Abstract
Purpose
The Segmental Assessment of Trunk Control (SATCo) provides a systematic method of assessing discrete levels of trunk control in children with motor disabilities. This study refined the assessment method and examined reliability and validity of the SATCo.
Methods
After refining guidelines, 102 video recordings of the SATCo were made on 8 infants with typical development followed longitudinally from 3–9 months of age and 24 children with neuromotor disability mean age 10 yr 4 mo. Eight researchers independently scored recordings.
Results
ICC values for inter-rater and intra- reliability were > .84 and >.98 across all data sets and all aspects of control. Tests of concurrent validity with the Alberta Infant Motor Scales resulted in coefficients ranging from .86 to .88.
Conclusion
The SATCo is a reliable and valid measure allowing clinicians greater specificity in assessing trunk control.
Introduction and Purpose
The ability to control sitting balance gradually emerges in children with typical development (TD) during the period from about two to nine months of age, with head control developing first, followed by progressive development of trunk control.1, 2, 3, 4, 5 In children with neuromotor disability development of sitting balance is delayed and, depending on the level of disability, children may continue to show constraints on sitting balance throughout their lives with some never gaining independent control of the trunk and head.6, 7, 8, 9, 10
Laboratory tests of sitting and standing balance control in both children with TD and in children with neuromotor disability have examined three aspects of balance control, all of which are important to mastering functional balance and reaching skills involved in activities of daily living. These include tests of 1) static or steady state balance, examining the child’s ability to maintain a steady posture without support, 2) active or anticipatory balance adjustments, examining the child’s ability to balance while reaching, and 3) reactive balance, examining the child’s ability to maintain or regain balance following a brief perturbation, such as a translation of the base of support.10, 11
Previous research on the development of sitting balance models the entire trunk as a single unit, ignoring the fact that the trunk is made up of many muscular and skeletal subunits.1, 3, 4, 5 This approach does not take account of the neuromuscular coordination that must be achieved to sit independently, including coordinating the many sacral, lumbar, abdominal, thoracic and cervical muscles used in maintaining equilibrium.12, 13
To determine the constraints on balance abilities in children with neuromotor disabilities and to determine if rehabilitation therapies are efficacious in improving balance in these populations, a variety of clinical movement assessments have been created. These assessment tools measure a wide range of balance functions, from sitting through walking abilities, and incorporate evaluation of trunk control as one of the subtests within the assessment.14, 15 Although these tools are helpful in assessing balance control as a broadly defined ability, they have limitations in the assessment of sitting balance. In common with the research studies on balance development, they also tend to model the entire trunk as a single unit. This approach ignores the fact that development of trunk control in sitting may occur in a progressive manner, with initial development of head control, followed by gradual incorporation of thoracic, lumbar and sacral segments. Treatment directed at improving control of the trunk as a whole, rather than identifying and addressing problems within the various trunk subsections, may contribute to the difficulty in improving trunk control and sitting balance that is seen in the child with more severe motor disability.16
For example, trunk stability is assessed in terms of the child’s general ability to sit quietly or to move in and out of a sitting position.17 Thus, the Gross Motor Function Measure (GMFM) 14, 15, 18 and Chailey Levels of Ability19, 20 include items for quiet sitting which include measures of trunk control but do not differentiate between different levels of trunk control. The Slump test21 is a more specific test of trunk control, evaluating treatment efficacy over time, yet it also limits trunk control measurement to sitting trunk angles, pelvic tilt and kyphosis. Similarly, the Seated Postural Control Measure (SPCM) includes assessment of pelvic, trunk and head inclination, but does not examine subunits of trunk control.22 A more recent assessment tool, the Spinal Alignment and Range of Motion Measure (SAROMM) evaluates the child’s ability to actively or passively achieve alignment of the cervical, thoracic or lumbar spine.23 However, most of these tests only assess static (steady state) and active (or anticipatory) balance control, through evaluation measures of ability to hold a particular posture or to reach for an object while in that posture. Thus they do not include the ability to recover balance (reactive control), an important aspect of balance control in activities of daily living.
A more recent clinical evaluation tool for assessing the degree of trunk control24, 25 was designed to approach the assessment of trunk control by considering the many subunits that must be coordinated to achieve control when sitting and to include tests of static, active and reactive control. It is based on the biomechanics of control of vertical trunk posture.26 It tests the child’s trunk control as the evaluator progressively changes the level of trunk support from a high level of support at the shoulder girdle to assess cervical (head) control, through support at the axillae (upper thoracic control), inferior scapula (mid thoracic control), lower ribs (lower thoracic control), below ribs (upper lumbar control), pelvis (lower lumbar control), and finally, no support, in order to measure full trunk control. This evaluation tool has the advantage over the previously mentioned tools of assessing all three aspects of trunk control: 1. static (or steady state) control 2. active (or anticipatory) control and 3. reactive control (maintaining or regaining trunk control following a threat to balance, produced by a brisk nudge). This test, now called the Segmental Assessment of Trunk Control (SATCo), enables a more in-depth analysis of a child’s trunk control abilities and, in turn, this enables a new perspective on the treatment of deficiencies of trunk control. Current tools that assess the trunk as a single unit will inevitably lead to treatment methods that address the trunk as a single unit. A test such as the SATCo allows close definition of the level at which trunk control difficulties present and leads to a ‘level by level’ treatment approach to the development of trunk control.
Although this test has been used clinically by staff at The Movement Centre for the past 13 years, consistent methods of administration and guidelines for scoring the SATCo were required for general use by clinicians. Although inter-rater reliability had previously been conducted (87% point-by-point agreement)24 the test has not previously been rigorously tested for inter- and intra-rater reliability, validity, or its sensitivity. Thus the purpose of this study is 1) to refine the SATCo to ensure consistent methods of administration and guidelines for scoring, 2) to examine its reliability and 3) to examine its concurrent validity.
Methods
The data for this study were generated as part of an ongoing study of trunk control which examined both typically developing infants and children with motor disabilities. As part of this larger study, parents were invited to share their child’s SATCo video data between 2 research teams, The Movement Centre and the other at the University of Oregon.
The assessment procedure and score form are detailed in Appendix 1 and also available online at www.the-movement-centre.co.uk. For each trunk segmental level static, active and reactive control are scored as present, absent or not tested (NT). Static control is credited if the child can maintain a neutral trunk posture above the level of hand support; active control is credited if the child can maintain a neutral posture during head movement; reactive control is credited if the trunk above the support remains stable during an external perturbation (a nudge). It should be noted that, depending on the child evaluated, these various aspects of control may or may not be simultaneously present at the same or even at adjacent levels. The child’s ability to maintain or quickly regain a vertical position of the unsupported trunk in all planes is assessed during static, active and reactive testing and control accordingly scored as present or absent. The nudge is applied once from each principal direction (front, back, left, right) and the point of nudge application remains at the same horizontal level throughout. This means that, as the support level is lowered, the number of joints free of support and which thus require voluntary control will increase. At the same time the disturbing moment increases at the joint directly above the support as the length of the moment arm increases. In cases when vertical collapse of the trunk (where the center of mass of the head remains within the base of support) is noted during administration of the test an additional true sagittal video view is recommended. This aspect is discussed in greater detail in ‘Results’.
Participants
Study participants were a cohort of 8 infants with TD each of whom was followed twice a month from 3 months to 9 months of age to capture the period during which independent sitting was acquired and 24 children with neuromotor disability (1 year 6 months to 17 years 1 month, mean age 10 years 4 months). The ratio of males: females was 1:1 for the infants with TD and for the children with neuromotor disability. Table 1 shows subject demographics for the children with neuromotor disability. Both the Gross Motor Function Classification System (GMFCS) and Manual Ability Classification System (MACS) levels are given in order to describe each child’s functional level more fully. The inclusion criterion was neuromotor disability resulting in problems of postural control in sitting. No specific exclusion criteria were applied. Infants with TD were recruited by advertisement within University of Oregon and the children with neuromotor disability were recruited by advertisement to medical and educational professionals throughout the state of Oregon. The study was conducted in accord with the Declaration of Helsinki guidelines and had Ethical Approval from the Human Subjects Committee at the University of Oregon. Written consent to participation in research and use of data in publication was obtained from participants and/or their legal guardians prior to data collection.
Table 1.
Subject Demographics
| Subject | Age (years/months) | Sex | Diagnosis | Mobility skill level (GMFCS)1 | Manual skill level (MACS)2 |
|---|---|---|---|---|---|
| 1 | 8y 5m | M | CP Spastic quadriplegia / dystonia | 5 | 5 |
| 2 | 11y 2m | F | CP Asymmetric quadriplegia | 5 | 4 |
| 3 | 1y 6m | F | Neurodevelopment delay / cortical visual impairment | Too young | 4 |
| 4 | 7y 8m | M | CP Spastic quadriplegia / dystonia | 4 | 3 |
| 5 | 15y 3m | M | CP Spastic triplegia / dystonia | 4 | 4 |
| 6 | 11y 8m | F | Neurological deficit post encephalitis Upper and Lower Motor Neuron lesions | 4 | 3 |
| 7 | 7y 6m | M | CP Spastic quadriplegia | 5 | 5 |
| 8 | 12y 4m | F | CP Spastic quadriplegia | 4 | 3 |
| 9 | 9y 10m | M | CP Spastic quadriplegia / extrapyramidal | 5 | 5 |
| 10 | 6y 6m | M | CP Hypotonia / seizure disorder | 5 | 4 |
| 11 | 6y 7m | F | CP Spastic quadriplegia | 5 | 4 |
| 12 | 16y 4m | F | CP Extrapyramidal Dystonia | 4 | 5 |
| 13 | 8y 10m | F | CP Spastic quadriplegia | 4 | 3 |
| 14 | 4y 3m | F | CP Hypotonia / mild dystonia / cortical visual impairment | 5 | 4 |
| 15 | 5y 0m | F | Aicardi syndrome, severe visual impairment | 5 | 5 |
| 16 | 8y 1m | M | CP Spastic quadriplegia / seizure disorder | 5 | 4 |
| 17 | 8y 7m | F | CP Spastic diplegia | 4 | 4 |
| 18 | 12y 5m | M | CP Spastic quadriplegia / seizure disorder | 4 | 4 |
| 19 | 8y 1m | M | CP Spastic diplegia / seizure disorder | 3 | 3 |
| 20 | 17y 1m | M | CP Spastic diplegia | 1 | 2 |
| 21 | 17y 0m | F | CP Spastic diplegia | 2 | 3 |
| 22 | 16y 7m | F | CP Extrapyramidal | 1 | 2 |
| 23 | 14y 1m | M | CP Asymmetric Diplegia | 1 | 1 |
| 24 | 14y 0m | M | CP Spastic Diplegia / seizure disorder | 3 | 2 |
Gross Motor Function Classification System (www.canchild.ca)
Manual Ability Classification System (www.macs.nu)
CP: cerebral palsy
Raters
Both research teams were involved as raters for the refinement phase and for the reliability evaluation. The raters thus included 3 physical therapists, 1 with 27 years of experience in pediatrics who had previous exposure to the test, 1 with 20 years experience in pediatrics and who originally created the assessment and the third with 6 years experience in pediatrics with clinical experience of the test, a physical therapy assistant with 7 years experience and experience in clinical use of the test, 1 graduate and 3 undergraduate research assistants studying Human Physiology, none of whom had clinical background, and a professor (Department of Human Physiology) with more than 30 years experience in posture control research.
Procedure
The refinement phase of the project consisted of an initial 6 month pilot period commencing October 2007, in which 30 video assessments, including both infants with TD and children with neuromotor disability, were used to aid the further development of this measure. These video assessments were additional to those used for reliability testing but drawn from the same groups of children. Numerous discussions occurred between the research teams at The Movement Centre and the University of Oregon about uncertainty and discrepancies in scoring particular children. This contributed to the process of ensuring consistent methods of administration and guidelines for scoring the SATCo. This process continued until consensus was reached. Interactive oral presentations were then given to pediatric therapists in both clinical and educational settings. Audience comments and suggestions were used to further refine the SATCo instructions.
The second phase of the project was to test the reliability of the refined measure. Data analysis for reliability purposes included a total of 102 assessments, all of which were evaluated by at least 6 of the raters over a 6 month period from April 2008. Forty-three of the assessments consisted of data collected from the 8 infants with TD. The remaining 59 assessments were from 24 children with neuromotor disability with 2 or 3 tests for each child spread evenly over a 6 month period. Raters had no knowledge of previous scores. Each assessment was recorded on video and scored retrospectively and independently by each rater. No video assessments generated for this study were excluded. The raters observed the physical behavior of the child during the SATCo and scored the assessment based on the set of detailed instructions (Appendix 1) and established rules (Appendix 2) that resulted from the refinement phase. Data and comments for each level of support were documented on the assessment form. At the end of the assessment, the rater produced a summary indicating the level(s) of trunk support at which static, active and reactive control were lost. For the purpose of reliability testing, each trunk level was assigned a numerical value by designating head control as ‘1’, upper thoracic control ‘2’, through to lower lumbar control ‘7’ and full trunk control ‘8’. Following reliability testing, video review was conducted for each assessment where either ‘present’ or ‘absent’ control was spread over 4 or more adjacent levels among the 6 to 8 raters. This was repeated for static, active and reactive control. As an example, one rater may thus have assessed loss of static control at upper thoracic level, another at lower thoracic level and a third at upper lumbar level. From this review possible sources of rater discrepancy were identified.
For the purposes of establishing validity the Alberta Infant Motor Scales (AIMS)27 were completed at the same time as SATCo video recordings were made in 52 sessions with the 8 infants with TD. The Sitting Dimension (B) of the Gross Motor Function Measure was video recorded at the same session as a SATCo recording of the 24 children with neuromotor disability. The Mobility Domain (Child Scores and Caregiver Scores) of the Pediatric Evaluation of Disability Inventory (PEDI)28 was also administered at this session.
Statistical analysis
The intraclass correlation coefficient (ICC) was chosen as the most appropriate for this inter-rater and intra-rater reliability study. The random set Shrout-Fleiss29 ICC (2,1) assumes that the variance of interest is the variance due to the difference in subjects, whereas the residual variance is the variance due to the difference in raters. The raters (minimum of 6 raters for each of the 102 videos) are assumed to be a random sample of a greater population of raters. An intraclass correlation ≥.75 was considered acceptable for all reliability coefficients and is comparable with other reported measures of motor function in children with neuromotor deficits.15 Intra-rater reliability was evaluated by 2 raters re-scoring the same 22 randomly selected videos (10 TD infant and 12 children with neuromotor disability). For this analysis time was considered a ‘fixed’ factor (time 1 and time 2) and subject and rater were considered random factors. An ICC analysis was then completed comparing time 1 to time 2 across both raters and all aspects of control. A Pearson’s product moment correlation coefficient was used to examine the concurrent validity between the SATCo and the functional tests of the AIMS and GMFM Dimension B. Correlation was also examined between the SATCo and the GMFCS and the PEDI Mobility Domain to establish whether the SATCo reflected the severity of neuromotor disability.
Results
Refinement Phase
The final version of the SATCo form and scoring guidelines are given in Appendix 1 and 2.
Reliability of the SATCo
Inter-rater reliability was excellent for the total data set at .84 and for both subsets of infants with TD and children with neuromotor disability (Table 2). Intra-rater reliability was also excellent at .98 across all data sets and aspects of control.
Table 2.
Reliability statistics
| Inter-rater reliability | |||
|---|---|---|---|
| Static ICC(2,1) | Active ICC(2,1) | Reactive ICC(2,1) | |
| Total data set | .84 | .84 | .84 |
| TD infant | .88 | .85 | .88 |
| Children with neuromotor disability | .80 | .82 | .80 |
Shrout Fleiss Intraclass Correlation Coefficient (ICC) for SATCo scores (static, active and reactive) for combined data set and for each subgroup (infants with TD, children with neuromotor disability). Raters: n=8. Videos: Infants with TD n=43, Children with neuromotor disability n=59
Complete agreement of raters occurred across each of the trunk levels defined in Appendix 1 for 17% of assessments. Review of those assessments with complete agreement in scores (present or absent) indicated that children with either definite control or total loss of control at a specific level were easier to score. Where control loss was disseminated throughout the trunk, for example with loss of reactive control at upper thoracic level but loss of static control at upper lumbar level, scoring was more subject to discrepancy between scorers. Inspection of those assessments in which scores spread over four or more adjacent levels (12% of videos), revealed similar sources of rater discrepancy in infants with TD and children with neuromotor disability. In these cases children used a variety of strategies to assist in balance when trunk control became compromised. Discrepancy in scores was attributed to poor methodology in conducting or scoring the SATCo (Appendix 2): 1) failure of the supporting therapist to adequately align and extend the trunk; 2) failure of raters to note compensatory strategies used by the child, particularly of trunk alignment and hand placement; 3) failure of raters to accurately determine the level of control being assessed in children with immaturity of the skeletal structure (ribs not yet elongated) and/or obscuration by adipose tissue. Two additional areas of disagreement occurred only in rating children with neuromotor disability: 1) discriminating loss of head control from habitual posture and 2) difficulty in discriminating between true loss of trunk control and head movement/posture related to cortical visual impairment (CVI). Children with CVI may use a preferred head posture such as side flexion of the cervical spine to maximize their visual abilities.
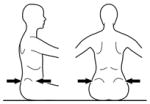
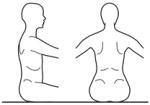
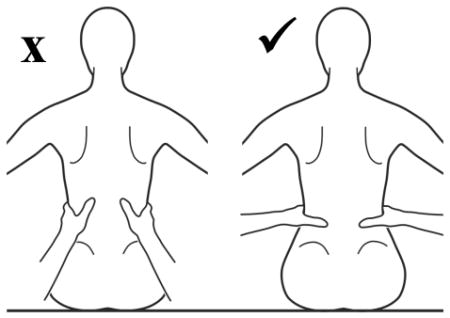
The use of compensatory strategies was noted when trunk control was compromised and, interestingly both groups of children used similar strategies with no strategy unique to either group (see Appendix 2). Strategies were incorporated by infants with TD primarily at the level where control became compromised with total loss of control occurring if support was further lowered. However, in some children with neuromotor disability, strategies incorporated at one level could be continued below this level giving an illusion of greater control than actually existed. The 3 most commonly observed strategies were 1) using hands for support (figure 1), 2) inclining the trunk forward or backward (figure 2) and 3) vertical collapse into excessive kyphosis and/or lordosis (center of mass of head remained centered over base of support) (figure 3).
Figure 1.
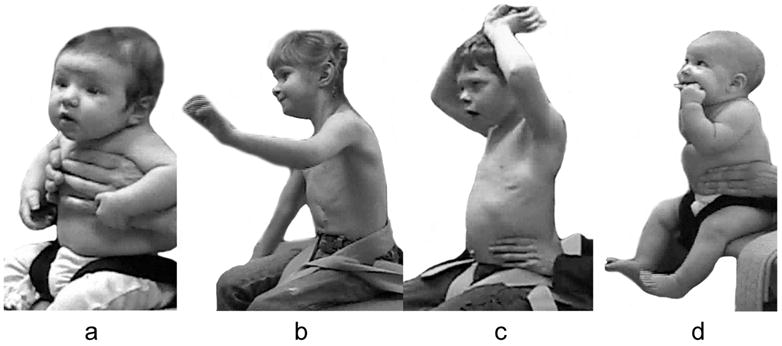
Strategies for gaining support from hands and arms included exerting pressure from arms or hands against the evaluator’s hands (a), placing a hand on the bench (b) or on any part of the child’s own body (legs, trunk, head / mouth or other arm) (c and d) which has the effect of cross-bracing the trunk.
Figure 2.
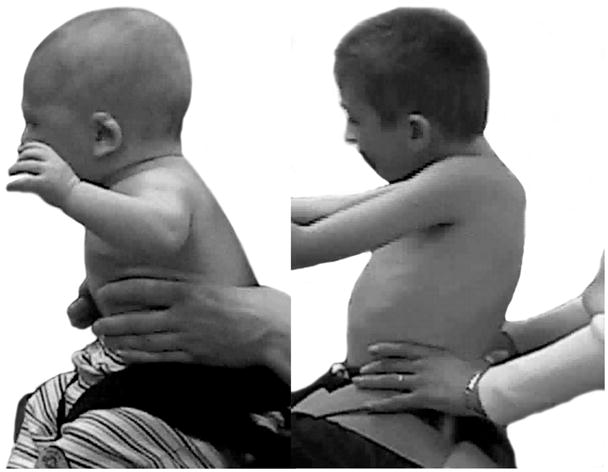
Strategies of inclining the trunk forward or backward allow a child to reduce the complexity of active control.
Figure 3.
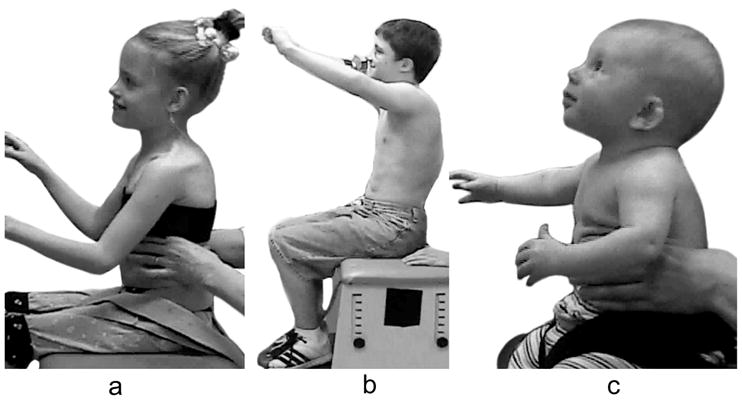
Collapsing the trunk over several specific segments was an active strategy rather than a passive collapse that presented either as a generalised rounding or with angulation. It can feature throughout the trunk as lordosis or kyphosis. This strategy was used more frequently in children with neuromotor disorder and seen briefly in only one infant with TD (c).
Validity of the SATCo
The Pearson’s product moment correlation coefficient showed high correlation with all three aspects of control and the AIMS and GMFM Dimension B (Table 3). The high correlation with the GMFCS and the Mobility Dimension of the PEDI show that the SATCo reflects the severity of disability and motor function.
Table 3.
Concurrent validity statistics
| Static Control | Active Control | Reactive Control | |
|---|---|---|---|
| Infants with TD (8 infants) | |||
| Alberta Infant Motor Scales (AIMS for infant videos) (sitting portion) | .883* | .860* | .868* |
| Children with Neuromotor disability (24 children, 21with cerebral palsy) | |||
| Gross Motor Function Measure (GMFM66) Dimension B (sitting) | .833* | .773* | .731* |
| GMFCS | −.817* | −.726* | −.705* |
| PEDI Mobility Domain Child | .803* | .717* | .695* |
| PEDI Mobility Domain Caregiver | .784* | .691* | .656* |
Pearson correlation is shown between SATCo scores (static, active and reactive) compared to functional tests of sitting behavior (for infants with TD and for children with neuromotor disability).
indicates p < .01
Discussion
The SATCo is a clinical evaluation tool that tests a child’s trunk postural control as the evaluator progressively reduces the level of trunk support from full support to free sitting. The purpose of this study is to ensure consistent methods of administration and guidelines for scoring the SATCo and to examine its reliability and concurrent validity. Evidence has been provided showing that the SATCo is a reliable and valid clinical measure that can be used with infants with TD or children with neuromotor disabilities. This level of reliability confirms that the administration and scoring instructions are clear and consistent. The SATCo has the advantage that it allows documentation of trunk control in children who have not yet achieved independent sitting as well as those who are able to sit. It has excellent reliability in infants with TD and in children with neuromotor disability. The high validity correlation strengthens the results presented showing that the SATCo reflects function and ability.
The limitations of this test are that specific equipment is necessary to administer the test accurately (see Appendix 1). This equipment is a bench of appropriate height and, preferably, a strapping system attached to the bench in order to ensure that the child’s pelvis is maintained in a neutral position. However, it is possible to support the smaller, lightweight child manually and maintain pelvic position without a strapping system. Two testers are needed and use of a video camera is helpful for later review of the test. Although the nudge used in the reactive component of the SATCo will vary between evaluators, provided that the nudge is brisk and not a gentle tap, the reaction of the child can still be assessed with confidence. When the test is used in the clinical situation, it is often the same evaluator who applies the nudge giving a more consistent assessment.
To assess the reliability of a scaled measure it is important to include assessments from children with a broad range of abilities. This data set had a minimum of 3 outcomes for each aspect of control (static, active, reactive) for each level of support and thus allowed reliability assessment across the full range of trunk control (Table 2). It should be noted that the subset of data from infants who were TD was secured from an ongoing longitudinal study which recruited infants at 3 months of age, by which time head control had been established. It was not felt reasonable to recruit infants below this age and thus infants with TD are less represented at the uppermost level of control.
Reliability studies commonly use a limited number of experienced or highly trained raters. Four of the raters in this study had no clinical background. All raters either had previous experience or were trained to administer SATCos during the pilot period of development of the test. Reliability might have been challenged by the inclusion of a large number of raters, some of whom had no clinical background but ensured the development of clear and definitive scoring guidelines. The fact that raters had some training to administer SATCos may be perceived as a limitation to generalization of this test since they have more experience with the assessment than clinicians who might choose to use the assessment. However, provided that clinicians are diligent in following the test protocol and instructions, it is anticipated that they could reach the same inter or intra-rater reliability as reported for this study without extensive training or practice.
The results of this study suggest that scoring might be improved by providing raters with prior information regarding the presence of visual impairment with recommendation to score the test based on trunk alignment without regard for head position. Although, in this study only vertical collapse into thoracic kyphosis was observed, the first author has noted a neutral thoracic posture with vertical collapse into lumbar lordosis or lumbar kyphosis as an alternative strategy, particularly in children who become ambulatory.
Although the SATCo is not looking at a ‘real-life’ situation of sitting ability nor does it take account of environmental or contextual influences, it contributes to the understanding of the child’s specific disability. Current functional assessments of static and dynamic ability to sit provide some inference of trunk control16, 20 while more specific tests of sitting posture21, 22, 23 document static and active trunk alignment. None of these tests has directly assessed the level of control within the trunk. The SATCo complements and extends currently available tests by allowing discrete assessment of static, active and reactive trunk control in children with and without the ability to sit independently. In addition, the SATCo allows reliable assessment of trunk control in very young children and/or children with cognitive deficits since little is required of the child in terms of cooperation.
In the context of the International Classification of Functioning, Disability and Health (ICF) the SATCo is a Body Function measure. A deficit in trunk control, defined by the SATCo, is likely to influence Activity Limitations and Participation Restriction. It will affect all Activities of Mobility and will extend to Major Life Areas such as education since poor trunk control will affect stability of the head in space thus affecting visual skills, eye-hand coordination, reach and hand function. Poor trunk control may also interfere with eye contact and efficient respiratory support for verbalization and thus may adversely influence social interaction. Although compensatory strategies such as trunk collapse or hand support can be of value in daily functioning, the SATCo seeks to define the true level of trunk control. This will help to explain some of the functional limitations seen. For example, a child with control problems in the lower lumbar level is likely to be able to sit independently, if insecurely, whereas a child with control problems in the upper thoracic level will have much greater functional loss and be unable to sit independently. The more detailed information about control of the trunk provided by the SATCo helps with treatment planning which can then be directed at improving control in the specific area of deficit rather than treating the trunk as a single unit. Once control has been acquired at the uppermost level of loss, therapy can then be directed at the next level of control loss in a caudal direction. This has been shown to be an effective strategy for gaining independent sitting ability in the severely neuromotor disabled child.24 The SATCo also allows a finer-grained documentation of progress, particularly if progress is slow.
A model of motor control will influence both assessment and treatment.11 The value of assessing trunk control in this way, level by level, is that it can influence treatment planning and this can apply not only to the treatment of deficiencies of trunk control but also to activities where trunk posture will be influential. The technique of analyzing trunk control on a level by level basis enhances observational skills so that knowledge of trunk control and trunk posture are incorporated into more generalized assessments, for example of gait or upper limb activities. At The Movement Centre the treatment of trunk control problems has co-evolved on a level by level basis with this assessment tool.24, 25 Specially designed therapy equipment is adjusted to give support at the appropriate level determined by the SATCo and progressed by lowering the top support height of equipment according to the change in SATCo. The clinical results achieved are most encouraging (see www.the-movement-centre.co.uk) and independent research into this method of treating trunk control problems is currently in process. As interest in this method of treatment increases, and manufacturers consider making the equipment generally available, knowledge and use of the SATCo by therapists will become increasingly important. The underlying philosophy of this assessment and treatment goes beyond specific therapy sessions and will affect, for example, classroom seating and/or standing frames in order to maximize trunk control training in a variety of situations.
The evidence presented here supports the use of the SATCo as a valid discriminative tool. To use it for evaluative purposes, sensitivity to change must be demonstrated. Testing for responsiveness in infants with TD and children with neuromotor disability is currently in progress.
Conclusions
The SATCo has been shown to be a reliable and valid clinical measure of trunk control in infants with TD as well as children with neuromotor disability, with overall scores for reliability of >.80. The assessment provides a discrete examination from head control through thoracic, lumbar and finally full trunk control and documents static, active and reactive control at each level tested. An advantage of the SATCo is that it can be used for children with a broad range of abilities including those with more severe motor and cognitive deficits. By discriminating the specific level and aspect of trunk control, this assessment provides therapists with valuable information for creating an effective rehabilitation program.
Acknowledgments
Grant Support: This research was funded by: 1) National Institutes of Health Grant 2R01 NS038714 (M.H. Woollacott, PI), National Institutes of Health National Research Services Award F31NS056726 (S. Saavedra) and The Movement Foundation (Charity Registration Number 1075549) (P.B. Butler and S.E. Jarvis).
We would like to acknowledge statistical consultation by Robin High, the assistance of the Medical Illustration Department at the Robert Jones and Agnes Hunt Orthopaedic and District Hospital NHS Trust, Oswestry, the contributions of the spinal control team (Michaela Krna, Francine Porter, Sarah Rajnus and Lynne Ford) and participation of the families and children who offered their time to the study. We would also like to thank the reviewers for their valuable comments and suggestions, not least in renaming of this assessment. Readers may previously have encountered it as the Segmental Assessment of Spinal Control (SASCo).
Appendix 1. Assessment of Trunk Control
| Client Name: Ref #: Tester Name: Date: |
Level of manual support Pelvic / thigh strap used except as indicated |
Functional Level Arms and hands in air except as indicated |
Static | Active | Reactive | Comments |
|---|---|---|---|---|---|---|
| Maintain vertical neutral position of head and trunk above manual support level | ||||||
| minimum of 5 seconds | while turning head with arms lifted | Maintain / quickly regain following brisk nudge | ||||
 |
Shoulder girdle Testers hand position may vary from horizontal |
Head control Arms may be supported throughout |
NOT Tested for Head Control | |||
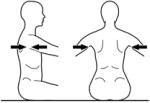 |
Axillae | Upper Thoracic Control | ||||
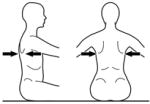 |
Inferior scapula | Mid Thoracic Control | ||||
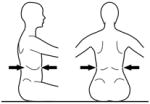 |
Over lower ribs | Lower thoracic Control | ||||
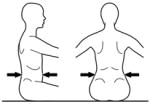 |
Below ribs | Upper lumbar Control | ||||
 |
Pelvis | Lower lumbar Control | ||||
 |
No support given and pelvic/thigh straps removed | Full trunk control | ||||
| Fixed spinal deformity? Yes_____No_____Comments__________ | ||||||
| Limitation of Cervical Rotation __Left__Right Comments__________ | ||||||
Instructions
Subject
The subject is seated on a bench, feet supported on the ground or on a stable surface and pelvis/thigh position controlled by the strapping system. The pelvis is orientated to neutral relative to vertical. The subject is supported in an upright posture “sitting up tall” with the presence of normal cervical, thoracic and lumbar curves. The head is upright. The subject’s hands and arms should be free of all external contact including with own trunk, thighs, bench or the tester’s arms/hands throughout the test except as indicated. The subject’s hands should not be joined together.
Tester
The tester applies firm manual support horizontally around the trunk at each of the designated levels in turn. The support given should be sufficient to ensure that the trunk is in a neutral vertical posture and that any collapse of the trunk is eliminated. The subject’s hands/arms should be lifted so that they there is no contact with the subject’s body or legs, the bench or the tester’s hands. Toys can be used to motivate a child ensuring that the child stretches/turns towards the toy but does not grasp it. At each support level the tester encourages the subject to sit up tall and lift the hands/arms during testing of a) static control, b) active control, by turning the head slowly to each side (>45° or to limitation of range) and c) reactive control by remaining stable during nudges. This requires an assistant to apply a single brisk nudge from front (manubrium/sternum), from behind (~C7), and from each side (acromion) using the fingertips, sufficient to briefly disturb balance. If a subject has minimal balance impairments they sway excessively but can return to vertical. If, however, they have moderate to severe balance impairments they lose balance and go to the limits of their range of motion. The test continues with lowering of support level until the subject clearly cannot maintain or quickly return to the starting posture. The tester should be behind the subject, usually in kneeling depending on the size of the subject and height of the bench and the assistant ideally out of line of the subject’s vision.
Scoring
At each level of support the presence (✓) or absence (−) of control is recorded. ‘NT’ indicates Not Tested. Presence of control is shown by:
Static
maintains a neutral vertical trunk posture in the sagittal and frontal planes for five seconds. If the subject’s attention is briefly lost, accompanied by a head turn, but a vertical position is maintained, this is still scored as presence of control.
Active
may be slight displacement from neutral (<20°) but realigns immediately by most direct route e.g. trunk flexion is corrected by extending to a neutral trunk posture rather than by circling through trunk side flexion.
Reactive
subject will move away from neutral vertical but quickly returns to upright by most direct route.
Optional Video Instructions
If video is available it is recommended that the assessment be videotaped. This secures visual documentation for future reference and also allows review of the test in case of ambiguity in scoring. If video tape is used, a camera set up at a 45 degree angle to the subject will usually allow movement to be judged from the front and side views sufficient to detect movement strategies.
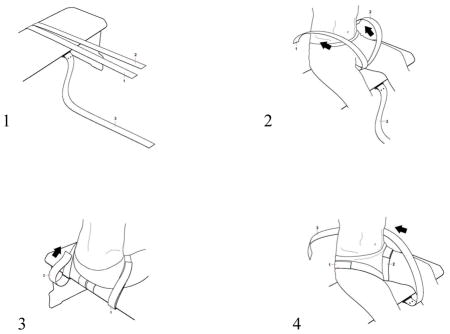
Strapping Instructions
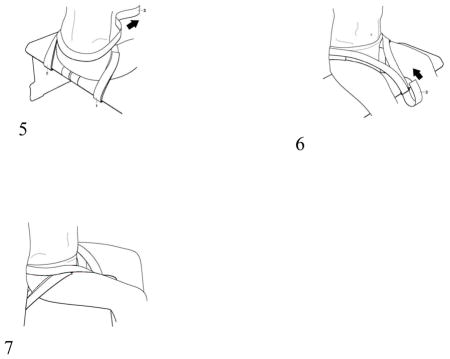
Three straps and three D rings should be firmly attached to the underside of a bench to allow the subject to be strapped to the bench as follows. Pull the thigh straps forward across the top of the bench. Subject should sit on the bench with the thigh straps underneath them. Pull each strap up from between the subject’s legs, over the top of the thigh through each D ring loop at the back of the bench and secure snugly. Next, pull the pelvic strap up from the front of the bench, wrap it behind the subject’s pelvis and back down through the D ring loop at the front of the bench. Keep the strap low enough to pull against the sacrum and do not allow it to slide up to the lumbar area. Adjust the tightness of this strap until the pelvis is aligned vertically. The purpose of the strap is only as ‘another pair of hands’ to ensure the pelvis is vertical.
Appendix 2: Scoring Guidelines
Definition of Control
stable neutral vertical alignment (brief deviation no more than 20°) in both frontal and sagittal planes (eyes level). Allow for normal cervical, thoracic and lumbar curves.
You score only what you see
If control is not demonstrated, score as absence of control (−) or not tested (NT). If you believe the child has control but performance demonstrating control cannot be elicited and a compensatory strategy persists during testing then it must be scored NT. Likewise if the tester made an error of alignment that prevents assessment of true vertical control it must be scored NT. NT should always contain a comment regarding the nature of the error for future reference.
Watch for compensatory strategies which may indicate a lack of normal control
-
Hand support
On bench
In mouth
On body (own or tester’s)
Together (on toy / object or clasped)
On toy / object held by tester
-
Trunk alignment
Leaning forward
Arching backward over manual support
Collapse beyond normal curves
-
Movement strategies
Stiffening (rigidity with lack of movement of the trunk above the level of support)
Rapid movement rather than a slower controlled movement e.g. of the head
Critical tester errors
-
Hand support
Not horizontal
Not stable
-
Trunk alignment
Trunk below support not held vertical and/or trunk collapse not eliminated
-
Movement
Poor placement and/or magnitude of nudge
Nudge during non-vertical alignment
Critical scorer errors leading to incorrect determination of control level
Immaturity of the skeletal structure (ribs not yet elongated)
Obscuration by adipose tissue
Discriminating loss of head control from habitual posture
Discriminating loss of control from head movement/posture related to cortical visual impairment
Level of Control Specification
The focus is to determine the highest level at which subject demonstrates loss of control and this is scored as absent control (−)
Not Tested (NT) at a level above a check mark (✓ control present) is counted as having control at that level
Not Tested (NT) at a level below check mark is counted as loss of control at that level
If static balance is NT but subject held the alignment during reactive or active then static is given credit as having control (✓)
References
- 1.Harbourne RT, Giuliani C, Neela JM. A kinematic and electromyographic analysis of the development of sitting posture in infants. Dev Psychobiol. 1993;26:51–64. doi: 10.1002/dev.420260105. [DOI] [PubMed] [Google Scholar]
- 2.Harbourne RT, Stergiou N. Nonlinear analysis of the development of sitting postural control. Dev Psychobiol. 2003;42:368–377. doi: 10.1002/dev.10110. [DOI] [PubMed] [Google Scholar]
- 3.Hadders-Algra M, Brogren E, Forssberg H. Development of postural control-differences between ventral and dorsal muscles? Neurosci Biobehav Rev. 1998;22:501–506. doi: 10.1016/s0149-7634(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 4.Woollacott M, Debu B, Mowatt M. Neuromuscular control of posture in the infant and child: is vision dominant? J Mot Behav. 1987;19(2):167–186. doi: 10.1080/00222895.1987.10735406. [DOI] [PubMed] [Google Scholar]
- 5.Hirschfeld H, Forssberg H. Epigenetic development of postural responses for sitting during infancy. Exp Brain Res. 1994;97:528–540. doi: 10.1007/BF00241546. [DOI] [PubMed] [Google Scholar]
- 6.Brogren E, Hadders-Algra M, Forssberg H. Postural control in children with spastic diplegia: muscle activity during perturbations in sitting. Dev Med Child Neurol. 1996;38:379–388. doi: 10.1111/j.1469-8749.1996.tb15095.x. [DOI] [PubMed] [Google Scholar]
- 7.Brogren E, Hadders-Algra M, Forssberg H. Postural control in sitting children with cerebral palsy. Neurosci Biobehav Rev. 1998;22:591–596. doi: 10.1016/s0149-7634(97)00049-3. [DOI] [PubMed] [Google Scholar]
- 8.Brogren E, Forssberg H, Hadders-Algra M. Influence of two different sitting positions on postural adjustments in children with spastic diplegia. Dev Med Child Neurol. 2001;43:534–546. doi: 10.1017/s0012162201000974. [DOI] [PubMed] [Google Scholar]
- 9.Liao SF, Yang TF, Hsu TC, Chan RC, Wei TS. Differences in seated postural control in children with spastic cerebral palsy and children who are typically developing. Am J Phys Med Rehabil. 2003;82:622–626. doi: 10.1097/01.PHM.0000073817.51377.51. [DOI] [PubMed] [Google Scholar]
- 10.Carlberg EB, Hadders-Algra M. Postural dysfunction in children with cerebral palsy: some implications for therapeutic guidance. Neural Plast. 2005;12:221–228. doi: 10.1155/NP.2005.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shumway-Cook A, Woollacott MH. Motor Control: Translating Research into Practice. Philadelphia: Lippincott/Williams and Wilkins; 2006. [Google Scholar]
- 12.Saavedra S, Woollacott M, van Donkelaar P. Effects of postural support on eye-hand interactions across development. Exp Brain Res. 2007;180:557–567. doi: 10.1007/s00221-007-0874-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preuss R, Fung J. Musculature and biomechanics of the trunk in the maintenance of upright posture. JElectromyogr Kinesiol. 2008;18:815–828. doi: 10.1016/j.jelekin.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Palisano RJ, Rosenbaum PL, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 15.Russell DJ, Rosenbaum PL, Avery LM, Lane M. Gross Motor Function Measure (GMFM-66 & GMFM-88) User’s Manual. New York: Mac Keith Press; 2002. [Google Scholar]
- 16.Rosenbaum P, Walter SD, Hanna SE, Palizano RJ, Russell DJ, Raina P, Wood E, Bartlett DJ, Galuppi BE. Prognosis for gross motor function in cerebral palsy: Creation of motor development curves. JAMA. 2002;288(11):1357–1363. doi: 10.1001/jama.288.11.1357. [DOI] [PubMed] [Google Scholar]
- 17.Msall M, Rogers B, Ribstein H, Lyon N, Wilczenski F. Measurements of functional outcomes in children with cerebral palsy. Ment Retard Dev Disabil Res Rev. 1997;8:194–203. [Google Scholar]
- 18.Russell DJ, Rosenbaum PL, Cadman DT, Gowland C, Hardy S, Jarvis S. The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol. 1989;31:341–352. doi: 10.1111/j.1469-8749.1989.tb04003.x. [DOI] [PubMed] [Google Scholar]
- 19.Pountney TE, Cheek L, Green E, Mulcahy C, Nelham R. Content and criterion validation of the Chailey levels of ability. Physiotherapy. 1999;85:410–416. [Google Scholar]
- 20.Pountney TE, Mulcahy CM, Clarke SM, Green EM. The Chailey Approach to Postural Management. North Chailey: Chailey Heritage Clinical Services; 2004. [Google Scholar]
- 21.White MA, Paper KE. The slump test. Am J Occup Ther. 1992;46:271–274. doi: 10.5014/ajot.46.3.271. [DOI] [PubMed] [Google Scholar]
- 22.Fife SE, Roxborough LA, Armstrong RW, Harris SR, Gregson JL, Field D. Development of a clinical measure of postural control for assessment of adaptive seating in children with neuromotor disabilities. Phys Ther. 1991;71:981–993. doi: 10.1093/ptj/71.12.981. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett D, Purdie B. Testing of the Spinal Alignment and Range of Motion Measure: a discriminative measure of posture and flexibility for children with cerebral palsy. Dev Med Child Neurol. 2005;47:739–743. doi: 10.1017/S0012162205001556. [DOI] [PubMed] [Google Scholar]
- 24.Butler PB. A preliminary report on the effectiveness of trunk targeting in achieving independent sitting balance in children with cerebral palsy. Clin Rehabil. 1998;12:281–293. doi: 10.1191/026921598667577442. [DOI] [PubMed] [Google Scholar]
- 25.Major RE, Johnson GR, Butler PB. Learning motor control in the upright position: a mechanical engineering approach. Proc Inst Mech Eng [H] 2001;215:315–323. doi: 10.1243/0954411011535911. [DOI] [PubMed] [Google Scholar]
- 26.Butler PB, Major RE. The learning of motor control. Biomechanical considerations. Physiotherapy. 1992;78:1–6. [Google Scholar]
- 27.Piper MC, Darrah J. Motor Assessment of the Developing Infant (AIMS) Philadelphia: WB Saunders Company; 1994. [Google Scholar]
- 28.Haley SM, Coster WJ, Ludlow LH, Haltiwanger JT, Andrellos PJ. Pediatric Evaluation of Disability Inventory: Development, Standardization, and Administration Manual, Version 1.0. Boston, MA: Trustees of Boston University, Health and Disability Research Institute; 1992. [Google Scholar]
- 29.Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]


