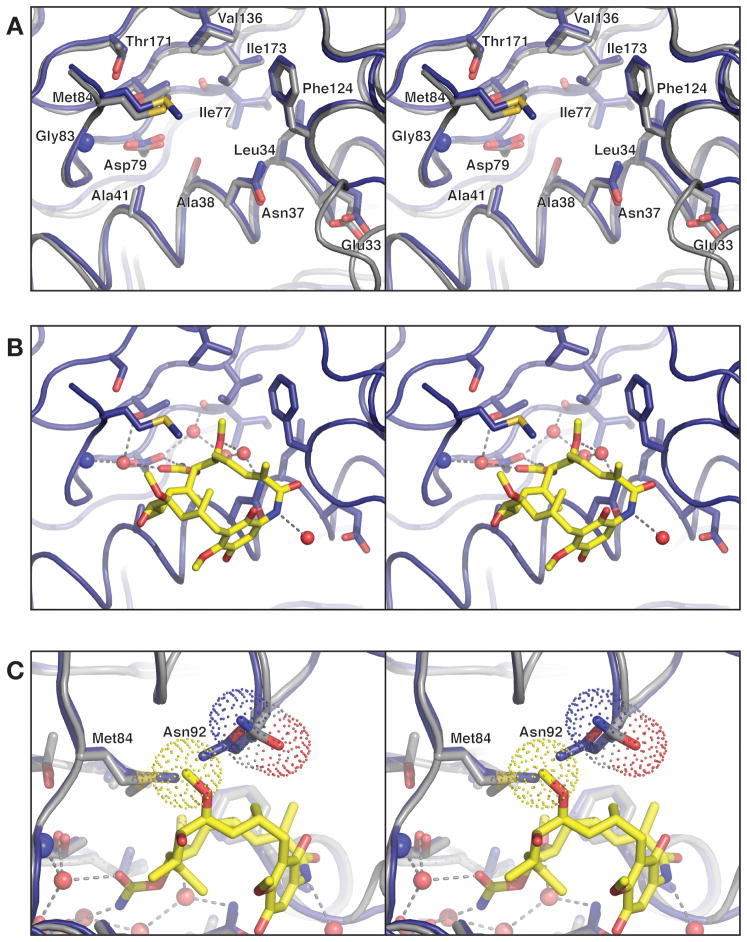
Figure 3. Prospective drug binding site of PfHsp90.
(A) Close-up of the Hsp90N nucleotide- binding site (blue) overlaid with the human Hsp90 N-terminal domain (gray), with residues surrounding the binding pocket show as sticks. The Cα r.m.s.d. for residues displayed is 0.23 Å. (B) Model for geldanamycin binding by PfHsp90, based on structural superposition of Hsp90N with the human Hsp90-geldanamycin complex (above). Geldanamycin and water molecules shown are present in the human Hsp90-geldanamycin complex structure. All chemical groups involved in hydrogen-bonding and Van der Waals interactions with geldanamycin are conserved between human and PfHsp90. (C) 90° rotated view from panel B, showing how Hsp90N residue Asn92 (blue model) clashes with C27 of modeled geldanamycin (yellow sticks, van der Waals surface for this atom is shown as yellow dots). The equivalent residue in human Hsp90, Asn106, is rotated slightly away to accommodate the ligand (gray model, van der Waals surface for the side chain is shown as dots).