Abstract
Background
The risk of tuberculosis (TB) in patients with rheumatoid arthritis (RA) is thought to be increased following anti-tumour necrosis factor (anti-TNF) therapy, with a proposed differential risk between the anti-TNF drugs etanercept (ETA), infliximab (INF) and adalimumab (ADA).
Objective
To compare directly the risk between drugs, to explore time to event, site of infection and the role of ethnicity.
Methods
Data from the British Society for Rheumatology Biologics Register (BSRBR), a national prospective observational study, were used to compare TB rates in 10 712 anti-TNF treated patients (3913 ETA, 3295 INF, 3504 ADA) and 3232 patients with active RA treated with traditional disease-modifying antirheumatic drugs.
Results
To April 2008, 40 cases of TB were reported, all in the anti-TNF cohort. The rate of TB was higher for the monoclonal antibodies ADA (144 events/100 000 person-years) and INF (136/100 000 person-years) than for ETA (39/100 000 person-years). After adjustment, the incidence rate ratio compared with ETA-treated patients was 3.1 (95% CI 1.0 to 9.5) for INF and 4.2 (1.4 to 12.4) for ADA. The median time to event was lowest for INF (5.5 months) compared with ETA (13.4 months) and ADA (18.5 months). 13/40 cases occurred after stopping treatment. 25/40 (62%) cases were extrapulmonary, of which 11 were disseminated. Patients of non-white ethnicity had a sixfold increased risk of TB compared with white patients treated with anti-TNF therapy.
Conclusion
The rate of TB in patients with RA treated with anti-TNF therapy was three- to fourfold higher in patients receiving INF and ADA than in those receiving ETA.
The introduction of anti-tumour necrosis factor (anti-TNF) therapy has significantly advanced the treatment of rheumatoid arthritis (RA). However, despite good efficacy, there have always been concerns about safety. A single case of tuberculosis (TB) was reported in the first anti-TNF randomised controlled trial.1 Since then, there has been accumulating evidence from spontaneous pharmacovigilance studies that anti-TNF therapy increases the risk of TB, with a possible differential risk between the three anti-TNF drugs: infliximab (INF) and adalimumab (ADA) (both monoclonal antibodies) having a higher risk than etanercept (ETA), a soluble TNF receptor.2–5 This proposed differential risk is supported by the report of multiple cases of TB in RA clinical trials and open-label extension studies of INF1 6–8 and ADA9–12 but only once within ETA publications.13
Although the published data suggest a differential risk between drugs, studies to date do not enable robust direct comparisons between drugs. The aim of this study was first, to compare directly the influence of the three licensed anti-TNF drugs upon the incidence of TB in patients with RA, and then to explore the magnitude of risk in anti-TNF treated patients compared with patients with RA treated with traditional disease modifying antirheumatic drug (DMARD) therapy.
Secondary aims included exploring the time to TB onset, the balance of pulmonary and extrapulmonary disease and the ethnicity of TB cases.
Methods
The subjects for this analysis were participating in a large national prospective observational study, the British Society for Rheumatology Biologics Register (BSRBR). The methods have been described in detail elsewhere.14 In brief, the study was established in 2001 in order to examine the long-term safety of biological drugs. UK national guidelines recommended that “any clinician prescribing these medications must (with the patient's permission) undertake to register the patient with the BSRBR and forward information on dosage, outcome and toxicity every 6 months”.15 Patients were recruited to the ETA and INF cohorts from 2001 onwards. Recruitment to the ADA cohort started later because of its more recent launch date. Recruitment targets of 4000 patients for the ETA cohort were met in 2005, for INF in 2007 and for ADA in 2008. Before recruitment targets were met, we estimated that >80% of anti-TNF-treated patients with RA in the UK were registered on the BSRBR.
Analysis was restricted to patients with a doctor's diagnosis of RA. All patients had to have at least one returned consultant follow-up questionnaire before 31 March 2008. The anti-TNF cohort comprised patients starting an anti-TNF drug as their first biological drug. A comparison cohort of biologic-naïve patients with active RA was recruited in parallel (see authorship list of the BSR Control Centre Consortium).14 These patients had active disease despite current treatment with a traditional DMARD and were biologic naïve.
Baseline assessment
Baseline information included demographics, disease duration, 28 swollen and tender joint counts, inflammatory markers and patient global assessment, which enables calculation of a Disease Activity Score (DAS28).16 Self-reported ethnicity was captured within the patient baseline questionnaire, then categorised as white or non-white. Details of all previous and current DMARD therapy were obtained, as well as smoking history, comorbidity and prior TB. Data on screening for latent TB were not requested. Patients completed a Health Assessment Questionnaire adapted for British use.17
Follow-up
Data on changes in treatment, disease activity and the occurrence of adverse events were captured in three ways: 6-monthly rheumatologist questionnaire, 6-monthly patient diary and by “flagging” with the UK Office for National Statistics which provided information on mortality, including cause of death. If active TB was reported from any source, further information, including site of infection and supporting evidence for diagnosis (clinical/radiological/microbiological/histopathological), was requested from the rheumatologist. Patient-reported cases of TB were only included in the analysis if later verified by a consultant.
Cases were categorised into “verified” or “unverified” TB by three clinicians, including a consultant in infectious diseases (WGD, JG and AU). Cases were “verified” if they were culture positive and/or acid fast bacillus smear positive, or TB was recorded on the death certificate. Cases of latent TB identified from pretreatment screening were not included. The site of infection was categorised as pulmonary (including pleural) or extrapulmonary. All cases of miliary or disseminated TB were categorised as extrapulmonary. Statistical analysis TB cases were attributed to anti-TNF therapy using two different models: “on drug” (if the patient was actively receiving that drug at the time of diagnosis) and “most recent drug”.18 Follow-up was censored at the most recently completed consultant follow-up or death, whichever came first. Patients contributed follow-up time to the “on drug” model only while they were actively receiving the drug (fig 1). The date of drug discontinuation was taken as the first missed dose. Patients could switch between different anti-TNF drugs and contribute person-years to more than one drug. Follow-up was not censored at the time of TB diagnosis. Patients could restart treatment following an episode of TB, either resuming prior treatment or switching drugs. Time following an episode of TB was included to estimate risk reflecting clinical practice. Sensitivity analyses were conducted excluding time after the first diagnosis of TB, and restricting analyses to the first anti-TNF drug.
Figure 1.
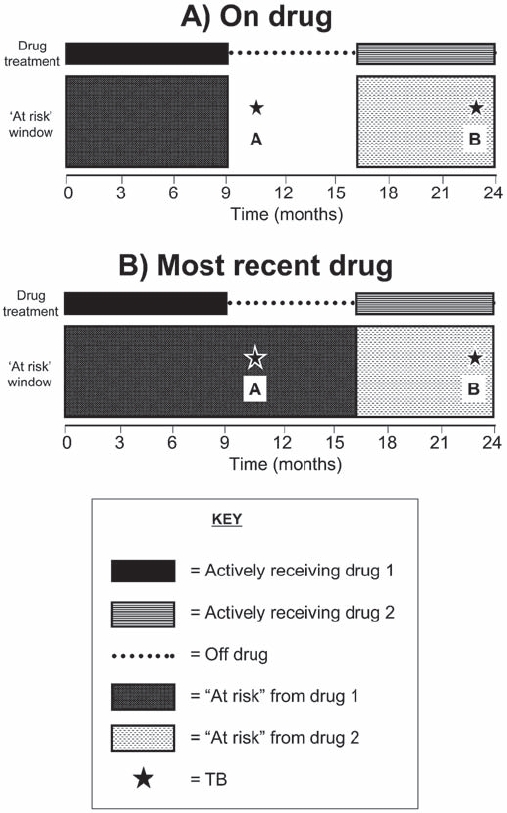
Models for attributing tuberculosis (TB) to drug treatment. (A) “On drug”. Patient-years and adverse events were attributed to each drug only while the patient was actively receiving that drug. Event A was not attributed to any drug, while event B was attributed to drug 2. (B) “Most recent drug”. Patient-years were accrued for each drug from the start date of that drug until the date of switching to the next anti-tumour necrosis factor drug, irrespective of drug discontinuation. Follow-up was censored at the most recently completed consultant follow-up or death, whichever came first, for all models.
Comparison patients also contributed person-years from their registration date until their most recent completed consultant follow-up or death, whichever came sooner. Patients in the comparison cohort who switched to an anti-TNF drug contributed person-years to the comparison cohort up to the date the anti-TNF drug was started and subsequent follow-up to the anti-TNF cohort. Conversely, patients initially registered in the anti-TNF cohort could not subsequently contribute person-years to the comparison cohort after stopping anti-TNF therapy.
Incidence rates of TB are presented as events/100 000 person-years with 95% confidence intervals (95% CI). Incidence rate ratios (IRRs) were calculated using Cox regression, comparing between anti-TNF drugs with ETA as the referent group. Comparison was then made between the anti-TNF cohort and the DMARD cohort. Adjustment was made for age, gender and calendar year of recruitment. In the absence of cases in the DMARD cohort, an “expected” number of cases was generated using indirect standardisation: assuming the DMARD cohort shared the same risk as the anti-TNF cohort, allowing for differences in age, gender and calendar year. Analyses examining the influence of ethnicity were performed after adjustment for age, gender and calendar year and after exclusion of patients with missing ethnicity data. Multiple variable regression was performed with additional confounders identified from an a priori list of possible confounders, including smoking, diabetes, chronic obstructive pulmonary disease/asthma, prior TB, disease severity (Health Assessment Questionnaire, DAS28 and disease duration as continuous variables), number of prior DMARDs, baseline steroid use, and methotrexate use (as a time-varying covariate). True confounders were identified by sequentially including each confounder in the regression model, and including in the multiple variable regression those confounders that individually changed the estimation after adjustment by more than 10%.19 All analysis was done using Stata 9.2 (StataCorp, College Station, Texas, USA).
Results
A total of 13 739 patients were included in the analysis: 3232 in the DMARD cohort and 10 712 in the anti-TNF cohort. Two hundred and five patients switched from the DMARD cohort to the anti-TNF cohort and contributed person-years to both cohorts. A total of 2552 (24%) patients received two anti-TNF drugs, and 416 (4%) received all three. Table 1 shows the baseline characteristics. The anti-TNF cohort was younger, comprised proportionally more women and, as expected, had more severe disease than the comparison cohort. Proportionally more patients treated with ETA had prior TB (2.5%) than those treated with INF or ADA (1.5%). Proportionally fewer in the DMARD cohort were of non-white origin.
Table 1.
Baseline characteristics
| First anti-TNF drug: | |||||||
|---|---|---|---|---|---|---|---|
| Characteristics | DMARD (n=3232)* | All anti-TNF (n=10 712)* | p Value† | ETA (n=3913) | INF (n=3295) | ADA (n=3504) | p Value‡ |
| Age (years), mean (SD) | 60 (12) | 56 (12) | <0.001 | 56 (12) | 56 (12) | 57 (12) | 0.012 |
| Women (%) | 72 | 76 | <0.001 | 77 | 76 | 75 | 0.108 |
| DAS28, mean (SD) | 5.1 (1.3) | 6.6 (1.0) | <0.001 | 6.6 (1.0) | 6.6 (1.0) | 6.5 (1.0) | <0.001 |
| HAQ, mean (SD) | 1.5 (0.8) | 2.0 (0.6) | <0.001 | 2.1 (0.6) | 2.1 (0.5) | 2.0 (0.6) | <0.001 |
| Disease duration (years) median (IQR) | 6 (1–15) | 11 (6–19) | <0.001 | 12 (6–19) | 12 (6–19) | 10 (5–18) | <0.001 |
| Number of prior DMARDs, median (IQR) | 2 (1–3) | 4 (3–5) | <0.001 | 4 (3–5) | 4 (3–5) | 3 (3–5) | <0.001 |
| Baseline steroid use, n (%) | 744 (23) | 4753 (44) | <0.001 | 1860 (48) | 1520 (46) | 1373 (39) | <0.001 |
| Diabetes, n (%) | 212 (6.6) | 596 (5.6) | 0.034 | 236 (6.1) | 156 (4.8) | 204 (5.9) | 0.042 |
| COPD/asthma, n (%) | 590 (18.4) | 1429 (13.5) | <0.001 | 558 (14.4) | 423 (13.0) | 448 (12.9) | 0.104 |
| Prior TB, n (%) | 74 (2.3) | 201 (1.8) | 0.193 | 96 (2.5) | 53 (1.5) | 52 (1.5) | 0.05 |
| Smoking, n (%) | |||||||
| Current | 751 (23) | 2334 (22) | 0.013 | 805 (21) | 718 (22) | 811 (23) | 0.057 |
| Former | 1273 (40) | 4067 (38) | 1485 (38) | 1247 (38) | 1335 (38) | ||
| Never | 1191 (37) | 4249 (40) | 1599 (41) | 1314 (40) | 1336 (38) | ||
| Ethnicity, n (%) | |||||||
| White | 2509 (78) | 8873 (83) | <0.001 | 3228 (82) | 2704 (82) | 2941 (84) | 0.363 |
| Non-white | 59 (2) | 366 (3) | 124 (3) | 124 (4) | 118 (3) | ||
| Missing | 664 (21) | 1473 (14) | 561 (14) | 467 (14) | 445 (13) | ||
| Entry year, n (%) | |||||||
| Before | 2003 11 (0) | 1322 (12) | <0.001 | 181 (5) | 1112 (34) | 29 (1) | <0.001 |
| 2003 | 306 (9) | 2924 (27) | 1440 (37) | 1063 (32) | 421 (12) | ||
| 2004 | 886 (27) | 3092 (29) | 1876 (48) | 485 (15) | 731 (21) | ||
| 2005 | 925 (29) | 1537 (14) | 412 (11) | 326 (10) | 799 (23) | ||
| 2006+ | 1104 (34) | 1837 (17) | 4 (0) | 309 (10) | 1524 (43) | ||
Two hundred and five patients switched from the DMARD cohort to the anti-TNF cohort and contributed person-years to both cohorts;
p value represents the significance of differences between the DMARD and anti-TNF cohorts using x2 tests for categorical outcomes and Wilcoxon rank sum tests for continuous variables;
p value represents the significance of differences between the three anti-TNF drugs using x2 tests for categorical outcomes and Kruskal–Wallis rank tests for continuous variables.
ADA, adalimumab; COPD, chronic obstructive pulmonary disease; DAS28, 28 joint count Disease Activity Score; DMARDs, disease-modifying antirheumatic drugs; ETA, etanercept;
HAQ, Health Assessment Questionnaire; INF, infliximab; TB, tuberculosis; TNF, tumour necrosis factor.
Total follow-up time was 7345 person-years for the DMARD cohort and 34 025 person-years for the anti-TNF cohort, with 28 447 person-years spent actively receiving anti-TNF therapy. The median duration of follow-up per patient was 3.21 years for the anti-TNF cohort and 2.30 years for the DMARD cohort. The median duration “on drug” was 2.48 years for ETA, 1.68 years for INF and 1.26 years for ADA.
There were 40 episodes of doctor-reported active TB in 39 patients, all in the anti-TNF cohort (see online supplementary table S1). There were no cases in the DMARD cohort. Thirteen of 40 episodes occurred after discontinuation of the anti-TNF drug. The numbers and rates for the two models of risk attribution are reported in table 2, with the cumulative incidence shown in fig 2. Of the 13 cases that occurred “off drug”, seven were diagnosed within 90 days of stopping treatment. In these cases, the drug was often stopped for symptoms of TB, although the diagnosis was made only after drug discontinuation. One case was diagnosed 6.0 months after stopping ETA, one 11.5 months after stopping INF, and four cases after stopping ADA (3.6, 7.3, 12.9 and 13.8 months).
Table 2.
Numbers and rates of incident tuberculosis, switchers included
| Number of patients ever received the drug | DMARD (n=3232) | All anti-TNF (n=10 712) | ETA (n=5521) | INF (n=3718) | ADA (n=4857) |
|---|---|---|---|---|---|
| On drug* | |||||
| Person-years | 7345 | 28 447 | 12 744 | 8069 | 7634 |
| Cases of TB | 0 | 27 | 5 | 11 | 11 |
| Rate/100 000 person-years (95% CI) | 0 | 95 (63 to 138) | 39 (13 to 92) | 136 (68 to 244) | 144 (72 to 258) |
| IRR, adjusted for age, gender and entry year (95% CI) | Referent | 3.1 (1.0 to 9.5) | 4.2 (1.4 to 12.4) | ||
| Most recent drug* | |||||
| Person-years | 7345 | 34 025 | 15 070 | 9730 | 9224 |
| Cases of TB | 0 | 40 | 8 | 12 | 20 |
| Rate/100 000 personyears (95% CI) | 0 | 118 (84 to 160) | 53 (23 to 105) | 123 (64 to 215) | 217 (132 to 335) |
| IRR, adjusted for age, gender and entry year (95% CI) | Referent | 2.2 (0.9 to 5.8) | 4.2 (1.8 to 9.9) | ||
Patients could switch between anti-TNF therapies, but all TB cases were attributable to one drug only
The two models of risk attribution are illustrated in fig 1.
ADA, adalimumab; DMARDs, disease-modifying antirheumatic drugs; ETA, etanercept; INF, infliximab; IRR, incidence rate ratio; TB, tuberculosis; TNF, tumour necrosis factor.
Figure 2.
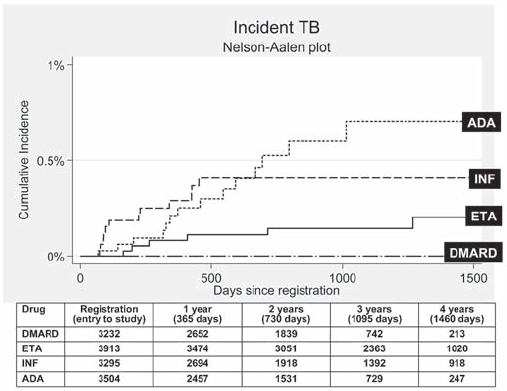
Cumulative incidence of tuberculosis (TB) following first exposure to anti-tumour necrosis factor (anti-TNF) therapy (most recent drug model, with person-years censored at death, last returned follow-up form, or date of switching to second anti-TNF). Numbers in table represent the number of patients eligible for follow-up at the specified follow-up time points. ADA, adalimumab; DMARD, disease-modifying antirheumatic drug; ETA, etanercept; INF, infliximab.
Between-drug comparisons
Using the “on drug” model of attributing risk (upper half of table 2), the rate of TB was highest for ADA (144 events/100 000 person-years), followed by INF (136/100 000 person-years) then ETA (39/100 000 person-years). Compared with ETA, the adjusted IRRs (aIRRs) and 95% CI for ADA and INF were 4.2 (1.4 to 12.4) and 3.1 (1.0 to 9.5), respectively. In the “most recent drug” model (lower half of table 2) the aIRRs remained higher for both INF and ADA compared with ETA, although the magnitude of the effect size fell for INF (aIRR=2.2 (0.9 to 5.8)). Censoring follow-up at the date of first TB diagnosis did not change the effect size (data not shown). None of the potential confounders changed the point estimate by >10%, and were therefore not included in the regression model. Age and ethnicity were the only potential confounders that were significantly associated with TB (irrespective of their influence upon change in the point estimate).
Comparison of the anti-TNF and DMARD cohorts
No direct comparison between the anti-TNF cohort and the DMARD cohort could be made owing to the absence of cases in the DMARD cohort. Indirect standardisation was performed, assuming the DMARD cohort shared the same risk as the anti- TNF cohort. After allowing for differences in age, gender and calendar year, the expected rate in the DMARD cohort was 136 events/100 000 person-years (65 to 250), equating to 10.0 cases. This was significantly more than the observed 0 cases (one-sided p value <0.001). Indirect standardisation was also performed assuming the DMARD cohort shared the same risk as the ETA-treated cohort. After allowing for differences in age, gender and calendar year, the expected rate was 41 cases/100 000 person-years (8 to 119), equating to 3.1 cases (also significantly increased, p=0.045).
Sensitivity analyses
Twenty-four of 40 (60%) cases were categorised as “verified” and 16 as “unverified”. Of the 24 verified cases, two were most recently treated with ETA, nine with INF and 13 ADA. Between-drug comparisons led to a statistically significantly increased rate of TB for both monoclonal antibodies compared with ETA, using the “most recent drug” model.
Seven cases of TB occurred after treatment with a second or third anti-TNF drug. Of these, two occurred with ADA, one with ETA, three after discontinuing ADA and one after discontinuing ETA. When sensitivity analyses were performed excluding time periods following switching (table 3), the between-drug aIRRs were largely unchanged.
Table 3.
Numbers and rates of incident tuberculosis, limited to first anti-TNF drug. (Follow-up censored at date of starting second anti-TNF drug)
| Number of patients ever received the drug | DMARD (n=3232) | All anti-TNF (n=10 712) | ETA (n=3913) | INF (n=3295) | ADA (n=3504) |
|---|---|---|---|---|---|
| On drug* | |||||
| Person-years | 7345 | 23 286 | 10 111 | 7459 | 5716 |
| Cases of TB | 24 | 4 | 11 | 9 | |
| Rate/100 000 person-years (95% CI) | 103 (66 to 153) | 40 (11 to 101) | 147 (74 to 264) | 157 (72 to 299) | |
| IRR, adjusted for age, gender and entry year (95% CI) | Referent | 3.7 (1.1 to 12.7) | 4.4 (1.3 to 15.2) | ||
| Most recent drug* | |||||
| Person-years | 7345 | 27 624 | 11 926 | 8963 | 6735 |
| Cases of TB | 33 | 6 | 12 | 15 | |
| Rate/100 000 personyears (95% CI) | 119 (82 to 168) | 50 (18 to 110) | 134 (69 to 234) | 223 (125 to 367) | |
| IRR, adjusted for age, gender and entry year (95% CI) | Referent | 2.7 (0.9 to 7.8) | 4.4 (1.6 to 12.1) | ||
The two models of risk attribution are illustrated in fig 1.
ADA, adalimumab; DMARDs, disease modifying antirheumatic drugs; ETA, etanercept; INF, infliximab; IRR, incidence rate ratio; TB, tuberculosis; TNF, tumour necrosis factor.
Time to event
The median time to TB diagnosis from the start of first anti-TNF drug was 13.4 months for cases most recently exposed to ETA, 5.5 months for INF and 18.5 months for ADA. Because patients could switch between drugs, the time from start date of first drug does not always reflect time since starting the most recent drug. In the sensitivity analysis excluding time periods after the start of a second anti-TNF drug, the median times to event were 11.0, 5.5 and 15.0 months for ETA, INF and ADA, respectively (fig 2). The differences in time to diagnosis between the three drugs for both analyses were statistically significant (p<0.05) using a Kruskal–Wallis test.
Site of TB
Fifteen of 40 cases (38%) were pulmonary and 25 (62%) extrapulmonary (table 4). Eleven (28%) were disseminated. A lower proportion (50%) of the TB cases after exposure to ETA was extrapulmonary than with INF (67%) and ADA (65%). Eight of 20 (40%) cases in the ADA cohort were disseminated, compared with 2/12 (17%) and 1/8 (13%) in the INF- and ETA-treated cohorts, respectively.
Table 4.
Classification and sites of TB infection, by drug
| ETA n=8 {5} | INF n=12 {11} | ADA n=20 {11} | All anti-TNF n=40 {27} | |
|---|---|---|---|---|
| Pulmonary, n=15 (38% total) | ||||
| Lower respiratory tract | 4 {2} | 2 {2} | 6 {3} | 12 {7} |
| Pleural | – | 2 {2} | 1 {1} | 3 {3} |
| Total pulmonary | 4 {2} | 4 {4} | 7 {4} | 15 {10} |
| Extra-pulmonary (including disseminated), n=25 (62% total) | ||||
| Bone and joint | 1 {1} | – | – | 1 {1} |
| Gastrointestinal | – | 3 {3} | – | 3 {3} |
| Lymph node | 2 {2} | 2 {2} | 2 {2} | 6 {6} |
| Central nervous system | – | 1 {1} | 2 {1} | 3 {2} |
| Pharyngeal wall | – | – | 1 {1} | 1 {1} |
| Disseminated | 1 {0} | 2 {1} | 8 {3} | 11 {4} |
| Total extrapulmonary | 4 {3} | 8 {7} | 13 {7} | 25 {17} |
Numbers represent number of cases attributable to most recent drug {number of cases while "on drug"}.
ADA, adalimumab; ETA, etanercept; INF, infliximab; TB, tuberculosis; TNF, tumour necrosis factor.
Influence of ethnicity
Ethnicity data were available for 32/39 patients who developed TB. Twenty-six (65%) were white and six (15%) non-white. This compared with around 80% patients who were white and 2–3% non-white in the original DMARD and anti-TNF populations. After excluding patients with missing ethnicity data, the age-, sex- and calendar year-adjusted IRR for active TBin non-white compared with white patients was 6.5 (2.8 to 15.3).
Ten of 39 patients who developed active TB died within 12 months of diagnosis date. Seven of these had TB listed on their death certificates as the underlying or contributory cause of death (see online supplementary table S1).
One patient had two discrete episodes of TB. A 30-year-old white woman developed verified TB in a cervical lymph node 10 months into treatment with ADA. Her ADA was discontinued and she was treated with Rifinah, pyridoxine and ethambutol for 6 months, with confirmed antibiotic sensitivity. Seventeen months later, without any additional anti-TNF therapy, she was diagnosed with verified pulmonary TB. Mycobacterial interspersed repetitive unit typing confirmed this second episode as a relapse of the first.
Discussion
We have confirmed that the monoclonal antibodies INF and ADA are associated with a three- to fourfold higher rate of TB compared with ETA. Although no direct comparison with the DMARD cohort was possible, the expected number of cases (n=10) in the DMARD cohort based on the rate seen in the anti-TNF cohort versus the observed (n=0) suggests that anti-TNF therapy confers a significant risk in patients with active RA. The combined strengths of the large size of the study with the accurate capture of time-dependent drug use and serious adverse event data enabled a robust direct comparison of the rates of TB between the three anti-TNF drugs.
Before concluding that these estimated relative risks represent a true differential risk between the drugs, we should explore alternative explanations. ADA was licensed later, and thus patients receiving ADA may have been more likely to have already received other anti-TNF drugs. Some risk of TB may be carried over from the previous drug, and sequential drug use may have a multiplicative risk. However, the sensitivity analysis censoring follow-up at switching did not change the results. Other factors that may influence the drug-specific rates include calendar year of drug start in parallel with the increasing background UK population rate of TB (12.3 to 14.7 events/100 000 person-years from 2001 to 200520) and increasing awareness of, and changing UK guidelines for, TB screening.21 This was addressed by adjusting all analyses for calendar year of recruitment. Nonetheless, there may be some residual confounding.
It has been suggested that there may be a time-varying risk of TB with anti-TNF therapy, with a higher early increased risk for INF than for ETA.5 Different average durations of follow-up by drug may influence our rate estimates if the risk of TB is nonlinear. However, limiting analysis to the first year of follow-up would result in too few cases to allow meaningful comparison between the drugs.
The early belief that the risk of TB was greater for INF-treated than for ETA-treated patients may have led to a selection bias, where clinicians preferentially prescribed ETA to patients at higher risk of TB. Indeed, proportionally more patients treated with ETA had prior TB, supporting this hypothesis. Assuming no effect of anti-TNF therapy, we might thus expect a higher rate of TB in ETA-treated patients. Our results show the opposite, meaning such a treatment bias does not account for our findings.
Our study did not capture data on all the known risk factors for TB. We did not have data on nutritional status, substance misuse, living environment (eg, residential care), contact with TB, vitamin D deficiency or immunodeficiency states such as HIV.[22] However, it is unlikely that these factors were more prevalent in the monoclonal antibody-treated patients than in the ETA cohort.
Comparing our results with the published literature, we found that there are few studies attempting to examine the question of drug-specific risk. Limitations of prior studies include imprecise estimates of rates in spontaneous pharmacovigilance studies, owing to under-reporting of cases and an unknown denominator4 5 and low numbers of events in observational studies.23 24 The French RATIO study recently attempted to examine drug-specific risk by calculating event “rates” from spontaneously reported cases of TB and estimates of national anti-TNF drug use across all indications.25 Our findings support the original suggestion that the rate of TB may be higher in INF-treated than in ETA-treated patients,3 26 although to a lesser extent than the French study.25 Our finding that TB rates are highest in patients treated with ADA is supported by a high rate of TB in the ADA open-label extension study.12 The possible mechanisms underlying this differential risk of TB have been elegantly reviewed elsewhere.27
Active TB was diagnosed only in patients treated with anti-TNF therapy. If patients in the DMARD cohort had an equal risk to those in the anti-TNF cohort, we would have expected to see 10 TB cases in the DMARD cohort. The magnitude of difference between the observed and expected cases strongly suggests that anti-TNF therapy is associated with significant risk of TB above and beyond the risk conferred by RA alone. The indirect standardisation data also support an increased risk for ETA, where the expected number of cases in the DMARD cohort would have been 3.1. This finding suggests that, although the rates of TB are higher in patients treated with the monoclonal antibodies, patients treated with ETA are not without risk. The average annual incidence of TB for the period of the study in the UK general population was 13.2 events/ 100 000 person-years.20 The anti-TNF rate was therefore eight times higher than in the UK general population. The magnitude of increased risk is higher than the estimated fourfold increased risk conferred by RA in the prebiologic era from non-UK sources.24 Unfortunately, there are no previously published rates of TB in UK RA populations from the pre-biologic era. Because of widely varying international TB incidence, it is not valid to compare our rates with those in other national RA cohorts. Nonetheless, the magnitude of risk in the anti-TNF cohort compared with the general population fits with prior estimates from other countries.23 28
All TB cases in the analysis were confirmed by a doctor. The “gold standard” for diagnosing TB is culture of Mycobacterium tuberculosis bacilli. However, in clinical practice, doctors diagnose and treat TB based on weaker criteria such as the presence of acid fast bacilli, caseating granulomata, suggestive radiological findings, or even clinical suspicion. We categorised our cases as verified or unverified. Our tight classification for verified TB led to the exclusion of 16 cases. Nonetheless, restriction to verified cases generated the same pattern of drug-specific risk.
Prior estimates of the frequency of extrapulmonary TB in patients treated with anti-TNF therapy have ranged from 28% to 75%, with most reporting >50% cases as extrapulmonary. Our study replicates these findings, but in addition shows a greater increased risk of extrapulmonary disease with the monoclonal antibodies, a finding not previously described. Our finding of a significant risk conferred by non-white ethnicity in patients treated with anti-TNF therapy also reflects similar findings in the RATIO study.25
There are a number of clinically important points to take from this analysis. First, although the relative risk of TB is three to four times higher for the monoclonal antibodies than for ETA, the actual number of cases is low. After a total follow-up time of nearly 35 000 person-years, we identified only 40 cases of TB. The “number needed to harm” for 1 year's therapy with ADA compared with ETA in this study is around 600. That said, the UK is a country with a relatively low prevalence of TB. Such a differential risk would probably have greater implications in countries with higher background prevalence. Second, the lower rate with ETA compared with the other anti-TNF drugs does not mean that there is a negligible risk with this drug. Although relatively safer than the monoclonal antibodies, clinicians should be aware that ETA still confers an increased risk. Third, the high prevalence of disseminated TB should remind clinicians that TB may present atypically in patients treated with anti-TNF therapy. Lastly, nearly half of the disseminated TB cases in patients most recently treated with ADA occurred after treatment had been stopped, with 13 of the total cases being diagnosed after stopping treatment (6/13 >3 months after stopping). This should remind clinicians to remain vigilant for TB even after discontinuing anti-TNF therapy.
Acknowledgments
The authors acknowledge the enthusiastic collaboration of all consultant rheumatologists and their specialist nurses in the UK in providing the data. In addition, we acknowledge the support from Dr Ian Griffiths (past) and Professor David Isenberg (current), Chairs of the BSRBR Management Committee, Professor Gabriel Panayi, Professor David G I Scott, Dr Andrew Bamji and Dr Deborah Bax, Presidents of the BSR during the period of data collection, for their active role in enabling the Register to undertake its tasks and from Samantha Peters (CEO of theBSR), Mervyn Hogg and members of the BSRBR Scientific Steering Committee. We also acknowledge the seminal role of the BSR Clinical Affairs Committee for establishing national biologic guidelines and recommendations for such a Register. Finally we would like to acknowledge the substantial contribution of Andy Tracey, Katie McGrother and Dr Mark Lunt in database design and manipulation and Professor Alan Silman in his prior role as a principal investigator of the BSRBR.
Footnotes
Competing interests: None.
Ethics approval: Ethical approval for this study was obtained in December 2000 from the Multicentre Research Ethics Committee (MREC) for the northwest of England.
Authors' contributions: WGD—study design, analysis, verification, interpretation, writing; KLH—study design, data management, interpretation, writing; KDW—study design, data management; ML—study design, data analysis; JG—verification, interpretation, writing; AU—verification; DPMS—study design, data management, interpretation, writing.
BSRBR Control Centre Consortium: Antrim Area Hospital, Antrim (Dr Nicola Maiden); Cannock Chase Hospital, Cannock Chase (Dr Tom Price); Christchurch Hospital, Christchurch (Dr Neil Hopkinson); Derbyshire Royal Infirmary, Derby (Dr Sheila O'Reilly); Dewsbury and District Hospital, Dewsbury (Dr Lesley Hordon); Freeman Hospital, Newcastle-upon-Tyne (Dr Ian Griffiths); Gartnavel General Hospital, Glasgow (Dr Duncan Porter); Glasgow Royal Infirmary, Glasgow (Professor Hilary Capell); Haywood Hospital, Stoke-on-Trent (Dr Andy Hassell); Hope Hospital, Salford (Dr Romela Benitha); King's College Hospital, London (Dr Ernest Choy); Kings Mill Centre, Sutton-In Ashfield (Dr David Walsh); Leeds General Infirmary, Leeds (Professor Paul Emery); Macclesfield District General Hospital, Macclesfield (Dr Susan Knight); Manchester Royal Infirmary, Manchester (Dr Ian Bruce); Musgrave Park Hospital, Belfast (Dr Allister Taggart); Norfolk and Norwich University Hospital, Norwich(Professor David Scott); Poole General Hospital, Poole (Dr Paul Thompson); Queen Alexandra Hospital, Portsmouth (Dr Fiona McCrae); Royal Glamorgan Hospital, Glamorgan (Dr Rhian Goodfellow); Russells Hall Hospital, Dudley (Professor George Kitas); Selly Oak Hospital, Selly Oak (Dr Ronald Jubb); St Helens Hospital, St Helens (Dr Rikki Abernethy); Weston General Hospital, Weston-super-Mare (Dr Shane Clarke); Withington Hospital, Manchester (Dr Paul Sanders); Withybush General Hospital, Haverfordwest (Dr Amanda Coulson).
Declaration: The British Society for Rheumatology (BSR) commissioned the Biologics Register (BSRBR) as a UK-wide national project to investigate the safety of biological agents in routine medical practice. DPMS and KLH are principal investigators on the BSRBR. BSR receives restricted income from UK pharmaceutical companies, presently Abbott Laboratories, Amgen, Schering Plough and Wyeth Pharmaceuticals. This income finances a wholly separate contract between the BSR and the University of Manchester who provide and run the BSRBR data collection, management and analysis services. The principal investigators and their team have full academic freedom and are able to work independently of pharmaceutical industry influence. All decisions concerning analyses, interpretation and publication are made autonomously of any industrial contribution. Members of the Manchester team, BSR trustees, committee members and staff complete an annual declaration in relation to conflicts of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999;354:1932–9 [DOI] [PubMed] [Google Scholar]
- 2.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. [see comment]. N Engl J Med 2001;345:1098–104 [DOI] [PubMed] [Google Scholar]
- 3.Mohan AK, Cote TR, Block JA, et al. Tuberculosis following the use of etanercept, a tumor necrosis factor inhibitor. [see comment]. Clin Infect Dis 2004;39:295–9 [DOI] [PubMed] [Google Scholar]
- 4.Wallis RS, Broder MS, Wong JY, et al. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. [see comment]. Clin Infect Dis 2004;38:1261–5 [DOI] [PubMed] [Google Scholar]
- 5.Wallis RS, Broder M, Wong J, et al. Granulomatous infections due to tumor necrosis factor blockade: correction. Clin Infect Dis 2004;39:1254–5 [DOI] [PubMed] [Google Scholar]
- 6.Shergy WJ, Isern RA, Cooley DA, et al. Open label study to assess infliximab safety and timing of onset of clinical benefit among patients with rheumatoid arthritis [see comment]. J Rheumatol 2002;29:667–77 [PubMed] [Google Scholar]
- 7.St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: arandomized, controlled trial. Arthritis Rheum 2004;50:3432–43 [DOI] [PubMed] [Google Scholar]
- 8.Westhovens R, Yocum D, Han J, et al. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum 2006;54:1075–86 [DOI] [PubMed] [Google Scholar]
- 9.Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 2004;50:1400–11 [DOI] [PubMed] [Google Scholar]
- 10.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37 [DOI] [PubMed] [Google Scholar]
- 11.Schiff MH, Burmester GR, Kent JD, et al. Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann Rheum Dis 2006;65:889–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burmester GR, Mariette X, Montecucco C, et al. Adalimumab alone and in combination with disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis in clinical practice: the Research in Active Rheumatoid Arthritis (ReAct) trial. Ann Rheum Dis 2007;66:732–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Heijde D, Klareskog L, Landewe R, et al. Disease remission and sustained halting of radiographic progression with combination etanercept and methotrexate in patients with rheumatoid arthritis. Arthritis Rheum 2007;56:3928–39 [DOI] [PubMed] [Google Scholar]
- 14.Watson K, Symmons D, Griffiths I, et al. The British Society for Rheumatology Biologics Register. Ann Rheum Dis 2005;64(Suppl 4):iv42–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NICE Guidance on the use of etanercept and infliximab for the treatment of rheumatoid arthritis.
- 16.Prevoo ML, van't Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8 [DOI] [PubMed] [Google Scholar]
- 17.Kirwan JR, Reeback JS. Stanford Health Assessment Questionnaire modified to assess disability in British patients with rheumatoid arthritis. Br J Rheumatol 1986;25:206–9 [DOI] [PubMed] [Google Scholar]
- 18.Dixon WG, Symmons DP, Lunt M, et al. Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum 2007;56:2896–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993;138:923–36 [DOI] [PubMed] [Google Scholar]
- 20.Health Protection Agency Tuberculosis in the UK. Annual report on tuberculosis surveillance and control in the UK. 2008. Available at http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1225268885969 (accessed November 2009).
- 21.British Thoracic Society Standards of Care Committee BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-{alpha} treatment. Thorax 2005;60:800–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose AM, Watson JM, Graham C, et al. Tuberculosis at the end of the 20th century in England and Wales: results of a national survey in 1998. Thorax 2001;56:173–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Reino JJ, Carmona L, Angel DM. Risk of tuberculosis in patients treated with tumor necrosis factor antagonists due to incomplete prevention of reactivation of latent infection. Arthritis Rheum 2007;57:756–61 [DOI] [PubMed] [Google Scholar]
- 24.Askling J, Fored CM, Brandt L, et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum 2005;52:1986–92 [DOI] [PubMed] [Google Scholar]
- 25.Tubach F, Salmon D, Ravaud P, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective french research axed on tolerance of biotherapies registry. Arthritis Rheum 2009;60:1884–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keane J. Tumor necrosis factor blockers and reactivation of latent tuberculosis. Clin Infect Dis 2004;39:300–2 [DOI] [PubMed] [Google Scholar]
- 27.Wallis RS. Tumour necrosis factor antagonists: structure, function, and tuberculosis risks. Lancet Infect Dis 2008;8:601–11 [DOI] [PubMed] [Google Scholar]
- 28.Wolfe F, Michaud K, Anderson J, et al. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum 2004;50:372–9 [DOI] [PubMed] [Google Scholar]


