Abstract
Objectives/Hypothesis
The vocal fold epithelium provides a barrier to the entry of inhaled and systemic challenges. However, the location of the epithelium makes it vulnerable to damage. Past research suggests, but does not directly demonstrate, that exposure to gastric reflux adversely affects the function of the epithelial barrier. Understanding the nature of reflux-induced epithelial barrier dysfunction is necessary to better recognize the mechanisms for vocal fold susceptibility to this disease. Therefore, we examined the effects of physiologically relevant reflux challenges on vocal fold transepithelial resistance and gross epithelial and subepithelial appearance.
Study Design
Ex vivo, mixed design with between-group and repeated-measures analyses.
Methods
Healthy, native porcine vocal folds (N = 52) were exposed to physiologically relevant acidic pepsin, acid-only, or pepsin-only challenges and examined with electrophysiology and light microscopy. For all challenges, vocal folds exposed to a neutral pH served as control.
Results
Acidic pepsin and acid-only challenges, but not pepsin-only or control challenges significantly reduced transepithelial resistance within 30 minutes. Reductions in transepithelial resistance were irreversible. Challenge exposure produced minimal gross changes in vocal fold epithelial or subepithelial appearance as evidenced by light microscopy.
Conclusions
These findings demonstrate that acidic environments characteristic of gastric reflux compromise epithelial barrier function without gross structural changes. In healthy, native vocal folds, reductions in transepithelial resistance could reflect reflux-related epithelial disruption. These results might guide the development of pharmacologic and therapeutic recommendations for patients with reflux, such as continued acid-suppression therapy and patient antireflux behavioral education.
Keywords: Vocal fold epithelium, gastric reflux, resistance, light microscopy
INTRODUCTION
Laryngopharyngeal reflux (LPR), a type of reflux-related disease, is the backflow of gastric contents from the stomach and esophagus into the pharynx and larynx.1 Estimated to affect approximately 20% of the US adult population, LPR represents a major health problem with huge societal costs2 and significant, adverse impact on patient quality of life.3 LPR is characterized by numerous symptoms including vocal hoarseness, dysphagia, chronic coughing, and throat clearing.4,5 Given the substantial negative effects of LPR, increased attention has focused on understanding how LPR affects vocal fold physiology, particularly the vocal fold epithelium. This emphasis on epithelium is critical given that in the true vocal folds this layer is first exposed to gastric reflux.
The vocal fold epithelium consists of stratified squamous cells connected by apical junctional complexes that together create a barrier to the entry of inhaled and systemic challenges. However, the epithelial barrier is not impervious to disruption. The vocal fold epithelium is susceptible to the contents of gastric reflux associated with LPR, particularly acid and pepsin.5,6 Pepsin, an acid-activated enzyme, when combined with acid is thought to be more damaging than acid alone.7 Research shows that acid and pepsin compromise the integrity of the vocal fold epithelial barrier.5,6 For example, laryngeal biopsy specimens from patients diagnosed with LPR are characterized by dilated paracellular spaces8 and decreased expression of E-cadherin, a paracellular junctional complex protein.5,6 These findings suggest, but do not directly demonstrate, that reflux adversely affects the function of the epithelial barrier. Disruptions of the epithelial barrier might increase vocal fold susceptibility to further reflux events that contact the vocal fold surface.
One marker of epithelial barrier function is transepithelial resistance (RT). RT measures the ability of the epithelial barrier to restrict movement of solute and solvents. RT has been extensively used as an indicator of barrier function in the stratified squamous epithelium of the esophagus7,9 and the vocal folds.10–12 High RT corresponds to a tight barrier, whereas low RT corresponds to a leaky barrier. In this investigation, we tested the hypothesis that LPR-like challenges (physiologically relevant acid and pepsin) would decrease RT in healthy, native vocal fold epithelium as measured by electrophysiology. Reduced RT following challenges would suggest increased epithelial leakiness and disruption in barrier function. We then investigated if these reductions in RT were accompanied by changes in the gross structural appearance of the vocal folds as examined by light microscopy. We hypothesized that epithelial and subepithelial damage would accompany reductions in RT. Findings from this investigation will serve to quantify the adverse effects of LPR on vocal fold epithelial barrier function and gross structural appearance.
MATERIALS AND METHODS
Tissue Preparation
Fresh, young adult porcine larynges (ages, 6–9 months) were procured in accordance with Purdue University protocol. A total of 52 vocal folds were utilized in these investigations. Larynges were obtained from each animal within 30 minutes of sacrifice and immediately immersed in fresh, cold saline for transport to the laboratory. Following validated procedures,11–13 each larynx was dissected into two hemilarynges along the mid-sagittal plane to reveal the true vocal folds. The vocal fold epithelium and superficial layer of the lamina propria, hereafter referred to as the vocal fold, were dissected from the underlying ligament using dissection instruments. During dissection, vocal folds were kept moist with Hank's Balanced Salt Solution (HBSS [mM: NaCl, 136.8; dextrose, 5.6; KCl, 5.6; NaHCO3, 4.2; CaCl2, 1.3; MgSO4, 0.8; KH2PO4, 0.4; Na2HPO4, 0.3]). Characteristics of dissected vocal fold were as follows: dimensions adequate to fit 9-mm Lucite chamber (see below) with thickness of 1.0 ± 0.2 mm. Following dissection, vocal folds were prepared for electrophysiology or light microscopy as detailed below.
Challenges
Vocal folds were exposed to one of four challenges. Challenges were as follows: 1) 1.0 mg/mL porcine pepsin with hydrochloric acid (HCl) at pH3, 2) HCl at pH3, 3) 1.0 mg/mL porcine pepsin at pH7, and 4) HBSS. Hereafter, these challenges will be referred to as acidic pepsin, acid-only, pepsin-only, and control, respectively. All challenges were placed on the luminal surface of the vocal folds. The pepsin concentration and pH values used here are characteristic of human gastric reflux.5 In addition, similar pepsin concentrations and pH values have been used to mimic gastric reflux in a porcine model.5 Fresh solutions were prepared immediately before each experiment and pH was verified. All chemicals were obtained from Sigma-Aldrich (St Louis, MO). The pH indicator strips were obtained from EMD Chemicals (Gibbstown, NJ).
Experiment 1. Electrophysiology
Protocol
In experiment 1, electrophysiology was utilized to examine RT. LPR is typically a mixture of pepsin and acid,14 and evidence suggests that pepsin and acid in combination produce more damage than acid alone.7 Therefore, experiment 1a sought to examine the effects of acidic pepsin on RT. Experiment 1b further sought to separate the effects of acid and pepsin on RT. An Ussing apparatus (model 15362; World Precision Instruments, Inc., Sarasota, FL) and associated voltage clamp (model DVC-1000) were used to measure RT. Prior to data collection, the Ussing apparatus was calibrated per validated procedures.12,13 Freshly dissected vocal folds were mounted on a removable Lucite chamber (World Precision Instruments, Inc.) and placed in the Ussing apparatus. To maintain tissue viability, the luminal and basal reservoirs of the Ussing apparatus were filled with 5 mL of warm, oxygenated HBSS. A circulating water bath maintained the tissue at 37°C. To measure open-circuit potential difference and short-circuit current, two voltage and two current electrodes (Ag+/AgCl electrodes with 3 mol/L KCl/agar salt bridges) were placed on either side of the mounted vocal fold. Vocal folds reached baseline in 45 to 60 minutes. Tissues that did not attain a baseline RT of at least 300 Ω·cm2 were discarded. Discarded tissue accounted for less than 10% of total examined vocal folds. Although the exact cause of low baseline RT is unknown, some reasons might include reduced freshness of tissue and dissection error (e.g., retention of the cartilaginous portion of one vocal fold after dissection, which precluded use of the entire larynx). On reaching baseline, vocal folds were treated with either acidic pepsin (n = 10), acid-only (n = 5), or pepsin-only (n = 5) challenge. For each vocal fold exposed to a challenge, the contralateral vocal fold from the same larynx served as a control. Overall, 20 vocal folds were used as controls. Ohm's Law was applied offline to determine RT. Specifically, RT was calculated from the current deflection to an imposed 2-mV pulse at baseline, and then at 15 minutes and 30 minutes following each challenge. Electrophysiological studies in esophageal epithelium suggest that similar challenges elicit the greatest change in RT within 15 minutes of treatment,7,9 providing the basis for the timeline of the current study. For challenges that reduced RT,effect reversibility was assessed as follows. Following 30 minutes of challenge exposure, the luminal reservoir was drained and immediately refilled with warm HBSS. RT was measured 30 minutes following tissue rinse. Last, to examine the epithelial response to varying degrees of acidic environments, vocal folds (n = 4) were treated with a series of acidic pH. Once each vocal fold reached baseline RT, pH of the luminal solution was sequentially lowered with HCl to pH4, then to pH3, and finally to pH2. RT was stable for 10 minutes at each pH level before the pH was further lowered.
Data analysis
To measure the change in RT from baseline, RT values at 15 minutes postchallenge and 30 minutes postchallenge were subtracted from baseline RT. To examine effect reversibility, RT values at 30 minutes postrinse were subtracted from baseline RT. Hereafter, these values will be referred to as ΔRT. A negative ΔRT indicates a decrease in RT from baseline (leaky epithelium), whereas a positive ΔRT indicates an increase in RT from baseline (tight epithelium).
Statistical analysis
For all analyses, an alpha level of .05 was considered statistically significant. Data were examined for normal distribution. When assumptions for normality were not met, a nonparametric statistical approach was utilized. To examine if acidic pepsin altered ΔRT as compared to control (experiment 1a), a repeated measures analysis of variance was performed with challenge (acidic pepsin/control) as the between-factor, and time (15 minutes, 30 minutes) as the within-factor. As the greatest change in RT following an acidic pepsin challenge was observed at 30 minutes (see Results), this time point was utilized for comparison in experiment 1b. Mann-Whitney tests were used to compare ΔRT for acid-only versus control challenge and pepsin-only versus control challenge. To examine if challenge effects were permanent, Wilcoxon signed-ranks tests were employed to compare ΔRT values from 30 minutes postchallenge to values at 30 minutes postrinse.
Experiment 2. Light Microscopy
Protocol
In experiment 2, light microscopy was used to evaluate if challenges that reduce RT disrupt the gross structural appearance of the vocal folds. Upon removal from the underlying vocal ligament (as described above), freshly dissected vocal folds were bisected longitudinally, resulting in four vocal fold samples per larynx. Four larynges, in total, were prepared for light microscopy. Samples were incubated in either acidic pepsin (n = 4), acid-only (n = 4), pepsin-only (n = 4), or HBSS control (n = 4) at 37° C for 30 minutes. Following incubation, vocal folds were processed for light microscopy. Samples were fixed in 10% buffered neutral formalin for 24 hours, dehydrated via an ethyl alcohol series, and embedded in paraffin. Sections (5-μm) were cut and stained with hematoxylin and eosin.
Vocal fold appearance was graded by a pathologist blinded to the challenge type. Vocal fold samples were rated on six criteria: epithelial shedding, epithelial erosion, epithelial intracellular or intercellular edema, epithelial intracellular or intercellular shrinkage, basilar to sub-basilar edema or vacuolization, and interstitial edema. Criteria were selected based upon histopathologic findings from previous investigations examining the effects of similar reflux-like challenges on esophageal or laryngeal epithelial and subepithelial tissue.4,7,9,15–17 Vocal fold appearance was graded on a 0 to 5 scale: 0 = none, 1 = minimal, 2 = mild, 3 = moderate, 4 = marked, and 5 = severe. Control tissues were assigned a 0 score for all criteria. The pathologist rated each challenge (acidic pepsin, pepsin-only, acid-only) in reference to the control tissue from each larynx. Statistical analyses were only performed for criteria given a score >0 for at least one larynx. Only four criteria received at least one score >0: epithelial shedding, epithelial intracellular or intercellular edema, basilar to sub-basilar edema or vacuolization, and interstitial edema.
Data and statistical analyses
An alpha level of .05 was considered statistically significant. Data was not normally distributed; therefore, nonparametric statistics were employed. Kruskal-Wallis tests were performed to compare the effects of acidic pepsin, acid-only, and pepsin-only challenges on gross vocal fold structure.
RESULTS
Experiment 1. Electrophysiology
Experiment 1a: Acidic pepsin decreased transepithelial resistance
RT decreased significantly following exposure to acidic pepsin but not control challenge (F [1, 18] = 5.03, P = .03) (Fig. 1A). This reduction in RT was observed at both 15 and 30 minutes as evident by a significant main effect for time (F [1, 18] = 5.03, P = .03). The greatest reduction in RT was observed at 30 minutes. Specifically, by 30 minutes, average RT declined by 77% of baseline.
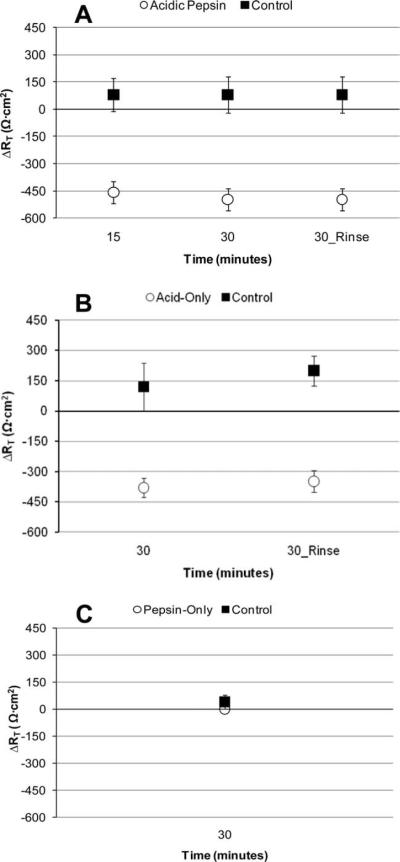
Fig. 1.
Change in transepithelial resistance from baseline (ΔRT) in response to simulated reflux and control challenges. (A) Acidic pepsin. (B) Acid-only. (C) Pepsin-only. Reversibility assessment of reductions in RT following acidic pepsin and acid-only challenges is also represented (30_Rinse) in (A) and (B). Negative ΔRT values suggest a decrease in resistance from baseline, whereas positive ΔRT suggest an increase from baseline. ΔRT for control challenges and pepsin-only challenge (C) remained positive, while ΔRT for acidic pepsin (A) and acid-only (B) challenges decreased. Error bars represent standard errors.
Because there was a significant reduction in RT from baseline following acidic pepsin challenge, reversibility of effect was examined. ΔRT at 30 minutes postchallenge did not significantly differ from ΔRT at 30 minutes postrinse (Wilcoxon Z = −0.50, P = .61) (Fig. 1A).
Experiment 1b: Acid-only but not pepsin-only challenge decreased transepithelial resistance
Because the greatest change in RT following acidic pepsin was observed at 30 minutes, this time point was utilized for analyses involving acid-only and pepsin-only challenges. ΔRT for vocal folds treated with acid-only challenge significantly differed from controls (Mann-Whitney U = 0.00, P = .007) (Fig. 1B). Specifically, at 30 minutes, average RT for acid decreased by 68% of baseline. On the other hand, ΔRT did not differ between pepsin-only and control challenges (Mann-Whitney U = 10.0, P = .31) (Fig. 1C).
Because there was a significant reduction in RT from baseline following acid-only challenge, reversibility of this effect was examined. ΔRT at 30 minutes postchallenge did not significantly differ from ΔRT at 30 minutes postrinse (Wilcoxon Z = −1.63, P = .10) (Fig. 1B), suggesting the reduction in RT following acid-only challenge is irreversible. Reversibility was not examined for pepsin-only challenges.
Experiment 2. Light Microscopy
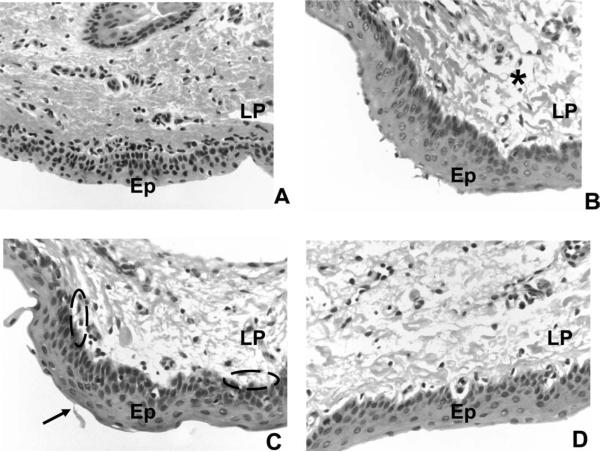
All epithelial samples were confirmed by the pathologist as stratified squamous type. Only four criteria received at least one score >0: epithelial shedding, epithelial intracellular or intercellular edema, basilar to sub-basilar edema or vacuolization, and interstitial edema. Means and standard deviations for these scoring criteria were obtained. Acidic pepsin, pepsin-only, and acid-only challenges exhibited a mean score of ≤1, for each of these criteria, suggestive of minimal damage. A nonparametric binomial test confirmed that challenged vocal folds were not significantly different from control vocal folds on scored criteria (P > .05). In addition, epithelial shedding (Kruskal-Wallis H = 1.12, P = .56), epithelial intracellular or intercellular edema (H = 0.16, P = .92), basilar to sub-basilar edema or vacuolization (H = 0.43, P = .80), and interstitial edema (H = 0.86, P = .65) scores did not significantly differ between acidic pepsin, acid-only, and pepsin-only challenges. Although some vocal folds demonstrated early, minimal-mild signs of damage (Fig. 2), overall these findings suggest that 30 minutes of challenge exposure produces minimal gross changes in vocal fold structure as determined by histological appearance.
Fig. 2.
Light microphotographs of representative vocal fold samples obtained after 30 minutes of challenge exposure (hematoxylin and eosin stain, 40× magnification). (A) Control. (B) Acidic pepsin (pH 3). (C) Acid-only (pH 3). (D) Pepsin-only (pH 7). Ep = stratified squamous epithelium; LP = lamina propria; arrowhead = epithelial shedding; dashed oval = sub-basilar edema or vacuolization; * = interstitial edema.
DISCUSSION
The larynx is situated in the upper airway in close proximity to the esophagus. This location places the vocal fold epithelium at high risk for exposure to both inhaled and systemic challenges. One such systemic challenge is LPR. It is well established that in this disease, the vocal folds are exposed to and damaged by reflux.5,18,19 In fact, less than three episodes of reflux per week can injure the vocal fold epithelium, whereas up to 50 reflux events per day in the esophagus are considered normal.4 In LPR, gastric reflux compromises the integrity of the vocal fold epithelium5,6 and has been suggested to adversely affect epithelial barrier function.6 However, this proposed disruption in barrier function has yet to be verified in the vocal fold epithelium. Pharmacologic and behavioral recommendations for patients with LPR necessitate an understanding of how epithelial barrier function is adversely affected by reflux. Therefore, we conducted a series of experiments to quantify the effects of physiologically relevant LPR on epithelial barrier function and gross structural appearance.
Acidic pepsin reduces RT in healthy, native vocal fold epithelium within 30 minutes of challenge. Decreases in RT are thought to reflect epithelial leakiness.20 Therefore, the findings of the current study suggest that acidic pepsin rapidly increases epithelial leakiness. RT measures the ability of the epithelial barrier to restrict movement through paracellular (apical junctional complexes) and/or transcellular (through cells) routes. In the rabbit esophagus, reductions in RT following reflux challenges similar to that employed in this study are accompanied by increases in paracellular permeability that allow molecules as large as 20 kD to diffuse across the epithelium.9 In the laryngeal epithelium of patients with LPR, there is decreased expression of E-cadherin5,6 and dilated paracellular spaces,8 which are markers of epithelial structural integrity and permeability, respectively. Although not directly examined, the rapid reduction in RT observed here might primarily result from increased paracellular permeability. If LPR-like challenges increase epithelial permeability, further reflux events will have easier access to the underlying lamina propria, thus amplifying the damaging effects of this disease.
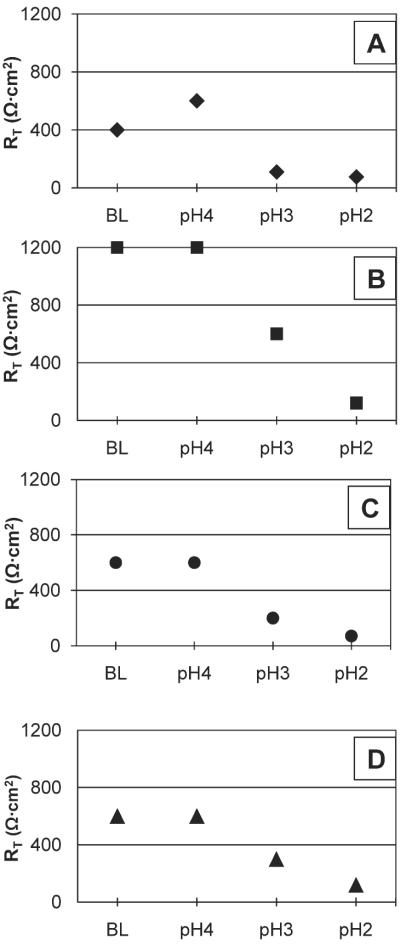
Given that acidic pepsin adversely affects vocal fold epithelial function as evidenced by reductions in RT, we questioned whether acid alone, pepsin alone, or a combination is responsible for this change. Therefore, we next sought to isolate the injurious potential of acid-only and pepsin-only challenges on the vocal fold epithelium. It has been suggested that acidic pepsin is primarily responsible for epithelial damage.6,7 However, recent research demonstrates that pepsin, in even nonacidic reflux, damages cultured hypopharyngeal epithelial cells.21 On theother hand, it is well established that acid in isolation also plays a key role in epithelial injury and the development of reflux disease.22 In this investigation, acid-only, but not pepsin-only challenges decreased RT. Data inspection reveals similar trends in RT reduction for acidic pepsin and acid-only challenges. Specifically, at 30 minutes postchallenge, RT was reduced by 77% following acidic pepsin challenge and by 68% following acid-only challenge. These findings suggest that rapid reductions in vocal fold RT appear to be a function of exposure to an acidic environment. Given these results, we decided to examine the effects of varying degrees of acidic environments on RT (Fig. 3). Reductions in RT were observed at pH3 and pH2 as compared to baseline pH and pH4. The greatest reduction in RT was seen at pH2. These pH response curves demonstrate that as the local environment becomes more acidic RT decreases, which suggests that vocal fold epithelium is sensitive to changes in ambient pH.
Fig. 3.
Raw transepithelial resistance (RT) data from vocal folds (n = 4) treated with a series of acidic pH. Each vocal fold is represented by a different icon: (A) = ◆, (B) = ■, (C) = ●, and (D) = ▲. RT decreased at pH3 and pH2 relative to baseline and pH4 for each vocal fold. The greatest reductions in RT are observed at pH2.
Mounting evidence demonstrates that the laryngeal epithelium is more susceptible to reflux than the esophageal epithelium.4 In rabbit esophageal epithelium, reductions in RT are minimal or absent at pH3, but are apparent at pH2.7,9 Here we show that even challenges at pH3 rapidly reduce vocal fold RT, suggesting that in porcine vocal folds less acidic reflux alters epithelial barrier function (Fig. 3). Further, these effects were irreversible. In rabbit esophageal epithelium, only acidic pepsin produces an irreversible decrease in RT.7,9 Although the use of different animal models precludes the direct comparison of laryngeal and esophageal findings, our results are noteworthy given that the esophagus might physiologically be better equipped than the larynx to withstand intermittent reflux events.4 Findings from the current investigation help elucidate the unique response patterns of porcine vocal fold epithelium to reflux and support the notion that the larynx might be inadequately protected from LPR.4,5
To examine if changes in gross structural appearance are associated with reductions in RT, vocal folds were examined with light microscopy. In general, vocal fold appearance did not significantly differ between acidic pepsin, acid-only, and pepsin-only challenges. Some challenged vocal folds showed early signs of damage, including epithelial shedding, epithelial intracellular or intercellular edema, basilar to sub-basilar edema or vacuolization, and interstitial edema (Fig. 2). However, there was considerable variability in appearance scores resulting in nonsignificant findings. Hence we conclude that 30 minutes of challenge exposure induces minimal alterations to vocal fold epithelial appearance as detected by light microscopy. In canine vocal fold epithelium, acidic (between pH1.5–pH4) and acidic pepsin (between pH1.5–pH4) challenges, designed to mimic intermittent reflux, fail to produce gross epithelial or subepithelial injury.4,16 On the other hand, canine studies designed to mimic chronic gastric and duodenal reflux (between pH1–pH2) do produce gross vocal fold injury.23 These findings suggest that the timeline used in this investigation might not have been sufficient to elicit considerable gross structural changes in ex vivo porcine vocal folds. It is, however, of note that significant changes in epithelial function occurred in healthy porcine vocal folds even with minimal changes in epithelial structure. Consequently, our results suggest that in the vocal folds, RT might be more sensitive to early reflux-related epithelial changes. Increased leakiness of the epithelial barrier might heighten vocal fold susceptibility to additional reflux events and future gross structural changes.
Although we observed no changes in gross epithelial structure or function following pepsin-only challenges, the contribution of pepsin in the development of laryngeal injury should not be discounted. Pepsin has been indicated in the breakdown of intrinsic epithelial defense mechanisms. For example, pepsin exposure reduces the expression of carbonic anhydrase III isoenzme19 and Sep7018 protective proteins, which have been indicated in acid neutralization and cellular defense pathways, respectively. Typically, pepsin is activated between pH2 and pH6.5.4 However, recent research demonstrates that pepsin at weakly acidic or nonacidic pH damages intracellular structures,21 thus providing the basis for our decision to also examine pepsin at a neutral pH. At least 1 hour of pepsin exposure is needed to elicit mitochondrial damage in hypopharyngeal epithelial cells.21 Consequently, it is possible the timeline of the current experiment might not have been adequate to capture nonacidic pepsin related changes in RT and gross vocal fold structure.
The current data offer clinical implications for both otolaryngologists and speech-language pathologists. Recently, it has been suggested that patients with reflux can be divided into two general groups: patients with primarily acidic reflux and patients with weakly acidic or nonacidic reflux.21 Here we show that rapid decreases in vocal fold RT are likely caused by the presence of an acidic environment. Consequently, this finding suggests the continued importance of pharmacologically based acid suppression treatments (e.g., H2 blockers, proton pump inhibitors) in patients with primarily acidic-based reflux. In addition, the results of this investigation offer therapeutic implications. As discussed, reductions in RT might place the vocal folds at higher risk for environmental insults (e.g., inhaled toxins, cigarette smoke) and additional reflux events. Up to 6 months is needed to fully repair LPR-caused vocal fold damage.1 During this period of repair, the vocal folds are even more susceptible to other injury. Unfortunately, a common misconception among patients and sometimes even healthcare professionals is that behavioral antireflux precautions and pharmacologic treatments actually reverse injury.24 Patients need to be educated regarding the importance of abiding by the prescribed pharmacologic regime and recommended lifestyle modifications to allow for vocal fold repair. Without this education, patients are at risk for further injuring an already more susceptible system.
In this investigation, we demonstrated the effects of simulated reflux on native vocal fold epithelial function and structure. However, some limitations warrant additional discussion. We recognize that the ability to generalize our results is somewhat limited due to use of a porcine model. However, research suggests that porcine vocal fold epithelium is similar to that of humans and appropriate for use in experimental investigations examining the effects of LPR on epithelial structure and function.25 Due to an unequal sample size, the effects of acidic pepsin and acid-only challenges were not directly compared. Although inspection of raw data revealed similar trends in RT reduction, future studies are needed that directly compare the effects of acidic pepsin and acid-only challenges on RT. In addition, only one acidic pH value (pH3) was extensively examined in the current investigation. This pH was chosen to represent the pH of typical human gastric contents and similar pH values have been used previously to mimic gastric reflux in a porcine model.5 Preliminary evidence presented here suggests that the vocal fold epithelium is sensitive to changes in acidic environments; therefore, vocal fold RT and gross structure in response to a range of acidic pH values should be further examined to better characterize laryngeal response to acidic reflux. Based on findings from the current study and previous investigations, we propose that the decrease in RT following acidic challenges might be associated with increased epithelial leakiness and permeability. However, we recognize that leakiness and permeability were not directly examined. Last, minimal changes in gross vocal fold appearance were noted following LPR-like challenges. In healthy porcine vocal folds, histological evaluation might not have been sensitive enough to capture structural reflux-related damage within 30 minutes. Future investigations would benefit from the use of confocal or electron microscopy techniques to further examine the effects of acid and pepsin on inter- and intracellular structure.
CONCLUSION
A series of experiments were conducted to examine the detrimental effects of physiologic relevant gastric reflux solutions on healthy, native vocal folds. Within 30 minutes, acidic pepsin and acid-only challenges induced an irreversible reduction in RT. Interestingly, these changes in epithelial function occurred with minimal changes in gross epithelial or subepithelial appearance. Decreased RT might predispose the vocal fold epithelium to further damage from reflux events. In summary, these findings help elucidate the unique response patterns of vocal fold epithelium to simulated reflux and might guide the development of pharmacologic and therapeutic recommendations for patients with this disease.
Acknowledgment
We thank Tracy Wiegand at the Purdue University Histopathology Service Laboratory for assistance with light microscopy. We also gratefully acknowledge Dr. Paul Snyder for his assistance with histology scoring. Last, we would like to thank Dakota Robinson and Grace Scott for their contributions to data collection.
This work was supported in part from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (grant no. 008690). The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Footnotes
Presented at the 2010 American Laryngological Association Annual Meeting in Las Vegas, Nevada, U.S.A., April 28, 2010.
BIBLIOGRAPHY
- 1.Rees C, Belfasky P. Laryngopharyngeal reflux: current concepts in pathophysiology, diagnosis, and treatment. Int J of Speech Lang Pathol. 2008;10:245–253. doi: 10.1080/17549500701862287. [DOI] [PubMed] [Google Scholar]
- 2.Rees L, Pazmany L, Gutowska-Owsiak D, et al. The mucosal immune response to laryngopharyngeal reflux. Am J Respir Crit Care Med. 2008;177:1187–1193. doi: 10.1164/rccm.200706-895OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connor N, Palazzi-Churas K, Cohen S, Leverson G, Bless D. Symptoms of extraesophageal reflux in a community-dwelling sample. J Voice. 2007;12:189–202. doi: 10.1016/j.jvoice.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101(4 pt 2suppl 53):1–78. doi: 10.1002/lary.1991.101.s53.1. [DOI] [PubMed] [Google Scholar]
- 5.Johnston N, Bulmer D, Gill GA, et al. Cell biology of laryngeal epithelial defenses in health and disease: further studies. Ann Otol Rhinol Laryngol. 2003;112:481–491. doi: 10.1177/000348940311200601. [DOI] [PubMed] [Google Scholar]
- 6.Gill G, Johnston N, Buda A, et al. Laryngeal epithelial defenses against laryngopharyngeal reflux: investigations of E-cadherin, carbonic anhydrase isoenzyme III, and pepsin. Ann Otol Rhinol Laryngol. 2005;114:913–921. doi: 10.1177/000348940511401204. [DOI] [PubMed] [Google Scholar]
- 7.Tobey N, Hosseini S, Caymaz-Bor C, Wyatt H, Orlando G, Orlando R. The role of pepsin in acid injury to esophageal epithelium. Am J Gastroenterol. 2001;96:3062–3070. doi: 10.1111/j.1572-0241.2001.05260.x. [DOI] [PubMed] [Google Scholar]
- 8.Franchi A, Brogelli B, Massi D, Santucci M, De Campora E, Gallo O. Dilation of intercellular spaces is associated with laryngopharyngeal reflux: an ultrastructural morphometric analysis of laryngeal epithelium. Eur Arch Otorhinolaryngol. 2007;264:907–911. doi: 10.1007/s00405-007-0295-z. [DOI] [PubMed] [Google Scholar]
- 9.Tobey N, Hosseini S, Argote C, Dobrucali A, Awayda M, Orlando R. Dilated intercellular spaces and shunt permeability in nonerosive acid-damaged esophageal epithelium. Am J Gastroenterol. 2004;99:13–22. doi: 10.1046/j.1572-0241.2003.04018.x. [DOI] [PubMed] [Google Scholar]
- 10.Sivasankar M, Fisher K. Vocal folds detect luminal ionic perturbations: an in vitro investigation. J Voice. 2008;22:408–419. doi: 10.1016/j.jvoice.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Sivasankar M, Erickson E, Rosenblat M, Branski R. Hypertonic challenge to the vocal folds: effects on barrier function. Otolaryngol Head Neck Surg. 2010;142:79–84. doi: 10.1016/j.otohns.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher K, Telser A, Phillips J, Yeates D. Regulation of vocal fold transepithelial water fluxes. J Appl Physiol. 2001;91:1401–1411. doi: 10.1152/jappl.2001.91.3.1401. [DOI] [PubMed] [Google Scholar]
- 13.Sivasankar M, Fisher K. Vocal fold epithelial response to luminal osmotic perturbation. J Speech Lang Hear Res. 2007;50:886–898. doi: 10.1044/1092-4388(2007/063). [DOI] [PubMed] [Google Scholar]
- 14.Vaezi M. Sensitivity and specificity of reflux-attributed laryngeal lesions: experimental and clinical evidence. Am J Med. 2003;115:97S–104S. doi: 10.1016/s0002-9343(03)00205-5. [DOI] [PubMed] [Google Scholar]
- 15.Lanas A, Royo Y, Ortego J, Molina M, Sainz R. Experimental esophagitis induced by acid and pepsin in rabbits mimicking human reflux esophagitis. Gastroenterology. 1999;116:97–107. doi: 10.1016/s0016-5085(99)70233-7. [DOI] [PubMed] [Google Scholar]
- 16.Sant'Ambrogio F, Sant'Ambrogio G, Chung K. Effects of HCl-pepsin laryngeal instillations on upper airway patency-maintaining mechanisms. J Appl Physiol. 1998;84:1299–1304. doi: 10.1152/jappl.1998.84.4.1299. [DOI] [PubMed] [Google Scholar]
- 17.Orlando R, Powell D, Carney C. Pathophysiology of acute acid injury in rabbit esophageal epithelium. J Clin Invest. 1981;68:286–293. doi: 10.1172/JCI110246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston N, Dettmar P, Lively M, et al. Effect of pepsin on laryngeal stress protein (Sep70, Sep53, and Hsp70) response: role in laryngopharyngeal reflux disease. Ann Otol Rhinol Laryngol. 2006;115:47–58. doi: 10.1177/000348940611500108. [DOI] [PubMed] [Google Scholar]
- 19.Johnston N, Knight J, Dettmar P, Lively M, Koufman J. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope. 2004;114:2129–2134. doi: 10.1097/01.mlg.0000149445.07146.03. [DOI] [PubMed] [Google Scholar]
- 20.Yeh P, Smith P, Ellens H. Effect of medium-chain glycerides on physiologic properties of rabbit intestinal epithelium in vitro. Pharm Res. 1994;11:1148–1154. doi: 10.1023/a:1018988832492. [DOI] [PubMed] [Google Scholar]
- 21.Johnston N, Wells C, Samuels T, Blumin J. Pepsin in nonacidic refluxate can damage hypopharyngeal epithelial cells. Ann Otol Rhinol Laryngol. 2009;118:677–685. doi: 10.1177/000348940911800913. [DOI] [PubMed] [Google Scholar]
- 22.Ylitalo R, Thibeault SL. Relationship between time of exposure of laryngopharyngeal reflux and gene expression in laryngeal fibroblasts. Ann Otol Rhinol Laryngol. 2006;115:775–783. doi: 10.1177/000348940611501011. [DOI] [PubMed] [Google Scholar]
- 23.Adhami T, Goldblum J, Richter J, Vaezi M. The role of gastric and duodenal agents in laryngeal injury: An experimental canine model. Am J Gastroenterol. 2004;99:2098–2106. doi: 10.1111/j.1572-0241.2004.40170.x. [DOI] [PubMed] [Google Scholar]
- 24.Hanson D, Jiang J. Diagnosis and management of chronic laryngitis associated with reflux. Am J Med. 2000;108:112S–119S. doi: 10.1016/s0002-9343(99)00349-6. [DOI] [PubMed] [Google Scholar]
- 25.Gill GA, Buda A, Moorghen M, Dettmar PW, Pignatelli M. Characterisation of adherens and tight junctional molecules in normal animal larynx; determining a suitable model for studying molecular abnormalities in human laryngopharyngeal reflux. J Clin Pathol. 2005;58:1265–1270. doi: 10.1136/jcp.2004.016972. [DOI] [PMC free article] [PubMed] [Google Scholar]