Abstract
Background
Elderly depressed patients have more vascular hyperintensities in frontal white matter and basal ganglia than elderly control subjects. Cell pathology that might be related to increased vascular hyperintensities has not been examined.
Methods
Postmortem samples from the orbitofrontal cortex (ORB) were collected in 15 elderly subjects with major depressive disorder (MDD) and 11 age-matched control subjects. Cell packing density of neurons and glia, density of pyramidal and nonpyramidal neurons, and cortical and laminar width were measured.
Results
The overall (layers I–VI) packing density of ORB neurons with pyramidal morphology was markedly decreased in MDD (by 30%) as compared with control subjects. Further laminar analysis of pyramidal neurons density revealed significant reductions in layers IIIc and V in MDD. In contrast, in MDD the density of nonpyramidal neurons and glia and cortical and laminar width were comparable to control values.
Conclusions
In elderly subjects with depression, the density of pyramidal neurons in the ORB was particularly low in cortical layers V and III, the origin of prefronto–striatal and prefronto–cortical and prefronto–amygdalar projections. Degeneration of neurons furnishing these projections might be related to the white matter hyperintensities previously observed. Neuronal pathology seems to be more severe in elderly than in younger subjects with MDD.
Keywords: Postmortem, morphometry, glial cells, major depression, late-life depression, aging
Growing clinical evidence indicates that depression in the elderly differs from that in younger patients by its etiology, phenomenology, and cerebrovascular pathology. Neuroimaging studies demonstrate that elderly (aged >60 years) patients with depression show reductions in the volume of prefrontal cortex (Kumar et al 1997, 1998) and orbitofrontal cortex (ORB) (Lai et al 2000; Lee et al 2003; Taylor et al 2003). Elderly depressed patients also display more frontal white matter hyperintensities than age-matched nondepressed control subjects, as revealed by structural magnetic resonance imaging studies (reviewed in Taylor and Krishnan 2003). The highest density of these hyperintensities in elderly depressives occurs in the white matter of the ORB and internal capsule of the left cerebral hemisphere (Greenwald et al 1998; MacFall et al 2001; Taylor et al 2001). In addition, hyperintensities are found in periventricular regions and the gray matter of the basal ganglia, although at a lower density (Coffey et al 1989, 1990; Krishnan et al 2004; Kumar et al 1997; MacFall et al 2001; Steffens and Krishnan 1998; Steffens et al 1999). More recently, an association between smaller ORB volume and a greater density of hyperintensities in the basal ganglia has been found in elderly patients with depression (Lee et al 2003). The results summarized above have led to the hypothesis that lesions of the white matter and other subcortical lesions disrupt white matter tracts and subsequently lead to a decrease in the volume of the ORB (Lee et al 2003). This disruption of fronto–striatal circuits caused by the white matter lesions leads to risk for late-life depression (Alexopoulos et al 1997; Taylor et al 2001). Other studies indicate that independent nonvascular factors, such as atrophy of fronto– limbic brain regions, impaired neuronal plasticity, or comorbidity with nonvascular medical conditions related to age, might also predispose for late-life depression (Alexopoulos et al 2004; Kumar et al 2000, 2002; Mattson et al 2004).
Despite these clinical studies suggesting neuronal pathology in the elderly with major depressive disorder (MDD), a quantitative analysis of cells in relevant cortical regions, including the ORB, has not been conducted in older subjects with MDD. A previous cell-counting study of postmortem ORB in a mixed population of younger and older adults with MDD (average age 53 years) revealed only subtle reductions in neuronal cell density and size but prominent reductions in the density of glial cells (Rajkowska et al 1999). In that study, overall neuronal density as measured across all six cortical layers did not significantly differ between subjects with MDD and the age-matched control group. When individual layers and size classes were examined, however, reductions in the density of large neurons were detected in supragranular layers II–IV of the rostral ORB in subjects with MDD. In addition, the mean size of neuronal cell bodies was smaller in layers II and III in MDD. This observation of unchanged overall neuronal density and smaller neuronal sizes in the ORB in MDD has been confirmed by independent studies in other postmortem frontal regions, such as dorsolateral prefrontal cortex, subgenual cortex, and anterior cingulate cortex (Cotter et al 2001, 2002b; Ongur et al 1998; Rajkowska et al 1999).
The above-mentioned reports of neuronal pathology in the frontal cortex were not designed to examine laminar or overall neuronal morphometric parameters specifically in the elderly subjects with MDD as compared with elderly nondepressed control subjects. On the other hand, studies conducted on the normal aging population of nondemented, psychiatrically normal subjects indicate that the frontal cortex is more vulnerable than other brain regions to age-related neuronal and white matter changes (Head et al 2004; Salat et al 2004; Tisserand et al 2002, 2004). Thus, aging-related dysfunction of the ORB might be a risk factor for depression in the elderly.
The goal of the present study was to assess neuronal pathology of the ORB in neurologically normal elderly patients with a diagnosis of MDD (age range, 63–87 years) and compare this pathology with age-matched nonpsychiatric control subjects (age range, 58–86 years). Moreover, we compared neuronal pathology in elderly depressed subjects from the present study with that described previously (Rajkowska et al 1999) in the same cortical region in younger subjects with MDD (aged <50 years). We hypothesized that neuronal pathology in ORB of elderly subjects with MDD is more severe than in age-matched control subjects or younger subjects with MDD. Furthermore, we hypothesized that ORB pathology will be most prominent in cortical layers III and V, the site of cells bodies of neurons giving rise to prefronto–striatal projections.
Methods and Materials
Postmortem samples from the left ORB were collected at autopsies performed at the Cuyahoga County Coroner's Office in Cleveland, Ohio. Brain tissue was collected from 15 elderly (average age, 75 ± 9 years [mean ± SD]; 8 male, 7 female) subjects with MDD and 11 age-matched control subjects (average age, 72 ± 8 years; 7 male, 4 female) (Table 1). In accordance with the policies of the institutional review board of the University of Mississippi Medical Center, written consent was obtained from the next-of-kin for all subjects included in the study. Retrospective, informant-based psychiatric assessments were performed for all depressed and control subjects. A trained interviewer administered the Structured Clinical Interview for DSM-IV Psychiatric Disorders (First et al 1996) to knowledgeable next-of-kin as previously described (Stockmeier et al 2004). Both lifetime and recent (last 2 weeks of life) Axis I psychopathology was assessed, and a consensus diagnosis was reached in conference according to DSM-IV criteria (American Psychiatric Association 1995) with information from the interview and medical records. In addition, information was also collected about prior psychoactive substance use, medication history, and postmortem toxicology (see Table 1 for information on subjects). Subjects were excluded from the study if they ever showed evidence of head trauma or neurologic disease (including Alzheimer's disease), established by postmortem neuropathological examination that included immunostaining for β-amyloid (neuritic plaques) or Tau protein (neurofibrillary tangles). Psychoactive substance use disorder within the last year of life was also an exclusion criterion. According to the clinical records, all control and nearly all depressed subjects exhibited signs of vascular disease, defined as the presence of one or more of the following conditions: a history of myocardial infarction, a diagnosis of coronary artery disease, myocardial infarction listed as the reason of death, atherosclerotic vascular disease by autopsy or other medical documentation, a history of hypertension, hypertensive cardiomyopathy, diabetes mellitus, amyloidosis, or renal stenosis. Thus, there were no differences in vascular risk between control and depressed subjects. There were no significant differences between the depressed and control groups in postmortem interval or time the tissue was kept in formalin (Table 1). The average postmortem interval (hours, mean ± SD) of the two groups was 21 ± 6 (MDD) and 22 ± 4 (control subjects). The average time in formalin was 23 ± 16 months (MDD) and 24 ± 9 months (control subjects). There was a small (2%) but significant [t(24) = 2.139, p = .043] difference in tissue pH between the groups. The average pH was 6.59 ± 0.18 (MDD) and 6.73 ± 0.14 (control subjects) (Table 1).
Table 1.
Characteristics of Subjects
| Subject No. |
Age (y)/ Race/ Gender |
PMI (h) |
TF (mo) |
pH | Cause of Death | Psychotropic Medication | Drugs/Alcohol | Toxicologya | Family History | Age at Onset of Depression (y) |
Duration of MDD (y) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MDD | |||||||||||
| 1 | 86/C/M | 21 | 5.6 | 6.23 | S/stabbing | Fluoxetineb | None | None detected | Suicide | 55 | 30 |
| 2 | 81/C/M | 33 | 28.5 | 6.78 | S/drowning | Sertralineb | None | None detected | AA, SA | 81 | 0.4 |
| 3 | 82/C/M | 12 | 60.3 | 6.46 | S/CO poisoning | Sertraline,b resperidoneb | None | CO | Depression | 22 | 60 |
| 4 | 73/C/M | 10 | 15.9 | 6.57 | S/hanging | Nortriptylineb | None | Nortriptyline, EtOH | None | 68 | 5 |
| 5 | 78/C/F | 25 | 6.4 | 6.94 | S/jumping | Lorazepam | None | None detected | None | 73 | 5 |
| 6 | 72/C/F | 19 | 46 | 6.61 | S/drowning | Trazodone,b lorazepam,b temazepamb | None | Propoxyphene | None | 72 | 0.75 |
| 7 | 78/A/M | 26.5 | 19 | 6.74 | S/hanging | Sertalineb | None | Sertralineb | None | 78 | 0.6 |
| 8 | 74/C/M | 25 | 17 | 6.67 | S/SIGSW head | Chlorpromazine, trazodone, clorazepate, methylphenidate | AA, SA | Diazepam, acetaminophen | AA | 50 | 24 |
| 9 | 72/C/F | 17 | 15.1 | 6.56 | N/CVD (heart attack) | Fluoxetine | None | None detected | None | 71 | 1 |
| 10 | 73/C/F | 17 | 25.1 | 6.57 | N/aortic aneurism | Lithium, nortriptyline,b temazepam, clonazepamb | AD for 40 y; stopped 8 y before death | None detected | AA, anxiety, depression | 22 | 51 |
| 11 | 78/C/M | 26 | 10.7 | 6.68 | N/severe coronary heart disease | Sertraline,b clonazepam,b carbamazepine,b amoxapine, imipramine, chlordiazepoxide, benztropine, nortriptyline, fluoxetine, temazepam, haloperidol, trazodone, loxapine, lithium | AA | Carbamazepine, chlorpheniramine, sertraline | Depression, AA, anxiety | 56 | 22 |
| 12 | 87/C/F | 24 | 42.9 | 6.56 | N/aortic aneurism due to heart disease | Trazodone, flurazepamb | None | Diphenyhydramine | None | 69 | 18 |
| 13 | 67/C/M | 17 | 23.5 | 6.52 | N/bronchopneumonia due to coronary sclerotic heart disease | Diazepamb | AD for 16 y, SA | Ethanol, diazipam | Depression, SA | 37 | 30 |
| 14 | 67/C/F | 17 | 13.8 | 6.69 | N/rupture of atheroselerothoracic aorta | Alprazolam, doxepinb | None | None detected | Anxiety, AA | 35 | 32 |
| 15 | 63/C/F | 24 | 11.9 | 6.32 | N/pulmonary thromboembolism | Chlorpromazine,b clonazepam,b amitriptyline,b amantadineb | SA (20 y) AA (25 y) | Amitriptyline, chlorpromazine, amantadine, nortrie, nortriptyline, lidocaine | AA, anxiety | 33 | 30 |
| Control Subjects | |||||||||||
| 16 | 71/C/M | 24 | 21.76 | 6.82 | N/cardiac rupture | None | AD for 20 y; stopped 32 y before death | None detected | AA | ||
| 17 | 58/C/M | 21.5 | 34.45 | 6.78 | N/heart disease | None | None | None detected | AA, SA | ||
| 18 | 69/C/M | 18 | 36.1 | 6.7 | N/dissecting aortic aneurism | None | None | None detected | AA, depression | ||
| 19 | 77/C/M | 24 | 30.2 | 6.56 | N/heart disease | None | None | None detected | SA | ||
| 20 | 70/C/M | 20 | 12.7 | 6.81 | N/heart complications | clonazepam (for leg twitch) | AA; stopped 30 y before death | None detected | AA | ||
| 21 | 80/C/F | 21 | 25.6 | 6.78 | N/hypertensive coronary sclerotic heart disease w/remote myocardial infarct | None | None | None detected | AD | ||
| 22 | 86/C/F | 18 | 18.7 | 6.81 | N/coronary sclerotic heart disease with myocardial fibrosis and cardiomegaly | None | None | Diltiazem | None | ||
| 23 | 67/C/M | 24 | 32.6 | 6.96 | N/atherosclerotic cardiovascular disease | None | None | None detected | AA, depression | ||
| 24 | 60/AAm/F | 19 | 10.2 | 6.74 | N/aortic aneurism | None | None | None detected | N/A | ||
| 25 | 73/AAm/M | 24 | N/A | 6.67 | N/heart | None | N/A | None detected | N/A | ||
| 26 | 75/C/F | 31.75 | 15.3 | 6.43 | N/severe hypertensive coronary sclerotic heart disease w/remote myocardial infarct, myocardial fibrosis and cardiomegaly | None | N/A | Caffeine | N/A |
MDD, major depressive disorder; PMI, postmortem interval, defined as the time between death and the beginning of formalin fixation; TF, time in formalin; C, Caucasian; A, Asian; AAm, African American; M, male; F, female; S, suicide; N, natural causes; CO, carbon monoxide; SIGSW, self-inflicted gunshot wound; CVD, cardiovascular disease; AA, alcohol abuse; SA, substance abuse; AD, alcohol dependence; N/A, not available.
Present in toxicology screen of blood.
Drugs that were prescribed in the last month of life.
The MDD group included 8 subjects with early onset of MDD (before age 60 years), and 7 subjects with late onset (after age 60 years). Of the 15 depressed subjects, 12 had a recent (within the last month of life) prescription for an antidepressant medication (Table 1). Only 3 of these 12 subjects were responders; 5 were nonresponders, 1 was noncompliant, and for 3 others there was no information available regarding whether they were a responder or nonresponder. An antidepressant medication was present in the blood of only 4 of the 12 with a recent prescription (Table 1). Of the 12 depressed subjects with a prescription for an antidepressant medication, 3 had also been treated with electroconvulsive therapy.
Celloidin-embedded sections were cut at 40 μm, stained with cresyl violet (final thickness after processing was 35–38 μm), and sampled in the rostral ORB region from Brodmann's area 47, located on the medial wall of the medial orbital sulcus (Figure 1A). This same area was targeted in our previous study of younger subjects (Rajkowska et al 1999), and it was identified according to the same cytoarchitectonic criteria. In each brain, three sections spaced evenly (800–1200 μm) were selected for cell counting. Neurons and glia were distinguished according to morphological criteria described previously (Selemon et al 1995). Pyramidal were distinguished from nonpyramidal neurons by the presence of a triangular cell body, a thick apical dendrite, and thinner basal dendrites.
Figure 1.
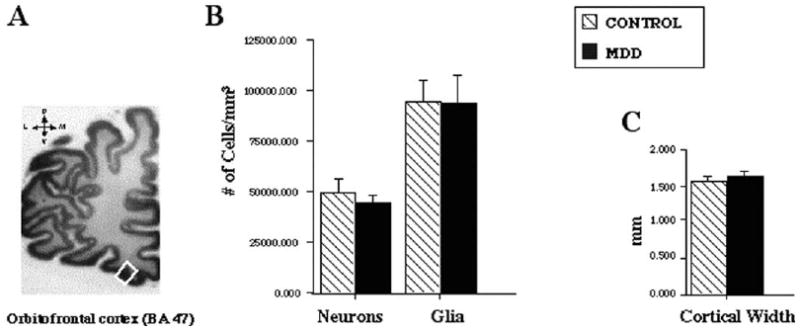
(A) The rostral orbitofrontal cortex (ORB), corresponding to Brodmann's area (BA) 47, is depicted in the white box. Sections from the ORB were used to measure (B) the overall neuronal and glial density and (C) cortical width. A 14% reduction in the density of the general population of neurons (pyramidal plus nonpyramidal) was found in the elderly group with major depressive disorder (MDD) as compared with age-matched nonpsychiatric control subjects. Values represent mean ± SD.
The overall and laminar density of neurons and glial cells, the density of pyramidal and nonpyramidal neurons, as well as the thickness of the cortex and relative width of each of its layers, were measured in three-dimensional counting boxes (120 μm × 100 μm × 25 μm; 5μm guard zone from the top) with the “Linear Optical Dissector” probe of Stereo Investigator software (5.05.4 MicroBrightField, Williston, Vermont). Mean overall cell density was compared between the groups by analysis of covariance (ANCOVA) (p < .05), with postmortem delay, time in formalin, pH, and age as covariates. Cell packing density in individual cortical layers was compared between the groups by multivariate repeated-measures ANCOVA (five cortical layers: I, II, IV, V, VI; and three sublayers: IIIa, IIIb, IIIc of layer III) followed by post hoc univariate contrast analyses. A Bonferroni-adjusted p value of .006 (.05/8) was considered statistically significant for laminar analyses. Correlations between age, age at onset, and duration of depression and neuronal densities were analyzed with Pearson correlation matrices, and the heterogeneity of covariance was also evaluated.
Results
Neuronal Packing Density
Analysis of variance without adjusting for covariates revealed that the overall density of the general population of ORB neurons (pyramidal plus nonpyramidal) in all cortical layers combined was significantly reduced [by 14%; F(1,24) = 7.547, p = .01] in the group of elderly depressed subjects as compared with the age-matched control group (Figure 1B); however, according to ANCOVA with postmortem delay, time in formalin, tissue pH, and age as covariates, the difference was not statistically significant [F (1,20) = 3.090, p = .089] (Figure 1B). The pH of the brain tissue was the only confounding variable, which was significantly correlated with the overall neuronal density in the control subjects (r = .791, p = .004) and at slightly less magnitude in the depressed group (r = .532, p = .041); however, pH was not correlated with either postmortem delay or age at the time of death.
Density of Pyramidal and Nonpyramidal Neurons
When the overall densities of pyramidal or nonpyramidal neurons were analyzed separately, the density of pyramidal neurons was significantly reduced (by 30%) in depressed as compared with control subjects [ANCOVA: F(1,20) = 14.633, p< .001], whereas the density of nonpyramidal neurons was unaffected in depression [ANCOVA: F (1,20) = .363, p = .554] (Figure 2A, C).
Figure 2.
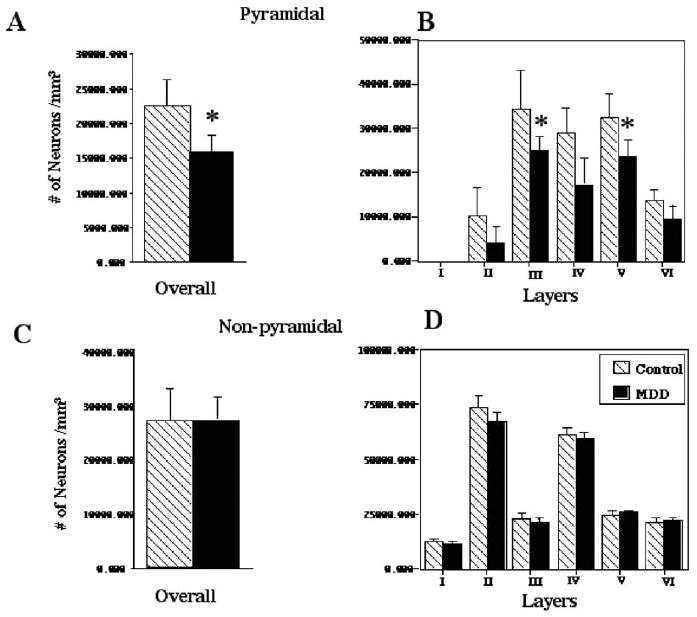
Histograms representing the overall and laminar mean density (±SD) of (A, B) pyramidal and (C, D) nonpyramidal neurons in the elderly group with major depressive disorder (MDD) and the elderly control group. Note that significant reductions (*.001 < p < .0001) in the mean neuronal density in MDD were observed only in pyramidal neurons, whereas the density of nonpyramidal neurons did not differ between cohorts.
Further analysis of the laminar density of pyramidal neurons revealed a significant effect of diagnosis on neuronal density [ANCOVA: F(1,20) = 11.202, p = .003] and a significant layer × diagnosis interaction [F(7,140) = 2.237, p = .035]. Post hoc analysis established that in MDD the density of pyramidal neurons in layers II–VI was reduced, as compared with control subjects. This reduction in MDD reached a significant 30% of the control values in layers IIIc (p = .006) and V (p < .002) (Figure 2B). Prominent 20%–60% reductions were also observed in layers II (p = .019), IIIb (p =.034), IV (p = .027), and VI (p = .038), although this reduction did not remain significant after Bonferroni adjustment. In contrast, in MDD the density of nonpyramidal neurons was not significantly different from that of the control group in any of the cortical layers [ANCOVA: F(1,21) = .101, p = .754) (Figure 2D).
Glial Cell Density
The overall density of glial cells in the depressed group was very similar to that of the control group [ANCOVA: F(1,20) = .321, p = .577] (Figure 1B). Similarly, glial density in individual cortical layers of ORB was unchanged in depression [ANCOVA: F(1,20) = 1.844, p = .190) (data not shown).
Cortical Thickness and Laminar Width
There was no statistically significant difference in the cortical thickness [ANCOVA: F(1,20) = .035, p = .854] or the relative width of any of the cortical layers [ANCOVA: F(1,20) = .028, p = .869] between the depressed and control groups (Figure 1C).
Neuronal Density in Young Depressed Versus Young Control Subjects
In our previous study of a mixed population of young and old depressed subjects (Rajkowska et al 1999), no significant differences in the overall or laminar packing density of the general population of (pyramidal plus nonpyramidal) neurons were found between depressed and control groups in the same region of rostral ORB. To assess whether the lack of differences was mainly attributable to young depressed subjects, we extracted the data on neuronal density from younger (aged <50 years) subjects with MDD (n = 7, aged 41 ± 8 years) and age-matched control subjects (n = 8, aged 34 ± 10 years) from our 1999 study and analyzed them here. Older subjects from that previous study (Rajkowska et al 1999) were recounted as part of the current study. Analysis of covariance revealed that there were no significant differences in these younger subjects between the depressed and control groups in either overall neuronal density [F (1,10) = .230, p = .642] or neuronal density in any of the individual cortical layers [F(1,10) = .411, p = .536] (data not shown).
Neuronal Density and Age in Elderly and Young Subjects
To better determine the relationship between neuronal density and age, neuronal density measurements from the ORB of older subjects (present study) were added to measurements previously obtained in younger subjects with a similar method (Rajkowska et al 1999; see the previous paragraph). Pearson correlation tests between neuronal density and age were carried out independently within the depressed and control groups to determine how age affects neuronal density in these groups. In the group with MDD, there was a significant negative correlation between age and overall neuronal density (r = −.826, p < .0001) and density in cortical layers II (r = −.760, p < .0001), IIIa (r = −.665, p = .001), IIIb (r = −.620, p = .002), IIIc (r = −.782, p < .0001), and IV (r = −.758, p < .0001) (Figure 3A). In the control group, there was also a significant negative correlation between age and overall neuronal density (r = −.610, p < .006), but the correlation between age and laminar density was significant only in layers II (r = −.710, p = .001) and IV (r = −.626, p = .004) and was not significant in sublayer IIIc (r = −.098, p = .689) (Figure 3B).
Figure 3.
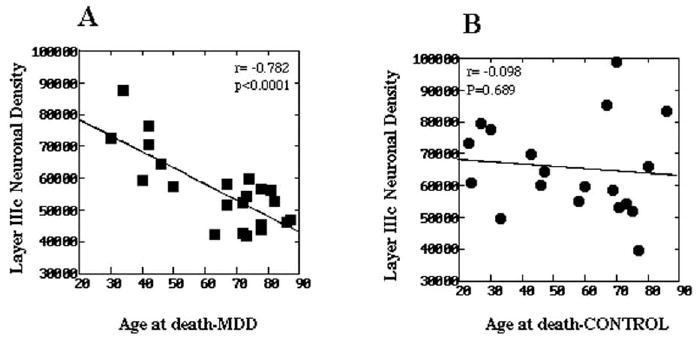
The relationship between neuronal density and age at the time of death in layer IIIc of the orbitofrontal cortex in (A) subjects with major depressive disorder (MDD) and (B) normal control subjects. Subjects aged <50 years were previously reported (Rajkowska et al 1999). Note that there is a statistically significant correlation between the neuronal density and age in MDD but not in the control group, indicating a greater age-related diminution of neuronal density in depressed subjects as compared with age-matched nondepressed control subjects.
To further assess whether age had a statistically similar effect in both cohorts on overall and laminar neuronal density, the heterogeneity of covariance was tested. In both cohorts, age had a statistically similar effect on overall neuronal density and neuronal density in all layers except sublayer IIIc. In this sublayer, there was a nearly significant trend [F(1,37) = 5.54, p = .024] for heterogeneity of covariance, suggesting a greater age-related diminution of neuronal density in the depressed group as compared with control subjects (Figure 3).
Neuronal Density, Duration of Depression, and Age at Onset of Depression in Elderly Subjects
In the group of elderly subjects with MDD, Pearson correlation matrices revealed that neither the overall density of all neurons nor the overall or laminar density of pyramidal neurons was correlated with the duration of depression (all neurons: r = .150, p = .594; pyramidal neurons: r = .295, p = .286) or with the age at onset of depression (all neurons: r = −.155, p = .582; pyramidal neurons: r = −.348, p = .204). To test whether later onset of depression is associated with lower neuronal density in elderly subjects with MDD, values for neuronal densities were compared between the following three subgroups: elderly subjects with a late onset of depression (first onset of depressed symptoms after age 60 years), elderly subjects with early onset of depression (aged <60 years), and elderly control subjects. There was a significant effect of diagnosis on overall [ANCOVA: F(2,19) = 7.237, p = .005] and laminar [F(2,19) = 5.993, p = .01] density of pyramidal neurons but not on the overall neuronal (pyramidal plus nonpyramidal) density [F(2,20) = 3.403, p = .053]. Post hoc Tukey testing of overall pyramidal neuronal density revealed that both the late-onset (p = .005) and early-onset (p = .04) depressives were significantly different from the control group but not different from each other (p = .816). Post hoc analysis of the laminar density of pyramidal neurons showed that the densities in the late-onset subgroup were significantly different from those in the control group in layers IIIc (p = .004) and V (p = .004). In the early-onset subgroup, however, the densities differed by a lower magnitude from those in the control group in layers V (p = .023) and II (p = .022). Density of pyramidal neurons was not different between the two MDD subgroups in any of the cortical layers.
Neuronal Density and Age at Onset of Depression in Elderly and Young Subjects
To further determine the relationship between neuronal density, age at onset of depression, and age at the time of death, correlations were examined between neuronal densities and age at the onset of depression for all MDD subjects. Younger subjects from the previous study (Rajkowska et al 1999) and elderly subjects from the present study were included. Significant negative correlations were observed between the age at onset of depression and the density of neurons in layers IIIb (r = −.611, p = .003) and IIIc (r = −.601, p = .003) (Figure 4A). There was also a marginally significant negative correlation between the overall neuronal density and the age at onset of depression (r = −.521, p = .013) (Figure 4B). The seven depressed subjects with the highest values for overall (Figure 4B) and layer IIIc (Figure 4A) neuronal density are the youngest subjects who died before age of 50 years. In contrast, the lowest values of overall and layer IIIc neuronal density belong to elderly MDD subjects with early and late onset of depression who died after age 60 years (Figure 4).
Figure 4.
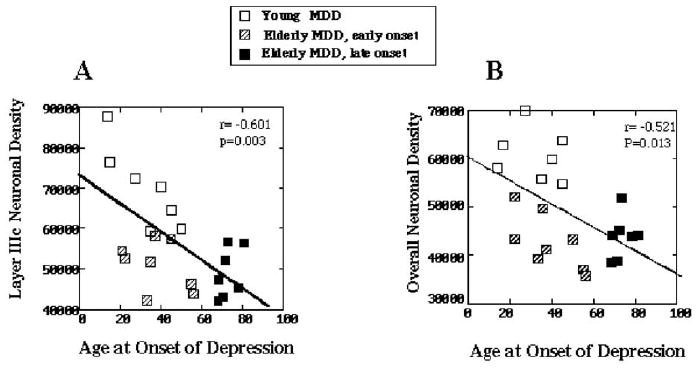
The relationship between neuronal density and age at the onset of depression. (A) Correlation between the neuronal density in layer IIIc and the age at onset of depression. (B) Correlation between the overall neuronal density and the age at onset of depression. Note that the highest values of neuronal densities belong to the youngest subjects with major depressive disorder (MDD) (open squares), who naturally also had an early onset of depression and died at a young age (<50 years). In contrast, the lowest neuronal densities are found in the subgroup of elderly subjects with early-onset depression (aged <60 years, striped squares) and elderly subjects with late-onset depression (aged >60 years, black squares) who died after age 60 years. Neuronal densities represent added values from the elderly subjects analyzed in the present study and younger subjects studied previously (Rajkowska et al 1999).
Discussion
The overall packing density of neurons with pyramidal morphology in the ORB was significantly decreased (by 30%) in elderly depressed subjects as compared with age-matched nonpsychiatric control subjects. This reduction in density in depression was particularly prominent in cortical layers V and IIIc. In contrast, no significant differences were found between the groups in the density of neurons with nonpyramidal morphology.
Prefronto–Striatal Pathology
A study tracing the pathway of connections of the ORB in nonhuman primates demonstrated that pyramidal neurons of layer V and deep layer III of the ORB give rise to glutamatergic prefrontal projections to the striatum (Haber et al 1995). If similar pathways are present in the human prefrontal cortex, a selective reduction in the packing density of pyramidal neurons in the ORB would suggest a depletion of prefronto–striatal projection fibers from the ORB neurons.
Diminished ORB cortico–striatal projections might play a role in the pathophysiology of depression in the elderly. Elderly depressed subjects have a higher density of vascular hyperintensities (areas of increased intensity in T2-weighted magnetic resonance imaging scans) in the gray matter of the basal ganglia and in white matter adjacent to the ORB containing fronto– striatal axons that course through the internal capsule (Coffey et al 1989, 1990; Greenwald et al 1998; Krishnan et al 2004; Kumar et al 1997; MacFall et al 2001; Steffens and Krishnan 1998; Steffens et al 1999; Taylor et al 2001). In fact, an association between smaller ORB cortical volume and greater density of hyperintensities in the basal ganglia has been demonstrated in elderly patients with depression as compared with age-matched nondepressed subjects (Lee et al 2003). These alterations might predispose toward cognitive changes that might render an individual more vulnerable to depression.
Increased white matter hyperintensities in elderly depressed subjects might be related to vascular disturbances or vascular lesions to the white matter tracts (Alexopoulos et al 1997; Taylor et al 2001; Thomas et al 2002). Recently, we investigated the morphology of blood vessels in white and gray matter of the ORB in the elderly depressed and control subjects used in the present report (unpublished data). An increased density of blood vessel profiles with abnormally enlarged perivascular spaces was noted in elderly depressed subjects as compared with elderly control subjects. These vascular changes were observed in the same Nissl-stained sections as used in the present study. In addition, on adjacent sections immunostained with antibodies to Von Willebrand factor, a specific endothelial marker, the density of enlarged vessels was increased in the deep white matter underlying the ORB. These observations suggest that either insufficient blood supply or simply a mechanical disruption of efferent and afferent ORB axons could contribute to the increased hyperintensities and degeneration of ORB neurons and thereby exacerbate the symptoms of depression in the elderly. Other putative mechanisms cannot be excluded, however: neuronal degeneration related to insufficiency in neurotrophic factors (Duman et al 1997; Mattson et al 2004; Sapolsky 2000) or comorbidity with nonvascular medical conditions related to age (Alexopoulos et al 2004; Kumar et al 2000, 2002).
Pathology in Other Neuronal Circuits
In the present study, in addition to significant reductions in the density of pyramidal neurons in cortical layers IIIc and V in the elderly MDD group, we detected 20%–60% marginally significant reductions in neuronal density in layers II, IIIb, IV, and VI. Studies in nonhuman primates indicate that whereas layer III is the main source of connections between prefrontal cortex (including ORB) and other associational and limbic cortical regions, ORB neurons of layers IV and VI establish reciprocal thalamo–cortical connections (Carmichael and Price 1995a, 1995b; Giguere and Goldman-Rakic 1988; Rempel-Clower and Barbas 2000; Schwartz and Goldman-Rakic 1984). In addition, neurons of layers III and V send axons to amygdala (Aggleton et al 1980), whereas layers II and VI are recipients of afferent projections from amygdala (Porrino et al 1981). Thus, several circuits, in addition to prefronto–striatal projections, might be compromised in elderly depressed patients. This is consistent with neuroimaging observations of reductions in the volume of frontal areas and other associated brain structures (Krishnan 1993; Kumar et al 1998, 2000; Lacerda et al 2004; Sheline et al 1996, 2003; Steffens et al 2002). Lower neuronal densities in elderly subjects with depression as compared with control subjects suggest a loss of ORB neurons, particularly in the absence of differences between cohorts in cortical thickness; however, the final confirmation of whether there is a loss of ORB neurons in elderly subjects with MDD awaits stereological estimation of the total cell number with a systematic, random sampling throughout the entire extent of the ORB.
Nonpyramidal Neurons
In the present study, pyramidal neuron density was selectively reduced in elderly subjects with MDD. In contrast, there was no difference between groups in the density of nonpyramidal neurons. This is consistent with another postmortem morphometric analysis of specific immunostained-types of nonpyramidal neurons in the anterior cingulate cortex in a mixed population of young and old subjects with MDD (Cotter et al 2002a). A characteristic morphological feature of nonpyramidal neurons is that dendritic and axonal trees are restricted to the close vicinity of cell bodies. In contrast to pyramidal neurons, the majority of nonpyramidal neurons do not send axons to the white matter. Therefore, the lesions of white matter in MDD might not have a direct effect on these nonpyramidal neurons, consistent with our findings of unchanged density of nonpyramidal neurons in elderly depressed subjects.
ORB Thickness
In the present study, it was not possible to estimate the ORB volume because only a portion of this region was available. Therefore, it was not possible to confirm at the microscopic level the previous neuroimaging observation of smaller ORB volume in elderly subjects with late-onset depression (Lai et al 2000; Lee et al 2003). The thickness of the ORB was measured, however (on a line perpendicular to the cortical surface spanning layers I through VI) at three selected levels in each subject, and no differences in the total thickness of the ORB or the width of any of its cortical layers were observed between the elderly depressed and control groups. In contrast, previous measurements of ORB thickness in subjects with younger average age and earlier onset of depression revealed a significant 12% narrowing of the ORB thickness in the MDD group as compared with the age-matched control group (Rajkowska et al 1999). Comparison of the cortical thickness values between elderly depressed (present study) and younger depressed (Rajkowska et al 1999) subjects reveals that the values for elderly depressed and elderly control subjects are lower than those for younger depressed and younger control subjects.
Glial Cells
We have recently observed reduced glial fibrillary acidic protein (GFAP, a marker of astrocytes) immunoreactivity (Miguel-Hidalgo et al 2000) and lower levels of GFAP protein (Si et al 2004) in younger depressed subjects as compared with younger control subjects and older depressed subjects. Moreover, the density of the general population of Nissl-stained glia was significantly reduced in younger depressed subjects as compared with younger control subjects (Rajkowska et al 1999), whereas there was no difference between elderly depressed subjects and elderly control subjects (present study). Interestingly, this suggests that active gliosis is not associated with the neuropathology of ORB in elderly depressed subjects, and other unique processes (e.g., neurotrophic and angiogenic factors) might be relevant. Alternatively, mild increases in glia are observed in older subjects with MDD when compared with younger subjects with MDD (Miguel-Hidalgo et al 2000; Si et al 2004). These increases in glial density and GFAP protein level might compensate for neuronal damage, which is more prominent in elderly than in younger subjects with MDD.
The pathophysiology of depression might be closely associated with cytomorphological and possibly glial changes. The thickness of the ORB is reduced in depressed subjects even with relatively early onset, and in control subjects the thinning of the cortex is only detectable late in life. Neuroimaging studies on nonpsychiatric subjects confirm that advanced age is associated with thinning of the prefrontal cortex (including ORB) and age-related cognitive decline (Head et al 2004; Salat et al 2004; Tisserand et al 2002, 2004). Processes involved in an earlier thinning of the cortex in depressed subjects might be a factor in producing further vulnerability of neurons in elderly depressed subjects (Kumar et al 2002). Thus, long exposure to depression in combination with normal aging might lead to prominent neuronal damage.
Early Versus Late-Onset Depression
It seems that both late-onset and early-onset subgroups of elderly depressed subjects contribute similarly to the lower neuronal density in elderly depressed subjects. There was no major difference in the overall or laminar density of pyramidal neurons between the early- and late-onset subgroups in MDD. This is consistent with some neuroimaging studies, which concluded that there is no substantial difference between early- and late-onset depression (Greenwald et al 1997; Krishnan et al 1995). On the other hand, it still might be argued that the late-onset subgroup contributes more than the early-onset subgroup because there was a significant difference in the density of pyramidal neurons in layers IIIc and V in this subgroup as compared with the control group, whereas the differences in the early-onset subgroup were of a lower magnitude. It is possible that in late-onset depression the factors resulting in low values of neuronal density are different from those acting in early-onset depression, despite the end result (i.e., lower neuronal density) being similar in both subgroups. Genetic susceptibility in combination with stress and a deficiency in neurotrophic factors have been proposed to play a role in the case of early-onset depression (Duman et al 1997; Sapolsky 2000), whereas vascular lesions, neurodegenerative changes related to atrophy of other brain regions connected to ORB, and nonvascular medical factors could contribute to lower neuronal density in late-onset depression (Alexopoulos et al 2004; Heun et al 2000; Krishnan 2002; Krishnan et al 2004; Kumar et al 2000, 2002; Van den Berg et al 2001). Interestingly, neurons of deep layer III and layer V, where the most prominent pathology is found in elderly depressed subjects, seem to be more vulnerable than other prefrontal neurons to pathological changes. For instance, neuronal pathology specific to these layers has been reported in Huntington's disease (Selemon et al 1995, 2004), Alzheimer's disease (Hof et al 1990), and schizophrenia (Pierri et al 2001; Rajkowska et al 1998). Nevertheless, our results have to be interpreted with caution because splitting the depressed group into late- and early-onset subgroups results in low sample size and a subsequent decrease in statistical power.
Confounding Variables
The reductions in the density of neurons with pyramidal morphology observed here in the depressed group are most likely attributable to the disorder itself because there is a significant difference in the density of pyramidal neurons between control and depressed groups after controlling for confounding variables, such as duration of postmortem interval, time in formalin, tissue pH, and age. Interestingly, only brain pH showed a small (2%) but significant difference between the groups. This difference can not be attributable to the length of the postmortem interval, age at the time of death, or circumstances surrounding death because these confounding variables were not different between groups, and there was no significant correlation between pH and any of these variables. Rather, our finding of a significant correlation between overall neuronal density and tissue pH in both MDD and control groups suggests that brain metabolic activity, which might be influenced by neuronal activity, might affect pH. Further studies are needed to investigate this relationship.
It is possible that psychotropic medications might have had an effect on neuronal density; however, it is unlikely in the present study because antidepressant drugs were present postmortem in only 4 of the 12 MDD subjects with a recent prescription for an antidepressant drug. Moreover, only 3 of these 12 subjects responded to antidepressant medication. Neuronal density values were comparable between all depressed subjects, regardless of medication history.
Neurons with pyramidal morphology in the ORB are selectively affected in elderly subjects with MDD as compared with age-matched control subjects and younger depressed subjects. This pathology is most severe in cortical layers that contain pyramidal neuron cell bodies giving rise to prefronto–striatal and prefronto–cortical projections. Degeneration of neurons furnishing these projections might be related to white matter hyperintensities observed in the ORB and basal ganglia in elderly depressed patients. To our knowledge, this is the first morphometric study in postmortem tissue in neurologically normal elderly subjects with MDD and the first comparison of neuronal pathology between elderly and younger depressed patients. As with other human postmortem morphometric studies, this is by definition not a longitudinal design to analyze directly the age-related progression of neuropathological changes in depression. Future neuroimaging studies with longitudinal protocols for acquisition of structural and functional data combined with postmortem morphometry will allow better insights into the neuropathology of late-life depression.
Acknowledgments
This study was supported by grants from the National Institute of Mental Health: MH60451, MH61578, MH63187, and RR17701.
We thank James C. Overholser, Ph.D., George Jurjus, M.D., and Lisa Konick for their work in establishing retrospective psychiatric diagnoses; the Cuyahoga County Coroner's Office, Cleveland, Ohio, for their excellent assistance; the next-of-kin of the deceased for their cooperation and support; Qingmei Shao for histological preparations; Jan Jiang, M.D., for evaluation of vascular risk in our studied subjects; and Gillian O'Dwyer for editorial reading of the manuscript.
References
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Murphy C, Heo M. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology. 2004;29:2278–2284. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1995. [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995a;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1995b;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Figiel GS, Djang WT, Saunders WB, Weiner RD. White matter hyperintensity on magnetic resonance imaging: clinical and neuroanatomic correlates in the depressed elderly. J Neuropsychiatry Clin Neurosci. 1989;1:135–144. doi: 10.1176/jnp.1.2.135. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Figiel GS, Djang WT, Weiner RD. Subcortical hyperintensity on magnetic resonance imaging: A comparison of normal and depressed elderly subjects. Am J Psychiatry. 1990;147:187–189. doi: 10.1176/ajp.147.2.187. [DOI] [PubMed] [Google Scholar]
- Cotter D, Landau S, Beasley C, Stevenson R, Chana G, MacMillan L, Everall I. The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry. 2002a;51:377–386. doi: 10.1016/s0006-3223(01)01243-4. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002b;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition (ver 2) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: Areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol. 1988;277:195–213. doi: 10.1002/cne.902770204. [DOI] [PubMed] [Google Scholar]
- Greenwald BS, Kramer-Ginsberg E, Bogerts B, Ashtari M, Aupperle P, Wu H, et al. Qualitative magnetic resonance imaging findings in geriatric depression. Possible link between later-onset depression and Alzheimer's disease? Psychol Med. 1997;27:421–431. doi: 10.1017/s0033291796004576. [DOI] [PubMed] [Google Scholar]
- Greenwald B, Kramer-Ginsberg E, Krishnan K, Ashtari M, Auerbach C, Patel M. Neuroanatomic localization of magnetic resonance imaging signal hyperintensities in geriatric depression. Stroke. 1998;29:613–617. doi: 10.1161/01.str.29.3.613. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, et al. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cereb Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Heun R, Kockler M, Papassotiropoulos A. Distinction of early- and late-onset depression in the elderly by their lifetime symptomatology. Int J Geriatr Psychiatry. 2000;15:1138–1142. doi: 10.1002/1099-1166(200012)15:12<1138::aid-gps266>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Hof PR, Cox K, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer's disease: I. Superior frontal and inferior temporal cortex. J Comp Neurol. 1990;301:44–54. doi: 10.1002/cne.903010105. [DOI] [PubMed] [Google Scholar]
- Krishnan K, Hays J, Tupler L, George L, Blazer D. Clinical and phenomenological comparisons of late-onset and early-onset depression. Am J Psychiatry. 1995;152:785–788. doi: 10.1176/ajp.152.5.785. [DOI] [PubMed] [Google Scholar]
- Krishnan KR. Neuroanatomic substrates of depression in the elderly. J Geriatr Psychiatry Neurol. 1993;6:39–58. doi: 10.1177/002383099300600107. [DOI] [PubMed] [Google Scholar]
- Krishnan KR. Biological risk factors in late life depression. Biol Psychiatry. 2002;52:185–192. doi: 10.1016/s0006-3223(02)01349-5. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Taylor WD, McQuoid DR, MacFall JR, Payne ME, Provenzale JM, Steffens DC. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;55:390–397. doi: 10.1016/j.biopsych.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bilker W, Jin Z, Udupa J. Atrophy and high intensity lesions: Complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264–274. doi: 10.1016/S0893-133X(99)00124-4. [DOI] [PubMed] [Google Scholar]
- Kumar A, Jin Z, Bilker W, Udupa J, Gottlieb G. Late-onset minor and major depression: Early evidence for common neuroanatomical substrates detected by using MRI. Proc Natl Acad Sci U S A. 1998;95:7654–7658. doi: 10.1073/pnas.95.13.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Mintz J, Bilker W, Gottlieb G. Autonomous neurobiological pathways to late-life major depressive disorder: Clinical and pathophysiological implications. Neuropsychopharmacology. 2002;26:229–236. doi: 10.1016/S0893-133X(01)00331-1. [DOI] [PubMed] [Google Scholar]
- Kumar A, Schweizer E, Jin Z, Miller D, Bilker W, Swan LL, Gottlieb G. Neuroanatomical substrates of late-life minor depression. A quantitative magnetic resonance imaging study. Arch Neurol. 1997;54:613–617. doi: 10.1001/archneur.1997.00550170085018. [DOI] [PubMed] [Google Scholar]
- Lacerda AL, Keshavan MS, Hardan AY, Yorbik O, Brambilla P, Sassi RB, Nicoletti M, et al. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol Psychiatry. 2004;55:353–358. doi: 10.1016/j.biopsych.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Lai T, Payne ME, Byrum CE, Steffens DC, Krishnan KR. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48:971–975. doi: 10.1016/s0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- Lee SH, Payne ME, Steffens DC, McQuoid DR, Lai TJ, Provenzale JM, Krishnan KR. Subcortical lesion severity and orbitofrontal cortex volume in geriatric depression. Biol Psychiatry. 2003;54:529–533. doi: 10.1016/s0006-3223(03)00063-5. [DOI] [PubMed] [Google Scholar]
- MacFall JR, Payne ME, Provenzale JE, Krishnan KR. Medial orbital frontal lesions in late-onset depression. Biol Psychiatry. 2001;49:803–806. doi: 10.1016/s0006-3223(00)01113-6. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2001;58:466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol. 1981;198:121–136. doi: 10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: A postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. The laminar pattern of connections between prefrontal and anterior temporal cortices in the Rhesus monkey is related to cortical structure and function. Cereb Cortex. 2000;10:851–865. doi: 10.1093/cercor/10.9.851. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: A primer on neuron death. Biol Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Schwartz ML, Goldman-Rakic PS. Callosal and intrahemispheric connectivity of the prefrontal association cortex in rhesus monkey: Relation between intraparietal and principal sulcal cortex. J Comp Neurol. 1984;226:403–420. doi: 10.1002/cne.902260309. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex: A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Evidence for progression in frontal cortical pathology in late-stage Huntington's disease. J Comp Neurol. 2004;468:190–204. doi: 10.1002/cne.10938. [DOI] [PubMed] [Google Scholar]
- Sheline Y, Wang P, Gado M, Csernansky J, Vannier M. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Si X, Miguel-Hidalgo JJ, O'Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology. 2004;29:2088–2096. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens D, Krishnan K. Structural neuroimaging and mood disorders: Recent findings, implications for classification, and future directions. Biol Psychiatry. 1998;43:705–712. doi: 10.1016/s0006-3223(98)00084-5. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Helms MJ, Krishnan KR, Burke GL. Cerebrovascular disease and depression symptoms in the cardiovascular health study. Stroke. 1999;30:2159–2166. doi: 10.1161/01.str.30.10.2159. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Payne ME, Greenberg DL, Byrum CE, Welsh-Bohmer KA, Wagner HR, MacFall JR. Hippocampal volume and incident dementia in geriatric depression. Am J Geriatr Psychiatry. 2002;10:62–71. [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Krishnan KR. Structural brain investigations in affective disorders. In: Soares JC, editor. Brain Imaging in Affective Disorders. New York: Marcel Dekker; 2003. pp. 53–78. [Google Scholar]
- Taylor WD, Payne ME, Krishnan KR, Wagner HR, Provenzale JM, Steffens DC, MacFall JR. Evidence of white matter tract disruption in MRI hyperintensities. Biol Psychiatry. 2001;50:179–183. doi: 10.1016/s0006-3223(01)01160-x. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Steffens DC, McQuoid DR, Payne ME, Lee SH, Lai TJ, Krishnan KR. Smaller orbital frontal cortex volumes associated with functional disability in depressed elders. Biol Psychiatry. 2003;53:144–149. doi: 10.1016/s0006-3223(02)01490-7. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, O'Brien JT, Davis S, Ballard C, Barber R, Kalaria RN, Perry RH. Ischemic basis for deep white matter hyperintensities in major depression: A neuropathological study. Arch Gen Psychiatry. 2002;59:785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB. Regional frontal cortical volumes decrease differentially in aging: An MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–669. [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex. 2004;14:966–973. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- Van den Berg MD, Oldehinkel AJ, Bouhuys AL, Brilman EI, Beekman AT, Ormel J. Depression in later life: Three etiologically different subgroups. J Affect Disord. 2001;65:19–26. doi: 10.1016/s0165-0327(00)00263-9. [DOI] [PubMed] [Google Scholar]


