Abstract
Objectives To examine the association between migraine and migraine aura status with risk of haemorrhagic stroke.
Design Prospective cohort study.
Setting Women’s Health Study, United States.
Participants 27 860 women aged ≥45 who were free from stroke or other major disease at baseline and had provided information on self reported migraine, aura status, and lipid values.
Main outcome measures Time to first haemorrhagic stroke and subtypes of haemorrhagic stroke.
Results At baseline, 5130 (18%) women reported any history of migraine; of the 3612 with active migraine (migraine in the previous year), 1435 (40%) described having aura. During a mean of 13.6 years of follow-up, 85 haemorrhagic strokes were confirmed after review of medical records. Compared with women without a history of migraine, there was no increased risk of haemorrhagic stroke in those who reported any history of migraine (adjusted hazard ratio 0.98, 95% confidence interval 0.56 to 1.71, P=0.93). In contrast, risk was increased in women with active migraine with aura (2.25, 1.11 to 4.54, P=0.024). The age adjusted increased risk was stronger for intracerebral haemorrhage (2.78, 1.09 to 7.07, P=0.032) and for fatal events (3.56, 1.23 to 10.31, P=0.02). Four additional haemorrhagic stroke events were attributable to migraine with aura per 10 000 women per year. Women who reported active migraine without aura had no increased risk for haemorrhagic stroke.
Conclusion Migraine with aura might, in addition to ischaemic events, also be a risk factor for haemorrhagic stroke. The relatively low number of events and attributable risk should caution against definitive conclusions and call for further confirmation of these observations.
Introduction
Migraine is an intermittent often severely disabling headache disorder that involves the neuronal and vascular system and has a prevalence of about 20% in middle aged women.1 2 3 Up to a third of affected patients experience migraine aura, which is characterised by transient neurological symptoms most often involving the visual system.
Several hospital and population based studies4 5 6 7 8 9 10 and meta-analyses11 12 have linked migraine, particularly migraine with aura, to an increased risk of ischaemic stroke. Potential explanations for this association include direct vascular involvement as part of the migraine pathophysiology,13 genetic polymorphisms,14 15 and shared risk factors, such as hypertension, smoking, raised cholesterol concentrations, and use of oral contraceptives.10 12 16 In addition, there is increasing evidence of pathology of the vessel wall in migraine, which might also increase the susceptibility to vascular events, including haemorrhagic stroke. Specifically, smooth muscle cell dysfunction,17 endothelial dysfunction,18 19 20 and involvement of genotypes of the angiotensin converting enzyme15 and the endothelin receptor21 22 have been suggested.
Data on the association between migraine and haemorrhagic stroke, however, are sparse, mainly because of the generally low incidence of haemorrhagic strokes. Two case-control studies suggested an association,6 23 and a large population based study with health insurance data indicated an association between peripartum migraine and increased risk of intracerebral haemorrhage.24
A previous report from the Women’s Health Study did not find any association between migraine and increased risk of haemorrhagic stroke.7 After the association between migraine with aura and ischaemic vascular events became more apparent with longer follow-up,25 26 however, we re-evaluated the association of migraine and migraine aura status with haemorrhagic stroke after a mean of over 13 years of follow-up.
Methods
We analysed data from participants in the Women’s Health Study, a completed randomised trial designed to test the benefits and risks of low dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer among apparently healthy women. The design, methods, and results have been described in detail previously.27 28 29 In brief, a total of 39 876 US female health professionals aged ≥45 years at baseline (1993-6) and without a history of cardiovascular disease, cancer, or other major illnesses were randomly assigned to active aspirin (100 mg on alternate days), active vitamin E (600 IU on alternate days), both active agents, or both placebos. Baseline information was self reported and collected by a posted questionnaire that asked about many cardiovascular risk factors and lifestyle variables. Twice in the first year and yearly thereafter participants were sent follow-up questionnaires asking about study outcomes and other information during the study period. For the current analysis, we included follow-up information from the time of randomisation to 2 March 2009.
Before randomisation, blood samples were collected from 28 345 participating women and stored in tubes containing EDTA. Of these, 27 939 samples could be assayed for a full lipid profile (Roche Diagnostics, Basel, Switzerland). We excluded 79 women for whom we had no information on migraine, leaving 27 860 women free from any self reported vascular disease at baseline.
Assessment of migraine
On the baseline questionnaire, participants were asked: “Have you ever had migraine headaches?” and “In the past year, have you had migraine headaches?” We distinguished between women with no history of migraine and those who reported any history of migraine, active migraine (migraine in the year before the baseline questionnaire), or previous migraine (those who reported ever having had a migraine but none in the year before completing the questionnaire). Those participants who reported active migraine were also asked details about their migraine attacks, including duration of attack, unilateral location and pulsating quality of pain, interference with daily activities, aggravation by routine physical activity, nausea or vomiting, and sensitivity to light or sound. Women who reported active migraine were asked whether they had an “aura or any indication a migraine is coming.” We used responses to classify affected women into active migraine with aura and active migraine without aura, as in other studies.7 25 30
We have previously shown25 that among participants in the Women’s Health Study who reported active migraine, over 83% fulfilled all but one of the first edition International Classification of Headache Disorders31 criteria (code 1.7, migrainous disorder) and around 47% fulfilled all criteria (code 1.1 migraine without aura). We also showed that in a subsample of women from the Women’s Health Study,32 according to second edition International Classification of Headache Disorders criteria,33 in the 88% with self reported active migraine the diagnosis was migraine without aura (72%) or probable migraine without aura (16%).
Stroke ascertainment
We asked participants who reported a stroke on a follow-up questionnaire for permission to review their medical records. An endpoints committee of physicians, including a board certified vascular neurologist, reviewed the medical records to confirm the diagnosis of stroke. Non-fatal stroke was confirmed if the participant had a new focal-neurological deficit of sudden or rapid onset attributable to a cerebrovascular event that persisted for more than 24 hours. Cases of fatal stroke were documented by evidence of a cerebrovascular cause of death obtained from all available sources, including death certificates and hospital records or information from next of kin. Clinical information and results from brain imaging were used to distinguish between major stroke subtypes, with excellent agreement between observers.34 Cases of haemorrhagic stroke were further subdivided into intracerebral haemorrhages and subarachnoid haemorrhages. We did not include in our analyses instances of ischaemic stroke with secondary haemorrhagic transformation into the affected area. The rate of positive imaging results for the diagnosis of haemorrhagic stroke in the Women’s Health Study was 97%.35
Statistical analysis
We compared baseline characteristics of participants according to migraine status. We used analyses of covariance to adjust continuous measures and direct standardisation to adjust categorical variables and incidence rates for haemorrhagic stroke for age.
We used Cox proportional hazard models to calculate age and multivariable adjusted hazard ratios (with 95% confidence intervals) for the association of migraine and migraine aura status with haemorrhagic stroke and subtypes of haemorrhagic stroke. We tested the proportionality assumption of the model by including an interaction term for the categories of migraine with time and found no significant violation.
As the number of outcome events was relatively low compared with the number of potential confounders, we used two approaches for multivariable adjustment. Firstly, we built a model that included only a limited set of potential confounders (parsimonious model). This model included information on age (continuous), history of hypertension (blood pressure of at least 140 mm Hg systolic or 90 mm Hg diastolic, or self reported diagnosis from a physician), smoking (never, past, current <15 cigarettes/day, ≥15 cigarettes/day), body mass index (BMI) (<25, 25-29.9, ≥30), alcohol consumption (rarely/never, 1-3 drinks/month, 1-6 drinks/week, ≥1 drink/day), and total cholesterol concentration (quarters of distribution).
Secondly, we used a propensity score36 method to adjust for confounding. This allowed us to simultaneously adjust for a large number of covariates, which otherwise would not have been possible because of general modelling constraints.37 38 The propensity score is the estimated probability of exposure (that is, migraine status) given a set of covariates (that is, the potential confounders). We distinguished between a propensity score for any migraine and a propensity score for migraine with aura. To estimate the propensity score, we used a logistic regression model that included age (as before), systolic blood pressure (increments of 10 mm Hg), antihypertensive treatment, smoking (as before), BMI (as before), alcohol consumption (as before), total cholesterol concentration (as before), exercise (rarely/never, less than once a week, once to three times a week, four or more times a week), menopausal status, postmenopausal use of hormone treatment (never, past, current), history of use of oral contraceptives, history of diabetes, family history of myocardial infarction before the age of 60, annual household income (<$50 000 (£33 000, €40 000), $50 000-<$100 000, ≥$100 000), highest level of education (less than university degree, university degree, masters degree, or doctorate), use of multivitamins (never, past, current), ethnicity (white versus other), interaction terms for age (<55, ≥55) with smoking, BMI, and oral contraceptive use, and randomised treatment assignments. Use of other lipid measures yielded comparable results. We weighted a Cox proportional hazards model based on propensity score values and used robust estimation of standard errors. We used standardised mortality ratio weights,39 40 which assign the value 1 for exposed and the propensity score odds for unexposed.
We incorporated an indicator variable for missing values if the number of women with missing information (maximum 1.9% missing) on potential confounding factors was 100 or more (this was the case for BMI and family history of myocardial infarction before the age of 60) or otherwise reassigned values of the reference group or past user group, as applicable.
In exploratory analyses, we evaluated potential effect modification of the association between migraine with aura (versus everybody else) and haemorrhagic stroke by age (<55, ≥55), history of hypertension (yes, no), smoking status (active, non-active), total cholesterol concentration (<6.2 mmol/l, ≥6.2 mmol/l), current postmenopausal hormone use (yes, no), estimated 10 year risk of coronary heart disease according to Framingham risk score (<5%, ≥5%),41 and randomised assignment to aspirin and vitamin E. Lastly, we ran a Cox proportional hazard model that adjusted for time varying frequency of non-steroidal anti-inflammatory drug (NSAID) intake and randomised aspirin assignment to explore the role of these drugs on the association between migraine with aura and haemorrhagic stroke.
All analyses were performed with SAS (SAS version 9.1, Cary, NC). All tests are two tailed, and we considered a P<0.05 as significant.
Results
Of the 27 860 participating women, 5130 (18%) reported any history of migraine, of whom 3612 (70%) reported active migraine (migraine in the year before the baseline questionnaire) and 1518 (30%) indicated previous migraine. Of the women with active migraine, 1435 (40%) were classified as having migraine with aura. Table 1 summarises the age adjusted baseline characteristics of participants according to migraine and aura status. Compared with women without a history of migraine, those with active migraine with aura were younger, were less likely to have a history of diabetes, smoked fewer cigarettes, and drank less alcohol. Women with migraine with aura were also more likely to be postmenopausal and to use postmenopausal hormones.
Table 1.
Age adjusted baseline characteristics according to migraine status in Women’s Health Study (n=27 860). Figures are percentages of women* unless stated otherwise
| Characteristics | No migraine history | Active migraine | Previous migraine† | |
|---|---|---|---|---|
| With aura | Without aura | |||
| No of participants | 22 730 | 1435 | 2177 | 1518 |
| Mean (SE) age (years) | 54.9 (0.05) | 53.2 (0.16) | 52.6 (0.12) | 55.5 (0.19) |
| Mean (SE) total cholesterol (mmol/l) | 5.5 (0.01) | 5.5 (0.03) | 5.5 (0.02) | 5.6 (0.03) |
| Mean (SE) BMI | 25.9 (0.03) | 25.8 (0.13) | 26.2 (0.11) | 26.1 (0.13) |
| History of hypertension‡ | 24.6 | 25.5 | 26.0 | 30.4 |
| Antihypertensive treatment | 13.0 | 13.9 | 14.3 | 17.2 |
| History of diabetes | 2.5 | 1.8 | 1.6 | 2.7 |
| Smoking: | ||||
| Never | 51.3 | 52.6 | 56.3 | 50.6 |
| Past | 37.0 | 37.3 | 34.5 | 35.4 |
| Current <15 cigarettes | 4.4 | 3.9 | 3.5 | 5.2 |
| Current ≥15 cigarettes | 7.3 | 6.2 | 5.8 | 8.9 |
| Alcohol consumption: | ||||
| Rarely/never | 43.5 | 48.3 | 47.4 | 45.1 |
| 1-3 drinks/month | 13.1 | 13.1 | 15.5 | 14.2 |
| 1-6 drinks/week | 32.7 | 30.3 | 30.1 | 30.4 |
| ≥1 drink/day | 10.8 | 8.3 | 7.1 | 10.3 |
| Physical activity: | ||||
| Rarely/never | 37.0 | 38.2 | 39.4 | 39.1 |
| <1/week | 19.2 | 20.5 | 21.7 | 20.1 |
| 1-3/week | 32.3 | 30.7 | 29.2 | 29.4 |
| ≥4/week | 11.6 | 10.6 | 9.7 | 11.3 |
| Premenopausal | 28.1 | 23.9 | 26.2 | 25.0 |
| Current postmenopausal hormone use | 41.7 | 51.0 | 46.7 | 43.7 |
| Family history of myocardial infarction before age 60 | 12.6 | 14.3 | 13.0 | 13.8 |
| Use of multivitamins | 13.1 | 13.2 | 11.2 | 11.5 |
| Randomised to aspirin | 50.2 | 49.1 | 50.5 | 47.8 |
| Randomised to vitamin E | 50.1 | 51.5 | 48.1 | 50.0 |
*Proportions might not add up to 100% because of rounding or missing values.
†History of migraine but no active migraine in year before completion of baseline questionnaire.
‡Systolic blood pressure ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg, or self reported diagnosis from physician.
During a mean of 13.6 years of follow-up (377 711 person years), there were 85 confirmed haemorrhagic strokes (44 intracerebral haemorrhages, 36 subarachnoid haemorrhages, and five without a clear distinction). After adjustment for age, the incidence of haemorrhagic stroke per 10 000 women per year was 2.3 for those without migraine, 2.5 for those with any history of migraine, 6.3 for those with active migraine with aura, 0.8 for those with active migraine without aura, and 1.3 for those who reported previous migraine. Compared with women without migraine, after adjustment for age and an assumption of causality, there were four additional haemorrhagic stroke events associated with migraine with aura per 10 000 women per year.
Table 2 summarises the age and multivariable adjusted hazards ratios for the association between migraine and risk of haemorrhagic stroke. Compared with women who did not report history of migraine, those who reported any history had no increased risk of haemorrhagic stroke (age adjusted hazard ratio 1.04, 95% confidence interval 0.59 to 1.82, P=0.90). When we made a distinction with regard to migraine aura, however, the results differed. After adjustment for age and compared with women without migraine history, women with active migraine with aura had increased risk of haemorrhagic stroke (2.31, 1.15 to 4.64, P=0.019). Adjustment for age, history of hypertension, smoking, BMI, alcohol consumption, and total cholesterol concentration (parsimonious model) yielded similar results. Also, adjustment for a large number of covariates via a regression model weighted for a propensity score did not substantially attenuate this finding (2.25, 1.11 to 4.54, P=0.024). Women who reported active migraine without aura and those who reported previous migraine had no increased risk of haemorrhagic stroke.
Table 2.
Hazard ratios (95% confidence intervals) for haemorrhagic strokes adjusted for age, multivariables, and propensity score according to migraine status in Women’s Health Study (n=27 860)
| No migraine history (n=22 730) | Any history of migraine (n=5130) | Active migraine | Previous migraine* (n=1518) | ||
|---|---|---|---|---|---|
| With aura (n=1435) | Without aura (n=2177) | ||||
| No with haemorrhagic stroke (n=85) | 70 | 15 | 9 | 3 | 3 |
| Age adjusted | 1.00 | 1.04 (0.59 to 1.82), P=0.90 | 2.31 (1.15 to 4.64), P=0.019 | 0.52 (0.16 to 1.66), P=0.27 | 0.63 (0.20 to 1.99), P=0.43 |
| Multivariable adjusted (parsimonious) model† | 1.00 | 1.03 (0.59 to 1.81), P=0.91 | 2.34 (1.16 to 4.70), P=0.017 | 0.54 (0.17 to 1.72), P=0.29 | 0.59 (0.19 to 1.88), P=0.37 |
| Propensity score adjusted‡ | 1.00 | 0.98 (0.56 to 1.71), P=0.93 | 2.25 (1.11 to 4.54), P=0.024 | 0.49 (0.14 to 1.70), P=0.26 | 0.45 (0.14 to 1.48), P=0.19 |
*History of migraine but no active migraine in year before completion of baseline questionnaire.
†Adjusted for age, history of hypertension, smoking, BMI, alcohol consumption, and total cholesterol concentration.
‡One propensity score calculated for overall migraine and one for having migraine with aura. Both models included information on age, systolic blood pressure, use of antihypertensive medication, smoking, BMI, alcohol consumption, exercise, total cholesterol concentration, postmenopausal status, postmenopausal hormone use, history of oral contraceptive, history of diabetes, family history of myocardial infarction before age 60, annual household income, level of education, multivitamin use, ethnicity, randomised treatment, and interaction terms for age with smoking, BMI, and oral contraceptives. Hazard ratios estimated by weighted Cox proportional hazard models.
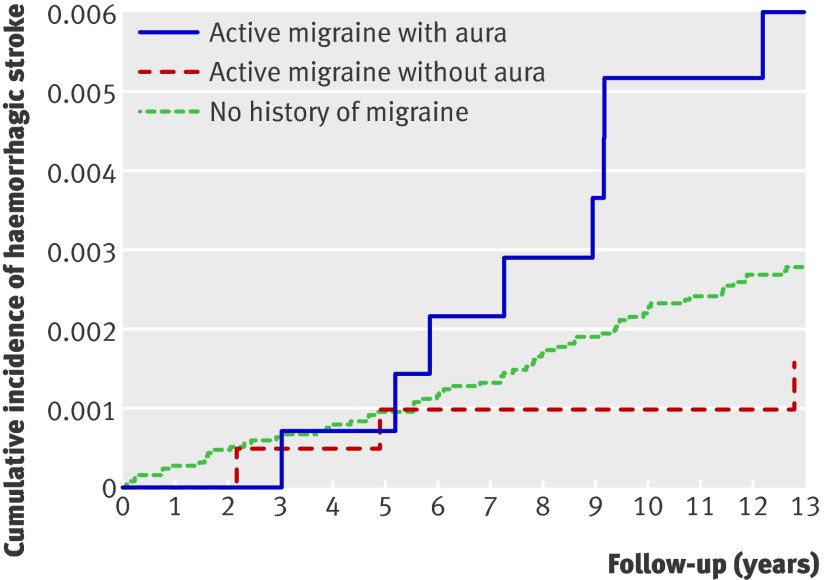
The figure shows the age adjusted incidence rates for haemorrhagic stroke for women without a history of migraine, women with active migraine with aura, and those with active migraine without aura. Compared with women with no history of migraine, the increased rates of haemorrhagic stroke for women with active migraine with aura were more apparent towards later years of follow-up.
Age adjusted cumulative incidence of haemorrhagic stroke according to migraine in women
To evaluate the association of migraine with aura with the few subtypes of haemorrhagic stroke (intracerebral haemorrhage n=44; subarachnoid haemorrhage n=36) as well as with fatal haemorrhagic stroke (n=28), we combined women who did not report migraine, reported migraine without aura, and reported previous migraine to form a reference group. The association between active migraine with aura and haemorrhagic stroke was stronger for intracerebral haemorrhages (hazard ratio adjusted for age 2.78, 1.09 to 7.07, P=0.032) than for subarachnoid haemorrhages (1.73, 0.53 to 5.65, P=0.37) and stronger for fatal (3.56, 1.23 to 10.31, P=0.02) than non-fatal haemorrhagic stroke (1.96, 0.78 to 4.93, P=0.15).
With regard to potential effect modification of the age adjusted association between active migraine with aura and haemorrhagic stroke, we found stronger effect estimates for age 55 and above (3.80, 1.61 to 8.97), no history of hypertension (3.23, 1.45 to 7.20), cholesterol concentrations <6.2 mmol/l (2.86, 1.36 to 6.01), current use of postmenopausal hormones (2.84, 1.19 to 6.76), non-active smokers (2.70, 1.29 to 5.67), Framingham risk score estimates of coronary heart disease of <5% (2.98, 1.41 to 6.30), randomisation to vitamin E (2.87, 1.21 to 6.80), and randomisation to aspirin placebo (3.27, 1.26 to 8.49). We did not, however, find any significant effect modification (smallest P value 0.19 for the interaction by age).
When we ran age adjusted Cox proportional hazards models that controlled for time varying frequency of NSAID use during the trial period and randomised aspirin assignment, there was no attenuation in the hazard ratio for haemorrhagic stroke for women with migraine with aura (2.34, 1.17 to 4.70).
Discussion
In this prospective study of middle aged and initially apparently healthy women, we found an association between migraine with aura and an increased risk of haemorrhagic stroke. Compared with women with no history of migraine and after adjustment for many potential confounders through a propensity score weighting, women who reported active migraine with aura had over twice the risk of haemorrhagic stroke. The low number of haemorrhagic strokes and the relatively low attributable risk, however, should caution against definitive conclusions about the association. Women who reported active migraine without aura or migraine in the more distant past had no increased risk of haemorrhagic stroke.
Our findings extend previous observations from the Women’s Health Study of an association between migraine with aura and increased risk of ischaemic vascular events, including ischaemic stroke, myocardial infarction, coronary revascularisation, angina, and death from ischaemic cardiovascular causes.25 The magnitude and pattern of the association between migraine with aura and haemorrhagic stroke are similar to those of migraine with aura and ischaemic vascular events. In contrast with the association between migraine with aura and ischaemic stroke, which is stronger among younger individuals,7 the association with haemorrhagic stroke seems more apparent in the older age group. We did not, however, find a significant effect modification.
Comparison with other studies
Our data contrast with a previous report from the Women’s Health Study in which migraine with aura was not associated with increased risk of haemorrhagic stroke after a mean of nine years of follow-up.7 The pattern of association suggests that this discrepancy is caused by the difference in length of follow-up. The increased risk for haemorrhagic stroke among women with migraine with aura becomes apparent only after longer follow-up (mean of 13.6 years in the present study), similar to a pattern observed for ischaemic vascular events.25 26
While several studies4 5 6 7 8 23 42 and two meta-analyses11 12 have evaluated the association of migraine, and specifically migraine with aura, with ischaemic stroke, data on the association between migraine and haemorrhagic stroke are sparse. One case-control study found an increased risk of haemorrhagic stroke for people with a family history of migraine (odds ratio 2.30, 95% confidence interval 1.35 to 3.90), but in those with active migraine the increased risk was more apparent for people with migraine without aura (1.84, 0.77 to 4.39).6 Another study of 430 women aged 15-44 with stroke used two control groups (their matched neighbours and hospital controls) to evaluate the association between migraine and haemorrhagic stroke.23 Compared with the neighbour control group, women with migraine who did not use oral contraceptives had an increased risk of haemorrhagic stroke (1.8, 1.2 to 2.7). The increased risk was not apparent when the hospital control group was used as reference, which might relate to biases when using hospital based control populations. Women with migraine who additionally used oral contraceptives had a significantly increased risk of haemorrhagic stroke regardless of the control group used for comparison.23 Information on migraine aura was not available in this study. In a large population based sample of inpatients, ICD-9 coding for migraine in the perinatal period was associated with various vascular events, including intracerebral haemorrhage (9.1, 3.0 to 27.8).24
Potential mechanisms linking migraine with haemorrhagic stroke
In addition to previous studies that have identified migraine with aura as a marker of ischaemic vascular events, our data suggest that it is also a marker of increased risk of haemorrhagic stroke. Potential mechanisms by which migraine with aura could be linked with increased risk of haemorrhagic stroke, however, are currently speculative. There could be several potential links. Firstly, one link might involve dysfunction or pathology of the vessel wall, which is supported by studies reporting smooth muscle cell dysfunction17 and endothelial dysfunction in migraine18 20 as well as pathological vascular resistance, already apparent in young people with migraine.19 This could ultimately increase the risk of intracerebral haemorrhage as well as the risk of lacunar infarcts.43 Secondly, there might be a synergistic effect between the vascular dysfunction in migraine and additional risk factors for haemorrhagic stroke. Previous results have suggested that the association between migraine with aura and ischaemic stroke is strongest among individuals with a low risk of vascular events30 as measured by the Framingham risk score for coronary heart disease, a pattern that is also suggested for haemorrhagic stroke in our data. Thirdly, both migraine and intracerebral haemorrhage could be related to a common cause, such as arteriovenous malformations,44 but such malformations are only a rare cause of migraine. Fourthly, migraine has been linked to platelet dysfunction,45 which could lead to an increased susceptibility to bleeding. Most studies, however, point towards an activation of the coagulation cascade,18 46 and there is no evidence of a generalised increased risk of bleeding among people with migraine. Finally, use of pain medication that inhibits platelet aggregation, such as aspirin and NSAIDs, could increase the risk of haemorrhagic stroke among patients with migraine. We believe this is an unlikely explanation of our findings as such medications are used by patients with migraine both with and without aura; in addition, the association between migraine with aura and haemorrhagic stroke in our study was stronger among those randomised to an aspirin placebo. Furthermore, when we adjusted for time varying NSAID use and randomised aspirin assignment the results were unchanged.
Strengths and limitations
Our study has several strengths, including its large sample size, haemorrhagic stroke cases confirmed by an endpoints committee of physicians with high agreement between raters in the classification of major subtypes of stroke,34 and homogeneity of the study participants, which should reduce confounding. Furthermore, we used a propensity score technique that allowed us to control for a large number of potential confounders, including socioeconomic and lifestyle factors that might play a role in the occurrence of haemorrhagic stroke47 48 and that have also been linked with migraine.49 50
Several limitations should, however, be considered. Firstly, and most importantly, the number of cases of haemorrhagic stroke was small (n=85) and only nine cases occurred among women with active migraine with aura. Thus, and despite statistical significance, our data should be interpreted with caution. Secondly, migraine status was self reported by participants and not classified according to strict International Headache Society criteria.33 Although previous reports from the Women’s Health Study have shown good agreement of migraine classification with modified International Headache Society criteria,25 26 misclassification is possible. As our study design is prospective, however, such misclassification would probably be random and unlikely to explain the observed association between active migraine with aura and haemorrhagic stroke and, if present, would probably lead to an underestimation of the effect. Thirdly, we had no further details on migraine aura in this cohort, which would have allowed us to classify participants according to International Headache Society criteria for aura or to take frequency of aura into account. Our prevalence of aura, however, is in the range reported in other population based studies.3 51 Fourthly, the Women’s Health Study was designed as a randomised controlled trial of aspirin and vitamin E, and specifically for aspirin there is a concern of increased risk of haemorrhagic stroke. In the Women’s Health Study, however, neither aspirin nor vitamin E assignment was associated with risk of haemorrhagic stroke,28 29 randomised treatment assignments were evenly distributed across migraine groups, and we found no significant effect modification of the association between migraine with aura and haemorrhagic stroke according to randomised treatment assignments. Fifthly, despite adjustment for many potential confounders through a propensity score technique, residual and unmeasured confounding remains possible as our study is observational. Finally, participants in the Women’s Health Study were middle aged health professionals, most of whom were white, and thus generalisability to other populations of women might be limited.
Clinical implications
Migraine with aura has been consistently linked with an increased risk of ischaemic stroke and might also be a marker of increased risk for other ischaemic events. Our results extend those previous findings and suggest that migraine with aura might also be a marker for increased risk of haemorrhagic stroke. Because of the relatively low attributable risk (four additional haemorrhagic strokes associated with migraine with aura per 10 000 women per year), however, there are no immediate implications for clinicians or patients other than the evaluation of prevalent cardiovascular risk factors that might influence ischaemic and haemorrhagic vascular events.
Unanswered questions and future research
There are many unanswered questions, particularly involving specifics on migraine aura and biological mechanisms linking migraine with aura to an increased risk of haemorrhagic stroke. The low attributable risk might mean that the increased risk of haemorrhagic stroke is limited to a subgroup of patients with migraine with aura. In addition to studies targeting potential biological mechanisms, future collaborative efforts are needed to study whether migraine aura specifics or the course of migraine influences risk of subsequent ischaemic and haemorrhagic vascular events.
What is already known on this topic
Migraine with aura has been associated with increased risk of ischaemic stroke and other ischaemic vascular events
Migraine with aura has also been linked to an adverse cardiovascular risk profile and endothelial dysfunction
What this study adds
In a large prospective study of female health professionals aged 45 years or older at study entry, migraine with aura was associated with increased risk of haemorrhagic stroke
Absolute numbers and attributable risk were low, arguing against definitive conclusions, and calling for further confirmation of these observations
We thank the participants in the Women’s Health Study for their outstanding commitment and cooperation and the entire staff of the Women’s Health Study for their expert and unfailing assistance.
Contributors: TK conceived and designed the study, analysed the data, and drafted the manuscript. CSK and JEB were responsible for the acquisition of the data. All authors interpreted the data, critically revised the draft for important intellectual content, and gave final approval of the version to be published. TK is guarantor.
Funding: The Women’s Health Study is supported by grants from the National Heart, Lung, and Blood Institute (HL-043851 and HL-080467), and the National Cancer Institute (CA-47988). The research for this work was supported by grants from the Donald W Reynolds Foundation, the Leducq Foundation, and the Doris Duke Charitable Foundation. The sponsors of the study played no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: TK has received investigator initiated research funding from the French National Research Agency, the National Institutes of Health, Merck, and the Migraine Research Foundation. He is a consultant to i3 Drug Safety and World Health Information Science Consultants, LLC (www.whiscon.com), and has received honorariums from Genzyme, Merck, and Pfizer for educational lectures. CK has received honorariums as a consultant for Sanofi-Aventis. MS has received investigator initiated research funds from the Migraine Research Foundation and honorariums from LEK. CT has received investigator initiated research funding from the French National Research Agency and received fees from Sanofi-Synthelabo for participating in a data safety monitoring board and from Merck Sharp & Dohme and the Servier company for participating in scientific committees. JEB has received investigator initiated research funding and support from the National Institutes of Health and Dow Corning Corporation and research support for pills and/or packaging from Bayer Heath Care and the Natural Source Vitamin E Association.
Ethical approval: This study was approved by the Institutional Review Board of Brigham and Women’s Hospital, Boston, MA, and all participants provided written informed consent.
Data sharing: No additional data available.
Cite this as: BMJ 2010;341:c3659
References
- 1.Goadsby PJ, Lipton RB, Ferrari MD. Migraine—current understanding and treatment. N Engl J Med 2002;346:257-70. [DOI] [PubMed] [Google Scholar]
- 2.Lipton RB, Bigal ME. The epidemiology of migraine. Am J Med 2005;118(suppl 1):3-10S. [DOI] [PubMed] [Google Scholar]
- 3.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41:646-57. [DOI] [PubMed] [Google Scholar]
- 4.Henrich JB, Horwitz RI. A controlled study of ischemic stroke risk in migraine patients. J Clin Epidemiol 1989;42:773-80. [DOI] [PubMed] [Google Scholar]
- 5.Tzourio C, Tehindrazanarivelo A, Iglesias S, Alperovitch A, Chedru F, d’Anglejan-Chatillon J, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ 1995;310:830-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ 1999;318:13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurth T, Slomke MA, Kase CS, Cook NR, Lee IM, Gaziano JM, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology 2005;64:1020-6. [DOI] [PubMed] [Google Scholar]
- 8.Stang PE, Carson AP, Rose KM, Mo J, Ephross SA, Shahar E, et al. Headache, cerebrovascular symptoms, and stroke: the Atherosclerosis Risk in Communities Study. Neurology 2005;64:1573-7. [DOI] [PubMed] [Google Scholar]
- 9.MacClellan LR, Giles WH, Cole J, Wozniak MA, Stern B, Mitchell B, et al. Probable migraine with visual aura and risk of ischemic stroke: the Stroke Prevention in Young Women Study. Stroke 2007;38:2438-45. [DOI] [PubMed] [Google Scholar]
- 10.Bigal ME, Kurth T, Santanello N, Buse D, Golden W, Robbins M, et al. Migraine and cardiovascular disease: a population-based study. Neurology 2010;74:628-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ 2005;330:63-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ 2009;339:b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bousser MG, Welch KM. Relation between migraine and stroke. Lancet Neurol 2005;4:533-42. [DOI] [PubMed] [Google Scholar]
- 14.Schurks M, Zee RY, Buring JE, Kurth T. Interrelationships among the MTHFR 677C>T polymorphism, migraine, and cardiovascular disease. Neurology 2008;71:505-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schurks M, Zee RY, Buring JE, Kurth T. ACE D/I polymorphism, migraine, and cardiovascular disease in women. Neurology 2009;72:650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scher AI, Terwindt GM, Picavet HS, Verschuren WM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology 2005;64:614-20. [DOI] [PubMed] [Google Scholar]
- 17.Napoli R, Guardasole V, Zarra E, Matarazzo M, D’Anna C, Sacca F, et al. Vascular smooth muscle cell dysfunction in patients with migraine. Neurology 2009;72:2111-4. [DOI] [PubMed] [Google Scholar]
- 18.Tietjen GE, Al-Qasmi MM, Athanas K, Utley C, Herial NA. Altered hemostasis in migraineurs studied with a dynamic flow system. Thromb Res 2007;119:217-22. [DOI] [PubMed] [Google Scholar]
- 19.Vanmolkot FH, Van Bortel LM, de Hoon JN. Altered arterial function in migraine of recent onset. Neurology 2007;68:1563-70. [DOI] [PubMed] [Google Scholar]
- 20.Lee ST, Chu K, Jung KH, Kim DH, Kim EH, Choe VN, et al. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology 2008;70:1510-7. [DOI] [PubMed] [Google Scholar]
- 21.Tzourio C, El Amrani M, Poirier O, Nicaud V, Bousser MG, Alperovitch A. Association between migraine and endothelin type A receptor (ETA -231 A/G) gene polymorphism. Neurology 2001;56:1273-7. [DOI] [PubMed] [Google Scholar]
- 22.Tikka-Kleemola P, Kaunisto MA, Hamalainen E, Todt U, Gobel H, Kaprio J, et al. Genetic association study of endothelin-1 and its receptors EDNRA and EDNRB in migraine with aura. Cephalalgia 2009;29:1224-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collaborative Group for the Study of Stroke in Young Women. Oral contraceptives and stroke in young women: associated risk factors. JAMA 1975;231:718-22. [DOI] [PubMed] [Google Scholar]
- 24.Bushnell CD, Jamison M, James AH. Migraines during pregnancy linked to stroke and vascular diseases: US population based case-control study. BMJ 2009;338:b664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA 2006;296:283-91. [DOI] [PubMed] [Google Scholar]
- 26.Cook NR, Bensenor IM, Lotufo PA, Lee IM, Skerrett PJ, Chown MJ, et al. Migraine and coronary heart disease in women and men. Headache 2002;42:715-27. [DOI] [PubMed] [Google Scholar]
- 27.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women’s Health Study. J Womens Health Gend Based Med 2000;9:19-27. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005;352:1293-304. [DOI] [PubMed] [Google Scholar]
- 29.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA 2005;294:56-65. [DOI] [PubMed] [Google Scholar]
- 30.Kurth T, Schurks M, Logroscino G, Gaziano JM, Buring JE. Migraine, vascular risk, and cardiovascular events in women: prospective cohort study. BMJ 2008;337:a636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia 1988;8(suppl 7):1-96S. [PubMed] [Google Scholar]
- 32.Schurks M, Buring JE, Kurth T. Agreement of self-reported migraine with ICHD-II criteria in the Women’s Health Study. Cephalalgia 2009;29:1086-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olesen J, Steiner TJ. The International classification of headache disorders. 2nd ed (ICDH-II). J Neurol Neurosurg Psychiatry 2004;75:808-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atiya M, Kurth T, Berger K, Buring JE, Kase CS. Interobserver agreement in the classification of stroke in the Women’s Health Study. Stroke 2003;34:565-7. [DOI] [PubMed] [Google Scholar]
- 35.Kurth T, Kase CS, Berger K, Gaziano JM, Cook NR, Buring JE. Smoking and risk of hemorrhagic stroke in women. Stroke 2003;34:2792-5. [DOI] [PubMed] [Google Scholar]
- 36.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41-55. [Google Scholar]
- 37.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87. [DOI] [PubMed] [Google Scholar]
- 38.Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol 2003;158:280-7. [DOI] [PubMed] [Google Scholar]
- 39.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology 2003;14:680-6. [DOI] [PubMed] [Google Scholar]
- 40.Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol 2006;163:262-70. [DOI] [PubMed] [Google Scholar]
- 41.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97. [DOI] [PubMed] [Google Scholar]
- 42.Kruit MC, van Buchem MA, Hofman PA, Bakkers JT, Terwindt GM, Ferrari MD, et al. Migraine as a risk factor for subclinical brain lesions. JAMA 2004;291:427-34. [DOI] [PubMed] [Google Scholar]
- 43.Scher AI, Gudmundsson LS, Sigurdsson S, Ghambaryan A, Aspelund T, Eiriksdottir G, et al. Migraine headache in middle age and late-life brain infarcts. JAMA 2009;301:2563-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monteiro JM, Rosas MJ, Correia AP, Vaz AR. Migraine and intracranial vascular malformations. Headache 1993;33:563-5. [DOI] [PubMed] [Google Scholar]
- 45.D’Andrea G, Cananzi AR, Perini F, Hasselmark L. Platelet models and their possible usefulness in the study of migraine pathogenesis. Cephalalgia 1995;15:265-71. [DOI] [PubMed] [Google Scholar]
- 46.Hering-Hanit R, Friedman Z, Schlesinger I, Ellis M. Evidence for activation of the coagulation system in migraine with aura. Cephalalgia 2001;21:137-9. [DOI] [PubMed] [Google Scholar]
- 47.Kurth T, Moore SC, Gaziano JM, Kase CS, Stampfer MJ, Berger K, et al. Healthy lifestyle and the risk of stroke in women. Arch Intern Med 2006;166:1403-9. [DOI] [PubMed] [Google Scholar]
- 48.Kurth T, Gaziano JM, Rexrode KM, Kase CS, Cook NR, Manson JE, et al. Prospective study of body mass index and risk of stroke in apparently healthy women. Circulation 2005;111:1992-8. [DOI] [PubMed] [Google Scholar]
- 49.Bigal ME, Liberman JN, Lipton RB. Obesity and migraine: a population study. Neurology 2006;66:545-50. [DOI] [PubMed] [Google Scholar]
- 50.Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache 2005;45(suppl 1):3-13S. [DOI] [PubMed] [Google Scholar]
- 51.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology 1999;53:537-42. [DOI] [PubMed] [Google Scholar]