Abstract
Na+/Cl−-dependent neurotransmitter transporters form a superfamily of transmembrane proteins that share 12 membrane-spanning regions. To gain information about the quaternary structure of these transporter proteins, we heterologously expressed the glial glycine transporter GlyT1 and its neuronal homolog GlyT2 in Xenopus oocytes. By using metabolic labeling with [35S]methionine or surface labeling with a plasma membrane impermeable reagent followed by affinity purification, we separately analyzed the total cellular pools of newly synthesized GlyTs and its functional plasma membrane-bound fractions. Upon blue native gel electrophoresis, the surface-localized transporter proteins were found to exist exclusively in complex-glycosylated monomeric form, whereas a significant fraction of the intracellular GlyT1 and GlyT2 was core-glycosylated and oligomeric. In contrast, even after treatment with the crosslinker glutaraldehyde, surface GlyTs failed to migrate as oligomeric proteins. These results indicate that plasma membrane-bound GlyT1 and GlyT2 are monomeric proteins. Thus, Na+/Cl−-dependent neurotransmitter transporters do not require oligomerization for substrate translocation.
Keywords: neurotransmitter transporters, quaternary structure, blue native PAGE
Neurotransmitter transporters play a key role in regulating the concentration of neurotransmitters in the synaptic cleft of central synapses and are believed to shape the time course of synaptic transmission (1, 2). Because of their similarities in primary structure and functional properties, the transporters for monoamines (serotonin, norepinephrine, and dopamine) and the inhibitory neurotransmitters γ-aminobutyric acid and glycine are classified into a common protein superfamily, the Na+/Cl−-dependent neurotransmitter transporters (1–3). These membrane proteins share a common transmembrane topology with 12 membrane-spanning segments each and cytoplasmic C- and N-terminal regions. Multiple N-glycosylation sites characterize the large extracellular loop connecting membrane segments 3 and 4; glycosylation at these sites is required for efficient plasma membrane insertion of newly synthesized transporter polypeptides (4).
Many efforts have been made to elucidate structure/function relationships for Na+/Cl−-dependent neurotransmitter transporters, including mapping of substrate and ion binding sites, determination of transmembrane domain orientation, as well as secondary and tertiary structure predictions (5–11). Little, however, is known about the quaternary structure of these membrane proteins. For the serotonin transporter (SERT) and the dopamine transporter (DAT), evidence for an oligomeric structure has been reported (12–15). Based on coexpression of mutant constructs, biochemical crosslinking, co-immunoprecipitation of differentially tagged proteins, and radiation inactivation, both the recombinant SERT and DAT have been proposed to exist in homodimeric and/or homotetrameric forms (12–15). On the other hand, SERT adducts have not been detected in native brain tissue (16). Also, for the structurally unrelated Na+-coupled glucose transporter containing 14 putative transmembrane domains, freeze-fracture electron microscopy (17) has yielded results that differ from those obtained by radiation inactivation (18). The proposal that Na+/Cl−-dependent neurotransmitter transporters function as oligomeric proteins (12–15) may therefore not be conclusive. Artefactual aggregation of intracellular proteins resulting from overexpression in heterologous cell systems as well as incomplete solubilization or oxidation during the detergent extraction of hydrophobic membrane proteins may severely bias conclusions based on crosslinking or immunoprecipitation approaches (19–22). Methods suitable for the selective detection of native plasma membrane-bound transporters appear essential for further clarification.
In this report, we used blue native (BN)/PAGE (23) to investigate the quaternary structure of the glial glycine transporter GlyT1 (24, 25) and its neuronal homolog GlyT2 (26). This method has proved suitable for analyzing under nondenaturing conditions the subunit composition and quaternary structure of different membrane proteins (27), including the P2X receptor, the glycine receptor, and the nicotinic acetylcholine receptor (28, 29). By using BN/PAGE in combination with selective surface-labeling methods, we show that both GlyT1 and GlyT2 function as monomeric plasma membrane proteins.
Experimental Procedures
Expression of Hexahistidyl (His6)-Tagged GlyT1 and GlyT2 in Xenopus Oocytes.
His6-tagged GlyT1 and GlyT2 cDNAs were constructed by PCR by using appropriate primers on either the mouse GlyT1a cDNA (25) subcloned in pCIS or on the rat GlyT2a cDNA (26) subcloned in pRC, respectively. For the His6-tagged GlyT1 constructs containing the tag attached to the N terminus (His-GlyT1) or C terminus (GlyT1-His), HindIII–XhoI fragments of the PCR products encoding the entire GlyT1 sequence and six histidines at the appropriate position were subcloned into the pNKS2 vector designed for cRNA synthesis for expression in Xenopus oocytes (30). For attaching the His6-tag to the N terminus of GlyT2 (His-GlyT2), a HindIII–NotI fragment of the respective PCR product was ligated to the 3′ NotI–PstI fragment of the GlyT2 cDNA, and after ligation the fragment was subcloned into the HindIII–PstI sites of the pNKS2 vector. A C-terminal His6 tag was introduced into GlyT2 (GlyT2-His) by ligating a NotI–PstI fragment of the corresponding PCR product to the 5′ HindIII–NotI fragment of the GlyT2 cDNA. After ligation, the tagged cDNA was subcloned into pNKS2 digested with HindIII and PstI. All constructs were verified by dideoxy sequencing of the entire coding sequences. Capped cRNAs were synthesized by SP6 RNA polymerase from templates linearized with EcoRI, purified by Sepharose G50 chromatography and phenol chloroform extraction, and dissolved in 5 mM Tris-HCl, pH 7.2, by using the optical density at 260 nm for quantitation (A260 of 1.0 = 0.04 μg/μl).
Oocytes were removed from Xenopus laevis females under anesthesia with ice-cold 1% (wt/vol) urethane, or 0.2% (wt/vol) tricaine, in water. Oocytes were defolliculated by overnight treatment with collagenase at 19°C. Washed oocytes were injected with 50-nl aliquots of cRNAs (0.5 μg/μl) and cultured at 19°C in ORi (oocyte Ringer's solution: 90 mM NaCl/1 mM KCl/1 mM CaCl2/1 mM MgCl2/5 mM Hepes, pH 7.4) until use.
[3H]Glycine Uptake.
Two days after the injection of cRNA, oocytes were assayed for glycine transport activity (24). Before uptake measurements, the oocytes were incubated for 15 min in 0.5 ml of a solution containing 102 mM KCl/1 mM CaCl2/1 mM MgCl2/10 mM Hepes, pH 7.5. The transport reaction was initiated by the addition of 100 mM NaCl/2 mM KCl/1 mM CaCl2/1 mM MgCl2/10 mM Hepes (pH 7.5)/0.05 μCi (10 μM) of [2-3H]glycine (1.52 GBq/μmol, Moravek Biochemicals, Brea, CA). At the end of a 45-min incubation period, the oocytes were washed three times with 0.5 ml of the same buffer without glycine and solubilized individually in 200 μl of 1% (wt/vol) SDS. Intracellular radioactivity was determined by scintillation counting.
Labeling Procedures and Affinity Purification by Ni2+-Nitrilotriacetic Acid (NTA) Chromatography.
His6-tagged GlyTs were purified by Ni2+-NTA agarose chromatography. cRNA-injected oocytes and noninjected controls were metabolically labeled by overnight incubation with L-[35S]methionine (>40 TBq/mmol, Hartman Analytic, Braunschweig) at 100 MBq/ml in ORi at 19°C. For labeling cell-surface proteins, [125I]sulfosuccinimidyl-3-(4-hydroxyphenyl)propionate ([125I]sulfo-SHPP), a membrane-impermeant amino-reactive reagent (31), was used exactly as described (28). Labeled oocytes were homogenized in detergent buffer consisting of 0.1 M sodium phosphate buffer, pH 8.0, containing 0.1 μM phenylmethylsulfonyl fluoride, the protease inhibitors antipain, leupeptin, and pepstatin A (10 μM, each; Biomol), and 1% (wt/vol) digitonin. After 15 min on ice, the detergent extracts were cleared by centrifugation (twice for 15 min at 30,000 × g and 4°C), diluted with detergent buffer, and incubated with Ni2+-NTA agarose (Qiagen) in the presence of 10 mM imidazole. After 30 min of rotary agitation at ambient temperature, the beads were washed four times with ice-cold detergent buffer containing 25 mM imidazole-HCl, pH 8.0. Bound protein was eluted with 20 mM Tris⋅HCl, pH 7.4, containing 200 mM imidazole, or with a mixture of 100 mM imidazole and 10 mM EDTA, and the desired detergent. Eluted proteins were kept at 0°C until analyzed.
BN/PAGE and SDS/PAGE.
BN/PAGE was carried out as described (28, 29). Samples were dissolved in 10% (wt/vol) glycerol, 0.2% (wt/vol) Serva blue G, and 20 mM sodium 6-amino-n-caproate and applied onto polyacrylamide gradient slab gels. Molecular mass markers (Combithek II, Boehringer Mannheim) were run in two different lanes of the gel and subsequently visualized by Coomassie staining. For SDS/PAGE, proteins were supplemented with SDS sample buffer to reach final concentrations of 60 mM Tris⋅HCl, pH 6.8/1% (wt/vol) SDS/10% (wt/vol) glycerol/0.2% (wt/vol) bromphenol blue/20 mM DTT and electrophoresed in parallel with 14C-labeled molecular mass markers (Rainbow, Amersham Pharmacia) on SDS/polyacrylamide gels. Endo-N-acetylglucosaminidase H (endo H) or peptide N-glycosidase F (PNGase F; New England Biolabs) treatment was performed in SDS-sample buffer containing 20 mM DTT for 2 h, or 24 h, at 37°C as detailed previously (28, 29). Gels were fixed, dried, and exposed to BioMax MS with BioMax TransScreen-LE (Kodak) at −80°C.
Crosslinking.
To crosslink surface-labeled protein, the 125I-SHPP-labeled oocytes were treated with 10 mM glutaraldehyde for the indicated time at 19°C. After incubation, the proteins were solubilized with 1% (wt/vol) digitonin and purified as described above. After elution with 200 mM imidazol buffer, pH 6.8, the protein samples were analyzed by SDS/PAGE on 4–10% gradient gels. Crosslinking of metabolically labeled polypeptides was performed with Ni2+-NTA agarose-bound protein. After washing, the bound proteins were treated with 1 mM glutaraldehyde for 60 min at room temperature before elution with 200 mM imidazole, pH 7.4.
Results
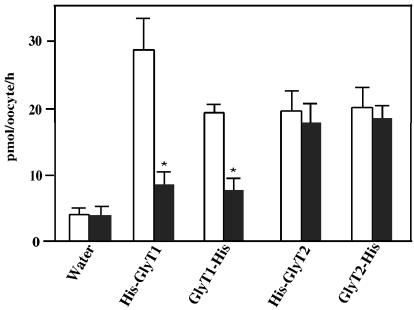
To allow affinity purification of recombinant GlyT1 and GlyT2 under nondenaturing conditions, we constructed N- and C-terminally His6-tagged versions of both transporter proteins. To examine whether the His6-tagged GlyT1 and GlyT2 proteins were functional in Xenopus oocytes, the respective cRNAs were injected, and expression was assessed by measuring [3H]glycine uptake activity. As shown in Fig. 1, oocytes injected with the different cRNAs all showed transport activities that were comparable with those of the unmodified wild-type transporters and similar to the uptake values reported by Liu et al. (24, 26). In accordance with the known substrate specificities of GlyT1 and GlyT2 (26, 32), His-GlyT1 and GlyT1-His mediated [3H]glycine uptake was inhibited by 1 mM sarcosine, in contrast to His-GlyT2 and GlyT2-His driven transport. These results show that the His6-tagged recombinant GlyT proteins are functionally active and therefore can be used for analyzing the quaternary structure of these membrane proteins.
Figure 1.
Transport activity of heterologously expressed His6-tagged GlyTs. Oocytes injected with 25 ng of the indicated cRNAs or water were kept at 19°C for 48 h. Groups of four oocytes each were then incubated with 10 μM [3H]glycine in the absence (open column) or presence (closed column) of 1 mM sarcosine for determination of [3H]glycine uptake. The data show the means ± SD from four groups of oocytes for each experimental condition, with uptake values of 28.7 ± 3.4 and 19.3 ± 1.2 pmol/oocyte/h for His-GlyT1 and GlyT1-His, and of 19.7 ± 2.9 and 20.1 ± 3.2 pmol/oocyte/h for His-GlyT2 and GlyT2-His, respectively. These values correspond to 60–129% of the uptake found with the wild-type GlyT1 and GlyT2 proteins under identical conditions. Statistically significant differences between oocytes incubated with and without sarcosine are indicated (*, P < 0.01).
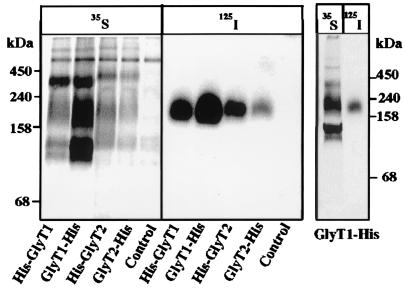
To evaluate whether the GlyT proteins might be oligomeric, we affinity-purified the His6-tagged transporters under nondenaturing conditions and analyzed them by BN/PAGE. To this end, oocytes injected with the GlyT cRNAs were either metabolically labeled with [35S]methionine or surface-labeled with [125I]sulfo-SHPP before extraction with 1% (wt/vol) digitonin and affinity purification on an Ni2+-NTA column. The purified GlyT1 and GlyT2 proteins were then analyzed by BN/PAGE (Fig. 2). After labeling with [125I]sulfo-SHPP, only single bands migrating at an apparent molecular mass of ≈200 kDa were detected with all GlyT constructs. In contrast, metabolic labeling consistently resulted in a ladder of 35S polypeptides corresponding to molecular masses of about 100, 200, and 400 kDa or higher, respectively. Apparently, the protein species other than the bands of ≈200 kDa were localized intracellularly and not accessible to surface iodination. Therefore, the bands of ≥400 kDa may represent oligomers or aggregates of intracellularly retained protein. Control experiments showed that detergent extraction with 1% (wt/vol) dodecylmaltoside produced essentially the same result as obtained with digitonin (data not shown). Thus, the different labeling patterns obtained with [125I]sulfo-SHPP and [35S]methionine are unlikely to result from detergent-specific protein aggregation. Furthermore, the failure to detect bands of ≥400 kDa in surface-labeled GlyT preparations could not be attributed to a loss of stabilizing substrate or dissociation by the Coomassie blue dye used in BN/PAGE. Neither purification and electrophoresis of the 125I-labeled GlyT1-His in the presence of 10 mM glycine nor native PAGE of this protein performed with Coomassie blue present at a 100-fold reduced concentration (0.002%, wt/vol) in the cathode buffer only produced bands other than the ≈200-kDa species (data not shown).
Figure 2.
BN/PAGE of metabolically and surface-labeled GlyTs. (Left) Oocytes injected with 25 ng of the indicated cRNAs were labeled metabolically with [35S]methionine or surface-labeled with [125I]sulfo-SHPP, respectively. After solubilization with 1% (wt/vol) digitonin, the His6-tagged GlyT proteins were isolated by Ni2+-NTA chromatography and resolved by 4–13% BN/PAGE. (Right) For direct comparison, the [35S]methionine-labeled and [125I]-labeled GlyT1-His preparations were separated on adjacent lanes of a native 4–13% polyacrylamide gradient gel.
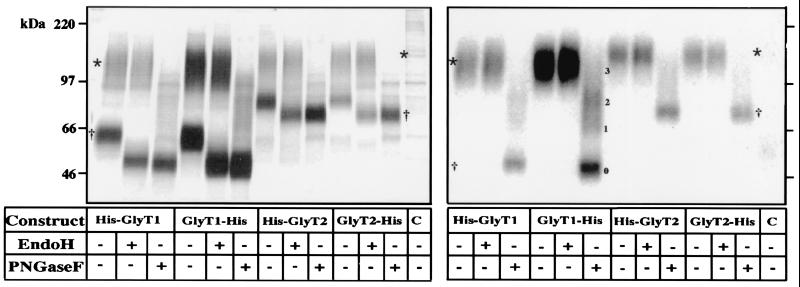
Next, the affinity-purified GlyT proteins shown in Fig. 2 were analyzed by SDS/PAGE (Fig. 3). This resolved the metabolically labeled His-GlyT1 and GlyT1-His preparations into two bands of 115 kDa and 63 kDa each. Upon glycosidase treatment, the 115-kDa bands proved resistant to endo H, whereas the 63-kDa bands displayed a remarkable size shift to about 49 kDa. In contrast, both polypeptide species migrated at 46 kDa after PNGase F treatment. These results indicate that the 115-kDa bands are complex-glycosylated, whereas the 63-kDa bands carry only core glycans. Similarly, the His-GlyT2 and GlyT2-His proteins also were resolved into two polypeptide species of about 120 kDa and 80 kDa, with both polypeptides being shifted to a core molecular mass of 73 kDa upon PNGase F treatment, whereas endo H increased only the mobility of the 80-kDa band. These characteristics in glycosylation state and accessibility to surface labeling are consistent with the 63-kDa and 80-kDa bands representing endoplasmic reticulum (ER) or early Golgi transport intermediates, whereas the 115-kDa and 120-kDa bands correspond to the complex-glycosylated plasma membrane transporters. Notably, three additional bands could be distinguished after PNGase F treatment of the surface-labeled GlyT1-His protein (Fig. 3, Right). These bands represent partially deglycosylated and nonglycosylated polypeptides that are expected to accumulate under conditions of incomplete PNGase F digestion, consistent with the presence of four putative N-glycosylation sites in this transporter (4).
Figure 3.
SDS/PAGE of metabolically (Left) and surface-labeled (Right) GlyTs before and after deglycosylation. Affinity-purified GlyT proteins were denatured in reducing SDS sample buffer, incubated for 2 h or 24 h, at 37°C with the enzymes indicated (5 units of endo H, 2.5 units of PNGase F), and analyzed on an 8% polyacrylamide gel. Different deglycosylation intermediates of GlyT1 and its fully deglycosylated form are indicated by 1–3 and 0, respectively. * and †, complex-glycosylated and core-glycosylated GlyT polypeptides.
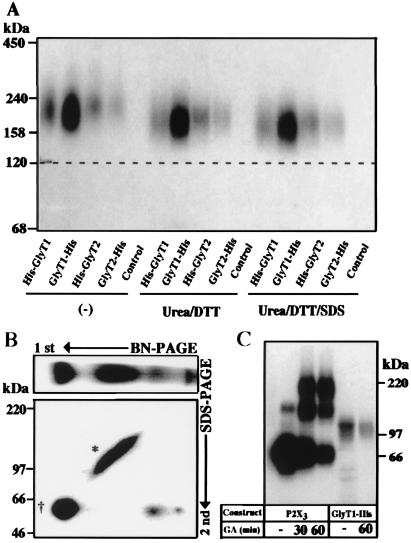
Upon surface labeling, all His6-tagged GlyT proteins exhibited a mobility in BN/PAGE that corresponded to an apparent molecular mass of ≈200 kDa (estimated by comparison with soluble marker proteins). This value is much higher than the calculated molecular masses of the GlyT1 (70.6 kDa) and GlyT2 (87.9 kDa) polypeptides and their even lower apparent molecular masses determined by SDS/PAGE, suggesting that these bands could represent dimers of the complex glycosylated forms that run at 115–120 kDa upon SDS/PAGE (Fig. 3). Alternatively, the apparent molecular masses of the GlyT monomers may be overestimated when using soluble proteins as molecular markers, as shown for the glycine receptor and the P2X3 receptor (28, 29). All of these membrane proteins might display a low electrophoretic mobility because of voluminous sugar side chains and/or bound detergent. Also, because BN/PAGE is a charge shift method (23), the excess of negative charges conferred by bound Serva blue G dye may not suffice to render the electrophoretic mobility of the His6-tagged GlyTs entirely independent of their intrinsic basic charges (23). To determine whether the 200-kDa band represents a monomer or an oligomer, we treated the affinity-purified GlyT samples with urea and DTT or urea, DTT, and SDS, respectively (Fig. 4A). Under both conditions, only minor shifts in the electrophoretic mobility of all His6-tagged GlyT proteins were observed. Thus, the low mobility resulting in an apparent molecular mass of ≈200 kDa of the surface-labeled GlyTs is likely because of a decreased electrophoretic mobility in the BN/PAGE system, but not because of oligomerization or formation of disulfide bonds between GlyT polypeptides. We therefore conclude that plasma membrane GlyTs are monomeric proteins. Consistent with this interpretation, the higher molecular mass complexes observed in BN/PAGE after metabolic labeling of GlyT1-His migrated at 63 kDa when subjecting the resolved polypeptides to SDS/PAGE on a two-dimensional polyacrylamide gel (Fig. 4B). Thus, these complexes are exclusively composed of the core-glycosylated intracellular GlyT1 protein.
Figure 4.
Surface GlyTs migrate as monomeric proteins. (A) Effect of reduction, urea, and SDS on the mobility of surface-labeled GlyT proteins. After surface labeling with [15I]sulfo-SHPP and affinity purification, the 125I-labeled GlyTs were incubated at room temperature either alone (Left) or with 100 mM DTT and 8 M urea in the absence (Center) or presence (Right) of 0.1% (wt/vol) SDS, respectively, before analysis by 4–10% BN/PAGE. Note that the mobility of the labeled proteins did not change significantly under the different conditions. (B) An aliquot of a detergent extract prepared from an GlyT1-His-injected oocyte after metabolic labeling with [35S]methionine as shown in Fig. 2 was subjected to two-dimensional PAGE. After BN/PAGE on a 4–10% acrylamide gradient gel, the separated proteins were resolved in the second dimension by 8% SDS/PAGE. * and †, the complex-glycosylated and core-glycosylated forms, respectively. (C) Surface-labeled oocytes expressing either the P2X3 receptor or GlyT1-His were incubated with 10 mM glutaraldehyde (GA) for the indicated periods before extraction and affinity purification. The purified proteins were then analyzed on a 4–10% SDS/polyacrylamide gradient gel. Note the formation of P2X3 dimers and trimers, whereas no adducts were seen with GlyT1-His.
To exclude the existence of oligomeric GlyT forms that are unstable under the conditions of BN/PAGE, we crosslinked the GlyT1-His protein with glutaraldehyde, both in intact oocytes before detergent extraction and after affinity purification. For crosslinking after purification, the metabolically labeled protein was immobilized on Ni2+-NTA beads before incubation with the crosslinker. This resulted in the formation of a ≈130-kDa band (not shown, but see ref. 12 for similar results with SERT). This band may represent a dimer of the core-glycosylated ER form (≈60 kDa) because its apparent molecular mass was reduced upon endo H digestion (data not shown). Alternatively, the injected oocytes were surface-labeled with [125I]sulfo-SHPP and incubated with glutaraldehyde for up to 60 min before extraction and affinity purification. In agreement with a previous report (28), these conditions resulted in the efficient crosslinking of surface-localized P2X3 receptor subunits after 30 min (Fig. 4C). In contrast, the plasma membrane GlyT1-His protein remained fully monomeric even after a 60-min treatment (Fig. 4C). These results further corroborate our conclusion that GlyTs do not form oligomers in the plasma membrane.
Discussion
The major finding of this study is that the recombinant glycine transporters GlyT1 and GlyT2 exist as monomeric proteins in the plasma membrane of Xenopus oocytes. In all of our surface-labeling experiments employing [125I]sulfo-SHPP, exclusively complex-glycosylated GlyT1 and GlyT2 monomers were detected. As [3H]glycine uptake studies showed that all of the His6-tagged GlyT proteins used in our experiments were functional in intact oocytes, we conclude that the substrate translocation and ion channel functions of Na+/Cl−-dependent neurotransmitter transporters do not depend on homo-oligomerization of these polytopic membrane polypeptides. This contrasts the stringent dependence on multisubunit assembly of different voltage- and ligand-gated ion channel proteins (19, 33) and suggests that the 12 membrane segments of individual Na+/Cl−-dependent neurotransmitter transporter molecules are sufficient to form both the neurotransmitter and ion translocation pathways.
Our conclusion is based on the following findings obtained upon surface iodination of GlyT1 and GlyT2 expressing oocytes with the membrane-impermeant reagent [125I]sulfo-SHPP. First, affinity purification of the epitope-tagged GlyT proteins invariably resulted in the isolation of single-labeled polypeptide species that migrated at apparent molecular masses of 200 kDa in BN/PAGE and 115 kDa, or 120 kDa, respectively, on SDS/polyacrylamide gels. The electrophoretic mobility of these polypeptides was not altered upon treatment with endo H but strikingly increased after incubation with PGNase F, an enzyme known to remove complex-glycosylated carbohydrate side chains. Second, the 200-kDa bands revealed by BN/PAGE proved resistant to treatment with SDS/urea and the reducing agent DTT. Thus, these bands did not represent complexes resulting from protein aggregation or cysteine oxidation. Third, incubation with the potent crosslinking reagent glutaraldehyde of [125I]sulfo-SHPP-labeled oocytes expressing the GlyT1-His protein failed to generate higher order adducts of this plasma membrane transporter, although another oligomeric membrane protein, the P2X3 receptor, was efficiently crosslinked under the same conditions. Also, native PAGE with Coomassie blue being added at a 100-fold reduced concentration to the cathode buffer only as well as purification and BN/PAGE in the presence of 10 mM glycine failed to generate GlyT1-His bands of >200 kDa. These results argue against the existence of GlyT oligomers that may not be stable under our conditions of detergent extraction and/or purification and confirm earlier observations indicating that digitonin is a rather mild detergent that has little or no denaturing effects on membrane proteins (34, 35).
Our results are consistent with previous sedimentation data on GlyT preparations solubilized from pig brainstem that resulted in an estimated molecular mass of 86 kDa (36). They contrast, however, several reports that indicate the existence of homo-oligomers for the recombinant monoamine transporters SERT and DAT (12–15). In particular, dimeric adducts of SERT have been identified by different investigators (12–14), and circumstantial evidence for the possible formation of both SERT and DAT tetramers has been reported (12, 13, 15). Interestingly, higher molecular weight forms of GlyT1 and GlyT2 were also observed in this study upon metabolic labeling of the transporter proteins with [35S]methionine, and their migratory behavior on BN/PAGE (≥400 kDa) is consistent with a homodimeric (and possibly homotetrameric) structure. However, both the inability to detect these complexes in the plasma membrane of intact oocytes and the endo H sensitivity of its constituent polypeptides clearly indicate that these high molecular weight GlyT proteins correspond to intracellular forms of the transporters. Consistent with this interpretation, two-dimensional electrophoresis of 35S-labeled affinity-purified GlyT1-His preparations showed that not only the monomeric ER form (migrating at ≈100 kDa in BN/PAGE) but also the higher order complexes (≥400 kDa) all displayed an apparent molecular mass of 63 kDa under denaturing conditions. This size is characteristic of the core-glycosylated polypeptide present in the ER. We therefore conclude that recombinant GlyT proteins form homo-oligomeric complexes. However, this process seems to be restricted to the incompletely processed transporter polypeptides found in intracellular membranes and thus may represent an overexpression artifact.
The possibility of artefactual transporter oligomerization was also noted by Quian et al. (16) when analyzing both recombinant and endogenous rat SERT preparations. These authors obtained an ≈200-kDa SERT oligomer upon heterologous expression in mammalian cells but failed to identify this adduct in membrane preparations from rat brain. Furthermore, enzymatic deglycosylation of the endogenous SERT found in platelets resulted in the formation of the 200-kDa adduct, indicating that nonspecific aggregation may occur in vitro. In contrast, experiments in which coexpression of a truncated SERT polypeptide was found to abolish substrate uptake by the wild-type protein have been interpreted as convincing evidence for an oligomeric structure of this transporter protein (13). Similarly, the identification of a 3′ splice variant of the norepinephrine transporter that behaved as a dominant-negative transporter isoform upon heterologous expression (37) also has suggested a functionally relevant interaction between individual transporter monomers. Moreover, concatenated SERT dimers and tetramers, but not trimers, have been shown to display transport activity upon transient expression in COS cells (13). Finally, radiation inactivation of DAT in canine striatal membrane preparations has yielded a target size of 278 kDa (15). All of these latter results seem consistent with monoamine transporters functioning as dimeric and/or tetrameric proteins. When applying BN/PAGE to the His-tagged SERT, however, dimeric adducts were only found with the affinity-purified metabolically labeled transporter protein, but not upon surface labeling with [125I]sulfo-SHPP (G. Schmalzing and A. Ashrafi, unpublished data). Again, the 35S-labeled dimers, but not the surface-labeled monomers, proved sensitive to endo H digestion. These preliminary data indicate that in Xenopus oocytes the major fraction of surface SERT also is monomeric. Our findings on both GlyTs and the SERT cannot, however, exclude that a small fraction of these membrane proteins actually forms dimers in the plasma membrane. Also, some Na+/Cl−-dependent neurotransmitter transporters may form transient oligomers, a hypothesis that could account for the cooperativity seen in substrate uptake studies with monoamine transporters like the SERT (38). Such transient oligomerization might not be essential for substrate and ion transport function but provide for regulation of neurotransmitter uptake at sites of high transporter density.
Acknowledgments
We thank Dr. Bodo Laube, Bernd Feiler, and Sven Sadtler for helpful advice and M. Baier for secretarial assistance. This work was supported by Deutsche Forschungsgemeinschaft (SFB 269), European Community Grant ERBFMR X-CT 98-0228, and the Fonds der Chemischen Industrie. M.H. was supported by a fellowship from the Alexander-von-Humboldt Foundation.
Abbreviations
- BN
blue native
- DAT
dopamine transporter
- GlyT
glycine transporter
- His
hexahistidyl
- SERT
serotonin transporter
- NTA
nitrilotriacetic acid
- ER
endoplasmic reticulum
- [125I]sulfo-SHPP
[125I]sulfosuccinimidyl-3-(4-hydroxyphenyl)propionate
- endo H
endo-N-acetylglucosaminidase H
- PNGase F
peptide N-glycosidase F
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.041329498.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.041329498
References
- 1.Amara S G. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- 2.Nelson N. J Neurochem. 1998;71:1785–1803. doi: 10.1046/j.1471-4159.1998.71051785.x. [DOI] [PubMed] [Google Scholar]
- 3.Schloss P, Püschel A, Betz H. Curr Opin Cell Biol. 1994;6:595–599. doi: 10.1016/0955-0674(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 4.Olivares L, Aragon C, Gimenez C, Zafra F. J Biol Chem. 1995;270:9437–9442. doi: 10.1074/jbc.270.16.9437. [DOI] [PubMed] [Google Scholar]
- 5.Bennett E R, Kanner B I. J Biol Chem. 1997;272:1203–1210. doi: 10.1074/jbc.272.2.1203. [DOI] [PubMed] [Google Scholar]
- 6.Bismuth Y, Kavanaugh M P, Kanner B I. J Biol Chem. 1997;272:16096–16102. doi: 10.1074/jbc.272.26.16096. [DOI] [PubMed] [Google Scholar]
- 7.Chen J-G, Liu-Chen S, Rudnick G. Biochemistry. 1997;36:1479–1486. doi: 10.1021/bi962256g. [DOI] [PubMed] [Google Scholar]
- 8.Norregaard L, Frederiksen D, Nielsen E O, Gether U. EMBO J. 1998;17:4266–4273. doi: 10.1093/emboj/17.15.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J-G, Sachpatzidis A, Rudnick G. J Biol Chem. 1997;272:28321–28327. doi: 10.1074/jbc.272.45.28321. [DOI] [PubMed] [Google Scholar]
- 10.Chen J-G, Liu-Chen S, Rudnick G. J Biol Chem. 1998;273:12675–12681. doi: 10.1074/jbc.273.20.12675. [DOI] [PubMed] [Google Scholar]
- 11.Ponce J, Biton B, Benavides J, Avenet P, Aragon C. J Biol Chem. 2000;275:13856–13862. doi: 10.1074/jbc.275.18.13856. [DOI] [PubMed] [Google Scholar]
- 12.Jess U, Betz H, Schloss P. FEBS Lett. 1996;394:44–46. doi: 10.1016/0014-5793(96)00916-7. [DOI] [PubMed] [Google Scholar]
- 13.Chang A S, Starnes D M, Chang S M. Biochem Biophys Res Commun. 1998;249:416–421. doi: 10.1006/bbrc.1998.9158. [DOI] [PubMed] [Google Scholar]
- 14.Kilic F, Rudnick G. Proc Natl Acad Sci USA. 2000;97:3106–3111. doi: 10.1073/pnas.060408997. . (First Published March 14, 2000; 10.1073/pnas.060408997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milner H E, Beliveau R, Jarvis S M. Biochim Biophys Acta. 1994;1190:185–187. doi: 10.1016/0005-2736(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 16.Qian Y, Melikian H E, Rye D B, Levey A I, Blakely R D. J Neurosci. 1995;15:1261–1274. doi: 10.1523/JNEUROSCI.15-02-01261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eskandari S, Wright E M, Kreman M, Starace D M, Zampighi G A. Proc Natl Acad Sci USA. 1998;95:11235–11240. doi: 10.1073/pnas.95.19.11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens B R, Fernandez A, Hirayama B, Wright E M, Kempner E S. Proc Natl Acad Sci USA. 1990;87:1456–1460. doi: 10.1073/pnas.87.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green W N, Millar N S. Trends Neurosci. 1995;18:280–287. [PubMed] [Google Scholar]
- 20.Milligan G. Science. 2000;288:65–66. doi: 10.1126/science.288.5463.65. [DOI] [PubMed] [Google Scholar]
- 21.Haugeto O, Ullensvang K, Levy L M, Chaudhry F A, Honore T, Nielsen M, Lehre K P, Danbolt N C. J Biol Chem. 1996;271:27715–27722. doi: 10.1074/jbc.271.44.27715. [DOI] [PubMed] [Google Scholar]
- 22.Coscoy S, Lingueglia E, Lazdunski M, Barbry P. J Biol Chem. 1998;273:8317–8322. doi: 10.1074/jbc.273.14.8317. [DOI] [PubMed] [Google Scholar]
- 23.Schägger H, Cramer W A, von Jagow G. Anal Biochem. 1994;217:220–230. doi: 10.1006/abio.1994.1112. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q-R, Nelson H, Mandiyan S, Lopez-Corcuera B, Nelson N. FEBS Lett. 1992;305:110–114. doi: 10.1016/0014-5793(92)80875-h. [DOI] [PubMed] [Google Scholar]
- 25.Adams R H, Sato K, Shimada S, Tohyama M, Püschel A W, Betz H. J Neurosci. 1995;15:2524–2532. doi: 10.1523/JNEUROSCI.15-03-02524.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Q-R, Lopez-Corcuera B, Mandiyan S, Nelson H, Nelson N. J Biol Chem. 1993;268:22802–22808. [PubMed] [Google Scholar]
- 27.Schägger H, Pfeiffer K. EMBO J. 2000;19:1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicke A, Baümert H G, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G. EMBO J. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffon N, Büttner C, Nicke A, Kuhse J, Schmalzing G, Betz H. EMBO J. 1999;18:4711–4721. doi: 10.1093/emboj/18.17.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gloor S, Pongs O, Schmalzing G. Gene. 1995;160:213–217. doi: 10.1016/0378-1119(95)00226-v. [DOI] [PubMed] [Google Scholar]
- 31.Thompson J A, Lau A L, Cunningham D D. Biochemistry. 1986;26:743–750. doi: 10.1021/bi00377a014. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Corcuera B, Martinez-Maza R, Nunez E, Roux M, Supplisson S, Aragon C. J Neurochem. 1998;71:2211–2219. doi: 10.1046/j.1471-4159.1998.71052211.x. [DOI] [PubMed] [Google Scholar]
- 33.Betz H. Biochemistry. 1990;29:3591–3599. doi: 10.1021/bi00467a001. [DOI] [PubMed] [Google Scholar]
- 34.Brown M H, Cantrell D A, Brattsand G, Crumpton M J, Gullberg M. Nature (London) 1989;339:551–553. doi: 10.1038/339551a0. [DOI] [PubMed] [Google Scholar]
- 35.Schmalzing G, Ruhl K, Gloor S M. Proc Natl Acad Sci USA. 1997;94:1136–1141. doi: 10.1073/pnas.94.4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Corcuera B, Alcantara R, Vazquez J, Aragon C. J Biol Chem. 1993;268:2239–2243. [PubMed] [Google Scholar]
- 37.Kitayama S, Ikeda T, Mitsuhata C, Sato T, Morita K, Dohi T. J Biol Chem. 1999;274:10731–10736. doi: 10.1074/jbc.274.16.10731. [DOI] [PubMed] [Google Scholar]
- 38.Sur C, Betz H, Schloss P. Proc Natl Acad Sci USA. 1997;94:7469–7644. doi: 10.1073/pnas.94.14.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]