Abstract
Increased neurogenesis in the dentate gyrus (DG) after brain insults such as excitotoxic lesions, seizures or stroke is a well known phenomenon in the young hippocampus. This plasticity reflects an innate compensatory response of neural stem cells (NSCs) in the young hippocampus to preserve function or minimize damage after injury. However, injuries to the middle-aged and aged hippocampi elicit either no or dampened neurogenesis response, which could be due to an altered plasticity of NSCs and/or the hippocampus with age. We examined whether the plasticity of NSCs to increase neurogenesis in response to a milder injury such as partial deafferentation is preserved during aging. We quantified DG neurogenesis in the hippocampus of young, middle-aged and aged F344 rats after partial deafferentation. A partial deafferentation of the left hippocampus without any apparent cell loss was induced via administration of Kainic acid (0.5 μg in 1.0 μl) into the right lateral ventricle of the brain. In this model, degeneration of CA3 pyramidal neurons and dentate hilar neurons in the right hippocampus results in loss of commissural axons which leads to partial deafferentation of the dendrites of dentate granule cells and CA1-CA3 pyramidal neurons in the left hippocampus. Quantification of newly born cells that are added to the dentate granule cell layer at post-deafferentation days 4-15 using 5′-bromodeoxyuridine (BrdU) labeling revealed greatly increased addition of newly born cells (~3 fold increase) in the deafferented young and middle-aged hippocampi but not in the deafferented aged hippocampus. Measurement of newly born neurons using doublecortin (DCX) immunostaining also revealed similar findings. Analyses using BrdU-DCX dual immunofluorescence demonstrated no changes in neuronal fate-choice decision of newly born cells after deafferentation, in comparison to the age-matched naive hippocampus in all age groups. Thus, the plasticity of hippocampal NSCs to increase DG neurogenesis in response to a milder injury such as partial hippocampal deafferentation is preserved until middle age but lost at old age.
Keywords: adult neurogenesis, aging, 5′-bromodeoxyuridine, dentate gyrus, dentate neurogenesis, doublecortin, kainic acid, neural stem cells, rat, stem cell proliferation, stem cell differentiation
Introduction
Hippocampal neurogenesis, epitomized by the insertion of new granule cells to the granule cell layer (GCL) of the dentate gyrus (DG) and hippocampal circuitry, takes place throughout life span in virtually all mammals including humans via division of neural stem/progenitor cells (NSCs) located in the subgranular zone (SGZ) of the DG (Kuhn et al., 1996; Gould et al., 1997, 1999a,b; Eriksson et al., 1998; van Praag et al., 2002). Slow proliferation of NSCs (a fraction of cells that express the glial fibrillary acidic protein) in this region produces a pool of transit amplifying cells, which proliferate rapidly and give rise to new neurons and glia (Cameron et al., 1993; Kornack and Rakic, 1999; Seri et al., 2001; Ihrie and Alvarez-Buylla, 2008). Newly differentiated granule cells mature, grow dendrites into the molecular layer, send axons into the CA3 region, and get incorporated into the hippocampal circuitry. Furthermore, a large number of studies imply that newly added granule cells play roles in hippocampal-dependent learning & memory functions and mood (Drapeau et al., 2003, 2007; Santarelli et al., 2003; Kempermann et al., 2004; Toni et al., 2007, 2008; Kee et al., 2007; Imayoshi et al., 2008; Jessberger et al., 2009). However, there is no universal consensus regarding this issue (Shors et al., 2002; Bizon & Gallagher, 2003; Bizon et al., 2004; Leuner et al., 2006).
Multiple factors regulate the proliferation and differentiation of NSCs and their progeny in the DG (Lichtenwalner et al., 2001; Kempermann et al., 2002; Jin et al., 2003; Monje et al., 2003; Sun et al., 2003, 2006; Sairanen et al., 2005; van Praag et al., 2005; Rai et al., 2007). Furthermore, DG neurogenesis is responsive to a variety of brain insults. For instance, brain insults such as excitotoxic lesions, acute seizures, status epilepticus, ischemia or stroke greatly enhance DG neurogenesis in the young adult hippocampus (Bengzon et al., 1997; Parent et al., 1997; Gray and Sundstrom 1998; Liu et al., 1998; Jin et al., 2001, 2004; Hattiangady et al., 2004, 2008; Gong et al., 2007; Rao et al., 2008). This plasticity likely reflects an innate compensatory response of NSCs in the young adult hippocampus to preserve function or minimize damage after injury. While the specific advantages and certain negative aspects of increased DG neurogenesis after hippocampal injury are yet to be understood (Parent, 2003; Jessberger et al., 2007; Scharfman and Gray 2007; Shetty and Hattiangady 2007), it is generally deemed that this NSC plasticity is constructive for diminishing cognitive dysfunction after brain injury (Kleindienst et al., 2005; Sun et al., 2007).
Previous studies examining the response of DG neurogenesis to focal hippocampal injury or acute seizures in different age groups of animals revealed substantial age-related impairment in the ability of hippocampal NSCs to augment DG neurogenesis in response to injury (Hattiangady et al., 2008; Rao et al., 2008). This was verified by the assessment that injuries to the middle-aged and aged hippocampi elicit either no or highly dampened neurogenesis response, which could be due to an altered plasticity of NSCs and/or the hippocampus with age. Thus, increased DG neurogenesis after hippocampal injury appears to be a phenomenon restricted to the young adult hippocampus (Hattiangady et al., 2008). These findings are consistent with studies in stroke models illustrating that NSCs in the aged hippocampus are only mildly proficient for increasing neurogenesis after brain injury (Jin et al., 2004; Darsalia et al., 2005). Nonetheless, it is unknown whether the plasticity of NSCs to increase neurogenesis in response to a milder injury such as partial deafferentation is conserved during aging. Bearing in mind that a partial deafferentation of the hippocampus is an event seen in the early stages of Alzheimer's disease (Rossner, 1997; Anderton et al., 1998; Scheff, 2003), it is of great interest to investigate how NSCs react to partial hippocampal deafferentation during younger, middle and advanced ages.
Therefore, we rigorously quantified DG neurogenesis in the hippocampus of young adult, middle-aged and aged F344 rats after partial deafferentation. A partial deafferentation of the left hippocampus devoid of any apparent cell loss was induced through administration of kainic acid (KA; 0.5 μg in 1.0 μl) into the right lateral ventricle of the brain. In this prototype, wide-ranging loss of CA3 pyramidal neurons and dentate hilar neurons in the right hippocampus prompts loss of commissural axons, which leads to a partial deafferentation of dendrites of dentate granule cells and CA1-CA3 pyramidal neurons in the left hippocampus. We quantified the numbers of new cells and neurons added to the SGZ-GCL of hippocampi contralateral to KA administration between post-deafferentation days 4 and 15 in all three age groups. This was accomplished through 5′-bromodeoxyuridine (BrdU) labeling of newly born cells, immunostaining for the early neuronal marker doublecortin (DCX), dual immunostaining for BrdU and DCX, and optical fractionator cell counting method.
Materials and Methods
Animals and induction of partial deafferentation
Animals purchased from the National Institute for Aging colony of male F344 rats maintained at Harlan Sprague-Dawley (Indianapolis, IN) were utilized in these studies. All experimentation were executed as per the animal protocol approved by the institutional animal care and use committee of the Duke University Medical Center and the animal studies subcommittee of the Durham Veterans Affairs Medical Center. This study encompassed six groups of rats, which include: intact young adult rats (4-months old, n = 5; Group 1), intact middle-aged rats (12-months old, n = 5; Group 2), intact aged rats (24-months old, n = 5; Group 3), young adult rats receiving unilateral intracerebroventricular kainic acid (ICV KA) administration (n = 5; Group 4), middle-aged rats receiving unilateral ICV KA (n = 5; Group 5), and aged rats receiving unilateral ICV KA (n = 5; Group 6). In animals belonging to groups 4-6, to induce partial deafferentation of the left hippocampus, KA was dispensed into the right lateral ventricle as explained in our earlier reports (Shetty and Turner, 1999a,b, 2000; Hattiangady et al., 2008). In brief, each rat was deeply anesthetized, fixed into a stereotaxic apparatus, the dorsal surface of the skull was exposed, a burr hole was drilled in the cranium using appropriate coordinates for the right lateral ventricle, and a 10 μl Hamilton syringe installed with a 25-gauge needle and loaded with KA liquid was lowered through the burr hole and brain into the lateral ventricle (equivalent to a depth of 4.5 mm from the surface of the brain). Subsequently, 1.0 μl of KA solution (containing 0.5 μg of KA) was infused gradually into the lateral ventricle, the needle was pulled out slowly after 15 minutes of injection, skin flaps were affixed through clips and animals were returned to their cages subsequent to the post-operative care.
5′-bromodeoxyuridine labeling of newly born cells in the DG, tissue processing, and analyses of hippocampal structure
Intraperitoneal injection of BrdU was performed daily to rats in all groups for 12 consecutive days at a dose of 100mg/kg b.w. (Sigma, St Louis, MO). In rats receiving ICV KA (Groups 4-6), daily BrdU injections were commenced on post-KA day 4, and ended on post-KA day 15. We commenced BrdU injections on day 4 after the KA injection to allow the recovery of animals after KA injections (performed through stereotaxic survival surgery using anesthesia) before starting the BrdU injections. At 24 hrs after the last BrdU injection, rats in all groups underwent intracardiac perfusion with 4% paraformaldehyde and brains harvested for histological investigation of newly born cells and neurons using cryostat sectioning. In brief, the brains were post-fixed in 4% paraformaldehyde overnight at 4°C, cryoprotected in 30% sucrose solution in phosphate buffer (PB), thirty-micrometer thick cryostat sections were cut coronally through the entire hippocampus and collected serially in PB. Serial sections (every 15th) through the entire hippocampus were chosen in each animal belonging to different groups and stained for Nissl to characterize changes in hippocampal structure on both ipsi- and contra- lateral sides of ICV KA administration.
Immunohistochemistry for visualization of BrdU+ newly born cells and DCX+ newly born neurons
Every 15th section through the entire hippocampus was selected in each rat and processed for BrdU immunohistochemistry using a monoclonal antibody to BrdU (Roche diagnostics; Indianapolis, IN). An additional series (every 15th) of sections from rats in all groups were processed for DCX immunostaining using a polyclonal antibody to DCX (Santa Cruz Biotechnology; Santa Cruz, CA) using avidin-biotin complex method. The protocols used for BrdU and DCX immunostaining are similar to that reported in our previous reports (Rao and Shetty, 2004; Hattiangady et al., 2004; Rao et al., 2005, 2006a). The peroxidase reaction was visualized by using diaminobenzidine as the chromogen for BrdU and Vector gray (Vector) as the chromogen for DCX.
Quantification of surviving neurons in different cell layers of the hippocampus on ipsi- and contra- lateral sides of ICV KA administration
Our previous studies have shown that an unilateral ICV KA injection effects a sizeable loss of hippocampal CA3 pyramidal and dentate hilar neurons in the hippocampus ipsilateral to ICV KA administration but leads to no apparent loss of neurons in the hippocampus contralateral to ICV KA administration in all three age groups (Shetty and Turner, 1999a,b, 2000, Hattiangady et al., 2008). However, in order to validate the above pattern of lesion size in this study, we quantified the numbers of surviving Nissl stained neurons in the dentate hilus and CA1 & CA3 pyramidal cell layers on both ipsi- and contra-lateral sides of the KA injection (n=4/age group). For comparison, Nissl stained neurons were also counted from age-matched naïve control animals (n=4). Every 15th section through the entire hippocampus and the optical fractionator cell counting method were employed for these measurements. We used the StereoInvestigator system (Microbrightfield Inc., Williston, VT) consisting of a color digital video camera (Optronics Inc., Muskogee, OK) interfaced with a Nikon E600 microscope for cell counting. Using a 100X oil immersion lens, Nissl stained neurons were measured from 50-100 randomly and systematically selected frames (each measuring 20 × 20 μm) in every 15th section.
The details of the optical fractionator cell counting method is described in our earlier reports (Rao and Shetty, 2004; Rao et al., 2005, 2006a). Briefly, the outline of the dentate hilus, and CA1 & CA3 pyramidal cell layers was first marked out in every section, the optical fractionator component was then turned on, and the number and location of counting frames and the counting depth for each section were established by keying in parameters such as the grid size, the thickness of the top guard zone (4 μm) and the optical dissector height (8 μm). A computer controlled motorized stage then facilitated the section to be evaluated at each of the counting frame sites. In each site, all Nissl+ neurons (exhibiting a clear nucleus and cytoplasm with Nissl bodies) that were located within the 8μm section depths were counted. The StereoInvestigator program then estimated the total number of Nissl+ neurons per cell layer by utilizing the optical fractionator formula, N = 1/ssf.1/asf.1/hsf.EQ−. The ellipsis ssf denotes the section sampling fraction, which was 15 in this study as every 15th section was sampled; asf is the area sampling fraction, which is calculated by dividing the area sampled with the total area of the cell layer (i.e. the sum of the cell layer areas in all sections used for counting); hsf is the height sampling fraction, which is calculated by dividing the height sampled (i.e. 8 μm in this study) with the section thickness at the time of counting (i.e. 18-20 μm in young adults and 14-16 μm in middle-aged and aged animals). The acronym EQ− denotes the total count of particles sampled for the entire cell layer.
Measurement of the numbers of BrdU+ newly born cells and DCX+ newly born neurons
In each animal belonging to different groups (n = 5 for each of 6 groups), BrdU+ and DCX+ cells in the dentate SGZ (two-cell thick region from the inner margin of the dentate GCL) and the GCL were measured in every 15th section through the whole antero-posterior extent of the hippocampus. Using a 100X oil immersion lens, BrdU+/DCX+ cells were measured from 50-500 randomly and systematically selected frames (each measuring 40 × 40 μm) in every 15th section. The detailed protocol used for counting is available in our earlier reports (Rao and Shetty, 2004; Rao et al., 2005, 2006a). In brief, the outline of the SGZ-GCL was first marked out in every section, the optical fractionator component was then turned on, and the number and location of counting frames and the counting depth for each section were established by keying in parameters such as the grid size, the thickness of the top guard zone (4 μm) and the optical dissector height (8 μm). A computer controlled motorized stage then facilitated the section to be evaluated at each of the counting frame sites. In each site, all BrdU+/DCX+ cells that were located within the 8μm section depths were counted. The StereoInvestigator program then estimated the total number of BrdU+/DCX+ cells per SGZ-GCL by utilizing the optical fractionator formula, N = 1/ssf.1/asf.1/hsf.EQ−, as described above. As aged animals exhibited much fewer numbers of BrdU+/DCX+ cells than younger and middle aged animals, we increased the numbers of frames for counting in every section to encounter more BrdU+/DCX+ cells during counting. The Gundersen coefficient of error (CE) values were less than 0.10 for young and middle aged animals but less than 0.15 for aged animals. The average number of cells actually counted in aged rats varied from 50-100. Whereas, in young and middle-aged animals, the numbers of cells actually counted varied from 200-800.
Measurement of neuronal differentiation of newly born cells via DCX and BrdU dual immunofluorescence and confocal microscopy
To measure the percentages of newly born cells (BrdU+ cells) that differentiate into neurons in the SGZ-GCL, sections from animals belonging to all groups were processed for DCX and BrdU dual immunofluorescence staining (Rao and Shetty, 2004). The sections were washed in PBS, blocked in 3% normal horse serum, incubated overnight at room temperature in the DCX antibody (1: 200; Sc-8066, Santa Cruz Biotechnology, Santa Cruz), washed in PBS, incubated in the biotinylated horse anti-goat IgG (Vector) for 1 h, washed in PBS, and incubated in the streptavidin fluorescein (or streptavidin Texas Red) solution for 1 hr. Following confirmation of the positive DCX immunofluorescence (green colored cells with the use of streptavidin fluorescein; red colored cells with the use of streptavidin Texas Red), sections were processed for BrdU immunofluorescence. To prevent the fading of DCX immunofluorescence, all subsequent incubations in different solutions were carried out in the dark. Briefly, sections were washed in Tris-buffered saline (TBS), incubated in formamide (50%) solution for 2 h at 65 °C, washed in TBS and incubated in 2 N HCl for 60 min at 37 °C. Following this, the sections were neutralized with borate buffer, washed in TBS, blocked in 3% normal goat serum, incubated overnight at 4 °C in the mouse monoclonal BrdU antibody and washed in PBS. The sections in which the DCX+ cells were visualized as green colored cells were then treated with goat anti-mouse IgG tagged with Alexa Fluor 594 for 1 h which resulted in red fluorescence for BrdU+ nuclei. On the other hand, the sections in which the DCX+ cells were visualized as red colored cells were treated with goat anti-mouse IgG tagged with Alexa Fluor 488 for 1 h which resulted in green fluorescence for BrdU+ nuclei. Sections were examined using a laser confocal microscope (LSM 510) to identify cells that exhibit BrdU and DCX co-expression. Fractions of BrdU+ cells that express DCX in each animal (~75-100 cells per animal) were then quantified by examination of individual BrdU+ cells at 400X. For this, one-micrometer thick optical Z-sections were sampled from different regions of the SGZ-GCL in all groups and the images were analyzed using LSM image browser.
Statistical analyses
For every parameter, the average value was first calculated separately for each animal before the means and standard errors were determined for the total number of animals included per group. The values from different age groups of animals were compared using two-way ANOVA.
Results
Animal behavior and hippocampal structure after unilateral ICV KA Administration
Observation of animals for 4 hours after unilateral ICV KA administration did not reveal apparent motor seizure activity in any of the age groups. This outcome is expected as KA was injected under anesthesia. This observation is also consistent with earlier reports that ICV KA administration, particularly at the low dose used in this study, does not produce motor seizures (Shetty and Turner 1999a, b; Shetty et al., 2003, 2004, 2005). Thus, there were no age-related differences in animal behavior following unilateral ICV KA administration.
Histological analyses of the tissue sections revealed significant neurodegeneration in the dentate hilus and the CA3 pyramidal cell layer of the hippocampus ipsilateral to the ICV KA administration (i.e. the lesioned hippocampus) in all three age groups (Fig. 1 [B1 & B2, E1 & E2, H1 & H2]), in comparison to the hippocampus of age-matched control animals (Fig. 1 [A1 & A2, D1 & D2, G1 & G2]). This is consistent with our observations in earlier studies (Hattiangady et al., 2008; Shetty et al., 2009). Quantification of the numbers of surviving neurons in all three age groups revealed that overall loss of neurons ranged from 48-58% in the dentate hilus (p < 0.05 to p < 0.001) and 80-83% in the CA3 pyramidal cell layer (Fig. 2 [A1, A3]; p < 0.001). The CA1 cell layer had minimal (or insignificant) loss of neurons in all three age groups [Fig. 2 [A2]; p > 0.05). Interestingly, the hippocampus contralateral to ICV KA administration displayed no apparent neuron loss and all hippocampal cell layers remained intact in all age groups of animals (Fig. 1 [C1 & C2, F1 & F2, I1 & I2]), which is consistent with our multiple earlier studies (Shetty and Turner, 1999a,b, 2000, 2001). Quantification of the numbers of surviving neurons in all three age groups revealed no significant loss of neurons in any of the hippocampal cell layers (Fig. 2 [B1, B2, B3]; p > 0.05). Thus, a unilateral ICV KA injection leads to significant loss of neurons in the hippocampus ipsilateral to KA administration but not in the hippocampus contralateral to KA administration. However, with the loss of CA3 pyramidal neurons and dentate hilar neurons in the hippocampus ipsilateral to KA administration, the contralateral hippocampus loses large amounts of commissural axons leading to a partial deafferentation of dentate granule cells and CA1-CA3 pyramidal neurons, as described in earlier studies (Nadler et al., 1980a,b). Thus, the hippocampus contralateral to ICV KA administration serves as an excellent model for analyzing neurogenesis in the deafferented hippocampus.
Figure 1.
Cytoarchitecture of the hippocampus at 16 days after kainic acid (KA) administration into the right lateral ventricle of young adult, middle-aged and aged rats, visualized by Nissl staining. A1, D1, and G1 respectively show structure of the intact hippocampus from the young, middle-aged and aged control rats. A2, D2 and G2 are magnified views of A1, D1 and G1 showing the dentate hilus and CA3c subregion. B1, E1 and H1 illustrate the hippocampus ipsilateral to the KA administration from the young, middle-aged and aged rats respectively. B2, E2 and H2 are magnified views of regions from B1, E1 and H1. Note the loss of dentate hilar neurons, and CA3 pyramidal neurons (indicated by asterisks in B1, E1 and H1) in all three age groups. C1, F1 and I1 show the hippocampus contralateral to the KA administration (i.e. the deafferented hippocampus) from the young, middle-aged and aged rats respectively. C2, F2, and I2 are magnified views of regions from C1, F1 and I1. Note that all cell layers are preserved in the hippocampus contralateral to the KA administration in all three age groups. DH, dentate hilus, Scale bar, A1, B1, C1, D1, E,1, F1, G1, H1, and I1 = 500 μm; A2, B2, C2, D2, E2, F2, G2, H2, I2 = 200 μm.
Figure 2.
Unilateral ICV KA injection leads to a significant loss of neurons in the hippocampus ipsilateral to KA administration but not in the hippocampus contralateral to KA administration. The bar charts on the left (A1-A3) show the average numbers of surviving neurons in the dentate hilus and the CA1 & CA3 pyramidal cell layers of hippocampi ipsilateral to the KA administration in different age groups, in comparison to age-matched intact hippocampi. Note that, the neuron loss is significant in the dentate hilus and the CA3 pyramidal cell layer in all three age groups. On the other hand, the CA1 cell layer exhibits no significant loss of neurons in all three age groups. The bar charts on the left (B1-B3) show the average numbers of surviving neurons in the dentate hilus and the CA1 & CA3 pyramidal cell layers of hippocampi contralateral to the KA administration (i.e. the deafferented hippocampi) in different age groups, in comparison to age-matched intact hippocampi. Note that the deafferented hippocampi exhibit no significant loss of neurons in any of the hippocampal cell layers.
Age-related changes in the extent of addition of new cells to SGZ-GCL after hippocampal deafferentation
Age-related alterations in the extent of addition of new cells to the SGZ-GCL after hippocampal deafferentation were assessed through BrdU immunostaining of hippocampal sections from animals belonging to all groups killed at 24 hours after the last of twelve daily BrdU injections (Fig. 3). In KA treated animals, the BrdU injections were administered during the post-KA days 4-15. Newly generated cells (BrdU+ cells) could be seen in the SGZ-GCL throughout their antero-posterior extent in the deafferented hippocampus of all age groups. Our earlier reports have described the distribution and addition of newly born cells to the SGZ-GCL of young adult, middle-aged and aged hippocampi under intact condition (Rao et al., 2005) and following ICV KA induced direct injury (Hattiangady et al., 2008). Therefore, in this study, our description is restricted to changes in the deafferented hippocampi (i.e. hippocampi contralateral to KA-injured hippocampi). The deafferented hippocampus of young adult rats exhibited a dramatic increase in the number of newly born cells in the SGZ-GCL (Fig. 3 [D1, D2]), in comparison to age-matched intact hippocampi (Fig. 3 [A1-A2]). Like young rats, the deafferented hippocampus of middle-aged rats showed considerable increase in the number of newly born cells in the SGZ-GCL (Fig. 3 [E1, E2]), in comparison to age-matched intact hippocampi (Fig. 3 [B1-B2]). In contrast, the deafferented hippocampus of aged rats exhibited density of BrdU+ cells in SGZ-GCL (Fig. 3 [F1-F2]) which is comparable to that observed in the age-matched intact hippocampi (Fig. 3 [C1-C2]). This suggests that no upregulation in the number of newly born cells occurs in these regions following deafferentation in aged rats.
Figure 3.
Newly generated cells in the dentate gyrus (DG) at 24 hours after 12 daily injections of 5′-bromodeoxyuridine (BrdU) in different groups, visualized with BrdU immunostaining and hematoxylin counterstaining. The groups include the dentate gyrus of naïve young adult (A1, A2), middle-aged (B1, B2) and aged (C1, C2) rats, and the dentate gyrus of deafferented hippocampi (i.e. hippocampi contralateral to kainic acid [KA] administration) from young adult (D1, D2), middle-aged (E1, E2) and aged (F1, F2) rats. A2, B2, C2, D2, E2, and F2 are magnified views of regions from A1, B1, C1, D1, E1, and F1 respectively. Note that the density of newly born cells in the subgranular zone-granule cell layer (SGZ-GCL) increases in the young adult and middle-aged rats after deafferentation (D1 & D2 and E1 & E2), in comparison to age-matched naïve rats (A1 & A2 and B1 & B2). In contrast, the density of newly born cells in these regions is unchanged in aged rats (F1, F2) after similar deafferentation, in comparison to the age-matched naïve group (C1, C2). The dentate hilus (DH) and CA3c sub region of the deafferented hippocampi however show increased density of BrdU+ cells in all age groups (D1, E1 and F1). Scale bar, A1, B1, C1 D1, E1, F1= 200 μm; A2, B2, C2, D2, E2, F2= 50 μm. The bar chart in G compares the absolute numbers of newly born cells added over a period of 12 days to the SGZ-GCL of intact and deafferented hippocampi in different age groups. Note that deafferentation greatly enhances the numbers of newly born cells in the SGZ-GCL of young adult and middle-aged rats but not in aged rats.
Quantification of the number of newly born cells added to the SGZ-GCL over a period of 12 days (i.e. during the post-deafferentation days 4-15) revealed a marked increase in the addition of new cells in the deafferented young adult and middle-aged hippocampi (Fig. 3 [G]). The deafferented young hippocampus exhibited 2.8 fold increase (p<0.001) whereas the deafferented middle-aged hippocampus displayed 2.6 fold increase (p<0.001), in comparison to numbers in respective age-matched intact hippocampi (Fig. 3 [G]). However, the deafferented aged hippocampus exhibited no increase in the number of newly born cells added to the SGZ-GCL during the post-deafferentation days 4-15 (Fig. 3 [G]). Thus, unlike the SGZ-GCL of the deafferented young and middle-aged hippocampi, the SGZ-GCL of the deafferented aged hippocampus displays no changes to the addition of new cells. Comparison of numbers across the deafferented groups reveals that the addition of new cells during the post-deafferentation days 4-15 in the deafferented young hippocampus is ~4 folds greater than that of the deafferented middle-aged hippocampus and ~14.0 folds greater than that of the deafferented aged hippocampus.
Neuronal fate-choice decision of newly born cells after hippocampal deafferentation
Neurons among newly born cells (BrdU+ cells) in the SGZ-GCL were characterized through dual immunofluorescence and confocal microscopic analyses for BrdU and DCX in tissues harvested at 24 hours after the last of twelve daily BrdU injections. This visualized BrdU expression in the nucleus and DCX expression within soma and dendrites of newly born neurons (Fig. 4). Doublecortin is an excellent marker of immature neurons in the DG, as DCX expression occurs within 3 hours after birth in neuronally committed newly born cells (Kempermann et al., 2003) and persists for at least two weeks (Rao and Shetty, 2004). In intact animals, our earlier studies have shown that aging does not impair the neuronal fate-choice decision of newly born cells in the SGZ-GCL, as similar fractions of newly born cells differentiated into DCX+ neurons in intact young, middle-aged and aged animals (Rao et al., 2005; Rao et al., 2006a). In close similarity, a vast majority of newly born cells (BrdU+ cells) expressed DCX in the SGZ-GCL of deafferented hippocampi, regardless of the age of the hippocampus at the time of deafferentation. In all groups, DCX+ neurons exhibiting the phenotype of differentiated granule cells (i.e. cells with vertically oriented dendrites projecting into the molecular layer) and DCX+ neurons displaying a relatively immature morphology (i.e. cells with horizontally oriented dendrites in the SGZ) were clearly positive for BrdU. Figure 4 illustrates examples of BrdU Immunopositivity in DCX+ neurons exhibiting vertically oriented dendrites projecting into the molecular layer (A1, A2) and in a DCX+ neuron displaying horizontally oriented dendrite running along the inner margin of the GCL (B1, B2).
Figure 4.
Figures A1 and B1 show examples of the differentiation of newly born cells into doublecortin (DCX) immunopositive neurons in the subgranular zone-granule cell layer (SGZ-GCL) at 24 hours after the last of twelve daily injections of 5′-bromodeoxyuridine (BrdU) and visualized through BrdU and DCX immunofluorescence and confocal microscopy. Figure A1 shows relatively mature newly born neurons with vertically oriented dendrites projecting into the molecular layer (ML) whereas A2 shown a newly born neuron with a horizontally oriented dendrite. Figures A2 & B2 show orthogonal views of newly born cells from A1 and B1 that are positive for both BrdU and DCX. Scale bar, = 10 μm. DH, dentate hilus. The bar chart in C compares the percentages of newly born cells (i.e. BrdU+ cells) that express the neuronal marker doublecortin (DCX) in intact and deafferented hippocampi of different age groups. Note that the rate of neuronal differentiation of newly born cells remains stable across the three age groups under both intact and deafferented conditions. The bar chart in D illustrates that similar percentages of DCX+ cells are immunoreactive for BrdU in different groups. This suggests that a vast majority of neurons visualized with DCX immunostaining in the SGZ-GCL of both intact and deafferented hippocampi are new granule cells that were generated during the preceding 12 days in all age groups.
The extent of neuronal differentiation of newly born cells in the SGZ-GCL was very similar across the three age groups after hippocampal deafferentation and did not differ significantly from that observed in age-matched intact rats (Fig. 4 [C]). The overall neuronal differentiation in the SGZ-GCL was 82 ± 2.6 % in the deafferented young adult hippocampus, 84.5 ± 4.9% in the deafferented middle-aged hippocampus, and 76.3 ± 2.6% in the deafferented aged hippocampus (Fig. 4 [C]). These percentages are closely comparable to percentages observed in intact age-matched hippocampi (Rao et al., 2005). Thus, majority of newly born cells in the SGZ-GCL of the DG differentiate into neurons in all age groups under both intact as well as deafferented conditions. This suggests that neither aging nor deafferentation interferes with neuronal fate-choice decision of newly born cells in the SGZ-GCL. Quantification of the percentages of DCX+ neurons having BrdU immunopositive nucleus at 16 days post-injury demonstrated that over 84% of all DCX+ neurons in the SGZ-GCL were born during the twelve daily BrdU injections (i.e. at post-injury days 4-15) in all three age groups (Fig. 4 [D]). This is similar to the earlier observation in age-matched intact rats (Rao et al., 2005). Thus, a vast majority of neurons visualized with DCX immunostaining in the SGZ-GCL of both intact and deafferented young adult, middle-aged and aged rats are new granule cells that were generated during the preceding 12 days (i.e. during the BrdU injection period). Interestingly, hippocampal deafferentation does not alter the DCX expression phase of newly born neurons in all groups examined in this study.
Hippocampal deafferentation induced changes in DCX+ newly born neuron population
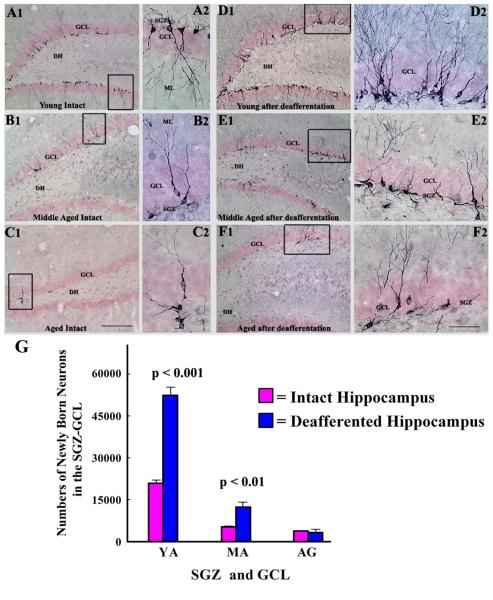
As an additional measure of the status of DG neurogenesis after hippocampal deafferentation, we characterized DCX+ newly born neurons in the SGZ-GCL and the dentate hilus of all age groups of rats (Fig. 5 [A1-F2]). An increased density of DCX+ newly born neurons was observed in the SGZ-GCL of the deafferented young hippocampus (Fig. 5 [D1, D2]) and the deafferented middle-aged hippocampus (Fig. 5 [E1, E2], in comparison to new neurons in respective regions of the age-matched intact hippocampi (Fig. 5 [A1-A2 and B1-B2]). However, the density of DCX+ newly born neurons in the SGZ-GCL of the deafferented aged hippocampus (Fig. 5 [F1-F2]) was comparable to new neurons in respective region of the age-matched intact hippocampus (Fig. 5 [C1-C2]). The dentate hilus showed only a few DCX+ neurons following deafferentation in all age groups. This suggests that the ectopic migration of newly born cells into the dentate hilus that occurs after direct hippocampal injury or seizures (Parent et al., 1997; Gray and Sundstrom 1998; Hattiangady et al., 2004, 2008) does not happen with the milder deafferentation insult to the hippocampus. Quantification of the numbers of DCX+ neurons in the SGZ-GCL of different groups revealed that hippocampal deafferentation considerably increases the number of new neurons in the SGZGCL of young adult and middle-aged rats but not in aged rats. In comparison to numbers in age matched intact hippocampi, the overall increase was 2.5 fold in the deafferented young hippocampus (p<0.001) and 2.3 fold in the deafferented middle-aged hippocampi (p<0.01; Fig. 5 [G]). In contrast, aged rats exhibited no significant changes in numbers of newly born neurons with deafferentation (p>0.05; Fig. 5 [G]).
Figure 5.
Newly born neurons in the subgranular zone-granule cell layer (SGZ-GCL) of different groups, visualized through doublecortin (DCX) immunostaining. The groups include DG of naïve young adult (A1, A2), middle-aged (B1, B2) and aged (C1, C2) hippocampi, and DG of deafferented hippocampi (i.e. hippocampi contralateral to KA) from young adult (D1, D2), middle-aged (E1, E2) and aged (F1, F2) rats at 16 days post-deafferentation. A2, B2, C2, D2, E2, and F2 are magnified views of boxed regions from A1, B1, C1, D1, E1, and F1. Following deafferentation, the density of newly born neurons in the SGZ-GCL increases in the young adult (D1, D2) and middle-aged (E1, E2), in comparison to age-matched naïve rats (A1, A2 and B1, B2). In contrast, the density of newly born neurons is unchanged in the deafferented aged hippocampus (F1, F2), in comparison to the age-matched naïve group (C1, C2). Newly born neurons were observed only occasionally in the dentate hilus of both intact and deafferented hippocampi in all age groups, suggesting that ectopic migration of newly born cells is minimal after deafferentation of the hippocampus. Scale bars, A1, B1, C1, D1, E1, F1 = 200 μm; A2, B2, C2, D2, E2, F2 = 50 μm. The bar chart in G illustrates absolute numbers of newly born neurons (i.e. DCX+ neurons) in the SGZ-GCL of intact and deafferented hippocampi belonging to different age groups. Note that deafferentation considerably enhances the numbers of newly born neurons in the SGZ-GCL of young adult and middle-aged rats but not in aged rats.
Discussion
The results provide novel evidence that the reaction of DG neurogenesis to a milder injury such as partial deafferentation varies depending on the age of the hippocampus. In this study, the left hippocampus was partially deafferented in young, middle-aged and aged animals through KA-induced degeneration of neurons in the right hippocampus (i.e. CA3 pyramidal neurons and dentate hilar neurons) that dispatch commissural axons to the left hippocampus. This partial deafferentation greatly enhanced neurogenesis in the young adult and the middle-aged hippocampi but effected no changes on DG neurogenesis in the aged hippocampus. This finding differs with the earlier observation that the reaction of DG neurogenesis to a direct hippocampal lesion is lost as early as middle age (Hattiangady et al., 2008). Taken together, these observations suggest that the plasticity of hippocampal NSCs to enhance DG neurogenesis in response to a milder injury such as partial deafferentation is preserved for longer periods during the lifespan.
Response of DG neurogenesis to deafferentation differs from its reaction to a direct injury in the middle-aged hippocampus
Earlier studies have shown that a direct hippocampal injury elicits an increased proliferative response from NSCs in the SGZ of the young adult hippocampus resulting in an enhanced DG neurogenesis (Parent et al., 1997; Gray and Sundstrom, 1998; Nakagawa et al., 2000; Hattiangady et al., 2004; and see Table 1). However, the middle-aged hippocampus fails to up-regulate DG neurogenesis after similar injury in most cases (Sivilia et al., 2008; Hattiangady et al., 2008; Rao et al., 2008; and see Table 1). Based on these results, it was thought that the ability to increase DG neurogenesis following an injury is restricted to NSCs in the young adult hippocampus. However, our observations in this study suggest that NSCs in the middle-aged hippocampus are capable of enhancing DG neurogenesis in response to milder insults. While the precise reasons underlying the diverged response of NSCs in the middle-aged hippocampus to a milder insult such as partial deafferentation vis-à-vis direct lesion are yet to be unraveled, it could be related to the differential response of the middle-aged hippocampus to a milder partial deafferentation in comparison to the much severe lesion. Particularly, it is plausible that certain factors that positively regulate neurogenesis such as brain-derived neurotrophic factor (BDNF), fibroblast growth factor-2 (FGF-2), vascular endothelial growth factor (VEGF) are up-regulated to greater levels after milder insults than after direct lesion in the middle-aged hippocampus. Although the deafferentation-induced changes in the above neurotrophic factors have not been quantified for the middle-aged hippocampus, studies on the adult hippocampus show up-regulation in some of these factors and their receptors at 3-15 days after hippocampal deafferentation induced by the entorhinal cortex lesion (Wang et al., 2005). A previous study has also shown induction of a neurite-promoting factor in the adult rat brain following deafferentation (Needels et al., 1986). On the other hand, a direct lesion to the middle-aged hippocampus does not seem to greatly increase the levels of these neurotrophic factors. For example, after KA-induced injury, the middle-aged hippocampus has 45% less BDNF than similarly injured young adult hippocampus (Shetty et al., 2004). Thus, it is possible that the middle-aged hippocampus is capable of up-regulating factors that enhance neurogenesis after milder injury such as deafferentation but not after a major lesion. However, additional studies that measure and compare the levels of different neurogenesis related genes and proteins between the deafferented and the lesioned middle aged hippocampi are needed in future to clearly address this issue.
Table 1.
Effect of Age on the Response of DG Neurogenesis to Different Types of Hippocampal Injury
| Type of Injury |
Young Hippocampus |
Middle Aged Hippocampus |
Aged Hippocampus |
|---|---|---|---|
| ICV KA Induced Direct Injury |
Enhanced Neurogenesis (Gray & Sundstrom, 1998 Hattiangady et al., 2004, 2008) |
No Change (Hattiangady et al., 2008) |
No Change (Hattiangady et al., 2008) |
| Status Epilepticus Induced Injury |
Enhanced Neurogenesis (Parent et al., 1997 Nakagawa et al., 2000 Hattiangady et al., 2004 Rao et al., 2008) |
No Change (Rao et al., 2008) |
No Change (Rao et al., 2008) |
| Stroke-Induced Injury |
Enhanced Neurogenesis (Jin et al., 2004 Darsalia et al., 2005) |
Slightly Increased Neurogenesis (Darsalia et al., 2005) |
No Change (Jin et al., 2004) |
| Hypoxic Ischemic Injury |
Enhanced Neurogenesis (Mattiesen et al., 2009) |
No Change (Sivilia et al., 2008) |
No Change (Mattiesen et al., 2009) |
| Partial Deafferentation Injury |
Enhanced Neurogenesis (Current Study) |
Enhanced Neurogenesis (Current Study) |
No Change (Current Study) |
Abbreviations; ICV KA, intracerebroventricular.
Potential reasons for loss of plasticity of NSCs in the aged hippocampus to various insults
Our prior neurogenesis studies in KA-injury prototypes have validated the incapability of the aged hippocampus to up-regulate DG neurogenesis after a direct hippocampal injury or status epilepticus induced damage (Hattiangady et al., 2008; Rao et al., 2008). Studies on the human brain samples after hypoxicischemic injury and on animal models of stroke also support the above conclusion (Jin et al., 2004; Mattiesen et al., 2009 and see Table 1). The current study now points out that the aged hippocampus is incapable of mounting a compensatory neurogenesis response even to a milder partial deafferentation.
The precise reasons for the lack of neurogenesis response to injury in the aged hippocampus are unclear. However, it is unlikely that it is a consequence of intrinsic modifications in NSCs themselves such as decreased telomerase levels and telomerase shortening during aging (Shay and Wright, 2000; Brazel et al., 2005) or reduction in their numbers in the SGZ. This is because NSCs in the aged hippocampus exhibit increased proliferation and neurogenesis in response to both administration of neurotrophic factors such as EGF, FGF-2 and IGF-1 (Lichtenwalner et al., 2001; Jin et al., 2003) and decreased levels of stress hormones induced through adrenalectomy (Cameron and McKay, 1999). Moreover, grafting of stem cells that potentially secrete neurotrophic factors enhance neurogenesis in the aged hippocampus (Bachstetter et al., 2008). Furthermore, the overall numbers of NSCs in the rodent SGZ remain constant during the course of aging though fractions of proliferating NSCs decline with aging (Olariu et al., 2007; Drapeau and Abrous, 2008; Hattiangady and Shetty, 2008; Aizawa et al., 2009).
Considering the above, it is possible that the microenvironment of the aged hippocampus plays a major role in the loss of NSC plasticity to various insults. For example, a number of studies demonstrate that injury to the young adult hippocampus is associated with enhanced concentration of neurotrophic factors that are known to stimulate the production of new neurons from NSCs. These comprise BDNF, NGF, FGF-2, EGF, VEGF and GDNF (Lowenstein et al., 1993; Shetty et al., 2003, 2004; Hagihara et al., 2005; Cheng et al., 2008). While injury increases the levels of BDNF in all age groups, the injured aged hippocampus contains considerably less BDNF than the lesioned young hippocampus (Shetty et al., 2004). Furthermore, unlike the injured young adult hippocampus, the IGF-1 does not seem to be up-regulated after injury in the aged hippocampus (Woods et al., 1998). Lower levels of neurotrophic factors in the injured aged hippocampus are also supported by the observation that NSCs in the injured aged hippocampus respond to exogenous applications of FGF-2 and BDNF through increased proliferation and neurogenesis (Hattiangady and Shetty, 2004, 2007). Additionally, grafting of NSCs into the injured aged hippocampus is associated with diminished differentiation of graft-derived cells (Shetty et al., 2008). On the other hand, pretreatment and grafting of neural cell grafts with neurotrophic factors FGF-2 and/or BDNF improves their survival in the injured aged hippocampus (Zaman and Shetty, 2002, 2003; Rao et al., 2006b). Taken together, it appears that subdued response of neurotrophic factors after injury in the aged hippocampus is likely one of the major reasons for loss of neurogenesis response to both milder and severe forms of injury. Additionally, changes in levels of factors that negatively regulate neurogenesis such as increased levels of TGF-β and caspase-1 activity likely also contribute to unchanged neurogenesis in the aged hippocampus after deafferentation or injury (Buckwalter et al., 2006; Gemma et al., 2007).
The unresponsiveness of neurotrophic factors to injury described above likely also contributes to the waning or loss of the plasticity in the aged hippocampus after injury. For example, the positive response of DG neurogenesis to deafferentation in the young adult hippocampus varies depending on the type of deafferentation. Earlier studies have shown that a partial hippocampal deafferentation induced through entorhinal cortex lesion (resulting in loss of perforant path axons) enhances neurogenesis in the adult hippocampus (Fontana et al., 2006). This is consistent with the current observation of increased neurogenesis in the young adult and middle-aged hippocampi following partial deafferentation inflicted through KA-mediated loss of CA3 pyramidal neurons and dentate hilar neurons in the opposite hippocampus (resulting in loss of commissural axons). In contrast, cholinergic deafferentation of the adult hippocampus via fimbria-fornix lesions has been found to diminish DG neurogenesis (Fontana et al., 2006). This suggests that NSC proliferation in the DG is very much dependent on the input from cholinergic neurons in the basal forebrain and septum. Thus, it is likely that increased neurogenesis observed after deafferentation inflicted through entorhinal cortex lesion (Fontana et al., 2006) or CA3 lesion in the opposite hippocampus (the current study) is a consequence of increased cholinergic input to the deafferented DG. This could result from sprouting of cholinergic axons and cholinergic neo-synaptogenesis on vacant synaptic sites on dendrites of granule cells after deafferentation. Indeed, previous studies suggest such axonal sprouting after deafferentation induced through both entorhinal cortex lesion (Forster et al., 1997; Naumann et al., 1997; Ramirez, 2001) and CA3 lesion in the opposite hippocampus (Nadler et al., 1980a,b). From these perspectives, it is possible that lack of DG neurogenesis plasticity in the aged hippocampus to partial deafferentation at least partially stems from the absence of cholinergic sprouting to deafferentation stimuli. This possibility is supported by the previous observation that deafferentation in the aged hippocampus is not generally followed by adequate sprouting response of spared axons (Cotman and Scheff, 1979; Ransmayr et al., 1989; Schauwecker et al., 1995; Woods et al., 1998; Shetty and Turner, 1999a; Crutcher, 2002).
Implications of loss of plasticity of NSCs in the aged hippocampus to various insults
Lack of neurogenesis response in the aged hippocampus to milder deafferentation as well as to direct lesion can have major consequences. This is because many studies show that the spatial memory performance in the aged rats predicts the level of dentate neurogenesis (van Praag et al., 2005; Drapeau et al., 2003, 2007; Bruel-Jungerman et al., 2005; Aimone et al., 2006; Siwak-Tapp et al., 2007) though universal consensus regarding this issue is lacking (Bizon & Gallagher, 2003; Bizon et al., 2004). Furthermore, recent studies imply that newly formed neurons in the DG get involved into learning and memory circuits (Kee et al., 2007, Toni et al, 2007, 2008; Imayoshi et al., 2008; Jessberger et al., 2009). The above perspectives help explain why cognitive impairments related to hippocampal deafferentation or injury are much severe in the elderly population (Ferrell and Tanev, 2002: Bruns and Hauser, 2003). Indeed, a previous study shows that a milder stroke-like hippocampal injury induced by endothelin-1 injections impairs learning & memory function in aged rats but not in young rats (Driscoll et al., 2008). Considering these findings and the improved cognitive function observed in rodent and primate models of Alzheimer's disease (AD) as well as AD patients following nerve growth factor (NGF) or BDNF gene delivery into the brain (Tuszynski et al., 2005; Nagahara et al., 2009), approaches that enhance neurogenesis-related neurotrophic factors appear highly beneficial for preserving cognitive function in the normal aged population and improving cognitive function in aged persons with both milder and moderate levels of hippocampal injury (Jessberger and Gage, 2008).
Acknowledgments
This research was supported by grants from the Department of Veterans Affairs (VA Merit Review Award to A.K.S.), the National Institute for Aging (R01 AG20924 to A.K.S.), and the National Institute of Neurological Disorders and Stroke (R01 NS054780 to A.K.S.).
References
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Anderton BH, Callahan L, Coleman P, Davies P, Flood D, Jicha GA, Ohm T, Weaver C. Dendritic changes in Alzheimer's disease and factors that may underlie these changes. Prog Neurobiol. 1998;55:595–609. doi: 10.1016/s0301-0082(98)00022-7. [DOI] [PubMed] [Google Scholar]
- Aizawa K, Ageyama N, Terao K, Hisatsune T. Primate-specific alterations in neural stem/progenitor cells in the aged hippocampus. Neurobiol Aging. 2009 Feb 5; doi: 10.1016/j.neurobiolaging.2008.12.011. [Epub ahead of print] PMID: 19201065. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Pabon MM, Cole MJ, Hudson CE, Sanberg PR, Willing AE, Bickford PC, Gemma C. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci. 2008;9:22. doi: 10.1186/1471-2202-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Elmer E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci USA. 1997;94:10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3:227–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- Brazel CY, Limke TL, Osborne JK, Miura T, Cai J, Pevny L, Rao MS. Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell. 2005;4:197–207. doi: 10.1111/j.1474-9726.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(Suppl 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- Buckwalter MS, Yamane M, Coleman BS, Ormerod BK, Chin JT, Palmer T, Wyss-Coray T. Chronically increased transforming growth factor-beta1 strongly inhibits hippocampal neurogenesis in aged mice. Am J Pathol. 2006;169:154–64. doi: 10.2353/ajpath.2006.051272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neurosci. 1993;56:337–44. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Di Liberto V, Caniglia G, Mudò G. Time-course of GDNFand its receptor expression after brain injury in the rat. Neurosci Lett. 2008;439:24–29. doi: 10.1016/j.neulet.2008.04.089. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Scheff SW. Compensatory synapse growth in aged animals after neuronal death. Mech Ageing Dev. 1979;9:103–117. doi: 10.1016/0047-6374(79)90124-6. [DOI] [PubMed] [Google Scholar]
- Crutcher KA. Aging and neuronal plasticity: lessons from a model. Auton Neurosci. 2002;96:25–32. doi: 10.1016/s1566-0702(01)00373-3. [DOI] [PubMed] [Google Scholar]
- Darsalia V, Heldmann U, Lindvall O, Kokaia Z. Stroke-induced neurogenesis in aged brain. Stroke. 2005;36:1790–1795. doi: 10.1161/01.STR.0000173151.36031.be. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Abrous ND. Stem cell review series: role of neurogenesis in age-related memory disorders. Aging Cell. 2008;7:569–89. doi: 10.1111/j.1474-9726.2008.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Montaron MF, Aguerre S, Abrous DN. Learning-induced survival of new neurons depends on the cognitive status of aged rats. J Neurosci. 2007;27:6037–6044. doi: 10.1523/JNEUROSCI.1031-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Hong NS, Craig LA, Sutherland LA, McDonald RJ. Enhanced cell death and learning deficits after a mini-stroke in aged hippocampus. Neurobiol Aging. 2008;29:1847–1858. doi: 10.1016/j.neurobiolaging.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ferrell RB, Tanev KS. Traumatic brain injury in older adults. Curr Psychiatry Rep. 2002;4:354–362. doi: 10.1007/s11920-002-0083-9. [DOI] [PubMed] [Google Scholar]
- Fontana X, Nacher J, Soriano E, del Rio JA. Cell proliferation in the adult hippocampal formation of rodents and its modulation by entorhinal and fimbria-fornix afferents. Cereb Cortex. 2006;16:301–312. doi: 10.1093/cercor/bhi120. Epub 2005 Jun 15. [DOI] [PubMed] [Google Scholar]
- Forster E, Naumann T, Deller T, Straube A, Nitsch R, Frotscher M. Cholinergic sprouting in the rat fascia dentata after entorhinal lesion is not linked to early changes in neurotrophin messenger RNA expression. Neurosci. 1997;80:731–739. doi: 10.1016/s0306-4522(97)00054-7. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Bizon JL, Hoyt EC, Helm KA, Lund PK. Effects of aging on the hippocampal formation in a naturally occurring animal model of mild cognitive impairment. Exp Gerontol. 2003;38:71–77. doi: 10.1016/s0531-5565(02)00159-6. [DOI] [PubMed] [Google Scholar]
- Gemma C, Bachstetter AD, Cole MJ, Fister M, Hudson C, Bickford PC. Blockade of caspase-1 increases neurogenesis in the aged hippocampus. Eur J Neurosci. 2007;26:2795–803. doi: 10.1111/j.1460-9568.2007.05875.x. [DOI] [PubMed] [Google Scholar]
- Gong C, Wang TW, Huang HS, Parent JM. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci. 2007;27:1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA. 1999a;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999b;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Gray WP, Sundstrom LE. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res. 1998;790:52–59. doi: 10.1016/s0006-8993(98)00030-4. [DOI] [PubMed] [Google Scholar]
- Hagihara H, Hara M, Tsunekawa K, Nakagawa Y, Sawada M, Nakano K. Tonic-clonic seizures induce division of neuronal progenitor cells with concomitant changes in expression of neurotrophic factors in the brain of pilocarpine-treated mice. Brain Res. 2005;39:258–266. doi: 10.1016/j.molbrainres.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK. Plasticity of hippocampal stem/progenitor cells to enhance neurogenesis in response to kainate-induced injury is lost by middle age. Aging Cell. 2008;7:207–224. doi: 10.1111/j.1474-9726.2007.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Subcutaneous administration of fibroblast growth factor-2 (FGF-2) enhances dentate neurogenesis in the injured aged hippocampus. Soc. Neurosci. Abstr. 2004:31.12. [Google Scholar]
- Hattiangady B, Shetty AK. Subcutaneous administration of BDNF dramatically enhances dentate neurogenesis in the injured aged hippocampus. Soc Neurosci Abstr. 2007:562.12. [Google Scholar]
- Ihrie RA, Alvarez-Buylla A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 2008;331:179–91. doi: 10.1007/s00441-007-0461-z. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Zhao C, Toni N, Clemenson GD, Jr, Li Y, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci. 2007;27:9400–9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Gage FH. Stem-cell-associated structural and functional plasticity in the aging hippocampus. Psychol Aging. 2008;23:684–691. doi: 10.1037/a0014188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Xie L, Sun Y, Mao XO, Wang Y, Simon RP, Greenberg DA. Ischemia-induced neurogenesis is preserved but reduced in the aged rodent brain. Aging Cell. 2004;3:373–377. doi: 10.1111/j.1474-9728.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kleindienst A, McGinn MJ, Harvey HB, Colello RJ, Hamm RJ, Bullock MR. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J Neurotrauma. 2005;22:645–655. doi: 10.1089/neu.2005.22.645. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci USA. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neurosci. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH, Seren MS, Longo FM. Prolonged increases in neurotrophic activity associated with kainate-induced hippocampal synaptic reorganization. Neuroscience. 1993;56:597–604. doi: 10.1016/0306-4522(93)90359-n. [DOI] [PubMed] [Google Scholar]
- Mattiesen WR, Tauber SC, Gerber J, Bunkowski S, Bruck W, Nau R. Increased neurogenesis after hypoxic-ischemic encephalopathy in humans is age-related. Acta Neuropathol. 2009;117:525–535. doi: 10.1007/s00401-009-0509-0. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Perry BW, Gentry C, Cotman CW. Degeneration of hippocampal CA3 pyramidal cells induced by intraventricular kainic acid. J Comp Neurol. 1980a;192:333–359. doi: 10.1002/cne.901920209. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Perry BW, Gentry C, Cotman CW. Loss and reacquisition of hippocampal synapses after selective destruction of CA3-CA4 afferents with kainic acid. Brain Res. 1980b;191:387–403. doi: 10.1016/0006-8993(80)91289-5. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needels DL, Nieto-Sampedro M, Cotman CW. Induction of a neurite-promoting factor in rat brain following injury or deafferentation. Neurosci. 1986;18:517–526. doi: 10.1016/0306-4522(86)90055-2. [DOI] [PubMed] [Google Scholar]
- Naumann T, Deller T, Bender R, Frotscher M. 192 IgG-saporin-induced loss of cholinergic neurons in the septum abolishes cholinergic sprouting after unilateral entorhinal lesion in the rat. Eur J Neurosci. 1997;9:1304–1313. doi: 10.1111/j.1460-9568.1997.tb01485.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa E, Aimi Y, Yasuhara O, Tooyama I, Shimada M, McGeer PL, Kimura H. Enhancement of progenitor cell division in the dentate gyrus triggered by initial limbic seizures in rat models of epilepsy. Epilepsia. 2000;41:10–18. doi: 10.1111/j.1528-1157.2000.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Olariu A, Cleaver KM, Cameron HA. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J Comp Neurol. 2007;501:659–667. doi: 10.1002/cne.21268. [DOI] [PubMed] [Google Scholar]
- Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai K, Hattiangady B, Shetty AK. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged and aged hippocampus following intracerebroventricular FGF-2 infusions. Eur J Neurosci. 2007;26:1765–1779. doi: 10.1111/j.1460-9568.2007.05820.x. [DOI] [PubMed] [Google Scholar]
- Ramirez JJ. The role of axonal sprouting in functional reorganization after CNS injury: lessons from the hippocampal formation. Restor Neurol Neurosci. 2001;19:237–262. [PubMed] [Google Scholar]
- Ransmayr G, Cervera P, Hirsch E, Ruberg M, Hersh LB, Duyckaerts C, Hauw JJ, Delumeau C, Agid Y. Choline acetyltransferase-like immunoreactivity in the hippocampal formation of control subjects and patients with Alzheimer's disease. Neurosci. 1989;32:701–714. doi: 10.1016/0306-4522(89)90291-1. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006a;5:545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. Fetal hippocampal CA3 cell grafts enriched with FGF-2 and BDNF exhibit robust long-term survival and integration and suppress aberrant mossy fiber sprouting in the injured middle-aged hippocampus. Neurobiol Dis. 2006b;21:276–290. doi: 10.1016/j.nbd.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. Status epilepticus during old age is not associated with enhanced hippocampal neurogenesis. Hippocampus. 2008;18:931–944. doi: 10.1002/hipo.20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rossner S. Cholinergic immunolesions by 192IgG-saporin--useful tool to simulate pathogenic aspects of Alzheimer's disease. Int J Dev Neurosci. 1997;15:835–850. doi: 10.1016/s0736-5748(97)00035-x. [DOI] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Gray WP. Relevance of seizure-induced neurogenesis in animal models of epilepsy to the etiology of temporal lobe epilepsy. Epilepsia. 2007;48(Suppl 2):33–41. doi: 10.1111/j.1528-1167.2007.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff S. Reactive synaptogenesis in aging and Alzheimer's disease: lessons learned in the Cotman laboratory. Neurochem Res. 2003;28:1625–1630. doi: 10.1023/a:1026048619220. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–60. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwecker PE, Cheng HW, Serquinia RM, Mori N, McNeill TH. Lesion-induced sprouting of commissural/associational axons and induction of GAP-43 mRNA in hilar and CA3 pyramidal neurons in the hippocampus are diminished in aged rats. J Neurosci. 1995;15:2462–2470. doi: 10.1523/JNEUROSCI.15-03-02462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1:72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B. Prospects of Stem Cell Therapy for Temporal Lobe Epilepsy. Stem Cells. 2007;25:2396–2407. doi: 10.1634/stemcells.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Zaman V, Shetty GA. Hippocampal neurotrophin levels in a kainate model of temporal lobe epilepsy: a lack of correlation between brain-derived neurotrophic factor content and progression of aberrant dentate mossy fiber sprouting. J Neurochem. 2003;87:147–159. doi: 10.1046/j.1471-4159.2003.01979.x. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Rao MS, Hattiangady B, Zaman V, Shetty GA. Hippocampal neurotrophin levels after injury: Relationship to the age of the hippocampus at the time of injury. J Neurosci Res. 2004;78:520–532. doi: 10.1002/jnr.20302. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Zaman V, Hattiangady B. Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. J Neurosci. 2005;25:8391–8401. doi: 10.1523/JNEUROSCI.1538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Rao MS, Hattiangady B. Behavior of hippocampal NSCs after grafting into the injured aged hippocampus. J Neurosci Res. 2008;86:3062–3074. doi: 10.1002/jnr.21764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Rao MS. Vulnerability of Hippocampal GABA-ergic Interneurons to Kainate Induced Excitotoxic Injury During Old Age. J Cell Mol Med. 2009 Feb 4; doi: 10.1111/j.1582-4934.2008.00675.x. [Epub ahead of print]. PMID: 19210578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Aging impairs axonal sprouting response of dentate granule cells following target loss and partial deafferentation. J Comp Neurol. 1999a;414:238–254. doi: 10.1002/(sici)1096-9861(19991115)414:2<238::aid-cne7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Vulnerability of the dentate gyrus to aging and intracerebroventricular administration of kainic acid. Exp Neurol. 1999b;158:491–503. doi: 10.1006/exnr.1999.7107. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Fetal hippocampal grafts containing CA3 cells restore host hippocampal glutamate decarboxylase-positive interneuron numbers in a rat model of temporal lobe epilepsy. J Neurosci. 2000;20:8788–8801. doi: 10.1523/JNEUROSCI.20-23-08788.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Glutamic acid decarboxylase-67-positive hippocampal interneurons undergo a permanent reduction in number following kainic acid-induced degeneration of ca3 pyramidal neurons. Exp Neurol. 2001;169:276–297. doi: 10.1006/exnr.2001.7668. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–84. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivilia S, Giuliani A, Del Vecchio G, Giardino L, Calza L. Age-dependent impairment of hippocampal neurogenesis in chronic cerebral hypopefusion. Neuropathol Appl Neuorbiol. 2008;34:52–61. doi: 10.1111/j.1365-2990.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol Learn Mem. 2007;88:249–259. doi: 10.1016/j.nlm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: evidence from knockout mice and growth factor administration. Dev Biol. 2006;289:329–335. doi: 10.1016/j.ydbio.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WY, Dong JH, Liu X, Wang Y, Ying GX, Ni ZM, Zhou CF. Vascular endothelial growth factor and its receptor Flk-1 are expressed in the hippocampus following entorhinal deafferentation. Neurosci. 2005;134:1167–1178. doi: 10.1016/j.neuroscience.2005.04.064. [DOI] [PubMed] [Google Scholar]
- Woods AG, Guthrie KM, Kurlawalla MA, Gall CM. Deafferentation-induced increases in hippocampal insulin-like growth factor-1 messenger RNA expression are severely attenuated in middle aged and aged rats. Neurosci. 1998;83:663–668. doi: 10.1016/s0306-4522(97)00539-3. [DOI] [PubMed] [Google Scholar]
- Zaman V, Shetty AK. Combined neurotrophic supplementation and caspase inhibition enhances survival of fetal hippocampal CA3 cell grafts in lesioned CA3 region of the aging hippocampus. Neurosci. 2002;109:537–553. doi: 10.1016/s0306-4522(01)00478-x. [DOI] [PubMed] [Google Scholar]
- Zaman V, Shetty AK. Fetal hippocampal CA3 cell grafts enriched with fibroblast growth factor-2 exhibit enhanced neuronal integration into the lesioned aging rat hippocampus in a kainate model of temporal lobe epilepsy. Hippocampus. 2003;13:618–632. doi: 10.1002/hipo.10091. [DOI] [PubMed] [Google Scholar]