Abstract
We tested the hypothesis that 17β-estradiol (E2) has dual effects on the heart, increasing levels of proteins thought to have beneficial cardiovascular effects (e.g. endothelial nitric oxide (NO) synthase (eNOS)) as well as those thought to have detrimental cardiovascular effects (e.g. type 1 angiotensin II (Ang II) receptor (AT1R)). Ovariectomized (OVX) Wistar rats consuming a high sodium diet received one of four treatments (n = 7 per group): group 1; placebo pellets, group 2; E2 (0.5 mg/pellet, 21-day release), group 3; NO synthase inhibitor, Nω-nitro-L-arginine-methyl-ester (L-NAME) (40 mg/kg/day for 14 days) plus Ang II (0.225 mg/kg/day on days 11–14), and group 4; E2 plus L-NAME/Ang II. E2 increased cardiac levels of estrogen receptor (ER)-α, ER-β, a ER-associated membrane protein caveolin-3, eNOS, and phosphorylated (p)eNOS, thus, exerting potentially beneficial cardiovascular effects on NO. However, E2 also increased cardiac levels of proteins associated with cardiovascular injury and inflammation including, AT1R, protein kinase C (PKC)δ, phosphorylated PKC and phosphorylated extracellular signal regulated kinase (pERK)1/2, plasminogen activator inhibitor-1 (PAI-1), osteopontin and ED-1, a monocyte/macrophage-specific protein. E2 treatment led to similar protein changes in hearts of L-NAME/AngII treated rats except that the increase in peNOS was prevented, and L-NAME/AngII and E2 had additive effects in increasing cardiac PKCδ and PAI-1. Thus, the highest levels of cardiac PAI-1 and PKCδ occurred in L-NAME/AngII treated rats receiving E2. In summary, E2 treatment increased cardiac expression of AT1R as well as the expression of pro-inflammatory and pro-thrombotic factors.
Keywords: 17β-estradiol, angiotensin II, Nω-nitro-L-arginine-methyl-ester, angiotensin II type 1 receptor, heart tissue and cardiovascular injury
Background
The incidence of cardiovascular disease among women is low before menopause and steadily increases after the onset of menopause (Mendelsohn ME & Karas RH 1999). This increase is believed to result in part from the loss of endogenous estrogen and its associated cardio-protective effects (Stampfer MJ et al. 1991). In observational human studies, estrogen replacement therapy in postmenopausal women is associated with a reduced risk of cardiovascular disease (Pinto S et al. 1997). However, the Women’s Health Initiative (WHI) Study (Rossouw JE et al. 2002) and the Heart and Estrogen/Progestin Replacement Study (HERS) (Hulley S et al. 1998) do not support the concept that hormone replacement therapy protects the cardiovascular system and, in fact, suggest the opposite view that such therapy may increase the risk of cardiovascular disease. Further, analysis of the WHI data suggests that estrogen plus progesterone therapy was beneficial in healthy, young postmenopausal women, but increased cardiovascular risk when treatment was initiated in older postmenopausal women with established coronary artery disease (Herrington DM et al. 2000; Manson JE et al. 2003). The reasons for the disparate results regarding the cardiovascular effects of estrogen are controversial in part due to an incomplete understanding of the mechanism underlying estrogen’s effects on the cardiovascular system.
Many experimental studies in animals and isolated cells support the belief that estrogen protects the cardiovascular system (Huang A et al. 2000) via activation of estrogen receptors (ERs)-α and -β (Medelsohn ME & Karas RH 1999). Animal studies show beneficial effects of 17β-estradiol (E2) on atherosclerosis (Hayashi T et al. 1992), inflammation (Koh KK 2002) and endothelial or vascular function (Gorodeski GI et al. 1995; Crew JK & Khalil RA 1999). Studies also demonstrate that estrogen modulation of endothelial nitric oxide (NO) synthase (eNOS) may be a mechanism of cardiac protection (Brunner F et al. 2003; Khalil RA 2005).
Other studies suggest that estrogen activates the renin angiotensin-aldosterone system (RAAS), which could be a mechanism of cardiac injury. In humans, estrogen increases circulating levels of Ang II (Schunkert H et al. 1997) and intra-renal Ang II activity (Seely EW et al. 2004), which is associated with a decrease in renal blood flow. In animal models of cardiovascular injury due to an activated RAAS, estrogen increases stroke and renal injury (Stier CT et al. 2003; Oestreicher EM et al. 2006). This increase in renal injury is associated with an increase in renal cortical levels of Ang II type 1 receptor (AT1R) protein and mRNA (Oestreicher EM et al. 2006).
As estrogen stimulates expression of some proteins that might have beneficial cardiovascular effects as well as others that might have detrimental effects, the goal of this study was to determine the balance of E2 effects on cardiac proteins involved in the early steps of cardiac injury. We examined the effects of E2 replacement in ovariectomized rats on cardiac levels of eNOS, AT1R, AT1R signaling pathways and inflammatory and pro-thrombotic proteins. Further, we tested the hypothesis that the adverse cardiac effects of E2 would predominate in a rodent model of cardiovascular injury induced by high Ang II and impaired nitric oxide production (Oestreicher EM et al. 2003; Rocha R et al. 2000; Martinez DV et al. 2002). In this rat model, treatment with Ang II and the nitric oxide synthase inhibitor Nω-nitro-L-arginine-methyl-ester (L-NAME) causes cardiac inflammation and increases in the pro-thrombotic factor PAI-1 (Oestreicher EM et al. 2003).
Materials and Methods
Experimental animals
Experiments used 10 week-old female Wistar rats (Charles River Lab, Wilmington, MA) that underwent bilateral ovariectomies (OVX). Rats had ad libitum access to drinking fluid. They were housed in individual metabolic cages in a climate-controlled environment (22 ± 1°C) with a 12-hour light, 12-hour dark cycle. All rats received 1% NaCl to drink. Rats were sacrificed at the end of the 14-day treatments without respect to timing of the 4-day estrous cycle and hearts were collected and frozen immediately. At this time blood was also collected for determination of E2 and aldosterone levels. All experimental procedures met guidelines of the Institutional Animal Care and Use Committee at Harvard University.
Experimental procedures
We examined the following groups of rats receiving Purina Lab Chow 5001 (Ralston Purina Co., St. Louis MO) and 1% NaCl to drink: 1) OVX rats implanted with pellets containing placebo and minipumps containing saline, n = 7; 2) OVX rats implanted with E2 pellets, n = 7; 3) OVX rats implanted with placebo pellets and receiving L-NAME/Ang II treatment, n = 7; 4) OVX rats implanted with E2 pellets and receiving L-NAME/Ang II treatment, n = 7. Pellets containing 17β-estradiol (#E121, Innovative Research of America, Sarasota, FL, 0.5 mg/pellet, 21-day release) or placebo (#C111, Innovative Research of America) were implanted subcutaneously in each rat 7 to 10 days after ovariectomy. These E2 pellets were designed to achieve plasma estradiol levels in the high-normal physiological range for cycling female rats (100–150 pg/ml). One week after implantation of the pellets, animals were treated with L-NAME/Ang II as previously described (Oestreicher EM et al. 2006). Briefly, rats received drinking water containing 1% NaCl. L-NAME (Sigma, St. Louis, MO, 40 mg/kg per day) was administered for 14 days via a subcutaneously implanted pellet (Innovative Research of America). Saline or Ang II (Sigma, 0.225 mg/kg per day) was administered via Alzet osmotic mini-pumps (Model 2001, Durect Corporation, Cupertino, CA) (1.0 μl/hour, 7 days) for the final 3 days. Pellets and mini-pumps were implanted under general anesthesia using isofluorane. On day 14, death was induced by administration of isofluorane followed by the immediate collection of blood and hearts.
Histological evaluation
Heart tissue for histological evaluation was processed into paraffin blocks. Heart sections (5 μm) were stained with hematoxylin and eosin and examined using light microscopy by a pathologist unaware of the treatment group assignment. The histologic sections of the hearts were scored for myocardial damage on a scale from 0–4 as follows: 0, normal histology; 1, focal interstitial inflammatory infiltrates without myocyte injury; 2, a single focus of interstitial inflammatory infiltrate associated with myocyte injury; 3, two or three foci of interstitial inflammatory infiltrates associated with myocyte injury; 4, four or more foci of inflammatory infiltrates associated with myocyte injury.
Measurements and assays
Daily food intake, water intake, body weight, and urine output were recorded. Systolic blood pressure (SBP) was measured in conscious animals by tail-cuff plethysmography (Blood Pressure Analyzer, Model 179, IITC Life Science, Woodland Hills, CA). Plasma estradiol was measured with the DPC Double Antibody Estradiol (analytical sensitivity of 1.4 pg/mL) as described previously (Oestreicher EM et al. 2006). Aldosterone was measured using the DPC Coat-A-Count Aldosterone radioimmunoassay as described previously (Turchin A et al. 2006) (DPC Diagnostic Products, Los Angeles, CA).
Western blot analysis
Heart tissues were homogenized in 1 ml ice-cold Lysing solution (Bio-Rad Cell Lysis Kit-Catalog #171-304012). The ground tissue was transferred to a clean microcentrifuge tube and frozen at −70°C. Homogenates were then thawed and sonicated on ice (Fisher Sonic Dismembrator, model 300, Fisher Scientific, Pittburgh, PA. Samples were then centrifuged at 6000 rpm for 4 minutes at 4°C. Supernatant was collected without disturbing the pellets. Protein concentration in the supernatant was determined using modified Lowry assay (RC DC Protein Assay, Bio-Rad Catalog #500-0119, BioRad, Hercules, CA). Supernatants (20 μg of protein concentration) were combined at least 1:2 with sample buffer (62.5 mM Tris, pH 6.8, 2% SDS, 25% glycerol, 0.01% bromophenol blue, 200mM β-Mercaptoethanol), heated at 95°C for 4 minutes, and size fractionated by electrophoresis on 12.5% SDS-polyacrylamide gels using 1X of the following 10X buffer: 250 mM Tris base, 1.92 M Glycine, 34.7 mM SDS. Proteins were electrophoretically transferred to Hybond-ECL nitrocellulose membranes (Amersham Bioscience, Piscataway, NJ) using following transfer buffer: 25 mM Tris, 192 mM glycine, 20% v/v methanol, pH 8.3. The membranes were blocked in 5% non-fat dried milk in PBS-T (80 mM Na2HPO4 anhydrous, 20 mM NaH2PO4, 100 mM NaCl, and 0.1 % Tween 20) for 1 hour at room temperature on an orbital shaker. Primary antibody incubation was incubated overnight at 4°C with antibody diluent consist of 1% non-fat dried milk in PBS-T. Equal loading was assessed by reprobing membranes with an antibody to β-actin (1:20,000; Clone AC-15, Sigma). After overnight incubation, the bound antibody was detected by enhanced chemiluminescence (Western Lightning Reagent Plus, Perkin Elmer Life Sciences, Boston, MA) with horseradish peroxidase-conjugated goat anti-rat IgG (sc-2006, Santa Cruz Biotechnology Inc., Santa Cruz, CA, dilution 1:3000) or goat anti-mouse IgG (sc-2005, Santa Cruz, dilution 1:5000) or goat anti-rabbit IgG (sc-2004, Santa Cruz, dilution 1:5000). Developed X-ray films were scanned and densitometric analysis was performed with the ImageQuant 5.2 software (Molecular Dynamics, Piscataway, NJ). To control for inter-gel variations we used the following procedure. On each 15-well mini-gels we analyzed 3 to 4 samples from each of the 4 treatment groups; two samples were used for normalization between mini-gels. All Western blots were re-probed once with anti-β-actin antibody and the protein of interest was normalized to β-actin to correct for loading variability. Samples were reanalyzed on a separate Western blot to confirm results. All values were expressed relative to the average of the OVX rats receiving control treatment.
Antibodies
We used the following antibodies to detect the proteins and receptors of interest by Western Blot: ER-α (#GR17, Calbiochem, San Diego, CA, dilution 1:1000); ER-β (#sc-8974, Santa Cruz Biotechnology Inc., Santa Cruz, CA, dilution 1:500); AT1R (#sc-1173, Santa Cruz, dilution 1:1000). The specificity of the antibody to AT1R was confirmed by receptor binding assays as described previously (Oestreicher EM et al. 2006); Cav3 (#RDI-CAVEOL3abrx, Research Diagnostics, Concord, MA, dilution 1:10000); eNOS (#N30030/L14, BD Transduction Laboratories, San Jose, CA, dilution 1:2500); PKCδ (#610397BD Transduction Laboratories, dilution 1:1000); PAI-1 (#612024, BD Transduction Laboratories, dilution 1:2500); ED-1 (#554954, BD Transduction Laboratories, dilution 1:1000); pPKC (#9371S, Cell Signaling, Danvers, MA, dilution 1:1000); pERK1/2 (#9101S, Cell Signaling, dilution 1:2000); peNOS (9571S, Cell Signaling, dilution 1:1000. The peNOS antibody is directed against phosphorylated serine 1177. This site is specific for eNOS activation.); OPN (#ab8448, ABCAM, Cambridge, MA, dilution 1:5000).
Statistical analysis
The statistical significance of the differences between group means for the data were determined by one-way ANOVA followed by Newman-Keuls post-hoc test for multiple comparisons. P values ≤ 0.05 were considered statistically significant. Values are expressed as mean ± standard error (SE).
Results
L-NAME, Ang II and E2 effects on cardiac histology
Treatment with L-NAME and Ang II caused a significant increase in cardiac damage compared with control treatment (Figures 1 and 2). Damaged hearts showed inflammatory infiltrates associated with myocyte injury (Figures 1C and 1D). E2 treatment had no significant effect on cardiac histology in the control NaCl treated OVX rats or in the L-NAME/AngII treated OVX rats (Figures 1B and 1D and 2).
Figure 1. Pathologic Assessment of Myocardial Injury.
Shown are representative histological sections of the myocardium stained with hematoxylin and eosin at 400X magnification. In the OVX rats treated with placebo (A) or estrogen (B), there is essentially normal histology. In the L-NAME/AngII-treated rats treated either with placebo (C) or estrogen (D) there are inflammatory infiltrates associated with myocyte injury.
Figure 2. Myocardial damage scores in ovariectomized rats receiving placebo (OVX), estrogen (E2), L-NAME/Ang II (LN/AII) and L-NAME/AngII plus E2 (LN/AII/E2).

E2 effects in healthy young ovariectomized rats
Ovariectomized rats receiving 1% NaCl in the drinking water were implanted with subcutaneous pellets containing either placebo or E2. After 14 days, E2 levels were significantly higher in the ovariectomized rodents receiving E2 as compared to those not receiving E2 (Table 1). Consistent with the known effects of E2 in rodents, ovariectomized rats receiving E2 had lower body weights, higher uterine weights, and higher uterine/body weight ratios than ovariectomized rats not receiving E2 (Table 1). Systolic blood pressure and heart weights were similar in the E2 and placebo treated ovariectomized rats (Table 1).
Table 1. Effects of E2 Treatment on Body, Heart Weights, Blood Pressure and E2 and aldosterone levels.
E2 indicated estradiol; ALDO, aldosterone; SBP, systolic blood pressure; UW, uterine weight. All values are mean ± SE. n = 7. The statistical significance of the differences between group means for the data was determined by one-way analysis of variance followed by Newman–Keuls post hoc test for multiple comparisons.
| OVX | OVX/L-NAME/AngII | |||
|---|---|---|---|---|
| Placebo | E2 | Placebo | E2 | |
| E2, pg/mL | 11.2 ± 2.1 | 212.6 ± 130.8* | 10.3 ± 0.9 | 168.0 ± 85.7# |
| ALDO, ng/dL | 15.3 ± 4.8 | 39.0 ± 5.0* | 11.51 ± 5.3 | 33.5 ± 5.7 # |
| SBP, mmHg | 111.0 ± 5.0 | 110 ± 4.0 | 153 ± 7.0** | 158 ± 5.0## |
| BW, g | 324.0 ± 6.6 | 255 ± 11.0* | 308 ± 3.4 | 251 ± 7.9# |
| HW, g | 1.1 ± 0.1 | 1.14 ± 0.03 | 1.14 ± 0.02 | 1.09 ± 0.04 |
| UW, g | 0.10 ± 0.0 1 | 0.70 ± 0.09* | 0.10 ± 0.01 | 0.70 ± 0.09# |
| UW/BW, mg/g | 0.31 ± 0.01 | 2.70 ± 0.3* | 0.33 ± 0.01 | 2.72 ± 0.32# |
P ≤ 0.05 for OVX/Placebo vs. OVX/E2,
P ≤ 0.05 for OVX/L-NAME/Ang II Placebo vs. OVX/L-NAME/Ang II/E2.
P ≤ 0.05 for OVX/Placebo vs. OVX/L-NAME/Ang II/Placebo,
P ≤ 0.05 for OVX/E2 vs. OVX/L-NAME/Ang II/E2.
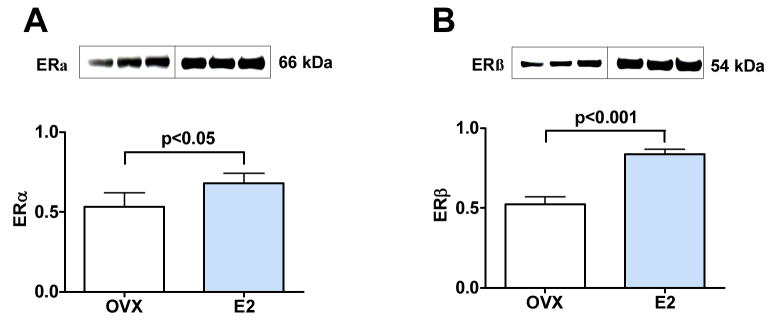
Protein levels of ER-α (Figure 3A) and ER-β (Figure 3B) were increased in heart tissues of ovariectomized rats receiving E2 treatment as compared with ovariectomized rats not treated with E2 (1.3 fold increase for ER-α, p < 0.05 and 1.6 fold increase for ER-β, p < 0.001). Further, E2 treatment increased cardiac levels of eNOS (p < 0.05) and phosphorylated peNOS (p < 0.05), the active form of eNOS, as compared with estrogen deficient ovariectomized rats (Figure 4A and 4B), a result consistent with the known effects of estrogen. E2 treatment also resulted in higher cardiac levels of Cav3 (1.55 fold increase, p < 0.01, Figure 4C), a caveolae protein that is part of the E2 signaling pathway in cardiomyocytes.
Figure 3. E2 treatment increases ER-α and ER-β protein expression in heart tissue of OVX rats.
Western blot of heart tissue showing results for three representative animals (20 ug of total protein per lane, each lane represent an individual animal) from OVX rats receiving placebo (OVX) and OVX E2-treated rats (E2). (A) 66 kDa band for ER-α. (B) 54 kDa band for ER-β.
Figure 4. E2 treatment increases eNOS, peNOS and Cav3 protein expression in heart tissue of OVX rats.
Western blot of heart tissue showing results for three representative animals (20 ug of total protein per lane, each lane represent an individual animal) from OVX rats receiving placebo (OVX) and OVX E2-treated rats (E2). (A) 140 kDa band for eNOS. (B) 140 kDa band for peNOS. (C) 22 kDa band for Cav3.
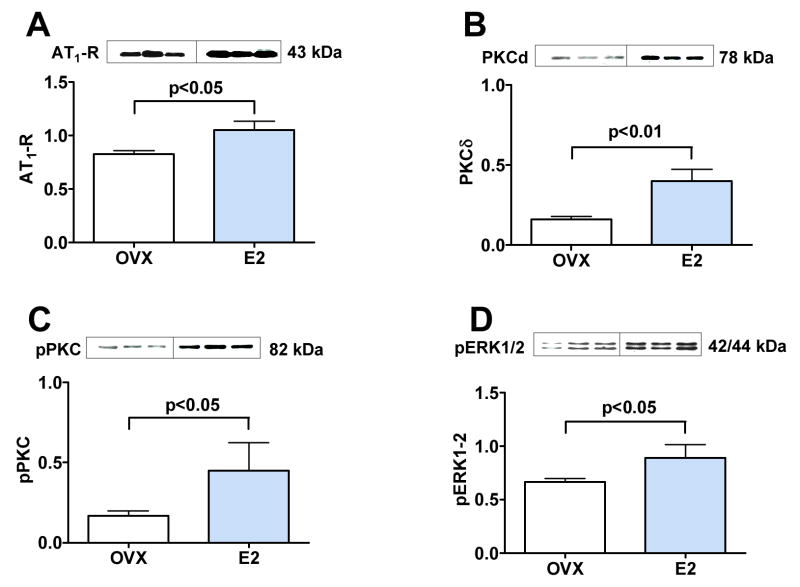
Plasma aldosterone levels were significantly elevated in rats receiving E2 when compared to animals that did not receive E2 (Table 1). Further, E2 treatment increased protein levels of AT1R in heart homogenates when compared with ovariectomized rats not receiving E2 (Figure 5A, p < 0.05).
Figure 5. Effect of estrogen on AT1R and intracellular PKC-ERK signaling pathways.
Western blot of heart tissue showing results for three representative animals (20 ug of total protein per lane, each lane represent an individual animal) from OVX rats receiving placebo (OVX) and OVX E2-treated rats (E2). (A) 43 kDa band for AT1R. (B) 78 kDa band for PKCδ. (C) 82 kDa band for pPKC. (D) 42/44 kDa band for pERK1/2.
Cardiac levels of PKCδ were increased 2.5 fold (Figure 5B, p < 0.01), pPKC were increased 2.2 fold (Figure 5C, p < 0.05) and pERK1/2 were increased 1.3 fold (Figure 5D, p < 0.05) in rats implanted with E2 pellets versus those implanted with placebo pellets.
We determined the effect of E2 on cardiac expression of PAI-1 (an E2-responsive pro-thrombotic factor (Smith LH et al. 2004), the chemokine osteopontin (OPN) and ED-1 (a protein expressed by monocytes/macrophages). The cardiac levels of PAI-1 protein were increased in rats receiving E2 as compared with those not receiving E2 (Figure 6A, p < 0.01). Further, E2 treatment significantly increased cardiac levels of ED-1 (Figure 6B, p < 0.01) and OPN (Figure 6C, p < 0.05). Thus, E2 increases pro-thrombotic and inflammatory factors in cardiac tissue in ovariectomized female rats that were otherwise healthy.
Figure 6. E2 effect on fibrinolytic and inflammatory proteins in heart.
Western blot of heart tissue showing results for three representative animals (20 ug of total protein per lane, each lane represent an individual animal) from OVX rats receiving placebo (OVX) and OVX E2-treated rats (E2). (A) 47 kDa band for PAI-1. (B) 150 kDa band for ED-1. (C) 66 kDa band for OPN.
E2 effects in ovariectomized rats receiving L-NAME/Ang II
OVX rats treated with L-NAME/AngII plus E2 had higher blood levels of E2, decreased body weight, increased uterine weight and increased uterine/body weight ratio compared with OVX rats receiving L-NAME/AngII (Table 1). E2 treatment did not affect heart weight or systolic blood pressure of L-NAME/Ang II treated rats. As occurred in rats drinking 1% NaCl, E2 treatment increased cardiac levels of ER-α and ER-β in rats receiving L-NAME/Ang II, a treatment which itself did not affect estrogen receptor levels (data not shown).
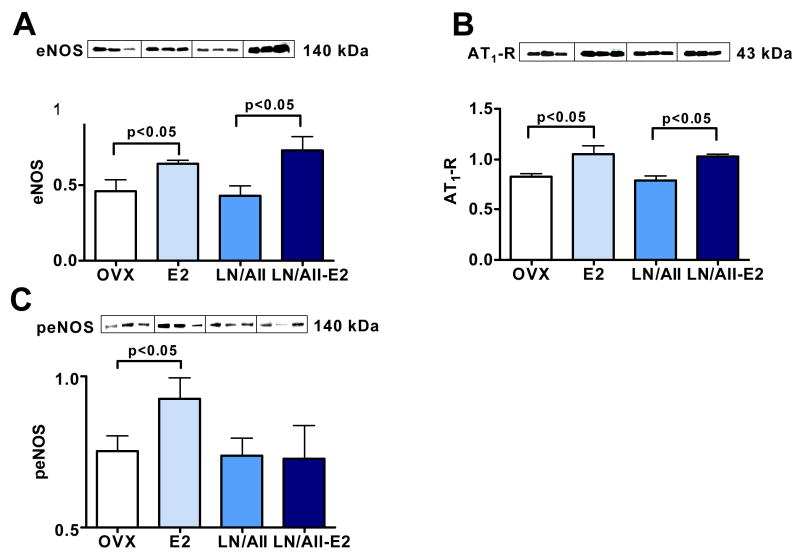
L-NAME/Ang II treatment alone did not alter cardiac levels of eNOS, peNOS or AT1R, nor plasma levels of aldosterone. In contrast, E2 treatment increased protein levels of eNOS (Figure 7A) in hearts from L-NAME/Ang II treated rats. The magnitude of this E2 effect in the L-NAME/Ang II/NaCl treated rats was similar to that observed in ovariectomized rats drinking 1% NaCl. However, E2 treatment did not increase peNOS levels in rats receiving L-NAME/Ang II (Figure 7C). As occurred in ovariectomized rats drinking 1% NaCl, E2 treatment increased plasma aldosterone and cardiac AT1R levels in rats receiving L-NAME/Ang II/NaCl.
Figure 7. Effect of E2 on eNOS, peNOS and AT1R expression in hearts of OVX animals treated with L-NAME/Ang II as compared with E2 effects in absence of L-NAME/Ang II.
Western blot of heart tissue showing results for three representative animals (20 ug of total protein per lane, each lane represent an individual animal) from OVX rats receiving placebo (OVX), OVX E2-treated rats (E2), L-NAME/Ang II and placebo (LN/AII) and L-NAME/Ang II and E2 treatments (LN/AII/E2). (A) 140 kDa band for eNOS. (B) 43 kDa band for AT1R. (C) 140 kDa band for peNOS. For ease of comparison OVX and E2 data from Figure 4A, 4B and 5A are reproduced in Figure 7.
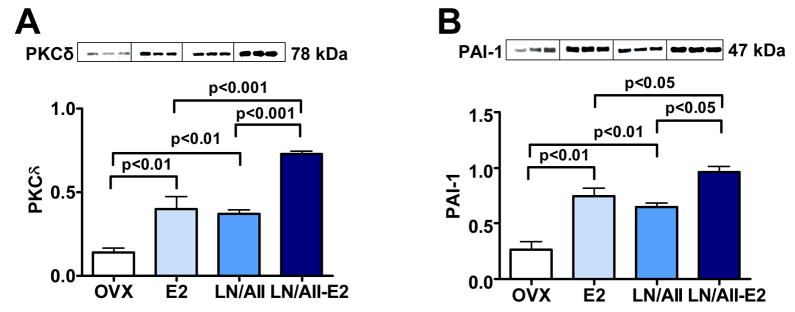
Both E2 and L-NAME/Ang II treatment increased cardiac levels of PKCδ and PAI-1, and these effects were additive (Figure 8A and Figure 8B). Finally, while E2 increased cardiac levels of pPKC, pERK-1/2, ED-1 and OPN in L-NAME/Ang II/NaCl treated animals as occurred in the rats receiving 1% NaCl alone, L-NAME/Ang II treatment did not affect these factors and there was no additive effect of these two treatments (data not shown).
Figure 8. Effect of E2 on PKCδ and PAI-1 expression in hearts of OVX animals treated with L-NAME/Ang II as compared with E2 effects in absence of L-NAME/Ang II.
Western blot of heart tissue showing results for three representative animals (20 ug of total protein per lane, each lane represent an individual animal) from OVX rats receiving placebo (OVX), OVX E2-treated rats (E2), L-NAME/Ang II and placebo (LN/AII) and L-NAME/Ang II and E2 treatments (LN/AII/E2). (A) 78 kDa band for PKCδ. (B) 66 kDa band for PAI-1. For ease of comparison OVX and E2 data from Figures 5B and 6A are reproduced in Figure 8.
Discussion
These studies determined the cardiac effects of E2 treatment in ovariectomized female rats. In ovariectomized, but otherwise healthy female rats, E2 increased cardiac expression of eNOS and peNOS, which would be expected to enhance NO production and, thus, have a beneficial cardiac effect. However, E2 also increased cardiac levels of AT1R and other factors (PAI-1, osteopontin, ED-1 and PKCδ) known to induce inflammation, thrombosis and/or cardiac damage. In the model of cardiovascular injury induced by Ang II and NO synthase inhibition, the E2-mediated increase in peNOS was lost, while E2-mediated increases in cardiac AT1R, PAI-1, osteopontin, ED-1 and PKCδ were maintained. Further, E2 acted additively with L-NAME/Ang II treatment to increase cardiac levels of PAI-1 and PKCδ. Thus, these data demonstrate that E2 increases expression of cardiac proteins which have beneficial cardiac effects (peNOS) as well as cardiac proteins which have detrimental cardiac effects (e.g. AT1R and PAI-1). When the beneficial effects of E2 on peNOS were blocked with an NO synthase inhibitor, the detrimental effects of E2 dominated.
Our results are consistent with the well-known beneficial effect of E2 to increase peNOS leading to increased NO and improved vasodilation (Collins P et al. 1995; Reis SE et al. 1994). We also demonstrated an increase in phosphorylation ERK1/2 with E2 treatment. This latter result is consistent with studies demonstrating activation of the ERK kinase pathway by estrogen in multiple cell types and tissues, including endothelial cells (Gorodeski GI et al. 1995), neuronal cells (Alexaki VI et al. 2006), smooth muscle cells (Keyes LE et al. 1996) and myocardium (Pedram A et al. 2005; Patten RD et al. 2004). These nongenomic effects of estrogen are mediated via its two receptors, ER-α, and ER-β (1). We demonstrated that E2 increased expression of both ER-α, and ER-β in heart tissues, consistent with other reports showing E2 replacement increases expression of ER-α, and ER-β in heart tissues from aged rats (Xu Y et al. 2003). In our study, the increase of ER-β was greater than that of ER-α, possibly due to differential effects of estradiol on synthesis and/or degradation of estrogen receptor subtypes. (Barchiesi F et al. 2004). Estrogen receptors interact with the caveolae anchoring protein Cav3 to mediate the rapid, nongenomic effects of estrogen and other steroids, and increasing Cav3 levels tends to inhibit eNOS activation (Hisamoto K & Bender JR 2005). Estrogen treatment increased Cav3 in our studies, raising the possibility that estrogen-mediated changes in caveolin levels modulate the effects of estrogens and other steroids on intracellular signaling pathways (Feron O & Kelly RA 2001; Williams TM & Lisanti MP 2004; Damy T et al. 2004).
The observation that E2 increases protein levels of AT1R in hearts of healthy, ovariectomized rats and L-NAME/Ang II treated rats is consistent with reports that E2 increases AT1R expression in uteri of healthy rats (Krishamurthi K et al. 1999) and in renal cortex of rats receiving L-NAME/Ang II (Oestreicher EM et al. 2006). In the latter study, the level of AT1R expression correlated with proteinuria (Oestreicher EM et al. 2006). Similarly, estrogen has been shown to increase cardiac and renal injury in other animal models characterized by an activated RAAS. In the stroke prone spontaneously hypertensive rat, ovariectomy reduced stroke and renal injury, while estrogen replacement increased this injury (Stier CT et al. 2003). The hypertensive mRen2.Lewis rat is a transgenic rat strain carrying the mouse ren-2 renin gene back-crossed into the inbred Lewis rat. In the hypertensive mRen2.Lewis female rat, ovariectomy reduced proteinuria, renal injury and blood levels of the inflammatory marker c-reactive protein in older, 64 week old mRen2.Lewis rats on a high salt diet (Yamaleyeva LM et al. 2007). Our observation that E2 and L-NAME/AngII treatment have additive effects on cardiac PAI-1 is consistent with these studies, and together these animal studies demonstrate that E2 promotes AngII-mediated cardiovascular injury. AT1R is expressed in endothelial cells, vascular smooth muscle cells and cardiomyocytes (Bueno OF et al. 2000). Additional studies are needed to determine which cell types within the heart demonstrate altered AT1R expression with estrogen treatment. Our observation that E2 increases AT1R protein provides a potential mechanism for the increase in intrarenal Ang II activity leading to a reduction in renal blood flow in postmenopausal women treated with estrogen (Seely EW et al. 2004).
In contrast to our observations, estrogen replacement was reported to decrease cardiac AT1R, increase cardiac angiotensin II type 2 receptor and improve heart remodeling in one year old ovariectomized rats (Xu Y et al. 2003). Estrogen also decreased AT1R levels in the adrenal and pituitary glands of ovariectomized rats (Wu Z et al. 2003). It is likely that the effects of estrogen on AT1R expression and AngII-mediated injury differ depending on the experimental animal model. Factors such as age, genotype, dietary sodium intake and underlying activity of the RAAS or NO system may modify the effects of estrogen. In our study, L-NAME treatment blocked the beneficial effects of E2 on peNOS levels. Additionally, as E2 increased cardiac levels of the Ang II receptor AT1R, the co-administration of Ang II in our rat model further amplified the adverse cardiac effects of E2 treatment.
In the current study, E2 treatment increased plasma aldosterone levels in ovariectomized rats receiving either placebo or L-NAME/AngII. It is unlikely that E2 increased systemic aldosterone levels through increases in adrenal AT1R as other investigators have shown that E2 decreases AT1R levels in adrenal tissue (Wu Z et al. 2003). However, this increase may result from E2-mediated increases in angiotensinogen leading to increases in Ang II and thus increased adrenal aldosterone production (Klett C et al. 1992; Gallagher PE et al. 1999).
It is now well-established that aldosterone causes cardiovascular injury with activation of the mineralocorticoid receptor causing increases in PAI-1, vascular injury and inflammation, as well as myocardial necrosis, inflammation and fibrosis (Oestreicher EM et al. 2003; Rocha R et al. 2000; Rocha R et al. 2002). Blockade of the mineralocorticoid receptor markedly reduces cardiovascular injury caused by L-NAME/Ang II treatment (Oestreicher EM et al. 2003; Rocha R et al. 2000). Given the effects of E2 on AT1R and aldosterone, it would be of interest to determine if mineralocorticoid receptor blockade prevents the adverse cardiovascular effects of E2. There are some limitations to these studies. Consistent with previous studies (Rocha R et al. 2000) we did not detect a significant effect of Ang II on aldosterone levels in animals receiving L-NAME and a high salt diet. This is likely due in part to our using a low dose of Ang II that is a sub-pressor dose in the absence of L-NAME treatment. However, this Ang II dose suppresses plasma renin activity (Rocha R et al. 2000) and it is possible that increases in aldosterone production would be detected using a more sensitive method such as 24-hour urinary aldosterone levels. Our studies used 0.5 mg, 21-day E2 pellets which are commonly used to assess effects of E2 and are designed to raise E2 levels into the range observed in pro-estrus (50–150 pg/mL) (Klett C et al. 1992). This experimental design did not allow us to determine if there are different E2 dose response characteristics for the beneficial and detrimental cardiac effects of E2. The wide range of E2 values in the E2 treated rats may have introduced variability and the presence of low E2 levels of ~10–12pg/mL in the ovariectomized rats may have limited to detect E2 effects. These levels are consistent with published reports of E2 (11.3 ± 3.6 pg/ml) in ovariectomized rats and are likely attributable to non-ovarian sources of E2 (Hugel S et al. 1999). In addition, our experimental approach did not allow us to determine whether the cardiac effects of E2 are due to direct effects of E2 or are mediated through other factors. For example, E2 treatment increased cardiac AT1R levels and activation of either ERs or AT1R can increase PKC-ERK pathways and PAI-1 (Alexaki VI et al. 2006; Smith LH et al. 2004). In our study we used cardiac PAI-1 levels as a marker of early cardiovascular injury as previously described (Oestreicher EM et al. 2003). While E2 treatment increased PAI-1 and other mediators of cardiovascular injury, the increase in these factors were not associated with a detectable increase in cardiac injury histopathology, possibly due to relative insensitivity of this method and to the relatively short duration of treatment.
These findings indicate that E2 has diverse effects on the heart some of which are beneficial (increases in eNOS and peNOS), and others of which are detrimental (increases in AT1R, PAI-1 and cardiac inflammation). The relative balance of these effects may determine whether the overall effect of E2 is beneficial or detrimental. Further elucidations of the factors that modify this balance are needed. The finding that E2 increases cardiac expression of AT1R has relevance to the mechanisms underlying the adverse cardiac effects of estrogen therapy in postmenopausal women.
Acknowledgments
This work was supported by NIH training grants 5T32HL007609 and DK07529, and NIH research grants HL-63423, HL-069208, HL-67332K24, RR018613, R01 HL67332 and HL-07718.
Footnotes
Publisher's Disclaimer: This is not the definitive version of record of this article. This manuscript has been accepted for publication in The Journal of Endocrinology, but the version presented here has not yet been copy edited, formatted or proofed. Consequently, the Society for Endocrinology accepts no responsibility for any errors or omissions it may contain. The definitive version is now freely available at 10.1677/JOE-08-0199.
References
- Alexaki VI, Charalampopoulos I, Kampa M, Nifli AP, Hatzoglou A, Gravanis A, Castanas E. Activation of membrane estrogen receptors induce pro-survival kinases. J Steroid Biochem Mol Biol. 2006;98:97–110. doi: 10.1016/j.jsbmb.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Barchiesi F, Jackson EK, Imthurn B, Fingerle J, Gillespie DG, Dubey RK. Differential regulation of estrogen receptor subtypes alpha and beta in human aortic smooth muscle cells by oligonucleotides and estradiol. J Clin Endocrinol Metab. 2004;89:2373–81. doi: 10.1210/jc.2003-030821. [DOI] [PubMed] [Google Scholar]
- Brunner F, Maier R, Andrew P, Wolkart G, Zechner R, Mayer B. Attenuation of myocardial ischemia/reperfusion injury in mice with myocyte-specific overexpression of endothelial nitric oxide synthase. Cardiovasc Res. 2003;57:55–62. doi: 10.1016/s0008-6363(02)00649-1. [DOI] [PubMed] [Google Scholar]
- Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. Embo J. 2000;19:6341–50. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P, Rosano GM, Sarrel PM, Ulrich L, Adamopoulos S, Beale CM, McNeill JG, Poole-Wilson PA. 17 beta-Estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease. Circulation. 1995;92:24–30. doi: 10.1161/01.cir.92.1.24. [DOI] [PubMed] [Google Scholar]
- Crews JK, Khalil RA. Gender-specific inhibition of Ca2+ entry mechanisms of arterial vasoconstriction by sex hormones. Clin Exp Pharmacol Physiol. 1999;26:707–15. doi: 10.1046/j.1440-1681.1999.03110.x. [DOI] [PubMed] [Google Scholar]
- Damy T, Ratajczak P, Shah AM, Camors E, Marty I, Hasenfuss G, Marotte F, Samuel JL, Heymes C. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet. 2004;363:1365–7. doi: 10.1016/S0140-6736(04)16048-0. [DOI] [PubMed] [Google Scholar]
- Feron O, Kelly RA. The caveolar paradox: suppressing, inducing, and terminating eNOS signaling. Circ Res. 2001;88:129–31. doi: 10.1161/01.res.88.2.129. [DOI] [PubMed] [Google Scholar]
- Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertension. 1999;33:323–8. doi: 10.1161/01.hyp.33.1.323. [DOI] [PubMed] [Google Scholar]
- Gorodeski GI, Yang T, Levy MN, Goldfarb J, Utian WH. Effects of estrogen in vivo on coronary vascular resistance in perfused rabbit hearts. Am J Physiol. 1995;269:R1333–8. doi: 10.1152/ajpregu.1995.269.6.R1333. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Fukuto JM, Ignarro LJ, Chaudhuri G. Basal release of nitric oxide from aortic rings is greater in female rabbits than in male rabbits: implications for atherosclerosis. Proc Natl Acad Sci U S A. 1992;89:11259–63. doi: 10.1073/pnas.89.23.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington DM, Reboussin DM, Brosnihan KB, Sharp PC, Shumaker SA, Snyder TE, Hugel S, Reincke M, Stromer H, Winning J, Horn M, Dienesch C, Mora P, Schmidt HH, Allolio B, Neubauer S. Evidence against a role of physiological concentrations of estrogen in post-myocardial infarction remodeling. J Am Coll Cardio. 1999;34:1427–34. doi: 10.1016/s0735-1097(99)00368-x. [DOI] [PubMed] [Google Scholar]
- Furberg CD, Kowalchuk GJ, Stuckey TD, Rogers WJ, Givens DH, Waters D. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522–9. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- Hisamoto K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2005;70:382–7. doi: 10.1016/j.steroids.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Huang A, Sun D, Koller A, Kaley G. 17beta-estradiol restores endothelial nitric oxide release to shear stress in arterioles of male hypertensive rats. Circulation. 2000;101:94–100. doi: 10.1161/01.cir.101.1.94. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. Jama. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Keyes LE, Moore LG, Walchak SJ, Dempsey EC. Pregnancy-stimulated growth of vascular smooth muscle cells: importance of protein kinase C-dependent synergy between estrogen and platelet-derived growth factor. J Cell Physiol. 1996;166:22–32. doi: 10.1002/(SICI)1097-4652(199601)166:1<22::AID-JCP3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Klett C, Ganten D, Hellmann W, Kaling M, Ryffel GU, Weimar-Ehl T, Hackenthal E. Regulation of hepatic angiotensinogen synthesis and secretion by steroid hormones. Endocrinology. 1992;130:3660–8. doi: 10.1210/endo.130.6.1597163. [DOI] [PubMed] [Google Scholar]
- Koh KK. Effects of estrogen on the vascular wall: vasomotor function and inflammation. Cardiovasc Res. 2002;55:714–26. doi: 10.1016/s0008-6363(02)00487-x. [DOI] [PubMed] [Google Scholar]
- Krishnamurthi K, Verbalis JG, Zheng W, Wu Z, Clerch LB, Sandberg K. Estrogen regulates angiotensin AT1 receptor expression via cytosolic proteins that bind to the 5′ leader sequence of the receptor mRNA. Endocrinology. 1999;140:5435–8. doi: 10.1210/endo.140.11.7242. [DOI] [PubMed] [Google Scholar]
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M Women’s Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- Martinez DV, Rocha R, Matsumura M, Oestreicher E, Ochoa-Maya M, Roubsanthisuk W, Williams GH, Adler GK. Cardiac damage prevention by eplerenone: comparison with low sodium diet or potassium loading. Hypertension. 2002;39:614–8. [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–11. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Oestreicher EM, Martinez-Vasquez D, Stone JR, Jonasson L, Roubsanthisuk W, Mukasa K, Adler GK. Aldosterone and not plasminogen activator inhibitor-1 is a critical mediator of early angiotensin II/NG-nitro-L-arginine methyl ester-induced myocardial injury. Circulation. 2003;108:2517–23. doi: 10.1161/01.CIR.0000097000.51723.6F. [DOI] [PubMed] [Google Scholar]
- Oestreicher EM, Guo C, Seely EW, Kikuchi T, Martinez-Vasquez D, Jonasson L, Yao T, Burr D, Mayoral S, Roubsanthisuk W, Ricchiuti V, Adler GK. Estradiol increases proteinuria and angiotensin II type 1 receptor in kidneys of rats receiving L-NAME and angiotensin II. Kidney Int. 2006;70:1759–68. doi: 10.1038/sj.ki.5001897. [DOI] [PubMed] [Google Scholar]
- Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, Force T, Mendelsohn ME, Karas RH. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95:692–9. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Aitkenhead M, Levin ER. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem. 2005;280:26339–48. doi: 10.1074/jbc.M414409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, Virdis A, Ghiadoni L, Bernini G, Lombardo M, Petraglia F, Genazzani AR, Taddei S, Salvetti A. Endogenous estrogen and acetylcholine-induced vasodilation in normotensive women. Hypertension. 1997;29:268–73. doi: 10.1161/01.hyp.29.1.268. [DOI] [PubMed] [Google Scholar]
- Reis SE, Gloth ST, Blumenthal RS, Resar JR, Zacur HA, Gerstenblith G, Brinker JA. Ethinyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in postmenopausal women. Circulation. 1994;89:52–60. doi: 10.1161/01.cir.89.1.52. [DOI] [PubMed] [Google Scholar]
- Rocha R, Martin-Berger CL, Yang P, Scherrer R, Delyani J, McMahon E. Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology. 2002a;143(12):4828–4836. doi: 10.1210/en.2002-220120. [DOI] [PubMed] [Google Scholar]
- Rocha R, Stier CT, Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871–8. doi: 10.1210/endo.141.10.7711. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Schunkert H, Danser AH, Hense HW, Derkx FH, Kurzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation. 1997;95:39–45. doi: 10.1161/01.cir.95.1.39. [DOI] [PubMed] [Google Scholar]
- Seely EW, Brosnihan KB, Jeunemaitre X, Okamura K, Williams GH, Hollenberg NK, Herrington DM. Effects of conjugated oestrogen and droloxifene on the renin-angiotensin system, blood pressure and renal blood flow in postmenopausal women. Clin Endocrinol (Oxf) 2004;60:315–21. doi: 10.1046/j.1365-2265.2004.01980.x. [DOI] [PubMed] [Google Scholar]
- Smith LH, Coats SR, Qin H, Petrie MS, Covington JW, Su M, Eren M, Vaughan DE. Differential and opposing regulation of PAI-1 promoter activity by estrogen receptor alpha and estrogen receptor beta in endothelial cells. Circ Res. 2004;95:269–75. doi: 10.1161/01.RES.0000136521.70093.f1. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325:756–62. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- Stier CT, Jr, Chander PN, Rosenfeld L, Powers CA. Estrogen promotes microvascular pathology in female stroke-prone spontaneously hypertensive rats. Am J Physiol Endocrinol Metab. 2003;285:E232–9. doi: 10.1152/ajpendo.00029.2003. [DOI] [PubMed] [Google Scholar]
- Turchin A, Guo CZ, Adler GK, Ricchiuti V, Kohane IS, Williams GH. Effect of acute aldosterone administration on gene expression profile in the heart. Endocrinology. 2006;147:3183–9. doi: 10.1210/en.2005-1674. [DOI] [PubMed] [Google Scholar]
- Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med. 2004;36:584–95. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- Wu Z, Zheng W, Sandberg K. Estrogen regulates adrenal angiotensin type 1 receptors by modulating adrenal angiotensin levels. Endocrinology. 2003;144:1350–6. doi: 10.1210/en.2002-221100. [DOI] [PubMed] [Google Scholar]
- Xu Y, Arenas IA, Armstrong SJ, Davidge ST. Estrogen modulation of left ventricular remodeling in the aged heart. Cardiovasc Res. 2003;57:388–94. doi: 10.1016/s0008-6363(02)00705-8. [DOI] [PubMed] [Google Scholar]
- Yamaleyeva LM, Pendergrass KD, Pirro NT, Gallagher PE, Groban L, Chappell MC. Ovariectomy is protective against renal injury in the high-salt-fed older mRen2.Lewis rat. Am J Physiol Heart Circ Physiol. 2007;293:H2064–71. doi: 10.1152/ajpheart.00427.2007. [DOI] [PubMed] [Google Scholar]