Abstract
Background
ALG-2 (a gene product of PDCD6) belongs to the penta-EF-hand (PEF) protein family and Ca2+-dependently interacts with various intracellular proteins including mammalian Alix, an adaptor protein in the ESCRT system. Our previous X-ray crystal structural analyses revealed that binding of Ca2+ to EF3 enables the side chain of R125 to move enough to make a primary hydrophobic pocket (Pocket 1) accessible to a short fragment of Alix. The side chain of F122, facing a secondary hydrophobic pocket (Pocket 2), interacts with the Alix peptide. An alternatively spliced shorter isoform, designated ALG-2ΔGF122, lacks Gly121Phe122 and does not bind Alix, but the structural basis of the incompetence has remained to be elucidated.
Results
We solved the X-ray crystal structure of the PEF domain of ALG-2ΔGF122 in the Ca2+-bound form and compared it with that of ALG-2. Deletion of the two residues shortened α-helix 5 (α5) and changed the configuration of the R125 side chain so that it partially blocked Pocket 1. A wall created by the main chain of 121-GFG-123 and facing the two pockets was destroyed. Surprisingly, however, substitution of F122 with Ala or Gly, but not with Trp, increased the Alix-binding capacity in binding assays. The F122 substitutions exhibited different effects on binding of ALG-2 to other known interacting proteins, including TSG101 (Tumor susceptibility gene 101) and annexin A11. The X-ray crystal structure of the F122A mutant revealed that removal of the bulky F122 side chain not only created an additional open space in Pocket 2 but also abolished inter-helix interactions with W95 and V98 (present in α4) and that α5 inclined away from α4 to expand Pocket 2, suggesting acquirement of more appropriate positioning of the interacting residues to accept Alix.
Conclusions
We found that the inability of the two-residue shorter ALG-2 isoform to bind Alix is not due to the absence of bulky side chain of F122 but due to deformation of a main-chain wall facing pockets 1 and 2. Moreover, a residue at the position of F122 contributes to target specificity and a smaller side chain is preferable for Alix binding but not favored to bind annexin A11.
Background
ALG-2 (apoptosis-linked gene 2) is a 22-kDa protein of 191 amino acid residues containing five serially repetitive EF-hand-type helix-loop-helix Ca2+-binding motifs (EF1 to EF5) and it belongs to the penta-EF-hand (PEF) family, including the calpain small subunit, sorcin, grancalcin and peflin in mammals [1]. ALG-2 is the most conserved protein among the PEF family and its homologues are widely found in eukaryotes. Despite the original report of a pro-apoptotic function of ALG-2 in T cell hybridomas [2], ALG-2-deficient mice develop normally with no obvious abnormalities in the immune system [3]. Nonetheless, potential physiological roles of ALG-2 in control of ER-stress-induced apoptosis, cancer and cell division have been reported [4-6]. Alix (also named AIP1) was the first protein identified as an ALG-2-interacting protein [7,8]. This cytoplasmic 95-kDa protein is now recognized as an auxiliary factor of the ESCRT (endosomal sorting complex required for transport) system, which is involved in endosomal sorting, retrovirus budding and cytokinesis [9-11]. In addition to roles in the ESCRT system, Alix functions in actin-cytoskeleton assembly, cell adhesion, signal transduction and apoptosis [12-15].
X-ray crystal structures of various PEF proteins including ALG-2 have common features: the presence of eight α-helices and dimer formation via paired EF5s that are positioned in anti-parallel orientation [16-20]. Previously, we solved the structures of Ca2+-free and -bound forms of N-terminally truncated human ALG-2 (des3-20ALG-2) and a Zn2+-bound form of full-length ALG-2 as well as the structure of the complex between des3-23ALG-2 and the peptide corresponding to Alix799-814 in the Zn2+-bound form. Although the four-EF-hand-region (EF1-EF4) of ALG-2 has a general structural resemblance to calmodulin, ALG-2 exhibits only a very small Ca2+-dependent conformational change. Binding of Ca2+ (or Zn2+) to EF3 enables the side chain of R125, present in the loop connecting EF3 and EF4, to move enough to make a primary hydrophobic pocket (Pocket 1) accessible to the critical PPYP motif found in Alix. This Ca2+/EF3-driven arginine switch mechanism explains how ALG-2 is activated by Ca2+ to bind to its target proteins [21,22]. The C-terminal half of the Alix peptide is also held in the second hydrophobic pocket (Pocket 2). On the other hand, in the case of calmodulin, each pair of EF1-EF2 (N-lobe) and EF3-EF4 (C-lobe) changes its conformation from "closed" to "open" state upon Ca2+ binding and exhibits a further gross change in relative stereotypic position by bending of the central helix connecting EF2 and EF3 in such a way that the two lobes grab the targeting peptide [23].
An isoform of ALG-2 was first reported in the mouse [24]. The isolated cDNA clone designated ALG-2,1 was shorter in six nucleotides corresponding to the two amino acids Gly121Phe122 in comparison with the full-length cDNA clone ALG-2,5. Both transcripts were present in mouse tissues at an approximate ratio of 2:1 (ALG-2,5 vs ALG-2,1). The same type of isoform lacking Gly121 Phe122 (designated ALG-2ΔGF122 in this article; non-deleted ALG-2 being regarded as wild type for convenience) is also registered in human DNA databases, such as GenBank under accession no. BC110291.1. Interaction of ALG-2ΔGF122 with Alix is significantly reduced or not detected in yeast two-hybrid or in vitro binding assays [24-27]. Although F122 interacts with the ALG-2-binding site (ABS) peptide of Alix in Pocket 2 in the crystal structure [21], the molecular basis for the defect of ALG-2ΔGF122 in binding to Alix has remained to be elucidated. In the present study, we crystallized des3-23ALG-2ΔGF122 and compared its X-ray crystal structure with that of the Ca2+-bound form of des3-23ALG-2. We found that deletion of the two residues causes shortening of an α-helix (α5) and leads to a change in the configuration of the R125 side chain. Surprisingly, however, the F122A mutant (ALG-2F122A, Phe substituted with Ala) exhibited an unexpected hyperactivity in Alix-binding. We also investigated effects of this mutation on the crystal structure, and we discuss the structural roles of F122 in this article.
Results
Structures of ALG-2ΔGF122 and ALG-2F122A
For determination of the 3D structures of ALG-2ΔGF122 and ALG-2F122A, we prepared recombinant proteins with deletion in the N-terminal Gly/Pro-rich region (des3-23ALG-2ΔGF122 and des3-20ALG-2F122A, respectively). Crystal structures were solved by the molecular replacement method using the previously solved structures of ALG-2 (PDB IDs 1HQV and 2ZN8) as a search model. Data collection, processing, and refinement statistics are summarized in Table 1. The structures of des3-23ALG-2ΔGF122 in the Ca2+-bound form (PDB ID, 3AAJ) and des3-20ALG-2F122A in the Zn2+-bound form (PDB ID, 3AAK) were solved at resolutions of 2.4 Å and 2.7 Å, respectively. Although the obtained data of 3AAK were processed to 2.5 Å, the refinement gave poor Rwork and Rfree values (near or greater than 30). Thus, we limited the resolution to 2.7 Å (Rwork/Rfree, 25.2/29.8). An asymmetric unit of the crystal of des3-23ALG-2ΔGF122 in the Ca2+-bound form contained two ALG-2 molecules (A and B) as a dimer (Additional file 1, Figure S1). The root-mean-square deviation (rmsd) value of the structures aligned between two molecules was calculated to be 0.73 Å for Cα atoms from residues L28 to V189. The structure of molecule A was used for further analysis. Crystals of the Ca2+-free and Ca2+-bound forms of des3-20ALG-2F122A suitable for X-ray diffraction were not obtained.
Table 1.
Data collection and refinement statistics
| des3-23ALG-2ΔGF122/Ca2+-bound | des3-20ALG-2F122A/Zn2+-bound | |
|---|---|---|
| PDB code | 3AAJ | 3AAK |
| Data collection | ||
| Beamline | PF BL-5A | PF AR NW-12 |
| Wavelength (Å)a | 1 | 1 |
| Space group | P21 | C2221 |
| a/b/c (Å)a | 42.6/78.9/60.3 | 71.3/170.2/47.0 |
| α/β/γ (°)a | 90.0/104.6/90.0 | 90.0/90.0/90.0 |
| Resolution (Å)a | 50.0-2.40(2.49-2.40) | 50.0-2.50(2.59-2.50) |
| Measured reflections | 55354 | 70399 |
| Unique reflections | 15185 | 10302 |
| Completeness (%)a | 97.9(95.1) | 97.3(88.4) |
| Rmerge (%)a | 5.3(18.8) | 4.3(12.0) |
| I/σa | 15.5(5.8) | 28.8(14.3) |
| Refinement | ||
| Resolution (Å) | 50.0-2.40 | 50.0-2.70 |
| Rwork/Rfree (%) | 19.6/26.6 | 25.2/29.8 |
| Rmsd bond length (Å) | 0.019 | 0.006 |
| Rmsd bond angle (°) | 1.68 | 0.86 |
| Average B-factor (Å2) | 36.8 | 56.3 |
| No. ALG-2 molecule | 2 | 1 |
a Values in parentheses are for highest-resolution shell.
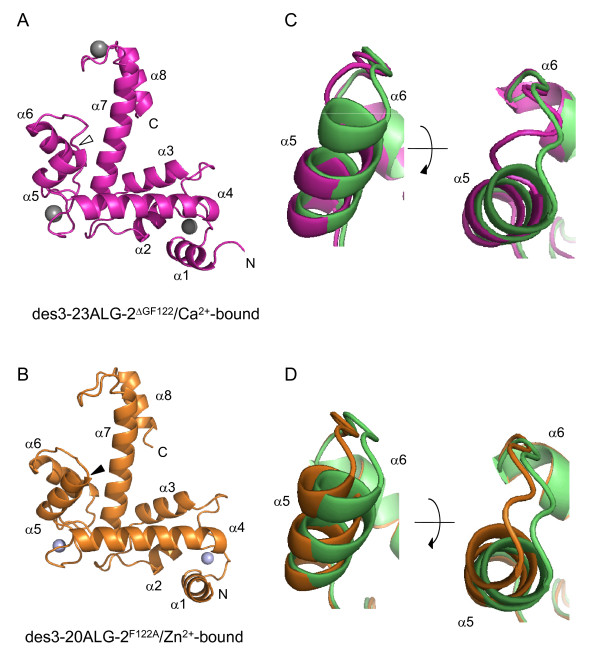
The basic architectures of the PEF domain containing eight α-helices (α1-α8), five EF-hand-like helix-loop-helix motifs (EF1-EF5), and pairing at EF5 as a dimer were maintained in the solved crystal structures (Figure 1 and Additional file 1, Figure S1), and the overall structures were very similar when compared with those of wild-type ALG-2 in the Ca2+-bound form of des3-20ALG-2 (PDB ID 2ZN9) and the Zn2+-bound form of full-length ALG-2 (PDB ID 2ZN8), respectively (rmsd values: des3-23ALG-2ΔGF122/Ca2+ vs des3-20ALG/Ca2+, 1.23 Å for Cα atoms from residues 24-189 of ALG-2; des3-20ALGF122A/Zn2+ vs ALG-2/Zn2+, 0.62 Å for Cα atoms from residues 26-191; des3-20ALG/Ca2+ vs ALG-2/Zn2+, 0.76 Å for Cα atoms from residues 26-189). In des3-23ALG-2ΔGF122, however, the deletion of Gly121Phe122 caused loss of the third turn in α5 that corresponds to the exiting helix of EF3 (Figure 1C, magenta). The loop connecting α5 of EF3 and α6 of EF4 started earlier, but it returned to a similar spatial position in the middle of the loop around Y122, corresponding to Y124 in wild-type ALG-2 (Figure 2A and 2B). This spatial position of Y122 was supported partly by hydrogen bonding between the nitrogen atom of Y122 (NY122) and the peptide carbonyl oxygen atom of L119 (OL119) and partly by hydrophobic interactions between Cβ of Y122 and Cδ1 of L124 as in the case of Y124 of wild-type ALG-2 (denoted CB and CD1, respectively, in the Latin alphabet in Table 2). While hydrogen bonding between the hydroxyl oxygen atom (Oη) of Y122 (OHY122) and the side chain amide nitrogen atom (Nε2) of Q157 (NE2Q157) in α7 was newly formed, hydrophobic interactions between side chains of Y122 and L156 were reduced in the ΔGF122 isoform. In the crystal structure of des3-20ALG-2F122A in the Zn2+-bound form, the axis of α5 inclined slightly outward from α4 and α7 (see Discussion section) (Figure 1D, orange).
Figure 1.
Structures of ALG-2ΔGF122 and ALG-2F122A. Structures of (A) Ca2+-bound des3-23ALG-2ΔGF122 (PDB ID 3AAJ) and (B) Zn2+-bound des3-20ALG-2F122A (PDB ID 3AAK) are shown in magenta and orange, respectively, in ribbon representations, and EF-hand-coordinated calcium and zinc ions are shown in gray and light cyan spheres. The deleted site (Gly121Phe122) and substituted site (F122A) are marked by an open arrowhead and closed arrowhead in (A) and (B), respectively. (C, D) The ALG-2 structures are compared with the previously resolved structures of wild-type ALG-2 of Ca2+-bound form (PDB ID 2ZN9) and Zn2+-bound form (PDB ID 2ZN8) by aligning at α-helix 4 (α4), and close-up views of segments from α5 to α6 are shown in ribbon representations. (C) The structures of Ca2+-bound des3-20ALG-2 and des3-23ALG-2ΔGF122 are shown in green and magenta, respectively. The view in the left panel is rotated approximately 90° to view from the top as shown in the right panel. (D) The structures of Zn2+-bound ALG-2 (green) and des3-20ALG-2F122A (orange) are presented similarly to (C).
Figure 2.
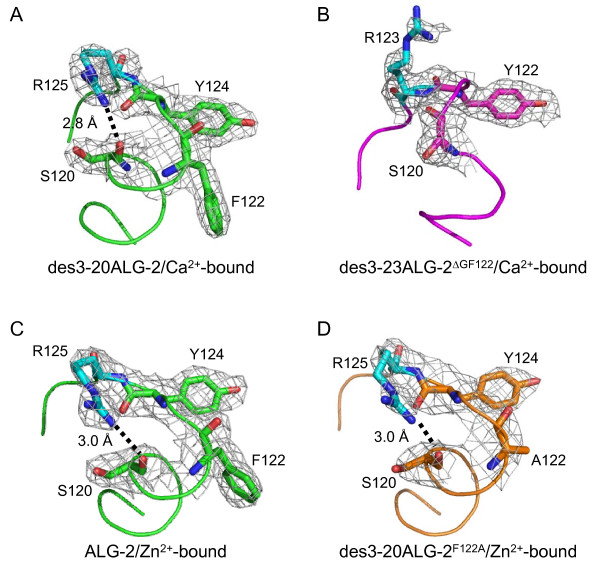
Differences in the side chain configurations of R125 and R123. Close-up views of a segment from L115 to D128 (D126 in des3-23ALG-2ΔGF122) in each ALG-2 structure are shown in ribbon representations and in stick models superimposed with electron densities of residues that are involved in the Ca2+/EF3-driven arginine switch mechanism. The structures of (A, C) wild-type ALG-2, (B) des3-23ALG-2ΔGF122 and (D) des3-20ALG-2F122A are colored green, magenta and orange, respectively. R125, a critical residue for the switch mechanism and interaction with Alix, and its corresponding residue in des3-23ALG-2ΔGF122 (R123) are colored cyan. The hydrogen bond between the guanidino nitrogen atom of R125 and carbonyl oxgen atom of S120 is shown by a dotted line and the distance is indicated in (A), (C) and (D).
Table 2.
Intramolecular interactions of ALG-2 in Ca2+/EF3-driven arginine switching loop
| WT | ΔGF122 | |||
|---|---|---|---|---|
| interacting atom | MF a | Ca b | Ca c | |
| S120 | ||||
| Hydrogen | Distance (Å) | |||
| N | OK116 | 2.8 | 3.2 | 3.1 |
| O | NG123/121 | 2.8 | 2.8 | NDd |
| NH2R125/123 | ND | 2.8 | ND | |
| OG | OL116 | 2.6 | ND | ND |
| Y124/122 | ||||
| hydrogen | ||||
| N | OL119 | 3.1 | 3.0 | 3.0 |
| OH | NE2Q159/157 | ND | ND | 2.8 |
| hydrophobic | ||||
| CB | CL119 | ND | 3.9 | ND |
| CD1L126/124 | 3.9 | 3.7 | 3.9 | |
| CGL126/124 | ND | ND | 3.7 | |
| CD1 | CBF122/- | 3.7 | 3.6 | -e |
| CD1L158/156 | ND | ND | 4.0 | |
| CD2 | CD2L158/156 | 3.9 | ND | ND |
| CE1 | CBF122/- | 3.8 | 3.8 | - |
| CD1F122/- | 3.9 | ND | - | |
| CD2L158/156 | 3.9 | ND | ND | |
| CE2 | CGL158/156 | 3.5 | ND | ND |
| CD2L158/156 | 3.7 | 3.8 | ND | |
| CZ | CD1L158/156 | ND | 4.0 | ND |
a Metal-free form of des3-20ALG-2
b Ca2+-bound form of des3-20ALG-2
c Ca2+-bound form of des3-23ALG-2ΔGF122
d Not detected in the range of 2.6-3.2 Å for hydrogen bonding and 3.2-4.0 Å for hydrophobic interaction
e Not present in des3-23 ALG-2ΔGF122
Configuration of R125 side chain
In the previously proposed Ca2+/EF3-driven arginine switch mechanism [21,22], binding of Ca2+ to EF3 enables formation of a hydrogen bond between one of the guanidino nitrogen atoms (Nη2) of R125 (NH2R125) and the peptide carbonyl oxygen atom of S120 (OS120), resulting in change in the configuration of the R125 side chain (Table 2, Figure 2, A and 2B; Additional file 1, Figure S2A). Binding of Zn2+ caused the same effect (Figure 2C) [21]. In the structure of des3-23ALG-2ΔGF122 in the Ca2+-bound form, however, NH2R123 (corresponding to NH2R125 in wild-type ALG-2) did not form such a hydrogen bond (Figure 2B). The side chain extended outwardly from the loop as in the case of the structure of the metal-free form, but the spatial positions of the side chains between R123 and Y122 were closer in the equivalent residues in the metal-free ALG-2 (R125 and Y124) (Additional file 1, Figure S2A). The lower electron densities corresponding to guanidino group atoms of R123 suggest flexibility of this side chain by solvent exposure. On the other hand, NH2R125 in the structure of Zn2+-bound des3-20ALG-2F122A formed a hydrogen bond with OS120, and the side chain of R125 showed a configuration similar to that of wild-type ALG-2 (Figure 2, C and 2D).
Metal coordination
Three calcium ions were found in EF1, EF3 and EF5 of des3-23ALG-2ΔGF122 at the canonical EF-hand coordination positions (x, y, z, -y, -x and -z), though water molecules at the -x positions in EF1 and EF3 were absent and no amino acid residue coordinated at the -z position in EF5 (Additional file 1, Figure S3; Additional file 2, Table S1). In addition to two zinc ions found in EF1 and EF3 at the canonical coordination positions (Additional file 2, Table S2), one zinc ion that was coordinated at a non-canonical position was found in EF5 in the structure of Zn2+-bound des3-20ALG-2F122A (Additional file 1, Figure S4; Additional file 2, Table S3). In this non-canonical EF-hand coordination, zinc ion was bonded to OD1D171 and OD2D173 in place of OD1D169 and OW175, respectively, at the x and -y positions. Interestingly, the Ca2+-bound form and the Zn2+-bound form displayed an opposite relationship regarding the presence of water molecules at -x positions in wild-type ALG-2 between EF1 and EF3 (water molecules at -x: EF1/Ca2+, present vs EF1/Zn2+, absent; EF3/Ca2+, absent vs EF3/Zn2+, present) (Additional file 2, Table S1; Additional file 2, Table S2). In the Zn2+-bound form of ALG-2F122A, a water molecule was found at this position in EF1. The difference in ion radius between calcium and zinc (0.99 Å and 0.74 Å, respectively) may influence the availability of coordinated water molecules at the -x position.
Comparison of surface structures
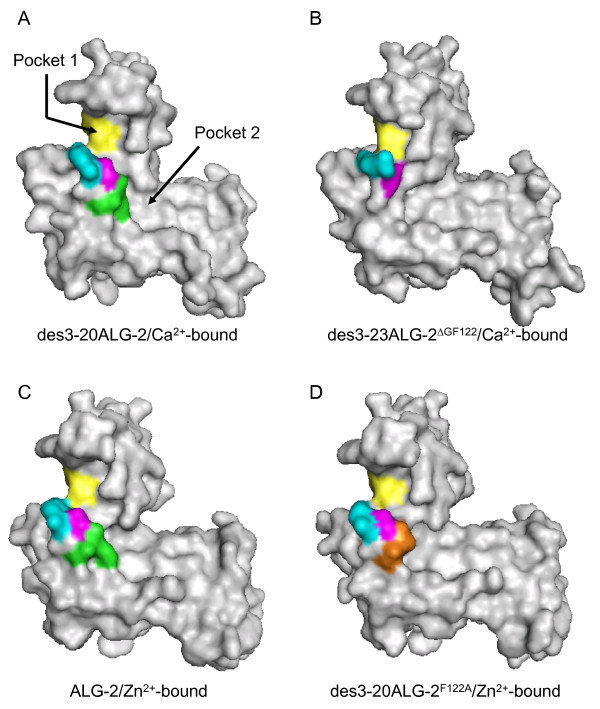
As shown in Figure 3, the deletion of two residues caused a noticeable change in the surface structure surrounding hydrophobic pockets (designated Pocket 1 and Pocket 2), which were shown to accommodate the Alix ABS peptide in our previous study [21]. The bottom of Pocket 1 was supported by Y180 derived from a dimerizing counterpart molecule of ALG-2 (Figure 3, yellow). A wall formed by Gly121Phe122 in des3-20ALG-2/Ca2+-bound (Figure 3A, green) disappeared in des3-23ALG-2ΔGF122/Ca2+-bound, and a surface structure represented by G123 was also changed (Figure 3B, magenta) The continuous wall formed by 121-GFG-123 was maintained in Zn2+-bound des3-20ALG-2F122A (Figure 3D, orange: Gly121Ala122; magenta, G123). The R125 side chain (Figure 3, cyan) was oriented away from Pocket 1 in the metal-bound ALG-2 proteins (Figure 3, A, C and 3D). On the other hand, the side chain directed toward Pocket 1 in Ca2+-bound des3-23ALG-2ΔGF122 (Figure 3B), but it did not fully block the entry path of Pocket 1 as in the case of metal-free ALG-2 (Additional file 1, Figure S2B).
Figure 3.
Loss of a wall surrounding hydrophobic pockets in ALG-2ΔGF122. Surface structures of (A) Ca2+-bound des3-20ALG-2, (B) Ca2+-bound des3-23ALG-2ΔGF122, (C) Zn2+-bound ALG-2, and (D) Zn2+-bound des3-20ALG-2F122A are presented in gray except for indicated residues of Gly121Phe122 (green), G123 (or G121 in des3-23ALG-2ΔGF122) (magenta), A122 in the F122A mutant (orange), R125 (or R123 in des3-23ALG-2ΔGF122) (cyan), Y180 (or Y178 of des3-23ALG-2ΔGF122) from a dimerized counterpart molecule (yellow).
Effects of amino acid substitutions of F122 on binding capacities and specificities
To investigate whether the side chain of F122 influences association between ALG-2 and Alix, we performed real-time surface plasmon resonance (SPR) interaction analyses using purified recombinant full-length ALG-2 and F122-substituted mutants as well as ALG-2ΔGF122 as analytes and an Alix ABS peptide as a ligand. The resonance signals at 10 s before the dissociation phase were compared with that of wild-type (WT) ALG-2 and expressed as relative binding capacities (Additional file 1, Figure S5). Higher binding capacities were observed for the ALG-2 mutants of F122A (240%) and F122G (140%), whereas lower binding capacities were observed for ALG-2ΔGF122 (18%) and the mutants of F122W (36%) and F122S (89%).
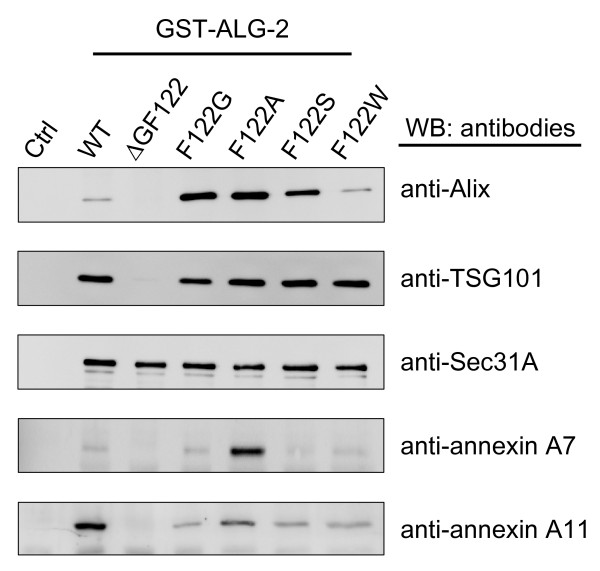
Next, we investigated whether substitutions of F122 equally influence binding to endogenous ALG-2-interacting proteins by pulldown assays using glutathione-S-transferase (GST)-fused ALG-2 proteins and unfused GST as a negative control (Ctrl) (Figure 4). Pulldown products were analyzed by Western blotting with specific antibodies against Alix, TSG101, Sec31A, annexin A7 and annexin A11. GST-ALG-2ΔGF122 gave essentially no specific immunoreactive signals except for Sec31A. For Alix interaction, the mutants of small side chain substitutions (F122G, F122A and F122S) gave stronger signals than those of WT and F122W mutant. Compared to the results obtained by SPR analyses (Additional file 1, Figure S5), GST-pulldown assays gave much more enhanced signals for mutants than for WT. Even F122W mutant gave a capacity similar to that of WT. Capacities of ALG-2 binding to TSG101 and Sec31A were not different from WT in all F122 mutants. Compared to WT, only F122A mutant gave significantly stronger signals for annexin A7, whereas signals for annexin A11 were decreased in all mutants.
Figure 4.
Effects of amino acid substitutions of F122 on binding specificities. ALG-2-interacting proteins in HEK293 cells were pulled down with GST-fused ALG-2 of wild type (WT), ALG-2ΔGF122 (ΔGF122), and F122-substituted mutants. Unfused GST was used as a negative control (Ctrl). Proteins bound to glutathione Sepharose beads were subjected to Western blotting with antibodies against Alix, TSG101, Sec31A, annexin A7, and annexin A11.
Augmentation of staurosporine-induced cell death by expression of ALG-2F122A
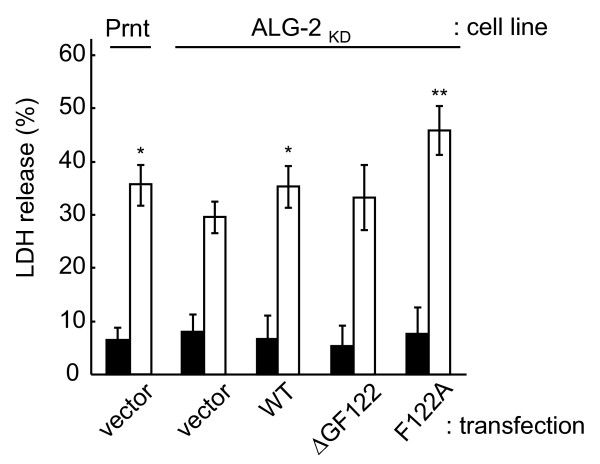
Staurosporine, a microbial alkaloid, acts as a non-selective protein kinase inhibitor with high potency by binding to ATP-binding pockets of kinases [28], and it induces cell death via caspase-dependent and -independent apoptotic pathways [29]. Previously, Vito et al. reported that ALG-2 and Alix (named AIP1 in the article) modulate staurosporine-induced cell death [8]. To investigate whether the enhanced Alix-binding capacity by F122A mutant exerts augmentation of cell death, we employed previously established ALG-2-knockdown (ALG-2KD) HeLa cells whose endogenous ALG-2 level was reduced by the RNA interference (RNAi) method [26]. After transfection with RNAi-resistant expression plasmids of either wild-type or mutant ALG-2 proteins or with a vector as a control, cells were treated with staurosporine for 24 h. The degree of cell death was estimated by measuring the amounts of lactate dehydrogenase (LDH) released into the culture medium. As shown in Figure 5, cells not treated with staurosporine released small amounts of LDH under the conditions used (closed columns, 6-8% of total LDH activities). On the other hand, dramatic increases were observed for the release of LDH from staurosporine-treated cells in all cases tested (open columns, 29-46%), but vector-transfected ALG-2KD HeLa cells (29%) showed lower values than vector-transfected parental HeLa cells (36%). The amounts of released LDH were increased slightly by exogenous expressions of wild-type (WT) ALG-2 (36%) and ALG-2ΔGF122 (33%) in ALG-2KD HeLa cells. The augmentation was statistically significant (P < 0.01) when ALG-2F122A was exogenously expressed (46%) and compared with the vector-transfected ALG-2KD HeLa cells (29%).
Figure 5.
Augmentation of staurosporine-induced cell death by ALG-2F122A. Parental (Prnt) HeLa cells and ALG-2-knockdown (ALG-2KD) HeLa cells were transfected with a vector (pcDNA3) as controls. ALG-2KD cells were transfected with plasmids that express wild-type ALG-2, ALG-2ΔGF122 or ALG-2F122A and cultured for 24 h. The transfected cells were treated with 1 μM staurosporine for 24 h. Cell death was monitored by measuring the amounts of lactate dehydrogenase (LDH) released into the culture medium as described in Materials and Methods. Total amounts of LDH released into the medium and retained in cells were expressed as 100%. Duplicate assays of five independent repetitive experiments were performed and data are presented as means +/- SD (n = 5). Closed column, not treated with staurosporine; open column, treated with staurosporine. Statistically significant differences among staurosporine-treated cells were evaluated by Student's t-test by comparing the measured values of samples with those of control ALG-2KD cells that were transfected with a control vector (*, P < 0.05; **, P < 0.01)
Discussion
A variant of ALG-2 cDNA lacking six nucleotides is found in human DNA databases. Since Gly121Phe122 is immediately followed by Gly in the amino acid sequence, the deletion of Phe122Gly123 is assigned in the results of BLAST searches using the blastp or tblastn program. In the nucleotide sequence of cDNAs, however, the deletion occurs in GGTTTC (Gly121Phe122) but not in TTCGGC (Phe122Gly123) in the sequence of TCAGGTTTCGGCTAC (corresponding to 120-SGFGY-124). In the current human EST database, the ratio of retrieved human ALG-2 cDNAs containing nucleotides corresponding to Gly121Phe122 and those lacking the corresponding hexanucleotides is approximately 3:1. In this article, we designate the major ALG-2 isoform as wild type (WT) to discriminate it from the shorter and minor isoform. Analysis of the human genome database revealed that the ALG-2 gene (symbol, PDCD6; location, 5 pter-p15.2) has six exons and that the boundary sequence of Exon 4 and Intron 4 contains an alternative splicing donor site (TCAGGTTTCGgtaactcactcactc: lower case, intron sequence; boldfaced sequence, missing by alternative splicing). The alternatively spliced ALG-2 isoform, designated ALG-2ΔGF122, is defective in Alix-binding [24-26]. These two residues (Gly121Phe122) comprise the last turn in α5 in the crystal structures [18,21]. However, the structural basis for the inability of ALG-2ΔGF122 to bind Alix has remained to be established. In the present study, we investigated the role of Gly121Phe122 by solving the X-ray crystal structure of the shorter isoform and by mutational analyses of F122.
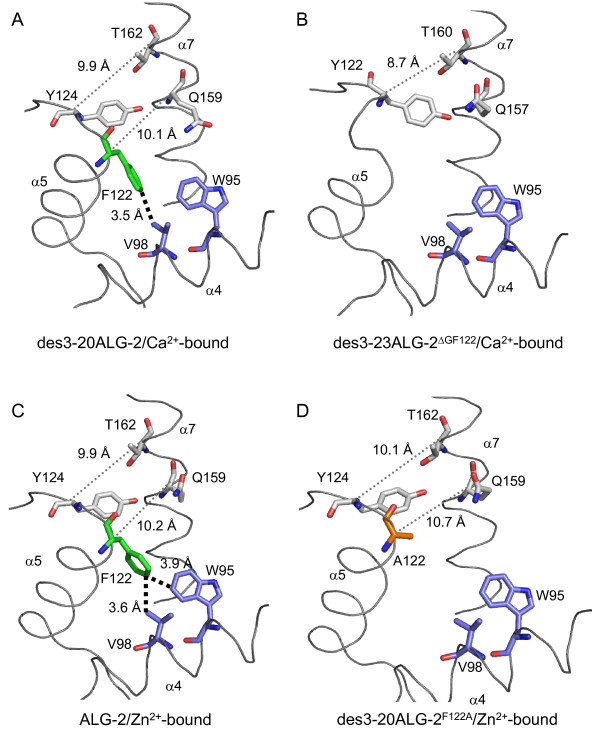
Comparison of the crystal structures of des3-20ALG-2 and des3-23ALG-2ΔGF122 in the Ca2+-bound forms revealed a significant difference in spatial positions of residues adjacent to Gly121Phe122 (Figures 1, 2, 3). Since S120 is located within α5 in ALG-2 but is placed at the end of the truncated helix in ALG-2ΔGF122 (Figure 1), an α-helix-supporting hydrogen bond between OS120 and NG121 (G123 in wild type) is disrupted (Table 2). This causes a change in the spatial orientation of OS120, and the hydrogen bond with NH2R125 in wild-type ALG-2 is lost in NH2R123 in ALG-2ΔGF122. Then, the side chain of R123 is placed to partially block Pocket 1, which is a primary acceptor site for the Alix ABS peptide (Figures 2 and 3) and resembles the topology of R125 in the Ca2+-unbound (metal-free) form of wild-type ALG-2 (Additional file 1, Figure S2). F122, present in α5 (exiting α-helix of EF3), interacts with residues W95 and V98 in α4 (entering α-helix of EF3) and stabilizes the EF-hand motif in metal-free des3-20ALG-2 (Table 3 and Additional file 1, Figure S2C,). This inter-helix interaction is maintained partly in the Ca2+-bound form (F122-V98 interaction) and disrupted in Ca2+-bound ALG-2ΔGF122 due to the absence of F122 (Figure 6, A and 6B, Table 3). The distance between the Cα atoms of Y122 and T160 (facing Pocket 1) is shorter in the crystal structure of des3-23ALG-2ΔGF122 (Figure 6B, 8.7 Å) than that between the corresponding Y124 and T162 in the Ca2+-bound (Figure 6A, 9.9 Å) and metal-free structure of des3-20ALG-2 (Additional file 1, Figure S2C, 9.0 Å), indicating a narrower Pocket 1 in the isoform. Moreover, the main chain of G123 in wild-type ALG-2 (Figure 3, magenta) is placed to face Pocket 1, and the main chain carbonyl carbon (C') interacts hydrophobically with the Alix ABS peptide [21], but this interaction is no longer possible because a continuous wall formed by Gly121Phe122 (facing Pocket 2) and G123 (facing Pocket 1) is destroyed in ALG-2ΔGF122 (Figure 3).
Table 3.
Loss of intramolecular interactions in F122A mutant
| WT | F122A | ||||
| interacting atom | MFa | Cab | Znc | Znd | |
| Distance (Å) | |||||
| F122 | A122 | ||||
| hydrogen | |||||
| N | OA118 | ND | 2.9 | 3.2 | NDe |
| OL119 | 3.0 | 3.1 | ND | 3.2 | |
| hydrophobic | |||||
| CB | CD1Y124 | 3.7 | 3.6 | 3.9 | ND |
| CE1Y124 | 3.8 | 3.8 | 4.0 | ND | |
| CD1 | CA118 | ND | ND | 4.0 | -f |
| CAL119 | 3.9 | ND | 4.0 | - | |
| CD2L119 | 3.9 | 3.7 | 4.0 | - | |
| CE1Y124 | 3.9 | ND | ND | - | |
| CE1 | CH2W95 | 3.9 | ND | ND | - |
| CZ3W95 | 3.9 | ND | ND | - | |
| CE2 | CG1V98 | ND | 4.0 | ND | - |
| CZ | CH2W95 | 3.9 | ND | ND | - |
| CZ3W95 | 3.5 | ND | 3.9 | - | |
| CG1V98 | 3.8 | 3.5 | 3.6 | - | |
a Metal-free form of des3-20ALG-2
b Ca2+-bound form of des3-20ALG-2
c Zn2+-bound form of ALG-2
d Zn2+-bound form of des3-20ALG-2F122A
e Not detected in the range of 2.6-3.2 Å for hydrogen bonding and 3.2-4.0 Å for hydrophobic interaction
f Not present in des3-20ALG-2F122A
Figure 6.
Close-up views of structural interrelations among α4, α5 and α7. Residues influencing space size in the hydrophobic pockets are shown in stick models and described in Discussion. Thick dotted lines represent hydrophobic interactions. Thin dotted lines indicate Cα-distances that show differences in the pockets between wild-type and mutant ALG-2 structures. In the stick model representation, carbons atoms are colored in blue for W95 and V98, green for F122, orange for A122 and gray for Y124, Q159 and T162.
Our previous X-ray crystal structure analyses of the complex between ALG-2 and the Alix ABS peptide (1-QGPPYPTYPGYPGYSQ-16, core motif boldfaced) revealed that an aromatic moiety of F122, constituting Pocket 2, interacts hydrophobically with Y11 of the Alix ABS peptide [21]. Surprisingly, however, substitution of F122 with Ala or Gly did not abolish the binding but instead increased the binding capacities as shown by SPR analyses, and substitution with Trp caused a decrease in the binding capacity (Additional file 1, Figure S5). Similar effects were observed by GST-pulldown assays of endogenous Alix (Figure 4). Thus, it is thought that the inability of ALG-2ΔGF122 to bind Alix is not due to the absence of a residue with a large hydrophobic side chain at residue No. 122 but is due to the unfavorable topology of the loop connecting α5 and α6 caused by deletion of the two residues. This idea may also explain the finding by Subramanian et al. that a single amino acid deletion of F122 caused a marked decrease in Alix-binding capacity [30]. On the other hand, the presence of an aromatic bulky side chain at this position (in Pocket 2) may be rather detrimental for binding to Alix due to steric hindrance. Interestingly, substitution of either M71 or Q159 (both present in Pocket 2 and interacting with the Alix ABS peptide in the complex crystal structure) with Ala resulted in higher Alix-binding capacities [21]. Thus, more open space or a deeper trough in Pocket 2 seems to favor interactions with Alix. The F122S mutant showed lower binding capacity (89%) than that of wild-type ALG-2 by SPR analyses (Additional file 1, Figure S5), but the GST-ALG-2F122S mutant exhibited a higher capacity than that of the wild type in the GST-pulldown assay. This discrepancy may arise from the differences in the ligands to be assayed (SPR: ABS oligopeptide corresponding to residue No. 799-814 of Alix; GST-pulldown: endogenous Alix protein of 868 residues) because residues 815-842 also contribute to interactions with ALG-2 to some extent [31].
EF-hand proteins have similar helix-loop-helix structures, but conformational states with respect to angles and distances between the two helices and their changes in response to Ca2+ binding are very diverse [32]. Unlike calmodulin, ALG-2 does not exhibit a significant change from the closed conformational state in the absence of Ca2+ to the open conformational state in the presence Ca2+ [21]. Nevertheless, binding of Ca2+ or Zn2+ to EF3 causes a small shift of α5 and leads to change in configuration of the R125 side chain [21]. Substitution of F122 with Ala disrupts the inter-helix interaction between α4 and α5 (Figure 6, C and 6D). Comparison of the estimated angles formed by α4 (entering helix) and α5 (exiting helix) in EF3 between the structures of wild-type ALG-2 (θ = 54.5°) and des3-20ALG-2F122A (θ = 62.6°) in the Zn2+-bound forms (Table 4) indicates inclination of α5 by 8.1° away from α4, resulting in a shift in the position of the Cα atom of F122A for 2.0 Å (Figure 1D). This more open conformation of the EF-hand motif in F122A mutant maintains distances or causes a small increase in distances between the Cα atoms of Y124 and T162 (facing Pocket 1) (WT, 9.9 Å; F122A, 10.1 Å) and between the Cα atoms of F122/A122 and Q159 (facing Pocket 2) (WT, 10.2 Å; F122A, 10.7 Å). On the other hand, regardless of the disruption of inter-helix interaction, ALG-2ΔGF122 gave only a small increase in the angle (WT, θ = 57.6°; ΔGF122, θ = 58.6°) in the Ca2+-bound form structure (Table 4), and the distance between the Cα atoms of Y124 and T160 was significantly decreased in ALG-2ΔGF122 (Figure 6, WT, 9.9 Å; ΔGF122, 8.7 Å). Consequently, the change in the relative spatial positions of α5 and the following loop may allow the side chains of A122, Y124 and adjacent R125 to place at more appropriate positions in the hydrophobic pockets of ALG-2F122A to interact with the Alix ABS peptide. To corroborate the above hypothetical mechanism of enhanced binding of ALG-2F122A to Alix, we attempted co-crystallization of des3-20ALG-2F122A with the Alix ABS peptide but could not obtain good crystals. The nature of N-terminal residues of ALG-2 influences qualities of crystals. In the present study, we used des3-20ALG-2F122A because X-ray diffraction resolutions were better in des3-20ALG-2 (2.4 Å) than in des3-23ALG-2 (3.1 Å) in the Ca2+-bound forms in our previous study [33]. It would be worthwhile, however, to test des3-23ALG-2F122A for crystal preparations of the complex.
Table 4.
Angles and distances calculated for ALG-2 EF3. ALG-2 EF3 has α4 (entering helix, residue No. 93-102) and α5 (exiting helix, residue No. 112-122), but a segment of α5 (residue No. 112-120, common to ALG-2ΔGF122) was used.
| Angle (°) | Distance (Å) | ||||||
|---|---|---|---|---|---|---|---|
| θ | φ | ω | inner ends | midpoint | outer ends | ||
| WT | MFa | 49.5 | 104 | 137 | 10.6 | 12.2 | 14.7 |
| Cab | 57.6 | 96.9 | 131 | 10.7 | 12.7 | 15.6 | |
| Znc | 54.5 | 98.0 | 130 | 10.7 | 12.6 | 15.4 | |
| ΔGF122 | Cad | 58.6 | 88.8 | 134 | 10.7 | 13.1 | 16.3 |
| F122A | Zne | 62.6 | 97.6 | 128 | 11.0 | 13.2 | 16.4 |
a Metal-free form of des3-20ALG-2
b Ca2+-bound form of des3-20ALG-2
c Zn2+-bound form of ALG-2
d Ca2+-bound form of des3-23ALG-2ΔGF122
e Zn2+-bound form of des3-20ALG-2F122A
Angles and distances were estimated by the Vector Geometry Mapping (VGM) software (Ref. 50)
ALG-2 interacts with various proteins with Pro-rich regions [34]. Previously, we classified ALG-2-interacting proteins into two groups (Alix type and non-Alix type) based on ability of binding to ALG-2ΔGF122 [27]. Alix-type proteins such as TSG101, annexin A7 and annexin A11 contain a consensus sequence of PPYPX3-5YP (where X is uncharged residues and PPYP is substitutable with PXYP or XPYP) similar to the core motif of Alix [21]. On the other hand, ALG-2ΔGF122 retains binding ability to non-Alix-type proteins such as Sec31A and phospholipid scramblase 3 (PLSCR3) that possess a common sequence of PXPGF (where X is P or A) but whose indispensability remains to be experimentally verified [26,27]. The mutation of F122 had different effects on capacities of binding to endogenous proteins among Alix-type proteins, even to two paralogous annexin family proteins (Figure 4). Although ALG-2-binding sites still remain to be identified, annexin A7 and annexin A11 have 4-PGYPPTGYPP-13 and 4-PGYPPPPGGYPP-15, respectively. A clear difference between the two sequences is the number of residues present between two YPs (annexin A7, 3 vs annexin A11, 5). Since F122 constitutes Pocket 2 and interacts with the aromatic side chain of the corresponding second YP in the Alix ABS peptide, mutation of F122 seems favored or disfavored for interaction depending on spatial positions of the concerned Tyr residues in the target proteins. Recently, mucolipin-1 has been shown to Ca2+-dependently interact with ALG-2 but not with ALG-2ΔGF122 [35]. The predicted binding site (37-EEDLRRRLKYFF-49) does not contain any YP or PXPGF motif, but charged residues as well as hydrophobic residues were shown to be important for interactions. Thus, mucolipin-1 may be recognized by a surface different from that for binding to Pro-rich target proteins. Ask1 and Raf-1, which are also known to interact with ALG-2 [36,37], do not possess conspicuous Pro-rich regions either, and the ALG-2-binding site has not been reported yet. It would be interesting to see whether F122A and other amino acid-substituted mutants of Pocket 1 and Pocket 2 present a different binding profile between Pro-rich type and non-Pro-rich type ALG-2-interacting proteins.
The biological significance of the occurrence of ALG-2ΔGF122 is not known. ALG-2 forms a dimer and each molecule of the dimer has capacity of Alix binding [21]. Recently, we demonstrated that ALG-2 (a longer isoform, wild type) functions as a Ca2+-dependent adaptor protein that bridges Alix and TSG101, whereas ALG-2ΔGF122, retaining dimerization ability, does not have such a function toward Alix and TSG101 [38]. Occurrence of ALG-2ΔGF122 in the cell should give chances to form dimers in different combinations at different ratios according to the expressed levels of wild-type ALG-2 and ALG-2ΔGF122 (WT/WT, WT/ΔGF122, ΔGF122/ΔGF122). Although ALG-2 dimers of WT/ΔGF122 and ΔGF122/ΔGF122 are inactive adaptors at least for the Alix-type ALG-2-interacting proteins, the WT/ΔGF122 dimer may still function for non-Alix-type proteins. Although the molecular mechanism of the ALG-2 function in staurosporine-induced cell death is not known yet, slight augmentation of staurosporine-induced cell death by ALG-2ΔGF122 (Figure 5) suggests that non-Alix type ALG-2-interacting proteins are also involved. Recent clinical investigations suggest that ALG-2 is a potential prognostic marker of certain lung and gastric cancers [39,40]. Expression analyses of ALG-2 by further distinguishing alternatively spliced isoforms would provide more reliable data in clinical studies. RBM22, a highly conserved RNA-binding protein functioning as an auxiliary factor of the spliceosome, was shown to interact with ALG-2 [41]. It would be interesting to see whether ALG-2 regulates its own splicing as well as Ca2+-dependent alternative splicing events [42]. Future studies are needed to clarify whether ALG-2ΔGF122 plays roles merely as a negative inhibitor of wild-type ALG-2 or positively functions by associating with ALG-2ΔGF122-specific interacting proteins.
Conclusions
Structural basis of the inability of the splicing isoform of human ALG-2, ALG-2ΔGF122, to bind to Alix was investigated by X-ray crystal structural analysis. Missing of two residues, Gly121Phe122, causes shortening of an α-helix (α5) and leads to a change in the configuration of the R125 side chain that resembles that of the metal-free form of ALG-2. Contrary to the expected importance of bulky side chain of F122, the F122A mutant exhibited a surprising hyperactivity in binding to Alix. The resolved structure of the F122A mutant showed that removal of the bulky F122 side chain not only created an additional open space in Pocket 2 but also abolished inter-helix interactions with W95 and V98 (present in α4) and that α5 inclined away from α4 to expand Pocket 2, suggesting acquirement of more appropriate positioning of the interacting residues to accept Alix. However, no hyperactivity against TSG101 or annexin A11 suggests that F122 partly restricts the recognition specificity to target proteins.
Methods
Bacterial expression and purification of recombinant ALG-2 proteins
Bacterial expressions of untagged ALG-2 by the T7 RNA polymerase system and GST-fused protein by pGEX vector were described previously [25,43]. Construction of the expression plasmid for an alternatively spliced ALG-2 isoform that lacks Gly121Phe122 (designated ALG-2ΔGF122) and GST-ALG-2ΔGF122 was described previously [25]. Single amino acid substitution mutation of ALG-2 was performed according to the instruction manual provided with a QuikChange Site-Directed Mutagenesis kit (Stratagene, USA) using specific oligonucleotide primers listed in Additional file 2, Table S4. Recombinant ALG-2 proteins of wild type and mutants (ALG-2, ALG-2ΔGF122, ALG-2F122A, ALG-2F122G, ALG-2F122S, ALG-2F122W, des3-23ALG-2ΔGF122, des3-20ALG-2F122A) were purified by affinity chromatography using a column immobilizing an ALG-2-binding site-2 (ABS-2) peptide of PLSCR3 as described previously [27]. GST fusion proteins were expressed and purified with glutathione Sepharose beads (GE Healthcare) according to the manufacturer's instructions.
Crystallization
Purified proteins were concentrated to about 10 mg/ml with a vacuum centrifuge evaporator (Sakuma, Japan). Concentrated proteins were dialyzed against 10 mM Tris-HCl, pH 7.5, containing 10 μM each of EDTA and EGTA. Crystallization conditions were first screened with an automated robotic system [44] and further optimized manually. Crystals were grown by the sitting or hanging drop vapor diffusion method at 20°C. Des3-23ALG-2ΔGF122 protein was crystallized with 25% (w/v) PEG-4000, 50 mM sodium cacodylate, pH 6.0, 300 mM ammonium acetate, and 10 mM calcium chloride. Des3-20ALG-2F122A protein was crystallized with 25% (w/v) 2-methyl-2,4-pentanediol (MPD), 100 mM sodium cacodylate, pH 6.5, and 50 mM zinc acetate.
Data collection, structure determination, refinement, and analyses
X-ray diffraction data were collected at beamlines BL-5A and NW-12 of Photon Factory (Tsukuba, Japan) under cryogenic conditions with crystals soaked in a cryoprotectant solution containing 20% glycerol and cooled to 100 K in a nitrogen gas stream. The diffraction data were integrated and scaled with the HKL2000 program package [45]. Crystal structures were solved by the molecular replacement method using the program MOLREP [46] with the published structure of ALG-2 (PDB ID 1HQV and 2ZN8) as a search model for des3-23ALG-2ΔGF122 and des3-20ALG-2F122A. All models were refined with the programs CNS [47] and REFMAC5 in the CCP4 package [48]. Manual adjustments of the model were performed with COOT [49]. All of the structural figures were generated with PyMol (DeLano Scientific LLC, Palo Alto, CA). Rmsd was calculated with the program lsqkab in the CCP4 package [48]. Inter-helix angles and distances of EF-hand motifs were estimated by using vector geometry mapping (VGM) software [50] downloaded from URL: http://calcium.uhnres.utoronto.ca/vgm/.
Binding assays
Real-time binding analyses were performed using an SPR biosensor (Biacore2000, GE Healthcare) at 25°C. A synthetic peptide of the ALG-2-binding site in Alix (kggsggsQGPPYPTYPGYPGYSQ, lower case residues indicating a linker, provided by Biosynthesis Inc., USA) was immobilized on the carboxymethylated dextran surface of a CM5 sensor chip (GE Healthcare) as described previously [21]. For interaction analyses, flow rate was maintained at 20 μl/min. Purified ALG-2 and mutants were diluted to 100 nM in HBS-P (10 mM HEPES-NaOH, pH 7.4, 150 mM NaCl, 0.005% surfactant P20) containing 100 μM CaCl2 and then injected and kept flowing over the immobilized sensor surface for 180 s. The sensor surface was then washed for 300 s with the same buffer and regenerated with the buffer containing 1 mM EGTA.
GST-pulldown assays of ALG-2 and its mutants were performed using cleared lysates of HEK293 cells as described previously [40]. Proteins bound to the beads (pulldown products) were analyzed by Western blotting using specific antibodies. Mouse monoclonal antibody (mAb) of anti-annexin VII (A-1) and goat polyclonal antibody (pAb) of anti-annexin XI (N-17) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-TSG101 mAb (4A10) and Sec31A mAb (clone32) were from Gene Tex (San Antonio, TX) and BD Transduction Laboratories (San Diego, CA), respectively. Preparation of anti-Alix pAb was described previously [25]. Signals of Western blotting were detected by the chemiluminescence method and analyzed with LAS-3000mini (Fujifilm, Japan).
Cell culture, DNA transfection and cell death assays
Constructs of pcDNA3/ALG-2/RNAiR and pcDNA3/ALG-2ΔGF122/RNAiR that express RNAi-resistant ALG-2 mRNAs were obtained by transferring EcoRI/XhoI fragments to the pcDNA3 vector from their previously constructed expression vectors of FLAG-tagged versions [37]. Construction of pcDNA3/ALG-2F122A/RNAiR was performed by site-directed mutagenesis using pcDNA3/ALG-2/RNAiR as a template and the primers (F122A) listed in Additional file 2, Table S4. An ALG-2 knockdown HeLa cell line was established by expression of the short hairpin RNA specific for ALG-2 mRNA as described previously [25]. One day after the cells had been seeded, they were transfected with the expression plasmid DNAs by using FuGENE6 (Roche, Basel, Switzerland). After 24 h, aliquots of cell suspensions (each 0.5 ml) were transferred to a 24-well plate, incubated for 24 h, and then treated with or without 1 μM staurosporine for 24 h. Cell mortality was measured by quantifying the amount of lactate dehydrogenase (LDH) released from dead cells using the CytoTox96® Non-Radioactive Cytotoxicity Assay (Promega, USA) according to the manufacturer's instructions. Total amount of LDH per sample (released and retained enzymes in dead and viable cells) was measured by lysing cells in 0.5 ml culture by adding the provided 10× Lysis buffer containing 9% Triton X-100.
List of abbreviations
ABS: ALG-2-binding site; LDH: lactate dehydrogenase; PEF: penta-EF-hand; RNAi: RNA interference; SPR: surface plasmon resonance; TSG101: tumor susceptibility gene 101; WT: wild type
Authors' contributions
TI and HSu carried out mutant preparations, purification of recombinant proteins, X-ray analyses of crystals. TI designed and performed cell death assays. MK and SW contributed in acquisition of X-diffraction data and interpretations of obtained data sets. TI, HSh and MM were involved in drafting and revising the manuscript. MM supervised the project. All authors read and approved the final version of the manuscript.
Supplementary Material
Supplementary figures. Showing structures of the calcium-bound dimeric form of des3-23ALG-2ΔGF122 (Figure S1), the metal-free form of des3-20ALG-2 (Figure S2), EF-hand Ca2+-coordination in des3-23ALG-2ΔGF122 (Figure S3), non-canonical Zn2+-coordination in EF5 (Figure S4), and SPR analyses of F122 mutants of ALG-2 for Alix-binding capacities (Figure S5).
Supplementary tables. Showing bond distances for the canonical EF-hand metal ion coordinates in ALG-2 for calcium (Table S1) and zinc (Table S2), bond distance for the non-canonical zinc ion coordinate in EF5 (Table S3), and primers used for site-directed mutagenesis performed in this study (Table S4).
Contributor Information
Tatsutoshi Inuzuka, Email: inuzuka.tatsutoshi@f.mbox.nagoya-u.ac.jp.
Hironori Suzuki, Email: suzuhiro@post.kek.jp.
Masato Kawasaki, Email: kawasaki@pfweis.kek.jp.
Hideki Shibata, Email: shibabou@agr.nagoya-u.ac.jp.
Soichi Wakatsuki, Email: soichi.wakatsuki@kek.jp.
Masatoshi Maki, Email: mmaki@agr.nagoya-u.ac.jp.
Acknowledgements
We thank Mr. Kakiuchi for earlier works related to this study and Dr. Hitomi for valuable suggestions. This work was partly supported by a Grant-in-Aid for Scientific Research (B) (to MM) and Grants-in-Aid for JSPS fellow (HSu and TI) from JSPS.
References
- Maki M, Kitaura Y, Satoh H, Ohkouchi S, Shibata H. Structures, functions and molecular evolution of the penta-EF-hand Ca2+-binding proteins. Biochim Biophys Acta. 2002;1600:51–60. doi: 10.1016/s1570-9639(02)00444-2. [DOI] [PubMed] [Google Scholar]
- Vito P, Lacanà E, D'Adamio L. Interfering with apoptosis: Ca2+-binding protein ALG-2 and Alzheimer's disease gene ALG-3. Science. 1996;271:521–525. doi: 10.1126/science.271.5248.521. [DOI] [PubMed] [Google Scholar]
- Jang IK, Hu R, Lacanà E, D'Adamio L, Gu H. Apoptosis-linked gene 2-deficient mice exhibit normal T-cell development and function. Mol Cell Biol. 2002;22:4094–4100. doi: 10.1128/MCB.22.12.4094-4100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RV, Poksay KS, Castro-Obregon S, Schilling B, Row RH, del Rio G, Gibson BW, Ellerby HM, Bredesen DE. Molecular components of a cell death pathway activated by endoplasmic reticulum stress. J Biol Chem. 2004;279:177–187. doi: 10.1074/jbc.M304490200. [DOI] [PubMed] [Google Scholar]
- la Cour JM, Høj BR, Mollerup J, Simon R, Sauter G, Berchtold MW. The apoptosis linked gene ALG-2 is dysregulated in tumors of various origin and contributes to cancer cell viability. Mol Oncol. 2008;1:431–439. doi: 10.1016/j.molonc.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høj BR, la Cour JM, Mollerup J, Berchtold MW. ALG-2 knockdown in HeLa cells results in G2/M cell cycle phase accumulation and cell death. Biochem Biophys Res Commun. 2009;378:145–148. doi: 10.1016/j.bbrc.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Missotten M, Nichols A, Rieger K, Sadoul R. Alix, a novel mouse protein undergoing calcium-dependent interaction with the apoptosis-linked-gene 2 (ALG-2) protein. Cell Death Differ. 1999;6:124–129. doi: 10.1038/sj.cdd.4400456. [DOI] [PubMed] [Google Scholar]
- Vito P, Pellegrini L, Guiet C, D'Adamio L. Cloning of AIP1, a novel protein that associates with the apoptosis-linked gene ALG-2 in a Ca2+-dependent reaction. J Biol Chem. 1999;274:1533–1540. doi: 10.1074/jbc.274.3.1533. [DOI] [PubMed] [Google Scholar]
- Odorizzi G. The multiple personalities of Alix. J Cell Sci. 2006;119:3025–3032. doi: 10.1242/jcs.03072. [DOI] [PubMed] [Google Scholar]
- Morita E, Sandrin V, Chung HY, Morham SG, Gygi SP, Rodesch CK, Sundquist WI. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007;26:4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton JG, Martin-Serrano J. The ESCRT machinery: new functions in viral and cellular biology. Biochem Soc Trans. 2009;37:195–199. doi: 10.1042/BST0370195. [DOI] [PubMed] [Google Scholar]
- Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- Schmidt MH, Hoeller D, Yu J, Furnari FB, Cavenee WK, Dikic I, Bögler O. Alix/AIP1 antagonizes epidermal growth factor receptor downregulation by the Cbl-SETA/CIN85 complex. Mol Cell Biol. 2004;24:8981–893. doi: 10.1128/MCB.24.20.8981-8993.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, Wang R, Zhou X, Corvera J, Kloc M, Sifers R, Gallick GE, Lin SH, Kuang J. Extracellular Alix regulates integrin-mediated cell adhesions and extracellular matrix assembly. EMBO J. 2008;27:2077–2090. doi: 10.1038/emboj.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahul-Mellier AL, Strappazzon F, Petiot A, Chatellard-Causse C, Torch S, Blot B, Freeman K, Kuhn L, Garin J, Verna JM, Fraboulet S, Sadoul R. Alix and ALG-2 are involved in tumor necrosis factor receptor 1-induced cell death. J Biol Chem. 2008;283:34954–34965. doi: 10.1074/jbc.M803140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki M, Narayana SV, Hitomi K. A growing family of the Ca2+-binding proteins with five EF-hand motifs. Biochem J. 1997;328:718–720. [PMC free article] [PubMed] [Google Scholar]
- Jia J, Han Q, Borregaard N, Lollike K, Cygler M. Crystal structure of human grancalcin, a member of the penta-EF-hand protein family. J Mol Biol. 2000;300:1271–1281. doi: 10.1006/jmbi.2000.3925. [DOI] [PubMed] [Google Scholar]
- Jia J, Tarabykina S, Hansen C, Berchtold M, Cygler M. Structure of apoptosis-linked protein ALG-2: insights into Ca2+-induced changes in penta-EF-hand proteins. Structure. 2001;9:267–275. doi: 10.1016/S0969-2126(01)00585-8. [DOI] [PubMed] [Google Scholar]
- Xie X, Dwyer MD, Swenson L, Parker MH, Botfield MC. Crystal structure of calcium-free human sorcin: a member of the penta-EF-hand protein family. Protein Sci. 2001;10:2419–2425. doi: 10.1110/ps.36701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilari A, Johnson KA, Nastopoulos V, Verzili D, Zamparelli C, Colotti G, Tsernoglou D, Chiancone E. The crystal structure of the sorcin calcium binding domain provides a model of Ca2+-dependent processes in the full-length protein. J Mol Biol. 2002;29:447–458. doi: 10.1006/jmbi.2002.5417. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kawasaki M, Inuzuka T, Okumura M, Kakiuchi T, Shibata H, Wakatsuki S, Maki M. Structural basis for Ca2+-dependent formation of ALG-2/Alix peptide complex: Ca2+/EF3-driven arginine switch mechanism. Structure. 2008;16:1562–1573. doi: 10.1016/j.str.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kawasaki M, Inuzuka T, Okumura M, Kakiuchi T, Shibata H, Wakatsuki S, Maki M. The mechanism of Ca2+-dependent recognition of Alix by ALG-2: insights from X-ray crystal structures. Biochem Soc Trans. 2009;37:190–194. doi: 10.1042/BST0370190. [DOI] [PubMed] [Google Scholar]
- Crivici A, Ikura M. Molecular and structural basis of target recognition by calmodulin. Ann Rev Biophys Biomol Struct. 1995;24:85–116. doi: 10.1146/annurev.bb.24.060195.000505. [DOI] [PubMed] [Google Scholar]
- Tarabykina S, Møller AL, Durussel I, Cox J, Berchtold MW. Two forms of the apoptosis-linked protein ALG-2 with different Ca2+ affinities and target recognition. J Biol Chem. 2000;275:10514–10518. doi: 10.1074/jbc.275.14.10514. [DOI] [PubMed] [Google Scholar]
- Katoh K, Suzuki H, Terasawa Y, Mizuno T, Yasuda J, Shibata H, Maki M. The penta-EF-hand protein ALG-2 interacts directly with the ESCRT-I component TSG101, and Ca2+-dependently co-localizes to aberrant endosomes with dominant-negative AAA ATPase SKD1/Vps4B. Biochem J. 2005;391:677–685. doi: 10.1042/BJ20050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H, Suzuki H, Yoshida H, Maki M. ALG-2 directly binds Sec31A and localizes at endoplasmic reticulum exit sites in a Ca2+-dependent manner. Biochem Biophys Res Commun. 2007;353:756–763. doi: 10.1016/j.bbrc.2006.12.101. [DOI] [PubMed] [Google Scholar]
- Shibata H, Suzuki H, Kakiuchi T, Inuzuka T, Yoshida H, Mizuno T, Maki M. Identification of Alix-type and Non-Alix-type ALG-2-binding sites in human phospholipid scramblase 3: differential binding to an alternatively spliced isoform and amino acid-substituted mutants. J Biol Chem. 2008;283:9623–9632. doi: 10.1074/jbc.M800717200. [DOI] [PubMed] [Google Scholar]
- Nakano H, Omura S. Chemical biology of natural indolocarbazole products: 30 years since the discovery of staurosporine. J Antibiot. 2009;62:17–26. doi: 10.1038/ja.2008.4. [DOI] [PubMed] [Google Scholar]
- Nicolier M, Decrion-Barthod AZ, Launay S, Prétet JL, Mougin C. Spatiotemporal activation of caspase-dependent and -independent pathways in staurosporine-induced apoptosis of p53wt and p53mt human cervical carcinoma cells. Biol Cell. 2009;101:455–467. doi: 10.1042/BC20080164. [DOI] [PubMed] [Google Scholar]
- Subramanian L, Crabb JW, Cox J, Durussel I, Walker TM, van Ginkel PR, Bhattacharya S, Dellaria JM, Palczewski K, Polans AS. Ca2+ binding to EF hands 1 and 3 is essential for the interaction of apoptosis-linked gene-2 with Alix/AIP1 in ocular melanoma. Biochemistry. 2004;43:11175–11186. doi: 10.1021/bi048848d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H, Yamada K, Mizuno T, Yorikawa C, Takahashi H, Satoh H, Kitaura Y, Maki M. The penta-EF-hand protein ALG-2 interacts with a region containing PxY repeats in Alix/AIP1, which is required for the subcellular punctate distribution of the amino-terminal truncation form of Alix/AIP1. J Biochem. 2004;135:117–128. doi: 10.1093/jb/mvh014. [DOI] [PubMed] [Google Scholar]
- Yap KL, Ames JB, Swindells MB, Ikura M. Diversity of conformational states and changes within the EF-hand protein superfamily. Proteins. 1999;37:499–507. doi: 10.1002/(SICI)1097-0134(19991115)37:3<499::AID-PROT17>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kawasaki M, Kakiuchi T, Shibata H, Wakatsuki S, Maki M. Crystallization and X-ray diffraction analysis of N-terminally truncated human ALG-2. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008;64:974–977. doi: 10.1107/S1744309108030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki M, Shibata H. The Penta-EF-hand protein ALG-2 and its interacting proteins. Calcium Binding Proteins. Calcium Binding Proteins. 2007;2:4–10. [Google Scholar]
- Vergarajauregui S, Martina JA, Puertollano R. Identification of the penta-EF-hand protein ALG-2 as a Ca2+-dependent interactor of mucolipin-1. J Biol Chem. 2009;284:36357–36366. doi: 10.1074/jbc.M109.047241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IS, Jung YS, Kim E. Interaction of ALG-2 with ASK1 influences ASK1 localization and subsequent JNK activation. FEBS Lett. 2002;529:183–187. doi: 10.1016/S0014-5793(02)03329-X. [DOI] [PubMed] [Google Scholar]
- Chen C, Sytkowski AJ. Apoptosis-linked gene-2 connects the Raf-1 and ASK1 signalings. Biochem Biophys Res Commun. 2005;333:51–57. doi: 10.1016/j.bbrc.2005.05.074. [DOI] [PubMed] [Google Scholar]
- Okumura M, Ichioka F, Kobayashi R, Suzuki H, Yoshida H, Shibata H, Maki M. Penta-EF-hand protein ALG-2 functions as a Ca2+-dependent adaptor that bridges Alix and TSG101. Biochem Biophys Res Commun. 2009;386:237–241. doi: 10.1016/j.bbrc.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Arao T, Gotoda T, Taniguchi H, Oda I, Shirao K, Shimada Y, Hamaguchi T, Kato K, Hamano T, Koizumi F, Tamura T, Saito D, Shimoda T, Saka M, Fukagawa T, Katai H, Sano T, Sasako M, Nishio K. Identification of prognostic biomarkers in gastric cancer using endoscopic biopsy samples. Cancer Sci. 2008;99:2193–2199. doi: 10.1111/j.1349-7006.2008.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviel-Ronen S, Coe BP, Lau SK, da Cunha Santos G, Zhu CQ, Strumpf D, Jurisica I, Lam WL, Tsao MS. Genomic markers for malignant progression in pulmonary adenocarcinoma with bronchioloalveolar features. Proc Natl Acad Sci USA. 2008;105:10155–10160. doi: 10.1073/pnas.0709618105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaville P, Dai Y, Cheung CY, Giller K, Becker S, Michalak M, Webb SE, Miller AL, Krebs J. Nuclear translocation of the calcium-binding protein ALG-2 induced by the RNA-binding protein RBM22. Biochim Biophys Acta. 2006;1763:1335–1343. doi: 10.1016/j.bbamcr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Krebs J. The influence of calcium signaling on the regulation of alternative splicing. Biochim Biophys Acta. 2009;1793:979–984. doi: 10.1016/j.bbamcr.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Satoh H, Nakano Y, Shibata H, Maki M. The penta-EF-hand domain of ALG-2 interacts with amino-terminal domains of both annexin VII and annexin XI in a Ca2+-dependent manner. Biochim Biophys Acta. 2002;1600:61–67. doi: 10.1016/s1570-9639(02)00445-4. [DOI] [PubMed] [Google Scholar]
- Hiraki M, Kato R, Nagai M, Satoh T, Hirano S, Ihara K, Kudo N, Nagae M, Kobayashi M, Inoue M, Uejima T, Oda S, Chavas LM, Akutsu M, Yamada Y, Kawasaki M, Matsugaki N, Igarashi N, Suzuki M, Wakatsuki S. Development of an automated large-scale protein-crystallization and monitoring system for high-throughput protein-structure analyses. Acta Crystallogr D Biol Crystallogr. 2006;62:1058–1065. doi: 10.1107/S0907444906023821. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. full_text. [DOI] [PubMed] [Google Scholar]
- Vagin A, Teplyakov A. MOLREP: an automated program for molecular replacement. J Appl Crystallogr. 1997;30:1022–1025. doi: 10.1107/S0021889897006766. [DOI] [Google Scholar]
- Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/S0907444998003254. [DOI] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson E. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Yap KL, Ames JB, Swindells MB, Ikura M. Vector geometry mapping. A method to characterize the conformation of helix-loop-helix calcium-binding proteins. Methods Mol Biol. 2002;173:317–324. doi: 10.1385/1-59259-184-1:317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures. Showing structures of the calcium-bound dimeric form of des3-23ALG-2ΔGF122 (Figure S1), the metal-free form of des3-20ALG-2 (Figure S2), EF-hand Ca2+-coordination in des3-23ALG-2ΔGF122 (Figure S3), non-canonical Zn2+-coordination in EF5 (Figure S4), and SPR analyses of F122 mutants of ALG-2 for Alix-binding capacities (Figure S5).
Supplementary tables. Showing bond distances for the canonical EF-hand metal ion coordinates in ALG-2 for calcium (Table S1) and zinc (Table S2), bond distance for the non-canonical zinc ion coordinate in EF5 (Table S3), and primers used for site-directed mutagenesis performed in this study (Table S4).