Physical activity confers beneficial metabolic effects by inducing anti-inflammatory activity in the hypothalamus region of the brain in rodents, resulting in a reorganization of the set point of nutritional balance and reduced insulin and leptin resistance.
Abstract
Overnutrition caused by overeating is associated with insulin and leptin resistance through IKKβ activation and endoplasmic reticulum (ER) stress in the hypothalamus. Here we show that physical exercise suppresses hyperphagia and associated hypothalamic IKKβ/NF-κB activation by a mechanism dependent upon the pro-inflammatory cytokine interleukin (IL)-6. The disruption of hypothalamic-specific IL-6 action blocked the beneficial effects of exercise on the re-balance of food intake and insulin and leptin resistance. This molecular mechanism, mediated by physical activity, involves the anti-inflammatory protein IL-10, a core inhibitor of IKKβ/NF-κB signaling and ER stress. We report that exercise and recombinant IL-6 requires IL-10 expression to suppress hyperphagia-related obesity. Moreover, in contrast to control mice, exercise failed to reverse the pharmacological activation of IKKβ and ER stress in C3H/HeJ mice deficient in hypothalamic IL-6 and IL-10 signaling. Hence, inflammatory signaling in the hypothalamus links beneficial physiological effects of exercise to the central action of insulin and leptin.
Author Summary
The hypothalamus is a brain region that gathers information on the body's nutritional status and governs the release of multiple metabolic signaling molecules such as insulin and leptin to maintain homeostasis. Overeating and obesity are associated with insulin and leptin resistance in the hypothalamus, and recent studies provide an intriguing link between inflammation and dysfunction of hypothalamic insulin and leptin signaling through activation of IKKβ, a key player in immune response, and endoplasmic reticulum (ER) stress. This means that strategies to reduce the aberrant activation of inflammatory signaling in the hypothalamus are of great interest to improve the central insulin and leptin action and prevent or treat related metabolic diseases. Using a combination of pharmacological, genetic, and physiological approaches, our study indicates that physical activity reorganizes the set point of nutritional balance through anti-inflammatory signaling mediated by interleukin (IL)-6 and IL-10 in the hypothalamus of rodents. Hence, IL-6 and IL-10 are important physiological contributors to the central insulin and leptin action mediated by exercise, linking it to hypothalamic ER stress and inflammation.
Introduction
Overnutrition and sedentary lifestyle are among the most important factors that lead to an unprecedented increase in the prevalence of obesity. In mammals, food intake and energy expenditure are tightly regulated by specific neurons localized in the hypothalamus. The hypothalamus can gather information on the body's nutritional status by integrating multiple signals, including potent hormonal signals such as insulin and leptin [1],[2]. The impairment of hypothalamic insulin and leptin signaling pathways is sufficient to promote hyperphagia, obesity, and type 2 diabetes (T2D) in different genetic rodent models with neuronal ablation of insulin and leptin signaling [1],[3],[4]. We and others have proposed that overnutrition induces the central insulin and leptin resistance through the aberrant hypothalamic activation of proinflammatory molecules, including TLR4 and IKK [5]–[7].
IKKβ is a key player in controlling both innate and adaptive immunity. Activation of IKKβ by phosphorylation at S177 and S181 induces phosphorylation, ubiquitination, and subsequent proteosomal degradation of its substrate IκBα. The degradation of IκBα allows NF-κB proteins to translocate to the nucleus and bind their cognate DNA binding sites to regulate the transcription of a large number of genes, including stress-response proteins and cytokines [8]. Growing evidence provides an intriguing link between metabolic inflammation and dysfunction of insulin and leptin signaling via activation of IKKβ and endoplasmatic reticulum (ER) stress [9]–[14]. Examination of ER stress markers in different tissues of dietary (high-fat diet-induced) and genetic (ob/ob) mouse models of obesity demonstrated increased levels of PERK phosphorylation and JNK and IKKβ activity [7],[12]. In addition, a recent study showed the activation of hypothalamic IKKβ/NF-κB, at least in part, through elevated endoplasmic reticulum stress in the hypothalamus and that these phenomena are associated with central insulin and leptin resistance, hyperphagia, and body weight gain in mice [7]. Thus, strategies to reduce the aberrant activation of inflammatory signaling and/or ER stress in hypothalamic neurons are of great interest to improve the central insulin and leptin action and prevent or treat obesity and related diseases.
Physical activity is considered a cornerstone of the treatment for obesity. Exercise has long been reported to reduce body weight and visceral adiposity, increasing the energy expenditure and improving glycaemic control in overweight or T2D patients [15],[16]. Since the discovery of interleukin (IL)-6 releases from contracting skeletal muscle, accumulating evidence indicates that exercise induces metabolic changes in other organs, such as the liver, the adipose tissue, and hypothalamus, in an IL-6 dependent manner. IL-6 is most often classified as a pro-inflammatory cytokine, although consistent data also demonstrate that IL-6 has an anti-inflammatory effect and may negatively regulate the inflammation of acute phase response by increasing IL-10, IL-1 receptor antagonist (IL-1ra), and soluble TNF-receptors (sTNF-R) [17]. Moreover, IL-6 appears to play a central role in the regulation of appetite, energy expenditure, and body composition [18],[19]. However, the effects of physical activity in the metabolic regulatory pathways in the central nervous system (CNS) remain unexplored. Thus, we hypothesized that exercise could exert its effects in the CNS by modulating the specific hypothalamic neurons responsible for the control of food consumption. In the present study, we investigated the effect of the anti-inflammatory response, mediated by IL-6, on hypothalamic IKKβ activation and ER stress, central insulin and leptin sensitivity, and food intake in diet-induced rats after physical activity.
Results
Exercise Suppresses Hyperphagia Mediated by Overnutrition
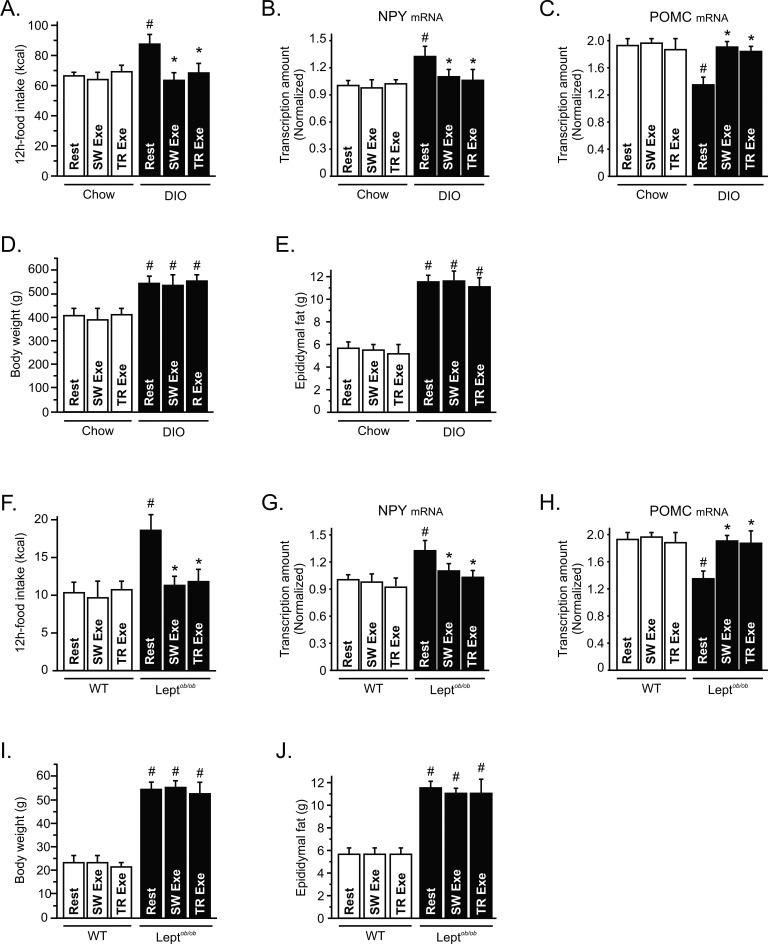
It has been demonstrated that physical activity may contribute to the energy balance by increasing energy expenditure. Although the energy expenditure aspects of such exercise may contribute to the effects of weight loss, the effect of exercise on the control of energy intake remains unclear. To evaluate the impact of physical activity on food consumption, we measured the 12-h total energy intake in lean and diet-induced obese (DIO) rats after one bout of swimming (SW Exe) and treadmill running (TR Exe) exercise. Neither of the exercise protocols changed the energy intake in lean animals; however, exercise suppressed the hyperphagic response, mediated by chronic overnutrition, restoring the energy intake to the levels of lean animals (Figure 1A). To assess whether the effects of exercise on food intake are dependent on the neuropeptides modulation, we performed a real time PCR assay to determine the mRNA levels of Neuropeptide-Y (NPY) and Proopiomelanocortin (POMC). After 9 h of fasting, we found that chronic overnutrition increased NPY mRNA and reduced POMC mRNA levels, while physical activity restored the NPY (Figure 1B) and POMC mRNA levels (Figure 1C) in obese animals; on the other hand, exercise did not change the NPY and POMC mRNA levels in lean rats (Figure 1B and C).
Figure 1. Exercise induces appetite-suppressive actions in different models of obesity.
(A) 12 h of food intake (kcal) in lean and diet-induced obesity (DIO) Wistar rats under resting conditions or after swimming exercise (SW Exe) or treadmill running (TR Exe) (n = 20–35 animals per group). Rats were fasted during 9 h and the hypothalamic levels (B) NPY and (C) POMC mRNA were examined using real time PCR assay. (D) Body weight, (E) epididymal fat pad weight, (F) 12-h food intake of leptin-deficient mice (Leptob/ob) and respective wild type group. (G) NPY and (H) POMC mRNA were examined using real time PCR assay. (I) Body weight and (J) epididymal fat pad weight of wild type and leptin-deficient mice under resting conditions or immediately after the exercise protocols (n = 10 animals per group). Data are the means ± SEM. # p<0.05 versus respective lean group at rest; * p<0.05 versus respective obese group at rest. Lean animals (white bars) and obese animals (black bars).
Chronic overnutrition increased body weight, epididymal fat (Figure 1D and E), serum insulin, leptin, triglycerides, and free fatty acid levels (Table 1), compared to age-matched controls. No significant variations were found in body weight, epididymal fat serum leptin, triglycerides, and urinary corticosterone levels between exercised and obese animals under resting conditions (Figure 1D, E and Table 1). The insulin levels were lower in both lean and obese rats after the exercise protocols (Table 1) and exercise increased the free fatty acid in obese animals (Table 1). To determine whether lean and obese rodents were swimming or running in the same fashion, we evaluated lactate production every 15 min during the SW Exe and TR Exe. We did not find any difference in the lactate production between lean and obese rats. Table 1 depicts the final values obtained in this test. These results reinforce the negative relationship between body weight change and stress related with the appetite-suppressive actions mediated by exercise.
Table 1. Metabolic parameters of lean and DIO rats after acute exercise protocols.
| Groups | Glucose (mg/dL) | Insulin (ng/mL) | Leptin (ng/mL) | Cholesterol (mg/dL) | TG (mg/dL) | FFA (mmol/L) | Corticost. (ng/mL) | Lactate (mmol/L) |
| Chow rest | 97±5 | 4.0±0.2 | 2.0±0.2 | 129.3±8.5 | 94.0±1,4 | 0.64±0.2 | 11.1±0.6 | ND |
| Chow SW exe | 108±9 | 2.8±0.3# | 2.1±0.1 | 123.7±6.4 | 93,7±7,2 | 0.81±0.1 | 11.0±0.4 | 3.6±0.6 |
| Chow TR exe | 118±12 | 2.9±0.2# | 2.1±0.2 | 121.8±6.3 | 97.3±7.5 | 0.79±0.2 | 11.4±0.5 | 4.20±0.4 |
| DIO rest | 115±5 | 7.8±0.4# | 3.6±0.3# | 141.0±10.1 | 152.5±7.8# | 1.75±0.5# | 11.2±0.7 | ND |
| DIO SW exe | 117±9 | 6.1±0.3#* | 3.7±0.2# | 141.6±9.5 | 141.7±9.5# | 2.89±0.3#* | 10.4±0.8 | 4.0±0.5 |
| DIO TR exe | 112±15 | 6.2±0.2#* | 3.6±0.3# | 145.2±12.5 | 150.3±8.0# | 2.65±0.4#* | 10.5±0.7 | 3.9±0.3 |
# p<0.05 versus chow rest and * p<0.05 versus DIO rest (n = 8–10).
To extend our hypothesis, we investigated food intake in leptin-deficient mice (ob/ob) after physical activity. Acute SW Exe and TR Exe did not change the food intake in wild type (WT) mice, however the food consumption was reduced in ob/ob mice (Figure 1F). After 9 h of fasting, we found that NPY mRNA was increased and POMC mRNA levels were reduced in ob/ob mice, while physical activity restored the NPY (Figure 1G) and POMC mRNA levels (Figure 1H) in obese animals; on the other hand, exercise did not change the NPY and POMC mRNA levels in control mice (Figure 1G and H). Exercise did not change the total body weight and epididymal fat pad weight in WT and ob/ob mice (Figure 1I and J). In addition, we observed that the exercise protocols did not change the triglycerides and free fatty acid levels but reduced the insulin levels in WT and ob/ob mice (Table 2). The lactate production was similar between lean and obese mice during the respective exercise protocols (Table 2). These exercise protocols did not evoke any significant stressful effect in these animals, as demonstrated by urinary corticosterone levels (Table 2). Thus, our data demonstrate that exercise modulates hypothalamic neuropeptides (NPY and POMC) and suppresses food intake in obese, but not in lean, rodents without changing the adipose tissue content and corticosterone levels.
Table 2. Metabolic parameters of control and ob/ob mice after acute exercise protocols.
| Groups | Glucose (mg/dL) | Insulin (ng/mL) | Leptin (ng/mL) | Cholesterol (mg/dL) | TG (mg/dL) | Corticosterone (ng/mL) | Lactate (mmol/L) |
| WT rest | 94±3 | 3.9±0.4 | 1.9±0.2 | 126.7±6.1 | 73.3±12.6 | 11.0±0.7 | ND |
| WT SW exe | 93±2 | 2.5±0.3# | 2.1±0.3 | 123.5±3.5 | 79.0±11.5 | 11.1±0.5 | 4.2±0.3 |
| WT TR exe | 94±2 | 2.7±0.3# | 2.0±0.3 | 128.5±3.5 | 77.33±12.7 | 11.5±0.8 | 5.3±1.3 |
| Leptob/ob rest | 284±18# | 8.0±0.4# | ND | 156.7±3.8# | 194.5±32.9# | 11.2±0.4 | ND |
| Leptob/ob SW exe | 154±8#* | 6.2±0.4#* | ND | 154.7±2.5# | 176.7±14.9# | 11.4±0.5 | 4.9±0.7 |
| Leptob/ob TR exe | 175±17#* | 6.5±0.3#* | ND | 153.2±2.7# | 173.7±16.5# | 11.2±0.6 | 5.3±0.8 |
# p<0.05 versus WT rest and *p<0.05 versus Leptob/ob rest (n = 6–8). ND, no detected.
Exercise Restores Insulin and Leptin Sensitivity in the Hypothalamus
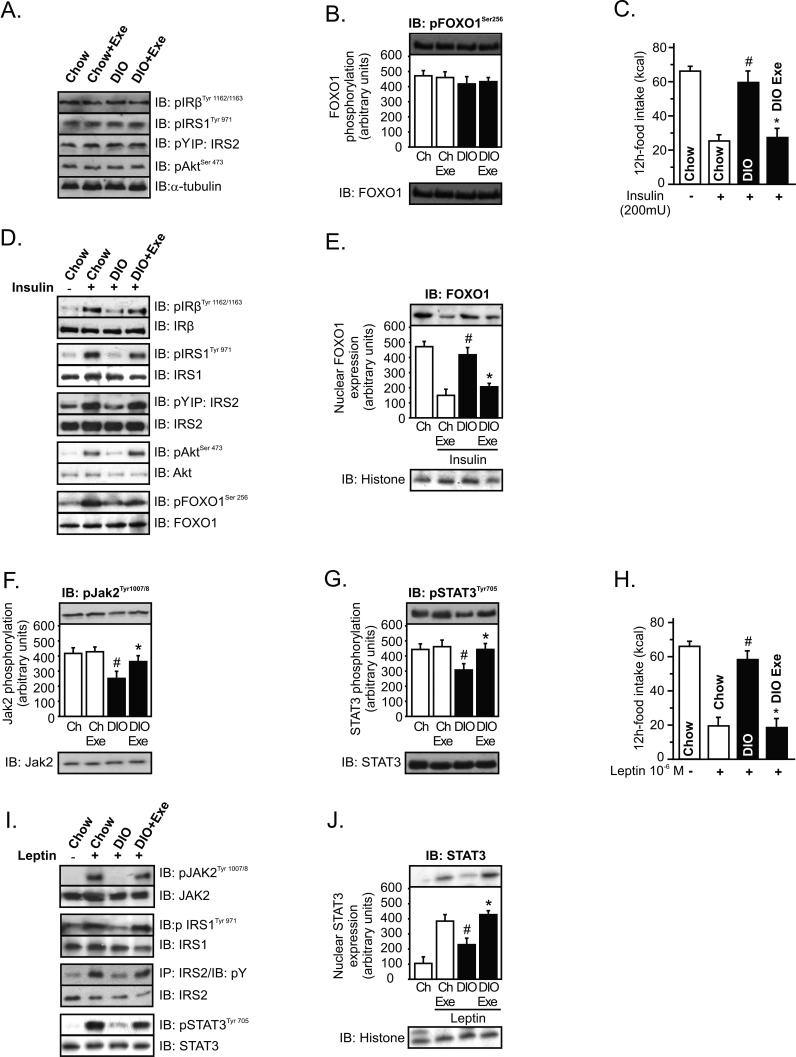
Next, we evaluated whether exercise modulates insulin signaling in the hypothalamus. Western blot analysis revealed that IRβ, IRS-1, IRS-2, Akt, and FOXO1 phosphorylation were similar between the groups (Figure 2A and B). Although exercise did not change the basal levels of insulin signaling, we next performed intrahypothalamic insulin (200 mU) or its vehicle injection to evaluated food intake and insulin sensitivity after the SW Exe protocol. Overnutrition markedly reduced the ability of intrahypothalamic insulin infusion to reduce food intake, when compared to chow-fed animals; however, exercise restored the central effects of insulin on reduced food intake (Figure 2C). Using Western blotting analysis, we determined the effects of exercise on the insulin sensitivity in hypothalamic tissue. The high-fat diet impaired insulin-induced tyrosine phosphorylation of insulin receptor β (IRβ), insulin receptor substrate-1 (IRS-1), and IRS-2 in the hypothalamus (Figure 2D). Similar results were observed for the serine phosphorylation of Akt and FOXO1 (Figure 2D). Physical activity was able to restore insulin-induced hypothalamic IRβ, IRS-1, and IRS-2 tyrosine phosphorylation and insulin-induced hypothalamic Akt and FOXO1 serine phosphorylation in DIO rats (Figure 2D). Subcellular fraction of hypothalamic extract was then performed to evaluate the nuclear FOXO1 expression. Intrahypothalamic infusion of insulin reduced the nuclear FOXO1 expression in control rats, but insulin failed to reduce the nuclear FOXO1 expression in rats after overnutrition (Figure 2E). After exercise, insulin reduced the nuclear FOXO1 expression in neuronal cells of obese animals (52%), when compared to DIO at rest (Figure 2E).
Figure 2. Hypothalamic insulin and leptin signaling after exercise.
Western blots showing hypothalamic lysates from Wistar rats; (A) Hypothalamic IRβ, IRS-1, IRS-2, and Akt phosphorylation, (B) Hypothalamic Foxo1 phosphorylation. (C) 12-h food intake (kcal) after intrahypothalamic infusion of insulin in lean and diet-induced obesity (DIO) Wistar rats under resting conditions or after exercise (n = 6–8 animals per group). Western blots of five independent experiments showing hypothalamic lysates from Wistar rats; (D) Insulin-induced IRβ, IRS-1, IRS-2, Akt, and Foxo1 phosphorylation in the hypothalamus. (E) Subcellular fractionation was performed to evaluate the nuclear Foxo1 expression in the hypothalamus of lean and obese rats at 30 min after insulin infusion. (F) Hypothalamic Jak-2 and (G) STAT-3 tyrosine phosphorylation. (H) 12-h food intake (kcal) after intrahypothalamic infusion of leptin (n = 6–8 animals per group). Western blots showing hypothalamic lysates from Wistar rats; (I) Leptin-induced Jak2, IRS-1, IRS-2, and STAT3 tyrosine phosphorylation in the hypothalamus. (J) Subcellular fractionation was performed to evaluate the nuclear STAT3 expression in the hypothalamic cells of lean and obese rats 30 min after leptin infusion. Data are the means ± SEM. # p<0.05 versus respective lean group at rest; * p<0.05 versus obese group at rest. Lean animals (white bars) and obese animals (black bars). The Figure 2F IB: pJak2Tyr1007/8 panel is excluded from the article's copyright license. See the accompanying retraction notice for more information.
We then explored the effects of exercise on hypothalamic leptin action, monitoring Janus Kinase-2 (Jak-2) and STAT-3 tyrosine phosphorylation. Exercise did not change the Jak-2 and STAT-3 phosphorylation in lean animals; however, overnutrition reduced Jak-2 and STAT-3 phosphorylation when compared to lean animals. Interestingly, physical activity was able to increase the neuronal Jak-2 and STAT-3 tyrosine phosphorylation in obese animals (Figure 2F and G). In addition we investigated the effects of exercise on leptin sensitivity. Intrahypothalamic infusion of leptin markedly reduced the 12-h total energy intake in control rats; however, the anorexigenic effects of leptin were attenuated in obese rats. In contrast, exercise restored the central effects of leptin on reduced food intake (Figure 2H). We noted that leptin modestly promoted the hypothalamic tyrosine phosphorylation of Jak-2, IRS-1, IRS-2, and STAT-3 after high-fat diet treatment. Conversely, exercise restored leptin-induced hypothalamic Jak-2, IRS-1, IRS-2, and STAT-3 tyrosine phosphorylation in obese animals (Figure 2I).
We also evaluated nuclear STAT3 expression after intrahypothalamic leptin infusion. After overnutrition, leptin failed to increase the expression of nuclear STAT3 in the hypothalamus. On the other hand, exercise increased the ability of leptin to increase the nuclear expression of STAT3 (48%) in the hypothalamus of obese animals (Figure 2J).
Increasing Hypothalamic Levels of IL-6 Reverses IKKβ and ER Stress Caused by Obesity
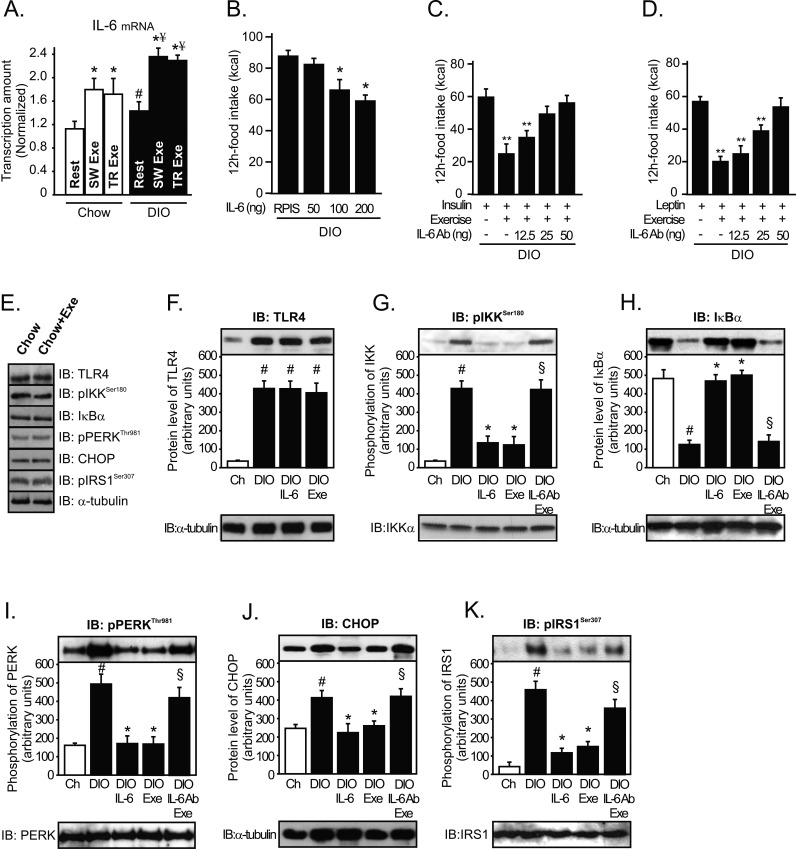
Recently, IL-6 was reported as the first myokine that is produced and released by contracting skeletal muscle fibers, exerting its effects on other organs of the body [20], including the hypothalamus [18],[21]. Thus, we evaluated the central role of IL-6 in the control of food intake. Firstly, the serum level of IL-6 was observed to be slightly up-regulated after high-fat diet treatment and was dramatically increased immediately after SW Exe and TR Exe, but we observed that, in exercised obese animals, the serum levels of IL-6 were higher when compared to exercised lean ones (Figure S1A). Similar results were found when IL-6 protein expression in the hypothalamic tissue was evaluated (Figure S1B). To investigate whether neuronal cells were producing IL-6 in response to exercise, we performed real time PCR to evaluate IL-6 mRNA levels in the hypothalamic tissue. IL-6 mRNA levels were slightly up-regulated after the high-fat diet treatment and were increased by about 53% and 64% immediately after physical activity in lean and obese rats, respectively (Figure 3A). Thus, these data demonstrate that exercise increases the serum and hypothalamic levels of IL-6.
Figure 3. Anti-hyperphagic response mediated by IL-6.
(A) IL-6 mRNA in the hypothalamus of lean or diet-induced obesity (DIO) rats under resting conditions and lean obese rats immediately after the swimming exercise (SW Exe) or treadmill running (TR Exe). (B) 12 h of food intake in obese rats under resting conditions following intrahypothalamic infusion of different doses of recombinant IL-6. Counter-regulatory effects of anti-IL-6 antibody on food intake in exercised obese rats after (C) insulin or (D) leptin infusion. Western blots of five independent experiments showing hypothalamic lysates from Wistar rats; (E) Expression and activity of protein involved in the inflammatory signaling or ER stress in control animals at rest condition or after acute exercise (F) TLR4 expression, (G) IKKβ phosphorylation, (H) IκBα expression, (I) PERK phosphorylation, (J) CHOP expression, and (K) IRS-1Ser307 phosphorylation from lean, obese, obese injected with recombinant IL-6, obese after exercise, and obese pretreated with anti-IL-6 antibody before the exercise protocol. Data are the means ± SEM. # p<0.05 versus lean group; * p<0.05 versus obese group at rest; ¥ p<0.05 versus respective exercised control rats; ** p<0.01 versus stimulated obese group at rest; § p<0.05 versus obese group injected with recombinant IL-6 and exercised obese rats (n = 8–10 animals per group). Swimming Exercise (SW Exe) or Treadmill Running (TR Exe). Lean animals (white bars) and obese animals (black bars).
Next, we sought to determine whether exercise requires IL-6 to mediate the anti-hyperphagic response. First we showed that the infusion of recombinant IL-6 into the third ventricle of obese animals under resting conditions reduced the food intake in a dose-dependent manner (Figure 3B) and restored the anorexigenic effects of insulin and leptin (Figure S2A and B). Although we used recombinant IL-6 to mimic the effects of exercise, in obese rats, the dose of recombinant IL-6 used (200 ng) is relatively high and this pharmacological approach does not reflect the same physiological conditions observed after exercise. Thus, we hypothesized that if exercise requires hypothalamic IL-6 activity to reduce food intake, inhibiting the hypothalamic effects of this cytokine, under physiological conditions, should diminish the appetite suppressive action mediated by exercise. To address this hypothesis, we developed an experimental strategy aimed at antagonizing the central action of IL-6 in the presence of a systemic elevation in plasma IL-6 concentration after physical activity. For this, we injected an anti-IL-6 antibody into the third-hypothalamic ventricle in obese animals at 15 min before the exercise protocol. Interestingly, pretreatment with anti-IL-6 antibody blocked the anorexigenic effects of insulin and leptin in exercised DIO rats (Figure 3C and D).
We then explored the mechanism by which IL-6 improves insulin and leptin signaling in the hypothalamus, evaluating the pro-inflammatory pathway. Firstly, we demonstrated that acute exercise did not change the expression or activity of the proteins involved in inflammatory signaling and in an ER stress in the hypothalamus of lean rats, when compared to control animals at rest (Figure 3E). However, high-fat diet consumption induced the aberrant activation of the NF-κB pathway components in the hypothalamic tissue, increasing the TLR4 expression, IKKβ serine phosphorylation, and the IκBα degradation (Figure 3F–H). We also monitored PERK phosphorylation and CHOP protein expression in the hypothalamus to evaluate ER stress. High-fat diet also activated ER stress, increasing PERK phosphorylation and CHOP protein expression in the hypothalamus (Figure 3I and J). In addition, high-fat diet increased IRS-1 serine 307 phosphorylation (Figure 3K). Neither acute exercise nor the single injection of recombinant IL-6 was able to reduce the TLR4 expression in the hypothalamic tissue of obese animals (Figure 3F). On the other hand, exercise and the intrahypothalamic injection of recombinant IL-6, in obese rats at rest, markedly reduced the hypothalamic IKKβ serine phosphorylation (∼60%) and prevented IκBα degradation in obese animals (Figure 3G and H). The recombinant IL-6 injection and exercise reduced PERK phosphorylation by about 60% and CHOP protein expression by about 45% (Figure 3I and J) and IRS-1 serine phosphorylation by about 60% (Figure 3K) in the hypothalamic tissue of hyperphagic animals. In addition, recombinant IL-6 and exercise restored insulin-induced Akt and leptin-induced and STAT-3 phosphorylation in the hypothalamus of obese animals (Figure S3A and B). Interestingly, our results show that the intrahypothalamic injection of anti-IL-6 antibody before the exercise protocol attenuated the ability of exercise to reduce the IKKβ/IκBα pathway, ER stress, and IRS1 serine phosphorylation in the hypothalamus (Figure 3G–K). The pretreatment with anti-IL6 antibody also blocked insulin-induced Akt and leptin-induced and STAT-3 phosphorylation, mediated by exercise in the hypothalamus of obese animals (Figure S3A and B).
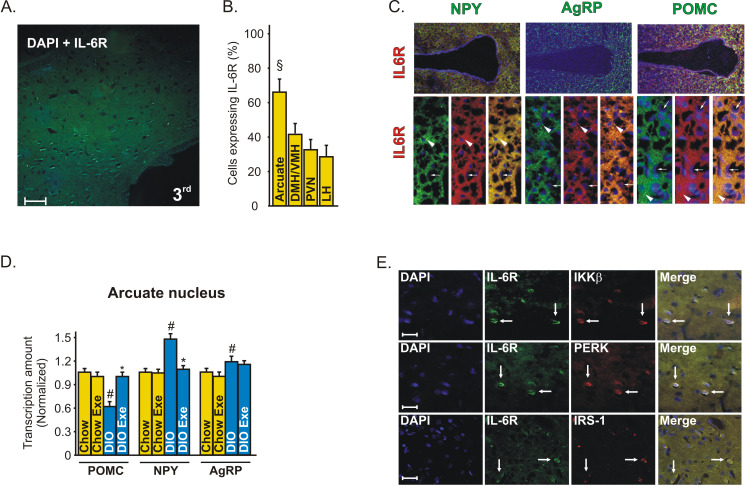
Immunohistochemistry with an anti-IL-6 Receptor (IL-6R)-specific antibody showed that IL-6R is expressed in a majority of neurons in the arcuate nucleus (Figure 4A). These data were confirmed when we quantified the positive cells in arcuate (Arc), dorsomedial and ventromedial (DMH/VMH), paraventricular (PVN), and lateral (LH) nuclei of hypothalamus (Figure 4B). The in situ hybridization experiment revealed that IL-6R is expressed in both anorexigenic and orexigenic neurons of rats (Figure 4C).
Figure 4. IL-6R localization in the hypothalamus of rats.
(A) Immunohistochemistry was performed in the hypothalamic tissue of control rats, using IL-6 receptor (IL-6R)-specific antibody (green) and DAPI (blue), with 50× magnification. (B) Positive cells were quantified in different hypothalamic nuclei, § p<0.05 versus the other nuclei. (C) In situ hybridization showing the co-localization of IL-6R (red) with POMC, NPY, and AgRP (green) neuropeptides in the hypothalamus of control rats. Head arrows show neurons and arrows show endothelial cells using 20× and 63× magnification. (D) The dissection of hypothalamic arcuate nucleus of lean and obese rats was obtained as described in Experimental Procedures to evaluate the mRNA of POMC, NPY, and AgRP, using the real time PCR. Data are the means ± SEM. # p<0.05 versus respective control group at rest; * p<0.05 versus obese rats at rest. Lean animals (yellow bars) and obese (blue bars). (E) Confocal microscopy was performed to evaluate the co-localization of IL-6R (green) and IKKβ, PERK, and IRS-1 (red) in the arcuate nuclei of obese rats, with 200× magnification (scale bar, 20 µm).
Since IL-6R is expressed in a majority of neurons in the arcuate nucleus, we dissected this specific hypothalamic region to evaluate the modulation of the neuropeptides in response to exercise in lean and obese rats. We found that exercise did not change the POMC, NPY, and AgRP mRNA in the arcuate nucleus of lean rats but increased the POMC and reduced the NPY mRNA levels in the arcuate nucleus of obese animals (Figure 4D).
Double-staining confocal microscopy showed that most neurons expressing IL-6R in the arcuate nucleus were shown to possess IKKβ, PERK, and IRS-1 in obese rats, showing a possible interaction between these molecules (Figure 4E).
Pharmacological Activation of IKKβ and ER Stress Is Suppressed by IL-6
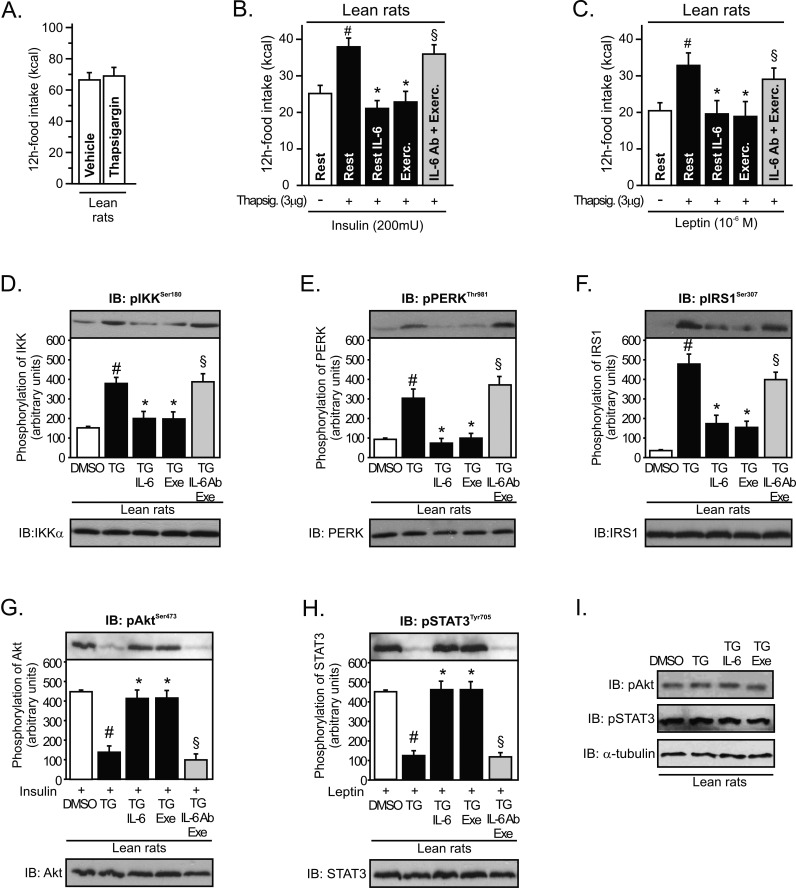
To further support data indicating that IL-6 may modulate ER stress, we performed an acute intrahypothalamic injection of an ER stress inducer, thapsigargin (TG), in lean rats. Acute intrahypothalamic infusion of thapsigargin did not change food intake in lean animals by itself (Figure 5A). However, our results revealed that intrahypothalamic infusion of thapsigargin blocked the anorexigenic effects mediated by insulin and leptin in lean rats and that the injection of recombinant IL-6 and exercise restored the suppressive appetite action of insulin and leptin (Figure 5B and C). In addition, the infusion of anti-IL6 antibody blocked the improvement in insulin and leptin action mediated by exercise (Figure 5B and C).
Figure 5. IL-6 reversed pharmacological endoplasmatic reticulum stress induction in the hypothalamus.
(A) 12 h of food intake in lean rats after thapsigargin infusion (3 µg). (B) Anorexigenic effects of insulin in the hypothalamus of lean rats pretreated with thapsigargin. (C) Anorexigenic effects of leptin in the hypothalamus of lean rats pretreated with thapsigargin. Western blots showing hypothalamic lysates from Wistar rats; (D) IKKβ, (E) PERK, and (F) IRS-1Ser307 phosphorylation from lean rats pretreated with thapsigargin. (G) Insulin-induced Akt serine phosphorylation, (H) leptin-induced STAT3 tyrosine phosphorylation in the hypothalamus of lean animals pretreated with thapsigargin, and (I) basal levels of Akt and STAT3 phosphorylation. Data are the means ± SEM. # p<0.05 versus DMSO group; * p<0.05 versus lean plus thapsigargin; § p<0.05 versus thapsigargin plus recombinant IL-6 or thapsigargin plus exercised (n = 8–10 animals per group).
In accordance with previous studies [7],[14],[22], we observed that thapsigargin markedly activated inflammatory signaling and ER stress in lean rats, as reflected by increased levels of hypothalamic IKKβ and PERK phosphorylation, respectively (Figure 5D and E), and induced central insulin and leptin resistance, increasing IRS-1 serine phosphorylation (Figure 5F) and reducing insulin-induced Akt serine phosphorylation and leptin-induced STAT-3 tyrosine phosphorylation (Figure 5G and H). Intrahypothalamic infusion of recombinant IL-6 and physical activity were sufficient to reverse all these phenomena (Figure 5D–H). Conversely, the infusion of intrahypothalamic anti-IL6 antibody before exercise protocol blocked these effects mediated by exercise (Figure 5D–H). There were no differences in the basal levels of Akt and STAT-3 phosphorylation between the groups (Figure 5I).
Low dose TNF-α has been reported to induce insulin and leptin resistance in the hypothalamus [23]. We injected a low dose of TNF-α into the hypothalamus of lean rats to investigate the effects of IL-6 on low-grade inflammation. First we observed that acute intrahypothalamic infusion of TNF-α did not change the food consumption in lean rats (unpublished data); however, TNF-α infusion blocked the anorexigenic actions of insulin and leptin in these animals (Figure S4A and B). The anorexigenic actions of these hormones were restored with the central infusion of recombinant IL-6 or after exercise in lean rats injected with TNF-α. In addition, the pretreatment with anti-IL6 antibody into the third ventricle blocked the improvement in insulin and leptin action mediated by exercise (Figure S4A and B).
The single injection of TNF-α also induced IKKβ serine, PERK threonine, and IRS-1 serine phosphorylation and reduced insulin-induced Akt serine phosphorylation and leptin- induced STAT-3 tyrosine phosphorylation in the hypothalamus of lean rats (Figure S4C–G). Intrahypothalamic infusion of recombinant IL-6 and physical activity were also sufficient to reverse all these phenomena. On the other hand, the central infusion of anti-IL6 antibody before the exercise protocol blocked the effects of physical activity (Figure S4C–G). There were no differences in the basal levels of Akt and STAT-3 phosphorylation between the groups (Figure S4H).
IL-6 Requires IL-10 to Reduce IKKβ and ER Stress in the Hypothalamus
Next, we sought to determine how IL-6 reduces the inflammatory response and ER stress in the hypothalamus after exercise. Several studies have reported that exercise-induced increases in plasma IL-6 levels are followed by increased circulating levels of well-known anti-inflammatory cytokines such as the IL-1ra and IL-10 [24],[25]. We found that the IL-1ra protein level was not changed in the hypothalamus after chronic overnutrition or after acute exercise protocols (Figure 6A); however, IL-10 protein expression was slightly increased in the hypothalamus in obese animals; both of the exercise protocols increased IL-10 expression in a similar fashion, but the induction of IL-10 expression, mediated by exercise, was higher in the hypothalamus of obese when compared to exercised lean animals (Figure 6B). The increase in hypothalamic IL-10 levels mediated by physical activity was confirmed by real time PCR assay (Figure 6C).
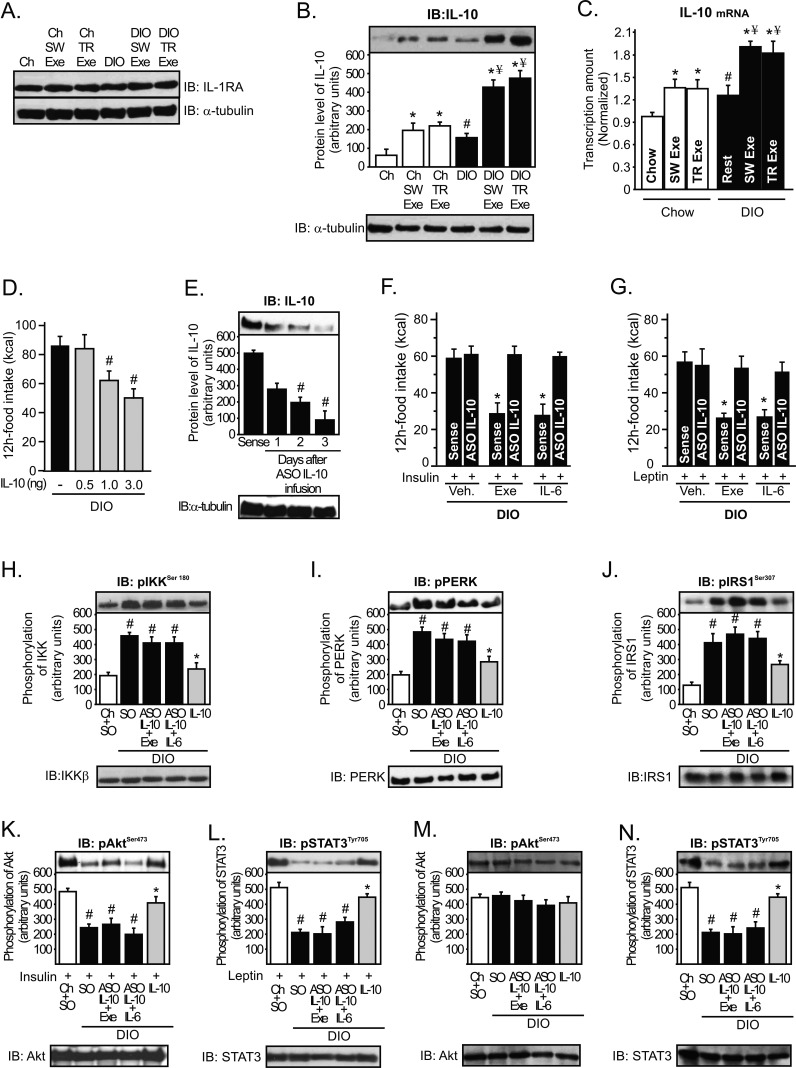
Figure 6. Role of hypothalamic IL-10 in the control of energy intake during obesity.
Western blots showing hypothalamic lysates from Wistar rats; (A) IL-1ra and (B) IL-10 expression in the hypothalamus. (C) IL-10 mRNA in the hypothalamus was examined using real time PCR assay. (D) 12 h food intake (kcal) in obese rats under resting conditions after intrahypothalamic infusion of different doses of recombinant IL-10. Western blots showing hypothalamic lysates from Wistar rats; (E) IL-10 expression after ASO IL-10 treatment in obese animals. (F) Intrahypothalamic treatment with ASO IL-10 blocked the anorexigenic response mediated by (F) insulin and (G) leptin in exercised obese animals or obese animals at rest injected with recombinant IL-6. Western blots showing hypothalamic lysates from Wistar rats; (H) IKKβ, (I) PERK, and (J) IRS-1Ser307 phosphorylation after ASO IL-10 treatment or after acute recombinant IL-10 infusion. (K) Insulin-induced Akt serine phosphorylation and (L) leptin-induced STAT3 tyrosine phosphorylation in the hypothalamus after ASO IL-10 treatment or after acute recombinant IL-10 infusion. (M) Basal levels of Akt serine phosphorylation and (N) STAT3 tyrosine phosphorylation in the hypothalamus after ASO IL-10 treatment or after acute recombinant IL-10 infusion. Data are the means ± SEM. # p<0.05 versus chow group; * p<0.05 versus DIO; ¥ p<0.05 versus exercised control animals; n = 8–10 animals per group. Lean animals (white bars), obese animals (black bars), and exercised obese plus recombinant IL-10 (grey bars). SO, sense oligonucleotide; ASO, antisense oligonucleotide.
We then investigated whether IL-10 reduced the energy intake in rodents. Intrahypothalamic injection of recombinant IL-10 reduced food intake in obese animals in a dose-dependent manner (Figure 6D). To explore whether IL-6 requires IL-10 expression to improve insulin and leptin action in the hypothalamus, we used an IL-10 antisense oligonucleotide (ASO IL-10) in the hypothalamus of obese rats to keep the expression levels of IL-10 low, even in the presence of high levels of IL-6 in the hypothalamus. Three days after ASO IL-10 treatment, IL-10 protein expression was reduced by about 75% in the hypothalamus of obese animals (Figure 6E). Thereafter, exercise and recombinant IL-6 infusion failed to improve the anorexigenic effects of insulin and leptin in obese animals treated with ASO IL-10 (Figure 6F and G).
IL-10 is a pleiotropic cytokine that controls inflammatory processes by suppressing the production of proinflammatory cytokines and blocking IKK/NF-κB signaling and ER stress [26],[27]. Thus, we investigated whether exercise and IL-6 requires IL-10 expression to reduce IKKβ activation and ER stress in the hypothalamus of obese animals. As demonstrated above, recombinant IL-6 infusion and exercise reduced IKKβ, PERK, and IRS-1Ser307 phosphorylation (Figure 3G, I, and K) and restored insulin and leptin signaling in the hypothalamus of obese animals (Figure S3), but the intrahypothalamic IL-10 ASO treatment abolished all these parameters mediated by recombinant IL-6 and exercise (Figure 6H–L). Conversely, the injection of recombinant IL-10 in the hypothalamus of obese animals at rest markedly reduced IKKβ, PERK, and IRS-1Ser307 phosphorylation and increased insulin-induced Akt and leptin-induced STAT-3 phosphorylation in the hypothalamic tissue of obese rats (Figure 6H–L). There were no differences in the basal levels of Akt (Figure 6M). However, STAT3 tyrosine phosphorylation was reduced in the hypothalamus of obese rats, but neither exercise nor IL-6 intrahypothalamic injection was able to increase the STAT-3 phosphorylation after IL-10 ASO treatment (Figure 6N).
Attenuating TLR-4-Dependent IL-6 and IL-10 Production Abolishes Exercise Sensitization of Insulin and Leptin in the Hypothalamus
Several studies showed that Toll-like receptor inactivation results in an attenuation of the secretion of several cytokines. TLR4- and MyD88-deficient mice sustain significantly lower levels of serum cytokines such as IL-1β, IL-6, TNFα, and IL-10 after different pro-inflammatory stimuli [28]–[30]. Since TLR4 mediates IL-6 transcriptional responses in myocytes and in the skeletal muscle of C3H/HeJ mice [31], we investigated whether exercise restores insulin and leptin signaling in the hypothalamus of TLR4-deficient mice (C3H/HeJ) injected with thapsigargin (TG, an endoplasmic reticulum stress inducer).
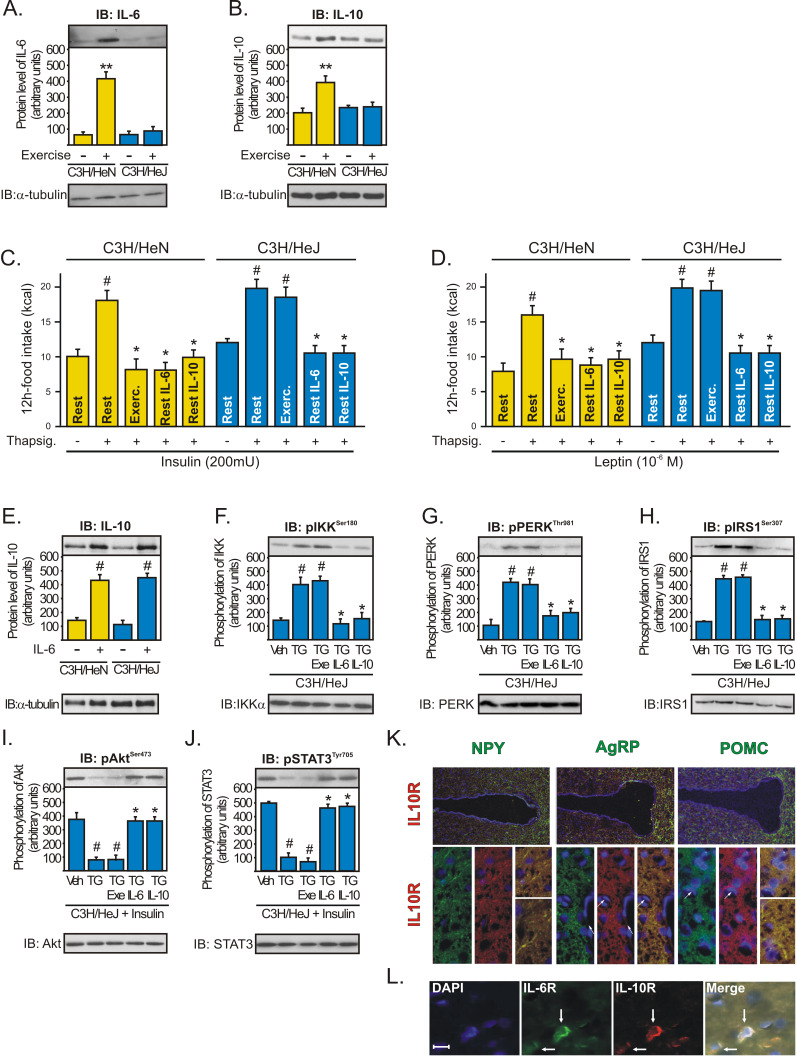
In contrast to WT mice, TLR4-deficient mice were found to sustain significantly lower hypothalamic levels of IL-6 (Figure 7A) and IL-10 (Figure 7B) after exercise. The food consumption was similar between C3H/HeN and C3H/HeJ under basal conditions, and acutely, thapsigargin alone did not affect the food intake in these mice (unpublished data); however, the intrahypothalamic administration of TG impaired the anorexigenic effects of insulin and leptin in WT (C3H/HeN) and in TLR4-deficient mice; while physical activity restored the appetite suppressive actions of insulin and leptin in WT but not in TLR4-deficient mice (Figure 7C and D). Furthermore, the intrahypothalamic injection of either recombinant IL-6 or IL-10 restored the anorexigenic actions of insulin and leptin in both WT and TLR4-deficient mice injected with TG (Figure 7C and D). We also observed that the intrahypothalamic infusion of recombinant IL-6 was able to increase the IL-10 protein expression in the hypothalamus of WT and TLR4-deficient mice (Figure 7E). Moreover, exercise failed to reduce inflammation and ER stress and failed to improve insulin and leptin sensitivity in the hypothalamus of TLR4-deficient mice injected with TG (Figure 7F–J). On the other hand, the intrahypothalamic injection of recombinant IL-6 or IL-10 reduced IKKβ, PERK, and IRS-1Ser307 phosphorylation and restored insulin and leptin signaling in the hypothalamus of TLR4-deficient mice injected with TG (Figure 7F–J). There were no differences in the basal levels of Akt and STAT-3 phosphorylation between the groups (unpublished data). The in situ hybridization experiment revealed that IL-10R is expressed in NPY, POMC, and AgRP neurons of rats (Figure 7K). Finally, immunohistochemistry with anti-IL-6R and anti-IL-10 Receptor (IL-10R)-specific antibodies revealed that IL-6R and IL-10R are expressed in the same specific neuronal subtypes in the arcuate nucleus (Figure 7L).
Figure 7. The central anti-inflammatory response mediated by exercise requires augmented hypothalamic levels of IL-6 and IL-10.
Western blots showing hypothalamic lysates from C3H/NeN and C3H/HeJ mice under resting conditions or after physical activity; (A) IL-6 and (B) IL-10 expression. Anorexigenic effects of insulin (C) or leptin (D) in C3H/NeN and C3H/HeJ mice under resting conditions, after thapsigargin, thapsigargin plus exercise, and thapsigargin plus recombinant IL-6 or IL-10. Western blots showing hypothalamic lysates from mice; (E) IL-10 expression at 2 h after intrahypothalamic injection of recombinant IL-6 (200 ng) in C3H/NeN and C3H/HeJ mice under resting conditions. (F) IKKβ, (G) PERK, and (H) IRS-1Ser307 phosphorylation and (I) Insulin-induced Akt serine phosphorylation and (J) leptin-induced STAT3 tyrosine phosphorylation in the hypothalamus of C3H/HeJ mice after intrahypothalamic infusion of DMSO, thapsigargin, thapsigargin plus exercise, and thapsigargin plus recombinant IL-6 or IL-10. Data are the means ± SEM. ** p<0.05 versus respective control group at rest; # p<0.05 versus respective control group non-stimulated or stimulated with DMSO; * p<0.05 versus thapsigargin; n = 5–6 animals per group. C3H/NeN (yellow bars) and C3H/HeJ (blue bars). (K) Co-localization of IL-10R (red) with NPY, AgRP, and POMC (green) was evaluated using in situ hybridization technique in the hypothalamus of lean rats, with 20× and 63× magnification. (L) Co-localization of IL-6R (green) and IL-10R (red) in the arcuate nuclei of lean rats, with 200× magnification (scale bar, 10 µm).
Effects of Chronic Exercise on Food Intake and Body Weight
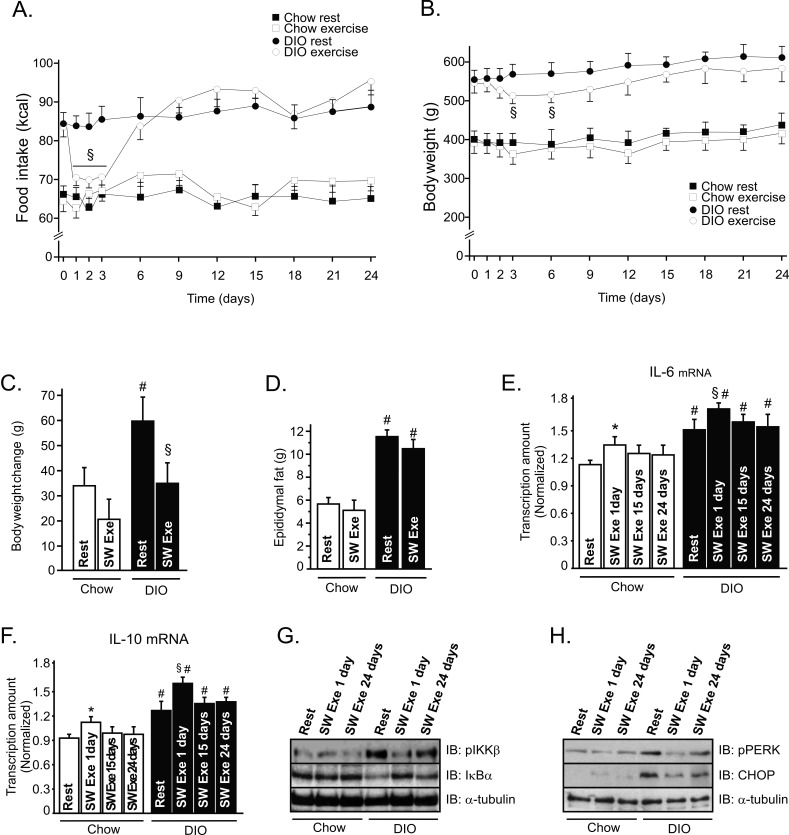
We then investigated the effects of chronic SW Exe on food intake and body weight in lean and obese rats. As observed in acute exercise, the chronic exercise protocol did not change the food consumption in lean animals; however, we observed that the food intake was reduced in obese animals after onset of the chronic exercise protocol, for 3 d, but thereafter, the food intake returned to basal levels on the sixth day and was maintained similar to that of obese rats at rest (Figure 8A). Exercised obese animals showed a significant reduction of the total body weight between the third and the sixth days, but this phenomenon was not observed in control animals (Figure 8B). We also evaluated the weight gain by analyzing the variation of the body weight between the 1st and 24th days. We observed a slight weight gain in control animals at rest, but the chronic exercise protocol did not attenuate the weight gain in lean animals (Figure 8C). On the other hand, overnutrition induced a great weight gain in the group under resting conditions, while chronic exercise attenuated the weight gain in obese animals (Figure 8C). We did not observe a statistical difference in the absolute values of the epididymal fat mass between the exercised obese animals and the obese animals at rest at the end of chronic exercise protocol (Figure 8D).
Figure 8. Effects of chronic exercise on food consumption, body weight, and IL-6 and IL-10 production.
Evaluation of (A) food intake (kcal) and (B) body weight in control and obese animals during chronic exercise protocol. Chow rest (black square), chow exercise (white square), DIO rest (black ball), and DIO exercise (white ball). (C) Body weight change between the 1st and 24th day. (D) Epididymal fat pad weight after chronic exercise, (E) IL-6 and (F) IL-10 mRNA levels in the hypothalamus of lean and obese rats at rest or after chronic exercise. Western blots showing hypothalamic lysates from lean and obese Wistar rats; (G) IKKβ phosphorylation and IκBα expression and (H) PERK phosphorylation and CHOP expression 1 and 24 d after the chronic exercise protocol. Data are the means ± SEM. * p<0.05 versus chow at rest; § p<0.05 versus DIO at rest; # p<0.05 versus chow group (rest); n = 8–10 animals per group. Lean animals (white bars) and obese animals (black bars).
Chronic overnutrition increased serum insulin, leptin, triglycerides, and free fatty acid levels, compared to age-matched controls; however, chronic exercise reduced serum insulin, triglycerides, and free fatty acid levels in obese animals (Table 3). To determine whether lean and obese rodents were swimming or running in the same fashion, we evaluated lactate production every 15 min during the SW Exe. We did not find any difference in the lactate production between lean and obese rats. Table 3 depicts the final values obtained in this test. We also determined that this exercise protocol did not change the corticosterone levels in lean and obese animals 3 d after the onset of this exercise protocol (Table 3).
Table 3. Metabolic parameters of lean and DIO rats after chronic exercise.
| Groups | Glucose (mg/dL) | Insulin (ng/mL) | Leptin (ng/mL) | Cholesterol (mg/dL) | TG (mg/dL) | FFA (mmol/L) | Corticost. (ng/mL) | Lactate (mmol/L) |
| Chow rest | 98±4 | 4.0±0.2 | 2.0±0.2 | 132.9±9.3 | 94.0±1,4 | 0.64±0.2 | 11.1±0.6 | ND |
| Chow SW exe | 99±8 | 3.1±0.4# | 2.2±0.2 | 134.5±6.2 | 92.3±6,3 | 0.64±0.2 | 11.6±0.7 | 5.2±0.5 |
| DIO rest | 115±5 | 7.8±0.4# | 3.6±0.3# | 149.6±10.8 | 152.5±7.8# | 1.75±0.5# | 11.2±0.7 | ND |
| DIO SW exe | 114±7 | 5.1±0.5#* | 3.1±0.3# | 144.6±10.1 | 102.3±10.7#* | 0.89±0.3#* | 11.5±0.9 | 5.3±0.7 |
# p<0.05 versus chow rest and *p<0.05 versus DIO rest (n = 8–10).
We also evaluated IL-6 and IL-10 mRNA levels in the hypothalamic tissue during the chronic exercise protocol. Interestingly, we observed that the levels of IL-6 mRNA in the hypothalamus were higher on the first day of exercise, when compared to the 15th and 24th days of exercise; this phenomenon was observed in lean and obese exercised rats (Figure 8E). Similar results were found when we analyzed the levels of IL-10 mRNA during chronic exercise (Figure 8F). Finally, the chronic exercise protocol reduced IKKβ phosphorylation and increased IκBα expression in the hypothalamus of obese rats; however, this anti-inflammatory response was more evident on the first day of exercise (Figure 8G). Similar results were found when we analyzed the ER stress markers, such as PERK phosphorylation and CHOP expression (Figure 8H).
Discussion
Exercise as a Potential Target for Countering Hyperphagia and Obesity
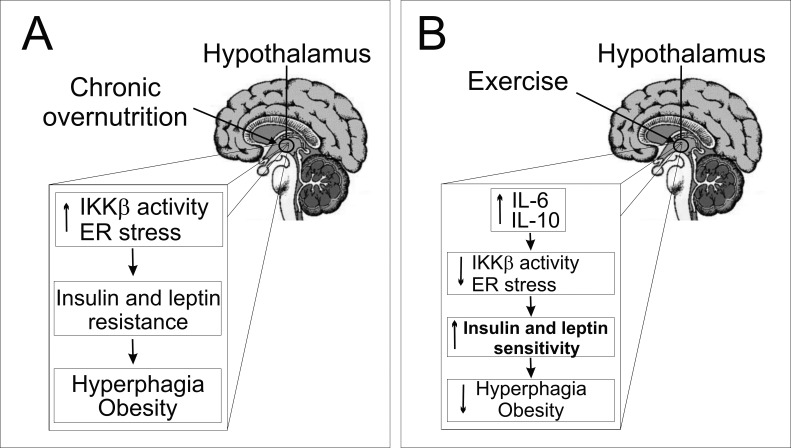
Physical activity is a cornerstone in the prevention of obesity and related diseases. Although the energy expenditure aspects of such exercise may contribute to the effects of weight loss, it has been suggested that physical exercise may also contribute to negative energy balance by altering appetite and reducing food intake in rodents [21],[32] and humans [33],[34]. Our study shows that acute exercise per se did not evoke any meaningful effect, in terms of food intake in lean animals, but interestingly, it was crucial for suppressing hyperphagia mediated by overnutrition, reducing hypothalamic IKKβ/NF-κB activation and ER stress, thus improving insulin and leptin action in an IL-6- and IL-10-dependent manner (Figure 9).
Figure 9. Schematic diagrams of the proposed role of the hypothalamic anti-inflammatory response mediated by exercise.
(A) Overnutrition induces hypothalamic IKKβ activation and endoplasmatic reticulum stress, leading to central insulin and leptin resistance, hyperphagia, and obesity. (B) We propose that exercise increases the central anti-inflammatory response, increasing hypothalamic IL-6 and IL-10 expression. This phenomenon is crucial for reducing hypothalamic IKKβ activation and endoplasmatic reticulum stress and turn, restoring insulin and leptin signaling, and reorganizing the set point of nutritional balance.
In the absence of obesity, exercise does not affect food behavior, as the anorexigenic or orexigenic pathways remain unchanged in rats. Several experimental studies have demonstrated that physical activity does not activate anorexigenic pathways, such as PI3-K or mTOR/p70S6K [18],[21], and does not inhibit the orexigenic pathways, such as AMPK signaling in the hypothalamus of control rodents [35]. On the other hand, the present study provides substantial evidence that physical activity could help to reorganize the set point of nutritional balance and, therefore, aid in counteracting the energy imbalance induced by overnutrition-related obesity. These data are in accordance with Park and colleagues [36], who showed that exercise improved insulin and leptin signaling, increased STAT3, and reduced AMPK phosphorylation in the cerebral cortex and hypothalamus of diabetic rats, contributing to the regulation of body weight and glucose homeostasis. These data demonstrate that exercise increases the anorexigenic pathways and attenuates the orexigenic signals, only in obese and diabetic animals, changing the anorexigenic and orexigenic signaling pathways in the hypothalamus. We also reported that physical activity reduced the hyperphagic response by reducing NPY mRNA and increasing POMC mRNA predominantly in the arcuate nucleus of obese animals. It is important to emphasize that acute exercise did not change the total body weight or epididymal fat pad weight, showing that physical activity can induce the anorexigenic response in the hypothalamus, independently of the body weight change. Our data showed that the reduction on food intake observed in obese animals after both exercise protocols was not related to stress as demonstrated by costicosterone levels. In opposite fashion, it has been demonstrated that NPY mediates stress-induced exacerbation of diet-induced obesity and metabolic syndrome after different stressor agents such as exposure to cold water or aggression in mice [37]. Thus, we hypothesized that some factors, produced during the exercise session, could be involved in this anorexigenic response.
IL-6 Is a Crucial Cytokine for Exercise to Restore Hypothalamic Insulin and Leptin Signaling
Skeletal muscle is an endocrine organ that, upon contraction, stimulates the production and release of cytokines, also called myokines, which can influence metabolism and modify cytokine production in tissue and organs. IL-6 is the first cytokine present in the circulation during exercise [17]. IL-6 can elicit proinflammatory or anti-inflammatory effects, depending on the in vivo environmental circumstances. Although IL-6 has been associated with low-grade inflammation and insulin resistance, it has been demonstrated that acute IL-6 treatment enhances insulin-stimulated glucose disposal in humans [38].
Centrally acting IL-6 appears to play a role in the regulation of appetite, energy expenditure, and body composition. Wallenius and colleagues elegantly showed that long-term peripheral IL-6 treatment to IL6−/− mice caused a decrease in body weight. In addition to increasing energy expenditure, IL-6 may prevent obesity by inhibiting feeding as obese IL-6−/− mice had increased absolute food intake [39]. In accordance with these data, mice fed on a high-fat diet with sustained circulating human IL-6 secreted predominantly from brain and lung (hIL6tg) had low leptin concentrations, consumed less food, and expended more energy than wild-type mice [40]. In addition, the intercrossing of hIL6tg and ob/ob mice increased the leptin sensitivity in these mice, when compared to ob/ob mice [40]. Recently, we demonstrated that exercise requires IL-6 to increase hypothalamic insulin and leptin sensitivity [18] and increase the effects of leptin on the AMPK/mTOR pathway in the hypothalamus of rodents [21]. Furthermore, IL-6 is also released from the brain during prolonged exercise in humans [41]. In the present study, we showed that the increment of IL-6 expression in the hypothalamus was crucial to exercise for reducing the inflammation and ER stress activation induced by overnutrition. However, these effects, promoted by exercise, were not observed when we used an intrahypothalamic infusion of anti-IL-6 antibody before the exercise protocol. In addition, the infusion of recombinant IL-6 into the third hypothalamic ventricle reduced the energy intake in obese animals under resting conditions, in a dose-dependent manner, and reduced hypothalamic IKKβ and ER stress activation.
In another approach, we used an ER stress inducer in lean rats to evaluate the effects of exercise/IL-6 on hypothalamic ER stress. We demonstrated that acute thapsigargin injection increased IKKβ and PERK phosphorylation and reduced insulin and leptin action in the hypothalamus and that exercise and the infusion of recombinant IL-6 were able to reduce thapsigargin-induced inflammation, ER stress, and insulin and leptin resistance, whereas the IL-6 antibody pretreatment reversed the effects of exercise. Although thapsigargin increased the hypothalamic IKKβ and PERK phosphorylation, we did not observe any difference in the basal levels of Akt serine 473 and STAT3 tyrosine 705 phosphorylation and in food intake in rats injected with thapsigargin alone. These data are in accordance with a previous study that reported that the ER-stress inhibitor, tauroursodeoxycholic acid (TUDCA), acutely reduced the hypothalamic PERK phosphorylation and NF-kB activation but did not change the food intake in mice fed on a high-fat diet [7]. Thus, our data demonstrate that IL-6 plays an important role in the control of the ER stress effects in the hypothalamus of rats.
All these results are significant, since IKKβ and ER stress activation were strongly associated with insulin and leptin resistance in the hypothalamic tissue. Although we showed a consistent anti-inflammatory effect, mediated by IL-6, in the hypothalamus, we cannot exclude the possibility that IL-6 acts directly as an anorexigenic factor.
Hypothalamic IL-10: A Core Anti-Inflammatory Cytokine Induced by IL-6
Although our findings clearly show that IL-6 diminished hypothalamic IKKβ and ER stress activation and restored the central insulin and leptin action in an animal model of obesity, the question remains as to how IL-6 promotes these events in the hypothalamus. Following exercise, the high circulating levels of IL-6 are followed by an increase in two anti-inflammatory molecules, IL-1ra and IL-10 [25]. Therefore, IL-6 induces an anti-inflammatory environment by inducing the production of IL-1ra and IL-10. In our study, we found that exercise increased the hypothalamic levels of IL-10 but did not change IL-1ra expression in this tissue. Thus, we showed that the anti-inflammatory response mediated by IL-6 involves the increase of IL-10 expression in the hypothalamus.
IL-10 is an important immunoregulatory cytokine with multiple biological effects. In the cytoplasm, it has been demonstrated that IL-10 blocks NF-κB activity at two levels: suppressing IKK activity and NF-κB DNA binding activity [26]. Moreover, IL-10 reduced ER stress in intestinal eptithelial cells, whereas IL-10−/− mice demonstrated that the expression of the ER stress response protein grp-78/BiP was increased in intestinal eptithelial cells under conditions of chronic inflammation [27].
In the CNS, the anti-inflammatory role of IL-10 has been extensively studied in experimental autoimmune encephalomyelitis, an animal model of human multiple sclerosis. The increase in IL-10 expression in the CNS during recovery from brain inflammation and the inability of IL-10 null mice to recover from acute CNS inflammation suggests that the presence of IL-10 within this target organ is required for disease remission [42],[43]. However, the role of hypothalamic IL-10 in the control of low-grade inflammation generated during obesity was unknown. Here, we discovered that intrahypothalamic infusion of recombinant IL-10 blocked IKK/NF-κB signaling and ER stress and restored Akt and STAT3 phosphorylation, promoting a re-balance in the energy intake in obese animals. On the other hand, the selective decrease in IL-10 expression in discrete hypothalamic nuclei of obese animals mediated by ASO treatment blunted the effects of both exercise and the intrahypothalamic infusion of recombinant IL-6 in the restoration of central insulin and leptin actions. In addition, we demonstrated that in mice that sustained significantly lower hypothalamic levels of IL-6 and IL-10 after exercise (C3H/HeJ), there was no reduction in pharmacological ER stress activation, in contrast to WT mice. These data are intriguing as IL-10 represents an important cytokine that may reduce both inflammation and ER stress in the hypothalamus. Thus, the modulation of hypothalamic IL-10 expression could be considered the direct target of exercise/IL-6 and constitutes a promising alternative to reduce hypothalamic inflammation and ER stress related to obesity.
The decrease in food intake induced by IL-10 in obese rats is not in accordance with the effects observed in IL-10 KO. It has been reported that mice with combined deficiency of leptin and IL-10 gain less body weight than mice lacking leptin only [44]. However, these discrepancies may be a consequence of methodological differences related to physiological versus genetic approaches and acute versus chronic situation investigated, and most important it may be consequence of IL-10 effects in the regulation of energy expenditure, likewise observed in mice lacking TNF-α receptor [45]; thus, the role of IL-10 in the control of food intake and energy expenditure deserves further exploration.
The long-term reversal effects on body composition, mediated by exercise alone, are controversial. It should be acknowledged that it is often difficult to find long-term reversal effects on body fat in both experimental animals and humans by exercise alone without restrained diet [46]. In the chronic experiments, we observed that the obese animals lost weight during the same period in which a reduction in food intake was observed. After this period, no significant difference was observed in the body weight of exercised animals, although the obese animals presented a significant improvement in metabolic parameters after the chronic exercise protocol.
Since IKKβ/NF-κB inhibition in the CNS represents a potential target therapy to combat obesity and most anti-inflammatory therapies have limited direct effects on IKKβ/NF-κB and a limited capacity for concentration in the CNS, our study provides substantial evidence that physical activity could help to reorganize the set point of nutritional balance and therefore aid in counteracting the energy imbalance induced by overnutrition through the anti-inflammatory response in hypothalamic neurons. Hence, IL-6 and IL-10 are important physiological contributors to the central insulin and leptin action mediated by physical activity, linking it to hypothalamic ER stress and inflammation.
Materials and Methods
Antibodies and Chemicals
Protein A-Sepharose 6 MB and Nitrocellulose paper (Hybond ECL, 0.45 µm) were from Amersham Pharmacia Biotech United Kingdom Ltd. (Buckinghamshire, United Kingdom). Ketamin was from Parke-Davis (São Paulo, SP, Brazil) and diazepam and thiopethal were from Cristália (Itapira, SP, Brazil). Anti-phospho-JAK2 (rabbit polyclonal, AB3805) antibody was from Upstate Biotechnology (Charlottesville, VA, USA). Anti-JAK2 (rabbit polyclonal, SC-278), anti-STAT3 (rabbit polyclonal, SC-483), anti-phospho-IRβ (rabbit polyclonal, SC-25103), anti-IRβ (rabbit polyclonal, SC-711), anti-phospho-IRS-1 (rabbit polyclonal, SC-17199), anti-IRS-1 (rabbit polyclonal, SC-559), anti-IRS-2 (rabbit polyclonal, SC-1556), anti-phosphotyrosine (mouse monoclonal, SC-508), anti-Foxo1 (rabbit polyclonal, SC-11350), anti-IL-1ra (goat polyclonal, SC-8481), anti-TNF-α (rabbit polyclonal, SC-8301), anti-IKKβ (goat polyclonal, SC-34673), anti-PERK (rabbit polyclonal, SC-13073), anti-phospho-PERK (rabbit polyclonal, SC-32577), anti-CHOP (GADD 153) (rabbit polyclonal, SC-575), anti-IL-10 (goat polyclonal, SC-1783), and anti-IL-6 (rabbit polyclonal, SC-7920) antibodies were from Santa Cruz Biotechnology, Inc. Anti-phospho-STAT3 (rabbit polyclonal, #9131), anti-phospho-Akt (rabbit polyclonal, #9271), anti-phospho-Foxo1 (rabbit polyclonal, #9461), anti-beta tubulin (rabbit polyclonal, #2146), anti-phospho-IKKα/β (rabbit polyclonal, #2687), anti-IκBα (rabbit polyclonal, #9242), anti-TLR4 (rabbit polyclonal, #2219), anti-phospho-IRS-1 307 (rabbit polyclonal, #2381), and anti-Akt (rabbit polyclonal, #9272) were from Cell Signalling Technology (Beverly, MA, USA). Leptin, thapsigargin, and recombinant IL-6 and -10 were from Calbiochem (San Diego, CA, USA). Routine reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise specified.
Serum Insulin, Leptin, and IL-6 Quantification
Blood was collected from the cava vein 15 min after the exercise protocols. Plasma was separated by centrifugation (1,100 g) for 15 min at 4 °C and stored at −80 °C until assay. RIA was employed to measure serum insulin. Leptin and IL-6 concentrations were determined using a commercially available Enzyme Linked Immunosorbent Assay (ELISA) kit (Crystal Chem Inc., Chicago, IL). Blood lactate was measured using Accutrend Plus equipment (Roche); sample blood was obtained from the tails every 15 min during the exercise protocols. Serum cholesterol and triglycerides were measured in control and exercised animals after 8 h of fasting using Accutrend Plus equipment (Roche). Serum free fatty acids (FFA) levels were analyzed in rats using the NEFA-kit-U (Wako Chemical GmBH, Neuss, Germany).
Corticosterone levels were determined using urine samples obtained from rats and mice using specific metabolic cage during 24 h after the exercise protocols. The corticosterone level was determined using an EIA kit from Cayman chemical (Ann Arbor, MI).
Animals
Male 4-wk-old Wistar rats were obtained from the University of Campinas Breeding Center. The investigation was approved by the ethics committee and followed the University guidelines for the use of animals in experimental studies and experiments conform to the Guide for the Care and Use of Laboratory Animals, published by the U.S. National Institutes of Health (NIH publication no. 85-23 revised 1996). The animals were maintained on 12h∶12h artificial light-dark cycles and housed in individual cages. Rats were randomly divided into two groups: control, fed on standard rodent chow (3,948 kcal.Kg−1), and DIO, fed a fat-rich chow (5,358 kcal.Kg−1) ad libitum for 3 mo. This diet composition has been previously used [47].
Male (10-wk-old) ob/ob mice and their respective controls C57BL/6J background were obtained from The Jackson Laboratory and provided by the University of São Paulo. The mice were bred under specific pathogen-free conditions at the Central Breeding Center of University of Campinas.
Male C3H/HeJ (10-wk-old) mice and their respective controls C3H/HeN were obtained from The Jackson Laboratory and provided by the University of São Paulo. The mice were bred under specific pathogen-free conditions at the Central Breeding Center of the University of Campinas.
Intracerebroventricular Cannulation
The animals were stereotaxically instrumented under intraperitoneal injection of a mix of ketamin (10 mg) and diazepam (0.07 mg) (0.2 ml/100 g body weight) with a chronic 26-gauge stainless steel indwelling guide cannula aseptically placed into the third ventricle at the midline coordinates of 0.5 mm posterior to the bregma and 8.5 mm below the surface of the skull of rats and 1.8 mm posterior to the bregma and 5.0 mm below the surface of the skull of mice.
Exercise Protocols
Animals were acclimated to swimming for 2 d (10 min per day). Water temperature was maintained at 34–35 °C. Rats performed two 3-h exercise bouts, separated by one 45-min rest period. The rats swam in groups of three in plastic barrels of 45 cm in diameter that were filled to a depth of 50 cm. This protocol was conducted between 11:00 a.m. and 6:00 p.m., as previously described [48], and mice performed four 30-min exercise bouts, separated by one 5-min rest period. The mice swam in groups of four in plastic barrels of 40 cm in diameter that were filled to a depth of 20 cm. This protocol was conducted between 3:00 p.m. and 6:00 p.m. Both exercise protocols finished at 6:00 p.m. for evaluation of food intake and analysis of hypothalamic tissue.
The chronic exercise protocol consisted of daily swimming sessions (1 h/d, 5 d/wk, for 4 wk) with an overload (2.0% of the body weight). The hypothalamic tissues and the metabolic parameter were evaluated 36 h after the last exercise session. Rats also performed a single bout of treadmill (Insight LTDA - Ribeirão Preto, SP) running (60 min, speed of 10–15 m/min at a 5% incline) and mice performed a single bout of treadmill running (90 min, speed of 7–10 m/min at a 5% incline).
Intracerebroventricular Treatments
Rats or mice were deprived of food for 2 h with free access to water and received 3 µl of bolus injection into the third ventricle, as follows:
Insulin and leptin treatments
Animals received intrahypothalamic infusion of vehicle, insulin (200 mU), or leptin (10−6 M) at 6:00 p.m. to evaluate the food intake or insulin and leptin signaling. Food intake was determined by measuring the difference between the weight of chow given and the weight of chow at the end of a 12-h period.
Recombinant IL-6 and IL-10 treatments
Animals received intrahypothalamic infusion of vehicle, or recombinant IL-6 (50, 100, or 200 ng) or recombinant IL-10 (0.5, 1.0, or 3.0 ng) at 6:00 p.m. to evaluate the food intake. For Western blot analysis, we injected recombinant IL-6 or IL-10 2 h after DMSO or thapsigargin into the third ventricle and the hypothalamus was excised 2 h later.
Thapsigargin treatments
Animals received intrahypothalamic infusion of vehicle, or thapsigargin (3.0 µg). To evaluate the energy intake and for Western blot analysis, thapsigargin was infused 40 min before the exercise protocol and 2 h before the recombinant IL-6 infusion. Immediately after exercise or 2 h after IL-6 infusion, animals received intrahypothalamic infusion of insulin (200 mU) or leptin (10−6 M).
IL-6 neutralizing antibody
Animals were randomly selected for treatment with saline, rabbit pre-immune serum (RPIS) or rabbit antiserum against IL-6 (IL-6 Ab) in different doses. IL-6 Ab was injected into the third ventricle of the rats 15 min before the exercise protocol.
ASO IL-10 treatments
Phosphorthioate-modified sense and antisense oligonucleotides (produced by Invitrogen Corp., Carlsbad, CA, USA) were diluted to final concentration of 1 nmol/µl in dilution buffer containing 10 mmol/l Tris–HCl and 1.0 mmol/l EDTA. The oligonucleotides were designed according to the Mus musculus IL-10 sequence deposited at the NIH-NCBI (http://www.ncbi.nlm.nih.gov/entrez) under the designation NM 010548 and were composed of 5′-GCC AGT CAG TAA GAG CAG-3′ (sense) and 5′-TGA GAT CTG CAA TGC A-3′ (antisense). Obese Wistar rats were injected into the third ventricle with two daily doses of 3 µl of dilution buffer containing, or not, sense (Sense IL-10) or antisense oligonucleotides (ASO IL-10) for 3 d. For Western blotting analysis, after ASO IL-10 treatment, obese animals were submitted to the exercise protocol or intrahypothalamic infusion of recombinant IL-6. In some experiments, the rats also received intrahypothalamic infusion of insulin (200 mU) or leptin (10−6 M) for the determination of food intake and Akt and STAT3 phosphorylation.
Recombinant of TNF-α treatments
Animals received intrahypothalamic infusion of vehicle, or TNF-α (10−12). To evaluate the energy intake and for Western blotting analysis, TNF-α was infused 40 min before the exercise protocol and 2 h before the recombinant IL-6 infusion. Immediately after exercise or 2 h after IL-6 infusion, animals received intrahypothalamic infusion of insulin (200 mU) or leptin (10−6 M).
Food Intake Determination
Intrahypothalamic infusions were performed between 5:00 and 6:00 p.m. Thereafter standard chow or high-fat diet was given and food intake was determined by measuring the difference between the weight of chow given and the weight of chow at the end of a 12-h period. Similar studies were carried out in animals after exercise.
Western Blot Analysis
After exercise and/or i.c.v. treatments, the animals were anaesthetized, and the hypothalamus was quickly removed, minced coarsely, and homogenized immediately in a freshly prepared ice-cold buffer (1% Triton X-100, 100 mmol/l Tris pH 7.4, 100 mmol/l sodium pyrophosphate, 100 mmol/l sodium fluoride, 10 mmol/l EDTA, 10 mmol/l sodium vanadate, 2 mmol/l phenyl methylsulphonyl fluoride, and 0.1 mg aprotinin) suitable for preserving phosphorylation states of enzymes, and Western blot was performed, as previously described [1].
Nuclear Extract
Foxo1 and STAT-3 nuclear expression were obtained as described [49]. Fragments of hypothalamic tissue from untreated rats or rats treated with insulin or leptin were obtained 30 min after insulin or leptin infusion and were minced and homogenized in 2 vol. of STE buffer (0.32 M sucrose, 20 mM Tris–HCl (pH 7.4), 2 mM EDTA, 1 mM DTT, 100 mM sodium fluoride, 100 mM sodium pyrophosphate, 10 mM sodium orthovanadate, 1 mM PMSF, and 0.1 mg aprotinin/ml) at 4 °C with a Polytron homogenizer. The homogenates were centrifuged (1,000×g, 25 min, 4 °C) to obtain pellets. The pellet was washed once and suspended in STE buffer (nuclear fraction). The nuclear fraction was solubilized in Triton buffer [1% (v/v) Triton X-100/150 mM NaCl/10 mM Tris/HCl (pH 7.4)/1 mM EGTA/1 mM EDTA/0.2 mM sodium orthovanadate/20 µM leupeptin A/0.2 mM PMSF/50 mM NaF/0.4 nM microcystin LR]. The fraction was centrifuged (15,000 g, 30 min, 4 °C), and the supernatant (nuclear extract) was stored at −80 °C.
Confocal Microscopy
Paraformaldehyde-fixed hypothalami were sectioned (5 µm). The sections were obtained from the hypothalami of six rats per group in the same localization (antero-posterior = −1.78 from bregma) and used in regular single- or double-immunofluorescence staining using DAPI, anti-IL6 receptor alpha (rabbit IgG, SC-13947), anti-IL-10 receptor (rabbit IgG, SC-987), anti-IKKβ (goat IgG, SC-34673), anti-PERK (rabbit IgG, SC-32577), anti-POMC (rabbit IgG, FL-267), and rabbit anti-IRS-1 (rabbit IgG, SC-559) (1∶200; Santa Cruz Biotechnology) antibodies. After incubation with the primary antibody, sections were washed and incubated with specific biotinylated anti-rabbit or anti-goat secondary antibodies (1∶150 dilution) for 2 h at room temperature, followed by incubation with Streptoavidin reagent (containing avidin-conjugated peroxidase) and color reaction using the DAB substrate kit (Vector Laboratories, Burlingame, CA, USA), according to recommendations of the manufacturer. Analysis and photodocumentation of results were performed using a LSM 510 laser confocal microscope (Zeiss, Jena, Germany). The anatomical correlations were made according to the landmarks given in a stereotaxic atlas [50]. The frequency of positive cells was determined in 100 randomly counted cells using Analysis software (Version 2.4).
mRNA Isolation and Real Time PCR
Hypothalamic total RNA was extracted using Trizol reagent (Life Technologies, Gaithersburg, MD, USA), according to the manufacturer's recommendations. Total RNA was rendered genomic DNA free by digestion with Rnase-free Dnase (RQ1, Promega, Madison, WI, USA). Rats were deprived of food for 9 h after for real time PCR analysis. Real time PCR and mRNA isolation were performed using a commercial kit, as follows: IL-6: Rn00561420_m1 IL-10: Rn00563409_m1, POMC: Rn00595020_m1, NPY: Rn00561681_m1, AgRP: Rn01431703_g1, GAPD, #4352338E, for rat and RPS-29 (NCBI: NM012876), sense: 5′-AGGCAAGATGGGTCACCAGC-3′, antisense: 5′-AGTCGAATCATCCATTCAGGTCfG-3′.
Dissection of the Arcuate Nucleus
After 9 h of fasting, rats were killed by decapitation and hypothalamic nuclei were quickly dissected and homogenized in Trizol reagent (Life Technologies, Gaithersburg, MD, USA), according to the manufacturer's recommendations. Later on, each region of the hypothalamus was dissected from 1 mm thick sagittal sections of fresh brain. Arcuate nucleus was dissected from the first sections from the midline of the brain. Coordinates for the arcuate nucleus is ventral part of the medial hypothalamus with anterior and dorsal margin and posterior margin (border with mammilary body).
In Situ Double mRNA Hybridization
For mRNA localization all solutions and materials utilized were RNAse free. The probes were determined and designed using the program Gene Runner 3.05 (Hastings Software, Inc., USA) according to mRNA sequences in NCBI: POMC (NM_139326.2), NPY (NM_012614.1), AgRP (XM_574228.2), IL6ra (NM_017020.1), and IL10ra (AJ_305049.1). Two probes were synthesized for each mRNA and were 5′-end labeled with Alexa Fluor 488 or 546 by Invitrogen Life Technologies (Carlsbad CA, USA). See details in the supplemental data (Table S1). Frozen sections were air dried for 30 min at 37 °C, fixed using cold acetone for 10 min, and washed twice in PBS for 5 min and twice in 2× SSC for 2 min. The sections were incubated with Proteinase K (20 µg/mL) for 10 min at room temperature and then washed twice for 5 min with 2× SSC. The sections were incubated in 0.1 M triethanolamine pH 8 (TEA Buffer) for 10 min and then with 0.25% acetic anhydride in TEA buffer for 10 min under magnetic stirring and then washed with 2× SSC. The pre-hybridization solution was composed by 50% formamide, 5× SSC, Denhardt's solution (1× final concentration), and completed with DEPC-treated water. The sections were pre-hybridized for 4 h without the probe at 50 °C in humidified chamber with 50% formamide in SSC. The probe mix (including two probes for each mRNA; i.e., IL6ra or IL10ra with POMC, AgRP, or NPY) was composed (for each tissue section) of 20 µL of pre-hybridization solution plus 500 µg/mL of torula RNA, 500 µg/mL of salmon sperm DNA, and 50 ng of riboprobe mix (anti-sense or sense). The mixture was placed over the sections and incubated at 52 °C overnight in a humidified chamber. After 18 h hybridization, the sections were washed four times with 4× SSC buffer for 10 and 5 min in PBS. The sections were visualized in Zeiss 510 confocal microscope.
Statistical Analysis
All numeric results are expressed as the means ± SEM of the indicated number of experiments. The results of blots are presented as direct comparisons of bands or spots in autoradiographs and quantified by optical densitometry (Scion Image). Statistical analysis was performed by employing the ANOVA test with Bonferroni post test. Significance was established at the p<0.05 level.
Supporting Information
Serum levels and hypothalamic expression of IL-6. (A) Serum levels of IL-6 and (B) protein expression of IL-6 in the hypothalamic tissue from lean and obese rats under rest condition or after exercise. Data are the means ± SEM. # p<0.05 versus respective control at rest; * p<0.05 versus respective lean plus exercise; § p<0.05 versus control at rest, n = 8 animals per group.
(1.22 MB DOC)
Effects of IL-6 on leptin and insulin action. Intrahypothalamic infusion of recombinant IL-6 improves the anorexigenic effects of insulin (A) or leptin (B) in obese Wistar rats. Data are the means ± SEM. * p<0.05 versus obese non-stimulated; ** p<0.01 versus obese stimulated with insulin or leptin alone, n = 6–8 animals per group.
(0.88 MB TIF)
IL-6 improves insulin and leptin signaling. Western blots of five independent experiments showing hypothalamic lysates from Wistar rats; (A) Insulin-induced Akt serine phosphorylation and (B) leptin-induce STAT3 tyrosine phosphorylation in lean, obese, obese plus recombinant IL-6, obese plus exercise, and exercise obese pretreated with anti-IL-6 antibody before the exercise protocol. Data are the means ± SEM. # p<0.05 versus lean group; * p<0.05 versus obese group at rest; § p<0.01 versus exercised obese group; n = 6–8 animals per group.
(1.43 MB TIF)
IL-6 suppresses TNF-α induced insulin and leptin resistance. Anorexigenic effects of insulin (A) and leptin (B) in the hypothalamus of lean rats injected with TNF-α, TNF-α plus IL-6, TNF-α plus exercise, and TNF-α in exercised lean animals pretreated with anti-IL-6 antibody before the exercise protocol. Western blots showing hypothalamic lysates from Wistar rats; (C) IKKβ, (D) PERK, (E) IRS-1Ser307, (F) insulin-induced Akt serine phosphorylation, and (G) leptin-induced STAT3 tyrosine phosphorylation and (H) basal levels of Akt and STAT3 phosphorylation in the hypothalamus of lean animals injected with TNF-α, TNF-α plus IL-6, TNF-α plus exercise, and TNF-α in exercised lean animals pretreated with anti-IL-6 antibody before the exercise protocol pretreated with TNF-α. Data are the means ± SEM. # p<0.05 versus DMSO group; * p<0.05 versus lean plus TNF-α; § p<0.05 versus TNF-α plus recombinant IL-6 or TNF-α plus exercised; n = 6–8 animals per group.
(2.44 MB TIF)
mRNA and probes sequences used in double mRNA hybridization. The probes were determined and designed according to mRNA sequences in NCBI: POMC (NM_139326.2), NPY (NM_012614.1), AgRP (XM_574228.2), IL6ra (NM_017020.1), and IL10ra (AJ_305049.1). Two probes were synthesized for each mRNA and were 5′-end labeled with Alexa Fluor 488 or 546.
(0.03 MB DOC)
Acknowledgments
We thank Mr. Luiz Janeri and Ms. Janine Sabino for the technical assistance and Nicola Conran for the English language editing.
Abbreviations
- ASO IL-10
IL-10 antisense oligonucleotide
- CNS
central nervous system
- DIO
diet-induced obese
- ER
endoplasmatic reticulum
- IL
interleukin
- IL-1ra
IL-1 receptor antagonist
- IL-6R
IL-6 Receptor
- IL-10R
IL-10 Receptor
- IRS-1
insulin receptor substrate-1
- Jak-2
Janus Kinase-2
- NPY
Neuropeptide-Y
- POMC
Proopiomelanocortin
- sTNF-R
soluble TNF-receptors
- SW Exe
swimming exercise
- T2D
type 2 diabetes
- TG
thapsigargin
- TR Exe
treadmill running exercise
- WT
wild type
Funding Statement
This study was supported by grants from Fundacao de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP) and Conselho Nacional de desenvolvimento científico e tecnológico (CNPq). The funders had no role in study design, data lection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Carvalheira J. B, Ribeiro E. B, Araujo E. P, Guimaraes R. B, Telles M. M, et al. Selective impairment of insulin signalling in the hypothalamus of obese Zucker rats. Diabetologia. 2003;46:1629–1640. doi: 10.1007/s00125-003-1246-x. [DOI] [PubMed] [Google Scholar]
- 2.Niswender K. D, Morton G. J, Stearns W. H, Rhodes C. J, Myers M. G, Jr, et al. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 3.Bruning J. C, Gautam D, Burks D. J, Gillette J, Schubert M, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 4.El-Haschimi K, Pierroz D. D, Hileman S. M, Bjorbaek C, Flier J. S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Souza C. T, Araujo E. P, Bordin S, Ashimine R, Zollner R. L, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 6.Milanski M, Degasperi G, Coope A, Morari J, Denis R, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Zhang G, Zhang H, Karin M, Bai H, et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayden M. S, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Hosoi T, Sasaki M, Miyahara T, Hashimoto C, Matsuo S, et al. Endoplasmic reticulum stress induces leptin resistance. Mol Pharmacol. 2008;74:1610–1619. doi: 10.1124/mol.108.050070. [DOI] [PubMed] [Google Scholar]
- 10.Martinez de Morentin P. B, Varela L, Ferno J, Nogueiras R, Dieguez C, et al. Hypothalamic lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:350–361. doi: 10.1016/j.bbalip.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Ozcan L, Ergin A. S, Lu A, Chung J, Sarkar S, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Ozcan U, Cao Q, Yilmaz E, Lee A. H, Iwakoshi N. N, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 13.Tsiotra P. C, Tsigos C. Stress, the endoplasmic reticulum, and insulin resistance. Ann N Y Acad Sci. 2006;1083:63–76. doi: 10.1196/annals.1367.007. [DOI] [PubMed] [Google Scholar]
- 14.Won J. C, Jang P. G, Namkoong C, Koh E. H, Kim S. K, et al. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity (Silver Spring) 2009;17:1861–1865. doi: 10.1038/oby.2009.194. [DOI] [PubMed] [Google Scholar]
- 15.Misra A, Alappan N. K, Vikram N. K, Goel K, Gupta N, et al. Effect of supervised progressive resistance-exercise training protocol on insulin sensitivity, glycemia, lipids, and body composition in Asian Indians with type 2 diabetes. Diabetes Care. 2008;31:1282–1287. doi: 10.2337/dc07-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuomilehto J, Lindstrom J, Eriksson J. G, Valle T. T, Hamalainen H, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 17.Petersen A. M, Pedersen B. K. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 18.Flores M. B, Fernandes M. F, Ropelle E. R, Faria M. C, Ueno M, et al. Exercise improves insulin and leptin sensitivity in hypothalamus of Wistar rats. Diabetes. 2006;55:2554–2561. doi: 10.2337/db05-1622. [DOI] [PubMed] [Google Scholar]
- 19.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen B. K, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol. 2001;536:329–337. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ropelle E. R, Fernandes M. F, Flores M. B, Ueno M, Rocco S, et al. Central exercise action increases the AMPK and mTOR response to leptin. PLoS ONE. 2008;3:e3856. doi: 10.1371/journal.pone.0003856. doi: [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Hu P, Han Z, Couvillon A. D, Kaufman R. J, Exton J. H. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moraes J. C, Amaral M. E, Picardi P. K, Calegari V. C, Romanatto T, et al. Inducible-NOS but not neuronal-NOS participate in the acute effect of TNF-alpha on hypothalamic insulin-dependent inhibition of food intake. FEBS Lett. 2006;580:4625–4631. doi: 10.1016/j.febslet.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen B. K, Fischer C. P. Physiological roles of muscle-derived interleukin-6 in response to exercise. Curr Opin Clin Nutr Metab Care. 2007;10:265–271. doi: 10.1097/MCO.0b013e3280ebb5b3. [DOI] [PubMed] [Google Scholar]
- 25.Steensberg A, Fischer C. P, Keller C, Moller K, Pedersen B. K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 26.Schottelius A. J, Mayo M. W, Sartor R. B, Baldwin A. S., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- 27.Shkoda A, Ruiz P. A, Daniel H, Kim S. C, Rogler G, et al. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132:190–207. doi: 10.1053/j.gastro.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 28.Bruns B, Maass D, Barber R, Horton J, Carlson D. Alterations in the cardiac inflammatory response to burn trauma in mice lacking a functional Toll-like receptor 4 gene. Shock. 2008;30:740–746. doi: 10.1097/SHK.0b013e318173f329. [DOI] [PubMed] [Google Scholar]
- 29.Kobbe P, Kaczorowski D. J, Vodovotz Y, Tzioupis C. H, Mollen K. P, et al. Local exposure of bone components to injured soft tissue induces Toll-like receptor 4-dependent systemic inflammation with acute lung injury. Shock. 2008;30:686–691. doi: 10.1097/SHK.0b013e31816f257e. [DOI] [PubMed] [Google Scholar]
- 30.Naugler W. E, Sakurai T, Kim S, Maeda S, Kim K, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 31.Frost R. A, Nystrom G. J, Lang C. H. Multiple Toll-like receptor ligands induce an IL-6 transcriptional response in skeletal myocytes. Am J Physiol Regul Integr Comp Physiol. 2006;290:R773–R784. doi: 10.1152/ajpregu.00490.2005. [DOI] [PubMed] [Google Scholar]
- 32.Patterson C. M, Bouret S. G, Dunn-Meynell A. A, Levin B. E. Three weeks of postweaning exercise in DIO rats produces prolonged increases in central leptin sensitivity and signaling. Am J Physiol Regul Integr Comp Physiol. 2009;296:R537–R548. doi: 10.1152/ajpregu.90859.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martins C, Robertson M. D, Morgan L. M. Effects of exercise and restrained eating behaviour on appetite control. Proc Nutr Soc. 2008;67:28–41. doi: 10.1017/S0029665108005995. [DOI] [PubMed] [Google Scholar]
- 34.Martins C, Truby H, Morgan L. M. Short-term appetite control in response to a 6-week exercise programme in sedentary volunteers. Br J Nutr. 2007;98:834–842. doi: 10.1017/S000711450774922X. [DOI] [PubMed] [Google Scholar]
- 35.Andersson U, Treebak J. T, Nielsen J. N, Smith K. L, Abbott C. R, et al. Exercise in rats does not alter hypothalamic AMP-activated protein kinase activity. Biochem Biophys Res Commun. 2005;329:719–725. doi: 10.1016/j.bbrc.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 36.Park S, Jang J. S, Jun D. W, Hong S. M. Exercise enhances insulin and leptin signaling in the cerebral cortex and hypothalamus during dexamethasone-induced stress in diabetic rats. Neuroendocrinology. 2005;82:282–293. doi: 10.1159/000093127. [DOI] [PubMed] [Google Scholar]
- 37.Kuo L. E, Kitlinska J. B, Tilan J. U, Li L, Baker S. B, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 38.Carey A. L, Steinberg G. R, Macaulay S. L, Thomas W. G, Holmes A. G, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- 39.Wallenius K, Wallenius V, Sunter D, Dickson S. L, Jansson J. O. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem Biophys Res Commun. 2002;293:560–565. doi: 10.1016/S0006-291X(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 40.Sadagurski M, Norquay L, Farhang J, D'Aquino K, Copps K, et al. Human IL6 enhances leptin action in mice. Diabetologia. 2010;53:525–535. doi: 10.1007/s00125-009-1580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nybo L, Nielsen B, Pedersen B. K, Moller K, Secher N. H. Interleukin-6 release from the human brain during prolonged exercise. J Physiol. 2002;542:991–995. doi: 10.1113/jphysiol.2002.022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy M. K, Torrance D. S, Picha K. S, Mohler K. M. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- 43.Samoilova E. B, Horton J. L, Chen Y. Acceleration of experimental autoimmune encephalomyelitis in interleukin-10-deficient mice: roles of interleukin-10 in disease progression and recovery. Cell Immunol. 1998;188:118–124. doi: 10.1006/cimm.1998.1365. [DOI] [PubMed] [Google Scholar]
- 44.Siegmund B, Sennello J. A, Lehr H. A, Batra A, Fedke I, et al. Development of intestinal inflammation in double IL-10- and leptin-deficient mice. J Leukoc Biol. 2004;76:782–786. doi: 10.1189/jlb.0404239. [DOI] [PubMed] [Google Scholar]
- 45.Romanatto T, Roman E. A, Arruda A. P, Denis R. G, Solon C, et al. Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J Biol Chem. 2009;284:36213–36222. doi: 10.1074/jbc.M109.030874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Sodlerlund A, Fischer A, Johansson T. Physical activity, diet and behaviour modification in the treatment of overweight and obese adults: a systematic review. Perspect Public Health. 2009;129:132–142. doi: 10.1177/1757913908094805. [DOI] [PubMed] [Google Scholar]
- 47.Pauli J. R, Ropelle E. R, Cintra D. E, Carvalho-Filho M. A, Moraes J. C, et al. Acute physical exercise reverses S-nitrosation of the insulin receptor, insulin receptor substrate 1 and protein kinase B/Akt in diet-induced obese Wistar rats. J Physiol. 2008;586:659–671. doi: 10.1113/jphysiol.2007.142414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ropelle E. R, Pauli J. R, Prada P. O, de Souza C. T, Picardi P. K, et al. Reversal of diet-induced insulin resistance with a single bout of exercise in the rat: the role of PTP1B and IRS-1 serine phosphorylation. J Physiol. 2006;577:997–1007. doi: 10.1113/jphysiol.2006.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ropelle E. R, Pauli J. R, Prada P, Cintra D. E, Rocha G. Z, et al. Inhibition of hypothalamic Foxo1 expression reduced food intake in diet-induced obesity rats. J Physiol. 2009;587:2341–2351. doi: 10.1113/jphysiol.2009.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mai J. K, Assheuer J, Paxinos G. Atlas of the human brain. San Diego; London: Academic.; 1997. p. viii, 336. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum levels and hypothalamic expression of IL-6. (A) Serum levels of IL-6 and (B) protein expression of IL-6 in the hypothalamic tissue from lean and obese rats under rest condition or after exercise. Data are the means ± SEM. # p<0.05 versus respective control at rest; * p<0.05 versus respective lean plus exercise; § p<0.05 versus control at rest, n = 8 animals per group.
(1.22 MB DOC)
Effects of IL-6 on leptin and insulin action. Intrahypothalamic infusion of recombinant IL-6 improves the anorexigenic effects of insulin (A) or leptin (B) in obese Wistar rats. Data are the means ± SEM. * p<0.05 versus obese non-stimulated; ** p<0.01 versus obese stimulated with insulin or leptin alone, n = 6–8 animals per group.
(0.88 MB TIF)
IL-6 improves insulin and leptin signaling. Western blots of five independent experiments showing hypothalamic lysates from Wistar rats; (A) Insulin-induced Akt serine phosphorylation and (B) leptin-induce STAT3 tyrosine phosphorylation in lean, obese, obese plus recombinant IL-6, obese plus exercise, and exercise obese pretreated with anti-IL-6 antibody before the exercise protocol. Data are the means ± SEM. # p<0.05 versus lean group; * p<0.05 versus obese group at rest; § p<0.01 versus exercised obese group; n = 6–8 animals per group.
(1.43 MB TIF)
IL-6 suppresses TNF-α induced insulin and leptin resistance. Anorexigenic effects of insulin (A) and leptin (B) in the hypothalamus of lean rats injected with TNF-α, TNF-α plus IL-6, TNF-α plus exercise, and TNF-α in exercised lean animals pretreated with anti-IL-6 antibody before the exercise protocol. Western blots showing hypothalamic lysates from Wistar rats; (C) IKKβ, (D) PERK, (E) IRS-1Ser307, (F) insulin-induced Akt serine phosphorylation, and (G) leptin-induced STAT3 tyrosine phosphorylation and (H) basal levels of Akt and STAT3 phosphorylation in the hypothalamus of lean animals injected with TNF-α, TNF-α plus IL-6, TNF-α plus exercise, and TNF-α in exercised lean animals pretreated with anti-IL-6 antibody before the exercise protocol pretreated with TNF-α. Data are the means ± SEM. # p<0.05 versus DMSO group; * p<0.05 versus lean plus TNF-α; § p<0.05 versus TNF-α plus recombinant IL-6 or TNF-α plus exercised; n = 6–8 animals per group.
(2.44 MB TIF)
mRNA and probes sequences used in double mRNA hybridization. The probes were determined and designed according to mRNA sequences in NCBI: POMC (NM_139326.2), NPY (NM_012614.1), AgRP (XM_574228.2), IL6ra (NM_017020.1), and IL10ra (AJ_305049.1). Two probes were synthesized for each mRNA and were 5′-end labeled with Alexa Fluor 488 or 546.
(0.03 MB DOC)