Abstract
Parkinson disease is characterized by a complex neuropathological profile that primarily affects dopaminergic neural pathways in the basal ganglia, including pathways that modulate cranial sensorimotor functions such as swallowing, voice and speech. Prior work in our lab has shown that the rat model of unilateral 6-hydroxydopamine infusion to the medial forebrain bundle that has been useful for studying limb sensorimotor deficits also yields vocalization deficits that may be amenable to treatment with intensive exercise. This affords us an opportunity to explore the potential mechanisms underlying behavioral and neural recovery as a result of intervention for cranial sensorimotor deficits associated with Parkinson disease (PD). Our methods include recording and acoustic analysis of male rat ultrasonic vocalizations in a control condition, after neurotoxin infusion (Parkinson disease model), and after targeted vocalization training. We also use well-established behavioral and immunohistochemical methods to assess the level of neurochemical recovery in the striatum of the basal ganglia after our interventions. Our findings, although preliminary, prompt us to look in other brain regions extraneous to the striatum for potential underlying mechanisms of recovery. Thus, our future work will focus on the underlying mechanisms of behavioral recovery in a PD model in the hope that this will lead to improved understanding of brain function and improved treatment for voice and swallowing disorders.
1. Introduction
Parkinson disease is characterized by a complex neuropathological profile that primarily affects dopaminergic neural pathways in the basal ganglia (Bergman & Deuschl, 2002; Braak, Ghebremedhin, Rub, Bratzke & Del Tredici, 2004), including pathways that modulate cranial sensorimotor functions such as swallowing, voice and speech. Thus, PD leads to dysphagia, dysphonia and dysarthria that compromise health and quality of life (Fox, Morrison, Ramig & Sapir, 2002; Ho, Iansek, Marigliani, Bradshaw & Gates, 1998; Plowmann-Prine et al., 2009). Common voice deficits associated with PD include breathiness, hoarse vocal quality, decreased vocal loudness, and decreased frequency variability (Darley, Aronson & Brown, 1969a, 1969b; Fox, et al., 2002; Ho, et al., 1998; Logemann, Fisher, Boshes & Blonsky, 1978). Speech and voice therapy in the form of intensive exercise has been shown to improve these deficits and quality of life in patients with PD (Ramig et al., 2001; Ramig, Sapir, Fox & Countryman, 2001; Sapir, Ramig & Fox, 2006). However, the underlying mechanisms of these behavioral interventions are not well understood. Data from a human imaging study after intensive intervention for parkinsonian dysarthria (Lee Silverman Voice Therapy) showed a more ‘normalized’ pattern of activation in the cortical motor and premotor areas of the brain and also additional recruitment of right anterior insula, dorsolateral prefrontal cortex, and basal ganglia (caudate head, putamen)(Liotti et al., 2003). Therefore, although data are limited to one study, it appears that behavioral intervention can alter neuronal patterns of activity.
In contrast to limited clinical data regarding the effects of behavioral therapy on neural pathways underlying cranial functions, there is a body of animal research demonstrating that intensive limb exercise leads to sparing of striatal dopamine neurons if started early in the disease process (Anstrom, Schallert, Woodlee, Shattuck & Roberts, 2007; Mabandla, Kellaway, St. Clair Gibson & Russell, 2004; Tillerson et al., 2001; Woodlee & Schallert, 2004). However, it is not known if behavioral intervention for vocalization deficits has these same effects. Thus, there is limited is knowledge of the underlying mechanisms for improvement in cranial sensorimotor control with treatment for PD.
It is tempting to use knowledge gained from behavioral studies of animal limb sensorimotor systems as explanations for the positive effects observed after voice and speech treatment in PD. However, common drug and surgical interventions that clearly benefit limb function do not appear to benefit cranial motor function to the same extent (Ciucci, Barkmeier-Kraemer & Sherman, 2008; Fuh, et al., 1997; Hunter, Crameri, Austin, Woodward & Hughes, 1997; Narayana et al., 2009; Potulska, Friedman, Krolicki, Jedrzejaowski & Spychala, 2002). There is not yet an adequate explanation or empirical data to explain the differential effects of pharmacologic and surgical treatments for PD on limb versus cranial systems. Perhaps cranial sensorimotor systems are modulated by dopamine in a different manner or by other non-dopaminergic neurotransmitters than those employed for limb actions. Putative mechanisms such as these should be investigated in future research.
Exploring potential mechanisms for cranial deficits in persons with PD requires invasive procedures and control of age, disease severity, medications, and environment. To overcome these methodological limitations, animal models can be used as a translational approach to studying interventions for PD. It is essential that we investigate vocalization deficits in awake animals with appropriate behavioral assays. While it is intuitively appealing to apply well-used measurements made in humans to the study of animals, our measures must be relevant to the animal in terms of vocalization, and sensitive to changes with PD and intervention. This concept is summarized by Cenci, Wishaw and Schallert (2002):
…the modeling of human-like symptoms in animals should be made primarily on the basis of an expectation of functional similarity, rather than on one of physical identity. The first question to ask is not whether a rat would show a given neurological symptom, but rather, how that neurological symptom would manifest itself in a rat. (p. 574)
Thus, although we formulate different measures than what we use in human evaluation, these measures can provide important insights into sensorimotor control processes relevant to humans when interpreted in the appropriate context.
To study the effects of PD and behavioral treatment in animal, the first step is to model the effects of the disease. These models are often based on a systemic pharmacologic administration or brain lesion. A model, in the best of circumstances, can only serve as an approximation to the human situation it is designed to reflect. As such, findings will be interpreted with the appropriate caution that should always be applied to all experimentation in the medical and social sciences. Albeit imperfect, an appropriate animal model can provide critical insights into putative mechanisms underlying health and disease in humans.
One widely used technique for creating a model of PD in the rat is depleting dopamine unilaterally with micro-infusion of the neurotoxin 6-hydroxydopamine (6-OHDA) to the medial forebrain bundle, a major dopaminergic pathway affected by PD. The medial forebrain bundle consists of neurons that originate in the substantia nigra and synapse in the striatum. The neurotoxin 6-OHDA leads to the degeneration of dopamine cells and quantifiable deficits that mimic those found in humans in the early stages of PD (Cenci, Whishaw & Schallert, 2002; Fulceri et al., 2006; Marshall, 1979; Meredith & Kang, 2006; Ungerstedt & Arbuthnott, 1970). This model has been used in prior examinations of PD-related sensorimotor deficits and neural modulation associated with intervention (Cenci et al., 2002; Fleming, Delville & Schallert, 2005; Meredith & Kang, 2006). Therefore the 6-OHDA model can be useful in examining some aspects of the deficits associated with PD.
Because of its extensive prior use, the 6-OHDA model of PD is associated with a wealth of behavioral tests to estimate lesion severity and recovery with intervention. The “forelimb asymmetry test,” also called the “cylinder test,” capitalizes on the unilateral lesion model because the affected limb can be compared with the intact limb during spontaneous movement. To perform this test, the awake rat is placed into a tall, clear plexiglass cylinder and observed while engaging in natural behaviors. Specifically, the use of the each forelimb is counted while the rat rears and explores. Rats with unilateral dopamine depletion preferentially use the unimpaired forelimb for support during exploration and show little or no use of the impaired forelimb (Schallert & Woodlee, 2005; Tillerson et al., 2001, 2002). Using a simple formula, the counted data are converted to a score that correlates highly with the amount of dopamine depletion found in the striatum (Woodlee & Schallert, 2004). Thus, this behavioral measure provides an accurate estimate of the extent of brain lesion and the associated sensorimotor deficit. Behavioral tests of this kind have been very useful in the study of brain regions and pathways underlying limb motor impairments in models of PD. The extent to which these tests and resultant measures apply to brain mechanisms underlying cranial sensorimotor disruption is unknown.
Most of the work in the 6-OHDA model of PD has been done in the limbs, but there are preliminary data concerning cranial motor systems from our laboratories. Specifically, we have found that a unilateral 6-OHDA lesion also leads to vocalization deficits (Ciucci, et al., 2007, 2009; Ciucci, Ma, Kane, Ahrens & Schallert, 2008). Under normal conditions, rats produce calls in the ultrasonic frequency range. A subset of these ultrasonic calls have frequencies that center around 50-kHz and are used to locate other rats, during play, in mother-pup interactions, and during sexual encounters (Bialy, Rydz, & Kaczmarek, 2000; Brudzynski, 2005; Brudzynski & Ociepa, 1992; Brudzynski & Pniak, 2002; McGinnis, & Vakulenko, 2003). Thus, these calls are thought to be semiotic, or convey meaning (Brudzynski, 2005). In our work, which utilizes sexual encounters to elicit calls, we determined that 3 different types of calls are produced while a male rat calls to a female rat in estrous: simple, frequency modulated, and harmonic (Fig. 1) (Ciucci et al., 2009). A rat in the control (unlesioned) condition primarily produces frequency modulated calls, but produces mostly simple calls after a unilateral 6-OHDA lesion (Ciucci et al., 2008). Within each call type, we also analyzed duration, bandwidth, and intensity and found that bandwidth and intensity are also vulnerable to striatal dopamine depletion (Ciucci et al., 2007, 2009). Our data suggest that similar to humans, striatal dopamine loss is associated with vocalization deficits and that the 6-OHDA model is useful for studying appropriately formulated paradigms regarding brain changes associated with PD and recovery with intervention. Thus, with the unilateral 6-OHDA model of PD, we study the nature of recovery for vocalization deficits with and without behavioral interventions. Specifically, we are addressing whether there is a behavioral improvement of vocalization following intensive vocal exercise and whether this recovery involves rescue or regeneration of striatal dopamine neurons.
Figure 1.
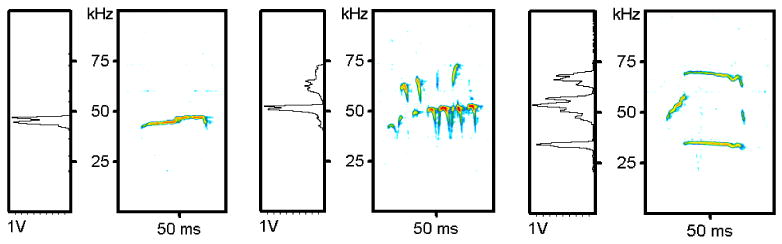
Reprinted with permission from Elsevier. Representative examples of the three different types of calls produced by a male rat calling to a receptive female rat: simple (left), frequency modulated (center), and harmonic (right). Time is on the x-axis and frequency on the y-axis. Relative intensity is represented with color. Boxes on the left represent the amplitude spectrum of the call expressed in volts.
2. Methods
2.1 Animals
Male and female Long-Evans rats are used in our experiments (Charles River) because they are known to vocalize extensively under normal conditions. Female rats are used to provide sexual experience and odor cues to elicit mate calling in the male rats. Male rats are typically 4-6 months old at the time of testing. Animals are housed two per cage in standard polycarbonate cages with sawdust bedding, and food and water are provided ad libitum, except during training, which requires a water restriction paradigm. Lights are maintained on a reverse 12:12 hour light: dark cycle. Because rats are nocturnal, the light cycle reversal ensures that exercise is provided at the time of most activity for the rats. Upon arrival at the animal care facility all of the animals are subjected to a light cycle reversal protocol to change the light cycle by two hours everyday until it reaches the desired time frame of 8 p.m. to 8 a.m. All experimentation and training is performed in the dark, with partial red illumination. Rats are handled for 14 days prior to behavioral testing and habituated to the recording environment for three days prior to vocalization recording sessions. All procedures are approved by the University of Wisconsin Institutional Animal Care and Use Committee.
2.2 Parkinson disease model
We infuse 6-OHDA to the medial forebrain bundle, which causes a moderate to severe degeneration of dopamine neurons (Fulceri et al., 2006; Marshall, 1979; Tillerson et al., 2001; Ungerstedt & Abuthnott, 1970). During the surgical procedure, rats are anesthetized with 4% isoflurane, and placed in a stereotaxic frame. An incision is made in the posterior aspect of the scalp and a small burhole is drilled in the skull. Rats receive unilateral infusions of 7 μg 6-OHDA hydrobromide (free base weight) dissolved in 3 μl artificial cerebrospinal fluid (composition: NaCl, KCl, CaCl2, MgCl2*6H20) containing 0.05% (w/v) ascorbic acid. Infusion coordinates are measured from a skull suture landmark (bregma: -3.3 AP; ±1.7 ML; -8.0 DV from dural surface), and infusions are delivered into the medial forebrain bundle at a rate of .3 μl/min for 10 minutes. Following surgery, animals are placed on a heating pad to prevent hypothermia, and upon waking are returned to their home cages.
To estimate the degree of 6-OHDA induced degeneration and the severity of parkinsonism, the forelimb asymmetry test (cylinder test) is performed by placing the rat in an upright acrylic cylinder (diameter 20 cm, height 30 cm) to encourage rearing and exploratory movements with the forepaws (Schallert & Tillerson, 2000; Schallert & Woodlee, 2005). The number of wall contacts made by either forelimb or by both forelimbs simultaneously is recorded (Fig. 2). The percentage of contacts made by the non-impaired forelimb relative to the total number of contacts is calculated using the formula: (nonimpaired limb contacts + both limb contacts)/ total number of contacts. Scores above 70% indicate a greater reliance on the unimpaired limb and are well correlated with the degree of nigrostriatal dopamine depletion.
Figure 2.

Forelimb asymmetry test on a rat with a severe lesion. View is from below. The rat is exploring the cylinder and contacts wall with the intact (left) forelimb and does not use impaired forelimb.
2.3 Ultrasonic vocalization recordings and analysis
Rats are tested in a 15 × 40 × 15 cm glass container with the top open to the microphone that is located approximately 15 cm from the rat's mouth. The ultrasonic microphone has a frequency response range of 10-180 kHz and a flat frequency response of up to 150 kHz (CM16, Avisoft, Germany) directed at the rat to record ultrasonic vocalizations. Prior to recording, each rat is placed in their homecage with a female rat who is sexually receptive, allowed to mount 2 times and then placed in the recording container. Ultrasonic vocalizations are recorded for 180 seconds.
Ultrasonic vocalizations are recorded on a computer and transferred to an external hard drive for storage and analysis. Analog recordings are digitized at 200-kHz sampling rate with 16-bit resolution and are analyzed with SasLab Pro (Avisoft, Germany). Sonograms are generated under a 512 FFT-length and 75% overlap frame setup. Generally, calls that are free from extraneous noise are analyzed for bandwidth, relative intensity, frequency variability, intensity variability and duration with automated software (SasLab Pro, Avisoft, Germany). Video recordings with a Panasonic PV-DV800 Infrared Camcorder are made with each session to ensure that behavior, rearing, and distance from the microphone are similar among all rats.
2.4 Vocalization training
Male rats are trained to increase the amount, complexity and intensity of calls after neurotoxin injection in the following manner. The male rats are water restricted overnight and paired with estrous females to elicit calls. A traditional learning paradigm is used where calls from the male are then reinforced with a water reward on a variable ratio 5 schedule and paired with an audible click. This means that on the average rats receive a water reward for every 5 calls produced, with some variance around the 5 call criterion. The water reward is associated with the click and over time, water restriction is diminished and the animals are reinforced with a click alone. Parameters of calls (type/complexity, bandwidth, relative intensity) are assessed by the trainer in real-time from the spectrogram output to provide an immediate reward to the rat. Rats are trained 5 days a week for 4 weeks. They are reinforced for producing complex calls with increasing intensity and bandwidth compared to their post-lesion call characteristics. A specific criterion is set for each animal based on the pre-lesion performance, meaning we attempt to restore the quality of their vocalizations to their pre-lesion status.
2.5 Tyrosine hydroxylase staining
At the end of the experiment, the animals are euthanized and undergo transcardial perfusion. Brains are extracted, fixed, sliced at 60 microns, and free floating sections are stained with immunoreactivity for the dopaminergic marker tyrosine hydroxylase (see Anstrom et al., 2007 and Tillerson, et al., 2001 for details of staining protocol). The brain slices mounted onto slides and are imaged and then analyzed with optical density measures (ImageJ, National Institutes of Health, Bethesda, MD) to quantify the degree of striatal dopamine loss (in percent loss) relative to the non-injured side. In this manner, the potential recovery of striatal dopaminergic neurons can be detected.
3. Preliminary data and discussion
Spectrograms of ultrasonic vocalizations from one rat in three different time periods are shown in Fig. 3: the pre-lesion condition, post-lesion condition, and post-training. Notice that the complexity, intensity and bandwidth diminish after 6-OHDA infusion (post-lesion) and recover to some degree after 4 weeks of intensive vocalization training. Fig. 4 shows brain slices of 2 different rats with severe lesions as determined by the forelimb asymmetry test and tyrosine hydroxylase immunoreactivity and varying degree of recovery of ultrasonic vocalization. The rat, whose brain image is shown on the left, produced primarily frequency modulated calls with bandwidth and intensity similar to that of pre-lesion measures. The rat with ‘poor’ recovery produced a mix of frequency modulated and simple calls with bandwidth and intensity that had improved from the post-lesion status, but did not return to baseline measures. However, there are no appreciable differences in terms of immunoreactivity for tyrosine hydroxylase (recovery of striatal dopaminergic neurons) in these 2 rats. Both rats appear to have nearly 100% loss of striatal dopaminergic neurons on the lesioned side, even though the rat on the left had better behavioral (vocalization) outcomes. Interestingly, when rats undergo intensive training for the forelimb, both behavioral recovery and striatal recovery occur (Anstrom et al., 2007; Tillerson et al., 2001).
Figure 3.
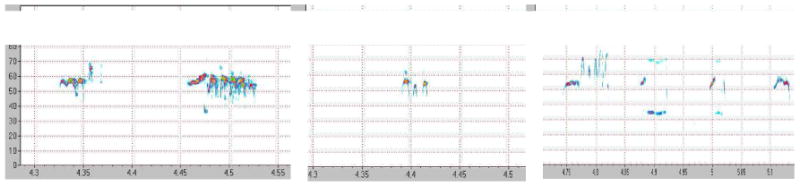
Spectrograms of ultrasonic vocalization from a male rat calling to a female rat in the pre, post-lesion, and post-exercise conditions.
Figure 4.
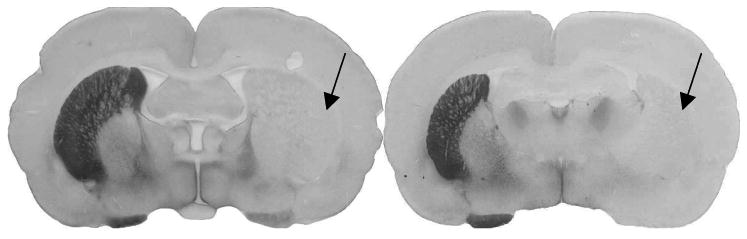
Representative brain slices of 2 rats that both underwent vocalization training, but recovered to varying degrees. The rat with good vocalization recovery is shown on the left and the rat with poor vocalization recovery is shown on the right. The brown color on left hemisphere of each brain slice is 3,3′-diaminobenzidine (DAB) chromogen that ‘marks’ the enzyme tyrosine hydroxylase, indicating neurons in the striatum that are positive for dopamine. Arrows represent areas in the right hemisphere where dopamine neurons have died and are not positive for tyrosine hydroxylase.
4. Conclusions
The rat model of unilateral 6-OHDA lesions that has been useful for studying limb sensorimotor deficits also yields vocalization deficits that may be amenable to treatment with intensive exercise. This affords us an opportunity to explore the potential mechanisms underlying the behavioral and neural recovery as a result of intervention for cranial sensorimotor deficits associated with PD. Our findings, although preliminary, prompt us to look in other brain regions extraneous to the striatum within other areas of the basal ganglia, brainstem, and cortical pathways for potential underlying mechanisms of recovery. Thus, our future work will focus on the underlying mechanisms of behavioral recovery in a PD model in the hope that this will lead to improved understanding of brain function and improved treatment for voice and swallowing disorders.
Acknowledgments
We would like to acknowledge Dr. Timothy Schallert for his ongoing scientific guidance, Jaime Shier for her technical assistance and Aaron Johnson for assistance with image analysis. This work was supported by grants: from the National Institute on Deafness and Other Communication Disorders F32 DC009363-01A1, 1P30DC010754-01, R01DC005935 and R01DC008149 and the Davis Phinney Foundation.
Appendix A. Continuing education
-
Parkinson disease leads to which of the following deficits:
Dysarthria
Dysphonia
Dysphagia
All of the above
-
Which of the following interventions has been shown to reliably and consistently improve voice deficits and quality of life:
Medication
Surgery
Intensive voice treatment
All of the above
-
A major pathway in the brain that uses dopamine, is primarily affected by Parkinson disease and is used in animal models of Parkinson disease is called the
Medial forebrain bundle
Striatum
Dorsolateral prefrontal cortex
Basal ganglia
-
The 6-OHDA model of Parkinson disease affects the following in the rat:
Limb use
Ultrasonic vocalizations
Dopamine levels
All of the above
-
Other brain regions outside of the basal ganglia may be involved in recovery from intensive treatment.
True
False
Answer key: 1:d; 2:c; 3:a; 4:d; 5:a
Footnotes
Learning outcomes: Readers will gain an understanding of how a rat model of Parkinson disease is used to study vocalization deficits and interventions.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anstrom KK, Schallert T, Woodlee MT, Shattuk A, Roberts DC. Repetitive vibrissae-elicited forelimb placing before and immediately after unilateral 6-hydroxydopamine improves outcome in a model of Parkinson's disease. Behavioral Brain Research. 2007;179:183–191. doi: 10.1016/j.bbr.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Bergman H, Deuschl G. Pathophysiology of Parkinson's disease: From clinical neurology to basic neuroscience and back. Movement Disorders. 2002;17(3):S28–S40. doi: 10.1002/mds.10140. [DOI] [PubMed] [Google Scholar]
- Bialy M, Rydz M, Kaczmarek L. Procontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behavioral Neuroscience. 2000;114:983–990. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Research. 2004;318:121–34. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Principles of rat communication: Quantitative parameters of ultrasonic calls in rats. Behavioral Genetics. 2005;35:85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Ociepa D. Ultrasonic vocalization of laboratory rats in response to handling and touch. Physiology and Behavior. 1992;52:655–660. doi: 10.1016/0031-9384(92)90393-g. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Pniak A. Social contacts and production of 50-kHz short ultrasonic calls in adult rats. Journal of Comparative Psychology. 2002;116:73–82. doi: 10.1037/0735-7036.116.1.73. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nature Reviews Neuroscience. 2002;3:574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- Ciucci MR, Barkmeier-Kraemer JM, Sherman SJ. Subthalamic nucleus deep brain stimulation improves deglutition in Parkinson's disease. Movement Disorders. 2008;23:676–683. doi: 10.1002/mds.21891. [DOI] [PubMed] [Google Scholar]
- Ciucci MR, Ahrens AM, Ma ST, Kane J, Cormack L, Schallert T. Reduction of dopamine synaptic activity: Degradation of 50-kHz ultrasonic vocalization in rats. Behavioral Neuroscience. 2009;123(2):328–36. doi: 10.1037/a0014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Ma TS, Fox C, Kane JR, Ramig L, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol. Behavioral Brain Research. 2007;182:284–289. doi: 10.1016/j.bbr.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Ma ST, Kane JR, Ahrens A, Schallert T. Limb-use and complex ultrasonic vocalization in a rat model of Parkinson's disease: Deficit-targeted training. Parkinsonism and Related Disorders. 2008;2:S172–175. doi: 10.1016/j.parkreldis.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. Journal of Speech and Hearing Research. 1969a;12:246–269. doi: 10.1044/jshr.1202.246. [DOI] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Clusters of deviant speech dimensions in the dysarthrias. Journal of Speech and Hearing Research. 1969b;12:462–496. doi: 10.1044/jshr.1203.462. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Delville Y, Schallert T. An intermittent, controlled-rate, slow progressive degeneration model of Parkinson's disease: Antiparkinson effects of Sinemet and protective effects of methylphenidate. Behavioral Brain Research. 2005;156:201–213. doi: 10.1016/j.bbr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Fox CM, Morrison CE, Ramig LO, Sapir S. Current perspectives on the Lee Silverman Voice Treatment (LSVT) for individuals with idiopathic Parkinson disease. American Journal of Speech Language Pathology. 2002;11:111–123. [Google Scholar]
- Fuh J, Lee R, Wang SJ, Lin C, Wang PN, Chiang J, et al. Swallowing difficulty in Parkinson's disease. Clinical Neurology and Neurosurgery. 1997;99:106–112. doi: 10.1016/s0303-8467(97)00606-9. [DOI] [PubMed] [Google Scholar]
- Fulceri F, Biagioni F, Lenzi P, Falleni A, Gesi M, Ruggieri S, et al. Nigrostriatal damage with 6-OHDA: Validation of routinely applied procedures. Annals of the New York Academy of Sciences. 2006;1074:344–348. doi: 10.1196/annals.1369.032. [DOI] [PubMed] [Google Scholar]
- Ho AK, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of patients with Parkinson's disease. Behavioral Neurology. 1998;11:131–137. [PubMed] [Google Scholar]
- Hunter PC, Crameri J, Austin S, Woodward MC, Hughes AJ. Response of Parkinsonian swallowing dysfunction to dopaminergic stimulation. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;63:579–583. doi: 10.1136/jnnp.63.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Ramig LO, Vogel D, New P, Cook CI, Ingham RJ, et al. Hypophonia in Parkinson's disease: neural correlates of voice treatment revealed by PET. Neurology. 2003;60(3):432–440. doi: 10.1212/wnl.60.3.432. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and cooccurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. Journal of Speech and Hearing Disorders. 1978;43:47–57. doi: 10.1044/jshd.4301.47. [DOI] [PubMed] [Google Scholar]
- Marshall JF. Somatosensory inattention after dopamine-depleting intracerebral 6-OHDA injections: Spontaneous recovery and pharmacological control. Brain Research. 1979;177:311–324. doi: 10.1016/0006-8993(79)90782-0. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Vakulenko M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiology & Behavior. 2003;80:81–88. doi: 10.1016/s0031-9384(03)00227-0. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Kang UJ. Behavioral models of Parkinson's disease in rodents: A new look at an old problem. Movement Disorders. 2006;21:1595–1606. doi: 10.1002/mds.21010. [DOI] [PubMed] [Google Scholar]
- Mabandla M, Kellaway L, St Clair Gibson A, Russell VA. Voluntary running provides neuroprotection in rats after 6-hydroxydopamine injection into the medial forebrain bundle. Metabolic Brain Disease. 2004;19:43–50. doi: 10.1023/b:mebr.0000027416.13070.c3. [DOI] [PubMed] [Google Scholar]
- Narayana S, Jacks A, Robin DA, Poizner H, Zhang W, Franklin C, et al. A noninvasive imaging approach to understanding speech changes following deep brain stimulation in Parkinson's disease. American Journal of Speech Language Pathology. 2009;18(2):146–61. doi: 10.1044/1058-0360(2008/08-0004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowmann-Prine EK, Sapienza CM, Okun MS, Pollock SL, Jacobson C, Wu SS, et al. The relationship between quality of life and swallowing in Parkinson's disease. Movement Disorders. 2009;24(9):1352–1358. doi: 10.1002/mds.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potulska A, Friedman A, Krolicki L, Spychala A. Swallowing disorders in Parkinson's disease. Parkinsonism Related Disorders. 2003;9:349–353. doi: 10.1016/s1353-8020(03)00045-2. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Sapir S, Countryman S, Pawlas AA, O'Brien C, Hoehn M, et al. Intensive voice treatment (LSVT) for patients with Parkinson's disease: A 2 year follow up. Journal of Neurology, Neurosurgery & Psychiatry. 2001;71:493–498. doi: 10.1136/jnnp.71.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig LO, Sapir S, Fox C, Countryman S. Changes in vocal loudness following intensive voice treatment (LSVT®) in individuals with Parkinson's disease: A comparison with untreated patients and normal age-matched controls. Movement Disorders. 2001;16:79–83. doi: 10.1002/1531-8257(200101)16:1<79::aid-mds1013>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Sapir S, Ramig LO, Fox C. The Lee Silverman Voice Treatment (LSVT®) for voice, speech, and other orofacial disorders in people with Parkinson's disease. Future Neurology. 2006;1:563–570. [Google Scholar]
- Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. Journal of Neuroscience. 2001;21:4427–4435. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillerson JL, Cohen AD, Caudle WM, Zigmond MJ, Schallert T, Miller GW. Forced non-use in unilateral parkinsonian rats exacerbates injury. Journal of Neuroscience. 2002;22:6790–6799. doi: 10.1523/JNEUROSCI.22-15-06790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U, Arbuthnott G. Quantitative recording of rotational behavior in rats after 6-0HDA lesions of the nigrostriatal dopamine system. Brain Research. 1970;24:485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- Schallert T, Tillerson JL. Intervention strategies for degeneration of dopamine neurons in parkinsonism: Optimizing behavioral assessment of outcome. In: Emerich DF, Dean RL III, Sanberg PR, editors. Central nervous system diseases: Innovative models of CNS diseases from molecule to therapy. Totowa, NJ: Humana Press; 2000. pp. 131–151. [Google Scholar]
- Schallert T, Woodlee MT. Orienting and placing. In: Whishaw IQ, Kolb B, editors. The behavior of the laboratory rat. New York, NY: Oxford University Press; 2005. pp. 129–140. [Google Scholar]
- Woodlee MT, Schallert T. The interplay between behavior and neurodegeneration in rat models of Parkinson's disease and stroke. Restorative Neurology & Neuroscience. 2004;22:153–161. [PubMed] [Google Scholar]


