Abstract
Individuals with schizophrenia have relative deficits in cognition, although little is known regarding the course of such deficits across the lifespan and at various stages of the illness. Furthermore, the relationship between psychosis and cognition has not been adequately explored to this point. Prospective, longitudinal, multi-assessment studies of the same patients across time are rare in the field and provide a unique opportunity to examine long-term changes in cognition among individuals with schizophrenia. As part of The Chicago Follow-up Study, we prospectively assessed 244 psychiatric inpatients, including individuals with schizophrenia, other psychotic disorders, and non-psychotic depression. Assessments were conducted seven times (once at index hospitalization, and then 6 times subsequently over the next 20 years) to provide longitudinal data about cognition and symptoms, with a focus on two aspects of cognition: processing speed and the ability to access general knowledge. The Digit Symbol-Coding and Information subtests from the Wechsler Adult Intelligence scale were used to measure the two cognitive domains at each assessment. At all 7 assessments, individuals with schizophrenia performed more poorly than the other diagnostic groups on the two cognitive measures. However, following the acute phase (index hospitalization), individuals with schizophrenia demonstrated significant improvements in cognition and did not show evidence of cognitive decline over the remaining six assessments spanning 20 years. Our data support the presence of relative cognitive impairment in schizophrenia, as well as a pattern of stability in some cognitive areas following the acute phase. Additionally, we find evidence for an association between relative cognitive impairment and psychosis.
The current research prospectively investigated the longitudinal course of two neurocognitive measures in individuals with schizophrenia and other mental disorders over a 20-year follow-up period. Cognitive deficits are now acknowledged to be one of the central aspects of the schizophrenia syndrome [1], and there is evidence of cognitive impairments among individuals with schizophrenia in a variety of domains [2–4]. Furthermore, cognitive deficits in individuals with schizophrenia have been identified prodromally during childhood, during the first episode of psychosis [5], and many years after the initial onset of illness [6]. However, despite the strides that have been made in characterizing the cognitive profile of schizophrenia at different stages of the illness, most studies have been cross-sectional or have used relatively short follow-up intervals. Thus, the longitudinal course and trajectory of cognitive function in schizophrenia is not as well described.
Existing research is mixed regarding the temporal stability of cognitive deficits in schizophrenia. Several studies in this area support the hypothesis that cognition in schizophrenia remains stable over time and does not decline more rapidly than in healthy controls [7–9]. For example, a longitudinal study by Russell and colleagues [9] found no differences between IQ scores of individuals with schizophrenia during childhood and at follow-up 19 year later. These findings suggest that global intellectual functioning in schizophrenia does not deteriorate at a greater rate than would be expected in healthy aging.
In contrast, other research has documented significant declines from premorbid intellectual capacity among individuals with schizophrenia [10–12]. Sheitman and colleagues [12] reported significantly lower current IQ scores (mean = 83) among a sample of schizophrenia participants relative to pre-morbid IQ scores (mean = 93). Harvey and co-workers [13] also found significant declines in cognitive functioning in schizophrenia patients over a period of two and a half years. However, that sample comprised an important but relatively rare group of geriatric chronic schizophrenia patients with many years of continuous hospitalization. Thus, the stability of neurocognitive functioning in young individuals with acute schizophrenia over time remains an important issue yet to be assessed prospectively over a long follow-up period.
Additionally, the longitudinal relationship between psychosis and cognition in schizophrenia has not been fully characterized. Untreated psychosis has been hypothesized to influence cognition negatively [14–15], and psychotic disorders (e.g., schizophrenia) have been associated with more prominent neuropsychological impairment than non-psychotic disorders [16]. Thus, the available literature may implicate psychosis as a factor contributing to cognitive deficits in schizophrenia. However, the nature of this relationship in individuals with schizophrenia across time at various stages of the illness has not been explored.
More recently, attention has been paid to processing speed as it relates to schizophrenia. For example, recent investigations suggest that deficits in processing speed may be somewhat specific to schizophrenia and more severe than deficits in other cognitive domains [17]. A recent meta-analysis [18] reported that the effect size for deficits in processing speed (as measured by the Digit Symbol-Coding task) in individuals with schizophrenia (effect size = −1.57) exceeded those typically found on standard measures of episodic memory (effect size = −1.25) and executive function (effect size = −1.00). The authors suggested that this result emphasizes the centrality of processing speed deficits in the schizophrenia syndrome [18]. To our knowledge, however, no studies have prospectively investigated processing speed longitudinally over a long follow-up period.
With these issues in mind, the current research was designed to address the following questions: 1) Do individuals with schizophrenia perform more poorly than individuals with other psychotic disorders or non-psychotic depression on two key measures of cognitive functioning at the acute phase of illness? 2) Does cognition improve or decline as patients emerge from the acute phase for all groups on both cognitive measures? 3) Does cognition decline among individuals with schizophrenia over time as they age? 4) Is psychosis associated with greater cognitive decline?
Methods
Patient Characteristics
The data from the present study are derived from the Chicago Follow-Up Study, a prospective longitudinal research program studying major dimensions of psychopathology, including symptoms, longitudinal course of illness, and global outcome [19–24]. This study was approved by the Human Subjects Review Committee, and subjects participated with informed, voluntary, written consent. The sample, diagnosed according to the Research Diagnostic Criteria (RDC) [25], included 244 patients: 84 patients with schizophrenia (52 male, 32 female), 63 patients with other types of psychotic disorders (30 male, 33 female), and 97 patients who at the acute phase of hospitalization had non-psychotic depression (36 male, 61 female). Patients with other psychotic disorders included 15 with psychotic depression, 35 with psychotic bipolar disorders, and 13 with other types of psychotic disorders, including unspecified functional psychosis and psychosis related to drug abuse.
Research diagnoses were based on structured diagnostic research interviews conducted at index hospitalization, with these interviews including the Schedule for Affective Disorders and Schizophrenia (SADS) [26] and/or the Schizophrenia State Inventory [27], plus admission clinical diagnostic interviews and detailed inpatient observations. Inter-rater reliability for the diagnosis of schizophrenia, using kappa, was K=.88. Subjects were assessed prospectively at index hospitalization and at follow-ups six times over the next 20 years post index hospitalization (at 2 years, 4.5 years, 7.5 years, 10 years, 15 years, and 20 years).
The groups were matched for age at index hospitalization. Mean ages for the schizophrenia, other psychotic disorders, and non-psychotic depression groups were 22.8, 23.1, and 23.2 years, respectively (F < 1, p = 0.76). The non-psychotic depression group had significantly more years of education (mean = 13.8 years) than their counterparts with schizophrenia (mean = 12.6 years). However, the schizophrenia participants did not differ from the other psychotic disorders group (mean = 13.3 years) in years of education (F = 5.82, p < .005). Forty-eight percent of the patients were male, with a higher percentage of male schizophrenia patients (62%) and a higher percentage of female patients with non-psychotic depression (63%). This type of sex ratio difference is typical for young patients with schizophrenia and depression [28].
Post-hospital assessments at the 20-year follow-up were completed for 75% of the sample. One hundred thirteen patients (46%) were studied at all 6 follow-ups over 20 years. An additional 87 patients were studied at 5 of the 6 follow-ups, 49 of whom (56%) were studied at the 20-year follow-up. Overall, 200 patients (92% of the current sample) were studied at 5 or 6 of the 6 follow-ups. The 84 patients with schizophrenia in the current research were compared with a subsample of schizophrenia patients who were assessed at index hospitalization and at the first follow-up but who were not reassessed at the 20-year follow-up. These 2 groups did not differ significantly on major demographic variables or variable related to pathology at the 2-year follow-up.
At the 20-year follow-up, 55% of schizophrenia patients were being treated with anti-psychotic medications. A significantly smaller percentage of the patients with other psychotic disorders (18%) were also being treated with anti-psychotics (x2 = 18.58, p < .001). An additional 10% of the schizophrenia patients and 37% of the patients with other psychotic disorders who were not being treated with anti-psychotics were being treated with other medications. Our patient data are consistent with studies, including work by M. Bleuler [29] and by the World Health Organization [30], reporting that a moderate percentage of individuals with chronic schizophrenia are not taking anti-psychotic medications a number of years into their illness.
To reduce the potential effects of long-term treatment, this sample was relatively young when first assessed: the mean age of all patients at index was 23.1 years (SD = 3.9). Patients entered the study as a young, non-chronic group: 53% of the patients were experiencing their first hospitalization at index. Seventy-six percent of the sample had either one or no previous hospitalizations. At the 20-year follow-up, 21% of the schizophrenia patients were 39 years old or younger, 42% were 40–44, and 36% were 45–54.
Measures
Processing Speed
We were interested in measuring processing speed, which has been suggested to represent one of the most significant cognitive impairments in schizophrenia [18]. Processing speed was measured at index hospitalization and at each of the six follow-up evaluations using the Digit Symbol-Coding subtest of the Wechsler Adult Intelligence Scale (WAIS) [31]. The total number of correct items was the index of performance used in subsequent analyses.
General Knowledge
Since we hypothesized that processing speed would be sensitive to longitudinal changes in cognition and mental status, we also analyzed an additional cognitive domain which was thought to be more stable over time in order to quantify and compare potential changes across time. Therefore, we also measured patients’ ability to access general knowledge, which was assessed using the Information subtest from the WAIS. Similar to the processing speed measure, this subtest was administered at index hospitalization and at each of the six subsequent follow-up evaluations. The total number of correct items on the Information subtest was the index of performance.
Results
Longitudinal Measures of Cognition
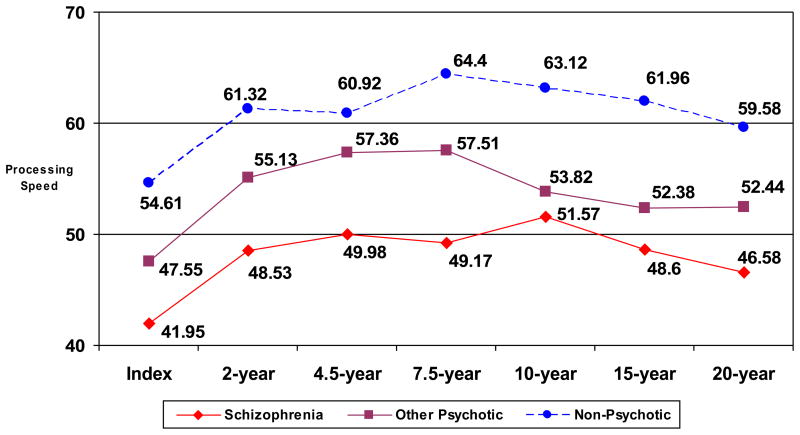
A one-way analysis of variance (ANOVA) was conducted to determine whether the diagnostic groups showed significant differences in processing speed at the acute phase of hospitalization. A priori findings suggest that the groups differed significantly in processing speed performance at Index Hospitalization [F (2,156) = 12.29, p < .001). A Newman-Keuls post-hoc analysis revealed that individuals with schizophrenia (mean DS = 41.9) had the slowest processing speed and were most impaired, while individuals with non-psychotic depression (mean DS = 54.6) had the fastest processing speed and individuals with other psychotic disorders (mean DS = 47.6) had processing speed that was intermediate between the other two groups. Each group differed significantly from the others (non-psychotic depression > other psychotic > schizophrenia) at Index Hospitalization.
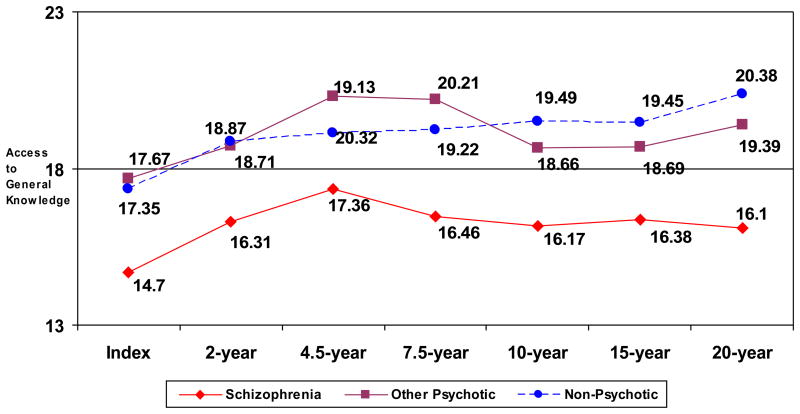
We also conducted a one-way ANOVA of performance on our measure of access to general knowledge to examine differences between diagnostic groups at Index Hospitalization. Results of the ANOVA revealed significant between group differences on this measure [F(2, 170) = 5.23, p < .01]. Post-hoc analysis revealed that individuals with schizophrenia (mean = 14.7) had the lowest scores on the general knowledge measure and were significantly lower than the non-psychotic depression (mean = 17.4, p < .01) and other psychotic disorders groups (mean = 17.7, p < .01) on this measure at Index Hospitalization. The other psychotic disorders and non-psychotic depression groups did not differ significantly from each other.
Next, differences in cognition over time as a function of diagnosis were assessed using a 3 (schizophrenia, other psychotic disorders, non-psychotic depression) × 6 (6 follow-up assessments) mixed design repeated measures ANOVA. Performance on the processing speed measure for each diagnostic group at each time point is shown in Figure 1. The diagnostic groups differed significantly in processing speed performance [F (2,35) = 4.09, p < .05]. In contrast to diagnostic differences, the effect of time of assessment after the acute phase was not significant [F (5,175) = .63, p > .67], suggesting that after improvement from the acute phase, none of the three diagnostic groups improved or declined significantly over time. Post-hoc analyses of potential diagnostic differences revealed that the non-psychotic depression group always had the fastest processing speed, followed by the other psychotic disorders group, with the schizophrenia group slowest at each follow-up (see Table 1). Additionally, while schizophrenia patients were significantly slower than patients with other psychotic disorders at the first three follow-ups, at the later follow-ups (10 years, 15 years, and 20 years post-hospitalization), the other psychotic disorders and schizophrenia groups did not differ significantly from each other. They were both significantly slower than the non-psychotic depression group at all three of the later follow-ups.
Figure 1.
Processing speed at Index Hospitalization and six follow-up evaluations in each diagnostic group
Table 1.
Differences in processing speed between diagnostic groups at each time point
| F | p-value | Direction | |
|---|---|---|---|
| 2-Year Follow-Up | 13.22 | < .001 | DEP > OPD > SCZ |
| 4.5-Year Follow-Up | 10.22 | < .001 | DEP = OPD > SCZ |
| 7.5-Year Follow-Up | 17.27 | < .001 | DEP > OPD > SCZ |
| 10-Year Follow-Up | 8.07 | < .001 | DEP > OPD = SCZ |
| 15-Year Follow-Up | 8.86 | < .001 | DEP > OPD = SCZ |
| 20-Year Follow-Up | 7.40 | < .002 | DEP > OPD = SCZ |
SCZ = Schizophrenia; OPD = Other Psychotic Disorders; DEP = Non-Psychotic Depression
Note: A = symbol in the Direction column indicates that groups do not differ significantly
We also examined the data measuring access to general knowledge at all follow-ups following index hospitalization in the three diagnostic groups. As shown in Figure 2, schizophrenia participants performed significantly more poorly than participants with non-psychotic depression on this measure at 5 of the 6 follow-ups (all p’s < .02) and more poorly than participants with other psychotic disorders at 4 of the 6 follow-ups (all p’s < .05). Participants with other psychotic disorders and non-psychotic depression did not differ from each other at any of the follow-ups.
Figure 2.
Performance on measure of accessing general knowledge at Index Hospitalization and six follow-up evaluations in each diagnostic group
Cognitive Changes Following the Acute Phase
To examine differences in cognition between index hospitalization and follow-up 2 years later, we conducted paired-samples t-tests within each diagnostic group comparing processing speed and access to general knowledge at the acute phase and at the 2-year follow-up. Data are presented in Table 2. Results of the analysis for processing speed indicated that schizophrenia participants improved significantly between the acute phase and 2-year follow-up (p < .001). Participants with other psychotic disorders and participants with non-psychotic depression also improved significantly from the acute phase to 2-year follow-up (p’s < .001). As shown in Figure 3, the majority of patients in each group showed some improvement in processing speed between Index Hospitalization and the 2-year follow-up. Regarding the data for access to general knowledge, schizophrenia participants again demonstrated significant improvements (p < .03), but neither of the other two groups showed significant change between the acute phase and 2-year follow-up (other psychotic disorders: p > .95; non-psychotic depression: p = .07).
Table 2.
Comparison of processing speed performance and access to general knowledge at Index Hospitalization and 2-Year Follow-Up within each diagnostic group
| Processing Speed | Initial Hospitalization | 2-Year Follow-Up | t | p-value |
|---|---|---|---|---|
| Schizophrenia | 40.7 (13.1) | 48.5 (13.7) | 4.03 | < .001 |
| Other Psychotic | 47.7 (13.9) | 56.1 (14.1) | 4.41 | < .001 |
| Non-Psych Dep | 56.0 (13.6) | 62.2 (15.4) | 3.94 | < .001 |
| Access to General Knowledge | Initial Hospitalization | 2-Year Follow-Up | t | p-value |
| Schizophrenia | 15.1 (6.3) | 16.1 (6.0) | 2.42 | < .05 |
| Other Psychotic | 17.6 (5.4) | 17.6 (5.9) | .05 | NS |
| Non-Psych Dep | 17.8 (5.0) | 18.4 (5.1) | 1.82 | NS |
Figure 3.
Percentage of patients from each diagnostic group showing improvement in processing speed from Index Hospitalization to 2-year follow-up.
Cognitive Decline Across Time
To examine longitudinal changes on measures of neurocognition, we conducted repeated measures ANOVAs within each diagnostic group (schizophrenia, other psychotic disorders, non-psychotic depression) assessing processing speed and access to general knowledge at each of the successive follow-up evaluations. Schizophrenia participants did not demonstrate significant changes in processing speed across the six follow-ups [F (5,50) = 0.76, p > .58], suggesting that cognition is somewhat stable in schizophrenia over time after the acute phase. Processing speed in participants with other psychotic disorders [F (5,45) = 0.90, p > .48] and participants with non-psychotic depression [F (5,80) = .73, p > .60) also did not change significantly across the six follow-ups following the acute phase.
Association between Psychosis and Cognition
We next examined the association between psychosis and measures of cognitive functioning among individuals with schizophrenia and other psychotic disorders. To do this, we compared individuals who were psychotic to individuals who were non-psychotic at the time of each follow-up evaluation. Schizophrenia patients who were psychotic had significantly slower processing speed than those who were non-psychotic at 3 of the 6 evaluations (i.e., the 7.5-year, 10-year, and 20-year follow-ups), whereas significant differences in retrieval of general knowledge (Non-Psychotic > Psychotic) were observed at 1 time point only (i.e., the 15-year follow-up). When similar analyses were performed for the other psychotic disorders group, we found significant differences in processing speed (Non-Psychotic > Psychotic) at 2 follow-up evaluations (i.e., the 10- and 20-year follow-ups), while no differences between psychotic and non-psychotic individuals were observed for retrieval of general knowledge at any of the 6 follow-up evaluations.
Discussion
The field currently has little information on the long-term course of neurocognitive functioning among individuals with schizophrenia and other disorders. To address this need, the present study was undertaken to investigate the longitudinal course of two measures of neurocognition over a 20-year period following index hospitalization in individuals with schizophrenia, other psychotic disorders, and non-psychotic depression. Data collections such as the current one, involving multiple assessments of patients beginning early in the illness and studied prospectively over a 20-year period, have not previously been available to the field. Although other investigators have made important contributions in this area [32–33], there have been very few prospective studies of cognitive stability in schizophrenia with follow-up intervals greater than 10 years. Our results provide a unique opportunity to examine long-term changes in cognition among individuals with schizophrenia as they age. Below, we discuss our findings for each research question in further detail.
Do individuals with schizophrenia perform more poorly than other groups on measures of cognition?
We measured processing speed and access to general knowledge in each group at index hospitalization and at six subsequent follow-up evaluations over 20 years. At every time point, schizophrenia participants had slower processing speed than the other diagnostic groups, although differences did not always reach statistical significance. At certain time points, processing speed in the schizophrenia group was significantly slower than in both of the other groups, whereas at other time points the schizophrenia group only differed significantly from the non-psychotic depression group. In general, the most dramatic differences were observed at the earlier follow-up evaluations within the first 7.5 years, whereas after this point differences between schizophrenia participants and the other groups were somewhat diminished. Regardless, individuals with schizophrenia showed significantly slower processing speed than one or both of the two psychiatric comparison groups at all evaluations. Our data in this regard support mounting evidence of relative processing speed deficits in schizophrenia [17,18,34]. However, such deficits do not appear to be unique to schizophrenia, as individuals with other psychotic disorders also show similar impairments when they are compared to individuals with non-psychotic disorders.
Similar to the processing speed findings, schizophrenia participants showed significantly poorer performance than one or both of the other groups on the retrieval of general knowledge measure at every follow-up evaluation. Once again, performance deficits in the schizophrenia group were most pronounced at earlier follow-ups, whereas impairments among schizophrenia participants relative to other diagnostic groups were somewhat diminished at later follow-ups.
Does cognition in schizophrenia significantly improve (or decline) following the acute phase?
Some controversy exists in the literature as to the course of cognition in schizophrenia as patients emerge from the acute phase of illness [7–12]. We observed significant improvements in processing speed among schizophrenia participants (as well as the other diagnostic groups) between index hospitalization and the first follow-up evaluation two years later. Indeed, the two-year improvement in processing speed among schizophrenia participants from acute phase to the 2-year follow-up represented the largest performance gain between any two evaluations for all diagnostic groups. Additionally, we found significant improvement in retrieval of general knowledge between index hospitalization and the 2-year follow-up in individuals with schizophrenia, whereas the other two diagnostic groups did not demonstrate such dramatic improvements over this time period.
Regarding the long-term trajectory of cognition in schizophrenia, two models have been proposed. The neurodegenerative model posits a pattern of progressive cognitive decline in schizophrenia following the first psychotic episode [35]. In contrast, the neurodevelopmental model supports a notion of mild to moderate developmental impairment in individuals with schizophrenia at the time of the first psychotic episode, after which cognition is relatively static [36–37]. Still other researchers endorse a model that hypothesizes the presence of both degenerative and neurodevelopmental processes [38]. Overall, there is general agreement in the literature for the neurodevelopmental theory of schizophrenia, although it remains unclear whether certain aspects of cognition decline over time.
Our data in this regard are supportive of a neurodevelopmental model of cognition in schizophrenia and are in line with recent studies examining this question using longitudinal research designs [7,39]. For example, Gold and colleagues [7] tested schizophrenia participants at index hospitalization and at a 5-year follow-up evaluation. They reported longitudinal improvements in a number of cognitive domains, including full scale IQ, memory, and executive functioning. In accordance with the findings of Gold et al [7], we found significant enhancements in cognition just two years after index hospitalization, suggesting that the time course for cognitive improvement following the acute phase of schizophrenia is relatively rapid. Our data provide a replication and extension of this work by demonstrating that the cognitive improvement shown by schizophrenia participants includes improvements in processing speed. Furthermore, our data demonstrate that such improvements are maintained over a 20-year time period.
The acute phase of schizophrenia itself is often associated with considerable cognitive impairment. However, once individuals with schizophrenia (and other psychiatric disorders) emerge from the period of destabilization, their cognitive performance improves substantially. Our data are consistent in this regard for all three patient groups. Our findings support previous research suggesting that most individuals with schizophrenia experience considerable cognitive impairment at the acute phase of hospitalization, with improvement and relative stability as they recover [40]. Furthermore, we present evidence that this stability lasts up to 20 years after initial hospitalization.
Does cognition in individuals with schizophrenia decline over time as they age?
The current study also explored longitudinal changes in two aspects of neurocognition over a 20-year period in individuals with schizophrenia. Following the index hospitalization, both processing speed and access to general knowledge in schizophrenia participants remained relatively stable throughout the course of six follow-up evaluations conducted over 20 years. Our findings are in line with a number of previous studies that have found evidence of cognitive stability over time in schizophrenia [7–9, 41–43]. A review of longitudinal studies of this type [37] found no evidence of decline in cognitive function in schizophrenia and concluded that following illness onset, and after patients emerge from and improve from the decline associated with acute psychopathology, relative cognitive impairments in schizophrenia appear to be stable over long periods of time.
Notably, there are indications in the literature that the longitudinal stability of cognition in schizophrenia is dependent upon the age of the individuals under study. Specifically, recent findings suggest that cognitive functioning is more stable over time for younger individuals with schizophrenia, whereas older individuals with schizophrenia tend to show more pronounced cognitive decline between assessments [6,42]. For example, a prospective longitudinal study of cognition among schizophrenia participants of various ages reported that older schizophrenia patients experienced greater levels of cognitive decline over a 6-year follow-up period than younger patients, and the pattern of cognitive decline among schizophrenia patients differed from the pattern found in individuals with Alzheimer’s disease [44]. The authors suggested that cognitive decline may accelerate when patients are older (i.e., over 65), a factor which has been supported by other work in this field [13] and has bearing on our findings. As our initial patient sample was young at index hospitalization (mean age = 23 years) and was still relatively young at the most recent 20-year follow-up assessment (mean age = 43 years), they may have been protected from such decline to this point. However, one cannot exclude the possibility that our sample of schizophrenia participants will experience a more rapid and pronounced cognitive decline later in life. Additional follow-up information and research is called for to clarify this question. Specifically, psychiatric and healthy control groups are needed to determine whether the rate of cognitive decline among individuals with schizophrenia is equivalent to or more rapid than that of other diagnostic groups. Such findings would have public policy implications and would be relevant for clinicians who educate patients and their families regarding long-term course of the illness.
Lastly, the question of practice effects on neurocognitive measures across time has been debated. Dikmen and colleagues [45] found a change in scaled scores of less than 1 point on the Digit Symbol-Coding subtest when assessed at a one-year interval. Furthermore, McCaffrey et al. [46] reported no practice effects when this measure was administered four times over varying intervals (1 week to 3 months). Notably, the shortest follow-up interval in the present study was 2.5 years, which may minimize any practice effects. Additionally, schizophrenia patients showed the same performance trajectory as the other groups, suggesting that any practice effects present were demonstrated by all participants. Overall, however, there is currently no consensus about this issue.
Is Psychosis Related to Measures of Neurocognitive Functioning in Schizophrenia?
We conducted analyses examining the relationship between psychosis and measures of neurocognition to address the questions of whether cognitive deficits were related to psychotic state, whether this relationship was similar in both groups with psychosis, and whether this relationship was similar for different neurocognitive measures. Overall, performance on measures of processing speed and general knowledge at the time of evaluation was variably successful in differentiating psychotic from non-psychotic individuals and depended on the measures used. Specifically, we found that at three of the six follow-up evaluations, individuals with schizophrenia who were non-psychotic at the time of assessment showed significantly better processing speed than individuals with schizophrenia who were psychotic at the time of assessment. A similar result was obtained for the individuals with other psychotic disorders, who showed significant processing speed differences (non-psychotic > psychotic) at two of six follow-ups. Performance on the measure of access to general knowledge only showed a limited relationship to psychosis. Specifically, neurocognitive performance differentiated psychotic from non-psychotic individuals on access to general knowledge at one of the follow-up evaluations in the schizophrenia group. However, psychotic state did not differentiate psychotic from non-psychotic individuals in the group with other psychotic disorders to a significant degree at any of the follow-ups. We offer these findings as evidence of the relationship between psychosis and processing speed (particularly in schizophrenia), as well as the sensitivity of processing speed to psychosis.
Furthermore, at later follow-up evaluations (10 years, 15 years, and 20 years) both the schizophrenia participants and participants with other psychotic disorders showed significantly slower processing speed than the non-psychotic depression group, although the two groups with psychosis did not differ significantly from each other at these time points. These data suggest that psychosis, in addition to other factors related to schizophrenia and processes of normal aging, is often associated with poorer neurocognitive functioning, particularly as individuals with psychosis grow older and have had the disease longer [47]. Additionally, it appears that at later follow-up evaluations, performance on two neurocognitive measures is capable of differentiating the groups with psychosis from the group without psychosis. Indeed, as described above we found differences on the two measures of neurocognition (mostly in processing speed) between individuals with schizophrenia who were psychotic and non-psychotic at the time of study. Furthermore, the literature in this area contains evidence documenting the detrimental effect of psychosis (particularly when untreated) on cognition [14,15], a phenomenon that some researchers have attributed to putatively toxic effects of psychosis on the brain [48], although this hypothesis has been challenged [49]. Studies directly comparing neuropsychological performance of schizophrenia participants and participants with non-psychotic disorders (such as depression) have also consistently found more severe impairments in schizophrenia [16], implicating psychosis as a contributing factor to the more pronounced cognitive deficits observed in this population. We suggest the possibility of a reciprocal effect between vulnerability to slower processing speed (and poorer cognition) and vulnerability to psychosis, with each having some negative effects on the other area.
Conclusions
The present study examined the longitudinal course of two neurocognitive functions in individuals with schizophrenia and individuals with other psychiatric diagnoses prospectively over a 20-year period. Our findings suggested that performance on two measures of neurocognition was impaired in schizophrenia relative to other psychiatric groups, particularly at evaluations earlier in the disease process. However, as patients emerged from the acute phase, performance on neurocognitive measures improved significantly in the schizophrenia group (as well as the other diagnostic groups), with significant differences in cognition between the acute phase and the first follow-up evaluation two years later for schizophrenia patients. Acute phase psychopathology impairs cognition in schizophrenia, as well as more sensitive measures of cognition (such as processing speed) in all types of psychiatric groups. Performance on measures of processing speed and general knowledge remained stable for long periods of time following the acute phase of illness and individuals with schizophrenia did not show greater cognitive decline over time than other diagnostic groups as they aged, at least up to the age of 45 years. Lastly, psychosis was associated with greater deficits in processing speed, particularly among individuals with schizophrenia. The present study reported longitudinal performance on two major aspects of neurocognition, although we did not have data on other cognitive abilities over an extended period of time and cannot draw conclusions about cognition in general in schizophrenia. Further research should be conducted to more fully characterize long-term function in other cognitive domains in schizophrenia. Additionally, performance on the Digit Symbol-Coding task measures multiple cognitive functions in addition to processing speed. Thus, we cannot conclude that processing speed alone was changing across time. It is also possible that neuroleptic medications had an effect on the cognitive performance of the patient groups, particularly for the Digit Symbol-Coding task, which is heavily reliant on processing speed. However, it is also possible that some patients would have been impaired to a greater degree had they not been taking neuroleptic medications. Overall, our findings in this regard lend additional clarity to the course of cognitive functioning in schizophrenia as well as the relationship between cognition and psychosis in this population.
Acknowledgments
Funding: The authors have no conflict of interest or financial relationships to disclose. This research was supported in part by USPH grants MH-26341 and MH-068688 by NIMH (Dr. Harrow).
The authors wish to acknowledge Robert Faull for his technical assistance with data preparation and analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 2.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;166:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 3.Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychology. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 4.Velligan DI, Bow-Thomas CC. Executive function in schizophrenia. Semin Clin Neuropsychiatry. 1999;4:24–33. doi: 10.1053/SCNP00400024. [DOI] [PubMed] [Google Scholar]
- 5.Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, et al. Neuropsychological deficits in neuroleptic naïve patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz MM. Neurocognitive impairment across the lifespan in schizophrenia: an update. Schizophr Res. 2005;74:15–26. doi: 10.1016/j.schres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Gold S, Arndt S, Nopoulos P, O’Leary DS, Andreasen NC. Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. Am J Psychiatry. 1999;156:1342–1348. doi: 10.1176/ajp.156.9.1342. [DOI] [PubMed] [Google Scholar]
- 8.Hoff AL, Sakuma M, Wieneke M, Horon R, Kushner M, DeLisi LE. Longitudinal neuropsychological follow-up study of patients with first-episode schizophrenia. Am J Psychiatry. 1999;156:1336–1341. doi: 10.1176/ajp.156.9.1336. [DOI] [PubMed] [Google Scholar]
- 9.Russell AJ, Munro JC, Jones PB, Hemsley DR, Murray RM. Schizophrenia and the myth of intellectual decline. Am J Psychiatry. 1997;154:635–639. doi: 10.1176/ajp.154.5.635. [DOI] [PubMed] [Google Scholar]
- 10.David A. Schizophrenia and intellectual decline. Am J Psychiatry. 1998;155:1634–1635. [PubMed] [Google Scholar]
- 11.Kremen WS, Buka SL, Seidman LJ, Goldstein JM, Koren D, Tsuang MT. IQ decline during childhood and adult psychotic symptoms in a community sample: a 19-year longitudinal study. Am J Psychiatry. 1998;155:672–677. doi: 10.1176/ajp.155.5.672. [DOI] [PubMed] [Google Scholar]
- 12.Sheitman BB, Murray MG, Snyder JA, Silva S, Goldman R, Chakos M, et al. IQ scores of treatment-resistant schizophrenia patients before and after the onset of the illness. Schizophr Res. 2000;46:203–207. doi: 10.1016/s0920-9964(00)00034-7. [DOI] [PubMed] [Google Scholar]
- 13.Harvey PD, Silverman JM, Mohs RC, Parrella M, White L, Powchik P, et al. Cognitive decline in late-life schizophrenia: a longitudinal study of geriatric chronically hospitalized patients. Biol Psychiatry. 1999;45:32–40. doi: 10.1016/s0006-3223(98)00273-x. [DOI] [PubMed] [Google Scholar]
- 14.Amminger GP, Edwards J, Brewer WJ, Harrigan S, McGorry PD. Duration of untreated psychosis and cognitive deterioration in first-episode schizophrenia. Schizophr Res. 2002;54:223–230. doi: 10.1016/s0920-9964(01)00278-x. [DOI] [PubMed] [Google Scholar]
- 15.Lappin JM, Morgan KD, Morgan C, Dazzan P, Reichenberg A, Zanelli JW, et al. Duration of untreated psychosis and neuropsychological function in first episode psychosis. Schizophr Res. 2007;95:103–110. doi: 10.1016/j.schres.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Rund BR, Sundet K, Asbjornsen A, Egeland J, Landro NI, Lund A, et al. Neuropsychological test profiles in schizophrenia and non-psychotic depression. Acta Psychiatr Scand. 2006;113:350–359. doi: 10.1111/j.1600-0447.2005.00626.x. [DOI] [PubMed] [Google Scholar]
- 17.Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64:823–827. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 19.Burdick KE, Goldberg JF, Harrow M, Faull RN, Malhotra AK. Neurocognition as a stable endophenotype in bipolar disorder and schizophrenia. J Nerv Ment Dis. 2006;194:255–260. doi: 10.1097/01.nmd.0000207360.70337.7e. [DOI] [PubMed] [Google Scholar]
- 20.Harrow M, Grossman LS, Jobe TH, Herbener ES. Do patients with schizophrenia ever show periods of recovery? A 15-year multi-follow-up study. Schizophr Bull. 2005;31:723–734. doi: 10.1093/schbul/sbi026. [DOI] [PubMed] [Google Scholar]
- 21.Harrow M, Jobe TH, Herbener ES, Goldberg JF, Kaplan KJ. Thought disorder in schizophrenia: working memory and impaired context. J Nerv Ment Dis. 2004;192:3–11. doi: 10.1097/01.nmd.0000105994.78952.b6. [DOI] [PubMed] [Google Scholar]
- 22.Harrow M, Jobe TH. How frequent is chronic multiyear delusional activity and recovery in schizophrenia: A 20-year multi-follow-up. Schizophr Bull. doi: 10.1093/schbul/sbn074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed RA, Harrow M, Herbener ES, Martin EM. Executive function in schizophrenia: is it linked to psychosis and poor life functioning? J Nerv Ment Dis. 2002;190:725–732. doi: 10.1097/00005053-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Strauss GP, Harrow M, Grossman LS, Rosen C. Periods of recovery in deficit syndrome schizophrenia: a 20-year multi-follow-up longitudinal study. Schizophr Bull. doi: 10.1093/schbul/sbn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Arch Gen Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- 26.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 27.Grinker R, Harrow M. Clinical research in schizophrenia: A multidimensional approach. Springfield, IL: Charles C. Thomas; 1987. [Google Scholar]
- 28.McGrath JJ. Myths and plain truths about schizophrenia epidemiology – the NAPE lecture 2004. Acta Psychiatr Scand. 2005;111:4–11. doi: 10.1111/j.1600-0447.2004.00467.x. [DOI] [PubMed] [Google Scholar]
- 29.Bleuler M. The Schizophrenic Disorders: Long-Term Patient and Family Studies. New Haven: Yale University Press; 1978. [Google Scholar]
- 30.Harrison G, Hopper K, Craig T, Laska E, Siegel C, Wanderling J, et al. Recovery from psychotic illness: a 15- and 25-year international follow-up study. Br J Psychiatry. 2001;178:506–517. doi: 10.1192/bjp.178.6.506. [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D. Manual for the Wechsler Adult Intelligence Scale. New York: The Psychological Corporation; 1955. [Google Scholar]
- 32.Putnam KM, Harvey PD. Memory performance of geriatric and nongeriatric chronic schizophrenic patients: a cross-sectional study. J Int Neuropsychol Soc. 1999;5:494–501. doi: 10.1017/s1355617799566022. [DOI] [PubMed] [Google Scholar]
- 33.Zorilla LTE, Heaton RK, McAdams LA, Zisook S, Harris MJ, Jeste DV. Cross-sectional study of older outpatients with schizophrenia and healthy comparison subjects: no differences in age-related cognitive decline. Am J Psychiatry. 2000;157:1324–1326. doi: 10.1176/appi.ajp.157.8.1324. [DOI] [PubMed] [Google Scholar]
- 34.Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, et al. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bilder RM, Lipschutz-Broch L, Reiter G, Geisler SH, Mayerhoff DI, Lieberman JA. Intellectual deficits in first-episode schizophrenia: evidence for progressive deterioration. Schizophr Bull. 1992;18:437–448. doi: 10.1093/schbul/18.3.437. [DOI] [PubMed] [Google Scholar]
- 36.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 37.Rund BR. A review of longitudinal studies of cognitive functions in schizophrenia patients. Schizophr Bull. 1998;24:425–435. doi: 10.1093/oxfordjournals.schbul.a033337. [DOI] [PubMed] [Google Scholar]
- 38.Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenic mechanism. Am J Psychiatry. 1998;155:1661–1670. doi: 10.1176/ajp.155.12.1661. [DOI] [PubMed] [Google Scholar]
- 39.Zipparo L, Whitford TJ, Redoblado Hodge MA, Lucas S, Farrow TF, Brennan J, et al. Investigating the neuropsychological and neuroanatomical changes that occur over the first 2–3 years of illness in patients with first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:531–538. doi: 10.1016/j.pnpbp.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Hoff AL, Svetina C, Shields G, Stewart J, DeLisi LE. Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res. 2005;28:27–34. doi: 10.1016/j.schres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Albus M, Hubmann W, Scherer J, Dreikorn B, Hecht S, Sobizack N, et al. A prospective 2-year follow-up study of neurocognitive functioning in patients with first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2002;252:262–267. doi: 10.1007/s00406-002-0391-4. [DOI] [PubMed] [Google Scholar]
- 42.Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58:24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- 43.Hyde TM, Nawroz S, Goldberg TE, Bigelow LB, Strong D, Ostrem JL, et al. Is there cognitive decline in schizophrenia? A cross-sectional study. Br J Psychiatry. 1994;164:494–500. doi: 10.1192/bjp.164.4.494. [DOI] [PubMed] [Google Scholar]
- 44.Friedman JI, Harvey PD, Coleman T, Moriarty PJ, Bowie C, Parrella M, et al. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: a comparison with Alzheimer’s disease and normal aging. Am J Psychiatry. 2001;158:1441–1448. doi: 10.1176/appi.ajp.158.9.1441. [DOI] [PubMed] [Google Scholar]
- 45.Dikmen SS, Heaton RK, Grant I, Temkin NR. Test-retest reliability and practice effects of expanded Halstead-Reitan Neuropsychological Test Battery. J Int Neuropsychol Soc. 5:346–356. [PubMed] [Google Scholar]
- 46.McCaffrey RJ, Ortega A, Haase RF. Effects of repeated neuropsychological assessments. Arch Clin Neuropsychol. 8:519–524. [PubMed] [Google Scholar]
- 47.Bonner-Jackson A, Grossman LS, Harrow M, Rosen C. Neurocognitive predictors of future recovery in schizophrenia: A 20-year follow-up. Schizophr Bull. 2009;35:292. [Google Scholar]
- 48.Loebel AD, Lieberman JA, Alvir JM, Mayerhoff DI, Geisler SH, Szymanski SR. Duration of psychosis and outcome in first-episode schizophrenia. Am J Psychiatry. 1992;149:1183–1188. doi: 10.1176/ajp.149.9.1183. [DOI] [PubMed] [Google Scholar]
- 49.McGlashan T. Is active psychosis neurotoxic? Schizophr Bull. 2006;32:609–613. doi: 10.1093/schbul/sbl032. [DOI] [PMC free article] [PubMed] [Google Scholar]