Summary
Carbohydrate catabolite repression (CCR) in Streptococcus mutans does not require CcpA and is exerted through a network of phosphoenolpyruvate-dependent sugar:phosphotransferase system (PTS) permeases. To probe the molecular mechanisms of CCR in S. mutans, the effects of various ptsH (HPr) and hprK (HPr kinase/phosphatase) mutations on growth and CCR were evaluated. An hprKV265F mutation, which enhanced phosphorylation of HPr at Ser46, inhibited growth on multiple PTS sugars. A ptsHS46A mutation reversed the effects of hprKV265F in most cases. A strain carrying a ptsHS46D mutation, which mimics HPr(Ser-P), presented with more severe growth defects than the hprKV265F mutant. The hprKV265F mutant displayed reduced expression of the fruA and levD operons, a phenotype reversible by the introduction of the ptsHS46A mutation. The effects of the hprKV265F mutation on fruA and levD expression were independent of CcpA, but dependent on ManL (IIABMan) and, to a lesser extent, on the FruI (IIABCFru), in a sugar-specific manner. The hprKV265F mutation inhibited growth on cellobiose and lactose, but only the transcription of the cel operon was decreased. Thus, in S. mutans, serine-phosphorylated HPr functions in concert with particular PTS permeases to prioritize carbohydrate utilization through modulation of sugar transport activity and the transcription of catabolic operons.
Keywords: Catabolite repression, HPr, Carbohydrate transport, Biofilm, Virulence
Introduction
The most common etiological agent of human tooth decay, Streptococcus mutans, is equipped with a phosphoenolpyruvate (PEP)-dependent sugar:phosphotransferase system (PTS) dedicated to the internalization and phosphorylation of an array of carbohydrates (Ajdic et al., 2002; Ajdic and Pham, 2007; Vadeboncoeur and Pelletier, 1997). This system consists of the general components Enzyme I (EI) and a phosphocarrier protein (HPr) that participate in uptake of all PTS sugars using an array of substrate-specific enzyme II (EII) complexes (Postma et al., 1993). S. mutans lacks a complete TCA cycle and respiratory chain, so the organism depends entirely on glycolysis to generate sufficient energy for growth. Carbohydrate catabolism is also intimately associated with pathogenesis by this organism, since the organic acids generated by glycolysis are directly responsible for the damage to tooth mineral. Thus, the ability to coordinate the utilization of the wide variety of carbohydrates that are encountered continually or transiently in the oral cavity is essential to the persistence and pathogenesis of S. mutans in oral microbial communities. A comprehensive transcriptome analysis has demonstrated that the majority of the 14 PTS EII operons in S. mutans are indeed subject to complex regulation in response to carbohydrate sources (Ajdic and Pham, 2007).
The primary mechanism by which bacteria regulate the utilization of non-preferred carbohydrates in the presence of preferred carbon sources is known as carbon catabolite repression (CCR) (Deutscher et al., 2006; Deutscher, 2008; Gorke and Stulke, 2008). A variety of mechanisms exist to modulate CCR, but in low G+C Gram-positive bacteria, HPr is perhaps the most critical component. When HPr becomes phosphorylated at serine residue 46 (Ser46) by an ATP-dependent HPr kinase/phosphatase (HprK), it can function as a co-repressor for catabolite control protein A (CcpA) to promote the binding of CcpA to conserved catabolite response elements (CREs) found in promoter regions of CCR-sensitive genes and operons (Deutscher, 2008). HPr(Ser-P) also regulates carbohydrate utilization by affecting PTS activity, since HPr(Ser-P) can be a poorer substrate for the PEP-dependent phosphorylation of HPr at His15, the first step in sugar transport and phosphorylation by the PTS (Deutscher, 1984; Reizer et al., 1989). Other functions of HPr(Ser-P) include inhibition of the activity of certain non-PTS permeases, which can block induction of transcription of the corresponding catabolic genes; a phenomenon known as inducer exclusion (Deutscher, 2008).
Initial analysis of the role of an apparent CcpA homologue in S. mutans led to the conclusion that this protein may not be required for CCR of a number of catabolic operons, since inactivation of the ccpA gene did not alleviate glucose-mediated repression of utilization of polymers of fructose (fructan) (Wen and Burne, 2002), nor did it enhance expression of the genes for catabolism of agmatine (Griswold et al., 2004; Griswold et al., 2006) or the levels of hydrolase enzymes for certain non-preferred carbohydrates when glucose was present (Simpson and Russell, 1998). Subsequently, it was shown that there is indeed a functional CcpA pathway in S. mutans, that CcpA-dependent CCR could be observed under certain conditions, and that loss of CcpA had a significant impact on the transcriptome of S. mutans during exponential growth in repressing or non-repressing carbohydrate sources (Abranches et al., 2008). However, CcpA-independent pathways for CCR in S. mutans are clearly dominant. In particular, deletion of the manL gene, encoding the A and B domains of a glucose/mannose PTS Enzyme II complex (IIABMan), relieved CCR of several carbohydrate catabolic operons, including the exo-β-fructosidase (fruA) required for hydrolyzing fructans (Burne et al., 1987; Burne et al., 1999; Wen and Burne, 2002; Zeng et al., 2006b) and the cel operon encoding a phospho-β-glucosidase (celA) and a cellobiose-PTS EII complex (Abranches et al., 2003; Abranches et al., 2006). Inactivation of manL in S. mutans had a major impact on the transcriptome, and particularly on genes for carbohydrate catabolism, as well as resulting in impaired biofilm formation and competence development (Abranches et al., 2006). Notably, several studies of the mannose-PTS system in Streptococcus salivarius have indicated an important role for IIABMan in CCR (Gauthier et al., 1997; Pelletier et al., 1998). However, in a number of other streptococci, e.g., Streptococcus gordonii (Dong et al., 2004; Zeng et al., 2006a), Streptococcus pneumoniae (Giammarinaro and Paton, 2002) and group A streptococcus (Almengor et al., 2007; Shelburne et al., 2008), CcpA does appear to play major roles in CCR. Thus, important differences exist in the basis for CCR between S. mutans and other streptococci, including other oral streptococci.
Transcription of the fruA gene in S. mutans requires an unusual four-component regulatory system (LevQRST), consisting of a classical two-component sensor kinase (LevS) and response regulator (LevR) that are co-transcribed with genes for two predicted membrane-associated sugar-binding proteins (LevQT). A complete LevQRST system is required for activation by fructose or mannose of the fruA operon and an operon encoding a fructose/mannose-PTS permease (LevDEFGX) (Zeng et al., 2006b) that is located adjacent to the levQRST operon. We also reported that three PTS permeases, ManL, FruI (IIABCFru) and LevDEFG (EIILev), participate in carbohydrate-specific catabolite repression of the fruA and levD operons (Zeng et al., 2006b). Specifically, ManL is required for efficient CCR in cells growing in its preferred substrates, glucose or mannose, whereas FruI and EIILev contribute to CCR of the target operons in cells growing in the preferred substrates for these permeases, i.e. fructose or fructose or mannose, respectively. Thus, in S. mutans, the PTS permeases play a dominant role in CCR. The functions of CcpA in S. mutans are also critically important, and include globally regulating pyruvate metabolism, fine-tuning the expression of certain PTS permeases, and influencing the expression of a variety of virulence attributes, including virulence gene expression, biofilm formation and acid tolerance (Abranches et al., 2008; Zeng and Burne, 2008). Clearly, though, there are fundamental differences in how S. mutans coordinates carbohydrate catabolism compared with the usual bacterial paradigms, and even with closely-related Gram-positive cocci.
Given the functional interrelationships of EII complexes, EI and HPr, we began to explore the potential roles of the general PTS proteins in EII-mediated CCR. In this report, using an HprK kinase-constitutive mutant (Monedero et al., 2001) and mutants containing various HPr replacement mutations, we identify multiple roles for HPr(Ser-P) and sugar-specific EII permeases in CcpA-independent CCR. Our findings reveal a complex regulatory system responsible for monitoring intracellular energy and extracellular pools of carbohydrate to optimize expression of genes for carbohydrate catabolism.
Results
Creation of S. mutans strains with mutations in HPr and HPr kinase/phosphatase
In S. mutans, the genes for HPr (ptsH) and Enzyme I (ptsI) are co-transcribed in a bi-cistronic operon. A variety of approaches were employed to try to obtain a ptsH-null mutant of S. mutans, but it appears that such a mutant is not viable. In contrast, we successfully constructed an EI deletion mutant that grew normally on certain carbohydrates, including glucose, but that showed no alteration in the ability to effect CCR of the fru or lev operons. As presented in Table 1, when CAT activities from the fruA or levD promoter:cat fusion were assayed under conditions that elicit CCR, the ΔEI strain showed only a small increase in fruA or levD promoter activity relative to the wild-type. The expression levels in the ΔEI mutant were far below what we routinely observe when CCR of these operons is relieved in various EII mutant backgrounds (Zeng and Burne, 2008). For example, CAT activities for PfruA-cat fusion in the manL mutant are 25-fold higher than those observed in the ΔEI mutant.
Table 1.
CAT specific activities of PfruA-cat and PlevD-cat promoter fusions strains measured in mid-exponential phase cultures (OD600 = 0.3~0.4) growing in TV medium with a mixture of 0.5% each of glucose and fructose. The values are (nmol of chloramphenicol acetylated) (mg protein)−1 min−1 and are the average (standard deviations) of results from three independent cultures.
| PfruA-cat | PlevD-cat | |
|---|---|---|
| UA159 | 0.28 (0.04) | 308.2 (14.1) |
| ΔEI | 0.99 (0.26) | 506.1 (18.5) |
In a few cases, strains of bacteria that are relatively closely-related to S. mutans have been isolated that were shown to contain mutations in the ptsH gene that alter HPr functionality (Gauthier et al., 1997; Reizer et al., 1989), including ptsHS46D and ptsHS46A. Based on these studies, mutants of S. mutans containing single amino acid changes in HPr that replaced Ser46 with aspartic acid (ptsHS46D), which has been shown to mimic serine-phosphorylated HPr, and a Ser46 to alanine mutation, which prevents HprK-dependent phosphorylation of HPr, were created using a co-transformation strategy and mismatch amplification mutational analysis (MAMA) (Cha et al., 1992) to screen for mutants (see Experimental procedures for details).
The HPr kinase/phosphatase homologue (hprK) of S. mutans shares up to 74% sequence similarity with the HprK enzyme of Lactobacillus casei, including the Walker domain and a conserved C-terminal sequence important for phosphatase activity. A replacement of valine residue 267 with phenylalanine (V267F) in the HprK of L. casei resulted in constitutive kinase and diminished phosphatase activity (Monedero et al., 2001). When the V267F variant of the Lactobacillus HprK was expressed in B. subtilis, marked decreases in catabolism of PTS and non-PTS sugars were observed (Monedero et al., 2001). A single amino acid change equivalent to the hprKV267F of L. casei was created in the S. mutans HprK enzyme (hprKV265F) using the mutagenesis strategy described above. An hprKV265F/ptsHS46A double mutant was also constructed.
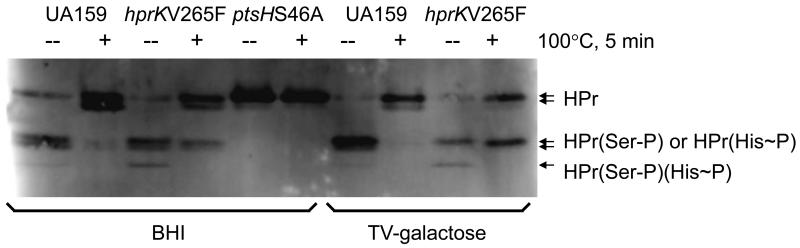
Enhancement of the levels of Ser46-phosphorylated HPr in S. mutans producing the HprKV265F variant was confirmed by immuno-blotting with a polyclonal antiserum directed against the purified HPr protein of S. mutans strain DR0001 (Fig. 1; a gift from C. Vadeboncouer). When separated on a non-denaturing polyacrylamide gel, HPr in the wild-type strain presented 5 bands, corresponding to 2 species of the non-phosphorylated molecule, 2 species with either His15 or Ser46 phosphorylation that migrate at the same rate, and 1 species with dual phosphorylation. The reason for the doublets seen with both non-phosphorylated and singly-phosphorylated HPr is likely due to an N-terminal modification of HPr by a methionine aminopeptidase, as suggested by previous studies in S. mutans and related streptococci (Dubreuil et al., 1996; Sutcliffe et al., 1993). Treatment of protein samples for 5 minutes at 100°C efficiently removes the phosphate group from His15, but not Ser46 (Cvitkovitch et al., 1995; Thevenot et al., 1995). As shown in Fig. 1, the introduction of an hprKV265F mutation resulted in significant increases in the intensity of the bands corresponding to serine-phosphorylated HPr, especially when cells were cultivated on the non-repressing sugar galactose (Abranches et al., 2008), as opposed to the glucose-containing medium BHI.
Figure 1.
Western blot showing various forms of HPr in wild type strain UA159 and isogenic mutants. Bacterial cells were harvested from mid-exponential phase cultures growing on BHI or TV-galactose, resuspended in 10 mM Tris buffer (pH 7.8), lysed by bead-beating and clarified by centrifugation. To distinguish histidine- and serine-phosphorylated forms, an aliquot out of each sample was treated at 100°C for 5 min before centrifugation for 5 min. Cell lysates (20 μg) were then subjected to non-denaturing PAGE using a 12.5% gel, followed by immunoblotting with an anti-HPr antiserum.
Growth phenotypes of the hprK and ptsH mutants on various sugars
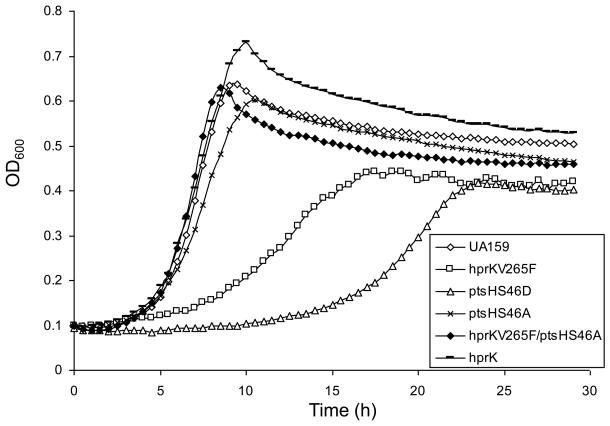
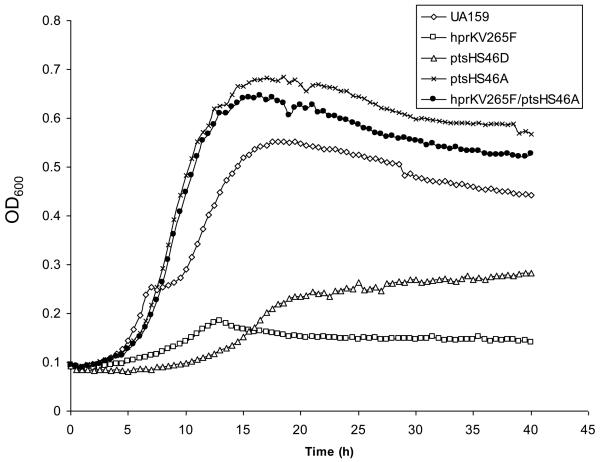
The effects of the ptsH and hprK mutations on growth on a variety of common carbohydrates were monitored. The hprKV265F mutant strain showed substantially slower growth, as well as significantly reduced final yields after 24 h, compared to the wild-type strain growing in TV-base medium with 10 mM glucose (Fig. 2), or with 10 mM fructose, mannose, galactose or lactose (supplementary Fig. S1), as the sole carbohydrate. Also, this mutant was unable to grow in TV supplemented with cellobiose, which is also transported by the PTS (Zeng and Burne, 2009). The ptsHS46D mutation is believed to mimic the effect of Ser46-phosphorylation by HprK by introducing a negative charge at that residue. It has been shown that the Ser46-phosphorylated form of HPr, while stimulating CcpA binding to CREs, also reduces the ability of EI to phosphorylate His15 of HPr, which is required for sugar transport by the PTS (Reizer et al., 1989). Consistent with the behavior of the hprKV265F mutant, the ptsHS46D mutant displayed even more severe reductions in growth on glucose (Fig. 2), fructose, mannose, and lactose, and no growth was detected on galactose or cellobiose after 24 h of incubation (supplementary Fig. S1). Consistent with the observed growth phenotypes, PTS-dependent transport of glucose, fructose or mannose by these mutants was significantly reduced in the hprKV265F and the ptsHS46D mutants (Fig. 3).
Figure 2.
Growth curves of UA159 and various mutants on TV-base medium containing 10 mM glucose.
Figure 3.
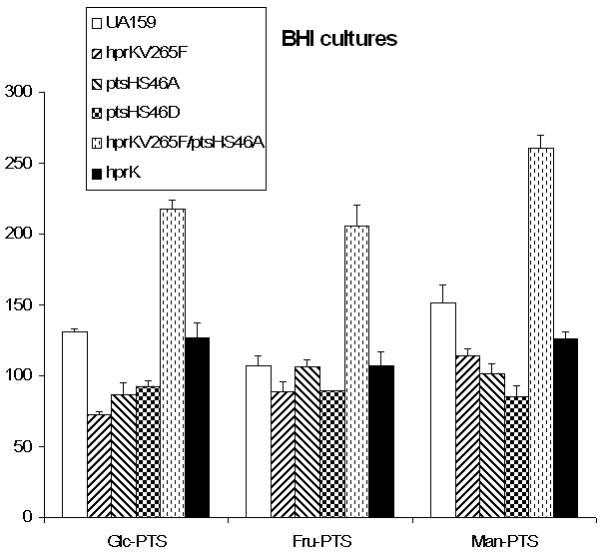
PEP-dependent PTS activities of UA159 and various mutants grown in BHI, measured using 10 mM glucose, fructose or mannose as a substrate. An asterisk represents a P value of less than 0.05, and two asterisks represent a P value of less than 0.001 (by the Student t-test).
In contrast to the behavior of the ptsHS46D and hrpKV265F strains, the ptsHS46A mutant, in which HPr cannot be phosphorylated by HprK (Fig. 1), grew at a rate that was only modestly slower than the wild-type strain and achieved similar final yields after 24 h, with the exception of when the strain was grown with cellobiose as the sole carbohydrate (Fig. 2 and Fig. S1). Interestingly, while the hprKV265F/ptsHS46A double mutant presented with markedly higher PTS activities than the hprKV265F and the wild-type strains, the presence of only the ptsHS46A mutation resulted in significantly lower glucose and mannose PTS activities, but wild-type levels of fructose PTS activity (Fig. 3) A mutant carrying a complete deletion of the hprK gene also showed decreases in the rates of growth in TV-medium with fructose, mannose, cellobiose or lactose, compared with the parental strain (Fig. S1); albeit the effects were not as severe as for the hprKV265F mutant. In contrast, little difference was seen between the hprK deletion and wild-type strains when growing on glucose (Fig. 2) or galactose (Fig. S1). Notably, there were also no differences in PTS activity for glucose or fructose between the hprK deletion strain and UA159 when cells were cultivated in BHI broth (data not shown). Also of interest, growth of the hprK strain was significantly slower than the ptsHS46A strain when lactose was the growth carbohydrate.
CCR of the fructan hydrolase gene
The fruA gene, encoding an exo-β-fructosidase, has proven to be a useful model for dissecting CCR in S. mutans (Abranches et al., 2008; Wen and Burne, 2002; Zeng et al., 2006b; Zeng and Burne, 2008). To probe the role of HPr in CCR of fruA, we examined the effects of the ptsH and hprK mutations during growth on a mixture of a preferred and a non-preferred carbohydrate source. As shown in Fig. 4, the wild-type strain UA159 displayed a classical diauxic growth curve on a combination of 0.05% glucose and 0.5% inulin, a homopolymer of fructose. The hprKV265F mutant, which displayed reduced growth on TV-glucose, produced limited growth on the mixture of glucose and inulin, with the final OD600 reaching only 0.2, suggestive of a loss of capacity to catabolize inulin. Similarly, the ptsHS46D mutant was unable to grow with inulin as the primary carbohydrate. Since the fruA gene, which encodes the enzyme for inulin hydrolysis, contains a functional CRE in its promoter region and is under the control of CcpA, the simplest explanation for these results is that elevated levels of HPr(Ser-P) in the hprKV265F mutant or the presence of the HPr(Ser-P) mimic (HPrS46D) resulted in constitutive repression of the fruA operon. Consistent with this idea, the ptsHS46A mutant displayed no evidence of diauxie, and the final OD600 of the culture was nearly 0.7, indicative of a loss of sensitivity to CCR and constitutive expression of the fruA operon. Furthermore, a ptsHS46A/hprKV265F double mutant also presented with non-diauxic growth and showed good growth on inulin, demonstrating that the effects of the hprK mutation in this case were exerted primarily through HPr. As an additional control, when these mutants were tested in TV medium supplemented with inulin alone, only the wild-type, ptsHS46A and ptsHS46A/hprKV265F strains were able to grow (data not shown). Interestingly, as we detail below, these results are, in fact, not attributable to repression of the fruA gene by CcpA.
Figure 4.
Growth of UA159 and various mutants as indicated in TV-base medium containing a mixture of 0.05% of glucose and 0.5% of inulin.
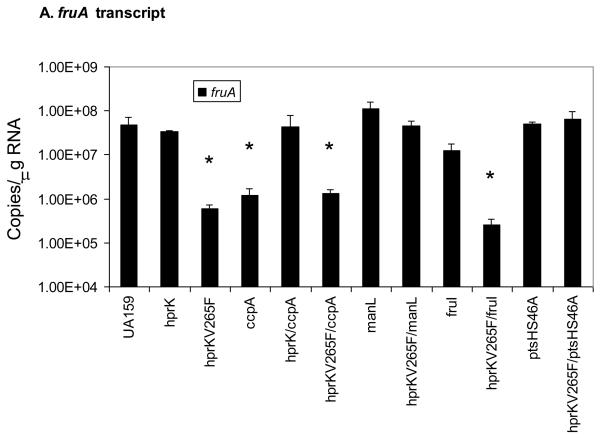
To further explore the basis for the growth phenotypes of the mutants on a mixture of glucose and inulin, cells were cultivated in BHI medium to exponential phase, RNA was extracted and quantitative Real-time RT-PCR was used to compare the amount of fruA mRNA in the mutants and wild-type strains. BHI broth was used for growth in this case because it was the medium in which the mutants showed the least difference in growth rates while still allowing for induction of expression of the fruA and levD genes (Zeng et al., 2006b). As presented in Fig. 5A, fruA mRNA levels were reduced in the hprKV265F mutant nearly 100-fold relative to the wild-type strain. However the hprKV265F/ptsHS46A double mutant showed wild-type levels of fruA mRNA, confirming that the effect of the hprKV265F mutation is exerted through enhanced serine phosphorylation of HPr. Also consistent with the growth data, the presence of the ptsHS46A mutation or hprK deletion alone had little impact on fruA mRNA levels. We do not present gene expression data for the ptsHS46D strain because this strain grows remarkably slower than the other strains utilized in this study and, more importantly, we noted a very high reversion frequency of this mutation. In particular, there was a rapid appearance of large-colony variants from the ptsHS46D mutant and DNA sequencing showed that these variants carried the wild-type allele ptsH. Consistent with this high reversion frequency, the limited gene expression data we did collect suggested a phenotype intermediate to the ptsHS46A derivative and the hprKV265F mutant, as would be expected from a mixed population of wild-type and mutant strains.
Figure 5.
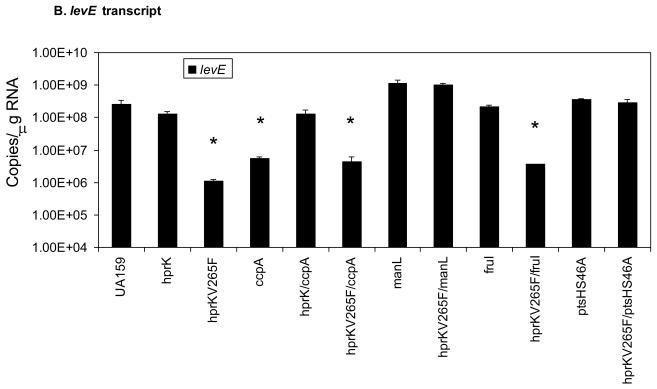
The levels of fruA (A) and levE (B) mRNA measured by Real-time quantitative RT-PCR. Error bars represent standard deviations. An asterisk indicates statistically significant differences from the parental strain (UA159) as assessed by the Student t-test (P < 0.05).
Role of CcpA and EII permeases
As reported previously, deletion of ccpA did not alleviate expression of fruA in cells growing in a combination of glucose and inulin (Wen and Burne, 2002), in spite of the fact that CcpA can repress fruA transcription though direct binding to CREs in the fruA promoter region (Abranches et al., 2008). Notably, a significant repression (~ 40 fold) of fruA was noted in a ccpA mutant when cells were grown in BHI (Fig. 5A), and this phenomenon was attributable to elevated expression of the genes for the EII permeases ManL and FruI (Zeng and Burne, 2008), which appear to play a dominant role in CCR of fruA. Interestingly, when an hprK deletion mutation was introduced into a strain carrying a ccpA deletion, fruA expression was restored to the same level as in the wild-type strain. Therefore, HPr(Ser-P) can exert CCR over the fruA gene independently of CcpA. Further support for this observation was obtained using an hprKV265F/ccpA double mutant, where it was shown that loss of CcpA did not relieve the repressive effects of the hprKV265F mutation (Fig. 5A).
We next investigated whether HPr(Ser-P) could exert its effect on fruA gene expression independently of PTS permeases. The manL mutant of S. mutans shows a dramatic derepression of fruA expression in the presence of glucose, compared to the wild-type strain (Zeng and Burne, 2008). However, when the hprKV265F was introduced into a strain carrying a manL deletion, expression of fruA remained high and was not significantly different than for the manL mutant alone. Therefore, these findings support that HPr(Ser-P)-mediated CCR of fruA requires the presence of a specific PTS permease and its cognate carbohydrate substrate(s). Further evidence of the carbohydrate-specificity of CCR was obtained when deletion of the fructose permease fruI, which we previously showed to alleviate fructose-specific CCR of fruA, did not lead to relief of fruA repression in a strain carrying the hprKV265F mutation when glucose, which is not a substrate for FruI, was the primary carbohydrate source (Fig. 5A). Also of interest, the manL/hprKV265F double mutant had a growth phenotype similar to that of hprKV265F mutant on BHI (data not shown), so it appears that the negative effect of elevated levels of HPr(Ser-P) on growth on glucose is not solely related to effects exerted on or by ManL.
Expression of levD and the role of LevR
In addition to fruA, another operon that is activated by the LevQRST four-component system is the levDEFGX operon, which encodes a fructose/mannose EII permease, EIILev. Unlike for fruA, there is no evidence for a direct interaction of CcpA with the levD promoter region, although similar to fruA CCR of levD-X does require ManL, FruI or LevD, depending on the growth carbohydrates (Zeng and Burne, 2008). To evaluate the contribution of HPr to CCR of the lev operon, cDNA samples used for measuring fruA transcript levels were subjected to quantitative Real-time PCR with levE-specific primers. The results (Fig. 5B) showed a pattern of lev expression that was nearly identical to fruA across all strains. When cells were cultivated in BHI, in which the repressing sugar is glucose, the hprKV265F mutant had drastically reduced expression of levE. The effects of the hprKV265F mutation could be reversed by the introduction of the ptsHS46A mutation or deletion of manL, but not by deletion of ccpA or fruI, when glucose was the growth carbohydrate. As noted previously, loss of CcpA alone led to reduced expression of levE, but this occurs because manL expression is elevated in a ccpA mutant (Zeng and Burne, 2008). Fig. 5B also shows that repression of levD requires an intact hprK gene. As additional controls, the transcript levels of manL were probed in these mutants (Zeng and Burne, 2008). The results (Supplementary Figure S2) confirmed our previous observations that manL expression is indeed elevated in the ccpA mutant and added further support to the notion that both HPr and ManL are required for CCR of the fruA and levD operons by glucose.
We previously demonstrated that CCR of fruA and levD mediated by the PTS permeases could not be observed in strains lacking the transcriptional regulator LevR, but the response was retained in strains deficient in the LevS, LevQ or LevT components of the signaling complex required for activation of fruA and levD expression via LevR (Zeng and Burne, 2008). These results implied that a direct interaction with LevR was, at least in part, the basis for CCR of fruA and levD. Drawing from a study with Agrobacterium in which a mutant response regulator that did not require kinase activity for activation was described (Jin et al., 1993), a levR mutant (levRR56D) was engineered that gives constitutive activation of the LevQRST pathway in the absence of inducing signal. As shown in Table 2, the presence of the levRR56D mutation led to increased expression of the levD promoter when cells were cultured in TV-galactose, which is neither a repressing sugar nor an inducing substrate for levD expression (Zeng and Burne, 2008), indicating that the LevRR56D protein did not require activation by the sensing complex to activate levD transcription. When the hprKV265F mutation was transformed into the levRR56D background, levD promoter expression was reduced to levels observed in strains carrying the wild-type copy of levR growing under the same conditions. When cultured in TV-glucose, the levD promoter fusion strain carrying the levRR56D mutation expressed over 20-fold higher CAT activity than found in the wild-type background. However, the hprKV265F/levRR56D double mutant gave CAT activity close to the wild-type level. Introduction of a manL deletion into the levRR56D mutant resulted in very high levels of expression (~60-fold) of CAT from the levD promoter in glucose-grown cells. Also, the introduction of the hprKV265F mutation into a strain carrying the manL/levRR56D mutations yielded higher levels of expression than in the wild-type background (~10-fold), but lower than that found in the manL/levRR56D mutant strain alone. Thus, the presence of elevated levels of HPr(Ser-P), in a strain expressing a functional ManL protein, are sufficient to interfere with LevR-dependent activation of its targets. Since the LevRR56D mutant protein can bypass the requirement for activation by the signaling complex, it raises the possibility that a direct interaction between LevR and HPr(Ser-P), alone or in concert with ManL, is a mechanism for effecting CCR of the levD and fruA operons. However, the results do not exclude that CCR could involve interactions of the signaling complex with HPr(Ser-P) and/or ManL, as well.
Table 2.
CAT specific activities of PlevD-cat measured in the background of UA159 and various mutant strains in mid-exponential phase cultures growing in TV with 0.5% glucose or galactose. Values are as in Table 1 and are derived from at least three independent cultures.
| Glucose | Galactose | |
|---|---|---|
| UA159 | 2.80 (0.36) | 9.63 (0.64) |
| levRR56D | 53.12 (2.35) | 86.32 (6.00) |
| hprKV265F | 0.33 (0.29) | 0 (0) |
| levRR56D/hprKV265F | 6.63 (0.21) | 12.44 (3.11) |
| levRR56D/manL | 161.01 (25.1) | NT |
| levRR56D/hprKV265F/manL | 34.38 (6.17) | NT |
NT, not tested.
Effect of the hprKV265F mutation on CCR in cells growing on fructose or mannose
One of the more interesting aspects of CcpA-independent CCR in S. mutans is its sugar specificity, mediated through certain PTS enzymes. For example, alleviation of CCR due to loss of manL is most significant when cells are cultivated on glucose or mannose, the preferred substrates for ManL transport, whereas the effects of deletion of fruI are most obvious when fructose is the repressing carbohydrate (Zeng and Burne, 2008). These results could arise from CCR being mediated by the sugar-specific permeases themselves, or relief of CCR may simply arise from the slower rate of uptake or catabolism of the preferred carbohydrates in the absence of their permeases, which would in turn affect the phosphorylation state of HPr or EI (Deutscher et al., 2006).
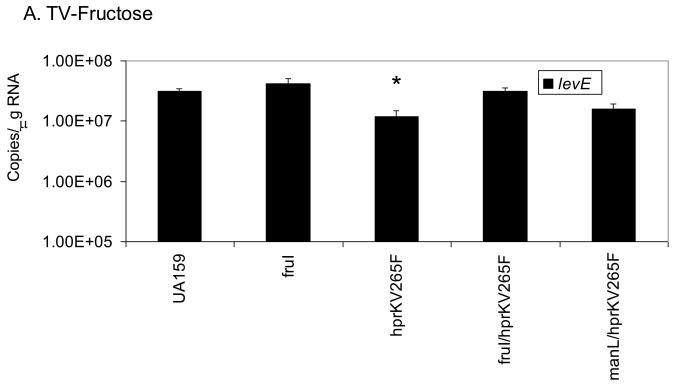
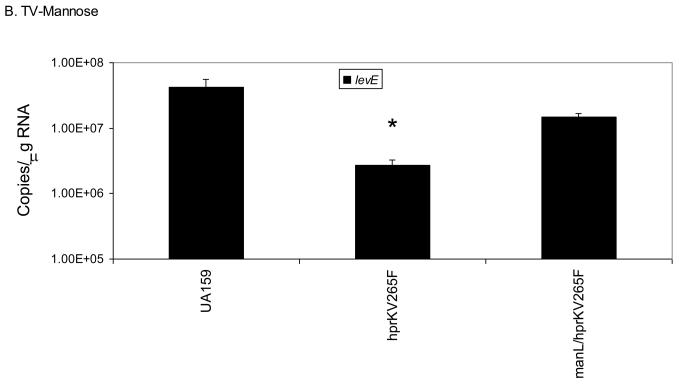
We have already shown that ManL is required to observe CCR of the fruA and levD operons in the hprKV265F mutant when grown in BHI medium (Fig. 5). To further explore if CCR of these operons requires specific EII components in the presence of preferred sugars other than glucose, we grew the hprKV265F mutant in TV with 0.5% fructose or mannose, which are both inducing substrates via LevQRST, then used quantitative RT-PCR to monitor the level of levE transcript. When cells were cultured in fructose, expression of levE (Fig. 6A) in the hprKV265F mutant was only 2.5-fold lower than in the wild-type background, a difference much smaller than when cells were grown in the glucose-containing medium BHI (> 200-fold). However, inactivation of the gene for an inducible fructose permease (fruI) in the hprKV265F mutant restored levE expression in the presence of fructose to wild-type levels. When the cells were grown on TV-mannose (Fig. 6B), mannose being a very effective inducer of fruA and levD through the LevQRST system and a substrate for ManL, nearly a 15-fold reduction was noted in the hprKV265F mutant relative to the wild-type strain. In contrast, an hprKV265F/manL double mutant produced levE mRNA at levels comparable to that found in the wild-type background when grown with mannose as the sole carbohydrate. Thus, CCR exerted on the fruA and levD operons by HPr(Ser-P) occurs optimally in the presence of a functional ManL (IIABMan) protein and one of the preferred substrates for ManL (glucose or mannose), whereas the effect of FruI on CCR of genes under the control of LevQRST appears minor. These results indicate that CCR involves a specificity imparted by the permeases and is not due solely to a diminution in the rate of carbohydrate transport. This observation is also consistent with the fact that loss of Enzyme I of the PTS did not affect CCR.
Figure 6.
Real-time quantitative RT-PCR measuring the expression levels of levE in various strains cultured on TV-fructose (A) or TV-mannose (B). An asterisk indicates statistically significant differences from the parental strain (UA159) as assessed by the Student t-test (P < 0.05).
To further confirm the requirement for ManL in CcpA-independent CCR and to ensure that the observed effects were not simply due to a general decrease in carbohydrate transport, a cross-species complementation strategy was adopted. A DNA fragment containing glcT and ptsG sequences originated from Bacillus subtilis strain 168, encoding a transcription antiterminator and a glucose-PTS enzyme II (A, B and C components), respectively (Schmalisch et al., 2003; Stulke et al., 1997), was cloned into an E. coli/S. mutans shuttle vector pDL278 and introduced into the manL mutant carrying the PlevD-cat reporter fusion. PtsG of B. subtilis is known not to function in CCR as proposed for ManL. Expression of the Bacillus EIIGlc in the manL mutant clearly restored the ability of the strain to grow well on glucose and to internalize glucose via the PTS nearly as well as the wild-type strain (Fig. S3). However, when CAT activities from the levD promoter were measured in cultures growing in TV supplemented with 0.5% each of glucose and fructose, expression of ptsG failed to reverse the relieved CCR of the levD promoter that occurred as a results of the manL mutation (Table 3). Thus, it is not simply slower growth and PTS transport of glucose that accounts for alleviation in the manL mutant. Interestingly, for reasons that are not entirely clear, CAT activity in the manL mutant expressing glcT-ptsG was nearly 3-fold higher than that in the same mutant carrying the empty vector pDL278.
Table 3.
CAT specific activities of PlevD-cat measured in cells growing exponentially in TV with 0.5% glucose and fructose (and 300 μg/ml spectinomycin). Values are as in Table 1 and are derived from at least three independent cultures.
| UA159 | 374.1 (33.1) |
| manL | 666.2 (35.5) |
| manL/pDL278 | 557.5 (20.7) |
| manL/glcT-ptsG | 1459.1 (175.4) |
Effects of hprK and ptsH mutations on utilization of other PTS sugars
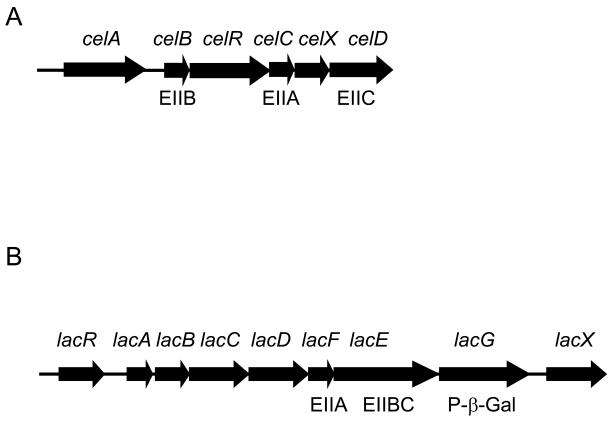
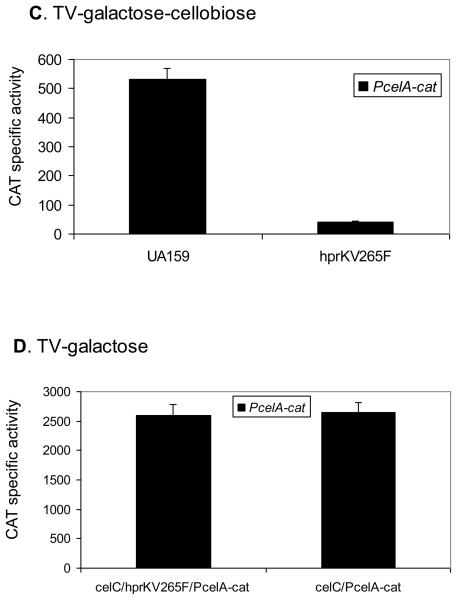
We previously reported that the expression of the cellobiose operon of S. mutans requires active transport of the substrates for the cellobiose PTS permease, EIICel, which include cellobiose and glucose (Zeng and Burne, 2009)(Fig. 7A). In particular, EIICel can phosphorylate two PTS regulatory domains (PRDs) in the CelR protein to inhibit CelR activation of the cel operon. In the presence of cellobiose, CelBCD instead direct the phosphate from HPr to the incoming carbohydrate, resulting in dephosphorylation of CelR at His284 and His391. Activation of cel gene expression by CelR also requires phosphorylation at His226, His332 and His576, presumably by EI and HPr. Transcriptional analysis using a PcelA-cat fusion (Zeng and Burne, 2009)(Fig. 7C) demonstrated a dramatic reduction in cel operon expression in the hprKV265F mutant. However, a direct role of HPr(Ser-P) on cel transcriptional regulation appeared unlikely, since an hprKV265F/celC double mutant, which lacks the IIACel protein and is therefore unable to phosphorylate CelR (Zeng and Burne, 2009), displayed constitutively high levels of expression from the celA promoter (Fig. 7D). Therefore, we hypothesize that the reduced transcription of the cel operon in the hprKV265F mutant is due to decreased uptake of cellobiose through the cellobiose-PTS, which would be consistent with the inability of this mutant to grow on cellobiose (Fig. S1). Of note, there are no CREs in the cel operon promoter region, nor is there any evidence for direct regulation by CcpA of this operon (Zeng and Burne, 2009).
Figure 7.
Schematic diagrams of the cellobiose (A) and lactose (B) operons of S. mutans UA159, and the expression of the celA promoter:cat fusion in various mutants (C, D). (A) The celA gene encodes cellobiose-6-phosphate hydrolase and celB, C, and D code for the B, A and C domains of the cellobiose PTS permease. The function of CelX has not been determined. (B) LacR is a transcriptional repressor of the lac operon. LacA and LacB are the subunits of galactose-6-phosphate isomerase. LacC is a tagatose-6-phosphate kinase and LacD a tagatose-1,6-bisphosphate aldolase. LacEF constitute the lactose PTS permease (IILac) and the function of LacX is unknown. Also presented are PcelA-cat activity in (C) UA159 and the hprKV265F mutant cultivated in TV supplemented with 0.5% galactose and cellobiose, and in (B) celC/hprKV265F and celC mutants grown in TV with 0.5% galactose.
Our previous work revealed that the lac operon of S. mutans (Fig. 7B) is also regulated by CcpA-independent CCR (Zeng and Burne, 2008). The lac operon encodes proteins required for utilization of both lactose and galactose and is controlled by the DeoR-like negative regulator LacR (Zeng and Burne, manuscript in preparation)(Ajdic et al., 1996; Ajdic and Ferretti, 1998; Rosey and Stewart, 1992). Loss of manL has been shown to markedly increase expression of lacG, encoding a phospho-β-D-galactosidase responsible for cleavage of lactose-6-phosphate internalized by the lactose-PTS (Zeng and Burne, 2008). Inactivation of CcpA has no effect on lac gene expression (Simpson and Russell, 1998), nor are there any CREs in the lac promoter region (Rosey and Stewart, 1992). Although growth on lactose was clearly reduced in the hprKV265F mutant (Fig. S1), wild-type levels of lacG expression were present in this mutant, as measured by Real-time PCR, both in the absence and presence of the inducing carbohydrate galactose (supplementary Fig. S4). Thus, we postulate that the primary mechanism by which serine phosphorylated HPr interferes with lactose utilization is by blocking internalization of lactose, or galactose, through the PTS permease encoded in the lac operon (IILac). Another intriguing finding about the effects of mutations in the gene for HPr on lactose utilization was that the ptsHS46A mutation did not restore rapid growth on lactose when introduced into the hprKV265F mutant, as it did for growth on all other sugars. In fact, the ptsHS46A/hprKV265F double mutant could not grow at all on lactose, whereas this strain was able to grow on cellobiose (Fig. S1). However, the hprK deletion mutant was able to grow slowly on lactose. Thus, it appears that either some level of HPr(Ser-P) is required for growth on lactose or that the HPr kinase/phosphatase plays another role in regulation of lactose catabolism.
Discussion
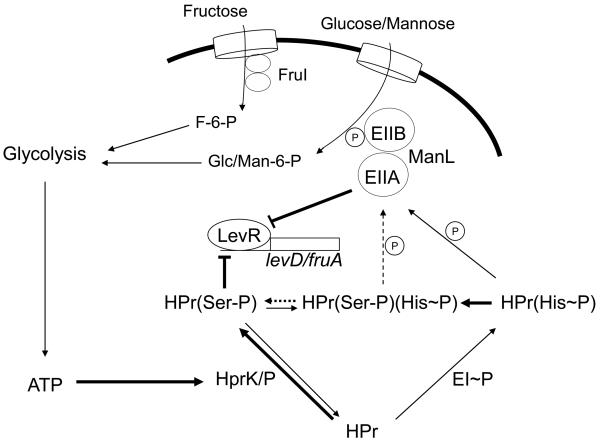
CCR of the fruA, levD, cel, and lac operons does not require the direct participation of CcpA. Instead, three PTS permeases that catalyze the transport of glucose and mannose (EIIMan) or fructose (FruI and EIILev) play essential roles in CCR, although CcpA influences the transcription of manL and fruI (Zeng and Burne, 2008). In this communication, we begin to explore the involvement of HPr of the PTS in CCR and show that HPr(Ser-P) can confer repression on the fruA and levD operons without the direct involvement of CcpA. We propose that a discrete network of PTS components in S. mutans governs sugar-specific catabolite repression of non-preferred carbohydrate sources by integrating the intracellular energy levels via HPr(Ser-P) with the monitoring of the availability of preferred carbohydrates through EII permeases (Fig. 8). By contrasting CCR of LevR-controlled operons with the lactose and cellobiose operons, we show that multiple mechanisms for HPr- and ManL-dependent CCR are operable in S. mutans.
Figure 8.
Working model of CcpA-independent catabolite repression in S. mutans. For simplicity, only the regulation of LevR activity is depicted here. The transport of the preferred sugars, glucose, mannose and fructose, via the PTS and their subsequent catabolism through glycolysis result in activation of the kinase activity of HprK, enhancing Ser-46-phosphorylation of HPr. Concurrently, transport of glucose/mannose leads to under-phosphorylation of ManL at its conserved His residues. HPr(Ser-P) may allosterically regulate the DNA-binding activity of LevR, a process that may require or be enhanced by the presence of under-phosphorylated ManL. It is also proposed that Ser-46-HPr can modulate inducer exclusion to prevent uptake and induction of certain catabolic operons.
LevR is a classical response regulator that requires the concerted action of the membrane-associated sensor kinase (LevS) and carbohydrate binding proteins (LevQT) to activate fruA and levD operon expression via binding to a conserved direct repeat in the promoter regions of these genes (Zeng et al., 2006b) (some data not shown). Since replacement of the conserved phosphorylation site Asp54 of LevR with Asn causes complete loss of activation of the fruA and levD operons (data not shown), it seems that modulation of LevR activity occurs via the classical pathway for two-component systems. Yet LevR is required to observe CCR of the fruA and levD operons, whereas strains lacking LevQST still show evidence of responsiveness to CCR. Computer algorithms were unable to identify any PRDs (PTS regulatory domains), which are targets for PTS-dependent phosphorylation (van Tilbeurgh and Declerck, 2001), in the LevR protein. Consequently, the simplest explanation for CCR of fruA and levD by ManL and HPr(Ser-P) is that one or both of these proteins is involved in allosteric regulation of LevR DNA binding activity. A direct interaction between HPr(Ser-P) and RbsR, a transcriptional regulator of the B. subtilis ribose operon, has been shown to enhance RbsR binding to its target sequence in vitro (Muller et al., 2006). More recently, a novel mechanism for CcpA-independent CCR in B. subtilis was shown to involve HPr and HPr(His~P) interacting with the transcriptional regulator YesS, which is required for the expression of pectin/rhamnogalacturonan utilization genes (Poncet et al., 2009). Thus, precedent for allosteric interaction of HPr(Ser-P) or HPr(His~P) and transcriptional regulators for particular operons exist. Why ManL is also required to observe CCR of LevR-regulated operons remains to be determined, but preliminary studies using a 6×histidine-tagged ManL in pull-down experiments indicate that ManL has a variety of potential binding partners (data not shown).
In contrast to the LevR system, the cellobiose operon is controlled by the transcriptional activator CelR, which contains at least five histidine residues, present in two PRDs in the central portion of the protein and an EIIA-like domain at the C-terminus (Zeng and Burne, 2009). Phosphorylation of CelR by EIIACel or EIIBCel negatively regulates CelR activity, so in the absence of cellobiose the operon is off. When extracellular cellobiose is present, the EIICel complex is engaged in phosphotransfer to the incoming disaccharide and the operon can be activated. In addition, transcriptional activation by CelR requires phosphorylation at three other His residues via the EI-HPr pathway, so if preferred carbohydrates are titrating phosphate from EI, then CCR is evident. In this study, a strain lacking EIIACel (celC) did not show decreased expression of the cel operon when the hprKV265F mutation was also present (Fig. 7). Since the presence of an intact EIICel was required to observe CCR, the most logical explanation for these findings is that the ptsHS46D or hprKV265F mutations result in reduced cellobiose-PTS activity, leading to a failure to induce cel gene expression; consistent with the observed inability of these mutants to grow at all on cellobiose (Fig. S1). Lactose operon expression, including the genes for the tagatose-6-phosphate pathway and a lactose PTS permease, is repressed by the LacR protein, a classical LacI-type transcriptional repressor with no obvious PRDs. In contrast to the other operons, there was very little change in the expression of the lac genes in the hprKV265F mutant, although utilization of lactose was apparently reduced. In this case, we propose that the relatively low levels of lactose that are internalized are sufficient to induce operon expression, but are insufficient to support growth on this carbohydrate.
Overall, the findings that the hprKV265F and ptsHS46D mutants showed slower growth on preferred carbohydrates and significant decreases in their transport rates via the PTS were consistent with current knowledge of PTS permease gene expression in S. mutans. Our laboratory has demonstrated that CcpA negatively regulates manL and fruI expression (Zeng and Burne, 2008), so increases in HPr(Ser-P) or its mimic should cause a CcpA-dependent decrease in expression of the genes for these porters, which could lead to the observed slower growth rates on glucose and fructose. Likewise, the combination of decreased expression of manL and fruI and diminished production of levD, via LevR, in the hprKV265F background is consistent with the slow growth phenotype on mannose that was observed in this mutant, since all three of these porters contribute to various degrees to internalization of mannose (Zeng and Burne, 2008). We also recognize that decreased carbohydrate uptake via ManL, FruI, FruCD or LevD could lead to alleviation of CCR via decreased carbohydrate flow through glycolysis, causing accumulation of phosphorylated HPr at His15 and decreased phosphorylation on Ser46 (Deutscher et al., 2006). That this pathway is one component of CCR in S. mutans is evident in the cellobiose pathway, where phosphorylation by EI/HPr appears required for the activity of CelR (Zeng and Burne, 2009).
Collectively, though, our data do not support the notion that change of CCR of the operons studied herein is due simply to an overall diminution in the rate of PTS transport of preferred carbohydrates related to down-regulation or loss of PTS porters. Obviously, the retention of CCR in strains lacking EI is powerful evidence for this notion. Further, as reported previously, the manL mutant actually grows faster than the wild-type strain on mannose (Abranches et al., 2003), as opposed to the slower growth on glucose. This behavior is explained by the fact that expression of the genes for EIILev, which is able to transport mannose at high affinity, is much higher in a strain lacking ManL. Importantly, though, alleviation of CCR of the fru and lev operons is seen in the manL mutant regardless of whether glucose or mannose is the growth substrate, consistent with mannose PTS measurements (Zeng and Burne, 2008). Finally, the cross-species complementation of the manL mutant with the glcT-ptsG genes from B. subtilis demonstrates unequivocally that restoration of growth on, and rapid uptake of, glucose is not sufficient to reverse the effects of the loss of ManL.
It is also apparent from this study that an optimal level of HPr-Ser46 phosphorylation is required for efficient growth of S. mutans on a wide range of sugars. The markedly increased levels of HPr-Ser46 phosphorylation in the hprKV265F mutant cause decreased expression of critical sugar permeases, including manL, fruI and levD-G and a corresponding reduction in PTS transport, explaining the slow growth phenotype of this mutant. Likewise, the ptsHS46D mutant, which would lead to all HPr molecules in the cell existing as a mimic of the serine-phosphorylated form of HPr, causes extremely poor growth and the strain displays a high frequency of reversion, indicative of a strong selective pressure against aberrantly high HPr(Ser-P) pools. However, when the level of HPr-Ser46 phosphorylation is pushed to the other extreme, as occurs in the ptsHS46A or hprK deletion mutants, slower growth on most of the sugars tested also resulted. Perhaps more significant is the observation that the ptsHS46A mutation resulted in substantial reductions in the pools of His15-phosphorylated HPr (Fig. 1). Clearly, then, some intermediate level of HPr-Ser46 phosphorylation in S. mutans is desirable for optimal growth. We propose that one factor that contributes to the behavior of these mutants may be related to the requirement to maintain proper NAD+/NADH balances, which are critical for glycolysis and anabolic processes. Despite a diminished role in CCR in S. mutans, CcpA appears to be the primary genetic control point for directing carbon flow in the cell by exerting dominant control over expression of the pyruvate dehydrogenase complex, pyruvate-formate lyase and the partial tricarboxylic acid cycle (TCA) enzymes (Abranches et al., 2008; Ajdic et al., 2002). Thus, alterations in the level of HPr(Ser-P) could affect the regulation of these key enzymes and, in turn, the flow of carbon in S. mutans, leading to sub-optimal ratios of NAD+ to NADH.
In conclusion, utilizing a set of engineered point and deletion mutants in the ptsH and hprK genes, we provide new mechanistic insights into the molecular basis for CcpA-independent CCR in S. mutans. Our results display a remarkable diversity in the ways that preferred carbohydrates override the utilization of non-preferred substrates. Optimization of carbohydrate utilization is particularly crucial for oral streptococci, not only because it is the overwhelmingly dominant pathway by which these organisms generate ATP, but also because of the unique demands imposed by the oral environment and the diversity and intermittent nature of the host diet. Clearly, CCR in this organism is a culmination of responses to multiple signals, with CcpA regulating overall carbohydrate flow, particularly pyruvate metabolism; HPr(Ser-P) integrating the general availability for carbohydrate flowing through glycolysis; and a collection of PTS permeases that fine tune gene expression in response to the source and availability of various sugars in the environment. Our results also reaffirm the critical role that the ManL protein plays in orchestrating internalization of preferred carbohydrate sources, particularly glucose. We believe these studies point to novel mechanisms for direct involvement and cooperation of ManL and HPr in governance of gene expression and carbohydrate transport. Studies are ongoing to determine how these constituents of the PTS work in concert to ensure optimal persistence and virulence of S. mutans.
Experimental procedures
Bacterial strains, growth conditions, and reagents
S. mutans UA159 and mutants were maintained in brain heart infusion broth (BHI, Difco Laboratories, Detroit, Mich.) at 37°C in 5% CO2 and 95% air with antibiotics used at the following concentrations: kanamycin (700 μg ml−1), erythromycin (5 μg ml−1), and spectinomycin (500 μg ml−1), when necessary. S. mutans cultures for growth rate tests were done in tryptone-vitamin-base (TV) medium (Burne et al., 1999) with 10 mM of glucose, fructose, mannose, galactose, cellobiose, lactose and inulin (at 0.5%), as the carbohydrate source. All chemical reagents and antibiotics were obtained from Sigma Chemical Co. Growth curves of S. mutans UA159 and mutant strains were generated using a Bioscreen C (Oy Growth Curves AB Ltd., Helsinki, Finland), with cultures covered by 50 μl of mineral oil and readings taken every 30 minutes. Chloramphenicol acetyltransferase (CAT) (Shaw, 1979; Wen and Burne, 2002) and PEP-dependent PTS assays (Abranches et al., 2004; LeBlanc et al., 1979) were performed as previously described.
DNA manipulation
Standard recombinant DNA techniques were performed to engineer plasmids. All restriction and modifying enzymes were purchased from New England Biolabs (Beverly, Mass.) and used as recommended by the supplier. DNA purification was carried out using Qiaquick DNA purification kits (Qiagen, Inc., Valencia, CA). All primers were synthesized by Integrated DNA Technologies (IDT), Inc. (Coralville, IA). Inactivation of hprK was carried out using the PCR-ligation-transformation technique, according to previously published protocols (Lau et al., 2002). In some cases, strains carrying multiple mutations were generated by transformation with chromosomal DNA isolated from other mutant strains of S. mutans, followed by selection for the appropriate antibiotic resistance and PCR confirmation. The plasmid used in the manL cross-species complementation experiment was constructed using an E. coli- streptococcus shuttle vector pDL278 (Dunny et al., 1991) and a PCR product containing both glcT and ptsG sequences that was amplified using B. subtilis strain 168 chromosomal DNA as the template. After restriction enzyme digestion and ligation, the ligation product was used to transform competent UA159 culture. Spectinomycin-resistant clones were identified by PCR and faithful amplification was confirmed by sequencing.
Mismatch amplification mutation analysis (MAMA) PCR
A “MAMA” primer, designed for the detection of site-directed mutations, usually bears 1 mismatch with the wild-type allele and 2 mismatches with the mutant allele within the last 3 nucleotides of the primer sequence (Cha et al., 1992). In addition to a paring primer A, a MAMA primer was used in a PCR reaction along with a control primer that anneals at a distal site (> 0.5 kbp) on the same strand. Mutation in the MAMA primer allows for preferential amplification of a shorter DNA fragment, only from the wild-type allele of the target gene. Amplification of a larger control product in place of the shorter fragment indicates the presence of the desired mutation in the template. S. mutans colonies were directly used in a 50-μl reaction that contained 0.6 μM primer A, 0.4 μM MAMA primer and 0.2 μM control primer, treated at 95°C for 5 min, followed by 30 cycles as following: 95°C for 25 sec, 55°C for 25 sec and 72°C for 2 min.
To engineer a point mutation in the S. mutans genome, a 1-2-kbp DNA fragment containing the desired mutation, usually positioned in the center of the fragment, was generated using a recombinant PCR reaction, then used to transform wild-type strain UA159 along with an indicator plasmid that carries a PlevD-cat reporter fusion (Zeng and Burne, 2008). The indicator plasmid was used at concentrations 50- to 100-fold lower than the mutating DNA fragment. When integrated into the gtfA site of the chromosome, transformants become resistant to kanamycin (Km), which allowed for selection of competent bacteria that likely had taken up the mutating DNA. After allowing colonies to form on BHI/Km plates, MAMA PCR was performed on each isolate to screen for the desired mutation. Typically, 70-80% of the colonies that were screened contained the desired mutation(s). Putative mutants were then further confirmed by DNA sequencing using the large control PCR product as templates.
Western blot analysis
S. mutans cells were grown to the mid-exponential phase (OD600 = 0.3~0.4) in BHI (50 ml), harvested via centrifugation and immediately frozen and stored at −80°C overnight. Total cell lysates were prepared by bead-beating of cells after resuspending in 750 μl of 10 mM Tris-Cl (pH 7.8), followed by centrifugation at 16,000×g at 4°C for 10 min. The supernatant fluid was recovered and used directly for further analysis or an aliquot was removed, treated for 5 min in boiling water, and centrifuged at 16,000×g at 4°C for 5 min. Equivalent concentrations of boiled and untreated samples were loaded on a 12.5% non-denaturing polyacrylamide gel. Western blot analysis was carried out and the presence of HPr was detected using an antiserum directed against a purified HPr protein of S. mutans DR0001.
RNA isolation, RT-PCR and quantitative real-time RT-PCR
Total RNA was extracted from S. mutans cultures by using the RNeasy mini kit (Qiagen) as described previously (Ahn et al., 2005). cDNA templates were then generated from 2 μg of total RNA with random hexamers using the Superscript III first-strand synthesis system (Invitrogen) according to instructions from the supplier. Real-time PCR reactions were carried out using an iCycler iQ real-time PCR detection system and iQSYBR green supermix, according to the standard protocol provided by the supplier (Bio-Rad, Hercules, CA). Three individual cultures from each strain were used to prepare total RNA and cDNA, and triplicates were included in subsequent reactions for each cDNA sample along with appropriate control, as previously described (Ahn et al., 2005). Transcript levels of the following genes were probed using gene-specific primers: for fruA, 5′- GGG ACT TGG GAA GTA CGA GAA G -3′ (forward) and 5′- AAA CAA GCG CTG CTG CAC CG -3′ (reverse); for levE, 5′- AGA AGC CTT TGC TAA ATC AC -3′ (forward) and CGG AAC AAA ACC TTG GTC AAC C -3′ (reverse); for 16S rRNA, 5′- CAC ACC GCC CGT CAC ACC - 3′ (forward) and 5′- CAG CCG CAC CTT CCG ATA CG -3′ (reverse); for manL, 5′- TGG CTA TCG GAA TCG TTA TCG C -3′ (forward) and 5′- ATC ATC AGG TCC TTC ACT TGG C -3′ (reverse); for lacG, 5′- ATT GGA TGC GTG CTT TTG ATG G -3′ (forward) and 5′- CGA CCG ACA CCC TTA ATC TGG -3′ (reverse). All expression data obtained using Real-time PCR were normalized against the levels of 16S rRNA transcripts measured in the same cDNA samples.
Supplementary Material
Acknowledgments
This work was supported by DE12236 from the National Institute of Dental and Craniofacial Research. The anti-HPr antiserum was a gift from C. Vadeboncoeur of Universite Laval, Quebec City, Canada. We also thank Meghan Keskar for technical assistance.
References
- Abranches J, Chen YY, Burne RA. Characterization of Streptococcus mutans strains deficient in EIIAB Man of the sugar phosphotransferase system. Appl Environ Microbiol. 2003;69:4760–4769. doi: 10.1128/AEM.69.8.4760-4769.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abranches J, Candella MM, Wen ZT, Baker HV, Burne RA. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. J Bacteriol. 2006;188:3748–3756. doi: 10.1128/JB.00169-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abranches J, Nascimento MM, Zeng L, Browngardt CM, Wen ZT, Rivera MF, Burne RA. CcpA regulates central metabolism and virulence gene expression in Streptococcus mutans. J Bacteriol. 2008;190:2340–2349. doi: 10.1128/JB.01237-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SJ, Lemos JA, Burne RA. Role of HtrA in growth and competence of Streptococcus mutans UA159. J Bacteriol. 2005;187:3028–3038. doi: 10.1128/JB.187.9.3028-3038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajdic D, Sutcliffe IC, Russell RR, Ferretti JJ. Organization and nucleotide sequence of the Streptococcus mutans galactose operon. Gene. 1996;180:137–144. doi: 10.1016/s0378-1119(96)00434-9. [DOI] [PubMed] [Google Scholar]
- Ajdic D, Ferretti JJ. Transcriptional regulation of the Streptococcus mutans gal operon by the GalR repressor. J Bacteriol. 1998;180:5727–5732. doi: 10.1128/jb.180.21.5727-5732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajdic D, McShan WM, McLaughlin RE, Savic G, Chang J, Carson MB, Primeaux C, Tian R, Kenton S, Jia H, Lin S, Qian Y, Li S, Zhu H, Najar F, Lai H, White J, Roe BA, Ferretti JJ. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A. 2002;99:14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajdic D, Pham VT. Global transcriptional analysis of Streptococcus mutans sugar transporters using microarrays. J Bacteriol. 2007;189:5049–5059. doi: 10.1128/JB.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almengor AC, Kinkel TL, Day SJ, McIver KS. The catabolite control protein CcpA binds to Pmga and influences expression of the virulence regulator Mga in the Group A streptococcus. J Bacteriol. 2007;189:8405–8416. doi: 10.1128/JB.01038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne RA, Schilling K, Bowen WH, Yasbin RE. Expression, purification, and characterization of an exo-beta-D-fructosidase of Streptococcus mutans. J Bacteriol. 1987;169:4507–4517. doi: 10.1128/jb.169.10.4507-4517.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne RA, Wen ZT, Chen YY, Penders JE. Regulation of expression of the fructan hydrolase gene of Streptococcus mutans GS-5 by induction and carbon catabolite repression. J Bacteriol. 1999;181:2863–2871. doi: 10.1128/jb.181.9.2863-2871.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha RS, Zarbl H, Keohavong P, Thilly WG. Mismatch amplification mutation assay (MAMA): application to the c-H-ras gene. PCR Methods Appl. 1992;2:14–20. doi: 10.1101/gr.2.1.14. [DOI] [PubMed] [Google Scholar]
- Cvitkovitch DG, Boyd DA, Thevenot T, Hamilton IR. Glucose transport by a mutant of Streptococcus mutans unable to accumulate sugars via the phosphoenolpyruvate phosphotransferase system. J Bacteriol. 1995;177:2251–2258. doi: 10.1128/jb.177.9.2251-2258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev. 2006;70:939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J. The mechanisms of carbon catabolite repression in bacteria. Curr Opin Microbiol. 2008;11:87–93. doi: 10.1016/j.mib.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Deutscher J, Kessler U, Alpert CA, Hengstenberg W. Bacterial phosphoenolpyruvate-dependent phosphotransferase system: P-Ser-HPr and its possible regulatory function. Biochemistry (Wash.) 1984;23:4455–4460. doi: 10.1021/bi00314a033. [DOI] [PubMed] [Google Scholar]
- Dong Y, Chen YY, Burne RA. Control of expression of the arginine deiminase operon of Streptococcus gordonii by CcpA and Flp. J Bacteriol. 2004;186:2511–2514. doi: 10.1128/JB.186.8.2511-2514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil JD, Jacques M, Brochu D, Frenette M, Vadeboncoeur C. Surface location of HPr, a phosphocarrier of the phosphoenolpyruvate: sugar phosphotransferase system in Streptococcus suis. Microbiology. 1996;142(Pt 4):837–843. doi: 10.1099/00221287-142-4-837. [DOI] [PubMed] [Google Scholar]
- Dunny GM, Lee LN, LeBlanc DJ. Improved electroporation and cloning vector system for gram-positive bacteria. Appl Environ Microbiol. 1991;57:1194–1201. doi: 10.1128/aem.57.4.1194-1201.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier M, Brochu D, Eltis LD, Thomas S, Vadeboncoeur C. Replacement of isoleucine-47 by threonine in the HPr protein of Streptococcus salivarius abrogates the preferential metabolism of glucose and fructose over lactose and melibiose but does not prevent the phosphorylation of HPr on serine-46. Mol Microbiol. 1997;25:695–705. doi: 10.1046/j.1365-2958.1997.4981870.x. [DOI] [PubMed] [Google Scholar]
- Giammarinaro P, Paton JC. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect Immun. 2002;70:5454–5461. doi: 10.1128/IAI.70.10.5454-5461.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorke B, Stulke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol. 2008;6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- Griswold AR, Chen YY, Burne RA. Analysis of an agmatine deiminase gene cluster in Streptococcus mutans UA159. J Bacteriol. 2004;186:1902–1904. doi: 10.1128/JB.186.6.1902-1904.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold AR, Jameson-Lee M, Burne RA. Regulation and physiologic significance of the agmatine deiminase system of Streptococcus mutans UA159. J Bacteriol. 2006;188:834–841. doi: 10.1128/JB.188.3.834-841.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau PC, Sung CK, Lee JH, Morrison DA, Cvitkovitch DG. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J Microbiol Methods. 2002;49:193–205. doi: 10.1016/s0167-7012(01)00369-4. [DOI] [PubMed] [Google Scholar]
- Monedero V, Poncet S, Mijakovic I, Fieulaine S, Dossonnet V, Martin-Verstraete I, Nessler S, Deutscher J. Mutations lowering the phosphatase activity of HPr kinase/phosphatase switch off carbon metabolism. Embo J. 2001;20:3928–3937. doi: 10.1093/emboj/20.15.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier M, Lortie LA, Frenette M, Vadeboncoeur C. The phosphoenolpyruvate:mannose phosphotransferase system of Streptococcus salivarius. Functional and biochemical characterization of IIABL(Man) and IIABH(Man) Biochemistry. 1998;37:1604–1612. doi: 10.1021/bi9721647. [DOI] [PubMed] [Google Scholar]
- Poncet S, Soret M, Mervelet P, Deutscher J, Noirot P. The transcriptional activator YesS is stimulated by histidine-phosphorylated HPr of the Bacillus subtilis phosphotransferase system. J Biol Chem. 2009 doi: 10.1074/jbc.M109.046334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma PW, Lengeler JW, Jacobson GR. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J, Sutrina SL, Saier MH, Stewart GC, Peterkofsky A, Reddy P. Mechanistic and physiological consequences of HPr(ser) phosphorylation on the activities of the phosphoenolpyruvate:sugar phosphotransferase system in Gram-positive bacteria: studies with site-specific mutants of HPr. Embo J. 1989;8:2111–2120. doi: 10.1002/j.1460-2075.1989.tb03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosey EL, Stewart GC. Nucleotide and deduced amino acid sequences of the lacR, lacABCD, and lacFE genes encoding the repressor, tagatose 6-phosphate gene cluster, and sugar-specific phosphotransferase system components of the lactose operon of Streptococcus mutans. J Bacteriol. 1992;174:6159–6170. doi: 10.1128/jb.174.19.6159-6170.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalisch MH, Bachem S, Stulke J. Control of the Bacillus subtilis antiterminator protein GlcT by phosphorylation. Elucidation of the phosphorylation chain leading to inactivation of GlcT. J Biol Chem. 2003;278:51108–51115. doi: 10.1074/jbc.M309972200. [DOI] [PubMed] [Google Scholar]
- Shaw WV. Chloramphenicol acetyltransferase activity from chloramphenicol-resistant bacteria. Methods Enzymol. 1979;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Keith D, Horstmann N, Sumby P, Davenport MT, Graviss EA, Brennan RG, Musser JM. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc Natl Acad Sci U S A. 2008;105:1698–1703. doi: 10.1073/pnas.0711767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CL, Russell RR. Identification of a homolog of CcpA catabolite repressor protein in Streptococcus mutans. Infect Immun. 1998;66:2085–2092. doi: 10.1128/iai.66.5.2085-2092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulke J, Martin-Verstraete I, Zagorec M, Rose M, Klier A, Rapoport G. Induction of the Bacillus subtilis ptsGHI operon by glucose is controlled by a novel antiterminator, GlcT. Mol Microbiol. 1997;25:65–78. doi: 10.1046/j.1365-2958.1997.4351797.x. [DOI] [PubMed] [Google Scholar]
- Sutcliffe IC, Hogg SD, Russell RR. Identification of Streptococcus mutans antigen D as the HPr component of the sugar-phosphotransferase transport system. FEMS Microbiol Lett. 1993;107:67–70. doi: 10.1016/0378-1097(93)90355-6. [DOI] [PubMed] [Google Scholar]
- Thevenot T, Brochu D, Vadeboncoeur C, Hamilton IR. Regulation of ATP-dependent P-(Ser)-HPr formation in Streptococcus mutans and Streptococcus salivarius. J Bacteriol. 1995;177:2751–2759. doi: 10.1128/jb.177.10.2751-2759.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C, Pelletier M. The phosphoenolpyruvate:sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol Rev. 1997;19:187–207. doi: 10.1111/j.1574-6976.1997.tb00297.x. [DOI] [PubMed] [Google Scholar]
- van Tilbeurgh H, Declerck N. Structural insights into the regulation of bacterial signalling proteins containing PRDs. Curr Opin Struct Biol. 2001;11:685–693. doi: 10.1016/s0959-440x(01)00267-6. [DOI] [PubMed] [Google Scholar]
- Wen ZT, Burne RA. Analysis of cis- and trans-acting factors involved in regulation of the Streptococcus mutans fructanase gene (fruA) J Bacteriol. 2002;184:126–133. doi: 10.1128/JB.184.1.126-133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Dong Y, Burne RA. Characterization of cis-acting sites controlling arginine deiminase gene expression in Streptococcus gordonii. J Bacteriol. 2006a;188:941–949. doi: 10.1128/JB.188.3.941-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Wen ZT, Burne RA. A novel signal transduction system and feedback loop regulate fructan hydrolase gene expression in Streptococcus mutans. Mol Microbiol. 2006b;62:187–200. doi: 10.1111/j.1365-2958.2006.05359.x. [DOI] [PubMed] [Google Scholar]
- Zeng L, Burne RA. Multiple sugar: phosphotransferase system permeases participate in catabolite modification of gene expression in Streptococcus mutans. Mol Microbiol. 2008;70:197–208. doi: 10.1111/j.1365-2958.2008.06403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Burne RA. Transcriptional regulation of the cellobiose operon of Streptococcus mutans. J Bacteriol. 2009;191:2153–2162. doi: 10.1128/JB.01641-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.