vaccines showed significant variability in ovalbumin content among vaccines of different manufacturers (2,3). Recently, Waibel and Gomez (4) used a commercial ovalbumin ELISA kit to assay ovalbumin concentrations in a sampling of 12 2009–2010 seasonal and H1N1 influenza vaccines. We extend the results of Waibel and Gomez by reporting the ovalbumin content of additional 2009 seasonal and 2009 H1N1 influenza vaccines. Further, we report our initial results of lot-to-lot variability.
Multiple lots from different vendors of Seasonal and H1N1 Flu vaccines were received from several clinics across the United States and Japan. Vaccines were collected over a 2 month period. These were tested at 2 or more dilutions.
Purified rabbit polyclonal anti-ovalbumin antibodies were purchased from Novus Biologicals (Littleton, CO). A HRP-conjugated rabbit polyclonal antibody to ovalbumin was purchased from Abcam (Cambridge, MA) and used as a detection antibody. The capture antibody (Novus NB600-922) was coated to a 96-well microtiter plate (Immulon 4HBX) at 3 μg/mL in Carbonate-Bicarbonate buffer (Pierce #28382) overnight at room temperature. After washing with wash buffer (0.5% Tween-20 in PBS, pH 7.4), wells were blocked with 1% normal rabbit serum in PBS for one hour at room temperature. Standards (chicken ovalbumin, Grade VI, Sigma, MO), controls and samples were added to the microtiter plate in duplicate and incubated for two hours at room temperature. After washing, detection antibody labeled with HRP (Abcam ab20415, diluted 1:50,000) were added and incubated at room temperature for one hour. The plate was washed and the TMB substrate was added for 5 to 10 minutes. The reaction was stopped with 2 N H2SO4 and absorbance at 450 nm was measured with a microplate reader (SpectraMax Plus; Molecular Devices, Sunnyvale, CA). The lowest point of the standard curve was 0.19 ng/mL. The recovery of ovalbumin, which was spiked into the vaccine specimens, was 95±6% (mean±SEM, n=16).
Fifty-eight samples (various manufacturers and lots) of 2009 influenza vaccines were assayed for ovalbumin concentration: 35 seasonal influenza vaccines and 23 H1N1 vaccines. Our study included a larger number of samples than the Waibel and Gomez report, which permitted an initial assessment of lot-to-lot variability of 2 vaccines. Our study also differs somewhat from Waibel and Gomez’s study as our assay was capable of measuring ovalbumin concentrations as low as 0.19 ng/ml. Finally, in our study, each sample was tested at 2 or more dilutions.
The median ovalbumin concentration of seasonal vaccine was 350 ng/ml (range 0.5–1,002). The median ovalbumin concentration of H1N1 vaccine was 21 ng/ml (range <1–76). One seasonal influenza vaccine (Fluzone, Sanofi Pasteur) showed higher levels compared with other seasonal influenza vaccines (Figure). Likewise, one H1N1 vaccine (Sanofi Pasteur) showed higher levels compared with other H1N1 vaccines (Figure). There were sufficient samples to show significant lot-to-lot variability for the 2 Sanofi Pasteur vaccines. The 2 samples of intranasal live attenuated virus seasonal influenza vaccine (Flumist) contained less than 1 ng/ml ovalbumin.
Figure 1.
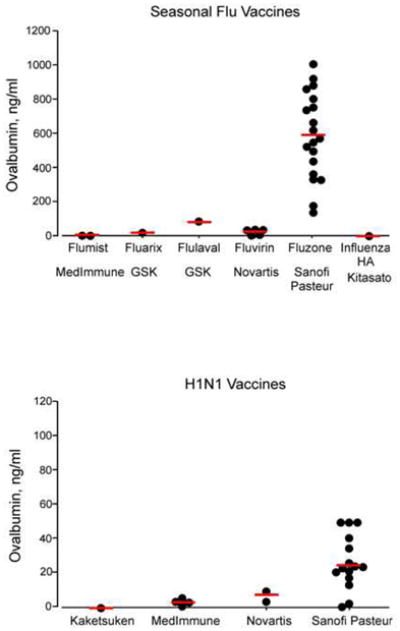
Ovalbumin levels in different lots of seasonal and H1N1 vaccines. Each dot represents the ovalbumin level in each lot. Figures show 29 lots from 6 products, 5 manufacturers (seasonable) and 22 lots from 4 manufactures (H1N1). Please note the difference in y-axis scale for seasonal and H1N1 vaccines. Horizontal bars represent medians.
A threshold of ovalbumin concentration for “safe” administration of influenza vaccine to patients with egg allergy has not been established. One study of 83 study subjects with egg allergy showed that seasonal influenza vaccine with ovalbumin concentrations up to 1.2 μg/ml did not result in any serious systemic reactions (5).
The measurement of ovalbumin content in seasonal and H1N1 influenza vaccines can lead to safer administration of these vaccines to patients with egg allergy. Vaccine lots with low ovalbumin content can be selected for patients with egg allergy; vaccine lots with high ovalbumin content can be avoided. All H1N1 vaccine lots tested showed low levels of ovalbumin (up to 76 ng/ml in our study, up to 64 ng/ml in the Waibel study). It is certainly possible that these vaccines can be administered safely to patients with egg allergy, but clinical confirmation is necessary. It is possible that all seasonal vaccines have sufficiently low ovalbumin content for safe administration to egg-allergic patients. However, with the higher ovalbumin concentration in some seasonal vaccines (up 1,002 ng/ml in our study, up to 1,421 ng/ml in the Waibel study), caution is warranted. Further, with manufacturer-manufacturer, lot-to-lot and likely year-to-year variability, measurement of ovalbumin content of each manufactured lot would seem advantageous. As found by Waibel and Gomez, intranasal live attenuated seasonal influenza vaccine contained very low levels of ovalbumin, and may be suitable for administration to egg allergic patients who do not have asthma.
A limitation of the study is the limited number of vaccine samples available for study. The samples were collected by solicitation of allergists and scientists in a short time frame. A larger and more systematic study would be better able to assess ovalbumin content variability by manufacturer and lot.
Acknowledgments
The authors thank Daniel G. Steinberg and Chitra Dinakar for their invaluable assistance in obtaining vaccine samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rank MA, Li JT. Clinical pearls for preventing, diagnosing, and treating seasonal and 2009 H1N1 influenza infection in patients with asthma. J Allerg Clin Immunol. 2009;124:1123–6. doi: 10.1016/j.jaci.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 2.Chaloupka I, Schuler A, Marschall M. Meier-Ewert H Comparative analysis of six European influenza vaccines. Eur J Clin Microbiol Inf Dis. 1996;15:121–7. doi: 10.1007/BF01591484. [DOI] [PubMed] [Google Scholar]
- 3.Zeiger RS. Current issues with influenza vaccination in egg allergy. J Allerg Clin Immunol. 2002;110:834–40. doi: 10.1067/mai.2002.129372. [DOI] [PubMed] [Google Scholar]
- 4.Waibel KH, Gomez R. Ovalbumin content in 2009 to 2010 seasonal and H1N1 monovalent influenza vaccines. J All Clin Immunol. 2010 doi: 10.1016/j.jaci.2009.12.015. in press. [DOI] [PubMed] [Google Scholar]
- 5.James JM, Zeiger RS, Lester MR, Fasano MB, Gern JE, Mansfield LE, et al. Safe administration of influenza vaccine to patients with egg allergy. J Pediatr. 1998;133:624–8. doi: 10.1016/s0022-3476(98)70101-5. [DOI] [PubMed] [Google Scholar]


