Abstract
Background
Transiently low levels of thyroid hormones occur in ∼50% of neonates born 24–28 weeks' gestation and are associated with higher rates of cerebral palsy and cognitive impairment. Raising hormone levels shows promise for improving neurodevelopmental outcome.
Objective
To identify whether any of 4 thyroid hormone supplementation regimens could raise T4 and FT4 without suppressing TSH (biochemical euthyroidism).
Methods
Eligible subjects had gestational ages between 240/7 and 276/7 weeks and were randomized <24 hours of birth to one of six study arms (n = 20–27 per arm): placebo (vehicle: 5% dextrose), potassium iodide (30 μg/kg/d) and continuous or bolus daily infusions of either 4 or 8 μg/kg/d of T4 for 42 days. T4 was accompanied by 1 μg/kg/d T3 during the first 14 postnatal days and infused with 1 mg/mL albumin to prevent adherence to plastic tubing.
Results
FT4 was elevated in the first 7 days in all hormone-treated subjects; however, only the continuous 8 μg/kg/d treatment arm showed a significant elevation in all treatment epochs (P < .002 versus all other groups). TT4 remained elevated in the first 7 days in all hormone-treated subjects (P < .05 versus placebo or iodine arms). After 14 days, both 8 μg/kg/d arms as well as the continuous 4 μg/kg/d arm produced a sustained elevation of the mean and median TT4, >7 μg/dL (90 nM/L; P < .002 versus placebo). The least suppression of THS was achieved in the 4 μg/kg/d T4 continuous infusion arm. Although not pre-hypothesized, the duration of mechanical ventilation was significantly lower in the continuous 4 μg/kg/d T4 arm and in the 8 μg/kg/d T4 bolus arm (P < .05 versus remaining arms). ROP was significantly lower in the combined 4 thyroid hormone treatment arms than in the combined placebo and iodine arms (P < .04). NEC was higher in the combined 8 μg/kg/d arms (P < .05 versus other arms).
Conclusions
Elevation of TT4 with only modest suppression of TSH was associated with trends suggesting clinical benefits using a continuous supplement of low-dose thyroid hormone (4 μg/kg/d) for 42 days. Future trials will be needed to assess the long-term neurodevelopmental effects of such supplementation.
Keywords: thyroxine, triiodothyronine, hypothyroxinemia, thyroid hormone, monodeiodinase, extremely low birth weight neonate, transient hypothyroxinemia, nonthyroidal illness, euthyroid sick syndrome, cerebral palsy, prematurity, randomized, controlled trial
Survival for extremely low gestational age neonates (ELGANs) (24–28 weeks' gestation) was negligible before the 1960s and now exceeds 80%.1 This extraordinary achievement is tempered by the intractable persistence of cerebral palsy (CP) and its accompanying disabilities affecting nearly 1 in 8 survivors at a prorated lifetime cost of nearly $1 million per handicapped child.2–4 An important priority in newborn medicine, therefore, is to translate gains in survival into survival without today's current high rates of neurodevelopmental impairments.5
Low levels of thyroid hormone are commonly found in the first weeks after birth, a hormone phenomenon referred to as transient hypothyroxinemia of prematurity (THOP). Although the etiology of CP and its underlying substrate of white matter injury is mutilfactorial,6–10 THOP is a strong independent risk factor for CP, white matter injury, and lower cognitive performance, suggesting an unmet preventable cause of neurologic injury.11–19
Low thyroid hormone levels in ELGANs arise from a combination of (1) loss of maternal and placenta-supplied hormones; (2) immaturity of the hypothalamic-pituitary-thyroid axis, including limited thyroidal capacity to increase hormone synthesis; and (3) accelerated inactivation (elevated reverse T3, sulfated forms, diiodothyronine, and monoiodothyronine).20–23 Iodine imbalance, medications, and adverse perinatal events (ie, nonthyroidal illness syndrome) all exacerbate the effects of these developmental and conditional limitations on thyroid homeostasis.20–23
Several trials have treated THOP in ELGANs by using a variety of dosage regimens. In all cases, intervention lowered plasma thyrotropin levels to values consistent with a significant overshoot in treatment.16,21,24,25 Because excessive thyroid hormone exposure during fetal brain growth may be as undesirable for normal development as is deficiency,26–28 it is important to identify how to supplement endogenous production without interfering with the maturing homeostatic control systems.
We used a masked randomized trial to identify a dosing regimen that would first elevate serum levels of free circulating thyroxine (FT4) and total circulating T4 (TT4) to target values (≥1.5 ng/dL [19 pM/L] and ≥6 μg/dL [77 nM/L], respectively) while secondarily minimizing suppression of thyrotropin levels (<0.4 mIU/L) over the period of supplementation (42 days) to achieve the most physiologic pattern of homeostasis.
Methods
Study Design and Intervention
Thyroid hormone (Bedford Pharmaceuticals, Mansfield, MA) was administered in 4 treatment arms within 24 hours of birth as thyroxine (T4) at 4 or 8 μg/kg per day delivered either as a bolus (1 mL/kg in 5% dextrose solution in water intravenously or per os [PO]) or via continuous infusion (0.5 mL/kg per hour intravenously or PO). The dosage was increased by 25% when converting to oral administration.29 Triiodothyronine (T3) was provided to all 4 T4-treated arms at 1.0 μg/kg per day for the first 14 postnatal days as immediately available active hormone.20,21 Subjects in the 2 continuous-infusion arms who began feedings every 2 hours had the appropriate amount of study drug added to ingested milk every 4 hours. T4 and T3 were infused along with 1 mg/mL albumin to prevent a measured 40% loss to plastic tubing.30 One arm of the study was provided with iodine (Upsher-Smith, Maple Grove, MN), 30 μg/kg per day in 1 mL of aqueous oral solution. The placebo arm received 5% dextrose solution in water (0.5 mL/kg per hour intravenously or PO).
Study Population and Exclusions
The study was approved by the institutional review board of the 3 clinical enrollment sites and the data center. Neonates of 240/7 to 276/7 weeks of gestation were enrolled <24 hours after birth. Exclusions included mothers <18 years of age or those with thyroid disease or reported substance abuse (ie, alcoholism or use of heroin or methadone)31 and newborns with major congenital malformations or if death was expected within 48 hours. If death or withdrawal of consent occurred in the first week of life, the subject was replaced in its study arm by the next available eligible newborn.
Clinical Definitions and Management Strategies
Gestational age was based on the best estimate of the attending neonatologist using obstetrical parameters and examination. Necrotizing enterocolitis (NEC) was recorded if ≥ Bell's stage 2,32 retinopathy of prematurity (ROP) as ≥ stage 3 in either eye,33 and chronic lung disease (CLD) as oxygen requirement at 36 weeks to keep oximeter saturations ≥88% to 90%.34 Fluids and nutrition were provided according to guidelines.35–38 Soy-based formulas were excluded because they are associated with prolonged increases in thyrotropin.39 Vasopressors (ie, dopamine/dobutamine) were avoided because these drugs suppress thyrotropin.31
Iodine Precautions
Iodinated skin cleansers (eg, Povidone Iodine, Aplicore, Inc, Meriden CT) were avoided because excess iodine suppresses hormone synthesis.31 Instead, Chlorhexidine (2% wt/vol 70% vol/vol ethanol; Enturia, Inc, Leawood, KS) was used for skin disinfection for 30 seconds followed by a sterile water rinse.
Data Monitoring
Clinical status was recorded daily for 2 weeks and weekly thereafter. Cranial ultrasounds were conducted on postnatal days 1 to 3, 7 to 10, and ≥4 weeks, and were interpreted by a masked radiologist (Paula Brill, MD, Cornell University Medical Center, New York, NY). Free T4 was measured by using the equilibrium dialysis technique and the other hormones were measured by standard immunoassay technology (Quest Diagnostics, Chantilly, VA).40
Data Safety Monitoring Board
A data safety monitoring board (DSMB) and a separate independent medical monitor reviewed study progress and evaluated potential adverse events on a quarterly basis.
Statistical Considerations
A randomization/minimization program (SAS software [SAS Institute, Inc, Cary, NC]) balanced 2 gestational age strata (240/7–260/7 and 261/7–276/7 weeks), gender, and center. Multiple births were treated as individual subjects, because ex utero experiences over the first 6 postnatal weeks were considered unique to individuals.
Power calculations were based on a sample size necessary to detect a 25% difference in mean FT4 levels between each pair of the 6 study arms at a 5% significance level with a power of 0.8 allowing for up to a 20% dropout rate by the date of final assessment. Hormone results were examined as area under the curve over time, normalized to weekly values for comparison (analysis of variance; SAS 9.1). For comparing categorical outcomes (eg, death), we used the χ2 test. When overall significance was attained, preplanned pairwise comparisons between study arms were conducted.
Results
Enrollment
From April 2005 to March 2007, 168 subjects participated: 46% (n = 77) were enrolled in New York, 31% (n = 52) in Amsterdam, and 23% (n = 39) in Madrid (Fig 1).
FIGURE 1.
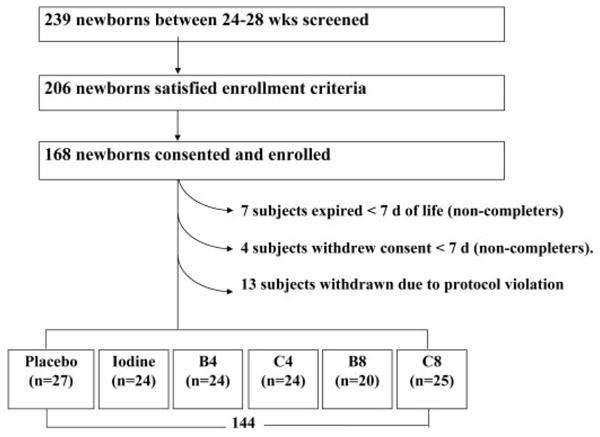
Flowchart of the THOP enrollment and treatment protocol. B4 indicates bolus 4 μg/kg per day; C4, continuous infusion 4 μg/kg per day; B8, bolus 8 μg/kg per day; and C8, continuous infusions 8 μ/kg per day.
Changes in Enrollment During the Trial
Eleven subjects were removed from the randomization/minimization file before 7 days by study design (7 deaths, 4 parental withdrawals). In addition, the DSMB interrupted trial enrollment for 2 reasons: (1) By the time 20 subjects had been entered in the bolus 8 μg/kg per day arm, that arm had experienced 6 deaths (postnatal ages: 10, 16, 25, 27, 28, and 31 days) at a time when no other arm had >3 deaths (not statistically significant); and (2) A pharmacy drug dilution error was discovered that resulted in a doubling of the dosage in the Madrid arm. From the onset of the trial until the error was discovered, infants slated to receive 4 and 8 μg/kg per day instead received 8 and 16 μg/kg per day of T4. Recipients of both 8 μg/kg per day in the initially designated 4 μg/kg group were retained in the 8 μg/kg per day arms. The 13 recipients of 16 μg/kg per day were excluded from the analysis and replaced by new enrollments. Adjusting the SAS randomization/minimization files and extending the enrollment period ensured that adequate numbers of subjects were assigned to each group.
Baseline Comparison of Study Arms
No mother in the study had abnormal T4 or FT4 values, but 8 mothers had serum thyrotropin levels of >5.0 mIU/L, suggesting subclinical hypothyroidism at delivery (Table 1). No arm of the study included infants from >2 of these women. Baseline newborn values of T4, FT4, T3, thyroid-binding globulin (TBG), thyrotropin, or cortisol did not differ across study arms (Tables 1 and 2).
TABLE 1.
Maternal Characteristics of THOP Study Subjects According to Treatment Arm
| Maternal Characteristic | Placebo (25 Women; 27 Subjects) | Iodine (24 Women; 24 Subjects) | Bolus 4 μg/kg per d (24 Women; 24 Subjects) | Continuous 4 μg/kg per d (14 Women; Subjects) | Bolus 8 μg/kg per d (19 Women; Subjects) | Continuous 8 μg/kg per d (24 Women; 25 Subjects) | Total (129 Women; 144 Subjects) |
|---|---|---|---|---|---|---|---|
| Maternal age, mean ± SEM, y | 31 ± 1 | 29 ± 1 | 28 ± 1 | 30 ± 1 | 30 ± 1 | 31 ± 1 | 30 ± 1 |
| Ethnicity, n (%) | |||||||
| White | 18 (67) | 15 (63) | 12 (50) | 13 (54) | 15 (75) | 15 (60) | 88 (61) |
| Black | 7 (26) | 6 (25) | 7 (29) | 8 (33) | 4 (20) | 4 (16) | 36 (25) |
| Hispanic | 2 (7) | 2 (8) | 2 (8) | 3 (12) | 1 (5) | 4 (16) | 14 (10) |
| Preeclampsia, n (%) | 5 (19) | 1 (4) | 3 (12) | 6 (25) | 1 (5) | 3 (12) | 19 (13) |
| Fever ≥ 100.4°F during pregnancy, n (%) | 3 (11) | 0 (0)a | 9 (38)a | 2 (8) | 1 (5) | 1 (4) | 16 (11) |
| Rupture of membrane > 24 h, n (%) | 4 (15) | 7 (30) | 10 (45) | 6 (29) | 6 (30) | 4 (19) | 37 (28) |
| Treatment before delivery, n (%) | |||||||
| Antibiotic | 11 (41) | 8 (33) | 18 (75)a | 13 (54) | 8 (40) | 10 (40) | 68 (47) |
| Antenatal steroids | 23 (85) | 21 (88) | 23 (96) | 21 (88) | 13 (65) | 20 (80) | 121 (84) |
| Hormone levels after delivery, mean ± SEM | |||||||
| FT4, ng/dL | 1.7 ± 0.4 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.5 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1 |
| TT4, μg/dL | 11.4 ± 0.6 | 11.5 ± 0.5 | 11.8 ± 0.6 | 11.2 ± 0.6 | 11.2 ± 0.8 | 11.1 ± 0.5 | 11.4 ± 0.2 |
| Thyrotropin, mIU/L | 2.5 ± 0.4 | 2.4 ± 0.3 | 2.7 ± 0.4 | 2.1 ± 0.4 | 2.0 ± 0.5 | 2.0 ± 0.2 | 2.3 ± 0.1 |
| Thyrotropin > 5.0 mIU/L | 2 (10) | 2 (9) | 1 (5) | 1 (5) | 2 (12) | 0 (0) | 8 (7) |
The total number of mothers is <144, because 1 mother can appear in up to 3 arms depending on her neonate's treatment assignment as a consequence of multiple gestation.
P < .05 for the occurrence of the sign or the mean of the variable in this arm is significantly different from that seen across all study arms.
TABLE 2.
Neonatal Characteristics of THOP Study Subjects According to Treatment Arm
| Neonatal Characteristic | Placebo (25 Women; 27 Subjects) | Iodine (24 Women; 24 Subjects) | Bolus 4 μg/kg per d (24 Women; 24 Subjects) | Continuous 4 μg/kg per d (24 Women; 24 Subjects) | Bolus 8 μg/kg per d (19 Women; 20 Subjects) | Continuous 8 μg/kg per d (24 Women; 25 Subjects) | Total (129 Women; 144 Subjects) |
|---|---|---|---|---|---|---|---|
| Birth weight, g | |||||||
| Mean ± SEM | 865 ± 35 | 870 ± 41 | 852 ± 44 | 888 ± 48 | 860 ± 39 | 819 ± 31 | 859 ± 16 |
| Median | 870 | 845 | 840 | 825 | 821 | 800 | 835 |
| Range | 558–1300 | 440–1325 | 460–1340 | 560–1550 | 530–1246 | 499–1190 | 440–1550 |
| Gestational age, wk | |||||||
| Mean ± SEM | 26 ± 0.2 | 26 ± 0.2 | 26 ± 0.2 | 26 ± 0.2 | 26 ± 0.2 | 26 ± 0.2 | 26 ± 0.1 |
| Median | 27 | 26 | 26 | 27 | 26 | 26 | 26 |
| Range | 24–28 | 24–28 | 24–28 | 25–28 | 24–28 | 24–27 | 24–28 |
| Hormone levels at birth, mean ± SEM | |||||||
| FT4, ng/dL | 1.5 ± 0.1 | 1.6 ± 0.2 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.6 ± 0.2 | 1.3 ± 0.1 | 1.5 ± 0.1 |
| TT4, μg/dL | 6.5 ± 0.5 | 6.3 ± 0.7 | 8.1 ± 0.7a | 6.5 ± 0.4 | 7.2 ± 0.7 | 6.0 ± 0.5 | 6.7 ± 0.2 |
| T3, ng/dL | 64 ± 7 | 61 ± 7 | 92 ± 9a | 65 ± 4 | 72 ± 10 | 62 ± 8 | 69 ± 3 |
| Thyrotropin, mIU/L | 5.8 ± 0.7 | 4.6 ± 0.9 | 5.2 ± 0.8 | 4.8 ± 0.5 | 3.9 ± 0.7 | 5.5 ± 1.0 | 5.0 ± 0.3 |
| Male, n (%) | 18 (67) | 13 (54) | 13 (54) | 10 (42) | 12 (60) | 16 (64) | 82 (57) |
| Cesarean delivery, n (%) | 13 (48) | 5 (21) | 13 (54) | 11 (46) | 11 (55) | 8 (32) | 61 (42) |
| Multiple birth, n (%) | |||||||
| Singleton | 20 (74) | 20 (83) | 18 (75) | 18 (75) | 16 (80) | 18 (72) | 110 (76) |
| Twins or triplets | 7 (26) | 4 (17) | 6 (25) | 6 (25) | 4 (20) | 7 (28) | 34 (24) |
| Apgar score at 5 min of < 7, n (%) | 4 (15) | 2 (8) | 6 (25) | 5 (21) | 6 (30) | 6 (24) | 29 (20) |
The total number of mothers is <144, because 1 mother can appear in up to 3 arms depending on her neonate's treatment assignment as a consequence of multiple gestation.
P < .05 for the occurrence of the sign or the mean of the variable in this arm is significantly different from that seen across all study arms.
Serum Hormone Results Over Time
Free T4
The median FT4 value for the placebo arm was <19 pM/L (1.5 ng/dL) throughout the entire period of observation (not shown), indicating that 50% of the control subjects at any given date had values below our predefined threshold (Fig 2A). Interestingly, 5% to 33% of subjects across treatment arms had at least 1 plasma FT4 value below the threshold of 19 pM/L, consistent with the severity of illness.41–43 Only the continuous 8 μg/kg per day treatment arm showed a significant elevation in free T4 at all time points during study drug intervention (P < .002 versus all other groups); this persisted after cessation of treatment (P < .01 versus placebo and bolus 4 μg/kg per day; day 56).
FIGURE 2.
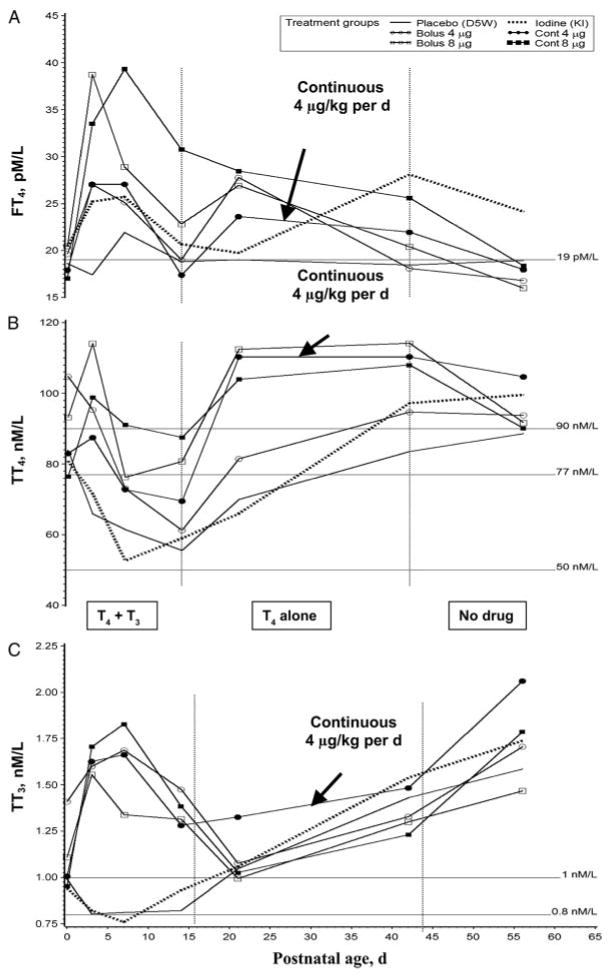
Thyroid hormone levels over time across the 6 study arms. A, Free T4 results across the 6 study arms Free T4 was elevated in the first 7 days in all hormone-treated subjects; however, only the continuous 8 μg/kg per day treatment arm showed a significant elevation in all treatment epochs (P < .002 versus all other groups), which persisted when off all drugs (P < .01 versus placebo and bolus 4 μg/kg per day; day 56). Note: reference line indicates the predefined lower threshold 1.5 ng/dL (19 pM/L); conversion to pM/L: 12.86 × ng/dL = pM/L. B, Total T4 results across the 6 study arms. Total T4 remained elevated in the first 7 days in all hormone-treated subjects (P < .05 versus placebo or iodine arms). After 14 days, both 8 μg/kg per day arms as well as the continuous 4 μg/kg per day arm produced a sustained elevation of the mean and median TT4 above 7 μg/dL (90 nM/L; hormone arms indicated are P < .002 versus placebo). Note: reference lines indicate desired level 7 μg/dL (90 nM/L), the predefined lower threshold: 6 μg/dL (77 nM/L), and severely low levels: 3.8 μg/dL (50 nM/L); conversion to nM/L: 12.86 × μg/dL = nM/L. C, Total T3 results across the 6 study arms. Total T3 remained significantly elevated relative to either placebo or iodine study arms in all hormone-supplemented subjects during the first 2 weeks' postnatal age while T3 was being administered (P < .001). No other differences were significant compared with either iodine or placebo arms. Note: reference lines indicate desired level 1 nM/L (65 ng/dL) and the predefined lower threshold 0.8 nM/L (52 ng/dL); conversion to nM/L: 65.1 × nM/L = ng/dL. Vertical lines demarcate treatment epochs as indicated in C. Data in each graph are derived from 20 to 27 values per point illustrated; see “Results” and “Discussion” for additional commentary. D5W indicates 5% dextrose in water.
Total T4
During the first 3 days, an elevation in TT4 was significant when compared with the iodine arm (Fig 2B; P < .03 for all hormone arms). After 14 days, both 8 μg/kg per day arms as well as the continuous 4 μg/kg per day arm produced a sustained elevation of TT4 above the desired level of 7 μg/dL (90 nM/L; hormone arms versus placebo, P < .002). This persisted after study drugs were discontinued in the continuous 4 μg/kg per day arm (P < .001 versus placebo control).
Total T3
Total circulating T3 (TT3) in both placebo and iodine arms fell below baseline and remained at ∼0.8 nM/L (52 ng/dL) (Fig 2C). In all hormone-supplemented subjects, TT3 remained significantly elevated relative to either placebo or iodine study arms from birth to day 14 (P < .001). After 14 days, all study arms showed a gradual rise in TT3 levels to above the desired values of 1 nM/L (65 ng/dL), becoming statistically indistinguishable from each other.
Thyrotropin
In all subjects, by the third postnatal day, thyrotropin levels fell below the cohort's mean value at birth (5.2 mIU/L; Fig 3, top) and remained low (<0.4 mIU/L) in all hormone-supplemented arms during the 2-week period of infusion of T3 (P < .001 versus placebo and iodine arms). Thyrotropin values of both 4 μg/kg per day arms were intermediate between both 8 μg/kg per day and placebo/iodine arms during days 15 to 42 (P < .001 for both comparisons). By 2 weeks after ending the hormone supplementation, the iodine arm and both 8 μg/kg per day arms had significantly lower thyrotropin levels than the placebo arm (P < .04).
FIGURE 3.
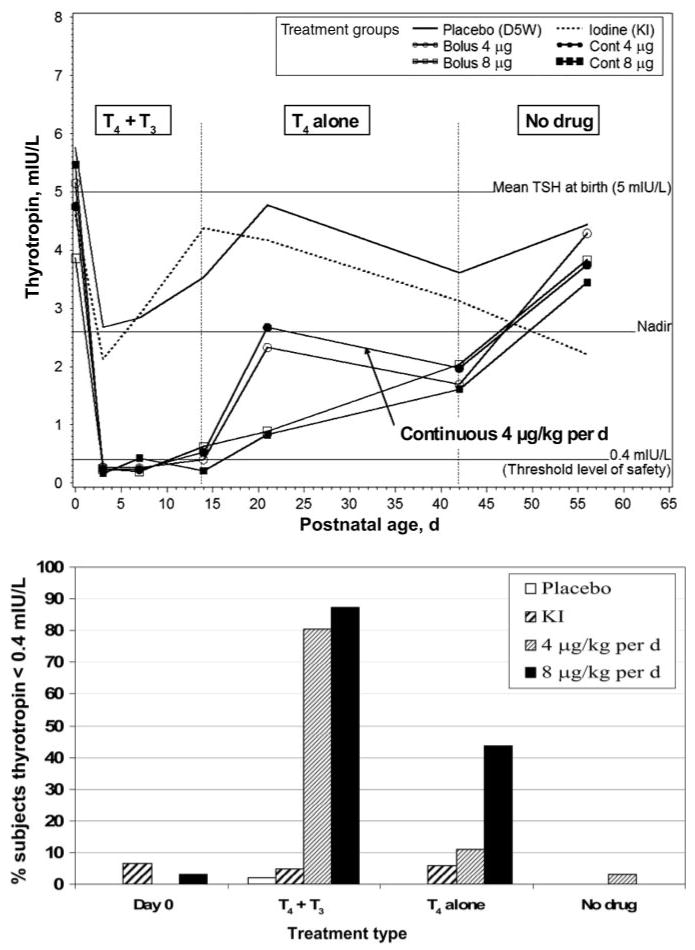
Upper, Thyrotropin results across the 6 study arms. After day 1, all thyrotropin levels fell in the hormone treatment arms to lower values than either placebo or iodine arms (P < .0001). Thyrotropin levels of both of the 4 μg/kg per day arms were intermediate between both 8 μg/kg per day and placebo arms during days 15 to 42 (P < .008 in either comparison). Similarly, on days 15 to 42, thyrotropin levels in the 8 μg/kg per day arms were both significantly lower than in the placebo and iodine arms (P < .001 in either comparison). Note: vertical lines demarcate treatment epochs as indicated. Data from 20 to 27 values per point are illustrated. Lower, Percent of subjects with a thyrotropin level of <0.4 mIU/L. Study arms are combined to illustrate the percentage of enrolled subjects with extremely low levels of thyrotropin (<0.4 mIU/L). During combined drug treatments (from birth to 14 days), >80% of hormone-treated subjects were affected (P < .001 versus placebo or iodine). Subsequently, when supplemented with only T4 (day 15–42), abnormally low thyrotropin levels were still observed in >40% of the combined 8 μg/kg per day arms but in just 11% of the combined 4 μg/kg per day arms (P < .002 vs 8 μg/kg per day). See “Results” and “Discussion” for additional commentary. D5W indicates 5% dextrose in water.
Few subjects had thyrotropin levels of <0.4 mIU/L at birth (Fig 3, bottom). In contrast, during combined drug treatments (birth to 14 days) over 80% of hormone-treated subjects experienced low thyrotropin (P < .001 versus placebo or iodine). When only T4 was given (days 15 to 42), abnormally low thyrotropin levels were still observed in >40% of the combined 8 μg/kg per day arms but in only 11% of the combined 4 μg/kg per day arms (Fig 3, bottom; P < .002 vs 8 μg/kg per day). Two weeks after completion of drug therapy nearly all subjects had thyrotropin values of >0.4 mIU/L.
Thyroid-Binding Globulin
The values for TBG did not differ significantly across study arms, reaching a nadir at day 7 and then gradually rising as a cohort from 15 mg/L at birth to 20 mg/L by study completion independent of interventions (not shown).
Cortisol
At baseline, average cortisol values ranged between 10 and 20 μg/dL across study arms, reflecting enrollment at all hours of the day of birth. Subsequently, samples were obtained at the diurnal nadir, thus becoming more tightly clustered. Cortisol values did not differ significantly by study arm; all values declined gradually and were 5 to 10 μg/dL by day 56 in all arms.
Neonatal Clinical Outcomes
At enrollment, the bolus 4 μg/kg per day arm had more mothers with fever or antibiotic use before delivery and higher neonatal T3 levels; the iodine arm had the fewest mothers with fever (both P < .05, Table 1). At discharge, the rate of NEC was highest in the continuous 8 μg/kg per day arm (P < .05 versus other groups). However, NEC-related mortality did not differ across treatment arms and overall was 66% (10 of 15) for these ELGANs.
The duration of mechanical ventilation was lowest in both the continuous 4 μg/kg per day T4 arm and the bolus 8 μg/kg per day T4 arm (P < .05 versus remaining groups; Table 3). ROP was lowest in the 8 μg/kg per day bolus arm (P < .05 versus other groups) and although not prehypothesized, ROP was considerably lower in the combined 4 thyroid hormone treatment arms than in the combined placebo and iodine arms (5% [5 of 93] vs 18% [9 of 51]; P < .04). There were no differences in intraventricular hemorrhage (IVH) across treatment arms, but white matter damage was lowest in the bolus 4 μg/kg per day arm (P < .05 versus other arms; Table 3).
TABLE 3.
Hospital Morbidities at Discharge for the THOP Trial According to Treatment Arm
| Placebo (N = 27) |
Iodine (N = 24) |
Bolus 4 μg/kg per d (N = 24) |
Continuous 4 μg/kg per d (N = 24) |
Bolus 8 μg/kg per d (N = 20) |
Continuous 8 μg/kg per d (N = 25) |
Total (N = 144) |
|
|---|---|---|---|---|---|---|---|
| Discharge weight or at death, mean ± SEM, g | 2973 ± 238 | 2653 ± 162 | 2346 ± 93 | 2596 ± 167 | 2134 ± 186 | 2338 ± 167 | 2521 ± 74 |
| Median | 2630 | 2620 | 2235 | 2302 | 2300 | 2400 | 2473 |
| Range | 1190–6335 | 750–4100 | 1235–3260 | 910–4600 | 850–3275 | 820–3840 | 750–6335 |
| Duration of ventilator, mean ± SEM, d | 38 ± 145 | 19 ± 5 | 14 ± 3 | 9 ± 2a | 13 ± 3a | 20 ± 4 | 19 ± 3 |
| Median | 11 | 9 | 5 | 5 | 10 | 12 | 8 |
| Range | 0–300 | 0–95 | 0–51 | 0–39 | 0–35 | 0–78 | 0–300 |
| Duration of CPAP, mean ± SEM, d | 23 ± 4 | 19 ± 3 | 22 ± 3 | 25 ± 3 | 24 ± 5 | 27 ± 4 | 23 ± 2 |
| Median | 18 | 19 | 23 | 27 | 20 | 25 | 23 |
| Range | 0–100 | 0–45 | 0–52 | 0–43 | 0–83 | 0–56 | 0–100 |
| Duration of hospital stay, mean ± SEM, d | 100 ± 12 | 88 ± 8 | 76 ± 6 | 83 ± 7 | 67 ± 8 | 82 ± 6 | 83 ± 4 |
| Median | 84 | 89 | 77 | 82 | 64 | 75 | 82 |
| Range | 18–343 | 15–210 | 7–116 | 10–146 | 10–133 | 25–132 | 7–343 |
| Death at >7 d, n (%) | 4 (15) | 4 (17) | 2 (8) | 2 (8) | 6 (30) | 4 (16) | 22 (15) |
| ROP ≥ stage 3, n (%) | 5 (19) | 4 (17) | 1 (4) | 2 (8) | 0 (0)a | 2 (8) | 14 (10) |
| NEC ≥ stage 2, n (%) | 2 (7) | 2 (8) | 1 (4) | 1 (4) | 3 (15) | 6 (24)a | 15 (10) |
| GM/IVH, n (%) | 9 (36) | 7 (29) | 3 (13) | 8 (33) | 7 (39) | 7 (32) | 41 (30) |
| White matter damage, n (%) | 3 (12) | 2 (8) | 0 (0)a | 2 (8) | 3 (17) | 2 (9) | 12 (9) |
| CLD, n (%) | 12 (44) | 8 (33) | 11 (46) | 5 (21) | 6 (30) | 9 (36) | 51 (35) |
| Combined arms: mortality, % (n/N) | 16 (8/51) | 8 (4/48) | 22 (10/45) | ||||
| Combined arms: ROP > stage 3, % (n/N) | 18 (9/51) | 6 (3/48) | 4 (2/45) | ||||
| Combined arms: NEC, % (n/N) | 8 (4/51) | 4 (2/48) | 20 (9/45)a | ||||
| Combined arms: GM/IVH, % (n/N) | 31 (16/51) | 23 (11/48) | 31 (14/45) | ||||
| Combined arms: white matter, % (n/N) | 10 (5/51) | 4 (2/48) | 11 (5/45) | ||||
| Combined arms: CLD, % (n/N) | 39 (20/51) | 33 (16/48) | 33 (15/45) | ||||
CPAP indicates continuous positive airway pressure; GM, germinal matrix.
The occurrence of the sign or the mean of the variable in this arm is significantly different from that across all study arms; P < .05.
Discussion
At birth, ELGANs experience a 50% increase in O2 consumption, a fall in environmental temperature, increased motor activity, increased metabolic and nutrient demands for ex utero growth, and a range of other organ-level maturational adjustments the transition of which proceeds optimally only in the euthyroid state.20,21,24,25,27,44 THOP occurs in nearly 50% of ELGANs because of an immature hypothalamic-pituitary-thyroid axis, increased in-activation of T4 and T3 hormones, attendant low plasma TT4, and in some cases of nutritional deprivation, decreased synthesis of TBG.20,21,24,25,44,45 Moreover, TT4 levels vary inversely with the severity of neonatal illness.41–43 Serum FT4 levels typically re-equilibrate to cord blood values or higher within 1 week after birth, yet TT4 can be delayed as long as 3 to 4 weeks.17,20,46 Considering these patterns, defining “normal” thyroid levels is difficult.
Because ELGANs frequently suffer severe systemic illness and experience changing biological requirements during a period of critical brain development,10,20–25,44,47,48 basing target hormone values on either healthy fetal levels or levels observed in term neonates seems imprudent.24 We argue that biochemical euthyroidism is the minimum thyroid hormone threshold associated with a reduced risk of neonatal illness or better long-term neurodevelopment.
To address the uncertainty, we targeted hormone values associated with favorable clinical outcomes.21,24,25 First, several large studies show that neuro-developmental outcome is generally better in ELGANs and other premature neonatal populations without THOP.11,49 Second, short-term improvement in clinical outcomes is reported after thyroid hormone treatment of sick newborns.16,18 Third, mortality is lower in neonatal cardiac surgical patients with euthyroid sick syndrome with hormone supplementation.50–52 Fourth, beneficial effects on IQ have been seen after thyroid hormone supplementation of newborns with other conditions (eg, trisomy 21)53 and congenital hypothyroidism.54–56 Thus, we identified our goal for a minimum threshold for FT4 concentration as ≥1.5 ng/dL (19 pM/L), for TT4 concentration as ≥6 μg/dL (77 nM/L), and for TT3 concentration as ≥52 ng/dL (0.8 nM/L).11,16,18,24,25,49–56
In addition to defining minimum target values to exceed, we sought to identify a clinical euthyroid state defined biochemically as a dose and mode of thyroid hormone supplementation that simultaneously raised the level of FT4 and TT4 without suppressing thyrotropin to nearly undetectable levels (a problem in all previous neonatal clinical trials).16,24 Each of these benchmarks was achieved by using a continuous infusion of thyroid hormone at 4 μg/kg per day of T4 for 42 days (Figs 2 and 3).
Supplementation of the prohormone T4 with the active hormone T3 for the first 14 postnatal days raised the TT3 blood level (Fig 2) but was coupled with thyrotropin values at or below the assay's level of detection, which is consistent with overtreatment during the period of dual therapy (Fig 3) suggesting that T3 supplementation is unnecessary. The fewest cases with a thyrotropin level of <0.4 mIU/L were seen in the 4 μg/kg per day arms and in untreated subjects (Fig 3, bottom). A reasonable conclusion is that 8 μg/kg per day is overtreatment and that 4 μg/kg per day can produce a biochemically euthyroid state with less frequent suppression of thyrotropin. Moreover, T3 production was not suppressed by using this lower dose regimen compared with previous reports.57 The current trial becomes the first to succeed in raising thyroid hormone levels to achieve the predefined threshold values of biochemical euthyroidism without suppressing thyrotropin values to <0.4 mIU/L in the majority of infants.
Although not prehypothesized, the mortality rate in the two 4 μg arms (8% [4 of 48]) was half that of the placebo/iodine arms (16% [8 of 51]) and also less than half that of higher dosage arms (22% [10 of 45]; Table 3). Although the pattern suggests a nadir in mortality when the lower thyroid hormone dose is used, these mortality differences failed to achieve statistical significance in this sample, which is underpowered for mortality analyses (Table 3). In a related analysis, when all hormone groups were combined, a statistically significant reduction in ROP stage ≥3 was found compared with the iodine/placebo arms (Table 3; P < .04). This is an intriguing result for future investigation, because thyroid hormone effects on vascular and retinal development in both animals and humans have been described.58 Although these correlations are clearly of interest to clinicians, future experimentation with an appropriately large sample size will be necessary to confirm the validity of these posthoc associations.
An important issue in our study was the dosage error in the Madrid pharmacy. We note that the ensuing dosage (16 μg/kg per day) is nearly the same as the 10 to 15 μg/kg per day starting dose range routinely used to treat congenital hypothyroidism55 and that earlier interventional trials used as high as 25 μg/kg per day.59 The Madrid team is following the 13 infants treated with the 16 μg/kg per day dose as outpatients, and their outcomes will be the subject of a separate report. At present, their initial hospital course did not differ significantly from the other hormone intervention groups. Another issue in the study is that the DSMB closed enrollment to the bolus 8 μg/kg per day group because of a perceived trend toward higher mortality. This interruption occurred after 20 of the intended 24 subjects had been enrolled, and thus did little to harm the power of the study. At completion of the trial, we found that death rates across arms were statistically indistinguishable (Table 3).55
Conclusions
The THOP 1 trial accomplished the goal of elevating thyroid hormone blood levels in extremely premature neonates to exceed a predefined target threshold without completely suppressing thyrotropin by using continuous 4 μg/kg of T4 as replacement therapy. The next step is to undertake a properly powered clinical trial that targets long-term neurodevelopmental outcomes and discerns whether thyroid hormone intervention will do more than simply elevate blood levels to target ranges in developing ELGANs.
What's Known on this Subject
Transient low levels of thryoid hormones in ELGANs are associated with cognitive delay and CP independent of other risk factors. It is not clear whether or not to treat, what target blood levels are desirable, or how to achieve them.
What this Study ADDS
Our study defines chemical euthyroidism and demonstrates a method of therapy to achieve this. A future trial will need to determine the impact on central nervous system outcomes.
Acknowledgments
The THOP 1 trial was supported by the National Institute of Neurological Disorders and Stroke grant NS45109, phase 1 trial of thyroid hormones in premature infants. The program director was Deborah Hirtz, MD.
We acknowledge the dedicated support of the subjects' families for allowing their children to participate in this trial; our NICU clinical programs and nursing personnel, Rita Daly, for administrative coordination; and our pharmacies for enabling the execution of this protocol. We are especially grateful to Dr Hirtz (program director, Clinical Trials Arm, National Institute of Neurological Disorders and Stroke) for her vision and organizational direction throughout this program. We also thank Ms Joanne Odenkirchen, our DSMB, and the independent medical monitor for program management.
Abbreviations
- CP
cerebral palsy
- CLD
chronic lung disease
- DSMB
data safety monitoring board
- D5W
5% dextrose water
- ELGAN
extremely low gestational age neonate
- FT4
free circulating thyroxine
- GM
germinal Matrix
- I2
Iodine
- IVH
intraventricular hemorrhage
- NEC
necrotizing enterocolitis
- PO
per os
- PVL
periventricular leukomalacia
- ROP
retinopathy of prematurity
- T3
triiodothyronine
- TT3
total circulating T3
- T4
thyroxine
- TT4
total circulating T4
- TBG
thyroid-binding globulin
- THOP
transient hypothyroxinemia of prematurity
Footnotes
During this trial Dr Fisher was an employee of Quest Diagnostics, Nichols Research Institute, San Juan Capistrano, CA. The assays were performed by Quest Diagnostics clinical laboratories in Chantilly, Virginia, as indicated in “Methods.” Dr Fisher served as a general consultant in this grant regarding interpretation of laboratory data quality and assay performance. The other authors are all full-time faculty at the universities indicated and have no financial relationships relevant to this article to disclose.
Reprints: Information about ordering reprints can be found online: http://www.pediatrics.org/misc/reprints.shtml
References
- 1.March of Dimes Foundation. PeriStats. [February 9, 2009]; Available at: www.marchofdimes.com/peristats.
- 2.Centers for Disease Control and Prevention. Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment: United States, 2003. MMWR Morb Mortal Wkly Rep. 2004;53(3):57–59. [PubMed] [Google Scholar]
- 3.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68(5):326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz JM, Wooliever DE, Jetton JR, Paneth N. A quantitative review of mortality and developmental disability in extremely premature newborns. Arch Pediatr Adolesc Med. 1998;152(5):425–435. doi: 10.1001/archpedi.152.5.425. [DOI] [PubMed] [Google Scholar]
- 5.Eichenwald EC, Stark AR. Management and outcomes of very low birth weight. N Engl J Med. 2008;358(16):1700–1711. doi: 10.1056/NEJMra0707601. [DOI] [PubMed] [Google Scholar]
- 6.Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Semin Perinatol. 2004;28(6):405–414. doi: 10.1053/j.semperi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346(3):149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 8.Kuban KC, Leviton A. Cerebral palsy. N Engl J Med. 1994;330(3):188–195. doi: 10.1056/NEJM199401203300308. [DOI] [PubMed] [Google Scholar]
- 9.Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108(6):1339–1348. doi: 10.1542/peds.108.6.1339. [DOI] [PubMed] [Google Scholar]
- 10.Sharlin DS, Tighe D, Gilbert ME, Zoeller RT. The balance between oligodendrocyte and astrocyte production in major white matter tracts is linearly related to serum total thyroxine. Endocrinology. 2008;149(5):2527–2536. doi: 10.1210/en.2007-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Den Ouden AL, Kok JH, Verkerk PH, Brand R, Verloove-Vanhorick SP. The relation between neonatal thyroxine levels and neurodevelopmental outcome at age 5 and 9 years in a national cohort of very preterm and/or very low birth weight infants. Pediatr Res. 1996;39(1):142–145. doi: 10.1203/00006450-199601000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Leviton A, Paneth N, Reuss ML, et al. Hypothyroxinemia of prematurity and the risk of cerebral white matter damage. J Pediatr. 1999;134(6):706–711. doi: 10.1016/s0022-3476(99)70285-4. [DOI] [PubMed] [Google Scholar]
- 13.Lucas A, Morley R, Fewtrell MS. Low triiodothyronine concentration in preterm infants and subsequent intelligence quotient (IQ) at 8 year follow up. BMJ. 1996;312(7039):1132–1133. doi: 10.1136/bmj.312.7039.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas A, Rennie J, Baker BA, Morley R. Low plasma triiodothyronine concentrations and outcome in preterm infants. Arch Dis Child. 1988;63(10):1201–1206. doi: 10.1136/adc.63.10.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meijer WJ, Verloove-Vanhorick SP, Brand R, van den Brande JL. Transient hypothyroxinaemia associated with developmental delay in very preterm infants. Arch Dis Child. 1992;67(7):944–947. doi: 10.1136/adc.67.7.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osborne D. Thyroid hormone for preventing neurodevelopment impairment in preterm infants. [December 16, 2008];Rev Cochrane Library. 2001 (4) doi: 10.1002/14651858.CD001070. Available at: http://mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD001070/abstract.html. [DOI] [PubMed]
- 17.Reuss ML, Leviton A, Paneth N, Susser M. Thyroxine values from newborn screening of 919 infants born before 29 weeks' gestation. Am J Public Health. 1997;87(10):1693–1697. doi: 10.2105/ajph.87.10.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Wassenaer AG, Kok JH, de Vijlder JJ, et al. Effects of thyroxine supplementation on neurologic development in infants born at less than 30 weeks' gestation. N Engl J Med. 1997;336(1):21–26. doi: 10.1056/NEJM199701023360104. [DOI] [PubMed] [Google Scholar]
- 19.Various A. Transient Hypothyroxinemia Prematurity. LaGamma EF, editor. Semin Perinatol. 2008 December;32(6):377–446. doi: 10.1053/j.semperi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Fisher DA. Thyroid function and dysfunction in premature infants. Pediatr Endocrinol Rev. 2007;4(4):317–328. [PubMed] [Google Scholar]
- 21.Kok JH, Briet JM, van Wassenaer AG. Postnatal thyroid hormone replacement in very preterm infants. Semin Perinatol. 2001;25(6):417–425. doi: 10.1053/sper.2001.27550. [DOI] [PubMed] [Google Scholar]
- 22.Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 2000;85(11):3975–3987. doi: 10.1210/jcem.85.11.6961. [DOI] [PubMed] [Google Scholar]
- 23.Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004;151(suppl 3):U25–U37. doi: 10.1530/eje.0.151u025. [DOI] [PubMed] [Google Scholar]
- 24.La Gamma EF, van Wassenaer AG, Golombek SG, et al. Neonatal thyroxine supplementation for transient hypothyroxinemia of prematurity: beneficial or detrimental? Treat Endocrinol. 2006;5(6):335–346. doi: 10.2165/00024677-200605060-00002. [DOI] [PubMed] [Google Scholar]
- 25.Rapaport R, Rose SR, Freemark M. Hypothyroxinemia in the preterm infant: the benefits and risks of thyroxine treatment. J Pediatr. 2001;139(2):182–188. doi: 10.1067/mpd.2001.116934. [DOI] [PubMed] [Google Scholar]
- 26.Anselmo J, Cao D, Karrison T, Weiss RE, Refetoff S. Fetal loss associated with excess thyroid hormone exposure. JAMA. 2004;292(6):691–695. doi: 10.1001/jama.292.6.691. [DOI] [PubMed] [Google Scholar]
- 27.Kempers MJ, van Trotsenburg AS, van Tijn DA, et al. Disturbance of the fetal thyroid hormone state has long-term consequences for treatment of thyroidal and central congenital hypothyroidism. J Clin Endocrinol Metab. 2005;90(7):4094–4100. doi: 10.1210/jc.2005-0197. [DOI] [PubMed] [Google Scholar]
- 28.Kopp P, van Sande J, Parma J, et al. Brief report: congenital hyperthyroidism caused by a mutation in the thyrotropin-receptor gene. N Engl J Med. 1995;332(3):150–154. doi: 10.1056/NEJM199501193320304. [DOI] [PubMed] [Google Scholar]
- 29.Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism: role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316(13):764–770. doi: 10.1056/NEJM198703263161302. [DOI] [PubMed] [Google Scholar]
- 30.Golombek SFM, Corbi D, LaGamma EF. Stability of thyroid hormones on a continuous infusion. Presented at the 13th International Thyroid Congress; October 30–November 4, 2005; Buenos Aires, Argentina. [Google Scholar]
- 31.Surks MI, Sievert R. Drugs and thyroid function. N Engl J Med. 1995;333(25):1688–1694. doi: 10.1056/NEJM199512213332507. [DOI] [PubMed] [Google Scholar]
- 32.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.An international classification of retinopathy of prematurity. Pediatrics. 1984;74(1):127–133. [PubMed] [Google Scholar]
- 34.Mishra R, Golombek SG, Ramirez-Tolentino SR, Das S, La Gamma EF. Low-birth-weight neonates exhibit a physiological set-point to regulate CO2: an untapped potential to minimize volutrauma-associated lung injury. Am J Perinatol. 2003;20(8):453–463. doi: 10.1055/s-2003-45388. [DOI] [PubMed] [Google Scholar]
- 35.Brumberg H, La Gamma EF. New perspectives on nutrition enhance outcomes for premature infants. Pediatr Ann. 2003;32(9):617–625. doi: 10.3928/0090-4481-20030901-10. [DOI] [PubMed] [Google Scholar]
- 36.La Gamma EF, Browne LE. Feeding practices for infants weighing less than 1500 g at birth and the pathogenesis of necrotizing enterocolitis. Clin Perinatol. 1994;21(2):271–306. [PubMed] [Google Scholar]
- 37.Omar SA, DeCristofaro JD, Agarwal BI, La Gamma EF. Effects of prenatal steroids on water and sodium homeostasis in extremely low birth weight neonates. Pediatrics. 1999;104(3 pt 1):482–488. doi: 10.1542/peds.104.3.482. [DOI] [PubMed] [Google Scholar]
- 38.Omar SA, DeCristofaro JD, Agarwal BI, LaGamma EF. Effect of prenatal steroids on potassium balance in extremely low birth weight neonates. Pediatrics. 2000;106(3):561–567. doi: 10.1542/peds.106.3.561. [DOI] [PubMed] [Google Scholar]
- 39.Conrad SC, Chiu H, Silverman BL. Soy formula complicates management of congenital hypothyroidism. Arch Dis Child. 2004;89(1):37–40. doi: 10.1136/adc.2002.009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson J, Yoo E, Wilcox R. Accuracy issues in free thyroxine testing methods. Semin Perinatol. 2008;32(6):403–406. doi: 10.1053/j.semperi.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Williams FL, Simpson J, Delahunty C, et al. Developmental trends in cord and postpartum serum thyroid hormones in preterm infants. J Clin Endocrinol Metab. 2004;89(11):5314–5320. doi: 10.1210/jc.2004-0869. [DOI] [PubMed] [Google Scholar]
- 42.Williams FL, Ogston SA, van Toor H, Visser TJ, Hume R. Serum thyroid hormones in preterm infants: associations with postnatal illnesses and drug usage. J Clin Endocrinol Metab. 2005;90(11):5954–5963. doi: 10.1210/jc.2005-1049. [DOI] [PubMed] [Google Scholar]
- 43.Simpson J, Williams FL, Delahunty C, et al. Serum thyroid hormones in preterm infants and relationships to indices of severity of intercurrent illness. J Clin Endocrinol Metab. 2005;90(3):1271–1279. doi: 10.1210/jc.2004-2091. [DOI] [PubMed] [Google Scholar]
- 44.Mercado M, Yu VY, Francis I, Szymonowicz W, Gold H. Thyroid function in very preterm infants. Early Hum Dev. 1988;16(2–3):131–141. doi: 10.1016/0378-3782(88)90093-x. [DOI] [PubMed] [Google Scholar]
- 45.Rapoport B, DeGroot LJ. Current concepts of thyroid physiology. Semin Nucl Med. 1971;1(3):265–286. doi: 10.1016/s0001-2998(71)80002-8. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell ML, Walraven C, Rojas DA, McIntosh KF, Hermos RJ. Screening very-low-birthweight infants for congenital hypothyroidism. Lancet. 1994;343(8888):60–61. doi: 10.1016/s0140-6736(94)90918-0. [DOI] [PubMed] [Google Scholar]
- 47.Anderson GW, Schoonover CM, Jones SA. Control of thyroid hormone action in the developing rat brain. Thyroid. 2003;13(11):1039–1056. doi: 10.1089/105072503770867219. [DOI] [PubMed] [Google Scholar]
- 48.Bernal J, Guadano-Ferraz A, Morte B. Perspectives in the study of thyroid hormone action on brain development and function. Thyroid. 2003;13(11):1005–1012. doi: 10.1089/105072503770867174. [DOI] [PubMed] [Google Scholar]
- 49.Reuss ML, Paneth N, Pinto-Martin JA, Lorenz JM, Susser M. The relation of transient hypothyroxinemia in preterm infants to neurologic development at two years of age. N Engl J Med. 1996;334(13):821–827. doi: 10.1056/NEJM199603283341303. [DOI] [PubMed] [Google Scholar]
- 50.De Groot LJ. Dangerous dogmas in medicine: the nonthyroidal illness syndrome. J Clin Endocrinol Metab. 1999;84(1):151–164. doi: 10.1210/jcem.84.1.5364. [DOI] [PubMed] [Google Scholar]
- 51.Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26(5):704–728. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- 52.Schönberger W, Grimm W, Emmrich P, Gempp W. Reduction of mortality rate in premature infants by substitution of thyroid hormones. Eur J Pediatr. 1981;135(3):245–253. doi: 10.1007/BF00442098. [DOI] [PubMed] [Google Scholar]
- 53.van Trotsenburg AS, Vulsma T, van Rozenburg-Marres SL, et al. The effect of thyroxine treatment started in the neonatal period on development and growth of two-year-old Down syndrome children: a randomized clinical trial. J Clin Endocrinol Metab. 2005;90(6):3304–3311. doi: 10.1210/jc.2005-0130. [DOI] [PubMed] [Google Scholar]
- 54.Köhler B, Schnabel D, Biebermann H, Gruters A. Transient congenital hypothyroidism and hyperthyrotropinemia: normal thyroid function and physical development at the ages of 6–14 years. J Clin Endocrinol Metab. 1996;81(4):1563–1567. doi: 10.1210/jcem.81.4.8636368. [DOI] [PubMed] [Google Scholar]
- 55.Rovet J. Congenital hypothyroidism: treatment and outcome. Curr Opin Endocrinol Diabetes Obes. 2005;12(1):42–52. [Google Scholar]
- 56.Tillotson SL, Fuggle PW, Smith I, Ades AE, Grant DB. Relation between biochemical severity and intelligence in early treated congenital hypothyroidism: a threshold effect. BMJ. 1994;309(6952):440–445. doi: 10.1136/bmj.309.6952.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Wassenaer AG, Kok JH, Dekker FW, Endert E, de Vijlder JJ. Thyroxine administration to infants of less than 30 weeks gestational age decreases plasma tri- iodothyronine concentrations. Eur J Endocrinol. 1998;139(5):508–515. doi: 10.1530/eje.0.1390508. [DOI] [PubMed] [Google Scholar]
- 58.Rovet J, Simic N. The role of transient hypothyroxinemia of prematurity in development of visual abilities. Semin Perinatol. 2008;32(6):431–437. doi: 10.1053/j.semperi.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Amato M, Pasquier S, Carasso A, Von Muralt G. Postnatal thyroxine administration for idiopathic respiratory distress syndrome in preterm infants. Horm Res. 1988;29(1):27–30. doi: 10.1159/000180961. [DOI] [PubMed] [Google Scholar]


