Abstract
Developmental control of proliferation relies on tight regulation of protein expression. Although this has been well studied in early embryogenesis, how the cell cycle is regulated during organogenesis is not well understood. Bruno-Like RNA binding proteins bind to consensus sequences in the 3′UTR of specific mRNAs and repress protein translation, but much of this functional information is derived from studies on mainly two members, Drosophila Bruno and vertebrate BrunoL2 (CUGBP1). There are however, six vertebrate and three Drosophila Bruno family members, but less is known about these other family members, and none have been shown to function in the endoderm. We recently identified BrunoL1 as a dorsal pancreas enriched gene, and in this paper we define BrunoL1 function in Xenopus endoderm development. We find that, in contrast to other Bruno-Like proteins, BrunoL1 acts to enhance rather than repress translation. We demonstrate that BrunoL1 regulates proliferation of endoderm cells through translational control of cyclin A2 mRNA. Specifically BrunoL1 enhanced translation of cyclin A2 through binding consensus Bruno Response Elements (BREs) in its 3′UTR. We compared the ability of other Bruno-Like proteins, both vertebrate and invertebrate, to stimulate translation via the cyclin A2 3′UTR and found that only Drosophila Bru-3 had similar activity. In addition, we also found that both BrunoL1 and Bru-3 enhanced translation of mRNAs containing the 3′UTRs of Drosophila oskar or cyclin A, which have been well characterized to mediate repression. Lastly, we show that it is the Linker region of BrunoL1 that is both necessary and sufficient for this activity. These results are the first example of BRE-dependent translational enhancement and are the first demonstration in vertebrates of Bruno-Like proteins regulating translation through BREs.
Keywords: Xenopus, pancreas, proliferation, endoderm, RNA binding protein, BrunoL1, cyclin A2, translational enhancement
Introduction
In Xenopus, the endoderm is derived from the vegetal hemisphere, and much is now understood about how the endodermal germ layer is formed and how regional specification of the endoderm is regulated (Chen et al., 2003; Chen et al., 2004; Horb, 2000; Horb and Slack, 2001; Li et al., 2008; McLin et al., 2007; Pan et al., 2007; Pearl et al., 2009). Yet the relationship between specification, differentiation and proliferation of the various endodermal organs at later stages is unclear (Blitz et al., 2006). Although much is known about cell cycle regulation during early Xenopus development, how differentiation is coupled to the cell cycle at tail bud and tadpole stages is unknown (Philpott and Yew, 2008). It is commonly assumed that exit from the cell cycle is required for differentiation to proceed, but exactly at what stage in the differentiation process this occurs has not been defined. Since many cell cycle regulators (cyclins, cdk) are not ubiquitously expressed, but rather localized to specific tissues, regulation of the cell cycle appears to be tissue specific (Vernon and Philpott, 2003). However, which cell cycle components control proliferation of the various endodermal organs is unknown.
RNA binding proteins have been implicated in post-transcriptional control of various mRNAs during embryogenesis, in particular through sequence elements located in the 3′UTR (Kuersten and Goodwin, 2003). One family of RNA binding proteins implicated in the control of cell differentiation and proliferation during embryonic development is the Bruno family (Good et al., 2000). These proteins contain three RRM motifs and regulate RNA translation and splicing. The founding member of this family, Bruno, was originally identified in Drosophila based on its ability to bind the 3′UTR of oskar mRNA and repress its translation (Kim-Ha et al., 1995). During oogenesis Bruno regulates germline stem cells by repressing cyclin A translation, thus permitting meiosis to proceed (Sugimura and Lilly, 2006); in bruno mutants germline stem cells overproliferate and never enter meiosis (Parisi et al., 2001). Bruno represses translation of specific mRNAs by binding consensus sequences in the 3′UTR called bruno response elements (BRE). Bruno-like genes are also found in other invertebrates. In planarians, a Bruno-like gene (Bruli) was found to be expressed in neoblasts (stem cells) and required for their maintenance, though exactly what mRNA(s) it regulates is not known (Guo et al., 2006).
In vertebrates there are six members in the Bruno family, and they are referred to as either BRUNOL (bruno-like) or CELF (CUG-BP1 and ETR-3 like factors) (Barreau et al., 2006; Good et al., 2000). In contrast to the work in Drosophila, these proteins are found to bind CUG repeats or AU rich elements in the 5′ or 3′UTRs of various mRNAs (Barreau et al., 2006). In Xenopus, five of the six Bruno-Like genes have been identified, but in most cases only their expression patterns are known (Amato et al., 2005; Wu et al., 2010). In Xenopus most research has focused on BrunoL2 (also known as EDEN-BP, Embryo Deadenylation Element Binding Protein), which binds AU rich sequences in the 3′UTR of various mRNAs and promotes deadenylation of these mRNAs (Gautier et al., 2004; Paillard et al., 1998). Xenopus BrunoL1 (etr-1) was originally identified as a neural-enriched gene with localized expression in the ventral neural tube and retina, though its function was not studied (Amato et al., 2005; Knecht et al., 1995; Knecht and Harland, 1997; Richter et al., 1988). In mammals, BrunoL1 (also known as TNRC4) is expressed in the developing embryo as early as E9.5; in the adult its expression is localized to the brain and testis. Though BrunoL1 was shown to regulate splicing of tau exon 10, mice lacking BrunoL1 do not display any overt morphological phenotypes (Chapple et al., 2007; Dev et al., 2007). Whether Bruno-like proteins are expressed in the endoderm or involved in endoderm differentiation has not been examined.
We recently identified BrunoL1 as a dorsal pancreas enriched gene in a microarray comparison of dorsal and ventral pancreatic buds (Horb et al., 2009; Jarikji et al., 2009). In this paper we describe the functional characterization of Xenopus BrunoL1 and show that it is directly involved in regulating cell proliferation in the endoderm. We show that Xenopus brunol1 is expressed throughout the gastrointestinal tract in a punctate fashion. Knockdown of BrunoL1 inhibited proliferation and subsequent differentiation of almost the entire endoderm; conversely overexpression of brunol1 resulted in an overproliferation phenotype. We demonstrate that BrunoL1 regulates proliferation of endodermal cells by enhancing translation of cyclin A2 mRNA by binding BREs in the 3′UTR. Last, we show that the linker region of BrunoL1 is not only necessary for this function, but is also sufficient to confer translational stimulation activity to a known repressor. These results are the first example of BRE-dependent translational enhancement and the first demonstration in vertebrates of Bruno-Like proteins regulating translation through BREs.
Materials and Methods
Nucleic Acids
Xenopus brunol1 (NM_001086467), brunol3 (NM_001086124) and cyclin A2 (NM_001088110) cDNAs were cloned by PCR from stage 42 whole gut cDNA based on published sequence information; brunol2 was obtained from XDB, clone XL171c18. Drosophila bruno (LD05405), bru-2 (LD19052) and bru-3 (LD31834) were obtained from the Drosophila Genomics Resource Center, and the ORF of each was then subcloned into pCS2+. For To clone brunol1-FLAG, the brunol1 cDNA was cloned by PCR amplifying brunol1 from pCS2+ using SP6 and a reverse primer with an NcoI site incorporated into the sequence 5′-atagcagccatggcgctgcct-3′. PCR product was inserted into flag-C107, both cut HindIII-NcoI. To clone FLAG-brunol1, brunol1-pCRII was cut EcoRV and cloned into FLAG-CS107 cut XhoI (blunt). All fusions were confirmed by sequencing. All mRNAs injected into Xenopus embryos were made from pCS2+ vector backbone using Ambion mMessage machine kit.
The 3′ end of Xenopus cyclin A2 (BC077260), which included part of the ORF, was cloned using the following primers: For 5′-gtgtcgattgtggatgaaga-3′ and Rev 5′-aatatgctaactgcctgg-3′. The 3′UTR was then cloned downstream of gfp in the pCS2+ vector as follows: CycA2 cut ClaI (blunt)-NotI and cloned into gfp-CS2 cut StuI-NotI. The BRE mutant cyclin A2 3′UTR was cloned by PCR- point mutations were included in primers used for amplification. Primer sequences used to introduce the mutations were For 5′-CgAatTCtgataaaagcacatgatgtacagttaattttttatcatataggctttggAtCGatatct-3′ and Rev 5′-ctaactgcctggttaattcaatctgtgcacaacagctttttaagagcaTCtaGaactaagattcaacatCTCGAGaattt -3′; changes indicated in capital letters. BREmut PCR product cloned EcoRI-blunt into gfp-bre cut EcoRI-EcoRV. The 3′UTR of Drosophila cyclin A (3329–3940 bp) was cloned from adult fly cDNA (gift of D. Hipfner) into pCRII; primers used were as previously described (Sugimura and Lilly, 2006). The 3′UTR of Drosophila oskar (2550–3670 bp) was cloned from adult fly cDNA into pCRII with the primers: For 5′-actagttgggttcttaatcaagatag-3′ and Rev 5′ctcgagcttcgatagcagggac-3′. Both oskar and cycA 3′UTRs (BamHI (blunt)-NotI) were then cloned downstream of gfp in pCS2 (StuI-NotI). For quantification of GFP fluorescence we calculated the average fluorescence intensity and pixel area of individual embryos for each group (n>10). Each experiment was reproduced 3 separate times and the graphs shown are of one representative experiment.
The various domain swap clones were constructed as follows. BrunoL1(-RRM2) was cloned by PCR as follows: the 5′ end (RRM1) was PCR isolated from BrunoL1-pCRII using T7 and BL1-rev360Xho primer 5′-gacagcgagagcactcgagaggaccgcaag-3′ and the 3′ end (contains linker and RRM3) using BL1-for600Xho 5′-cacggagaaggctcgaggactgagg-3′ and SP6 and cloned into pCS2+ (EcoRI-EcoRV). BrunoL1(R2L2) containing RRM2 from BrunoL2 was cloned by PCR isolating RRM2 from BrunoL2 using the following primers BL2-for650Xho 5′-gaataatgctcgagaagacagaaagctc-3′ and BL2-rev950Xho 5′-cgcttctgttctcgagctttctgagtg-3′ and cloning it into the XhoI site of BrunoL1(-R2). BrunoL1(-linker) was cloned by PCR as follows: the 5′ end (RRM1+RRM2) was PCR isolated from flag-brunol1 using SP6 primer and BL1rev600Xho 5′-cctcagtcctcgagccttctccgtg-3′ and the 3′ end (RRM3) using BL1for1200Xho 5′-gcaacagagagaggctcgagagggctgcaa-3′ and T3 and cloned into pCS2+ (EcoRI-SacII). BrunoL2(linker L1) was cloned by PCR as follows. First BrunoL2(-linker) was cloned; the 5′ end (RRM1+RRM2) was PCR cloned from BrunoL2-CS2 using SP6 and BL2rev650Xho 5′-gagctttctgtcttctcgagcattattc-3′ and the 3′ end(RRM3) using BL2-for1550Xho 5′-gtcagaaagaagctcgagaaggagcc-3′ and BL2-rev1810Xba 5′-cttcccagggctctagatttcagtacg-3′. These were ligated together into pCS2+ (EcoRI-XbaI). The linker region from BrunoL1 was then PCR cloned into the XhoI site of BrunoL2(-linker) using the following primers BL1-for600Xho 5′-cacggagaaggctcgaggactgagg-3′ and BL1-rev1200Xho 5′-ttgcagccctctcgagcctctctctgttgc-3′. All constructs were sequenced completely to confirm no mutations were present.
Whole mount in situ hybridization was performed as previously described (Horb et al., 2003). Information regarding probes can be found in our previous publications (Jarikji et al., 2009; Jarikji et al., 2007) and as indicated: brunol1-pCRII (HindIII, T7); chromogranin A-pCRII (XhoI, SP6); hnf6-pCRII (XhoI, SP6); ptf1a-pCRscript (NcoI, T7); gluc-pCRscript (NotI, T7); Ins-pCRscript (EcoRI, T3); elas-pCMVsport6 (EcoRI, T7); Hex-pBS (NotI, T7); secretIII-pCRscript (XhoI, T3); Insm1-pCRII (HindIII, T7); ngn3-pBS (EcoRI, T3); Nkx2.2-pBS (NotI, T7); pax6-pCDNA3 (SmaI, SP6); neurod-pBS (XhoI, T7); frp5-pCRII (NotI, SP6). Double in situ hybridizations were done as described (Horb and Slack, 2002; Horb and Thomsen, 1999). Whole mount immunostaining with anti-phosphohistone H3 antibody was carried out as described (Saka and Smith, 2001). Histology was performed as described (Horb and Thomsen, 1997). Expression of each marker was examined in brunol1 morphant whole guts from at least 3 separate experiments.
Morpholinos
Antisense morpholino oligonucleotides were designed by and purchased from Gene Tools, LLC. Specificity was confirmed by in vitro transcription and translation for each gene and morpholino. Morpholinos were injected into the dorsal vegetal blastomeres at the eight-cell stage, targeting the stomach, liver and pancreas. Proper targeting was confirmed by monitoring fluorescence from labeled morpholino oligonucleotides after dissection of whole guts. Only those showing even fluorescence in these organs were used for analysis. The brunol1 morpholino sequences were: brunol1-utr 5′-gagcagagaaggaacagactctcac-3′, brunol1-start 5′-gtttgatggcatctggttccttcat-3′ and brunol1-mismatch 5′-CagcaCagaaCgaaGagaGtctGaG-3′.
Protein
To confirm specificity of morpholino binding to mRNA we performed TnT assays using the TNT-coupled reticulate lysate system (Promega) with 500ng of cDNA (brunol1-flag) and 500ng of antisense or mismatch morpholino; 5ul of the reaction was then used for western blot analysis using a polyclonal anti-flag antibody (Sigma) to detect BrunoL1 protein. For co-immunoprecipitations neurula stage 15 embryos were homogenized in lysis buffer (150mM NaCl, 50mM Tris, 1% Triton, 10mM EDTA and 1X Complete Protease Inhibitor Tablet (Roche)), sonicated and cleared by centrifugation at 15,000 rpm at 4°C. M2 anti-Flag beads (Sigma) were then added to the cleared lysates and incubated with rotation for 2 hours at 4°C; the beads were then washed extensively in lysis buffer, and divided into two pools. One half was used for Western Blot Analysis to confirm immunoprecipitation, the other half was added to Trizol (Invitrogen) to isolate total bound RNA. cDNA was then synthesized using oligo-dT and superscript RT (Invitrogen), and PCR carried out with primers as follows: gfp (25 cycles) For 5′-tacagctcgtccatgccatg-3′ and Rev 5′-gaagtcaagttcgaaggtga-3′; cycA2 (25 cycles) For 5′-aagatggagcatctggtgct-3′ and Rev 5′-gagaggtaggtctggtatag-3′; edd primers were as previously described (Horb and Slack, 2001). Analysis of endogenous Cyclin A2 protein in brunol1 mRNA injected embryos was done as follows: 400pg of brunol1 was injected into 8 cell stage embryos and at stage 15 protein extract was isolated from groups of 4 embryos (control and brunol1). One embryo equivalent was loaded onto the gel and endogenous Cyclin A2 protein levels were determined using the monoclonal anti-cyclin A2 antibody (1:200; gift of Tim Hunt).
To quantify the total GFP fluorescence we acquired the color images (8-bits per channel) using a Color camera DFC450 from Leica. The green channel was extracted from the color image. Normalized fluorescence values were obtained by the following procedure. Pixels were initially selected from the 8 bits grayscale (green channel) image using a threshold value (set to 13). The sum of selected values was obtained from at least 10 embryos for each group. Sums of images were average along the same sample type. Average values were normalized against the control type giving Fold stimulation.
Results
Xenopus BrunoL1 was originally isolated as a neural enriched gene (named etr-1) localized to the ventral neural tube (Knecht et al., 1995); whether it was expressed in the endoderm was not examined. Since our recent microarray identified brunol1 as being expressed in the pancreas, we examined its endodermal expression in detail. Expression of brunol1 was first detected in the dorsal endoderm in only a few cells beginning at NF32 (Fig. 1A). By NF35 expression extended to more lateral endoderm, but was confined to the outer layer of cells (Fig. 1B). At NF39 expression was now detected throughout the endoderm in scattered cells (Fig. 1C). Within the pancreas, we found to be localized to the dorsal pancreas at NF40 (Fig. 1D). By NF42/44 brunol1 expression was now found in both dorsal and ventral portions of the pancreas (Fig. 1E). In addition to the pancreas, we found brunol1 to be expressed throughout the entire gastrointestinal tract at all stages, but absent from the liver (Fig. 1G,H). Although this punctate staining pattern is reminiscent of that seen with endocrine-specific markers, brunol1 was not expressed in differentiated endocrine cells. Brunol1 was not co-expressed with either insulin, which marks pancreatic beta cells, or with chromogranin A, which marks both gastrointestinal and pancreatic endocrine cells (Fig. 1F,I). Furthermore, the temporal distribution of brunol1 within the pancreas is vastly different from all other endocrine specific genes. Pancreatic endocrine differentiation markers remain localized to the dorsal pancreas until NF46/47, at which time their expression is also found within the portion of the pancreas derived from the ventral pancreatic bud (Horb and Slack, 2002). In contrast, ventral pancreas expression of brunol1 is detected quite early, beginning at NF42/43, several days before other endocrine differentiation markers.
Figure 1. Expression of brunol1 in developing endoderm.

(A–C) Expression of brunol1 in tadpoles at NF32, NF35 and NF39. A few scattered cells expressing brunol1 are detected at stage 32 in the extreme dorsal endoderm. By stage 35 expression can now be detected in more lateral endoderm. (D) NF41 isolated pancreas/liver tissue. At this early stage, brunol1 is expressed in a punctate pattern only in the dorsal pancreas. (E) NF44 isolated pancreas/liver. Expression of brunol1 is now detected throughout the entire pancreas. (F) Double in situ hybridization of brunol1 (purple, arrow) and insulin (red, arrowhead) in NF44 isolated pancreas/liver. No overlap in expression is seen. Insulin expression is confined to the dorsal pancreas, whereas brunol1 expression is also present in the ventral part of the pancreas. (G,H) NF41 and NF44 isolated whole gut. Expression is punctate throughout the entire gastrointestinal tract. (I) Double in situ hybridization of isolated whole gut for chromogranin A (red, arrowhead) and brunol1 (purple, arrow). No overlap in expression is seen. Chromogranin A is expressed in all differentiated endocrine cells. (J) pH3 staining at NF32. Expression in the endoderm is only detected in scattered cells (arrowhead). Mesodermal expression is also detected. (K) pH3 staining at NF39. Proliferating cells are found throughout the entire endoderm, similar to brunol1. (L) pH3 staining at NF40 in isolated liver/pancreas tissue. Proliferating cells are only found in the dorsal pancreas (line demarcates dorsal versus ventral portions of the pancreas). Proliferating cells are also found in the liver.
This lack of co-expression with known endocrine cell markers raised the question of which cell types in the pancreas and endoderm were expressing brunol1. Our results presented below led us to conclude that brunol1 was expressed in cells undergoing proliferation. Therefore, we compared the expression profiles of brunol1 and proliferating cells. Using a phosphohistone H3 antibody we stained for proliferating cells from early tadpole stages, NF32, through to late stages in isolated whole guts and liver/pancreas tissue, NF45. We first detected pH3 staining in the endoderm beginning at NF32, and only in a few scattered cells in the dorsal endoderm in the same region as brunol1 (data not shown). By NF35, scattered proliferating cells could now be detected in slightly more lateral regions (Fig. 1J). By NF39 abundant pH3 staining cells were now detected throughout the entire endoderm (Fig. 1K). Within the pancreas at NF40, proliferating cells were only detected within the dorsal pancreas (Fig. 1L). Shortly thereafter, by NF42 proliferating cells were also seen in the ventral pancreas derived tissue (data not shown). As expected pH3 staining marked a larger population of cells than brunol1 did. At early tadpole stages, mesoderm cells stained were pH3 positive, while in later stages the liver also contained abundant proliferating cells (see Fig. 1J,L). This temporal and spatial profile of pH3 staining within the early endoderm and in the pancreas was almost identical to that seen for brunol1 suggesting that brunol1 was perhaps expressed in progenitor cells undergoing proliferation, and our functional data below supports this possibility.
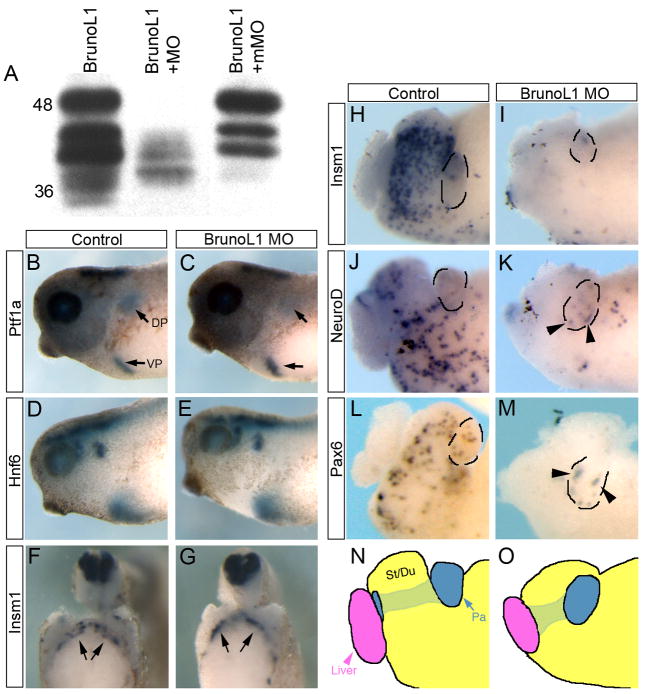
To investigate whether brunol1 was necessary for endoderm development we created a knockdown phenotype using antisense morpholinos. Two antisense morpholinos were designed to the 5′UTR and translation start and injected (20ng) into dorsal vegetal blastomeres at the eight-cell stage targeting the anterior endoderm. (Both morpholinos gave identical results, but only results from the 5′UTR morpholino are presented throughout the paper.) We only targeted the anterior endoderm since embryos injected into all four vegetal blastomeres were severely growth retarded and died at early tadpole stages, most likely due to neural tissue effects where brunol1 is highly expressed. A 7bp mismatch morpholino was used as a control and specificity was determined by in vitro transcription and translation (Fig. 2A). Embryos injected with the antisense brunol1 morpholino developed normally to tadpole stages with no overt defects. However, beginning at NF40/41 we noticed that the stomach, duodenum and pancreas all appeared smaller; this phenotype was observed in 100% of embryos where the morpholino was targeted to the anterior endoderm (n=60).
Figure 2. Endodermal patterning occurs normally in BrunoL1 knockdown tadpoles.
(A) In vitro transcription translation reactions. Translation of BrunoL1 is blocked in the presence of the antisense morpholino, but not the mismatch morpholino. (B,C) Ptf1a expression in control and BrunoL1 depleted tadpole showing normal induction of dorsal and ventral pancreatic buds (n=10). (Arrows point to the dorsal and ventral pancreatic buds that express Ptf1a.) (D,E) Hnf6 expression is also normal in BrunoL1 knockdown tadpoles (n=10). (F,G) Insm1 expression in the dorsal pancreas at stage 32 is normal in brunol1 morphants (n=10). (H,I) Expression of the endocrine progenitor marker insm1 (n=17) is reduced in both the pancreas and gastrointestinal tract. (J–M) Both neuroD (n=23) and pax6 (n=23) are also reduced, but some expression can be detected in the pancreas, most likely marking those beta cells that are present. Whole guts are from stages 41/42. In all panels the pancreas is outlined. (N,O) Trace drawings of panels J and K to illustrate the different organs.
At early tadpole stages, NF35, we found normal expression of ptf1a, hnf6 and pdx1 in brunol1 knockdown tadpoles indicating that patterning of the anterior endoderm was normal (Fig. 2B–E and data not shown). These results were also confirmed by real time PCR (data not shown). In addition, we found expression of the endocrine progenitor marker, insm1, to be normal in the pancreatic endoderm at NF32, suggesting that initial development of endocrine beta cells was unaffected (Fig. 2F,G). However, by NF40/41 expression of endocrine-specific progenitor markers (ngn3 and insm1) was completely absent in both the gastrointestinal tract and pancreas (Fig. 2H–K). Expression of endocrine differentiation transcription factors (neuroD and pax6) was also absent in the gastrointestinal tract, but some expression was still detected in the pancreas (Fig. 2L–O). The normal expression of early insm1 and the persistent expression of neurod and pax6 in the pancreas at later stages suggested that differentiation of beta cells (first pancreatic endocrine cells to develop in Xenopus) was normal.
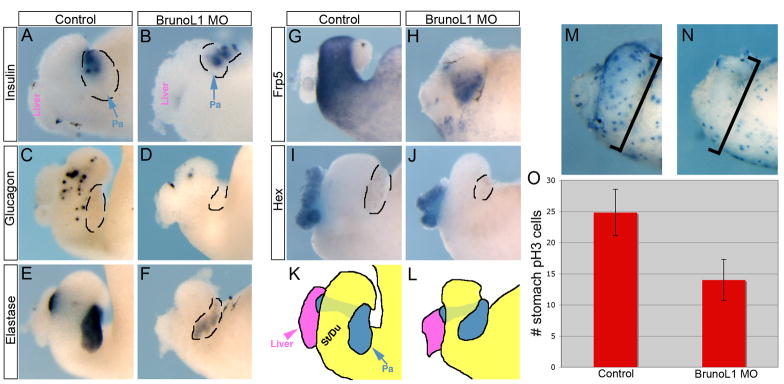
In agreement with this we found normal expression of the beta cell marker insulin at stage 40/41 (Fig. 3A,B). In contrast, we were unable to detect expression of glucagon (gcg) or somatostatin (som) in the stomach/duodenum (Fig. 3C,D and data not shown). And at later stages, when som and gcg are expressed in the pancreas, we also did not detect expression of either marker (data not shown). Similar to the endocrine pancreas, we found differentiation of the exocrine pancreas to also be inhibited. Expression of the late acinar differentiation marker elastase was reduced (Fig. 3E,F), whereas there was normal expression of the early acinar marker XPDIp (data not shown). Although we cannot explain the reason for these differential effects on acinar markers, we believe it may be due to the temporal distinctions seen between XPDIp and elastase. Since XPDIp is expressed earlier than other acinar differentiation markers and in a broader domain, it may mark acinar cells in an earlier state of differentiation. In addition to the defects in the pancreas, we also found diminished expression of the general stomach/duodenum marker frp5 (Fig. 3G,H). In agreement with the fact that brunol1 was not expressed in the liver we found normal expression of hex (Fig. 3I,J). These results showed that brunol1 was essential for differentiation of most of the endoderm, but not for liver or pancreatic beta cells.
Figure 3. BrunoL1 is essential for endodermal cell differentiation.
(A,B) Expression of insulin was normal in the pancreas of brunol1 morphants (n=15). (C,D) Expression of glucagon in the stomach/duodenum is reduced (n=23). Similar results were found for somatostatin (n=20). (E,F) elastase expression was reduced (n=16). (G,H) Similarly, there was reduced expression of the general stomach marker, frp5 (n=14). (I,J) Liver development was normal as assessed by expression of hex (n=12). (K,L) Trace drawings of panels G and H. The pancreas is outlined in panels A–D, F, and I–J. (M) Control NF41 whole gut stained for phosphohistone H3 to mark proliferating cells. (N) Whole gut from brunol1 morphant stained for phosphohistone H3. Note the large decrease in proliferating cells, especially apparent in the stomach and duodenum. (O) Average number of proliferating cells within the stomach of 10 different whole guts from control and morpholino injected. (Error bars represent standard deviation.) We only counted the number of cells in the stomach/duodenal area, even though the morpholino was targeted to a larger region, including more posterior endoderm. To ensure that equivalent areas were compared we counted cells in control and morpholino samples within the same defined total area. A reduction in pH3 staining was also seen in the other targeted regions, but for quantification we only focused on the stomach/duodenal region. Areas not targeted by the morpholino (i.e. no fluorescence) showed normal pH3 staining.
To examine how loss of BrunoL1 could affect differentiation of almost every endodermal cell population, we examined whether proliferation was affected in the endoderm of BrunoL1 knockdown tadpoles. In control whole guts at NF41, abundant phosphohistone H3 staining is seen throughout the gastrointestinal tract, while in endoderm lacking brunol1 we saw a large reduction in phosphohistone H3 (Fig. 3M,N). To quantify this effect, we counted the total number of phosphohistone H3 cells within the stomach/duodenum region of both control and brunol1 morphants (Fig. 3M,N brackets). In control whole guts, there were an average of 25 positive cells, while in the brunol1 morphants there were only 13; an almost 50% decrease (Fig. 3O, n=19). A similar reduction was also seen in more posterior endoderm that was targeted by the brunol1 morpholino, while areas not targeted by the morpholino showed normal pH3 staining (data not shown). These results demonstrated that loss of BrunoL1 resulted in reduced proliferation of endodermal cells. That proliferation of pancreatic beta cells is regulated differently than other endocrine cells is supported by the fact that cdk4 was found to be required only for beta cell proliferation, and not other endocrine cell types (Rane et al., 1999).
To confirm that the observed phenotype was directly related to inhibition of endogenous BrunoL1 we attempted to rescue the morpholino-induced phenotype through co-injection of a flag-brunol1 mRNA lacking the 5′UTR sequence to which the antisense morpholino was designed. Co-injection of 200–300pg of flag-brunol1 mRNA was sufficient to rescue the differentiation of anterior endoderm as judged by expression of elastase (elas). In control embryos, the pancreas is of normal size and abundant elas expression is detected in the pancreas (Fig. 4A). In morpholino-injected embryos, expression of elas was reduced in 100% of injected samples (Fig. 4B, n=43). In embryos injected with the morpholino and flag-brunol1 mRNA the size of the pancreas and elas expression were restored in 71% of injected embryos (Fig. 4C, n=38). When higher doses of flag-brunol1 were injected we observed an overexpression phenotype as discussed below. These results demonstrate that the morpholino-induced phenotype is directly due to the loss of BrunoL1.
Figure 4. Flag-BrunoL1 mRNA rescues the morpholino knockdown phenotype.
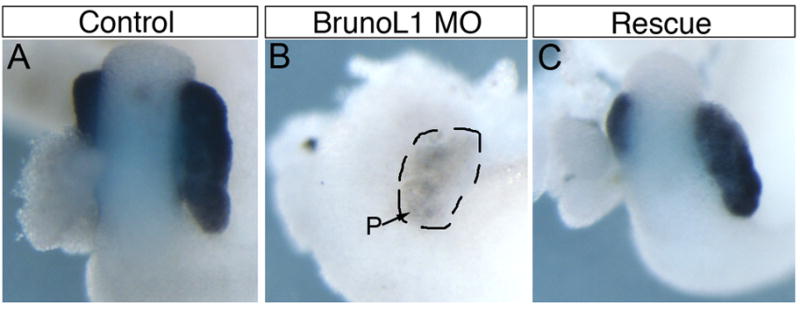
(A) Control NF42 whole gut stained for elastase mRNA showing expression in the pancreas. (B) Whole gut from embryo injected with 15ng of brunol1 morpholino. Elastase expression is almost completely lost in injected embryos (n=43). (C) Isolated whole gut stained for elastase expression from embryo injected with 15ng of brunol1 morpholino and 200pg of flag-brunol1 mRNA. Elastase expression was restored in 27/38 embryos.
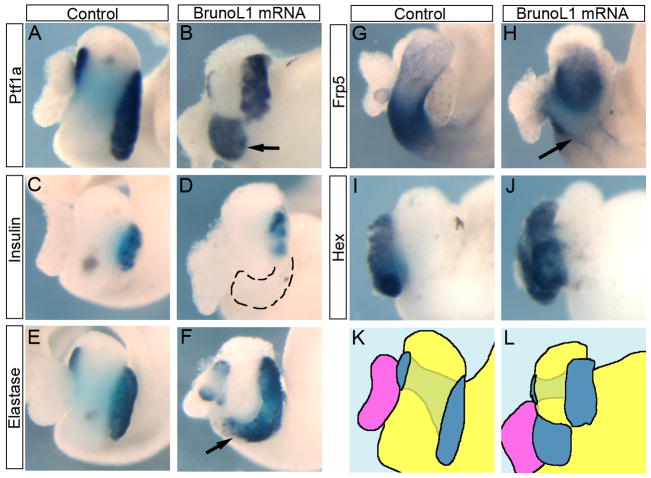
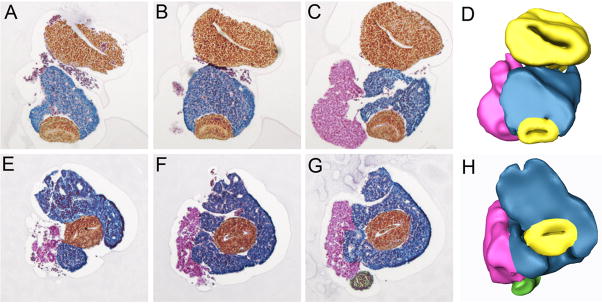
Based on these results, we concluded that if BrunoL1 was involved in regulating endodermal proliferation, then ectopic expression should produce a phenotype. To test this, we injected brunol1 mRNA into vegetal blastomeres of eight cell embryos. Beginning at NF40, when the pancreas, liver, and stomach are apparent, we noticed two different phenotypes: ectopic pancreas and overgrowth of more posterior foregut endoderm (Fig. 5). The ectopic pancreas was the most common phenotype that resulted in the pancreas growing on the ventral side of the stomach (Fig. 5A–F). These ectopic pancreatic cells expressed elastase, but not insulin or other general endocrine markers (Fig. 5C–F and data not shown). The overgrowth of more posterior endoderm appeared as large bulges growing out of the endoderm (Fig. 5G,H). We next examined histological sections to confirm the expansion of pancreatic tissue in these samples. Comparison of serial sections of whole guts from control and brunol1 mRNA injected samples confirmed the development of ectopic pancreas in brunol1-injected embryos. In control samples the pancreas is found to lie between the stomach/duodenum and intestine (Fig. 6A–D). At its longest point control pancreatic tissue extends 180° around the stomach/duodenum (Fig. 6B). In contrast, in brunol1-injected whole guts pancreatic tissue extends around the entire stomach/duodenum (Fig. 6E–H). These overexpression results agreed with the loss-of-function data and showed that BrunoL1 is not only necessary, but also sufficient for endodermal cell proliferation.
Figure 5. Overexpression of brunol1 results in an overproliferation phenotype.
Enlarged pancreas phenotype seen in embryos injected with brunol1 mRNA injected into 2 dorsal-vegetal blastomeres at the eight-cell stage. (A,B) ptf1a expression (n=12). Arrow points to the ectopic pancreas. (C,D) No expression of insulin is detected in the ectopic pancreas (n=24). Pancreas is outlined. (E,F) elastase is expressed in the ectopic pancreatic tissue (arrow, n=23). (G,H) frp5 expression is normal (n=10). Arrow points to overgrowth. (I,J) Expresison of the liver marker hex is normal, though the liver does appear a little larger (n=14). (K,L) Trace drawing of panels A and B illustrating the overgrowth of pancreatic tissue in the tadpoles injected with brunol1 mRNA.
Figure 6. 3-D reconstruction of brunol1 overexpression phenotype.
Serial histological sections were taken from control and brunol1 injected NF44 whole guts. Color overlays added to help visualize differences (Blue-pancreas, yellow-gut endoderm, pink-liver). (A–C) Representative sections of control whole guts. (D) 3-D reconstruction of control whole guts using Amira software. Notice that the pancreas does not encircle the duodenum. (E–G) Representative sections of whole guts isolated from tadpoles injected with brunol1 mRNA. (H) 3-D reconstruction reveals increased amounts of pancreatic tissue such that it now encircles the duodenum. Blue-pancreas, yellow-gut endoderm, pink-liver.
BrunoL1 controls proliferation through translational regulation of cyclin A2
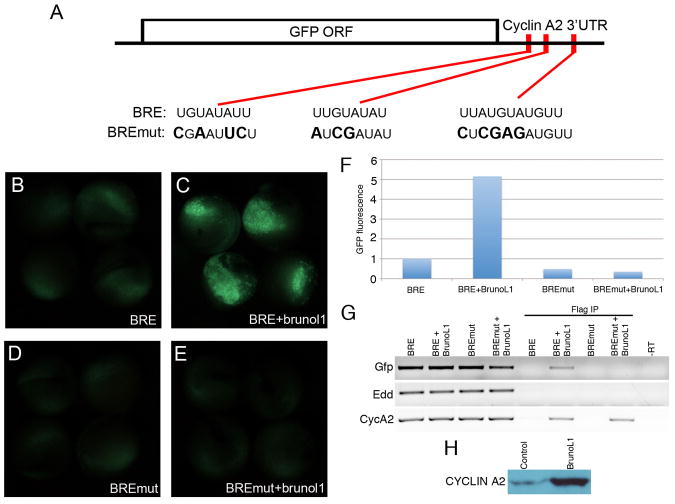
This effect on proliferation suggested that Xenopus BrunoL1 was regulating endodermal cell proliferation through translational control of specific cell cycle mRNAs, analogous to how Drosophila bruno regulates translation of cyclin A during oogenesis. However, since the loss-of-function effects of Drosophila bruno (overproliferation) and Xenopus brunol1 (decreased proliferation) were opposite, we hypothesized that Xenopus BrunoL1 was acting to stimulate translation of cell cycle mRNAs rather than repress. To test this hypothesis we examined whether BrunoL1 was involved in translational regulation of cyclin A. (In Xenopus there are two cyclin A homologues, but only cyclin A2 was found to be expressed in the gut endoderm (data not shown).) We cloned the cyclin A2 3′UTR downstream of the GFP open reading frame (gfp-bre) and injected this mRNA alone or with brunol1 mRNA into the vegetal hemisphere of Xenopus embryos (Fig. 7A). Injection of this gfp-bre mRNA alone resulted in low levels of GFP fluorescence at neurula stages (Fig. 7B). However, co-injection of brunol1 mRNA resulted in a 5-fold increase in GFP protein fluorescence (Fig. 7C,F). Since the ability of Drosophila Bruno to repress translation is mediated by consensus sequences located in the 3′UTR of these mRNAs called bruno response elements (BRE) we examined whether Xenopus BrunoL1 enhanced translation of cyclin A2 by binding BRE elements in the 3′UTR. We searched the cyclin A2 3′UTR for bruno response elements (BRE) and found three BREs (Fig. 7A). To determine whether the increased translation of gfp-bre by BrunoL1 was due to it binding these BREs, we made point mutations in all 3 BRE sites (gfp-bremut). Similar to gfp-bre mRNA, low level GFP fluorescence was observed in embryos injected with gfp-bremut mRNA alone (Fig. 7D). However, co-injection of brunol1 mRNA with gfp-bremut mRNA did not result in increased GFP protein expression (Fig. 7E,F). These results demonstrated that BrunoL1 was sufficient to increase translation of an heterologous mRNA containing the 3′UTR of cyclin A2, and that this activity was BRE-dependent.
Figure 7. BrunoL1 function is dependent on BREs in the 3′UTR.
(A) Schematic diagram illustrating the sequence of the 3 BREs in the 3′UTR of cyclin A2 and the respective point mutations made. (B) Injection of gfp-bre resulted in a low level of GFP fluorescence. (C) Coinjection of brunol1 increased GFP fluorescence in 78% of embryos (n=67). (D) Injection of the gfp-bremut mRNA produced a similar low-level fluorescence as seen with the gfp-bre mRNA. (E) No increase in GFP fluorescence was seen when brunol1 was coinjected with gfp-bremut mRNA (n=17). (F) Quantification of GFP fluorescence. Injection of brunol1 mRNA along with gfp-bre resulted in a 5 fold increase in GFP fluorescence. For details on how we calculated fold stimulation see materials and methods. Experiment repeated at least 3 times. (G) RT-PCR detection of gfp, edd and cyclin A2 from total RNA of injected embryos (1st four lanes) or from total RNA after immunoprecipitation using flag antibody (2nd four lanes). gfp mRNA was immunoprecipated in the presence of Flag-BrunoL1 protein, but not when the BRE sites were mutated (BREmut+BrunoL1). Edd was used as a control, and was detected in total RNA, but no in the imunnoprecipitated RNA. Endogenous cyclin A2 mRNA was detected in both lanes where BrunoL1 was immunoprecipitated. (H) Levels of endogenous Cyclin A2 protein in control and brunol1 injected embryos, demonstrating that there was increased Cyclin A2 protein levels in brunol1 injected embryos.
We next examined whether BrunoL1 was in a complex that bound to the 3′UTR of cyclin A2 mRNA. Flag-brunol1 mRNA was co-injected along with gfp-bre or gfp-bremut mRNAs into eight cell embryos and at neurula stages the BrunoL1-mRNA complex was immunoprecipated. Total RNA was then extracted from the immunoprecipitate and RT-PCR used to determine whether gfp-bre mRNA was associated with BrunoL1. When gfp-bre mRNA was injected with flag-brunol1, the gfp-bre mRNA co-immunoprecipitated with Flag-BrunoL1 protein (Fig. 7G). However, when gfp-bremut mRNA was co-injected with flag-brunol1 we did not detect an association with BrunoL1. Expression of the general endoderm marker, Edd, served as our control and was detected in the total RNA fractions, but not in the immunoprecipitated fractions (Fig. 7G). Since the gfp-bre mRNA we used was an exogenously injected mRNA we examined whether BrunoL1 associated with endogenous cyclin A2 mRNA. Using primers specific for endogenous cyclin A2 open reading frame, we were able to show that cyclin A2 mRNA was bound by Flag-BrunoL1 (Fig. 7G). Endogenous cyclin A2 was also detected in the BrunoL1 complex immunoprecipitated from the BREmut+BrunoL1 samples (Fig. 7G). We also found increased levels of endogenous Cyclin A2 protein in brunol1 injected embryos as compared to control (Fig. 7H). These results showed that BrunoL1 bound endogenous cyclin A2 mRNA and enhanced translation leading to increased amount of Cyclin A2 protein.
Two possible explanations for the increased translation of gfp-bre mRNA by BrunoL1 is either mRNA stability or translational activation. In the first instance BrunoL1 would bind gfp-bre mRNA and stabilize the mRNA, preventing degradation and leading to increased translation. In the second instance BrunoL1 would bind gfp-bre mRNA and promote increased translational efficiency without affecting gfp-bre mRNA degradation. To differentiate between these two possibilities we examined whether the degradation of gfp-bre mRNA was altered in the presence of BrunoL1. Equal amounts of gfp-bre (or gfp-bremut) mRNA were injected either alone or in combination with brunol1 mRNA. Immediately after injection embryos were collected to establish the initial amount of gfp-bre mRNA injected. At neurula stage 15 a second set of embryos was collected; at this stage increased GFP protein is evident in the brunol1 + gfp-bre embryos. Using RT-PCR, we compared the amounts of gfp-bre mRNA present in each fraction and normalized the levels to eEF1A, but found no difference in the levels of gfp-bre mRNA (data not shown). This demonstrated that BrunoL1 did not act to increase the stability of gfp-bre mRNA.
Linker region of BrunoL1 is responsible for its ability to increase translation
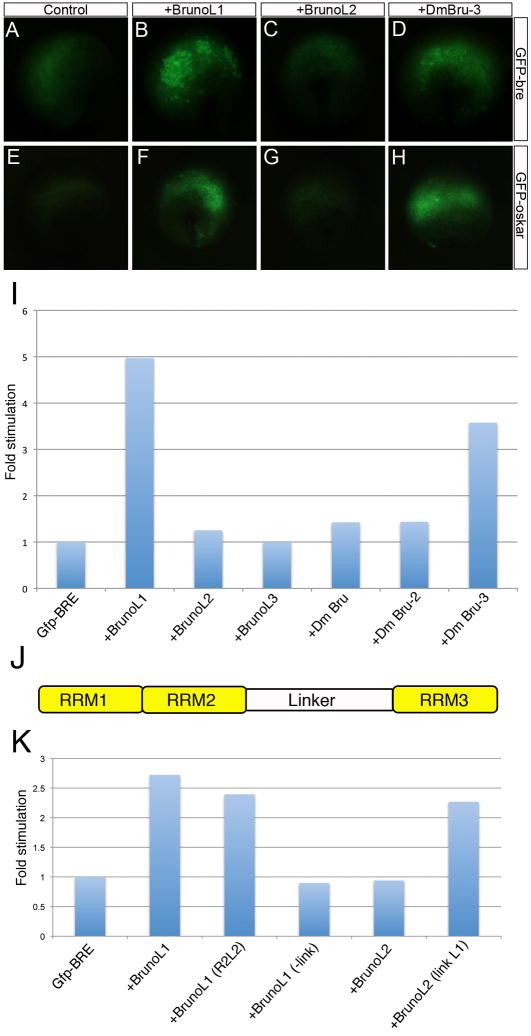
The ability of BrunoL1 to increase translation was surprising since all previous data on Bruno-like proteins in Drosophila and vertebrates showed that this family of proteins acted to repress translation. We therefore examined whether this ability to increase translation was a general characteristic of Bruno-Like proteins. We tested the ability of two other Xenopus Bruno-Like proteins (BrunoL2 and BrunoL3) and three Drosophila Bruno proteins (Bruno, Bru-2 and Bru-3). Of these, only Drosophila Bru-3 stimulated translation of gfp-bre mRNA, though it was not as efficient as BrunoL1 (Fig. 8A–I and data not shown). Similar to Xenopus BrunoL1 we found the ability of DmBru-3 to enhance translation was mediated by BRE sequences, as it was unable to enhance translation of the gfp-bremut mRNA (data not shown). Last, we examined whether this activity was specific to the cyclin A2 3′UTR or found with any BRE containing 3′UTRs. We tested the 3′UTRs of Drosophila cyclin A and oskar, both of which have been well characterized as targets for Drosophila Bruno repression (Kim-Ha et al., 1995; Sugimura and Lilly, 2006). Interestingly, we found both Xenopus BrunoL1 and Drosophila Bru-3 able to increase translation of gfp-Dmoskar and gfp-DmcycA mRNAs in Xenopus embryos (Fig. 8E–H and data not shown). These results demonstrate that the ability to increase translation of mRNA targets is not a general characteristic of every Bruno-Like protein, but is limited to BrunoL1 and Bru-3 and is BRE-dependent.
Figure 8. Linker region is essential for BrunoL1 to increase translation.
(A–H) Representative image of a single embryo injected with gfp mRNA alone or with Xenopus BrunoL1, BrunoL2 or Drosophila Bru-3 mRNA. Top row gfp mRNA contains the Xenopus cyclin A2 3′UTR (GFP-bre) and the bottom row gfp is fused to the Drosophila oskar 3′UTR (GFP-Oskar). (I) Fold stimulation of GFP fluorescence over gfp-bre mRNA alone. (J) Schematic diagram illustrating the various domains of Bruno-Like proteins. (K) Different domains of the Bruno-Like proteins were either removed or replaced with he corresponding region from other Bruno-Like proteins. BrunoL1 (R2L2) indicates that the RRM2 in BrunoL1 was replaced with the BrunoL2 RRM2. BrunoL1 (-link) indicates that the linker region was removed from BrunoL1. BrunoL2 (link L1) indicates that the linker region of BrunoL2 was replaced with the BrunoL1 linker domain. Fold stimulation was then calculated.
To delineate which domain of BrunoL1 was responsible for this effect, we individually removed and/or replaced different domains of BrunoL1 (RRM motifs and the linker region) with the corresponding region from BrunoL2, which did not increase translation of gfp-bre mRNA. We tested the ability of these new chimeric proteins to enhance translation of gfp-bre mRNA. As before, coinjection of brunol1 increased total GFP protein fluorescence over 2.5 fold (Fig. 8K). Replacement (or removal) of the second RRM with the BrunoL2 RRM (BrunoL1 (R2L2)) did not have any effect, whereas removal of the BrunoL1 linker region (BrunoL1 (-link)) completely abolished the translational enhancement activity of BrunoL1 (Fig. 8K and data not shown). When we replaced the BrunoL2 linker domain with the BrunoL1 linker (BrunoL2 (link L1)) we found that this new chimeric protein was able to stimulate translation of gfp-bre mRNA (Fig. 8K). In conclusion, we found that the linker region was both necessary and sufficient to stimulate translation.
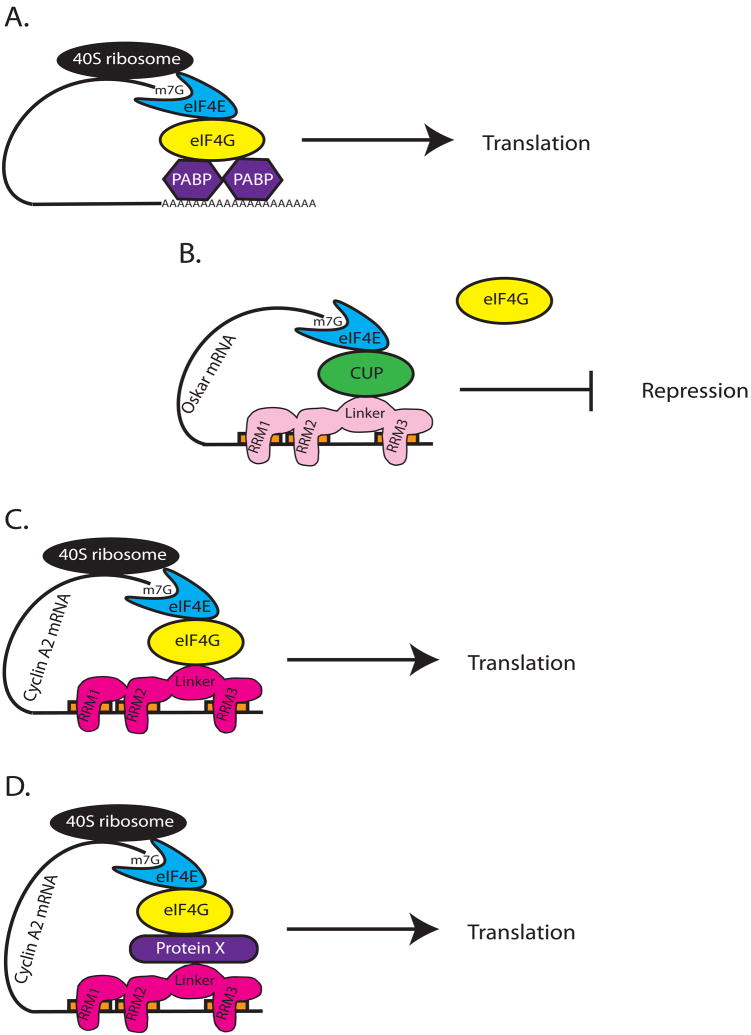
Based on our results we propose the following models to account for BrunoL1 function. The standard closed loop model of translational regulation via the 3′UTR by RNA binding proteins involves the establishment of preinitiation complex that then recruits the 40S ribosome resulting in translational activation (Fig. 9A). Several different mechanisms have been identified that block the formation of this preinitiation complex resulting in translational repression. For example, Drosophila Bruno represses translation by binding BRE sites in the 3′UTR of oskar and recruiting the protein Cup (through the linker domain), which then binds eIF4E (Fig. 9B). This action prevents eIF4G from binding. There are two possible models to explain our results with BrunoL1. In the first, BrunoL1 binds BREs in the 3′UTR of cyclin A2 and directly interacts with eIF4G through its linker domain leading to the establishment of the preinitiation complex and translational activation (Fig. 9C). In the second model, the linker domain of BrunoL1 interacts with an intermediary protein (protein X), which then recruits eIF4G (Fig. 9D). Since it is the linker domain that specifying translational repression versus activation it will be interesting to identify the proteins that bind the BrunoL1 linker domain.
Figure 9. Proposed mechanism of BrunoL1 regulation of translation.
(A) Generic closed loop model of translation. In this example, PABP (Poly(A) binding protein) binds long poly(A) tails and interacts directly with eIF4G, which then binds eIF4E leading to the recruitment of the 40S ribosome and active translation. (B) In Drosophila, Bruno (light pink) binds BREs (orange rectangles) located in the 3′UTR of oskar or cyclin A mRNAs to repress translation. It does this by interacting with Cup (through its linker domain); Cup in turn binds eIF4E preventing it from interacting with eIF4G, and thus blocking translation. The 3 RRMs and Linker region are shown separately to illustrate that Bruno binds 3 BREs. (C,D) Two proposed models for how BrunoL1 may lead to increased translation. In both models the 3 RRM domains of BrunoL1 bind the 3 BRE sites in the 3′UTR of cyclin A2 mRNA. Then BrunoL1 interacts (via the linker domain) either directly with eIF4G or through an intermediate protein (protein X) that binds eIF4G. By bringing eIF4G into close proximity with eIF4E, the two proteins can bind leading to the recruitment of the 40S ribosome and stimulation of translation.
Discussion
In this paper we identified a new function for BrunoL1 in endoderm development. We demonstrated that brunol1 is expressed throughout the endoderm in a punctate fashion and that it was required for proliferation of most endodermal cells. We show that BrunoL1 binding to the 3′UTR of cyclin A2 mRNA resulted in increased translation, which was dependent on three consensus Bruno Response Elements (BREs). Lastly, we identified the linker domain of BrunoL1 as necessary for its ability to stimulate translation, and we showed that this domain was also sufficient to confer this ability to BrunoL2. These results are the first example of BRE-dependent translational enhancement and the first to demonstrate BRE-dependent translational regulation in vertebrates.
BrunoL1 in endoderm development
Our study is the first to describe a function for Bruno-like genes in endoderm development. The function of other RNA binding proteins in the context of endoderm development has not been studied in detail though a few examples have been published. In Xenopus, the KOC (K-homologous domain containing protein overexpressed in cancer) homologue, Vg1RBP, is essential for pancreas development (Spagnoli and Brivanlou, 2006). And in zebrafish nil per os (npo) was shown to be required for exocrine pancreas development and gut morphogenesis (Mayer and Fishman, 2003). Exactly how these RNA binding proteins regulate endoderm development and their mRNA targets remains to be elucidated.
Whereas these RNA binding proteins were found to affect specific cell types, we found BrunoL1 to be a global regulator of endodermal cell proliferation. Of interest is the fact that BrunoL1 does not regulate proliferation of pancreatic beta cells and the liver. The reason for this difference is unclear, though it is known that beta cell proliferation is regulated differently than other endocrine cell types; cdk4 was shown to be required only for beta cell proliferation, and not other endocrine cell types (Rane et al., 1999). Yet, we did find that both pancreatic beta cells and liver cells required cyclin A2 for proliferation (data not shown). This suggests that another, yet to be defined RNA binding protein(s) regulates cyclin A2 translation in these cell types. In this regard, the related RNA binding protein HuR and Wilms’ tumor 1-associated protein (WTAP) have both been shown to stabilize cyclin A mRNA through its 3′UTR (Horiuchi et al., 2006; Wang et al., 2000). It will be interesting to ascertain whether other RNA binding proteins play a role in regulating cyclin A2 translation within the endoderm.
Dorsal pancreas specific genes
Since many of the other dorsal pancreas enriched genes identified in our dorsal-ventral pancreatic bud microarray were endocrine specific genes (Horb et al., 2009; Jarikji et al., 2009), we expected brunol1 to also be expressed in endocrine cells and play a role in endocrine cell differentiation, and its speckled expression pattern did initially suggest this. However, we did not find it to be co-expressed with any endocrine markers, and our functional data demonstrated that brunol1 regulated proliferation of a larger subset of endodermal cells. This implies that the dorsal pancreas is composed of more than just endocrine cells. The fact that endocrine specific genes are expressed in only a small population of the dorsal pancreas (speckled pattern) and not uniformly distributed agrees with this idea. Most likely there are several different populations of cells within the dorsal pancreas, which have yet to be characterized.
We believe that brunol1 marks one such population. In agreement with this, the temporal expression changes of brunol1 in the pancreas are quite different from that seen with other endocrine markers. Expression of other pancreatic endocrine markers is initially localized to the dorsal pancreas, and expression in the ventral portion of the pancreas is not seen until after stage 45. In contrast, brunol1 was found expressed in the ventral pancreas as early as stage 42. This lack of co-expression with endocrine markers suggested that brunol1 was marking a separate population of cells within the endoderm, most likely proliferating cells. In agreement with this, we found a direct correlation between the temporal and spatial expression patterns of brunol1 and proliferating cells (see Fig. 1). Initially phosphohistone H3 staining cells are only detected in scattered cells in the dorsal endoderm at NF32, with expression extending to more lateral and ventral regions by NF35, with abundant staining throughout the endoderm at NF39. This pattern was identical to what was seen with brunol1. Within the pancreas, we found proliferating cells initially only in the dorsal pancreas at NF40, with expression rapidly extending to the ventral pancreas by NF42. We believe that BrunoL1 is expressed in proliferating cells, and this would explain why knockdown of BrunoL1 has such drastic effects on the developing endoderm.
Differential effects of brunol1 loss on endodermal differentiation
Our results showed that knockdown of Brunol1 blocked differentiation of almost every endodermal cell type, except for liver and pancreatic beta cells. The lack of an effect on the liver is explained by the fact that brunol1 is not expressed in hepatic endoderm, while the effects on pancreatic beta cells seem contradictory. The reason for this apparent contradiction is, that at first glance, that expression of brunol1 seems to parallel insulin expression. Upon closer examination however, this is not the case. Expression of brunol1 in the dorsal pancreas only begins after beta cells have differentiated. Insulin expression is first detected in the dorsal endoderm as early as stage 28, whereas expression of brunol1 is only detected beginning at stage 32. In agreement with this, we found initial expression of endocrine progenitor marker insm1 in the dorsal endoderm at stage 32 to be normal in Brunol1 morphants, while at later stages its expression was absent. This difference is reflective of the fact that loss of BrunoL1 does not block differentiation of the first born endocrine cells (beta cells), but does block later endocrine cells, both in the pancreas and the gastrointestinal tract. The differences seen with Insm1 can be explained by the fact that insm1 is only expressed after endodermal cells have proliferated and cell fate established. Since beta cell fate and proliferation is regulated at an earlier stage, prior to brunol1 expression and thus independent of brunol1, initial expression of insm1 is therefore unaffected.
We believe BrunoL1 is expressed in endodermal progenitors and controls their proliferation, beginning from NF32. If BrunoL1 is indeed expressed in endodermal progenitor cells, then its loss of function should affect differentiation of all endodermal cells, and this is exactly what we see. Only after stage 30 does loss of BrunoL1 have any effect on endoderm development. BrunoL1 acts to control proliferation after organ domains have been specified, but prior to cell fate determination. Thus proliferation of endodermal cells occurs after specification of organ domains, but prior to cell fate determination.
Linker domain and regulation of translation
One of the key findings of our data was that the linker domain was both necessary and sufficient for BrunoL1 function. Given that the RRM domains garner most of the attention this result was surprising though not entirely unexpected. It was previously shown that Drosophila Bruno interacted with Cup through its linker domain (Nakamura et al., 2004). Since Bruno family members bind similar RNA sequences via their RRM domains (Good et al., 2000) this implies that specificity for function resides in the Linker region. Coupled with the fact that the linker domain of BrunoL1 is unique (i.e. does not show high amino acid sequence similarity with other members) our hypothesis is that this linker domain regulates translational enhancement function to BrunoL1 (Fig. 9). In agreement with this, we found that replacement of the linker domain of BrunoL2 with the BrunoL1 linker region conferred BrunoL2 the ability to increase translation through the cyclin A2 3′UTR.
The linker region has also been shown to be important for the ability of other RNA binding domain proteins to increase translation. For example, the ability of HuD, a member of the ELAV family of RNA binding domain proteins, to stimulate translation is dependent on its linker domain (Fukao et al., 2009). Similarly, the ability of BrunoL2 (CUGBP1) to increase translation via GC-rich sequences in the 5′ regions of p21 and C/EBPβ is dependent upon the phosphorylation of Ser-302, which is located in its Linker domain of CUGBP1 (Iakova et al., 2004; Timchenko et al., 2006; Timchenko et al., 1999). However, different mechanisms are used by each protein- CUGBP1 binds eIF2, while HuD binds eIF4A. Whether BrunoL1 interacts with either of these proteins has yet to be determined. The fact that the amino acid sequence of the linker domain of BrunoL1 is not similar to either CUGBP1 or HuD linker domains suggests that it binds different proteins to stimulate translation, either directly with eukaryotic translation initiation factors or through an intermediate (Fig. 9C,D).
Our demonstration that BrunoL1 can increase translation of mRNAs may explain the results obtained in planarians where a bruno-like gene (bruli) was found essential for stem cell (neoblasts) maintenance (Guo et al., 2006). Although no mechanism was described to account for their phenotype, they speculated that bruli might function to repress translation of specific targets. In light of our data we would argue that bruli function in neoblasts is to enhancement translation of specific target mRNAs involved in the regulation of proliferation. Indeed, it may be that this is a common mechanism (i.e. increasing translation of cell cycle components) used by other Bruno-like proteins to regulate proliferation of progenitor and stem cells in other tissues.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK077197). Special thanks go to Frédéric Bourque for his care of the frogs, Annie Vallée for the histological sections, Dr. Gregor Andelfinger and his lab for their help with the 3-D reconstruction, Dr. Esther Pearl for her help in drawing figure 9 and Dr. Dominic Filion for his help with the GFP quantification. We would also like to thank Dr. Tim Hunt for his generous gift of the cyclin A2 antibody.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amato MA, Boy S, Arnault E, Girard M, Della Puppa A, Sharif A, Perron M. Comparison of the expression patterns of five neural RNA binding proteins in the Xenopus retina. J Comp Neurol. 2005;481:331–9. doi: 10.1002/cne.20387. [DOI] [PubMed] [Google Scholar]
- Barreau C, Paillard L, Mereau A, Osborne HB. Mammalian CELF/Bruno-like RNA-binding proteins: molecular characteristics and biological functions. Biochimie. 2006;88:515–25. doi: 10.1016/j.biochi.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Blitz IL, Andelfinger G, Horb ME. Germ layers to organs: using Xenopus to study “later” development. Semin Cell Dev Biol. 2006;17:133–45. doi: 10.1016/j.semcdb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Chapple JP, Anthony K, Martin TR, Dev A, Cooper TA, Gallo JM. Expression, localization and tau exon 10 splicing activity of the brain RNA-binding protein TNRC4. Hum Mol Genet. 2007;16:2760–9. doi: 10.1093/hmg/ddm233. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jurgens K, Hollemann T, Claussen M, Ramadori G, Pieler T. Cell-autonomous and signal-dependent expression of liver and intestine marker genes in pluripotent precursor cells from Xenopus embryos. Mech Dev. 2003;120:277–288. doi: 10.1016/s0925-4773(02)00460-4. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pan FC, Brandes N, Afelik S, Solter M, Pieler T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev Biol. 2004;271:144–160. doi: 10.1016/j.ydbio.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Dev A, Nayernia K, Meins M, Adham I, Lacone F, Engel W. Mice deficient for RNA-binding protein brunol1 show reduction of spermatogenesis but are fertile. Mol Reprod Dev. 2007;74:1456–64. doi: 10.1002/mrd.20742. [DOI] [PubMed] [Google Scholar]
- Fukao A, Sasano Y, Imataka H, Inoue K, Sakamoto H, Sonenberg N, Thoma C, Fujiwara T. The ELAV protein HuD stimulates cap-dependent translation in a Poly(A)- and eIF4A-dependent manner. Mol Cell. 2009;36:1007–17. doi: 10.1016/j.molcel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Gautier C, Le Clainche C, Barreau C, Audic Y, Graindorge A, Maniey D, Osborne HB, Paillard L. EDEN-BP-dependent post-transcriptional regulation of gene expression in Xenopus somitic segmentation. Development. 2004;131:6107–6117. doi: 10.1242/dev.01528. [DOI] [PubMed] [Google Scholar]
- Good PJ, Chen Q, Warner SJ, Herring DC. A family of human RNA-binding proteins related to the Drosophila Bruno translational regulator. J Biol Chem. 2000;275:28583–28592. doi: 10.1074/jbc.M003083200. [DOI] [PubMed] [Google Scholar]
- Guo T, Peters AH, Newmark PA. A Bruno-like gene is required for stem cell maintenance in planarians. Dev Cell. 2006;11:159–69. doi: 10.1016/j.devcel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Horb LD, Jarkji ZH, Horb ME. Xenopus Insm1 is essential for gastrointestinal and pancreatic endocrine cell development. Dev Dyn. 2009;238:2505–2510. doi: 10.1002/dvdy.22071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horb ME. Patterning the endoderm: the importance of neighbours. Bioessays. 2000;22:599–602. doi: 10.1002/1521-1878(200007)22:7<599::AID-BIES2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Horb ME, Shen CN, Tosh D, Slack JM. Experimental conversion of liver to pancreas. Curr Biol. 2003;13:105–15. doi: 10.1016/s0960-9822(02)01434-3. [DOI] [PubMed] [Google Scholar]
- Horb ME, Slack JM. Endoderm specification and differentiation in Xenopus embryos. Dev Biol. 2001;236:330–43. doi: 10.1006/dbio.2001.0347. [DOI] [PubMed] [Google Scholar]
- Horb ME, Slack JM. Expression of amylase and other pancreatic genes in Xenopus. Mech Dev. 2002;113:153–7. doi: 10.1016/s0925-4773(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Horb ME, Thomsen GH. A vegetally localized T-box transcription factor in Xenopus eggs specifies mesoderm and endoderm and is essential for embryonic mesoderm formation. Development. 1997;124:1689–98. doi: 10.1242/dev.124.9.1689. [DOI] [PubMed] [Google Scholar]
- Horb ME, Thomsen GH. Tbx5 is essential for heart development. Development. 1999;126:1739–51. doi: 10.1242/dev.126.8.1739. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Umetani M, Minami T, Okayama H, Takada S, Yamamoto M, Aburatani H, Reid PC, Housman DE, Hamakubo T, Kodama T. Wilms’ tumor 1-associating protein regulates G2/M transition through stabilization of cyclin A2 mRNA. Proc Natl Acad Sci U S A. 2006;103:17278–83. doi: 10.1073/pnas.0608357103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iakova P, Wang GL, Timchenko L, Michalak M, Pereira-Smith OM, Smith JR, Timchenko NA. Competition of CUGBP1 and calreticulin for the regulation of p21 translation determines cell fate. Embo J. 2004;23:406–17. doi: 10.1038/sj.emboj.7600052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarikji Z, Horb LD, Shariff F, Mandato CA, Cho KW, Horb ME. The tetraspanin Tm4sf3 is localized to the ventral pancreas and regulates fusion of the dorsal and ventral pancreatic buds. Development. 2009;136:1791–800. doi: 10.1242/dev.032235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarikji ZH, Vanamala S, Beck CW, Wright CV, Leach SD, Horb ME. Differential ability of Ptf1a and Ptf1a-VP16 to convert stomach, duodenum and liver to pancreas. Dev Biol. 2007;304:786–99. doi: 10.1016/j.ydbio.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Ha J, Kerr K, Macdonald PM. Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell. 1995;81:403–12. doi: 10.1016/0092-8674(95)90393-3. [DOI] [PubMed] [Google Scholar]
- Knecht AK, Good PJ, Dawid IB, Harland RM. Dorsal-ventral patterning and differentiation of noggin-induced neural tissue in the absence of mesoderm. Development. 1995;121:1927–1935. doi: 10.1242/dev.121.6.1927. [DOI] [PubMed] [Google Scholar]
- Knecht AK, Harland RM. Mechanisms of dorsal-ventral patterning in noggin-induced neural tissue. Development. 1997;124:2477–2488. doi: 10.1242/dev.124.12.2477. [DOI] [PubMed] [Google Scholar]
- Kuersten S, Goodwin EB. The power of the 3′ UTR: translational control and development. Nat Rev Genet. 2003;4:626–37. doi: 10.1038/nrg1125. [DOI] [PubMed] [Google Scholar]
- Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22:3050–63. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AN, Fishman MC. Nil per os encodes a conserved RNA recognition motif protein required for morphogenesis and cytodifferentiation of digestive organs in zebrafish. Development. 2003;130:3917–28. doi: 10.1242/dev.00600. [DOI] [PubMed] [Google Scholar]
- McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–17. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Sato K, Hanyu-Nakamura K. Drosophila cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev Cell. 2004;6:69–78. doi: 10.1016/s1534-5807(03)00400-3. [DOI] [PubMed] [Google Scholar]
- Paillard L, Omilli F, Legagneux V, Bassez T, Maniey D, Osborne HB. EDEN and EDEN-BP, a cis element and an associated factor that mediate sequence-specific mRNA deadenylation in Xenopus embryos. Embo J. 1998;17:278–87. doi: 10.1093/emboj/17.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan FC, Chen Y, Bayha E, Pieler T. Retinoic acid-mediated patterning of the pre-pancreatic endoderm in Xenopus operates via direct and indirect mechanisms. Mech Dev. 2007;124:518–31. doi: 10.1016/j.mod.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Parisi MJ, Deng W, Wang Z, Lin H. The arrest gene is required for germline cyst formation during Drosophila oogenesis. Genesis. 2001;29:196–209. doi: 10.1002/gene.1024. [DOI] [PubMed] [Google Scholar]
- Pearl EJ, Bilogan CK, Mukhi S, Brown DD, Horb ME. Xenopus pancreas development. Dev Dyn. 2009;238:1271–1286. doi: 10.1002/dvdy.21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott A, Yew PR. The Xenopus cell cycle: an overview. Mol Biotechnol. 2008;39:9–19. doi: 10.1007/s12033-008-9033-z. [DOI] [PubMed] [Google Scholar]
- Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat Genet. 1999;22:44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- Richter K, Grunz H, Dawid IB. Gene expression in the embryonic nervous system of Xenopus laevis. Proc Natl Acad Sci U S A. 1988;85:8086–8090. doi: 10.1073/pnas.85.21.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka Y, Smith JC. Spatial and temporal patterns of cell division during early Xenopus embryogenesis. Dev Biol. 2001;229:307–18. doi: 10.1006/dbio.2000.0101. [DOI] [PubMed] [Google Scholar]
- Spagnoli FM, Brivanlou AH. The RNA-binding protein, Vg1RBP, is required for pancreatic fate specification. Dev Biol. 2006;292:442–456. doi: 10.1016/j.ydbio.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Sugimura I, Lilly MA. Bruno inhibits the expression of mitotic cyclins during the prophase I meiotic arrest of Drosophila oocytes. Dev Cell. 2006;10:127–35. doi: 10.1016/j.devcel.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Timchenko LT, Salisbury E, Wang GL, Nguyen H, Albrecht JH, Hershey JW, Timchenko NA. Age-specific CUGBP1-eIF2 complex increases translation of CCAAT/enhancer-binding protein beta in old liver. J Biol Chem. 2006;281:32806–19. doi: 10.1074/jbc.M605701200. [DOI] [PubMed] [Google Scholar]
- Timchenko NA, Welm AL, Lu X, Timchenko LT. CUG repeat binding protein (CUGBP1) interacts with the 5′ region of C/EBPbeta mRNA and regulates translation of C/EBPbeta isoforms. Nucleic Acids Res. 1999;27:4517–25. doi: 10.1093/nar/27.22.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon AE, Philpott A. The developmental expression of cell cycle regulators in Xenopus laevis. Gene Expr Patterns. 2003;3:179–92. doi: 10.1016/s1567-133x(03)00006-1. [DOI] [PubMed] [Google Scholar]
- Wang W, Caldwell MC, Lin S, Furneaux H, Gorospe M. HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J. 2000;19:2340–50. doi: 10.1093/emboj/19.10.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Li C, Zhao S, Mao B. Differential expression of the Brunol/CELF family genes during Xenopus laevis early development. Int J Dev Biol. 2010;54:209–14. doi: 10.1387/ijdb.082685jw. [DOI] [PubMed] [Google Scholar]