Full text
PDF
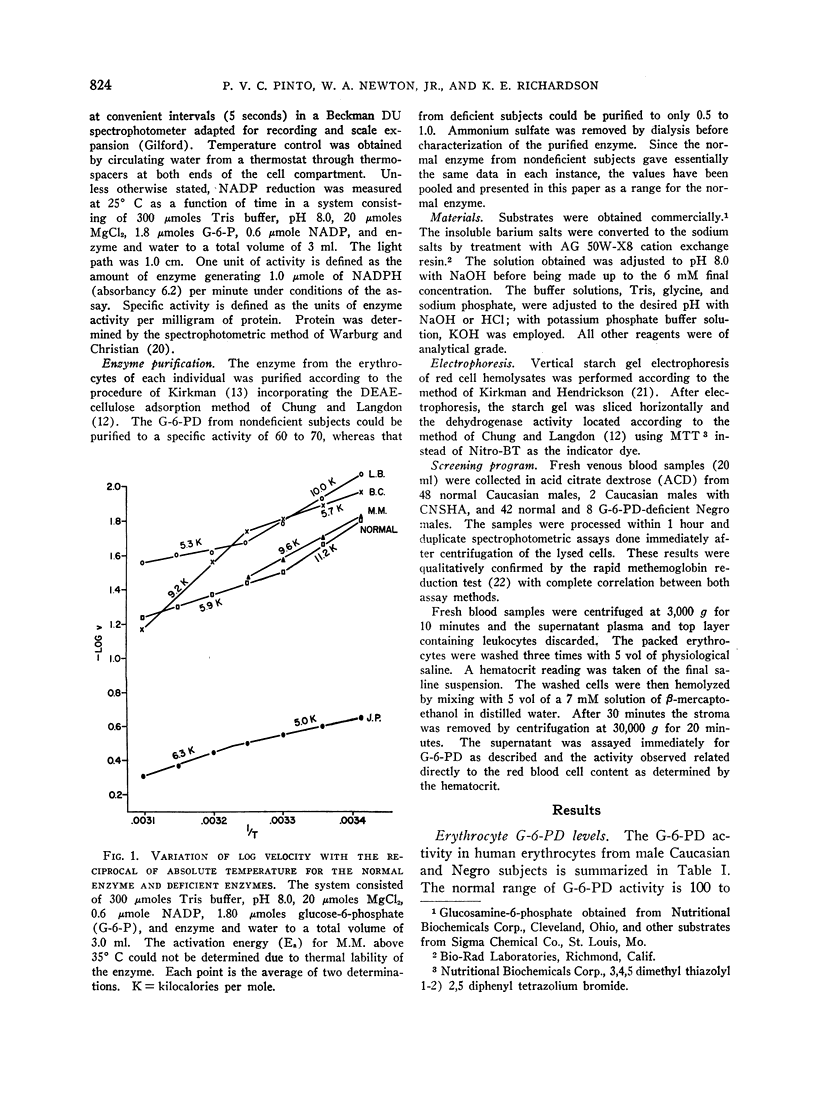
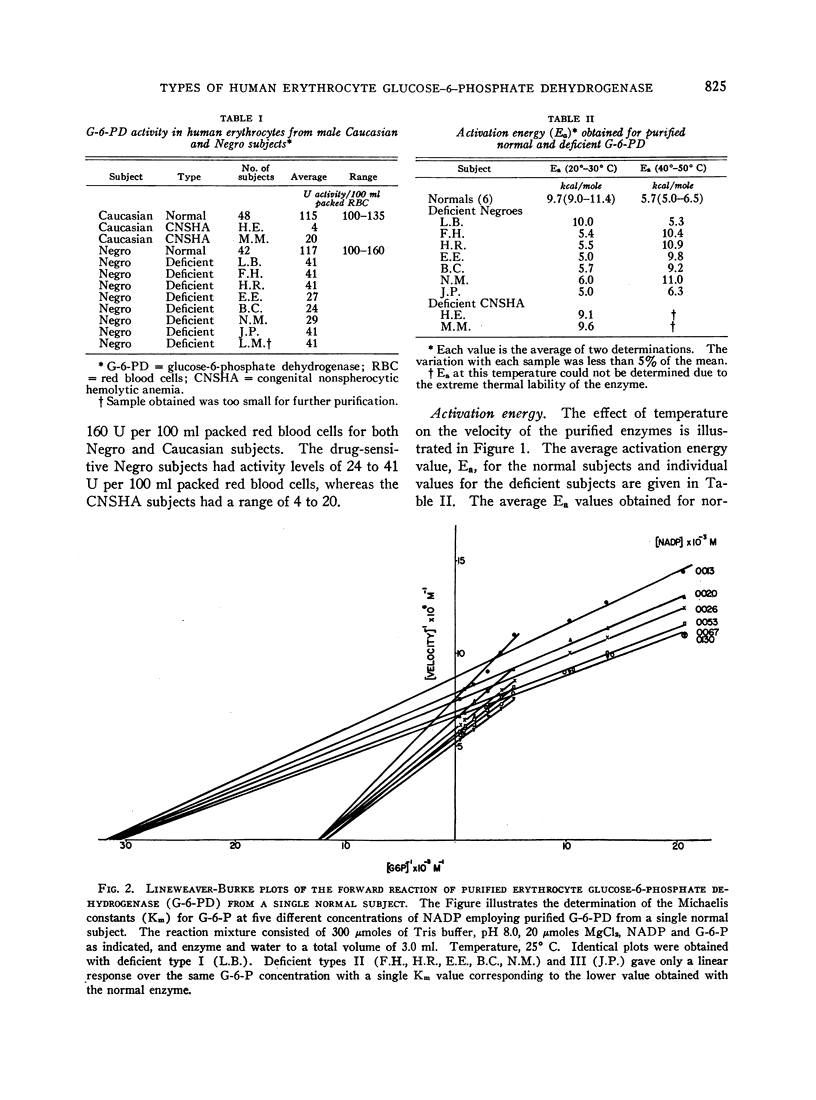
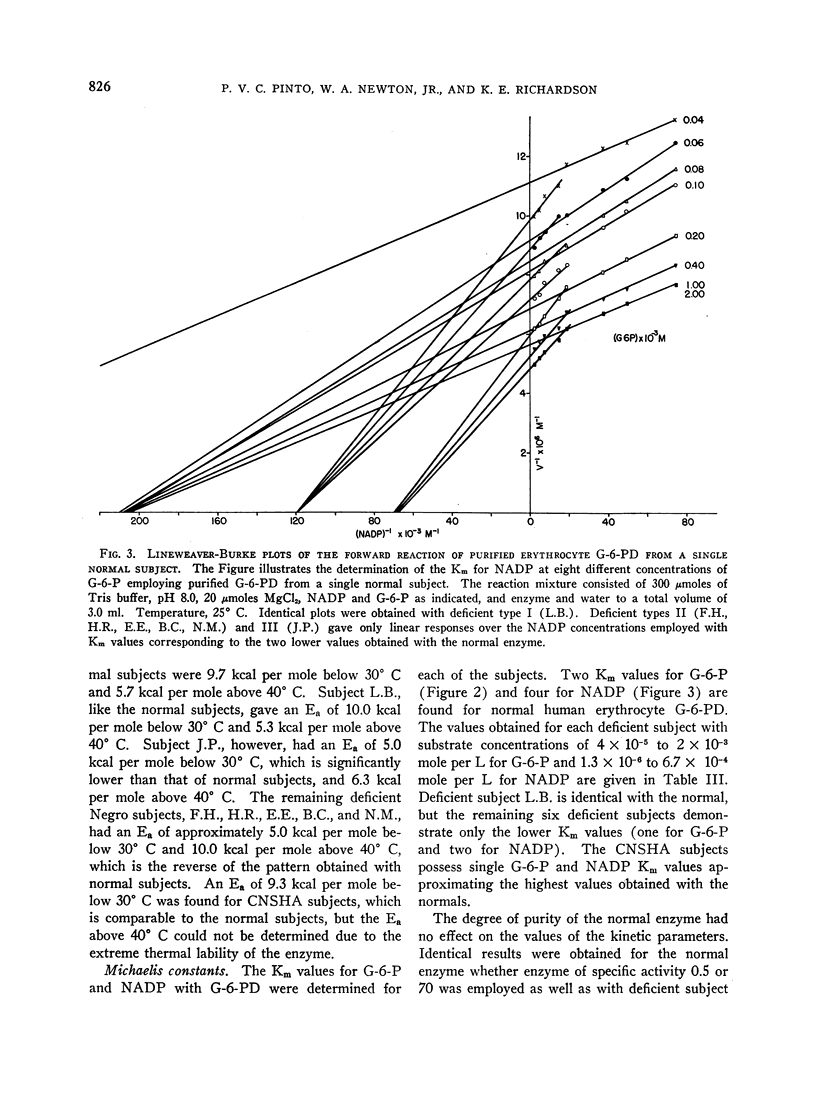


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALINSKY D., BERNSTEIN R. E. The purification and properties of glucose-6-phosphate dehydrogenase from human erythrocytes. Biochim Biophys Acta. 1963 Feb 12;67:313–315. doi: 10.1016/0006-3002(63)91828-6. [DOI] [PubMed] [Google Scholar]
- BEUTLER E. The hemolytic effect of primaquine and related compounds: a review. Blood. 1959 Feb;14(2):103–139. [PubMed] [Google Scholar]
- BOYER S. H., PORTER I. H., WEILBACHER R. G. Electrophoretic heterogeneity of glucose-6-phosphate dehydrogenase and its relationship to enzyme deficiency in man. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1868–1876. doi: 10.1073/pnas.48.10.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREWER G. J., TARLOV A. R., ALVING A. S. The methemoglobin reduction test for primaquine-type sensitivity of erythrocytes. A simplified procedure for detecting a specific hypersusceptibility to drug hemolysis. JAMA. 1962 May 5;180:386–388. doi: 10.1001/jama.1962.03050180032008. [DOI] [PubMed] [Google Scholar]
- CARSON P. E. Glucose-6-phosphate dehydrogenase deficiency in hemolytic anemia. Fed Proc. 1960 Dec;19:995–1006. [PubMed] [Google Scholar]
- CHUNG A. E., LANGDON R. G. Human erythrocyte glucose 6-phosphate dehydrogenase. I. Isolation and properties of the enzyme. J Biol Chem. 1963 Jul;238:2309–2316. [PubMed] [Google Scholar]
- CHUNG A. E., LANGDON R. G. Human erythrocyte glucose 6-phosphate dehydrogenase. II. Enzyme-coenzyme interrelationship. J Biol Chem. 1963 Jul;238:2317–2324. [PubMed] [Google Scholar]
- KIRKMAN H. N. Charasteristics of glucose-6-phosphate dehydrogenase from normal and primaquine-sensitive erythrocytes. Nature. 1959 Oct 24;184:1291–1292. doi: 10.1038/1841291a0. [DOI] [PubMed] [Google Scholar]
- KIRKMAN H. N. Glucose 6-phosphate dehydrogenase from human erythrocytes. I. Further purification and characterization. J Biol Chem. 1962 Jul;237:2364–2370. [PubMed] [Google Scholar]
- KIRKMAN H. N., HENDRICKSON E. M. Sex-linked electrophoretic difference in glucose-6-phosphate dehydrogenase. Am J Hum Genet. 1963 Sep;15:241–258. [PMC free article] [PubMed] [Google Scholar]
- KIRKMAN H. N., MCCURDY P. R., NAIMAN J. L. FUNCTIONALLY ABNORMAL GLUCOSE-6-PHOSPHATE DEHYDROGENASES. Cold Spring Harb Symp Quant Biol. 1964;29:391–398. doi: 10.1101/sqb.1964.029.01.041. [DOI] [PubMed] [Google Scholar]
- KIRKMAN H. N., ROSENTHAL I. M., SIMON E. R., CARSON P. E., BRINSON A. G. "CHICAGO I" VARIANT OF GLUCOSE-6-PHOSPHATE DEHYDROGENASE IN CONGENITAL HEMOLYTIC DISEASE. J Lab Clin Med. 1964 May;63:715–725. [PubMed] [Google Scholar]
- Kirkman H. N., Riley H. D., Crowell B. B. DIFFERENT ENZYMIC EXPRESSIONS OF MUTANTS OF HUMAN GLUCOSE-6-PHOSPHATE DEHYDROGENASE. Proc Natl Acad Sci U S A. 1960 Jul;46(7):938–944. doi: 10.1073/pnas.46.7.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKS P. A., SZEINBERG A., BANKS J. Erythrocyte glucose 6-phosphate dehydrogenase of normal and mutant human subjects: properties of the purified enzymes. J Biol Chem. 1961 Jan;236:10–17. [PubMed] [Google Scholar]
- PORTER I. H., BOYER S. H., WATSON-WILLIAMS E. J., ADAM A., SZEINBERG A., SINISCALCO M. VARIATION OF GLUCOSE-6-PHOSPHATE DEHYDROGENASE IN DIFFERENT POPULATIONS. Lancet. 1964 Apr 25;1(7339):895–899. doi: 10.1016/s0140-6736(64)91626-5. [DOI] [PubMed] [Google Scholar]
- RAMOT B., ASHKENAZI I., RIMON A., ADAM A., SHEBA C. Activation of glucose-6-phosphate dehydrogenase of enzyme-deficient subjects. II. Properties of the activator and the activation reaction. J Clin Invest. 1961 Mar;40:611–616. doi: 10.1172/JCI104291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVAL D. N., WOLFE R. G. Malic dehydrogenase. II. Kinetic studies of the reaction mechanism. Biochemistry. 1962 Mar;1:263–269. doi: 10.1021/bi00908a012. [DOI] [PubMed] [Google Scholar]
- TARLOV A. R., BREWER G. J., CARSON P. E., ALVING A. S. Primaquine sensitivity. Glucose-6-phosphate dehydrogenase deficiency: an inborn error of metabolism of medical and biological significance. Arch Intern Med. 1962 Feb;109:209–234. doi: 10.1001/archinte.1962.03620140081013. [DOI] [PubMed] [Google Scholar]