Abstract
Nitrotyrosine is a new biomarker of atherosclerosis and inflammation. The objective of this study was to determine the direct effects of free nitrotyrosine on human aortic smooth muscle cells (AoSMC) migration and molecular mechanisms. By a modified Boyden chamber assay, nitrotyrosine significantly increased AoSMC migration in a concentration-dependent manner. For example, nitrotyrosine at 300 nM increased AoSMC migration up to 152% compared with L-tyrosine-treated control cells (P<0.01). Cell wound healing assay confirmed this effect. Nitrotyrosine significantly increased the expression of some key cell migration-related molecules including PDGF receptor B, matrix metalloproteinase 2 (MMP2) and integrins αV and β3 at both mRNA and protein levels in AoSMC (P<0.01). In addition, nitrotyrosine increased reactive oxygen species (ROS) production in AoSMC by staining with fluorescent dyes including dihydroethidium and DCFHDA. Furthermore, nitrotyrosine induced transient phosphorylation of ERK2 by Bio-Plex luminex immunoassay and western blot analysis. AoSMC were able to uptake nitrotyrosine. Antioxidants including seleno-L-methionine and superoxide dismutase mimetic (MnTBAP) as well as ERK1/2 inhibitor PD98059 effectively blocked the promoting effect of nitrotyrosine on AoSMC migration and the mRNA expression of above cell migration-related molecules. Thus, nitrotyrosine directly increases AoSMC migration in vitro and the expression of migration-related molecules through overproduction of ROS and activation of ERK1/2 pathway. Nitrotyrosine may contribute to cardiovascular pathogenesis.
Keywords: Nitrotyrosine, Smooth muscle cell migration, Oxidative stress, Antioxidant, Atherosclerosis
1. Introduction
Increased levels of both circulating free nitrotyrosine and protein-combined nitrotyrosine are commonly found in many pathological conditions [1,2]. Many studies have demonstrated that protein nitration is evidenced in human atherosclerotic tissues, associated with different stages of atherosclerosis, and even correlated with plaque instability in patients [3,4]. Increased nitrotyrosine is also observed in the vascular remodeling with neointima formation [5]. More clinical studies have shown that plasma levels of nitrotyrosine can serve as an independent predictor of risk for coronary artery disease and atherosclerotic burden, and are modulated by known cardiovascular disease (CVD) risk–reducing therapies such as statins [6]. Thus, nitrotyrosine becomes a new biomarker for cardiovascular disease. However, there are few studies on the direct role of nitrotyrosine in the vascular system.
Migration and proliferation of vascular smooth muscle cells (VSMC) are the key processes of neointima formation in many vascular pathogenesis such as atherosclerosis, restenosis after angioplasty, and saphenous vein graft failure [7]. VSMC may contribute to the development of atherosclerosis through the production of inflammatory cytokines such as monocyte chemoattractant protein-1, and the synthesis of matrix proteins [8]. These processes involve the interaction of VSMCs with matrix and changes in intracellular signaling pathways that regulate cell migration. Platelet-derived growth factor-BB (PDGF-BB) is a major regulator of VSMC migration. Matrix metalloproteinases (MMP) and integrins provide permissive effects for VSMC migration by breaking down major extracellular barriers such as basal membranes, interstitial collagens, and proteoglycans [9], thereby playing an essential role in regulating VSMC migration.
Reactive oxygen species (ROS), generated by a variety of extracellular and intracellular mechanisms, have gained attention as novel signal mediators that regulate signal transduction events including mitogen-activated protein kinases (MAPKs) [10]. ROS integrate cellular signaling pathways involved in VSMC proliferation and migration associated with atherosclerosis [11]. Antioxidants are believed to counteract with ROS and reduce the incidence of coronary artery disease [12]. Seleno-L-methionine (SeMet) is an effective antioxidant by increasing the activity of glutathione peroxidase [13] and other mechanisms. Mn (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP) is a cell permeable superoxide dismutase (SOD) mimetic, which is used as a potent superoxide anion scavenger [14].
The objective of the current study was to investigate the biological functions of nitrotyrosine in vascular cell migration, which may contribute to vascular lesion formation. Human aortic smooth muscle cells (AoSMC) were treated with free nitrotyrosine, and the cell migration and the expression of PDGF receptor-B (PDGFR-B), MMP and integrins as well as involvement of ROS generation and MAPK activation were investigated. The data from this study provide experimental evidence of the direct effects of free nitrotyrosine on human AoSMC migration with unique molecular mechanisms.
2. Materials and methods
2.1. Chemicals and reagents
3-Nitrotyrosine (FW 226.2) was purchased from Cayman Chemical Company (Ann Arbor, Michigan). L-Tyrosine was purchased from Sigma-Aldrich (St. Louis, MO). Anti-human integrin β3 (CD61) and integrin αV were purchased from BD Biosciences Pharmingen (San Diego, CA). Anti-human β-actin antibody and SeMet were obtained from Sigma-Aldrich. Human PDGF-BB and anti-human PDGFR-B antibody were from R&D Systems, Inc. (Minneapolis, MN). Dihydroethidium (DHE) was obtained from Molecular Probes (Eugene, OR). Extracellular signal-regulated kinase (ERK) inhibitor (PD98059) and calcein-AM were obtained from Calbiochem Inc. (San Diego, CA). MnTBAP was purchased from A.G. Scientific (San Diego, CA).
2.2. Cell culture
AoSMC (Walkersville, MD) were routinely cultured in Smooth Muscle Medium-2 (SmGM-2) with growth factors and antibiotic (SmGM-2 Bullet Kit) supplemented with 10% fetal calf serum (FCS). Prior to each experiment, AoSMC were placed in the SmGM-2 medium with 1% FCS, without addition of growth factors, for 16 h (serum starvation). AoSMC were used between passages 3 and 7.
2.3. Cell migration assay
Cell migration was measured by using a modified Boyden chamber assay including Fluoroblock transwell migration plates (Becton Dickinson Labware, Franklin Lakes, NJ). Briefly, serum-starved cell suspension (250 μl, 1 × 105 cells/well) was added to the upper chamber of the FlouroBlock transwell insert (24-well plate). After 4 to 24 h incubation at 37°C in a 5% CO2 atmosphere, the chambers were incubated in Hank’s Balanced Salt Solution (HBSS) with 50 nM calcein-AM, a fluorescence dye to label living cells. The fluorescence of the cells migrated to the lower chamber was measured from the bottom using a fluorescence microplate reader at 485/535 nm wavelength. The migrated cells in the filters were also observed under a fluorescence microscope (Olympus, Tokyo, Japan).
2.4. Cell wound healing assay
AoSMC were grown to 90% confluence in six-well plates, a scratch was made with a sterile cell scraper. The starting point was marked with a marker pen at the bottom of the plate. Cells were washed with basal medium twice and were incubated with L-tyrosine (300 nM) or nitrotyrosine (300 nM), or PDGF-BB (10 ng/ml) in the basal medium. The cells were incubated for 16 h and stained with calcein-AM [15]. Photos were taken using fluorescence microscope.
2.5. RNA isolation and real time RT-PCR
Serum-starved AoSMC were treated with nitrotyrosine (300 nM) or L-tyrosine (300 nM) for 24 h, and total RNA was isolated using Trizol reagent and then treated with TURBO™ DNase (RNase-free) (Ambion, Austin, TX). One μg of total RNA was reverse-transcribed in 20 μl reaction solution using the iScript™ cDNA Synthesis Kit. Human PDGFR-B, MMP, and integrins as well as β-actin primers were designed using Beacon Designer software (Table 1). Real-time PCR reactions were performed with the iCycler iQ Real-Time PCR Detection System (Bio-Rad Laboratories) in 96-well reaction plates. Reaction volume was 25 μl, containing 2 μl cDNA and 100 μM of each pair of primers and iQ™ SYBR Green Supermix (Bio-Rad). Thermal cycling condition included pre-incubation 95°C for 3 min followed by 40 PCR cycles at 95°C for 20 sec and 60°C for 1 min. The iCycler software was used to analyze the calibration curve by plotting the threshold cycle (Ct) versus the logarithm of the number of copies for each calibrator. The quality and quantities of samples were normalized to the housekeeping gene β-actin as [2(Ct β-actin-Ct gene of interest)].
Table 1.
Sequence details of individual pairs of primers
| Gene | Gene bank No. | Forward primer | Reverse primer |
|---|---|---|---|
| PDGFR-B | NM_002609 | CCTTACCACATCCGCTCCATCC | CATTCACACTCTCCGTCACATTGC |
| MMP2 | AY738117 | CAACTACAACTTCTTCCCTCGCA | GGTCACATCGCTCCAGACTTG |
| Integrin αv | NM_002210 | GATTTCTTCGTGCCCAGCG | GCGGGTAGAAGACCAGTCAC |
| Integrin β3 | NM_000212 | ATGCTTCATCCATCATCGGTGTC | GGTCACCTGGTCAGTTAGCGT |
| β-actin | BC013835 | CTGGAACGGTGAAGGTGACA | GGAAGCACTTCCTGGACTTGAT |
2.6. ROS measurement
ROS generation was first measured by DHE staining with flow cytometry analysis. Cultured cells were treated with nitrotyrosine (300 nM) or L-tyrosine (300 nM) for 30 min. ROS production was detected by DHE staining. DHE is freely permeable to cells. In the presence of ROS such as superoxide anion, DHE is oxidized to ethidium bromide (EtBr) with red fluorescence, and it is trapped by intercalating with the DNA. Thus, the amount of EtBr detected by a fluorescence measurement instrument such as flow cytometer is well correlated to the level of cellular ROS. One milliliter of DHE (3 μM) in PBS was added into each well of six-well plates and incubated for 20 min at room temperature. One non-treated well was left as non-DHE stained control. Each analysis included 1 × 104 cell events. Fluorescence was monitored at 580/420 nm (red DHE). Data were analyzed using Cell Quest or Flow Jo software (Becton Dickinson, CA). Analyses were carried out on Becton Dickinson FACSCalibur equipped with an argon laser (488 nm). Data analysis was performed on fluorescence intensities. Cell Quest analysis software was used for fluorescence determination and data analysis. The data was presented by the percentage of positively stained cells compared with DHE non-treated cells.
ROS generation was also determined by flow cytometriy analysis of DCFHDA oxidation [16, 17], which is widely used to measure oxidative stress in cells due to the high sensitivity of fluorescence-based assays. The assay consists of the oxidation of DCFHDA (after hydrolysis of the diacetate form) to fluorescein by ferryl-type intermediates and/or oxygen and nitrogen reactive species, whose fluorescence can be measured at 522 nm. Following treatments with nitrotyrosine (300 nM) or L-tyrosine (300 nM), with/without SeMet (20 μM) or MnTBAP (3 μM) for 30 min, AoSMC were collected by trypsinization, washed, and incubated with 20 μM 2′,7′-dichlorofluorescin diacetate (DCFHDA, Invitrogen, CA) for 20 min. Intracellular ROS generation was assessed by flow cytometric monitoring of DCFHDA oxidation. DCFHDA oxidation was also directly measured in a fluorescent microplate reader using excitation and emission wavelengths of 485 and 535 nm.
2.7. MAPK activation
The MAPK phosphorylation state of AoSMC was analyzed by Bio-Plex phosphoprotein and total target assays (Bio-Rad) according to manufacturer’s instructions. Briefly, serum-starved AoSMC were treated with nitrotyrosine (300 nM) or L-tyrosine (300 nM), and the cell lysates were collected at different time points of 0, 5, 10, 20, 30, and 60 min with the Bio-Plex Cell Lysis Kit. The protein concentration was adjusted to 600 μg/ml, and 50 μl of cell lysate was used for each assay. Fifty μl of coupled beads, which recognize phosphorylated and total ERK2, p38, and JNK, respectively, were added to the 96-well filter plate. Same volume of the cell lysate was added and incubated with the beads for 15–18 h. Then, 25 μl of detection antibodies (1×) were added and incubated for 30 min. Fiftyμl of streptavidin-PE (1×) was added and incubated for 10 min in dark. The phosphoprotein and total proteins of MAPKs were analyzed by a Luminex 100TM analyzer (BioRad).
2.8. Western blotting
AoSMC were treated with nitrotyrosine (300 nM) or L-tyrosine (300 nM) for 24 h, and the cell lysates were collected using cell lysate buffer (Cell Signaling Technology, Danvers, MA). Fifteen micrograms of lysate proteins was loaded for Western blot analysis. Total cellular proteins were separated by 10% SDS-polyacrylamide gel electrophoresis and then transblotted overnight at 4°C onto Hybond-P PVDF membrane. After blocking the membrane with 5% nonfat dried milk, the membrane was probed with the respective primary antibodies against human integrin αV, MMP2, and PDGFR-B, p22phox, p47phox, phosphorylated ERK2, and total ERK2 respectively, at room temperature for 1 h. The membranes were then incubated in the horseradish peroxidase–linked secondary antibody for 50 min at room temperature. The immunoreactive bands were detected by enhanced chemiluminescent (ECL) plus reagent kit. The band density was measured with the use of AlphaEaseFC 3.1.2 software (Alpha Innotech Corporation, San Leandro, Ca).
2.9. MMP2 activity assay
MMP2 activity was determined by the EnzoLyte™ 490 MMP2 Assay Kit from AnaSpec Co. (San Jose, CA) in a 96-well plate. Briefly, the supernatants of AoSMC cultures were collected and concentrated in YM-10 columns for 30 min. One hundred microgram proteins in 50 μl of the concentrated supernatant were used for each assay. After activated by p-aminophenylmercuric acetate (APMA) for 15 min at 37°C, 50μl of a fluorogenic [an EDANS/DABCYL fluorescence resonance energy transfer (FRET) peptide] MMP2 substrate solution was added and the reaction was incubated for 30 min at room temperature. The fluorescence intensity representing the MMP2 activity was measured at 485/535 nm wavelength.
2.10. Immunocytochemistry
AoSMC were treated with nitrotyrosine (300 and 3000 nM) or L-tyrosine (3000 nM) for 24 h, and the cells were fixed in 4% paraformaldehyde, preincubated in 0.3% Trition-X in PBS at room temperature for 15 min. After blocking in 1.5% blocking serum for 1 h, the cells were incubated in rabbit anti-nitrotyrosine antibody (Millipore Corporation, TX) solution overnight at 4°C. Expression was detected using FITC-conjugated goat anti-rabbit IgG secondary antibody (R&D Systems, Inc., MN) and the cells were observed under a florescence microscope (Olympus, Tokyo, Japan).
2.11. Statistical analysis
Data are presented as mean±SEM in all experiments. Data from treated groups were compared with control groups by a Student’s t-test (two tailed). A value of P<0.05 was considered significant.
3. Results
3.1. Nitrotyrosine promotes human AoSMC migration
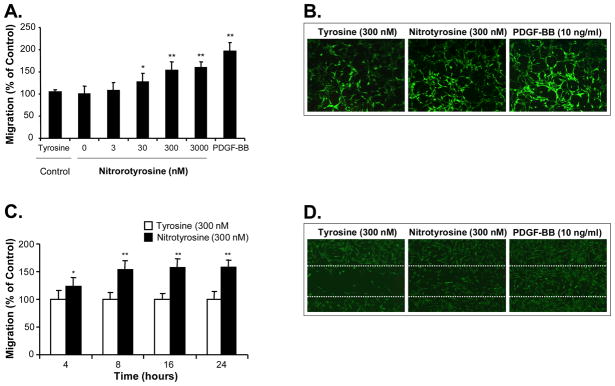
To investigate the effect of nitrotyrosine on AoSMC migration, cells were treated for 24 h with increasing concentrations of nitrotyrosine (0.3–3000 nM) or L-tyrosine (3000 nM) as a negative control in the upper chamber of a modified Boyden chamber assay. The cells migrated through a polystyrene-membrane with 8 μm-size pores were stained with calcein-AM, a fluorescence dye, and measured with a fluorescence reader. As shown in Fig. 1A, nitrotyrosine significantly increased AoSMC migration in a concentration-dependent manner with the maximum effect at 3000 nM (up to 157% compared with the L-tyrosine control, P<0.01, n=4). The L-tyrosine treatment had no effect on AoSMC migration compared with the medium alone control. As a positive control, PDGF-BB significantly increased AoSMC migration up to 198%. The cells that had migrated to the lower chamber were also visualized under a fluorescence microscope (Fig. 1B). To confirm the above finding, we performed a time course study at 4, 8, 16 and 24 h, and observed a detectable effect of nitrotyrosine (300 nM) on cell migration as early as 4 h after cell seeding, and the significant effect was observed at 8, 16 and 24 h (Fig. 1C). However, nitrotyrosine (300 and 3000 nM) had no effects on AoSMC viability and proliferation as tested by a non-radioactive MTS assay (data not shown).
Fig. 1.
Effect of nitrotyrosine on human AoSMC migration. (A) Concentration-dependent study in the Boyden chamber assay. Serum-starved AoSMC were seeded onto the transwell plate with different concentrations of nitrotyrosine (0–3000 nM). After 24 h incubation, AoSMC migrated to the lower chamber were measured by a fluorescence dye, calcein-AM, staining and quantitation. L-tyrosine was used as a negative control. Results are expressed as percentage of the control. (B) The representative photomicrographs of the migrated cells to the lower chamber after calcein-AM staining. L-tyrosine (300 nM), nitrotyrisine (300 nM) and PDGF-BB (10 ng/ml) as a positive control are shown. (C) Time course study by Boyden chamber assay. Serum-starved AoSMC were seeded onto the transwell plate with 300 nM nitrotyrosine or L-tyrosine. AoSMC migration was studied at 4, 8, 16, and 24 h. Each bar represents the mean ± SEM. *P<0.05 and **P<0.01 compared with the control (L-tyrosine). n = 4 per group. (D) The representative photomicrographs of cell wound healing assay. Confluent AoSMC were wounded by a scratch injury line made with a sterile cell scraper. Cells were then treated with L-tyrosine (300 nM); nitrotyrisine (300 nM); or PDGF-BB (10 ng/ml). Photos were taken at 16 h. Dotted white lines delimit the initially wounded regions. L-tyrosine (300 nM), nitrotyrisine (300 nM), and PDGF-BB (10 ng/ml) as a positive control are shown.
To further confirm the effect of nitrotyrosine on AoSMC migration, a cell wound healing assay was used to show the cell morphology and migration behavior after treatment of nitrotyrosine (300 nM) or L-tyrosine (300 nM). As shown in Fig. 1D, nitrotyrosine substantially promoted the cell migration during the cell wound healing process compared with the L-tyrosine control, although this effect was less than the positive control PDGF-BB (10 ng/ml).
3.2. Nitrotyrosine increases the expression of PDGFR-B, MMP2, and integrins in AoSMC
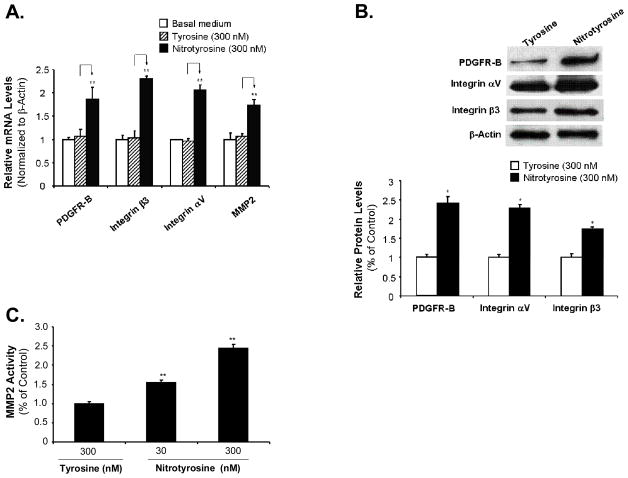
PDGF-BB mediated through its receptor PDGFR-B, is a major regulator of smooth muscle cell proliferation and migration. MMP and integrins play an essential permissive role in cell migration. Expression of PDGFR-B, MMP and integrins in AoSMC by nitrotyrosine, L-tyrosine treatment or medium alone treatment were evaluated by real-time PCR. As shown in Fig. 2A, L-tyrosine has no effect on the expression of these migration markers compared with medium alone treatments, which were consistent with the functional assay. However, nitrotyrosine significantly increased the mRNA levels of PDGFR-B, MMP2, integrin β3 and αV up to 174%, 162%, 221%, and 214%, respectively, compared with the L-tyrosine treated controls (100%; P<0.05; n = 3).
Fig. 2.
Effects of nitrotyrosine on the expression of VSMC migration-related molecules and MMP2 activity. Serum-starved AoSMC were treated with nitrotyrosine (300 nM) or L-tyrosine (300 nM) for 24 h. (A) Real time PCR analysis. The cDNA was synthesized from total mRNA of the treated cells by reverse transcription. The mRNA levels of PDGFR-B, integrin β3, integrin αV, and MMP2 were analyzed by real-time PCR and were normalized to the housekeeping gene β-actin as [2(Ct β-actin-Ct gene of interest)]. Values are expressed as percentage of the non-treated control. (B) Western blot analysis of protein levels of PDGFR-B, integrin αV, and integrin β3 in AoSMC. Equal amount of 15 μg cell lysate proteins were used to run western blotting against human PDGFR-B, integrin αV, and integrin β3. The band density was measured with AlphaEaseFC 3.1.2 software and normalized to β-actin levels. (C) MMP2 activity in the supernatants of AoSMC cultures. Serum-starved AoSMC were treated with nitrotyrosine (300 nM) or L-tyrosine (300 nM) for 24 h. One hundred microgram of proteins from each concentrated cell supernatant was analyzed by MMP2 activity assay. After activated by APMA for 15 min at 37°C, 50 μl of a fluorogenic FRET conjugated MMP2 substrate solution was added and incubated for 30 min at room temperature. The fluorescence intensity representing the MMP2 activity was measured at 485/535 nm wavelength. Results are expressed as percentage of controls (L-tyrosine). Data represent as mean ± SEM. *P<0.05. **P<0.01. n = 3.
Western blotting and quantitation demonstrated that nitrotyrosine increased protein levels of PDGFR-B, integrin αV, and integrin β3 expression up to 241%, 228% and 173%, respectively, compared with the L-tyrosine treatment (100%, Fig. 2B). Nitrotyrosine significantly increased MMP2 activity in a concentration-dependent manner with the maximal effect up to 245% compared with the L-tyrosine treated control (100%, P<0.01, n = 3, Fig. 2C). Thus, we demonstrated that nitrotyrosine upregulates the VSMC migration-related molecules such as PDGFR-B, MMP2, and integrins at both mRNA and protein levels.
3.3. ROS production is involved in nitrotyrosine-induced AoSMC migration
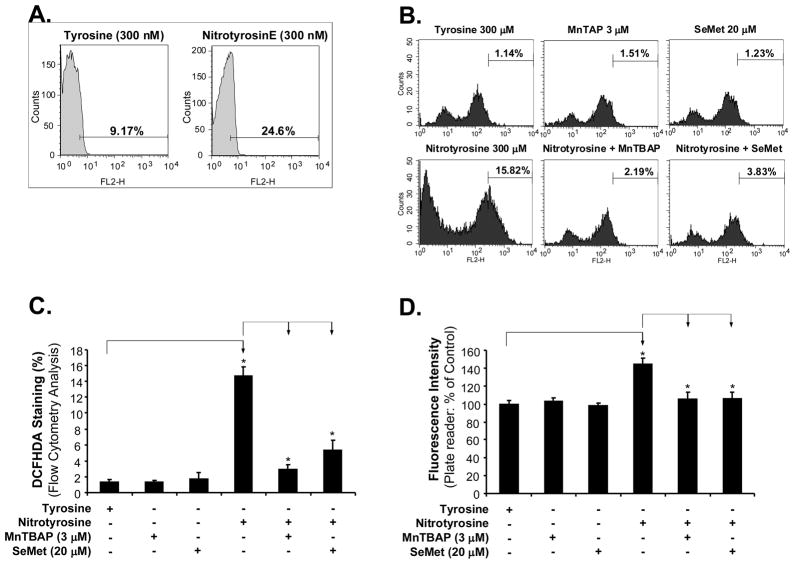
To explore whether ROS could be involved in the molecular mechanism of nitrotyrosine-induced AoSMC migration, we examined the effects of nitrotyriosine on ROS generation and antioxidant application. ROS levels were determined by fluorescence staining with DHE, a compound chemically oxidized to the fluorescent DNA intercalating agent ethidium in proportion to the amount of ROS present [18–20]. The fluorescence for ROS was analyzed by flow cytometry analysis. As shown in Fig. 3A, nitrotyrosine (300 nM) increased ROS production up to 25% of cell population compared with L-tyrosine (300 nM) (9%) after 30 min treatment. We did not detect any significant differences of ROS between treated and control groups after 24 h treatment (data not shown). In addition, ROS generation was also assessed by DCFHDA oxidation assay [16, 17]. With flow cytometery analysis, nitrityrosine treatment for 30 min significantly increased DCFHDA oxidation up to 14.66% of cell population compared to L-tyrosine treatment (1.32%, Fig. 3B and C). These data were also confirmed by a florescence plate reader showing that nitrityrosine significantly increased DCFHDA oxidation up to 145% compared with L-tyrosine controls (100%, Fig. 3D). These data indicate that nitrotyrosine can induce a transient ROS production, which might be involved in the signaling pathway of the nitrotyrosine-induced increase in cell migration.
Fig. 3.
Roles of ROS in nitrotyrosine-induced AoSMC migration. The cells were treated with nitrotyrosine (300 nM) or L-tyrosine (300 nM), with or without SeMet (20 μM) or MnTBAP (3 μM) for 30 min. Then the cells were stained with DHE or DCFHDA and analyzed by flow cytometry or a florescence plate reader. (A) Representative histograms of DHE staining and flow cytometry analysis. (B) Representative histograms of DCFHDA oxidation staining and flow cytometry analysis. (C) Quantitation of DCFHDA oxidation and flow cytometry analysis. (D) DCFHDA oxidation assay by a florescence plate reader. Each bar represents the mean ± SEM. *P<0.05 and **P<0.01 compared with the control (L-tyrosine). n = 4 per group.
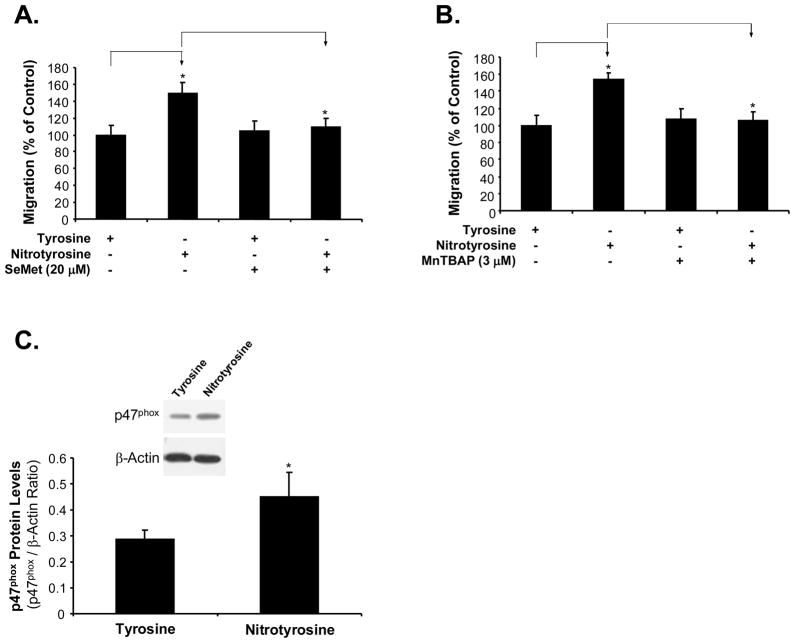
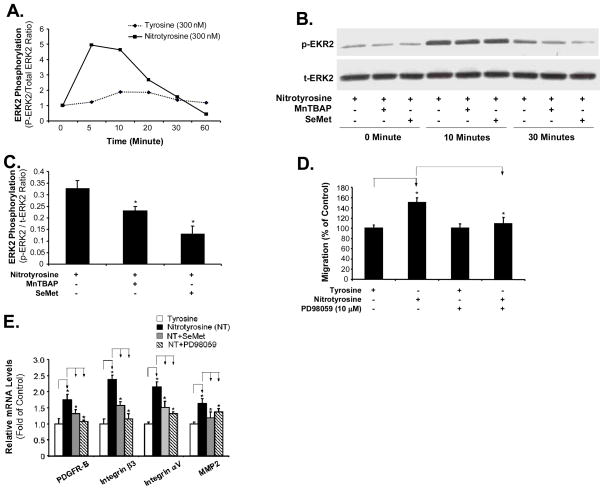
MnTBAP is a specific superoxide anion scavenger and SeMet is a well characterized antioxidant. Both antioxidants effectively blocked nitrotyrosine-induced ROS generation (Fig. 3B, C and D). Indeed, SeMet (20 μM) and MnTBAP (3μM) effectively blocked nitrotyrosine-induced AoSMC migration (P<0.01, n = 4, Fig. 4A and B). These data indicate that ROS plays an important role in nitrotyrosine-induced AoSMC migration.
Fig. 4.
Effects of antioxidants SeMet and MnTBAP on nitrotyrosine-induced AoSMC migration increase and NADPH oxidase expression. Serum-starved AoSMC were seeded onto the transwell plate and incubated with nitrotyrosine (300 nM) or L-tyrosine (300 nM), with the presence or absence of SeMet (20 μM) (A) or MnTBAP (3 μM) (B). After 24 h incubation, AoSMC migrated to the lower chamber were measured by calcein-AM staining and quantitation. Results are expressed as percentage of controls (L-tyrosine). n = 4. (C) Expression of NADPH oxidase subunits (p47 phox) in AoSMC after nitrotyrosine and L-tyrosine treatment by western blot analysis. The band density was measured with AlphaEaseFC 3.1.2 software and normalized to β-actin levels. Data represent as mean ± SEM. *P<0.05. n = 3.
3.4. Nitrotyrosine increases the expression of NADPH oxidase subunits in AoSMC
Oxidative stress could result from up-regulation or activation of ROS generating enzymes. To test whether these molecules could play a role in nitrotyrosine-induced oxidative stress, we determined expressions of NADPH oxidase subunits, a major ROS generating enzyme in AoSMC. Treatment with nitrotyrosine increased the expression of NADPH oxidase subunit p47phox in AoSMC compared with that with L-tyrosine (Fig. 4C). At 300 nM of nitrotyrosine, the protein level of p47phox was significantly increased by 60% compared with that of L-tyrosine (P<0.05, n=3, Fig. 4C). However, the expression of p22phox in protein levels did not show any obvious changes between the nitrotyrosine-treated and tyrosine-treated cells (data not shown).
3.5. ERK1/2 activiation is involved in nitrotyrosine-induced AoSMC migration
Since the activation of MAPKs can be triggered by oxidative stress through direct or indirect mechanisms [18, 21], we investigated whether MAPKs could be activated in nitrotyrosine-treated cells and involved in the nitrotyrosine-induced cell migration. Major MAPKs include ERK1/2, p38, and JNK. Serum-starved AoSMC were incubated with nitrotyrosine (300 nM) and L-tyrosine (300 nM), and the cell lysates were collected at different time points. A Bio-Plex luminex assay (Bio-Rad) was used to detect phospho- and total proteins of ERK2, p38 and JNK. The ratio of phosphoproteins to total proteins at each time point was used to evaluate the phosphorylation levels of ERK2, p38, and JNK. As shown in Fig. 5A, nitrotyrosine (300 nM) substantially increased ERK2 phosphorylation with a peak up to 5.11-fold at 5 min, whereas L-tyrosine (300 nM) had very limited effects on ERK2 phosphorylation. However, nitrotyrosine (300 nM) had no significant effects on p38 and JNK phosphorylation (data not shown). In addition, nitrotyrosine-induced ERK2 phosphorylation was confirmed by western blot analysis (Fig. 5B). These results suggest that nitrotyrosine can activate ERK1/2 signaling pathways on AoSMC. Two antioxidants, SOD mimetic (MnTBAP) and SeMet, effectively blocked nitrotyrosine-induced increase in ERK2 phosphorylation by 30% and 60%, respectively, in AoSMC at 30 minutes (Fig. 5B and C).
Fig. 5.
Role of ERK1/2 activation in nitrotyrosine-induced AoSMC migration. ERK2 phosphorylation. Serum-starved AoSMC were treated with nitrotyrosine (300 nM) or L-tyrosine (300 nM), with or without the presence of SeMet (20 μM) or MnTBAP (3 μM). The cell lysates were harvested at different time points as indicated. Phosphorylated and total ERK2 were determined by Bio-Plex luminex immunoassay (A) or Western blot (B). Ratio of phosphorylated and total ERK2 proteins in each sample was calculated. (C) Quantitation of the western blot protein bands. The band density was normalized to β-actin levels. n = 3. (D) Effect of ERK1/2 inhibitor on nitrotyrosine-induced AoSMC migration. Serum-starved AoSMC were seeded onto the transwell plate and incubated with nitrotyrosine (300 nM) or L-tyrosine (300 nM), with the presence or absence of ERK1/2 inhibitor (PD98059, 10 μM). After 24 h incubation, AoSMC migrated to the lower chamber were measured by calcein-AM staining and quantitation. Results are expressed as percentage of controls (L-tyrosine). n = 4. (E). Effects of antioxidant SeMet and ERK1/2 inhibitor on nitrotyrosine-induced mRNA expression of several key cell migration-related molecules. Serum-starved AoSMC were seeded onto the transwell plate and incubated with nitrotyrosine (300 nM) or L-tyrosine (300 nM), with the presence or absence of either SeMet (20 μM) or ERK1/2 inhibitor (PD98059, 10 μM). After 24 h incubation, cells were harvested. The cDNA was synthesized from total mRNA of the treated cells by reverse transcription. The mRNA levels of PDGFR-B, integrin β3, integrin αV, and MMP2 were analyzed by real-time PCR and were normalized to the housekeeping gene β-actin as [2(Ct β-actin-Ct gene of interest)]. Values are expressed as percentage of controls (L-tyrosine). Each bar represents the mean ± SEM. *P<0.05 compared with the control (L-tyrosine). n = 3 per group.
To further test the impact of ERK1/2 activation in the promoting effect of nitrotyrosine on AoSMC migration, we incubated AoSMC with an ERK1/2 specific inhibitor (PD98059) for 1 h prior and during nitrotyrosine or L-tyrosine treatment. Cell migration was evaluated by the modified Boyden chamber assay. As shown in Fig. 5D, PD98059 (10 μM) completely blocked the promoting effect of nitrotyrosine on AoSMC migration (P<0.01, n = 4).
We also found that PD98059 (10 μM) as well as SeMet significantly blocked nitrotyrosine-induced mRNA expression of migration-related molecules including PDGFR-B, integrins (αV and β3) and MMP2 (P<0.05, n=3, Fig. 5E). These findings suggest that nitrotyrosine promotes AoSMC migration through the ERK1/2 signaling pathway.
3.6. AoSMC uptake nitrotyrosine in a concentration-dependent manner
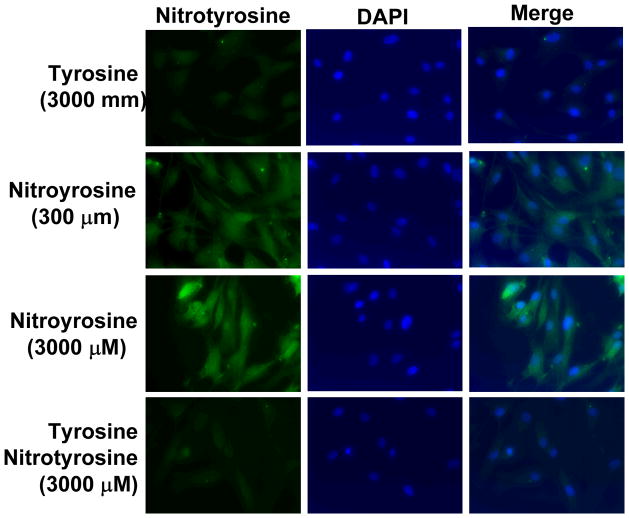
To address the cellular effect of nitrotyrosine on AoSMC, we detected nitrotyrosine in AoSMC by immunocytochemistry after nirotyrosine treatment (300–3000 nM) for 24 h. AoSMC was able to uptake nitrotyrosine in a concentration dependant manner (Fig. 6). The uptake was partially blocked by co-treatment with tyrosine. These data indicate that nitrotyrosine might act by the intracellular mechanism, which is consistent with previous observations in other types of cells [22].
Fig. 6.
Nitrotyrosine uptake by AoSMC. Cells were treated with nitrotyrosine (300, 3000 nM) or L-tyrosine (3000 nM) for 24 h, and nitrotyrosine was detected by immunocytochemistry using a nitrotyrosine antibody and recognized by FITC-conjugated secondary antibody (green). DAPI was used to stain nuclei of the cells (blue). The cells were observed under a florescence microscope at 40X magnification.
4. Discussion
Although there is a clinical association between nitrotyrosine plasma level and cardiovascular disease, the direct roles of nitrotyrosine in vascular functions have received limited attention. In the current study, we demonstrated that nitrotyrosine promotes human AoSMC migration in a concentration- and time-dependent manner. In addition, nitrotyrosine increases the expression of SMC migration-related molecules such as PDGFR-B, MMP2 and integrinsαV and β3 at both mRNA and protein levels. Furthermore, we have found that nitrotyrosine induces a transient ROS production in AoSMC and activates ERK1/2 signal pathway. These findings provide a direct link between nitrotyrosine and smooth muscle cell migration which may contribute to cardiovascular disease.
Although it is not clear how much free nitrotyrosine is present in plasma, increased levels of free nitrotyrosine are observed in patients with many pathological conditions such as rheumatoid arthritis [23], liver transplantation [24], septic shock [25], and amyotrophic lateral sclerosis [26]. Free nitrotyrosine was implied in the hemodynamic responses in vivo [27], and cell growth and morphological changes in vitro [28, 29]. In addition, an increase in the proportion of nitrotyrosine from 2% of net free tyrosine in controls to 6% in the mice carrying mutated SOD occurs at the onset of clinical symptoms [30]. Thus, free nitrotyrosine may play an important role in certain pathogenesis processes.
The human circulating free nitrotyrosine level varies depending on the detecting method. It is reported to be in a range between 7.6±1.4 nM and 21.2±4.6 nM in normal population [31, 32]. Although different detection methods reported different plasma levels of nitrotyrosine in normal individuals [33–35], patients with coronary artery disease showed a 1-fold increase of plasma levels of nitrotyrosine compared with normal controls [36]. The plasma level of nitrotyrosine correlates with the level of glycemia [37] and the presence of peripheral vascular disease [38]. In the current study, we found that nitrotyrosine promotes AoSMC at a concentration range of 30–300 nM (nmol/l), which are clinically relevant.
Migration and proliferation of VSMC are crucial events in the development of restenosis and atherosclerosis. Especially, VSMC migration from the media into the intima contributes to the formation of atherosclerotic plaque [39]. These vascular lesions require the interaction of cell surface integrin receptors with the surrounding extracellular matrix [40]. Integrins are a family of heterodimeric transmembrane glycoproteins consisting of noncovalently associated α and β chains. The integrin complexes αVβ3 and αVβ5 have been shown to be expressed on VSMC and to regulate their migration through interactions with the extracellular matrix [41]. In the current study, we found that free nitrotyrosine not only directly promotes human AoSMC migration but also upregulates the expression of several integrins including αV and β3, as well as PDGFR-B and MMP2. These molecules could be responsible for the nitrotyrosine-induced AoSMC migration.
Other in vitro studies showed that free nitrotyrosine (100–500 μM) induced alterations in cellular morphology and increased permeability in epithelial cell line [42], and led to cell cycle arrest with decreased DNA synthesis in rat aortic smooth muscle cells [43]. In the current study, we tested the effect of nitrotyrosine (0.3–3000 nM) or L-tyrosine (3000 nM) on AoSMC viability and proliferation by MTS assay, and however, we did not detect any changes of cell viability and proliferation in AoSMC compared with non-treated controls (data not shown). These discrepancy results may result from the different concentrations used in our study (3 μM) and in other publications (100–500 μM). Cell types or culture conditions may also differ between our studies and other publications.
Oxidative stress is a state in which excess ROS overwhelms endogenous antioxidant systems. The effects of oxidative stress on the endothelium and VSMC include direct oxidation and nitration of proteins and indirect modulation of kinase pathways, such as PKC, ERK, Src, and Pho [10]. Many factors such as cytokine, drugs, and extracellular inflammation factors could increase ROS generation to regulate cellular reaction such as cell proliferation and migration [11]. In the current study, we demonstrate that nitrotyrosine can increase the generation of ROS in AoSMC after 30 min treatment by two ROS generation assays. More importantly, antioxidants (MnTBAP and SeMet) were shown to be able to block nitrotyrosine-induced ROS generation and inhibit the promoting effect of nitrotyrosine on AoSMC migration. These findings indicate that ROS play an important role in the biological activities of free nitrotyrosine in the vascular cells. Our study also showed that the nitrotyrosine-induced ROS generation might be the result of upregulation of NADPH oxidase subunit p47phox in AoSMC.
MAPKs are activated by growth factors and other signaling molecules, and are critical in signaling pathways mediating cellular proliferation and migration [44]. Iwagaki et al. reported that nitrotyrosine induced significantly greater phosphorylation of ERK1/2 in rat lung lysates and phosphorylated ERK proteins co-immunoprecipitated with nitrotyrosine. It suggests tyrosine nitration might directly activate the ERK signaling pathway [45, 46]. In our current study, we found that nitrotyrosine increased transient phosphorylation of ERK2, but not p38 and JNK. Furthermore, we found ERK2 phosphorylation had a functional significance because the specific ERK1/2 inhibitor PD98059 completely inhibited the promoting effect of nitrotyrosine on AoSMC migration. However, ERK inhibitor did not affect tyrosine-treated control cells because EKR1/2 is not activated. Basic cell migration activity in tyrosine-treated cells may be mediated by other mechanisms, but not ERK1/2 MAPK [47]. These data indicate that the activation of the ERK signaling pathway is essential for nitrotyrosine-induced AoSMC migration. Our data are consistent with the other reports that ERK1/2 is involved in regulating VSMC proliferation and migration [40, 41, 48].
The current study was designed to simulate the clinical situation of increased circulation nitrotyrosine in inflammation conditions regardless of the source of nitrotyrosine. Our experimental data support our hypothesis that free nitrotyrosine may increase SMC migration through oxidative stress, which may contribute to the vascular lesion formation. However, we did not address the specific issue about the potential effect of intrinsic nitrotyrosine generated from SMCs under oxidative stress on the cell migration. In order to directly link increased superoxide anion to SMC migration, several studies have demonstrated that 6-anilino-5,8-quinolinequinone (LY83583), a well known superoxide generator [49, 50], can increase SMC migration [51, 52]. However, intrinsic levels of nitrotyrosine after LY83583 treatment are not clear. Further studies of the relationship between superoxide and intrinsic nitrotyrosine as well as their functional roles in SMC migration are warranted.
In summary, the results of the present study show that free nitrotyrosine exerts direct effects on human AoSMC by promoting cell migration and upregulating PDGFR-B, integrins αV and β3, and MMP2. The action of nitrotyrosine may be mediated by increasing ROS production and activating the ERK1/2 signal pathway. Our findings provide direct evidence that nitrotyrosine is a biologic active molecule, which may contribute to the pathogenesis of cardiovascular disease.
Acknowledgments
This work is partially supported by research grants from the National Institutes of Health (Peter Lin: HL076345; Qizhi Yao: DE15543 and AT003094; and Changyi Chen: HL65916, HL72716, EB-002436, and HL083471) and by the Michael E. DeBakey Department of Surgery, Baylor College of Medicine and Michael E. DeBakey VA Medical Center, Houston, Texas.
References
- 1.Turko IV, Murad F. Protein nitration in cardiovascular diseases. Pharmacol Rev. 2002;54:619–634. doi: 10.1124/pr.54.4.619. [DOI] [PubMed] [Google Scholar]
- 2.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 3.Buttery LD, Springall DR, Chester AH, Evans TJ, Standfield EN, Parums DV, Yacoub MH, Polak JM. Inducible nitric oxide synthase is present within human atherosclerotic lesions and promotes the formation and activity of peroxynitrite. Lab Invest. 1996;75:77–85. [PubMed] [Google Scholar]
- 4.Cromheeke KM, Kockx MM, De Meyer GR, Bosmans JM, Bult H, Beelaerts WJ, Vrints CJ, Herman AG. Inducible nitric oxide synthase colocalizes with signs of lipid oxidation/peroxidation in human atherosclerotic plaques. Cardiovasc Res. 1999;43:744–754. doi: 10.1016/s0008-6363(99)00148-0. [DOI] [PubMed] [Google Scholar]
- 5.Beller CJ, Radovits T, Kosse J, Gerö D, Szabó C, Szabó G. Activation of the peroxynitrite-poly(adenosine diphosphate-ribose) polymerase pathway during neointima proliferation: a new target to prevent restenosis after endarterectomy. J Vasc Surg. 2006;43:824–830. doi: 10.1016/j.jvs.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Shishehbor MH, Brennan ML, Aviles RJ, Fu X, Penn MS, Sprecher DL, Hazen SL. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426–431. doi: 10.1161/01.CIR.0000080895.05158.8B. [DOI] [PubMed] [Google Scholar]
- 7.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 8.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 9.Vigetti D, Moretto P, Viola M, Genasetti A, Rizzi M, Karousou E, Pallotti F, De Luca G, Passi A. Matrix metalloproteinase 2 and tissue inhibitors of metalloproteinases regulate human aortic smooth muscle cell migration during in vitro aging. FASEB J. 2006;20:1118–1130. doi: 10.1096/fj.05-4504com. [DOI] [PubMed] [Google Scholar]
- 10.Parinandi NL, Kleinberg MA, Usatyuk PV, Cummings RJ, Pennathur A, Cardounel AJ, Zweier JL, Garcia JG, Natarajan V. Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L26–L38. doi: 10.1152/ajplung.00123.2002. [DOI] [PubMed] [Google Scholar]
- 11.Madamanchi NR, Moon SK, Hakim ZS, Clark S, Mehrizi A, Patterson C, Runge MS. Differential activation of mitogenic signaling pathways in aortic smooth muscle cells deficient in superoxide dismutase isoforms. Arterioscler Thromb Vasc Biol. 2005;25:950–956. doi: 10.1161/01.ATV.0000161050.77646.68. [DOI] [PubMed] [Google Scholar]
- 12.Wassmann S, Laufs U, Stamenkovic D, Linz W, Stasch JP, Ahlbory K, Rösen R, Böhm M, Nickenig G. Raloxifene improves endothelial dysfunction in hypertension by reduced oxidative stress and enhanced nitric oxide production. Circulation. 2002;105:2083–2091. doi: 10.1161/01.cir.0000014618.91633.67. [DOI] [PubMed] [Google Scholar]
- 13.Jornot L, Junod AF. Differential regulation of glutathione peroxidase by selenomethionine and hyperoxia in endothelial cells. Biochem J. 1995;306:581–587. doi: 10.1042/bj3060581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuzzocrea S, Costantino G, Mazzon E, De Sarro A, Caputi AP. Beneficial effects of Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in zymosan-induced shock. Br J Pharmacol. 1999;128:1241–1251. doi: 10.1038/sj.bjp.0702826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mu H, Ohashi R, Yang H, Wang X, Li M, Lin P, Yao Q, Chen C. Thymosin beta10 inhibits cell migration and capillary-like tube formation of human coronary artery endothelial cells. Cell Motil Cytoskeleton. 2006;63:222–230. doi: 10.1002/cm.20117. [DOI] [PubMed] [Google Scholar]
- 16.Chakrabarti S, Blair P, Freedman JE. CD40-40L signaling in vascular inflammation. J Biol Chem. 2007;22:18307–18317. doi: 10.1074/jbc.M700211200. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchiya M, Suematsu M, Suzuki H. In vivo visualization of oxygen radical-dependent photoemission. Methods Enzymol. 1994;233:128–140. doi: 10.1016/s0076-6879(94)33015-8. [DOI] [PubMed] [Google Scholar]
- 18.Bleeke T, Zhang H, Madamanchi N, Patterson C, Faber JE. Catecholamine-induced vascular wall growth is dependent on generation of reactive oxygen species. Circ Res. 2004;94:37–45. doi: 10.1161/01.RES.0000109412.80157.7D. [DOI] [PubMed] [Google Scholar]
- 19.Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 20.Rothe G, Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescein. J Leukoc Biol. 1990;47:440–448. [PubMed] [Google Scholar]
- 21.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 22.Blanchard-Fillion B, Prou D, Polydoro M, Spielberg D, Tsika E, Wang Z, Hazen SL, Koval M, Przedborski S, Ischiropoulos H. Metabolism of 3-nitrotyrosine induces apoptotic death in dopaminergic cells. J Neurosci. 2006;26:6124–6130. doi: 10.1523/JNEUROSCI.1038-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur H, Halliwell B. Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett. 1994;350:9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- 24.Skinner KA, Crow JP, Skinner HB, Chandler RT, Thompson JA, Parks DA. Free and protein-associated nitrotyrosine formation following rat liver preservation and transplantation. Arch Biochem Biophys. 1997;342:282–288. doi: 10.1006/abbi.1997.0114. [DOI] [PubMed] [Google Scholar]
- 25.Fukuyama N, Takebayashi Y, Hida M, Ishida H, Ichimori K, Nakazawa H. Clinical evidence of peroxynitrite formation in chronic renal failure patients with septic shock. Free Radical Biol Med. 1997;22:771–774. doi: 10.1016/s0891-5849(96)00401-7. [DOI] [PubMed] [Google Scholar]
- 26.Beal MF, Ferrante RJ, Browne SE, Matthews RT, Kowall NW, Brown RH., Jr Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann Neurol. 1997;42:644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- 27.Kooy NW, Lewis SJ. Nitrotyrosine attenuates the hemodynamic effects of adrenoceptor agonists in vivo: relevance to the pathophysiology of peroxynitrite. Eur J Pharmacol. 1996;310:155–161. doi: 10.1016/0014-2999(96)00376-7. [DOI] [PubMed] [Google Scholar]
- 28.Riccardi VM, Maragos VA. The pathophysiology of neurofibromatosis. I. Resistance in vitro to 3-nitrotyrosine as an expression of the mutation. In Vitro. 1980;16:706–714. doi: 10.1007/BF02619200. [DOI] [PubMed] [Google Scholar]
- 29.MacLean SJ, Huber RE. The effects of DL-p-fluorophenylalanine and L-3-nitrotyrosine on the growth and biochemistry of the Taper liver tumor. Cancer Res. 1971;31:1669–1672. [PubMed] [Google Scholar]
- 30.Bruijn LI, Beal MF, Becher MW, Schulz JB, Wong JB, Price DL, Cleveland DW. Elevated free nitrotyrosine levels, but not protein-bound nitrotyrosine or hydroxyl radicals, throughout amyotrophic lateral sclerosis (ALS)-like disease implicate tyrosine nitration as an aberrant in vivo property of one familial ALS-linked superoxide dismutase 1 mutant. Proc Natl Acad Sci USA. 1997;94:7606–7611. doi: 10.1073/pnas.94.14.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang XL, Rainwater DL, Leone A, Mahaney MC. Effects of diabetes on plasma nitrotyrosine levels. Diabet Med. 2004;21:577–580. doi: 10.1111/j.1464-5491.2004.01211.x. [DOI] [PubMed] [Google Scholar]
- 32.Massy ZA, Fumeron C, Borderie D, Tuppin P, Nguyen-Khoa T, Benoit MO, Jacquot C, Buisson C, Drüeke TB, Ekindjian OG, Lacour B, Iliou MC. Increased pasma S-nitrosothiol concentrations predict cardiovascular outcomes among patients with end-stage renal disease: a prospective study. J Am Soc Nephrol. 2004;15:470–476. doi: 10.1097/01.asn.0000106716.22153.bb. [DOI] [PubMed] [Google Scholar]
- 33.Tsikas D, Schwedhelm E, Frolich JC. Methodological considerations on the detection of 3-nitrotyrosine in the cardiovascular system. Circ Res. 2002;90:E70. doi: 10.1161/01.res.0000014802.05780.ae. [DOI] [PubMed] [Google Scholar]
- 34.Tsikas D, Schwedhelm E, Stutzer FK, Gutzki FM, Rode I, Mehls C, Frölich JC. Accurate quantification of basal plasma levels of 3-nitrotyrosine and 3-nitrotyrosinoalbumin by gas chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784:77–90. doi: 10.1016/s1570-0232(02)00751-1. [DOI] [PubMed] [Google Scholar]
- 35.Schwedhelm E, Tsikas D, Gutzki FM, Frolich JC. Gas chromatographic-tandem mass spectrometric quantification of free 3-nitrotyrosine in human plasma at the basal state. Anal Biochem. 1999;276:195–203. doi: 10.1006/abio.1999.4361. [DOI] [PubMed] [Google Scholar]
- 36.Shishehbor MH, Aviles RJ, Brennan ML, Fu X, Goormastic M, Pearce GL, Gokce N, Keaney JF, Jr, Penn MS, Sprecher DL, Vita JA, Hazen SL. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289:1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 37.Ceriello A, Mercuri F, Quagliaro L, Assaloni R, Motz E, Tonutti L, Taboga C. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia. 2001;44:834–838. doi: 10.1007/s001250100529. [DOI] [PubMed] [Google Scholar]
- 38.Da Ros R, Quagliaro L, Gasparini D, Barillari G, Ceriello A. Nitrotyrosine in peripheral vascular disease. J Thromb Haemost. 2003;1:382–383. doi: 10.1046/j.1538-7836.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz SM, deBlois D, O’Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997;100:S87–89. [PubMed] [Google Scholar]
- 41.Kintscher U, Lyon C, Wakino S, Bruemmer D, Feng X, Goetze S, Graf K, Moustakas A, Staels B, Fleck E, Hsueh WA, Law RE. PPARalpha inhibits TGF-beta-induced beta5 integrin transcription in vascular smooth muscle cells by interacting with Smad4. Circ Res. 2002;91:e35–44. doi: 10.1161/01.res.0000046017.96083.34. [DOI] [PubMed] [Google Scholar]
- 42.Eiserich JP, Estévez AG, Bamberg TV, Ye YZ, Chumley PH, Beckman JS, Freeman BA. Microtubule dysfunction by posttranslational nitrotyrosination of alpha-tubulin: a nitric oxide-dependent mechanism of cellular injury. Proc Natl Acad Sci USA. 1999;96:6365–6370. doi: 10.1073/pnas.96.11.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phung AD, Soucek K, Kubala L, Harper RW, Chloë Bulinski J, Eiserich JP. Posttranslational nitrotyrosination of alpha-tubulin induces cell cycle arrest and inhibits proliferation of vascular smooth muscle cells. Eur J Cell Biol. 2006;85:1241–1252. doi: 10.1016/j.ejcb.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 44.Cospedal R, Abedi H, Zachary I. Platelet-derived growth factor-BB (PDGF-BB) regulation of migration and focal adhesion kinase phosphorylation in rabbit aortic vascular smooth muscle cells: roles of phosphatidylinositol 3-kinase and mitogen-activated protein kinases. Cardiovasc Res. 1999;41:708–721. doi: 10.1016/s0008-6363(98)00232-6. [DOI] [PubMed] [Google Scholar]
- 45.Iwagaki A, Choe N, Li Y, Hemenway DR, Kagan E. Asbestos Inhalation Induces Tyrosine Nitration Associated with Extracellular Signal-Regulated Kinase 1/2 Activation in the Rat Lung. Am J Respir Cell Mol Biol. 2003;28:51–60. doi: 10.1165/rcmb.2002-0013OC. [DOI] [PubMed] [Google Scholar]
- 46.Pinzar E, Wang T, Garrido MR, Xu W, Levy P, Bottari SP. Angiotensin II induces tyrosine nitration and activation of ERK1/2 in vascular smooth muscle cells. FEBS Lett. 2005;579:5100–5104. doi: 10.1016/j.febslet.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 47.Day RM, Lee YH, Park AM, Suzuki YJ. Retinoic acid inhibits airway smooth muscle cell migration. Am J Respir Cell Mol Biol. 2006;34:695–703. doi: 10.1165/rcmb.2005-0306OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jang JH, Surh YJ. AP-1 mediates beta-amyloid-induced iNOS expression in PC12 cells via the ERK2 and p38 MAPK signaling pathways. Biochem Biophys Res Commun. 2005;331:1421–1428. doi: 10.1016/j.bbrc.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 49.He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LM, Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 50.Hasegawa T, Bando A, Tsuchiya K, Abe S, Okamoto M, Kirima K, Ueno S, Yoshizumi M, Houchi H, Tamaki T. Enzymatic and nonenzymatic formation of reactive oxygen species from 6-anilino-5, 8-quinolinequinone. Biochem Biophys Acta. 2004;1670:19–27. doi: 10.1016/j.bbagen.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Yang M, Foster E, Kahn AM. Insulin-stimulated NAD(P)H oxidase activity increases migration of cultured vascular smooth muscle cells. Am J Hypertens. 2005;18:1329–1334. doi: 10.1016/j.amjhyper.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Dubey RK, Jackson EK, Lüscher TF. Nitric oxide inhibits angiotensin II-induced migration of rat aortic smooth muscle cell. Role of cyclic-nucleotides and angiotensin1 receptors. J Clin Invest. 1995;96:141–149. doi: 10.1172/JCI118014. [DOI] [PMC free article] [PubMed] [Google Scholar]