Abstract
Purpose
To describe the clinical and genetic findings in one Chinese family with juvenile-onset open angle glaucoma (JOAG).
Methods
One family was examined clinically and a follow-up took place 5 years later. After informed consent was obtained, genomic DNA was extracted from the venous blood of all participants. Linkage analysis was performed with three microsatellite markers around the MYOC gene (D1S196, D1S2815, and D1S218) in the family. Mutation screening of all coding exons of MYOC was performed by direct sequencing of PCR-amplified DNA fragments and restriction fragment length polymorphism (RFLP) analysis. Bioinformatics analysis by the Garnier-Osguthorpe-Robson (GOR) method predicted the effects of variants detected on secondary structures of the MYOC protein.
Results
Clinical examination and pedigree analysis revealed a three- generation family with seven members diagnosed with JOAG, three with ocular hypertension, and five normal individuals. Through genotyping, the pedigree showed a linkage to the MYOC on chromosome 1q24–25. Mutation screening of MYOC in this family revealed an A→T transition at position 1348 (p. N450Y) of the cDNA sequence. This missense mutation co-segregated with the disease phenotype of the family, but was not found in 100 normal controls. Secondary structure prediction of the p.N450Y by the GOR method revealed the replacement of a coil with a β sheet at the amino acid 447.
Conclusions
Early onset JOAG, with incomplete penetrance, is consistent with a novel mutation in MYOC. The finding provides pre-symptomatic molecular diagnosis for the members of this family and is useful for further genetic consultation.
Introduction
Primary open-angle glaucoma (POAG;OMIM 137760) is one of the leading causes of blindness in the world [1]. It is a neurodegenerative disorder characterized by progressive excavation of the optic discs due to loss of retinal ganglion cells. It is usually associated with elevation of intraocular pressure (IOP) [2]. Based upon the age of diagnosis, primary open-angle glaucoma can be sub-classified to either juvenile-onset primary open-angle glaucoma (JOAG) or adult-onset primary open-angle glaucoma. JOAG is a relatively rare form of primary open angle glaucoma that occurs in children and young adults. The exact age boundary for juvenile-onset varies from one study to the next, but it usually falls between 35 and 40 years of age [2].
Strong evidence indicates that genetic factors play a role in the pathogenesis of glaucoma. About 30%–56% of patients with glaucoma or ocular hypertension (OHT) have a positive family history; first-degree relatives of POAG patients are seven to ten times more likely to have POAG, compared with the general population [3,4]. Genetically, most POAG cases follow a complex (non-Mendelian) pattern of inheritance, which manifests clinically in adulthood (>40 years). However, juvenile-onset open-angle glaucoma typically shows an autosomal dominant inheritance [2-4]. To date, three genes, namely myocilin (MYOC), optineurin (OPTN), and WD repeat-containing protein 36 (WDR36), have been reportedly linked to POAG [5-10]. MYOC (OMIM 601652) was the first gene to be identified as responsible for POAG. Mutations in MYOC account for over 8% of JOAG and 3%–4% of adult-onset POAG [11,12].
MYOC, consisting of three exons, encodes 504 amino acid residues. Myocilin is an acidic protein that contains an NH2-terminal myosin-like domain and a COOH-terminal olfactomedin-like domain [6]. Almost 80 mutations have been found in MYOC and about 90% of the mutations are located in the olfactomedin-like domain encoded by exon3 [6,11-30].
In this study, we describe the clinical findings in a Chinese family with a novel MYOC mutation.
Methods
Patients and DNA sample collection
This study was performed according to the tenets of the Declaration of Helsinki for research involving human subjects. This study was approved by the Beijing Tongren Hospital Joint Committee on Clinical Investigation. After informed consent was obtained, all participants underwent ophthalmologic examination including bilateral best corrected visual acuity using E decimal charts, slit-lamp biomicroscopy inspection of the anterior chamber, intraocular pressure (IOP) measurement by applanation tonometry (Goldmann), anterior chamber angle evaluation by gonioscopy (Goldmann), and fundus examination with a 66-diopter VOLK lens. Most members were clinically followed for five years, from 2004 to 2009. Some individuals underwent Octopus’s perimeter examination. Diagnosis of POAG was based on the observation of at least two of the following abnormalities: characteristic glaucomatous optic disc changes, characteristic glaucomatous visual field defects, and high intraocular pressure (>21 mmHg) in the presence of a normal open anterior chamber angle. Characteristic glaucomatous optic disc changes include vertical cup-disc (c/d) ratio of 0.7 or more, notching of the neutral rim, and disc hemorrhage. Subjects were sub-classified JOAG if the diagnosis of POAG was made before 35 years of age. Individuals with intraocular pressure greater than 22 mmHg but with no characteristic optic disc damage or visual field impairment were defined as ocular hypertension. Unaffected people had IOP in the normal range (≤21 mmHg) and optic nerves presented normal in appearance.
Linkage analysis
Genotyping and linkage analysis were performed with three microsatellite markers (D1S196, D1S2185, and D1S218) around the MYOC gene in the family. The fine mapping primer sequences were obtained from the GDB Human Genome Database. LOD scores were calculated for the two markers by two-point linkage analysis using linkage package 5.2. We modeled the disease as an autosomal dominant trait with reduced penetrance. Pedigree and haplotype maps were constructed using Cyrillic version 2.0 software.
Mutation screening of MYOC
Peripheral blood was obtained by venipuncture and genomic DNA was extracted according to standard protocols. The entire coding region of MYOC was amplified by polymerase chain reaction (PCR) from genomic DNA. Primers for three exons and exon-intron boundaries of MYOC were designed by the Primer3 program. These primer sequences are presented in Table 1. For direct sequencing, PCR products were purified (Shenneng Bocai PCR purification kit; Shenneng, Shanghai, China). An automatic fluorescence DNA sequencer (ABI, Prism 373A; Perkin Elmer, Foster City, CA), used according to the manufacturer’s instructions, was used to sequence the purified PCR products in both forward and reverse directions. DNAssist Version 1.0 compared nucleotide sequences with the published DNA sequence of MYOC (GenBank NM_000261). For the MYOC gene, cDNA numbering +1 corresponded to the A in the ATG translation initiation codon of MYOC.
Table 1. PCR primers used in this study.
| Primer | Forward (5'-3') | Reverse (5'-3') | Tm (°C) | Product size (bp) |
|---|---|---|---|---|
| exon1 |
CTCTGTCTTCCCCCATGAAG |
AGCAGGTCACTACGAGCCATA |
62 |
785 |
| exon2 |
TAGTCAATCCTTGGGCCATT |
ACCACGTGGGCACAAAAG |
60 |
561 |
| exon3-1 |
CTTCCGCATGATCATTGT |
CTTCCGCATGATCATTGT |
58 |
352 |
| exon3-2 |
ATACTGCCTAGGCCACTGGAA |
CCGCTATAAGTACAGCAGCATGAT |
58 |
440 |
| exon3-3 | GCCTTCATCATCTGTGGCAC | CAGGCAGCTTTGACTGCTTT | 58 | 342 |
Restriction fragment length polymorphism (RFLP) analysis
To confirm the variations found in the sequencing, restriction endonuclease HindII (New England Biolabs, Ipswich MA) was used in all available family members and in 100 normal control subjects. The reaction was performed in a 10 μl volume containing 9.4 μl PCR product, 0.1 μl BSA (100 μg/ml), and 0.5 μl enzyme (10 U/μl). After incubating the reaction overnight at 37 °C, the entire digest was run on a 1% agarose gel and visualized under ultraviolet light.
Bioinformatics analysis
Garnier-Osguthorpe-Robson (GOR) software was used to predict the effect of the mutation on the secondary structure of MYOC [31]. This method infers the secondary structure of a sequence by calculating the probability for each of the four structure classes (helix, sheet, turn, and loop) based on the central residue and its neighbors from the calculated matrices.
Results
Clinical findings
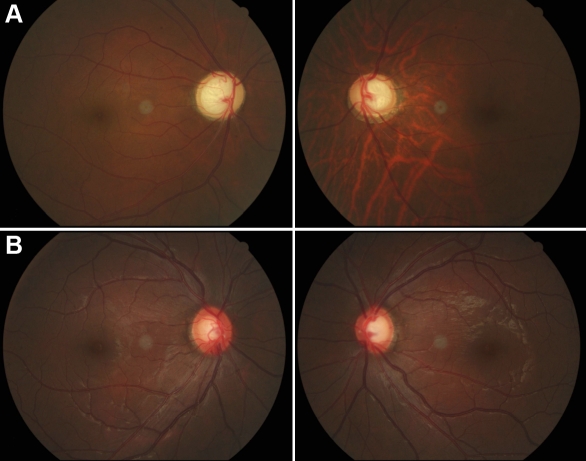
We have identified a three- generation family diagnosed with JOAG. The inheritance pattern in this family appeared to be autosomal dominant (Figure 1). After clinical examinations and hospital records reviewing, six individuals of this pedigree were found to have glaucoma in 2004. The patient in the first generation had not received any treatment and totally lost her sight before the age of 35. The remaining five patients underwent trabeculectomies in both eyes. The mean onset age of these patients was 27.42 years (ranging from 20 to 31 years old), which was consistent with juvenile glaucoma. All patients experienced elevated IOP (32–50 mmHg) and most of them presented typical late stage glaucoma changes in the optic disc and in the visual field (Figure 2A). In 2004, six members were diagnosed with ocular hypertension (IOPs were higher than 22 mmHg) but without optic disc or visual field changes. A five-year follow-up was conducted with fifteen of the seventeen individuals and their blood samples were collected for further genetic analysis. At the 5-year follow-up, two ocular hypertension patients (Figure 1; III:2 and III:7) were newly diagnosed with glaucoma due to their elevated IOP, enlarged cup/disc ratio of the optic disc, and early visual field changes in 2009 (Figure 2B) . Detailed clinical information of the pedigree is summarized in Table 2.
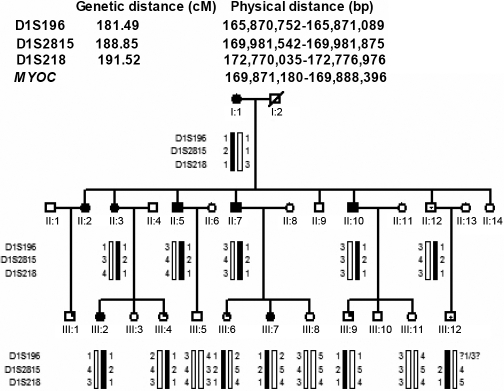
Figure 1.
Family structure and haplotype analysis of a Chinese family with JOAG. Pedigree and haplotype analysis of the family with JOAG showed segregation with three microsatellite markers on chromosome 1, listed in descending order from the centromeric end. Squares indicate males; circles indicate females; slashed symbols indicate deceased; solid symbols indicate affected; open symbols indicate unaffected; symbols with upper left filled-in quadrant indicate members with ocular hypertension; symbols with dot in the center indicate carriers.
Figure 2.
Fundus appearances of patients with JOAG. A: Fundus images of II:7 showed late-stage glaucomatous cupping of the optic disc. B: Fundus images of III:7, who was confirmed to have glaucoma in 2009, presented early glaucomatous appearances of the optic disc.
Table 2. Clinical features of individuals of this pedigree with JOAG.
| Pedigree number | Gender/ Age year | Onset Age year | BCVA OD/OS (2004) | BCVA OD/OS (2009) | Maxium IOP mmHg | IOP (OD/OS) (2004) mmHg | IOP (OD/OS) (2009) mmHg | Optic Disc (C/D) (OD/OS) (2004) | Optic Disc (C/D) (OD/OS) (2009) | Medical therapy (OD/OS) | Diagnosis (2004) | Diagnosis (2009) | N450Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| II:4 |
F/79 |
20 |
NLP |
NLP |
NA |
N/A |
NA |
1.0/1.0 |
1.0/1.0 |
NMT |
JOAG |
JOAG |
Yes |
| III:1 |
F/58 |
30 |
0.8/0.8 |
NA |
45/50 |
18/16 |
NA |
0.6/0.6 |
NA |
S/S |
JOAG |
NA |
NA |
| III:3 |
F/56 |
28 |
0.2/0.2 |
0.2/0.2 |
50/60 |
10/14 |
14/14 |
0.9/0.9 |
0.9/0.9 |
S/S |
JOAG |
JOAG |
Yes |
| III:5 |
M/52 |
31 |
0.2/0.2 |
0.8/0.8 |
53/40 |
20/20 |
16/15 |
0.8/0.4 |
0.8/0.4 |
S/S |
JOAG |
JOAG |
Yes |
| III:7 |
M/49 |
29 |
0.1/0.1 |
0.1/0.1 |
52/56 |
15/15 |
22/22 |
0.9/0.9 |
0.9/0.9 |
S/S |
JOAG |
JOAG |
Yes |
| III:10 |
M/45 |
28 |
0.1/0.1 |
0.1/0.1 |
55/55 |
31/21 |
35/28 |
0.9/0.9 |
0.9/0.9 |
S/S |
JOAG |
JOAG |
Yes |
| III:12 |
M/39 |
|
0.8/0.8 |
0.8/0.8 |
|
21/16 |
21/21 |
0.2/0.2 |
0.2/0.2 |
|
Normal |
Carrier |
Yes |
| IV:1 |
M/30 |
|
1.0/1.0 |
NA |
|
24/24 |
NA |
0.4/0.4 |
NA |
|
OHT |
NA |
NA |
| IV:2 |
F/22 |
22 |
1.0/1.0 |
1.0/1.0 |
30/32 |
22/22 |
28/26 |
0.4/0.4 |
0.7/0.5 |
M/M |
OHT |
JOAG |
Yes |
| IV:3 |
F/19 |
|
1.0/1.0 |
1.0/1.0 |
|
22/22 |
NA |
0.5/0.5 |
NA |
|
OHT |
OHT |
Yes |
| IV:4 |
M/21 |
|
1.0/1.0 |
1.0/1.0 |
|
18/18 |
16/16 |
0.4/0.4 |
0.4/0.4 |
|
Normal |
Normal |
No |
| IV:5 |
F/23 |
|
1.2/1.2 |
1.2/1.2 |
|
25/25 |
25/25 |
0.3/0.3 |
0.3/0.3 |
|
OHT |
OHT |
Yes |
| IV:6 |
F/22 |
22 |
1.0/1.0 |
1.0/1.0 |
34/32 |
26/26 |
34/32 |
0.5/0.5 |
0.7/0.7 |
M/M |
OHT |
JOAG |
Yes |
| IV:7 |
F/17 |
|
1.0/1.0 |
1.0/1.0 |
|
14/14 |
18/18 |
0.2/0.2 |
0.2/0.2 |
|
Normal |
Normal |
No |
| IV:9 |
M/22 |
|
1.0/1.0 |
1.0/1.0 |
|
24/20 |
26/20 |
0.5/0.5 |
05/0.5 |
|
OHT |
OHT |
Yes |
| IV:10 |
F/16 |
|
1.0/1.0 |
1.0/1.0 |
|
15/15 |
16/17 |
0.2/0.2 |
0.2/0.2 |
|
Normal |
Normal |
No |
| IV:12 | M/16 | 1.2/1.2 | 1.2/1.2 | 19/20 | 17/17 | 0.2/0.2 | 0.2/0.2 | Normal | Carrier | Yes |
Abbreviations: M, male; F, female; BCVA, best-correct visual aucuity; OD, right eye; OS, left eye; NLP, no light peception; IOP, introocular pressure; C/D, cup disc ratio; NMT, no medical therapy; NA, unavailable; S, surgery; M, medical therapy; OHT, ocular hypertension, JOAG, juvenile-onset angle glaucoma.
Genotyping results
The family was genotyped with three STRP markers located around the MYOC gene in the chromosome 1q24–25 region. The marker results for D1S218 and D1S2815 were fully informative for linkage. There was no affected (glaucomatous patients and ocular hypertension patients) recombinant for either of the two makers (Figure 1). Two clinical unaffected individuals (II:12 and III:12), however, were found to be carrying the affected haplotype. Therefore, the disease penetrance appeared incomplete in this pedigree. Two-point LOD scores for D1S2815 and D1S218 with 80% penetrance were 2.40 (θ=0.0) and1.63 (θ=0.0), respectively.
Mutation analysis
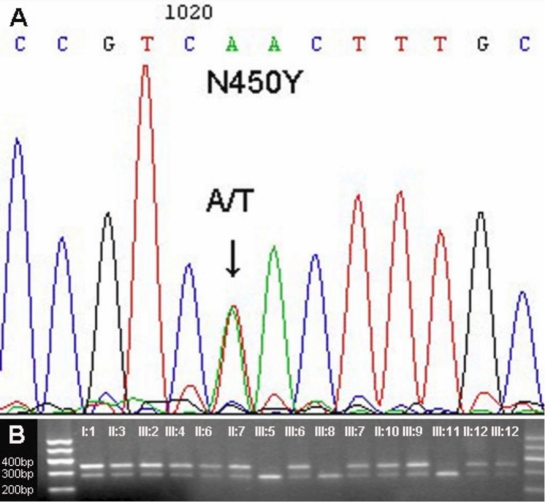
By direct sequencing of three exons of MYOC, we found a novel base change (A→T) at position 1348 of MYOC cDNA, replacing asparagine with tyrosine at amino acid 450 residue (Figure 3A). This heterozygous missense mutation abolished a HindII restriction site that segregated with all affected members and ocular hypertension individuals in this Chinese family, but that was not detected in 100 unrelated normal controls. As observed in the genotyping, two clinical unaffected individuals (II:12 and III:12) carried the mutation as well (Figure 3B).
Figure 3.
DNA sequence chromatograms and co-segregation analysis of the p.N450Y mutation with disease phenotype. A: Heterozygote sequence (sense strand) shows an A/T transition in codon 450 that changed asparagine (AAC) to tyrosine (TAC). B: Restriction fragment length analysis shows the p.N450Y mutation abolishing a HindII site co-segregated with JOAG patients, ocular hypertensions, and the carriers (342 and 279 bp), but not with unaffected individuals (279 bp).
Prediction of two-dimensional structure
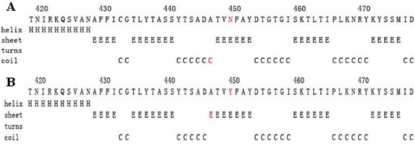
Using the GOR method, the results for secondary structure prediction suggested that the mutant MYOC450Y replace a coil “C” with a β sheet “E” at amino acid 447 Figure 4).
Figure 4.
The effect of p. N450Y on the secondary structure of MYOC using the GOR method. A: The secondary structure of wild type MYOC around the site N450. B: The secondary structure of mutant Y450 of MYOC of the corresponding region.
Discussion
This study described a Chinese family with clinically diagnosed juvenile-onset open angle glaucoma. By screening the MYOC gene, we identified a novel heterozygous missense mutation p. N450Y in the pedigree. The mutation p. N450Y co-segregated with all glaucoma patients and ocular hypertension individuals, but was not detected in 100 normal controls.
MYOC was the first disease-causing gene identified for POAG and almost 80 mutations have been reported [6,11-30]. Mutations in MYOC are racial/ethnic specific and some of them have been found only in a specific region [6,11-30]. So far, 11 MYOC mutations have been identified in Chinese patients or pedigrees and seven of them were Chinese specific (Table 3) [19,20,22,23,25,26,28].
Table 3. Mutations in MYOC identified in Chinese families or patients.
| Mutation | Location | Case control | Family-base | Phenotype | Proband age at diagnosis | Country/ethnicity | Reference |
|---|---|---|---|---|---|---|---|
| R91X |
Exon1 |
Yes |
|
NA |
|
China |
[19] |
| C245Y |
Exon3 |
Yes |
Yes |
JOAG |
16 |
China |
[22,23] |
| G252R |
Exon3 |
Yes |
Yes |
JOAG |
29 |
Caucasian, China |
[12,20] |
| E300K |
Exon3 |
Yes |
|
NA |
NA |
China |
[19,22] |
| S313F |
Exon3 |
Yes |
|
NA |
NA |
China |
[22] |
| Q337X |
Exon3 |
|
Yes |
JOAG |
40** |
China |
[28] |
| S341P |
Exon3 |
|
Yes |
JOAG |
24 |
China, Korean |
[14,26] |
| T353I* |
Exon3 |
Yes |
|
NA |
NA |
Asian |
[19,22] |
| P370L |
Exon3 |
Yes |
Yes |
JOAG |
11 |
Caucasian, Asian |
[12,27,32,33] |
| N450Y |
Exon3 |
|
Yes |
JOAG |
20 |
China |
Present study |
| T455K |
Exon3 |
|
Yes |
JOAG |
26 |
China |
[25] |
| Y471C | Exon3 | Yes | NA | NA | China | [19,22] |
The asterisk indicates uncertain pathogenicity and the double asterisk indicates the proband was in the end stage of glaucoma. NA refers date is unavailable.
The Asn450 residue, located in the olfactomedin-like domain, is highly conserved in humans, rats, mice, cattle, dogs, and zabrafish (Figure 5). The results of GOR suggested that p.N450Y lead to a secondary structure change by replacing a coil structure with a β sheet around the Asn450 residue, which might interfere with the correct folding of the protein. In a large case control study, another mutation (p. N450D) was also detected at the Asn450 residue in a sporadic Germany patient [18]. This may imply that the Asn450 residue is very important for the activity of the olfactomedin-like domain.
Figure 5.
Sequence alignment portion of the olfactomedin-like domain spanning the novel missense mutation p.N450Y of human MYOC and a comparison with other species.
Phenotype and genotype correlation has been well established in some MYOC mutations [11,12,27]. Patients carrying the P370L mutation usually developed glaucoma at a very early age, with high levels of IOP, which responds poorly to medical treatment [12,32,33]; while patients with the Q368X mutation were diagnosed with glaucoma at a later adult age and their maximum IOPs were around 30 mmHg, which could be well controlled by medical therapy [12,34,35]. One American family carrying the p.D380H MYOC mutation presented with an intermediate phenotype between juvenile and adult onset glaucoma [36]. In the current study, the onset age of glaucoma ranged from 20 to 31 years (mean 26 years). The mean highest IOP was 48.57 mmHg (range from 32 to 60 mmHg). One patient totally lost her sight before 35 years of age. Except for two patients newly diagnosed in 2009, the remaining five patients responded poorly to medical therapy and required filtration surgery for long-term IOP control. Five individuals diagnosed with ocular hypertension in 2004 carried the mutation p.N450Y and their mean age at diagnosis was 17.8 years. At the 5-year follow up, two of them presented glaucomatous optic disc change and were newly diagnosed with glaucoma. The phenotype and genotype correlation study on seven patients in this pedigree indicated that affected members carrying the mutation p.N450Y experienced more severe symptoms at an earlier age.
Incomplete penetrance has been observed in most families with MYOC mutations and the penetrances are age-dependent and mutation-specific [11,12,27]. The penetrance of pedigrees carrying p. P370L was 100% at age 30 years [12,32,33], while it was 0 for the pedigrees with Q368X [12,34,35]. In this pedigree, two clinically healthy individuals and three ocular hypertension patients were found harboring both mutation p.N450Y and the affected haplotype. The penetrance of this pedigree was 50% (6/12) at age 30 and almost 60% (7/12) at age 35 years. More than 80% (10/12) of the individuals carrying the p.N450Y mutation have developed glaucoma or ocular hypertension. Interestedly, one of the healthy members (II-12) was already 39 years old, which was ten years older than the average onset age of this family; this implied that other unidentified factors (genetic or environmental) might be associated with the JOAG of this pedigree. However, whole carriers should undergo ophthalmologic surveillance at regular intervals for the rest of their lives.
In summary, the report described a novel conserved tyrosine to asparagine substitution at exon 3 of MYOC associated with an early-onset and severe juvenile-onset open angle glaucoma pedigree. The results further expanded the mutation spectrum of MYOC and characterized the genotype-phenotype correlations of this pedigree. These results provide pre-symptomatic molecular diagnosis for the members of the pedigree and are useful for further genetic consultation with this family.
Acknowledgments
We thank the patients and their families for participation in this study. The study was supported by the Beijing National Science Foundation (No, 07G0069).
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma world wide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiggs JL, Damji KF, Haines JL, Pericak-Vance MA, Allingham RR. The distinction between juvenile and adult-onset primary open –angle glaucoma. Am J Hum Genet. 1996;58:243–4. [PMC free article] [PubMed] [Google Scholar]
- 3.Booth A, Churchill A, Anwar R, Menage M, Markham A. The genetics of primary open angle glaucoma. Br J Ophthalmol. 1997;81:409–14. doi: 10.1136/bjo.81.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budde WM. Heredity in primary open-angle glaucoma. Curr Opin Ophthalmol. 2000;11:101–6. doi: 10.1097/00055735-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Sheffield VC, Stone EM, Alward WL, Drack AV, Johnson AT, Streb LM, Nichols BE. Genetic linkage of familial open angle glaucoma to chromosome 1q21-q31. Nat Genet. 1993;4:47–50. doi: 10.1038/ng0593-47. [DOI] [PubMed] [Google Scholar]
- 6.Stone EM, Fingert JH, Alward WLM, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–70. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 7.Sarfarazi M, Child A, Stoilova D, Brice G, Desai T, Trifan OC, Ioinoosawmy D, Crick RP. Localization of the fourth locus (GLC1E) for adult-onset primary open-angle glaucoma to the 10p15-p14 region. Am J Hum Genet. 1998;62:641–52. doi: 10.1086/301767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Heon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–9. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- 9.Monemi S, Spaeth G, DaSilva A, Popinchalk S, Ilitchev E, Liebmann J, Ritch R, Heon E, Crick RP, Child A, Sarfarazi M. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Hum Mol Genet. 2005;14:725–33. doi: 10.1093/hmg/ddi068. [DOI] [PubMed] [Google Scholar]
- 10.Pasutto F, Mardin CY, Michels-Rautenstrauss K, Weber BH, Sticht H, Chavarria-Soley G, Rautenstrauss B, Kruse F, Reis A. Profiling of WDR36 missense variants in German patients with glaucoma. Invest Ophthalmol Vis Sci. 2008;49:270–4. doi: 10.1167/iovs.07-0500. [DOI] [PubMed] [Google Scholar]
- 11.Fingert JH, Stone EM, Sheffield VC, Alward WLM. Myocilin glaucoma. Surv Ophthalmol. 2002;47:547–61. doi: 10.1016/s0039-6257(02)00353-3. [DOI] [PubMed] [Google Scholar]
- 12.Gong G, Kosoko-Lasaki O, Haynatzki GR, Wilson MR. Genetic dissection of myocilin glaucoma. Hum Mol Genet. 2004;13:R91–102. doi: 10.1093/hmg/ddh074. [DOI] [PubMed] [Google Scholar]
- 13.Adam MF, Belmouden A, Binisti P, Brezin AP, Valtot F, Bechetoille A, Dascotte JC, Copin B, Gomez L, Chaventre A, Bach JF, Garchon HJ. Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Genet. 1997;6:2091–7. doi: 10.1093/hmg/6.12.2091. [DOI] [PubMed] [Google Scholar]
- 14.Kee C, Ahn BH. TIGR gene in primary open-angle glaucoma and steroid-induced glaucoma. Korean J Ophthalmol. 1997;11:75–8. doi: 10.3341/kjo.1997.11.2.75. [DOI] [PubMed] [Google Scholar]
- 15.Wiggs JL, Allingham RR, Vollrath D, Jones KH, De La Paz M, Kern J, Patterson K, Babb VL, Del Bono EA, Broomer BW, Pericak-Vance MA, Haines JL. Prevalence of mutations in TIGR/Myocilin in patients with adult and juvenile primary open-angle glaucoma. Am J Hum Genet. 1998;63:1549–52. doi: 10.1086/302098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fingert JH, Heon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, Stone EM. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8:899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu S, Lichter PR, Johnson AT, Zhou Z, Higashi M, Gottfredsdottir M, Othman M, Moroi SE, Rozsa FW, Schertzer RM, Clarke MS, Schwartz AL, Downs CA, Vollrath D, Richards JE. Age-dependent prevalence of mutations at the GLC1A locus in primary open angle glaucoma. Am J Ophthalmol. 2000;130:165–77. doi: 10.1016/s0002-9394(00)00536-5. [DOI] [PubMed] [Google Scholar]
- 18.Michels-Rautenstrauss K, Mardin G, Wakili N, Jünemann AM, Villalobos L, Mejia C, Soley GC, Azofeifa J, Özbey S, Naumann GO, Reis A, Rautenstrauss B. Novel mutations in the MYOC/GLC1A gene in a large group of glaucoma patients. Hum Mutat. 2002;20:479–80. doi: 10.1002/humu.9092. [DOI] [PubMed] [Google Scholar]
- 19.Pang CP, Leung YF, Fan BJ, Baum L, Tong WC, Lee WS, Chua JK, Fan DS, Liu Y, Lam DS. TIGR/MYOC gene sequence alterations in individuals with and without primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2002;43:3231–5. [PubMed] [Google Scholar]
- 20.Vincent AL, Billingsley G, Buys Y, Levin AV, Priston M, Trope G, Williams-Lyn D, Heon E. Digenic Inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am J Hum Genet. 2002;70:448–60. doi: 10.1086/338709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruttini M, Longo I, Frezzotti P, Ciappetta R, Randazzo A, Orzalesi N, Fumagalli E, Caporossi A, Frezzotti R, Renieri A. Mutations in the myocilin gene in families with primary open-angle glaucoma and juvenile open-angle glaucoma. Arch Ophthalmol. 2003;121:1034–8. doi: 10.1001/archopht.121.7.1034. [DOI] [PubMed] [Google Scholar]
- 22.Fan BJ, Wang DY, Fan DS, Tam PO, Lam DS, Tham CC, Lam CY, Lau TC, Pang CP. SNPs and interaction analyses of myocilin, optineurin, and apolipoprotein E in primary open angle glaucoma patients. Mol Vis. 2005;11:625–31. [PubMed] [Google Scholar]
- 23.Fan BJ, Leung DYL, Wang DY, Gobeil S, Raymond V, Tam POS, Lam DSC, Pang CP. Novel Myocilin mutation in a Chinese family with Juvenile-onset open-angle glaucoma. Arch Ophthalmol. 2006;124:102–6. doi: 10.1001/archopht.124.1.102. [DOI] [PubMed] [Google Scholar]
- 24.Hogewind BF, Gaplovska-Kysela K, Theelen T, Cremers FPM, Yam GHF, Hoyng CB, Mukhopadhyay A. Identification and functional characterization of a novel MYOC mutation in two primary open angle glaucoma families from The Netherlands. Mol Vis. 2007;13:1793–801. [PubMed] [Google Scholar]
- 25.Tian Q, Li FH, Zhao KX, Wang L, Shan XY, Pang YY, Li YX, Wu MJ, Qiu F, Li HY. A novel mutation in the myocilin gene identified in a Chinese primary open angle glaucoma family. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2007;24:629–34. [PubMed] [Google Scholar]
- 26.Qin L, Li J. Investigation on the mutation of MYOC in two family pedigrees with open angle glaucoma in Shanxi. Yan Ke Xue Bao. 2007;23:75–8. [PubMed] [Google Scholar]
- 27.Hewitt AW, Mackey DA, Craig J. Myocilin allele-specific glaucoma phenotype database. Hum Mutat. 2008;29:207–11. doi: 10.1002/humu.20634. [DOI] [PubMed] [Google Scholar]
- 28.Xie X, Zhou X, Qu X, Wen J, Tian Y, Zheng F. Two novel myocilin mutations in a Chinese family with primary open-angle glaucoma. Mol Vis. 2008;14:1666–72. [PMC free article] [PubMed] [Google Scholar]
- 29.Mengkegale M, Fuse N, Miyazawa A, Takahashi K, Seimiya M, Yasui T, Tamai M, Nakazawa T, Nishida K. Presence of myocilin sequence variants in Japanese patients with open-angle glaucoma. Mol Vis. 2008;14:413–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Avisar I, Lusky M, Robinson A, Shohat M, Dubois S, Raymond V, Gaton D. The novel Y371D myocilin mutation causes an aggressive form of juvenile open-angle glaucoma in a Caucasian family from the Middle-East. Mol Vis. 2009;15:1945–50. [PMC free article] [PubMed] [Google Scholar]
- 31.Garnier J, Gibrat JF, Robson B. GOR method for predicting protein secondary structure from amino acid sequence. Methods Enzymol. 1996;266:540–53. doi: 10.1016/s0076-6879(96)66034-0. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Jiang D, Wan B, Yu L, Sun X. Presymptomatic genetic diagnosis for consulters from a large Chinese family with juvenile open angle glaucoma. Mol Vis. 2006;12:360–6. [PubMed] [Google Scholar]
- 33.Zhuo YH, Wei Y, Bai Y, Duan S, Lin M, Saragovi HU, Ge J. Pro370Leu MYOC gene mutation in a large Chinese family with Juvenile-onset open angle glaucoma: correlation between genotype and phenotype. Mol Vis. 2008;14:1533–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Allingham RR, Wiggs JL, De La Paz MA, Vollrath D, Tallett DA. Gln368STOP myocilin mutation in families with late-onset primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2288–95. [PubMed] [Google Scholar]
- 35.Angius A, Spinelli P, Ghilotti G, Casu G, Sole G, Loi A, Totaro A, Zelante L, Gasparini P, Orzalesi N, Pirastu M, Bonomi L. Myocilin Gln368stop mutation and advanced age risk factors for late-onset primary open-angle glaucoma. Arch Ophthalmol. 2000;118:674–9. doi: 10.1001/archopht.118.5.674. [DOI] [PubMed] [Google Scholar]
- 36.Wirtz MK, Samples JR, Choi D. gaudette ND. Clinical features associated with an Asp380His myocilin mutation in a USA family with primary open angle glaucoma. Am J Ophthalmol. 2007;144:75–80. doi: 10.1016/j.ajo.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]