Abstract
The genetic pathways underlying shoulder blade development are largely unknown, as gene networks controlling limb morphogenesis have limited influence on scapula formation. Analysis of mouse mutants for Pbx and Emx2 genes has suggested their potential roles in girdle development. In this study, by generating compound mutant mice, we examined the genetic control of scapula development by Pbx genes and their functional relationship with Emx2. Analyses of Pbx and Pbx1;Emx2 compound mutants revealed that Pbx genes share overlapping functions in shoulder development and that Pbx1 genetically interacts with Emx2 in this process. Here, we provide a biochemical basis for Pbx1;Emx2 genetic interaction by showing that Pbx1 and Emx2 can bind specific DNA sequences as heterodimers. Moreover, the expression of genes crucial for scapula development is altered in these mutants, indicating that Pbx genes act upstream of essential pathways for scapula formation. In particular, expression of Alx1, an effector of scapula blade patterning, is absent in all compound mutants. We demonstrate that Pbx1 and Emx2 bind in vivo to a conserved sequence upstream of Alx1 and cooperatively activate its transcription via this potential regulatory element. Our results establish an essential role for Pbx1 in genetic interactions with its family members and with Emx2 and delineate novel regulatory networks in shoulder girdle development.
Keywords: Emx2, Girdle, Hox, Pbx, Mouse, Scapula
INTRODUCTION
The development of the shoulder blade (scapula) is poorly understood. In the chick, its proximal components, i.e. the blade and spine, derive from dermomyotomal mesenchyme, whereas its distal components, i.e. the glenoid cavity and coracoid, as well as the acromion, derive partly from the somatopleuric compartment of the lateral plate mesoderm (LPM) (Huang et al., 2000; Wang et al., 2005) (see Fig. 2). In the mouse, the bony structures of the scapula have dual neural crest-mesoderm origin, as shown by genetic lineage labeling (Matsuoka et al., 2005).
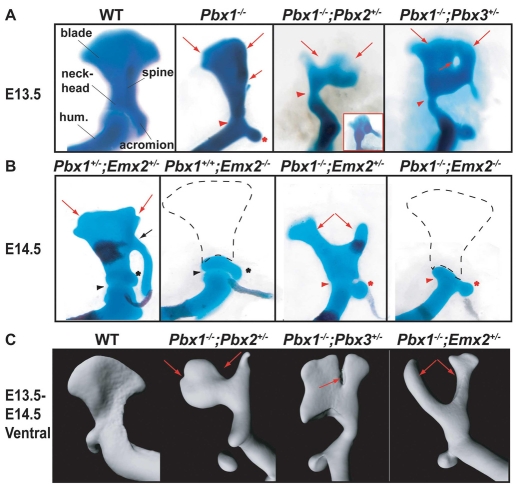
Fig. 2.
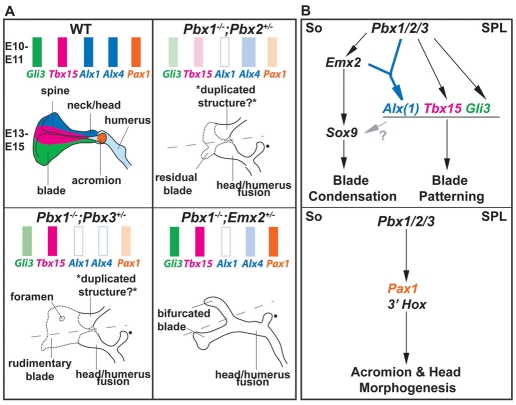
Hard-tissue phenotypes of compound Pbx and Pbx1;Emx2 mutants. Phenotypes were assessed in mutants versus WT littermates by skeletal preparation and OPT. (A) E13.5 Pbx1–/– scapulae exhibit blade dysmorphologies (long red arrows), shortened spines (short red arrow), head-humeral fusions (red arrowhead) and coracoid expansions (red asterisk). Pbx1–/–;Pbx2+/– scapulae exhibit proximal-distal blade reductions (long red arrows), whereas Pbx1–/–;Pbx3+/– have blade indentations (long red arrows) and foramina (short red arrow). Both mutants show scapular-humeral joint fusions (red arrowhead), potential head duplications (inset) and coracoid dysmorphologies. (B) E14-14.5 Pbx1+/–;Emx2+/– scapulae exhibit proximal blade indentations/reductions (red arrows), whereas Pbx1+/+;Emx2–/– embryos lack blades (dashed black line) but retain coracoids (black asterisk) and scapular-humeral joints (black arrowhead). Pbx1–/–;Emx2+/– embryos exhibit bifurcated blades (red arrows). Pbx1–/–;Emx2–/– exhibit blade absence (dashed line), and scapular-humeral joint fusions (red arrowhead) and coracoid malformations (red asterisk). (C) As assessed by OPT, Pbx1–/–;Pbx2+/– embryos display reduced blades along their central/lateral domains (red arrows), Pbx1–/–;Pbx3+/– embryos show reduced blades with proximal-to-distal indentations (red arrow) and Pbx1–/–;Emx2+/– embryos show blade bifurcations with rami appearing as rounded long bones (red arrows).
Scapula development is modestly affected by the genetic pathways that pattern the limb (e.g. Prahlad et al., 1979; Ros et al., 1996), and there is only rudimentary knowledge of the genes that act upstream of blade and head development (Huang et al., 2006). By contrast, much information has been gathered on genes that finely pattern these structures. For example, Pax1/Hoxa5 and Hoxc6 are involved in acromion and head development, respectively (Aubin et al., 1998; Timmons et al., 1994). Additionally, two distinct pathways have been suggested in blade patterning (Kuijper et al., 2005): one regulated by Emx2, the loss-of-function of which results in blade absence in mice (Pellegrini et al., 2001), and the other governed by genetic interactions among aristaless-related (Alx1, Alx3, Alx4), T-box (Tbx15) and Gli (Gli3) genes. It remains unknown whether these two blade-specific pathways are separate or integrated, whether Emx2 controls one or both, and which genes act upstream of Emx2 in blade and head morphogenesis.
Recent studies suggest that Pbx TALE homeoproteins control scapula development (Capellini et al., 2006; Selleri et al., 2001). Indeed, Pbx1-null (Pbx1–/–) embryos exhibit hypoplastic blades and scapular head-humeral fusions (Selleri et al., 2001), whereas compound Pbx1–/–;Pbx2+/– mutants present exacerbations of these phenotypes (Capellini et al., 2006; Capellini et al., 2008). Conversely, the single loss of Pbx2 or Pbx3 does not cause scapula mutant phenotypes (Rhee et al., 2004; Selleri et al., 2004). Scapula and proximal limb have thus been proposed to be `Pbx dependent', consistent with Pbx roles as Hox co-factors (reviewed by Moens and Selleri, 2006). However, loss of Hox genes, including entire Hox clusters, from pre-scapular tissues results, at best, in negligible scapula alterations, despite co-expression of Pbx and Hox genes in these domains (Fromental-Ramain et al., 1996; Aubin, 1997). Therefore, it currently appears unlikely that Pbx proteins function as Hox co-factors in scapula formation, suggesting instead cooperation with other factors.
Emx2 shares structural characteristics with Hox proteins, including: (1) a Gln (Q) in position 50 of the homeodomain, which is predicted to confer DNA-binding properties similar to those of Hox proteins; and (2) a YPWL sequence N-terminal to the homeodomain that resembles part of the Hox IYPWMK motif [`hexapeptide' (Bürglin, 1994)], a hallmark for heterodimerization with Pbx (reviewed by Moens and Selleri, 2006). It has never been addressed, though, whether Pbx factors and Emx2 interact genetically and/or physically in scapula development.
Here, we demonstrate that Pbx genes share overlapping functions in scapula development, as Pbx1;Pbx2 and Pbx1;Pbx3 compound mutants display more severe and novel phenotypes compared with those of Pbx1–/– embryos. We also show that the expression of genes crucial for scapular blade patterning or acromion and head development is altered in these mutants, placing Pbx genes upstream of pathways involved in scapula ontogeny. Additionally, we demonstrate that Pbx1 genetically interacts with Emx2 in blade and spine formation, as compound Pbx1;Emx2 mutants display novel defects. Moreover, we establish that Pbx1 and Emx2 heterodimerize on a specific DNA sequence, providing a molecular basis for their genetic interaction. Pbx1 and Emx2 proteins also bind in vivo to a conserved sequence upstream of the Alx1 (formerly Cart1) gene, and cooperatively activate transcription via this potential regulatory element. Our results thus delineate novel genetic networks in shoulder girdle development.
MATERIALS AND METHODS
Mice
Intercrosses between Pbx1+/– (Selleri et al., 2001), Pbx2+/– (Selleri et al., 2004), Pbx3+/– (Rhee et al., 2004) and Emx2+/– (Pellegrini et al., 2001) mice were performed to obtain Pbx1+/–;Pbx2+/–, Pbx1+/–;Pbx3+/–, Pbx2+/–;Pbx3+/– and Pbx1+/–;Emx2+/– mutants. On a C57BL/6 background, the double-heterozygous numbers obtained were below the expected Mendelian ratios. To increase numbers, C57BL/6 double-heterozygous males for each mutant line were crossed to Black-Swiss [NIH-BL(S)]. Next, double-heterozygous C57BL/6 females and mixed double-heterozygous C57BL/6-Black-Swiss males were intercrossed and their progeny analyzed for skeletal phenotypes. On mixed genetic backgrounds, marked ameliorations of C57BL/6 scapular phenotypes were observed, which led us to use C57BL/6 to Black-Swiss intercrosses. To generate more complex Pbx compound genotypes, Pbx1+/–;Pbx2+/– and Pbx1+/–;Pbx3+/– mutants were intercrossed to obtain Pbx1+/–;Pbx2+/–;Pbx3+/– mice; their in utero lethality prevented further intercrossing.
Skeletal preparations, in situ hybridization, cell proliferation and apoptosis immunohistochemistry and optical projection tomography (OPT)
Skeletons of E13.5-14.5 embryos were stained with Alcian Blue/Alizarin Red S (Depew et al., 1999; McLeod, 1980). Whole-mount in situ hybridizations were performed on somite-matched embryos using digoxygenin-labeled RNA probes (Di Giacomo et al., 2006). Alx1 (Kuijper et al., 2005), Alx4 (Beverdam and Meijlink, 2001), Emx2 (Pellegrini et al., 2001), En1 (Davidson et al., 1988), Gli3 (Beverdam and Meijlink, 2001), Hoxc6 (Sharpe et al., 1988), Pax1 (Chalepakis et al., 1991), Pbx1 (Brendolan et al., 2005), Pbx2 (Selleri et al., 2004), Pbx3 (Di Giacomo et al., 2006), Sox9 (Wright et al., 1995) and Tbx15 (Kuijper et al., 2005) probes were used. Section in situ hybridization was conducted as described (Di Giacomo et al., 2006). Cell proliferation and apoptosis assays were carried out as described (Capellini et al., 2008); for details, see Fig. S1 in the supplementary material. Skeletal preparations and whole-mount in situ hybridization embryos were processed for OPT according to published protocols (Sharpe, 2003; Sharpe et al., 2002).
Chromatin immunoprecipitation (ChIP)
As previously described (Salsi et al., 2008), chromatin was extracted from mouse proximal forelimb and flank dissected from wild-type E11.5 embryos. Cross-linked chromatin was immunoprecipitated with 5 μg anti-Pbx antiserum (C-20, Santa Cruz) or a specific anti-Emx2 monoclonal antibody (see below). Semi-quantitative PCR analysis was performed on three independent ChIPs for each genomic region analyzed, and primers were designed accordingly (Salsi and Zappavigna, 2006) (see Table S1 in the supplementary material).
Plasmid constructs
The pSG5Pbx1 and pETPbx1 expression constructs for the Pbx1a protein have been described (Di Rocco et al., 1997). The pSG5Emx2 expression construct was generated by cloning EcoRI/BglII-cut PCR-amplified Emx2 coding sequence into EcoRI/BamHI-cut pSG5 (Stratagene) vector. The pRSETEmx2 expression construct was generated by cloning the Emx2 coding sequence as an EcoRI fragment into the EcoRI site of the pRSETB vector. The pML(EP)6 reporter construct was obtained by cloning a tandem hexamer of the EP oligonucleotide 5′-GATCCGTCGACGGATCATTAAAGCCCTCGAGA-3′ into the BglII site of the pML luciferase reporter (Di Rocco et al., 1997). The pMLAlx1-EPBS(900) reporter was generated by cloning a 974 bp fragment containing the PCR-amplified Alx1 5′-7 genomic sequence [–4670 to –3699, relative to the transcription start site (TSS)] into the SmaI site of pML. The pMLAlx1-EPBS reporter was generated by cloning a 130 bp PCR-amplified fragment from the Alx1 5′-7 region containing Sites A, B and C of the EPBS sequence (–4270 to –4139) into the NheI/BglII sites of pML. The pMLAlx1-EPBS(900)A+Bmut and pMLAlx1-EPBS mutated reporters (Amut, Bmut or Cmut) were obtained by mutating Site A, B, A+B or C within the EPBS element by PCR-mediated mutagenesis and cloning the fragments into the NheI/BglII sites of pML. The mutations were identical to those of the electrophoretic mobility shift assay (EMSA) oligonucleotide probes (see below). All PCR-amplified fragments were verified by sequencing.
Protein production and purification, binding site selection, cell extracts and EMSAs
His-tagged Emx2 and Pbx1 proteins were produced in E. coli using pRSETEmx2 and pETPbx1 plasmids and purified using Ni-NTA Agarose (Qiagen). For binding site selection, purified Emx2 and Pbx1 proteins were incubated with 32P-labeled SelEP oligonucleotide pool (5′-GGCGAGATCTCTCGAGGGNNNNNATGATCCGTCGACGGATCCGCGG-3′) in binding buffer (Chang et al., 1995) for 30 minutes at 0°C and electrophoresed on a 6% polyacrylamide gel in 0.5× TBE. The retarded band corresponding to the Emx2-Pbx1 heterodimer was excised and eluted in 0.5 M ammonium acetate, 10 mM MgCl2, 1 mM EDTA, 0.1% SDS. Aliquots of the eluted band were PCR amplified for 30 cycles (95°C 1 minute, 55°C 30 seconds, 72°C 1 minute) with primers complementary to the flanking arms of SelPE, and the amplified DNA was used in further binding reactions. After six selection rounds, amplified DNA was cloned, sequenced and consensus sequences were aligned using CLUSTAL W (Thompson et al., 1994) (http://workbench.sdsc.edu).
EMSAs were performed (Di Rocco et al., 2001) using purified, bacterially produced Emx2 and Pbx1 proteins, or whole-cell extracts (10-20 μg each) from COS cells exogenously expressing both genes. To identify protein-DNA complexes, 200 ng of anti-Pbx antibody (C20, Santa Cruz) or 0.1 μl of an anti-Emx2 monoclonal antibody were added to binding reactions, which were separated by 6% PAGE in 0.5× TBE, dried and exposed to film. The anti-Emx2 monoclonal antibody was generated using the N-terminal portion of Emx2 and tested for its lack of cross-reaction with Pbx1 by immunoblotting. Oligonucleotides used in EMSAs were (mutated nucleotides are underlined):
Alx1-EPBS, 5′-TATAGCTTTGATGTAAGTAGAAGTATCTTTCATGTCCAAAATTAAAATTACATT-3′;
EPBS-Amut, 5′-TATAGCGGGGCGGGCAGTAGAAGTATCTTTCATGTCCAAAATTAAAATTACATT-3′;
EPBS-Bmut, 5′-TATAGCTTTGATGTAAGTAGAAGTATCTGGACGGTCCAAAATTAAAATTACATT-3′; and
EPBS-Cmut, 5′-TATAGCTTTGATGTAAGTAGAAGTATCTTTCATGTCCAAACGGCAACGGCCATT-3′.
Cell culture, transfections and luciferase assays
P19 mouse embryonal carcinoma cells were cultured in MEMα with 10% foetal calf serum. In typical transfections, performed by CaPO4 precipitation, reporter plasmid (5 μg), protein expression construct (2-4 μg), pCH110 β-galactosidase plasmid (0.5 μg) as an internal control, and pBluescript (variable quantities), were added to a total of 20 μg transfected DNA per 9-cm dish. Cells were harvested 48 hours post-transfection, and assayed for luciferase and β-galactosidase expression (Zappavigna et al., 1994).
RESULTS
Pbx genes are co-expressed in prospective scapular-forming domains
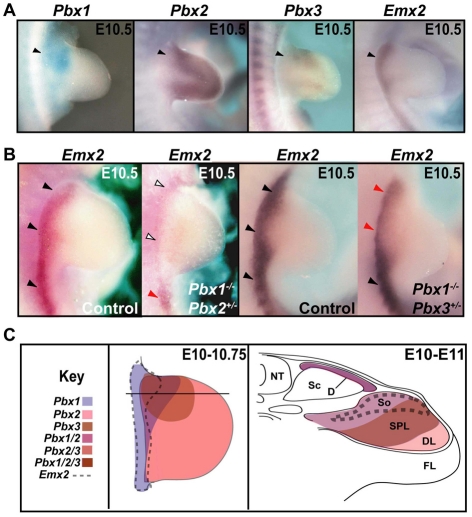
At E10.5, Pbx1 and Pbx2, but not Pbx3, were expressed in the dermomyotome and somatopleure (see Fig. S1A in the supplementary material). However, from E11 to E12, all three Pbx genes were detected, although Pbx3 was at lower levels (see Fig. S1B in the supplementary material). At E10.5-11.5, all three Pbx genes were co-expressed in proximal forelimb mesenchyme (Fig. 1A,C), corroborating previous reports (Capellini et al., 2006). This pattern persisted until E11.0-11.5, when the expression of Pbx1 remained proximal, whereas Pbx3 was confined to proximal-anterior limb domains and Pbx2 to distal domains (see Capellini et al., 2006; Di Giacomo et al., 2006). Emx2 was co-expressed with Pbx genes at E10.5 (Fig. 1A,C) (Theil et al., 1999; Tian and Lev, 2002).
Fig. 1.
Expression of Pbx and Emx2 genes in pre-scapular domains in wild-type mice and Pbx compound mutants. Expression was assessed by in situ hybridization. (A) Pbx1-3 and Emx2 co-expression in wild-type (WT) E10.5 proximal-superior forelimbs. (B) Emx2 expression in E10.5 Pbx compound mutants. Compared with WT (black arrowheads), Emx2 is severely reduced in Pbx1–/–;Pbx2+/– (white arrowheads) and reduced in Pbx1–/–;Pbx3+/– forelimbs (red arrowheads), but present in posterior flank mesoderm (black arrowheads). (C) Diagram of Pbx1-3 and Emx2 co-expression in E10-10.75 proximal limbs (left) and in a transverse section at the superior forelimb level in an E10-11 WT embryo. The differently shaded domains and dashed lines represent overlapping expression territories for Pbx1-3 and Emx2.
Pbx1 genetically interacts with Pbx2 and Pbx3 to form scapular structures
Given the overlapping expression of the Pbx genes in pre-scapular domains, compound Pbx mutants were generated to investigate the respective contributions of Pbx1-3. Scapula defects were evident in only three allelic combinations: Pbx1–/–, Pbx1–/–;Pbx2+/– and Pbx1–/–;Pbx3+/– (Fig. 2A,C; see Fig. S2A in the supplementary material); Pbx1–/–;Pbx2–/– and Pbx1–/–;Pbx3–/– mutants died in utero before scapula morphogenesis (Capellini et al., 2006).
At E13.5, Pbx1–/– embryos exhibited reduced blades and spines, obliterated glenoid cavities fused to humeral heads and expanded coracoids (Fig. 2A) (Selleri et al., 2001). In Pbx1–/–;Pbx2+/– mutants (Pbx1/2Mut), several Pbx1–/– scapula phenotypes were exacerbated and novel defects became evident. Cartilage preparations at E12.5 revealed that scapular condensations were disorganized and reduced, indicating disruption prior to mesenchyme formation (Capellini et al., 2006). Analysis of Sox9 expression, a mesenchymal condensation marker, revealed significant reductions across the superior-to-inferior blade-forming domains (Fig. 3A). OPT (Sharpe, 2003; Sharpe et al., 2002) of the three-dimensional expanse of Sox9 expression revealed a decrease in mesenchyme across entire pre-scapular domains (Fig. 3C; see Fig. S2B and Movie 1 in the supplementary material). As a result, reduced cartilages were observed at E12.5/E13.5 in skeletal preparations and by OPT. Pbx1/2Mut scapulae (Fig. 2A,C; see Fig. S2A and Movie 2 in the supplementary material) were dysmorphic, their blades were markedly reduced compared with those of Pbx1–/– and wild-type (WT) embryos, and their only intact aspects were near the head-neck junction. Furthermore, spines and acromia were absent. The remaining `scapular' mass was fused to the humeral head with an adjoining skeletal element, possibly a duplicated scapular/humeral head (Fig. 2A, inset, Fig. 2C; see Fig. S2A in the supplementary material). At E13.5, skeletal preparations and OPT revealed that Pbx1–/–;Pbx3+/– (Pbx1/3Mut) embryos also exhibited scapulae with proximal-to-distal blade reductions (Fig. 2A,C; see Fig. S2A in the supplementary material), although less severe than in Pbx1/2Mut embryos (Fig. 2A,C; see Fig. S2A and Movie 3 in the supplementary material). Additionally, Pbx1/3Mut scapulae showed blade indentations (Fig. 2C; see Fig. S2A in the supplementary material) and foramina (Fig. 2A), lacked well-developed spines and acromia, and their heads remained fused to humeri and were potentially duplicated (Fig. 2A,C; see Fig. S2A in the supplementary material). Sox9 expression was reduced in Pbx1/3Mut scapular domains (Fig. 3A; see Fig. S2B in the supplementary material), although OPT revealed that this reduction was not as drastic as in Pbx1/2Mut embryos (Fig. 3C; see Fig. S2B and Movie 4 in the supplementary material).
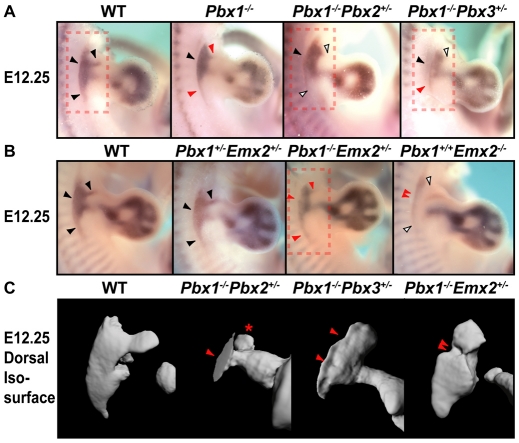
Fig. 3.
Mesenchymal phenotypes of compound Pbx1-3 and Pbx1;Emx2 mutants. Phenotypes were assessed by Sox9 in situ hybridization and OPT. (A) In specific E12.25 pre-scapular domains, Sox9 expression is reduced in Pbx1–/– (red arrowheads), significantly reduced in Pbx1–/–;Pbx2+/– (white arrowheads), and reduced in Pbx1–/–;Pbx3+/– (red and white arrowheads) embryos. (B) In E12.25 pre-scapular domains, Sox9 expression is normal in Pbx1+/–;Emx2+/– (black arrowheads), slightly reduced in Pbx1–/–;Emx2+/– (red arrowheads) and severely reduced (double red arrowheads) to nearly absent (white arrowheads) in Pbx1+/+;Emx2–/– embryos. (C) OPT of Sox9 expression at E12.25. Dorsal views of Sox9 OPT in the pre-scapular domains outlined by red dashed boxes in A and B reveal reductions in blade mesenchyme in Pbx1–/–;Pbx2+/– (red arrowheads), less severe reductions with indentations in Pbx1–/–;Pbx3+/– (red arrowheads), and strong central vertebral reductions in Pbx1–/–;Emx2+/– (double red arrowheads) embryos. The asterisk in Pbx1–/–;Pbx2+/– indicates expanded/duplicated humeral heads.
Scapular domains of Pbx2;Pbx3 mutants, particularly Pbx2–/–;Pbx3+/– and Pbx2+/–;Pbx3–/– embryos, appeared similar to those of WT (not shown), whereas Pbx2–/–;Pbx3–/– mutants died before shoulder development could be assessed (i.e. before E10.5). Finally, Pbx1;Pbx2;Pbx3 compound mutants could not be obtained owing to the perinatal lethality of Pbx1+/–;Pbx2+/–;Pbx3+/– embryos, which did not exhibit appendicular defects (not shown).
Pbx1 genetically interacts with Emx2 during scapular blade formation
In light of the blade defects of compound Pbx mutants, we examined the expression of Emx2, the absence of which results in blade agenesis (Pellegrini et al., 2001). Whereas Emx2 was unchanged in E10.5 Pbx1–/– embryos (not shown), it was severely and moderately reduced in Pbx1/2Mut and Pbx1/3Mut embryos, respectively (Fig. 1B). By contrast, Pbx1-3 expression remained grossly unperturbed in the proximal-superior forelimbs of Emx2–/– embryos (see Fig. S1C in the supplementary material).
Given that (1) Pbx and Emx2 genes are co-expressed in blade-patterning domains, (2) mice deficient for either of these genes exhibit blade phenotypes, and (3) both Pbx and Emx2 proteins possess structural features permitting their heterodimerization, potential genetic interactions of Pbx1 with Emx2 were assessed. At E14.5, Pbx1+/+;Emx2–/– embryos displayed blade loss, as previously reported (Fig. 2B) (Pellegrini et al., 2001), whereas Pbx1–/–;Emx2+/+ embryos exhibited blade alterations and head fusions (Fig. 2A) (Selleri et al., 2001). Genetic interaction was evident in Pbx1+/–;Emx2+/– embryos, which showed minor proximal blade reductions that were absent in single Pbx1+/– or Emx2+/– progeny (not shown), and in Pbx1–/–;Emx2+/– mutants (Pbx1/Emx2Mut), which exhibited centrally bifurcated blades with superior and inferior rami resembling individual bones (Fig. 2B). OPT uniquely revealed rounded, shaft-like rami, rather than flattened, blade-like structures (Fig. 2C; see Fig. S2A and Movie 5 in the supplementary material). These phenotypes were not observed in Pbx1–/– or Emx2+/– embryos (Fig. 2B,C). Additionally, Pbx1/Emx2Mut limbs displayed scapular-humeral head fusions, as in Pbx1–/– mutants (Fig. 2B,C; see Fig. S2A in the supplementary material) (Selleri et al., 2001). Analysis of Sox9 expression in Pbx1/Emx2Mut embryos at E12.5 revealed reduced mesenchymal condensations, and OPT revealed this mesenchyme to be markedly indented along its vertebral border, foreshadowing later scapular bifurcation (Fig. 3C; see Fig. S2B and Movie 6 in the supplementary material). Finally, Pbx1–/–;Emx2–/– mutants displayed blade agenesis, as in Emx2–/– embryos, and scapular-humeral head fusions, as in Pbx1–/– embryos (Fig. 2B). Given the additive phenotype of Pbx1–/–;Emx2–/– mutants, only Pbx1/Emx2Mut mice were studied for scapula marker gene expression.
Pbx factors are required for the expression of genes involved in scapular head formation
To investigate Pbx and Emx2 roles in patterning distal scapular structures, the expression of genes crucial for head and acromial development was examined in compound mutants. Pax1, a regulator of acromion formation (Aubin et al., 2002; Timmons et al., 1994; Wilm et al., 1998), was markedly reduced proximally (Fig. 4A, left) but was maintained within its dorsoventral domain (Fig. 4B, left) in Pbx1/2Mut and Pbx1/3Mut embryos, whereas it was only slightly decreased in Pbx1–/– mutants (Fig. 4A, left). Severe reductions were not present in Emx2–/– or Pbx1/Emx2Mut embryos (Fig. 4A, left). Although Hoxc6–/– mutants lack scapular head malformations (Garcia-Gasca and Spyropoulos, 2000), misexpression studies implicate this gene in its specification (Oliver et al., 1990). In Pbx1–/–, Pbx1/2Mut and Pbx1/3Mut embryos, Hoxc6 was posteriorly expanded in limbs, unlike in controls (Fig. 4A, right) and was dorsoventrally expanded in compound Pbx mutants (Fig. 4B, right), but unaltered in Emx2–/– or Pbx1/Emx2Mut embryos (Fig. 4A, right).
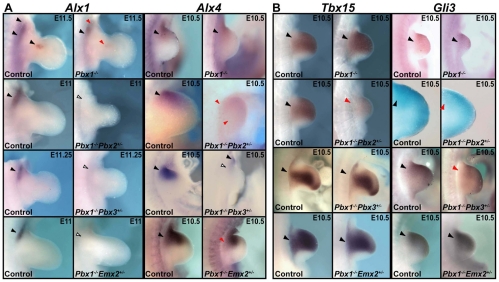
Fig. 4.
Expression of acromion and head markers in E11.5 compound Pbx and Pbx1;Emx2 mutants. Expression was assessed by in situ hybridization. (A) Compared with controls (black arrowheads), Pax1 expression (left panels) is reduced in Pbx1–/– and Pbx1–/–;Emx2+/– (red arrowheads), markedly reduced in Pbx1–/–;Pbx2+/– and Pbx1–/–;Pbx3+/– (double red arrowheads) and proximally expanded in Pbx1+/+;Emx2–/– (blue arrowhead) mutants. Compared with controls (black and white arrowheads), Hoxc6 expression (right panels) is expanded in Pbx1–/– and Pbx1–/–;Pbx3+/– (blue arrowheads), drastically expanded in Pbx1–/–;Pbx2+/– (double blue arrowheads) and unchanged in Pbx1+/+;Emx2–/– and Pbx1–/–;Emx2+/– (black arrowheads) mutants at E10.75/E11.5. (B) In Pbx1–/–;Pbx2+/– embryos, Pax1 expression (left panels) remains dorsoventrally restricted (red arrowheads) within the normal domain (black arrowheads). Hoxc6 (right panels) is dorsoventrally expanded across the midline of the forelimb (dashed line) in Pbx1–/–;Pbx2+/– (red arrowheads) compared with WT (black arrowheads) embryos. Magnification of WT and Pbx1–/–;Pbx2+/– Hoxc6 panels is 1.5× that of the other panels. D, dorsal; V, ventral.
Pbx factors and Emx2 are required for the expression of genes involved in blade patterning
Only the proximal forelimb expression of regulators of blade patterning was considered crucial (Fig. 5; see Figs S3 and S4 in the supplementary material), as distal domains typically correlate with limb development (Huang et al., 2006). The expression of Alx1, a gene that patterns the superior blade (Kuijper et al., 2005), was reduced in Pbx1–/– (Fig. 5A, left) and Emx2–/– (see Fig. S3 in the supplementary material) proximal forelimbs, whereas it was absent in E10.5-11.5 Pbx1/2Mut, Pbx1/3Mut and Pbx1/Emx2Mut proximal forelimbs (Fig. 5A, left). At E10.5, the expression of Alx4, which also patterns the superior blade, was only slightly reduced in Pbx1–/– and Pbx1/Emx2Mut proximal forelimbs, but markedly reduced and spatially altered in Pbx1/2Mut and Pbx1/3Mut forelimbs (Fig. 5A, right). However, this perturbation was not as extreme at E11.5 in all mutants (see Fig. S4 in the supplementary material), including Emx2–/– mutants (see Fig. S3 in the supplementary material). Expression of Tbx15, a gene that patterns the central blade, was only reduced in Pbx1/2Mut proximal forelimbs at E10.5 (Fig. 5B, left), whereas at E11.5 it was expanded in proximal forelimbs of Pbx1–/– embryos and all other mutants lacking Pbx1 (see Fig. S4 in the supplementary material) but was absent in Emx2–/– mutants (see Fig. S3 in the supplementary material). Expression of Gli3, which patterns the inferior blade, was nearly absent and reduced in Pbx1/2Mut and Pbx1/3Mut proximal forelimbs, respectively, from E10.5 (Fig. 5B, right) through E11.5, when only Pbx1/Emx2Mut (see Fig. S4 in the supplementary material) and Emx2–/– (see Fig. S3 in the supplementary material) embryos retained normal Gli3 expression. The expression of Gli3 and Alx4, along with engrailed 1 (En1), which demarcates the somitic dermomyotome (Davidson et al., 1988), was unaltered in all of these mutants (not shown). These results are summarized in Fig. 8A.
Fig. 5.
Expression of blade-patterning genes in E10.5-11.5 compound Pbx and Pbx1;Emx2 mutants. Expression was compared in mutants and control littermates (black arrowheads) by in situ hybridization. (A) (Left) Alx1 is reduced in Pbx1–/– (red arrowheads) and is undetected in Pbx1–/–;Pbx2+/–, Pbx1–/–;Pbx3+/– and Pbx1–/–;Emx2+/– (white arrowheads) mutants. (Right) Alx4 expression remains normal in Pbx1–/– (black arrowheads), whereas it is markedly (red arrowheads) and severely (white arrowheads) reduced in Pbx1–/–;Pbx2+/– and Pbx1–/–;Pbx3+/– mutants, respectively. In Pbx1–/–;Emx2+/– forelimbs, Alx4 is only slightly reduced (red arrowhead). (B) (Left) Tbx15 is markedly reduced only in Pbx1–/–;Pbx2+/– proximal forelimbs (red arrowhead). (Right) Gli3 is normal in Pbx1–/– and Pbx1–/–;Emx2+/– (black arrowheads), whereas it is markedly reduced in Pbx1–/–;Pbx2+/– (red arrowhead) and slightly reduced in Pbx1–/–;Pbx3+/– (red arrowhead) proximal-superior forelimbs. Magnification of Gli3 and Alx4 expression in WT and Pbx1–/–;Pbx2+/– panels is 2.5× that of other panels.
Fig. 8.
Pbx and Emx2 interaction and function in scapular development. (A) Summary of marker gene expression for blade and spine (top) and of phenotypes (bottom) in each compound Pbx and Pbx1;Emx2 mutant. Colored rectangles (top) at E10-11 depict normal expression levels and domains for genes specifying superior-to-inferior blade and acromial structures, color-coded in the E13-15 WT scapula (bottom). Lighter/unshaded rectangles depict reduced/nearly absent expression levels, and wider rectangles indicate expanded domains. Specific phenotypes for each mutant are represented versus WT (bottom). Changes in gene expression versus WT underlie the respective reductions in blades and spines in each mutant. A filled dot indicates what is most likely to be a malformed coracoid process. (B) Model of Pbx and Emx2 function in scapular development. (Top) Hierarchical genetic relationships between Pbx1-3, Emx2, Sox9 and blade markers in scapular blade condensation and patterning in the somatopleure (So) and superior proximal limb (SPL). A bifurcated blue arrow indicates that Pbx1 and Emx2 genetically and biochemically interact to regulate Alx1 transcription. It remains unknown whether blade-patterning genes control condensation and chondrogenesis (gray arrow with question mark). (Bottom) Pbx function upstream of Pax1 and acromion morphogenesis and of 3′ Hox gene and head morphogenesis within the So and SPL.
Finally, proliferation and apoptosis assays were carried out in Pbx1/2Mut and Pbx1/Emx2Mut embryos (see Fig. S5 in the supplementary material; data not shown). No marked differences in cell proliferation, as assessed by in vivo BrdU incorporation, and in apoptosis, as revealed by anti-caspase 3 immunohistochemistry, were identified in pre-scapular domains of Pbx1/2Mut versus WT embryos from E9.5 to E11.5 (see Fig. S5A in the supplementary material; data not shown). Similarly, there was no conspicuous increase in apoptosis in Pbx1/Emx2Mut embryos (data not shown). By contrast, Pbx1/Emx2Mut embryos displayed reductions in cell proliferation compared with WT (see Fig. S5B in the supplementary material), and more marked reductions were observed in Emx2–/– embryos, which lack the blade altogether (Pellegrini et al., 2001).
Emx2 and Pbx1 can form stable DNA-bound, transcriptionally active heterodimers
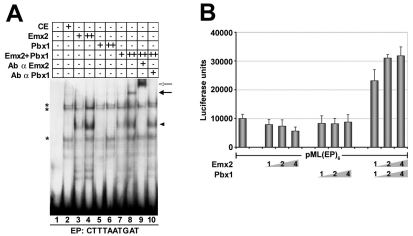
Given the Emx2 and Pbx1 genetic interaction, we determined the as yet unexplored optimal DNA-binding consensus sequence for a putative Emx2-Pbx1 heterodimer. An oligonucleotide containing random nucleotides in the five positions 5′ to the Pbx1 TGAT core consensus binding sequence (Lu et al., 1995; Van Dijk et al., 1993), along with purified Emx2 and Pbx1 proteins, were used in site-selection experiments (see Materials and methods). Emx2 and Pbx1 proved to bind DNA as a heterodimer, preferentially to sites matching the consensus sequence 5′-CTTTAATGAT-3′ (hereafter, the `EP' binding site) (not shown). To confirm that Emx2 and Pbx1 cooperatively bind EP, EMSAs were performed using whole-cell extracts from COS cells exogenously expressing Emx2 or Pbx1, alone or in combination. Extracts containing both Emx2 and Pbx1 showed a retarded band (Fig. 6A, lane 8) that was supershifted or abolished by the addition of an anti-Emx2 or anti-Pbx1 antibody, respectively (Fig. 6A, lanes 9 and 10). Whereas Pbx1 alone did not bind the EP site (lanes 5 and 6), Emx2 alone formed a faster-migrating shifted complex (lanes 3 and 4).
Fig. 6.
Pbx and Emx2 physically interact and cooperatively regulate transcription in cell culture. (A) EMSA using an oligonucleotide representing the consensus binding sequence of Emx2-Pbx1 heterodimers (EP site). Whole-cell extracts (20 and 40 μg) were used from COS cells transiently transfected with expression vectors for Emx2 and/or Pbx1. Co-expressed Emx2 and Pbx1 led to the formation of a retarded band (black arrow, lane 8) that was supershifted (white arrow) or abolished by the addition of an anti-Emx2 (lane 9) or anti-Pbx1 (lane 10) antibody, respectively. CE, control whole-cell extract of non-transfected COS cells. Black arrowhead indicates binding of Emx2 alone to the probe. Asterisks indicate non-specific complexes. (B) Luciferase activity, in arbitrary units, assayed from P19 cell extracts transiently transfected with the pML(EP)6 reporter, together with Pbx1 and/or Emx2 expression constructs. Numbers indicate amounts (μg) of transfected expression constructs. Bars represent mean luciferase activity ± s.e.m. of at least five independent experiments.
To test whether Emx2 and Pbx1 activate transcription via the EP site, a luciferase reporter construct containing six EP copies upstream of the adenovirus major late (AdML) minimal promoter [pML(EP)6] (Fig. 6B) was transiently transfected into P19 embryonal carcinoma cells with increasing amounts of Emx2 and/or Pbx1 expression constructs. Whereas Emx2 or Pbx1 alone could not activate transcription via EP, their co-expression led to a significant stimulation above reporter basal activity (Fig. 6B), indicating that they physically interact to form DNA-bound heterodimers on specific DNA sequences and cooperatively activate transcription.
Emx2 and Pbx1 bind the Alx1 locus in vivo and cooperatively activate transcription via a conserved Alx1 upstream element
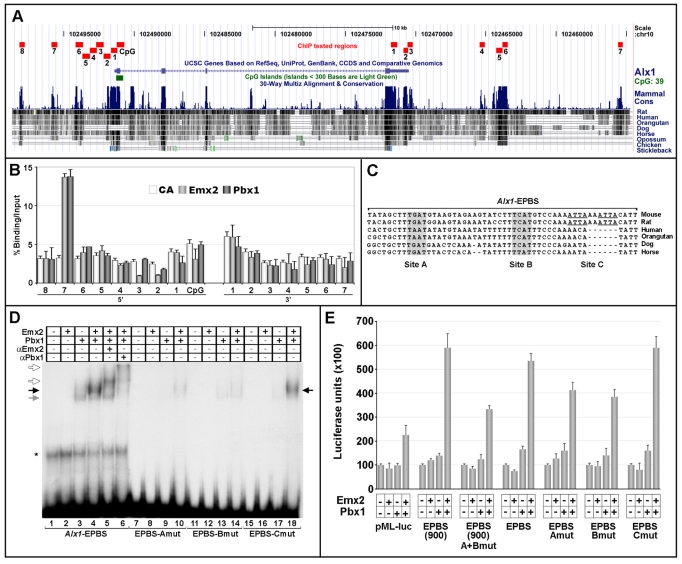
As Emx2 and Pbx1 act upstream of Alx1 (Fig. 5A, left), we examined whether Alx1 transcription is directly regulated by Emx2 and/or Pbx1. We searched for EP consensus sequences within a region spanning from ∼10 kb 5′ to the putative Alx1 transcription start site (TSS) to ∼20 kb 3′ to its polyadenylation signal (PAS), using MatInspector (Cartharius et al., 2005; Quandt et al., 1995). A binding site matrix representing the EP consensus was generated using MatInd (Cartharius et al., 2005), based on our site-selection results (not shown). Because an evolutionarily conserved motif similar to the EP consensus was not identified within this region, we determined whether Emx2 and/or Pbx1 were bound in vivo to putative Alx1 regulatory sequences. ChIP was performed on sixteen genomic regions surrounding Alx1 exons, chosen because of their evolutionary conservation and gene proximity (Fig. 7A). Nine regions were 5′ to the Alx1 TSS, with the most upstream (5′-8) 9 kb away (Fig. 7A), and seven were 3′ to the Alx1 PAS, with the most downstream (3′-7) 16 kb away (Fig. 7A). ChIP on E11.5 WT mouse proximal forelimb and flank chromatin using anti-Pbx and anti-Emx2 antibodies revealed that only region 5′-7 (Fig. 7A,B), comprising –4472 to –4075 from the TSS, displayed significant enrichments for both Emx2- and Pbx1-immunoprecipitated chromatin (Fig. 7B). This 400 bp region and its conserved flanking sequences were analyzed by EMSA for binding by Emx2 and Pbx1 (not shown). Only a highly conserved 54 bp sequence overlapping the 3′ end of this region showed significant binding (Alx1-EPBS, Fig. 7C). The Alx1-EPBS sequence was bound weakly by Pbx1 alone and not by Emx2 alone (Fig. 7D, left, lanes 2 and 3). However, addition of both Emx2 and Pbx1 led to the formation of a retarded band (Fig. 7D, lane 4) that was supershifted or abolished by an anti-Emx2 or anti-Pbx1 antibody, respectively (lanes 5 and 6), demonstrating that Emx2 and Pbx1 cooperatively bind Alx1-EPBS. The Alx1-EPBS sequence contains a conserved TGAT motif (Site A) representing the Pbx1 binding consensus, a conserved TCAT motif (Site B) found within the EP site (ATCATTAAAG, complementary strand), and two non-conserved ATTA motifs (Site C). We mutated (see Materials and methods) these three putative core binding motifs (EPBS-Amut, EPBS-Bmut and EPBS-Cmut, Fig. 7D) and tested them in EMSAs. Mutation of TGAT (Amut, lanes 7-10) or TCAT (Bmut, lanes 11-14) motifs strongly reduced Pbx1-Emx2 cooperative binding, whereas mutation of the ATTA motifs (Cmut, lanes 15-18) only weakly reduced the formation of the Pbx1-Emx2 retarded complex.
Fig. 7.
Pbx and Emx2 bind in vivo and cooperatively regulate transcription at a putative Alx1 regulatory element. (A) Interspecies comparison of genomic sequences spanning ∼25 kb around Alx1. Alignments to chr10:102498500-102455000 mouse genomic sequences (July 2007/mm9 assembly) were performed using the UCSC Genome Browser (Kent et al., 2002). The conserved genomic regions analyzed by ChIP, their sizes and positions are indicated in red. (B) ChIP of Emx2 or Pbx binding to genomic sequences spanning Alx1. The graph represents the average enrichment of control antibody (white), Emx2-bound (light gray) or Pbx-bound (dark gray) Alx1 genomic regions, as assessed by semi-quantitative PCR. Values indicate the percentage ratio between DNA amounts in immunoprecipitates (IP) and input chromatin. Error bars represent the mean percentage IP/input ratio ± s.e.m. of at least three independent ChIPs for each sample. (C) Interspecies alignment of a 54 bp sequence (Alx1-EPBS) within the Alx1 5′-7 region containing possible Emx2-Pbx binding sites. TGAT (Site A) and TCAT (Site B) core motifs are in gray. Two non-conserved ATTA motifs (Site C) are underlined. (D) Emx2 and Pbx1 cooperatively bind the Alx1-EPBS sequence via the TGAT and TCAT motifs. EMSA using the Alx1-EPBS, or the EPBS-Amut, EPBS-Bmut and EPBS-Cmut oligonucleotides as probes. Black arrow indicates the Emx2-Pbx1 heterodimer; gray arrow indicates a retarded complex formed by Pbx1 alone; white arrows indicate the supershifted Emx2-Pbx1 complexes. (E) Luciferase activity, in arbitrary units, assayed from P19 cells transiently transfected with the pMLAlx1-EPBS(900) [EPBS(900)] or pMLAlx1-EPBS (EPBS) reporters, or their mutated derivatives [EPBS(900)A+Bmut, EPBS-Amut, EPBS-Bmut, EPBS-Cmut] together with Pbx1 and/or Emx2 expression constructs. Bars represent mean luciferase activity ± s.e.m. of at least four independent experiments.
To test whether Pbx1 and/or Emx2 activated transcription via the Alx1-EPBS sequence, we generated luciferase reporter constructs by inserting a 974 bp fragment spanning region 5′-7 (Fig. 7A) including the Alx1-EPBS sequence [EPBS(900), Fig. 7E], or a 130 bp fragment that exclusively encompasses the Alx1-EPBS sequence (EPBS, Fig. 7E), upstream of the AdML promoter. These reporter constructs were transfected together with Emx2 and/or Pbx1 expression constructs into P19 cells. Whereas Emx2 and Pbx1 alone did not appreciably stimulate the EPBS(900), or the EPBS, reporter activity, their co-expression led to an increase in EPBS(900) (∼5.9-fold) and EPBS (∼5.4-fold) reporter activity (Fig. 7E). We then generated reporter constructs carrying the same mutations tested in EMSAs within the single Site A (EPBS-Amut), Site B (EPBS-Bmut) or Site C (EPBS-Cmut), and a compound mutation of Sites A and B [EPBS(900)A+Bmut] (Fig. 7E). Whereas the mutation of Site C did not cause any reduction in reporter transactivation by Emx2 and Pbx1, the mutation of Site A or Site B caused a decrease in reporter transactivation (Fig. 7E). A further decrease in Emx2-Pbx1-mediated transcriptional activation was caused by the compound mutation of Sites A and B (Fig. 7E). These results indicate that both Sites A and B are necessary for the assembly of a DNA-bound Emx2-Pbx1 complex and for full transcriptional activation by Emx2-Pbx1 via the Alx1-EPBS sequence, whereas Site C is dispensable.
DISCUSSION
Pbx genes control scapular blade and head development
In dermomyotome, in which Pbx3 is not expressed, the compound absence of Pbx1 and Pbx2 does not alter the expression of scapular blade-patterning genes (e.g. Gli3, Alx4). Of note, although we previously reported rostral shifts in Hox gene expression in Pbx1/2Mut paraxial mesoderm (Capellini et al., 2006), no known single or compound Hox mutant displays blade defects. Only Hox5 mutants exhibit rostral blade shifts, with normal blade morphology (Rancourt et al., 1995). Thus, we cannot yet invoke a mechanism whereby loss of Pbx proteins, as Hox co-factors, leads to the blade phenotypes of Pbx compound mutants via altered Hox function.
As in dermomyotome, the expression of Pbx1-3 does not colocalize in somatopleure. However, they do show overlapping expression in limb mesoderm (Fig. 1A,C). Indeed, Pbx1 and Pbx2 show the most extensive overlapping expression in E8.0-10.5 LPM and proximal limbs (Fig. 1C) (Capellini et al., 2006), whereas Pbx1 and Pbx3 colocalize only in proximal-anterior limbs after E9.5 and within somatopleure after E11.0 (Fig. 1) (Di Giacomo et al., 2006). Accordingly, blade defect severity in Pbx1/2Mut significantly exceeds that of Pbx1/3Mut embryos, and is supported by the accentuated reduction in blade-patterning gene expression in Pbx1/2Mut embryos (see below). These findings indicate that Pbx2 is more crucial than Pbx3 in scapular development, probably because it shares greater spatiotemporal domains with Pbx1, or is expressed at higher levels.
Importantly, most girdle and LPM limb derivatives are hypoplastic and malformed in compound Pbx mutants. Pbx1/2Mut and Pbx1/3Mut scapulae also exhibit markedly abnormal head morphologies. Analyses of known molecular markers of the head (Hoxc6) and acromion (Pax1) revealed that they are spatially diffused and reduced in these mutants, although they are still present in domains that lack detectable changes in cell proliferation and apoptosis. Furthermore, as Pax1 and Hoxc6 are reduced in their domains, other mesodermal genes such as Tbx15 are upregulated. These findings corroborate our previous reports on the disruption of Hox expression in Pbx1/2Mut forelimbs (Capellini et al., 2006). For example, we reported the upregulation of Hoxa9 and Hoxd9, genes that are involved in the growth and patterning of the stylopod in Pbx1/2Mut forelimbs (Capellini et al., 2006). Along with spatial expansions of other 3′ Hox genes (i.e. Hoxc6), such data support our finding of potential scapular head/humeral head duplications in these mutants (Fig. 8B). Thus, the hierarchical role of Pbx factors in Hox gene expression and/or their role as Hox co-factors in the LPM are likely mechanisms for assigning positional information to head progenitors.
Lastly, our results show that whereas Pbx1;Pbx2 and Pbx1;Pbx3 mutants exhibit severe blade and head defects, Pbx2;Pbx3 mutants are normal. Therefore, in light of the overlapping functions of Pbx genes in development (Capellini et al., 2006; Capellini et al., 2008; Stankunas et al., 2008), we establish that the dosage of Pbx1 is paramount also in scapula morphogenesis.
Pbx factors hierarchically control genes crucial for scapular blade development including Emx2, which, in turn, regulates blade progenitor cell proliferation
In mice, Emx2–/– embryos do not initiate mesenchymal condensation in pre-blade domains (Pellegrini et al., 2001), and in chick Emx2 functions upstream of Sox9 (Huang et al., 2006). Emx2 has thus been considered to maintain the positional identity of cells fated to form the blade (Bi et al., 2001; Prols et al., 2004). Here, we reveal that Emx2 controls cell proliferation in the pre-blade mesenchyme. Thus, the reduction of Sox9 and absence of the blade observed in Emx2–/– mutants (Pellegrini et al., 2001) might be partially due to this perturbed cellular behavior.
In Pbx1/2Mut forelimbs, Emx2 expression was severely reduced (Fig. 1B) and Sox9 expression was diminished across the pre-blade region, as shown by OPT (Fig. 3). Similar, although not as extreme, reductions were observed in Pbx1/3Mut domains (Fig. 3). These data suggest that Pbx genes cooperate and genetically regulate Emx2 (and Sox9). Interestingly, unlike Emx2–/– embryos, Pbx1/2Mut embryos did not exhibit proliferation defects. It remains unclear how Emx2 dosage influences cell proliferation, as Emx2+/– embryos retain normal cell proliferation (data not shown) and intact blades. Similarly, Emx2, although reduced in Pbx1/2Mut embryos, might still be present at sufficient levels to sustain cell division. Alternatively, the compound absence of all three Pbx genes might be required for the complete loss of Emx2 expression (Fig. 8B) and reduction of cell proliferation.
Pbx genes also control a second pathway involved in blade patterning, which includes Alx1/Alx4, Gli3, and Tbx15, genes that have specific roles in patterning the superior, central, and inferior blade domains, respectively (Fig. 8B) (Kuijper et al., 2005). From E10-11, they become co-expressed with Emx2 in the proximal limb and somatopleure, within a region fated to form the scapula (Huang et al., 2006). A seminal study (Kuijper et al., 2005) demonstrated that blade patterning is sensitive to gene dosage, with the most extreme reductions occurring when Tbx15 loss is coupled with reduced dosage of either at least one gene crucial for inferior blade patterning (i.e. Gli3), or of at least two genes essential for superior blade patterning (e.g. Alx1 and Alx4). We observed that the expression of these genes in Pbx1–/–, Pbx1/2Mut and Pbx1/3Mut embryos is disrupted proportionally to the severity of blade defects in each respective mutant and that such abnormalities occur without detectable perturbations in cell proliferation or apoptosis (Fig. 8A). Accordingly, at E10.5, the expression of all known blade-patterning genes was reduced to nearly undetectable levels in Pbx1/2Mut, whereas only a subset of these genes (Alx1, Alx4 and Gli3) was altered in Pbx1/3Mut, and, lastly, only Alx1 was modestly reduced in Pbx1–/– mutants (Fig. 5). Of interest, Pbx1/3Mut embryos additionally exhibit foramina, a phenotype resembling that reported in extra toes (Gli3) (Hui and Joyner, 1993; Johnson, 1967; Schimmang et al., 1992) and polycomb gene M33–/– (Cbx2 – Mouse Genome Informatics) (Core et al., 1997) mutant mice. Accordingly, Pbx genes have been shown to mediate polycomb expression in axial mesodermal tissues (Capellini et al., 2008).
Pbx1 is an interaction partner of Emx2 in scapular blade development
Our studies reveal that, unlike Pbx1-3, whose domains of genetic interaction include blade and head regions, Pbx1 and Emx2 interaction is restricted to the blade. Compound Pbx1+/–;Emx2+/– and Pbx1–/–;Emx2+/– (Pbx1/Emx2Mut) mutants exhibit novel blade defects (i.e. proximal indentations and blade bifurcations) that are absent from single Pbx1+/–, Pbx1–/–, Emx2+/– or Emx2–/– embryos (Fig. 2) and are likely to arise from reductions in Sox9-positive cells along the vertebral border of the blade. These defects are partially mediated by Emx2 control of cell proliferation, as documented in blade-forming domains of Pbx1/Emx2Mut embryos. Although one copy of Emx2 is present in the latter genotype and Pbx1–/– embryos lack changes in Emx2 expression, decreased levels of Emx2 might be sufficient to cause proliferation defects. Thus, the bifurcation and substantial blade loss in Pbx1/Emx2Mut embryos might be a partial consequence of reduced mesenchymal cell number and condensations.
Importantly, analyses of Pbx1/Emx2Mut embryos also showed that Pbx1 and Emx2 cooperatively control genes upstream of superior blade patterning (Alx1 and Alx4) (Fig. 8B). Concomitantly, they might govern genes patterning other blade domains, as Emx2–/– and compound Pbx1/2Mut embryos lack Tbx15 expression (Fig. 5; see Fig. S3 in the supplementary material). However, owing to in utero lethality of Pbx1–/–;Pbx2+/– mutants, the generation of more complex Pbx1-3;Emx2 genotypes was impossible.
We also uncovered that Pbx1 and Emx2 heterodimerize at specific DNA sequences. Indeed, the Emx2 homeodomain YPWL motif (Bürglin, 1994), which is identical to part of the `hexapeptide' required for Hox-Pbx interaction (reviewed by Moens and Selleri, 2006), is likely to mediate Emx2-Pbx1 heterodimerization. We found that the Emx2-Pbx1 heterodimer preferentially binds the CTTTAATGAT sequence (EP site), within which TGAT represents the Pbx1 half-site (Lu et al., 1995; Van Dijk et al., 1993). Interestingly, the tandem half-site configuration bound by Emx2-Pbx1 differs from that adopted by Hox-Pbx heterodimers, whereby Hox occupies a half-site 3′ to the TGAT Pbx1 binding sequence (Chang et al., 1996). This difference is not due to a TGAT position bias in the oligonucleotide used in site selection because a similar oligonucleotide, containing random nucleotides at five positions 3′ to the TGAT, did not promote Emx2-Pbx1 heterodimer binding (not shown).
Alx1, a regulator of scapular blade formation, is a target of Pbx and Emx2 factors
Among the aristaless-related genes involved in scapular development, Alx1 has a pre-eminent function (Kuijper et al., 2005). Expression of Alx1, loss-of-function of which results in superior blade defects (Kuijper et al., 2005), was undetectable in Pbx1/2Mut, Pbx1/3Mut and Pbx1/Emx2Mut embryos (Fig. 5A, left). Alx1 loss occurred in the absence of cell proliferation and apoptosis defects in Pbx1/2Mut embryos, whereas it was accompanied by a decrease in cell proliferation in Pbx1/Emx2Mut embryos. Interestingly, in Emx2–/– embryos, which exhibit blade loss and a marked decrease in cell proliferation, part of the Alx1 expression territory was maintained. Overall, these data strongly suggest that Pbx and Emx2 factors are required for Alx1 transcription. Indeed, we identified a conserved element (Alx1 5′-7) upstream of the putative Alx1 TSS that is bound in vivo by Emx2-Pbx1, suggesting that both proteins directly control Alx1 expression in pre-scapular domains. Although the possibility cannot be excluded that other sites more distant from Alx1 might recruit Emx2 and/or Pbx1, we did not detect additional binding by Emx2-Pbx1 within the 16 evolutionarily conserved regions that were analyzed, covering more than 25 kb around Alx1. Interestingly, the Alx1 5′-7 element lacks a DNA sequence identical to the EP site, but displays two nearby conserved motifs that are both required for cooperative Emx2-Pbx1 binding. Of these, one contains the TGAT Pbx half-site (Site A), and the other a TCAT motif (Site B), which is also present within the selected EP site (complementary strand). Additionally, other predicted Pbx1 and Emx2 sites within the conserved element are not bound by their respective proteins as assayed by EMSA. The Alx1-EPBS element furthermore mediated transactivation in transient transfections of cultured cells only when Emx2 and Pbx1 were co-expressed. Interestingly, whereas mutation of the TGAT (Site A) or the TCAT (Site B) motif alone within the Alx1-EPBS element caused a discrete decrease in Emx2-Pbx1-mediated transactivation, only the compound mutation of both motifs significantly reduced reporter activity, underscoring that both Sites A and B are necessary for full transcriptional activation by Emx2-Pbx1.
In conclusion, here we have addressed the hierarchical control of scapula development by Pbx genes and their interaction with Emx2. We have uncovered that Pbx genes act upstream of head development and control genetic pathways underlying blade formation (Fig. 8B) (Kuijper et al., 2005). Importantly, we have established that Pbx genes hierarchically control Emx2 and blade mesenchymal condensation, and that Pbx1 and Emx2 genetically and physically interact in blade patterning. Lastly, by highlighting a direct synergistic regulation of Alx1 by Pbx1-Emx2, we have delineated a novel genetic network underlying shoulder girdle development.
Supplementary Material
Acknowledgments
We are grateful to Matt Koss for apoptosis assays; Michael Cleary for anti-Pbx antibodies; Mouse Genetics Community researchers for in situ probes; and Liz Lacy and Ann Foley for discussions. T.D.C. was the recipient of the CUNY Carole and Morton Olshan Fellowship. Work was supported by grants from the NIH (2R01HD043997-06 [ARRA Suppl], 3R21DE018031-02S1 and 1R01HD061403-01 to L.S.); March of Dimes and Birth Defects Foundation (#6-FY03-071 to L.S.); and the Italian Telethon, ASM Foundation, and the Italian Association for Cancer Research to V.Z. L.S. is an Irma T. Hirschl Scholar, a recipient of grants from The Alice Bohmfalk Trust and The Frueauff Foundation, and a recipient of a research award for `Cleft Lip/Palate and Craniofacial Anomalies' from the Cleft Palate Foundation. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.048819/-/DC1
References
- Aubin J., Lemieux M., Tremblay M., Berard J., Jeannotte L. (1997). Early postnatal lethality in Hoxa-5 mutant mice is attributable to respiratory tract defects. Dev. Biol. 192, 432-445 [DOI] [PubMed] [Google Scholar]
- Aubin J., Lemieux M., Tremblay M., Behringer R. R., Jeannotte L. (1998). Transcriptional interferences at the Hoxa4/Hoxa5 locus: importance of correct Hoxa5 expression for the proper specification of the axial skeleton. Dev. Dyn. 212, 141-156 [DOI] [PubMed] [Google Scholar]
- Aubin J., Lemieux M., Moreau J., Lapointe J., Jeannotte L. (2002). Cooperation of Hoxa5 and Pax1 genes during formation of the pectoral girdle. Dev. Biol. 244, 96-113 [DOI] [PubMed] [Google Scholar]
- Beverdam A., Meijlink F. U. (2001). Expression patterns of group-I aristaless-related genes during craniofacial and limb development. Mech. Dev. 107, 163-167 [DOI] [PubMed] [Google Scholar]
- Bi W., Huang W., Whitworth D. J., Deng J. M., Zhang Z., Behringer R. R., de Crombrugghe B. (2001). Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc. Natl. Acad. Sci. USA 98, 6698-6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendolan A., Ferretti E., Salsi V., Moses K., Quaggin S., Blasi F., Cleary M. L., Selleri L. (2005). A Pbx1-dependent genetic and transcriptional network regulates spleen ontogeny. Development 132, 3113-3126 [DOI] [PubMed] [Google Scholar]
- Bürglin T. R. (1994). A comprehensive classification of homeobox genes. Guidebook to the Homeobox Genes (ed. D. Duboule), pp.25-72 Oxford, UK: Oxford University Press; [Google Scholar]
- Capellini T. D., Di Giacomo G., Salsi V., Brendolan A., Ferretti E., Srivastava D., Zappavigna V., Selleri L. (2006). Pbx1/Pbx2 requirement for distal limb patterning is mediated by the hierarchical control of Hox gene spatial distribution and Shh expression. Development 133, 2263-2273 [DOI] [PubMed] [Google Scholar]
- Capellini T. D., Zewdu R., Di Giacomo G., Asciutti S., Kugler J. E., Di Gregorio A., Selleri L. (2008). Pbx1/Pbx2 govern axial skeletal development by controlling Polycomb and Hox in mesoderm and Pax1/Pax9 in sclerotome. Dev. Biol. 321, 500-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartharius K., Frech K., Grote K., Klocke B., Haltmeier M., Klingenhoff A., Frisch M., Bayerlein M., Werner T. (2005). MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21, 2933-2942 [DOI] [PubMed] [Google Scholar]
- Chalepakis G., Fritsch R., Fickenscher H., Deutsch U., Goulding M., Gruss P. (1991). The molecular basis of the undulated/Pax-1 mutation. Cell 66, 873-884 [DOI] [PubMed] [Google Scholar]
- Chang C.-P., Shen W.-F., Rozenfeld S., Lawrence H. J., Largman C., Cleary M. (1995). Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox proteins. Genes Dev. 9, 663-674 [DOI] [PubMed] [Google Scholar]
- Chang C.-P., Brocchieri L., Shen W.-F., Largman C., Cleary M. (1996). Pbx modulation of Hox homeodomain amino-terminal arms establishes different DNA-binding specificities across the Hox locus. Mol. Cell. Biol. 16, 1734-1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core N., Bel S., Gaunt S. J., Aurrand-Lions M., Pearce J., Fisher A., Djabali M. (1997). Altered cellular proliferation and mesoderm patterning in Polycomb-M33-deficient mice. Development 124, 721-729 [DOI] [PubMed] [Google Scholar]
- Davidson D., Graham E., Sime C., Hill R. (1988). A gene with sequence similarity to Drosophila engrailed is expressed during the development of the neural tube and vertebrae in the mouse. Development 104, 305-316 [DOI] [PubMed] [Google Scholar]
- Depew M. J., Liu J. K., Long J. E., Presley R., Meneses J. J., Pedersen R. A., Rubenstein J. L. (1999). Dlx5 regulates regional development of the branchial arches and sensory capsules. Development 126, 3831-3846 [DOI] [PubMed] [Google Scholar]
- Di Giacomo G., Koss M., Capellini T. D., Brendolan A., Popperl H., Selleri L. (2006). Spatio-temporal expression of Pbx3 during mouse organogenesis. Gene Expr. Patterns 6, 747-757 [DOI] [PubMed] [Google Scholar]
- Di Rocco G., Mavilio F., Zappavigna V. (1997). Functional dissection of a transcriptionally active, target-specific Hox-Pbx complex. EMBO J. 16, 3644-3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rocco G., Gavalas A., Popperl H., Krumlauf R., Mavilio F., Zappavigna V. (2001). The recruitment of SOX/OCT complexes and the differential activity of HOXA1 and HOXB1 modulate the Hoxb1 auto-regulatory enhancer function. J. Biol. Chem. 276, 20506-20515 [DOI] [PubMed] [Google Scholar]
- Fromental-Ramain C., Warot X., Lakkaraju S., Favier B., Haack H., Birling C., Dierich A., Doll E. P., Chambon P. U. (1996). Specific and redundant functions of the paralogous Hoxa-9 and Hoxd-9 genes in forelimb and axial skeleton patterning. Development 122, 461-472 [DOI] [PubMed] [Google Scholar]
- Garcia-Gasca A., Spyropoulos D. D. (2000). Differential mammary morphogenesis along the anteroposterior axis in Hoxc6 gene targeted mice. Dev. Dyn. 219, 261-276 [DOI] [PubMed] [Google Scholar]
- Huang R., Zhi Q., Patel K., Wilting J., Christ B. (2000). Dual origin and segmental organisation of the avian scapula. Development 127, 3789-3794 [DOI] [PubMed] [Google Scholar]
- Huang R., Christ B., Patel K. (2006). Regulation of scapula development. Anat. Embryol. 211, 65-71 [DOI] [PubMed] [Google Scholar]
- Hui C. C., Joyner A. L. (1993). A mouse model of greig cephalopolysyndactyly syndrome: the extra-toes mutation contains an intragenic deletion of the Gli3 gene. Nat. Genet. 3, 241-246 [DOI] [PubMed] [Google Scholar]
- Johnson D. R. (1967). Extra-toes: a new mutant gene causing multiple abnormalities in the mouse. J. Embryol. Exp. Morphol. 17, 543-581 [PubMed] [Google Scholar]
- Kent W. J., Sugnet C. W., Furey T. S., Roskin K. M., Pringle T. H., Zahler A. M., Haussler D. (2002). The human genome browser at UCSC. Genome Res. 12, 996-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper S., Beverdam A., Kroon C., Brouwer A., Candille S., Barsh G., Meijlink F. U. (2005). Genetics of shoulder girdle formation: roles of Tbx15 and aristaless-like genes. Development 132, 1601-1610 [DOI] [PubMed] [Google Scholar]
- Lu Q., Knoepfler P., Scheele J., Wright D., Kamps M. (1995). Pbx1 and E2A-Pbx1 bind the DNA motif ATCAATCAA cooperatively with the products of multiple murine Hox genes, some of which are themselves oncogenes. Mol. Cell. Biol. 15, 3786-3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T., Ahlberg P. E., Kessaris N., Iannarelli P., Dennehy U., Richardson W. D., McMahon A. P., Koentges G. (2005). Neural crest origins of the neck and shoulder. Nature 436, 347-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod M. (1980). Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 22, 299-301 [DOI] [PubMed] [Google Scholar]
- Moens C. B., Selleri L. (2006). Hox cofactors in vertebrate development. Dev. Biol. 291, 193-206 [DOI] [PubMed] [Google Scholar]
- Oliver G., De Robertis E. M., Wolpert L., Tickle C. (1990). Expression of a homeobox gene in the chick wing bud following application of retinoic acid and grafts of polarizing region tissue. EMBO J. 9, 3093-3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M., Pantano S., Fumi M. P., Lucchini F., Forabosco A. U. (2001). Agenesis of the scapula in Emx2 homozygous mutants. Dev. Biol. 232, 149-156 [DOI] [PubMed] [Google Scholar]
- Prahlad K. V., Skala G., Jones D. G., Briles W. E. (1979). Limbless: a new genetic mutant in the chick. J. Exp. Zool. 209, 427-434 [DOI] [PubMed] [Google Scholar]
- Prols F., Ehehalt F., Rodriguez-Niedenfuhr M., He L., Huang R., Christ B. U. (2004). The role of Emx2 during scapula formation. Dev. Biol. 275, 315-324 [DOI] [PubMed] [Google Scholar]
- Quandt K., Frech K., Karas H., Wingender H., Werner T. (1995). MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 23, 4878-4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancourt D. E., Tsuzuki T., Capecchi M. R. (1995). Genetic interaction between hoxb-5 and hoxb-6 is revealed by nonallelic noncomplementation. Genes Dev. 9, 108-122 [DOI] [PubMed] [Google Scholar]
- Rhee J. W., Arata A., Selleri L., Jacobs Y., Arata S., Onimaru H., Cleary M. L. (2004). Pbx3 deficiency results in central hypoventilation. Am. J. Pathol. 165, 1343-1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros M. A., Lopez-Martinez A., Simandl B. K., Rodriguez C., Izpisua Belmonte J. C., Dahn R., Fallon J. F. (1996). The limb field mesoderm determines initial limb bud anteroposterior asymmetry and budding independent of sonic hedgehog or apical ectodermal gene expressions. Development 122, 2319-2330 [DOI] [PubMed] [Google Scholar]
- Salsi V., Zappavigna V. (2006). Hoxd13 and Hoxa13 directly control the expression of the EphA7 ephrin tyrosine kinase receptor in developing limbs. J. Biol. Chem. 281, 1992-1999 [DOI] [PubMed] [Google Scholar]
- Salsi V., Vigano M. A., Cocchiarella F., Mantovani R., Zappavigna V. (2008). Hoxd13 binds in vivo and regulates the expression of genes acting in key pathways for early limb and skeletal patterning. Dev. Biol. 317, 497-507 [DOI] [PubMed] [Google Scholar]
- Schimmang T., Lemaistre M., Vortkamp A., Ruther U. (1992). Expression of the zinc finger gene Gli3 is affected in the morphogenetic mouse mutant extra-toes (Xt). Development 116, 799-804 [DOI] [PubMed] [Google Scholar]
- Selleri L., Depew M. J., Jacobs Y., Chanda S. K., Tsang K. Y., Cheah K. S., Rubenstein J. L., O_Gorman S., Cleary M. L. (2001). Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development 128, 3543-3557 [DOI] [PubMed] [Google Scholar]
- Selleri L., DiMartino J., van Deursen J., Brendolan A., Sanyal M., Boon E., Capellini T., Smith K. S., Rhee J., Popperl H., et al. (2004). The TALE homeodomain protein Pbx2 is not essential for development and long-term survival. Mol. Cell. Biol. 24, 5324-5331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe J. (2003). Optical projection tomography as a new tool for studying embryo anatomy. J. Anat. 202, 175-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe J., Ahlgren U., Perry P., Hill B., Ross A., Hecksher-Sorensen J., Baldock R., Davidson D. (2002). Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science 296, 541-545 [DOI] [PubMed] [Google Scholar]
- Sharpe P. T., Miller J. R., Evans E. P., Burtenshaw M. D., Gaunt S. J. (1988). Isolation and expression of a new mouse homeo-box gene. Development 102, 397-407 [DOI] [PubMed] [Google Scholar]
- Stankunas K., Shang C., Twu K. Y., Kao S. C., Jenkins N. A., Copeland N. G., Sanyal M., Selleri L., Cleary M. L., Chang C. P. (2008). Pbx/Meis deficiencies demonstrate multigenetic origins of congenital heart disease. Circ. Res. 103, 702-709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil T., Alvarez-Bolado G., Walter A., Ruther U. (1999). Gli3 is required for Emx gene expression during dorsal telencephalon development. Development 126, 3561-3571 [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673-4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D., Lev S. (2002). Cellular and developmental distribution of human homologues of the Drosophilia rdgB protein in the rat retina. Invest. Ophthalmol. Vis. Sci. 43, 1946-1953 [PubMed] [Google Scholar]
- Timmons P. M., Wallin J., Rigby P. W., Balling R. (1994). Expression and function of Pax 1 during development of the pectoral girdle. Development 120, 2773-2785 [DOI] [PubMed] [Google Scholar]
- Van Dijk M. A., Voorhoeve P. M., Murre C. (1993). Pbx1 is converted into a transcriptional activator upon acquiring the N-terminal region of E2A in pre-B-cell acute lymphoblastoid leukemia. Proc. Natl. Acad. Sci. USA 90, 6061-6065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., He L., Ehehalt F., Geetha-Loganathan P., Nimmagadda S., Christ B., Scaal M., Huang R. (2005). The formation of the avian scapula blade takes place in the hypaxial domain of the somites and requires somatopleure-derived BMP signals. Dev. Biol. 287, 11-18 [DOI] [PubMed] [Google Scholar]
- Wilm B., Dahl E., Peters H., Balling R., Imai K. (1998). Targeted disruption of Pax1 defines its null phenotype and proves haploinsufficiency 95, 8692-8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E., Hargrave M. R., Christiansen J., Cooper L., Kun J., Evans T., Gangadharan U., Greenfield A., Koopman P. (1995). The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat. Genet. 9, 15-20 [DOI] [PubMed] [Google Scholar]
- Zappavigna V., Sartori D., Mavilio F. (1994). Specificity of HOX protein function depends on DNA-protein and protein-protein interactions, both mediated by the homeo domain. Genes Dev. 8, 732-744 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.