Abstract
Limb bone diaphyseal structure is frequently used to infer hominin activity levels from skeletal remains, an approach based on the well-documented ability of bone to adjust to its loading environment during life. However, diaphyseal structure is also determined in part by genetic factors. This study investigates the possibility that genetic variation underlying diaphyseal structure is influenced by the activity levels of ancestral populations and might also have functional significance in an evolutionary context. We adopted an experimental evolution approach and tested for differences in femoral diaphyseal structure in one-week-old mice from a line that had been artificially selected (45 generations) for high voluntary wheel running and unselected controls. As adults, selected mice are significantly more active on wheels and in home cages, and have thicker diaphyses. Structural differences at one week can be assumed to primarily reflect the effects of selective breeding rather than direct mechanical stimuli, given that the onset of locomotion in mice is shortly after day seven. We hypothesized that if genetically determined diaphyseal structure reflects the activity patterns of members of a lineage, then selected animals will have relatively larger diaphyseal dimensions at one week compared to controls. The results provide strong support for this hypothesis and suggest that limb bone cross sections may not always only reflect the activity levels of particular fossil individuals, but also convey an evolutionary signal providing information about hominin activity in the past.
Keywords: cross-sectional geometry, genes, activity, artificial selection
Anthropologists frequently use limb bone diaphyseal structure1 to infer behavior, particularly activity levels, from skeletal remains. Individuals with thick diaphyses are assumed to have been highly active during life, while individuals with slender diaphyses are assumed to have been more sedentary (Larsen, 1997; Ruff, 2000). This paradigm is based on the well-documented ability of diaphyseal bone to adjust its structure during life to its mechanical environment (Goodship and Cunningham, 2001 and references therein). Generally, increased loading shifts the balance between bone’s formative and resorptive activity towards net formation, while disuse causes net resorption. Although the mechanosensitivity of bone is undisputed, it is not the case that diaphyseal structure results solely from physiological adaptation to applied loads. Diaphyseal structure is also influenced by genetics (Eisman, 1999; Peacock et al., 2002; Middleton et al., 2008a), as well as nutrition, hormones, age, and other factors (for reviews in the anthropological literature, see Churchill, 1999; Chiu and Hamrick, 2002; Lovejoy et al., 2003; Pearson and Lieberman, 2004).
The effect of genetics on modulating structure, independent of mechanical signals, is implied by analyses of juvenile human remains from geographically and temporally dispersed contexts which have shown that populational differences in gross diaphyseal dimensions emerge very early in ontogeny (e.g., Cowgill and Hager, 2007; Robbins, 2007; Cowgill, 2008, 2009). For example, Cowgill (2008, 2009) recently analyzed the developmental trajectories of limb bone diaphyseal dimensions in seven diverse Holocene samples and found that substantial variation was present prior to one year of age; i.e., prior to the onset of walking (Burnett and Johnson, 1971; Hesinger, 1986; Stanitski et al., 2000), and when infants are probably carried (Tracer, 2009). Such variation prior to activity-related bone loading is likely to reflect genetic variability in diaphyseal structure and not simply differences in individual activity levels. The influence of genetics on diaphyseal structure is similarly evident from biomedical research on inbred mouse strains demonstrating that the significant interstrain differences in structure documented among adults (e.g., Akhter et al., 2000; Jepsen et al., 2003; Judex et al., 2004; Wergedal et al., 2005) are also evident during perinatal development (Price et al., 2005; Jepsen et al., 2009). Mapping studies in mice also clearly demonstrate the influence of genes on bone phenotypes, including diaphyseal dimensions (Saless et al., 2009). In humans, broad- and narrow-sense heritability estimates for diaphyseal size measures range from 27% to over 50% (Koller et al., 2001; Demissie et al., 2006; Havill et al., 2007).
Natural selection favoring particular activity levels (or any other complex trait) should engender an evolutionary response involving multiple changes in anatomy and physiology, as well as the underlying genetic architecture (Garland and Kelly, 2006). Given the critical role of the skeleton in locomotion, alleles influencing bone formation would presumably be among those especially affected (Middleton et al., 2008a). This idea has not been lost on anthropologists (e.g., Trinkaus et al., 1994; Churchill, 1999). For example, discussing the large diaphyseal dimensions characteristic of Pleistocene human limb bones, Churchill (1999: 51–52) hypothesized that “heightened skeletal strength … may be expected to positively co-occur in populations with an evolutionary history of high activity.” In this scenario, thick diaphyses among Pleistocene juveniles (e.g., Ruff et al., 1994; Trinkaus and Ruff, 1996; Kondo and Dodo, 2002; Trinkaus et al., 2002; Arsuaga et al., 2007) may be present even at ages prior to the onset of limb loading activities as an evolutionary response to selection for high activity acting on earlier members of a lineage. This may also explain some of the variation in growth patterns among Holocene populations. For functional morphologists, this would mean that limb bone cross sections may not necessarily reflect the activity levels of particular fossil individuals, but nevertheless convey an evolutionary signal providing information about the activity levels of their ancestral populations.
To examine the degree to which limb bone diaphyseal structure reflects the activity levels of members of a lineage, an experimental evolution approach was adopted (Garland and Rose, 2009). Since the early 1990s, Garland and colleagues have been conducting a selection experiment for high voluntary wheel-running behavior in house mice in order to study the correlated evolution of high levels of locomotor activity and various behavioral and physiological traits (Swallow et al., 1998; Garland, 2003; Rhodes et al., 2005). After 16 generations, selected High Runner mice (HR) were voluntarily completing, on average, 170–200% more daily revolutions than non-selected controls (C), a difference that has persisted and increased in subsequent generations. As adults, HR mice exhibit many differences relative to controls, including increased home-cage activity when housed without wheels (Malisch et al., 2008, 2009), wheel-running speed (Girard et al., 2001), maximum aerobic performance (Rezende et al., 2006a,b), endurance (Meek et al., 2009), increased circulating corticosterone and adiponectin (Vaanholt et al., 2007; Malisch et al., 2008, 2009), decreased circulating leptin (Girard et al., 2007), and decreased body mass and fat content (Swallow et al., 1999, 2001; Dumke et al., 2001; Nehrenberg et al., 2009). Several limb bone changes associated with selection for high activity have also been documented among adults, including increased diaphyseal thickness (Kelly et al., 2006), increased articular surface areas (Garland and Freeman, 2005; Kelly et al., 2006; Middleton et al., 2008a), and reduced directional asymmetry in limb lengths (Garland and Freeman, 2005). Importantly, when the activity levels of the HR and C mice are limited by denying them wheel access, HR mice continue to have thicker diaphyses (Kelly et al., 2006), possibly indicating genetic differences in diaphyseal growth patterns; however, the mechanical effects of elevated home-cage activity (Malisch et al., 2008, 2009) could not be ruled out. To determine if selective breeding for high activity alters diaphyseal structure independent of mechanical signals, we tested for differences in the femur of one-week-old animals. Differences in diaphyseal structure at one week can be assumed to primarily reflect the evolutionary effects of past selection rather than direct mechanical stimuli, given that the onset of locomotion in mice is shortly after day seven (Williams and Scott, 1954; Garland, pers. observation). We hypothesized that, if genetically determined diaphyseal structure reflects the activity patterns of members of a lineage, then HR mice will have larger gross diaphyseal dimensions and/or greater tissue strength at one week compared to mice from non-selected control (C) lines.
MATERIALS AND METHODS
Experimental design
The complete design of the artificial selection experiment for high voluntary wheel-running behavior in house mice has been described elsewhere (Swallow et al., 1998; Garland, 2003), and will only be briefly summarized here. From a base population of outbred mice of the Hsd:ICR strain (Harlan-Sprague-Dawley, Indianapolis, IN, USA), eight closed lines were established. In each successive generation, 6–8 week old mice are housed individually with access to Wahman-type activity wheels (1.12 m circumference) for six days. Daily revolutions are recorded with a computer-automated system in one minute bins. The selection criterion is the total number of revolutions on days five and six of the six-day test. In four selected High Runner (HR) lines, the male and female from each family that completes the greatest number of revolutions are chosen as breeders. In four control (C) lines, breeders are randomly chosen from each family. Chosen breeders are randomly paired, except that sibling matings are not allowed. Animals used in the analysis reported here are from a single selected line (laboratory designation line 8) and a single control line (laboratory designation line 2) from generation 45 of selection. At one week postnatal, one male and one female from each of 23 families were weighed, euthanized via decapitation, and frozen. The bones of three individuals were excluded from the study due to damage sustained during organ extraction. At a later date, carcasses were defrosted and right and left femora were extracted and preserved in 70% EtOH. Body and triceps surae muscle mass data for these individuals were reported in a previous study (Middleton et al., 2008b). No significant differences in body mass (females: P = 0.13; males: P = 0.35) or triceps surae mass (females: P = 0.17; males: P = 0.59) or triceps surae mass relative to body mass (females: P = 0.81; males: P = 0.98) were found between the HR and C lines at one week of age. Diaphyseal structure was assessed by microcomputed tomography (µCT) in the mid-diaphysis. All experimental procedures were reviewed and approved by the University of California, Riverside IACUC.
µCT
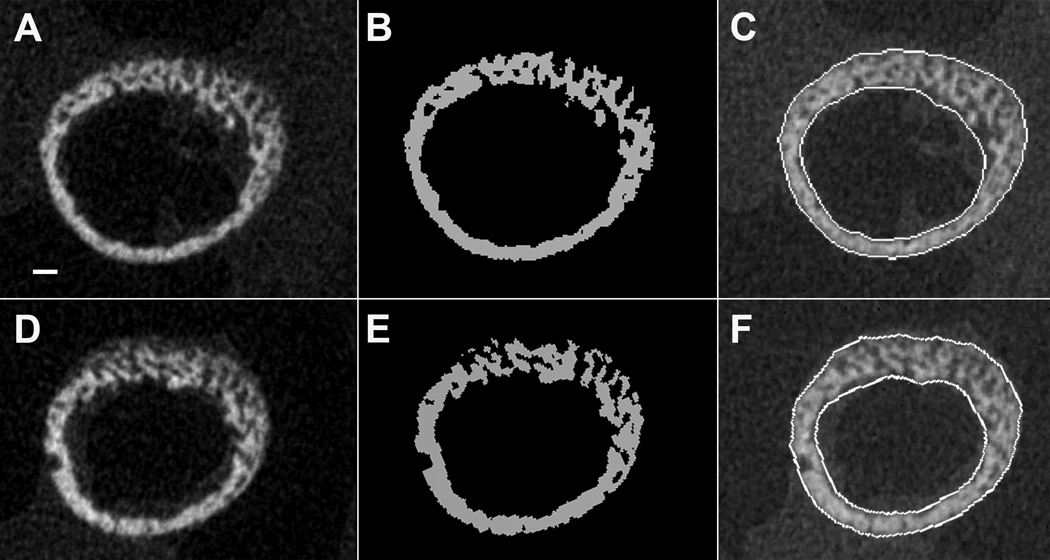
The µCT scanning protocol followed Miller et al. (2007) with slight modification. Femoral diaphyses were scanned at a resolution of 8µm (70 kV, 114µA, 300 ms integration time) with a µCT 40 scanner (Scanco Medical, Bassersdorf, Switzerland). Structural parameters were quantified in a 0.64 mm long volume of interest (VOI) that was defined at midspan between the growth plates, a region uniform in shape and with no apparent shape differences between HR and C bones (Fig. 1). Using an automated algorithm described in Lublinsky et al. (2007), raw gray scale images (Fig. 2a, d) were filtered using a constrained 3D Gaussian filter with a support value of 1 and sigma value of 0.1 to reduce noise, and segmented using a global threshold of 21% of maximum possible gray scale value (Fig. 2b, e). This threshold was determined with the help of density histograms, and visual comparison indicated that the 21% value rendered good concordance between the raw and thresholded images. Importantly, a single threshold was applied to the bones of both HR and C mice as to not introduce bias. The cortical mask of the diaphyseal VOI was defined with dilation and subsequent erosion operations, filling in holes and connecting fragments to produce continuous endosteal and periosteal contours. The cortical mask edges were checked by overlay on the raw images (Fig. 2c, f).
Fig. 1.
Three-dimensional reconstruction of the midshaft region of a one-week-old mouse femur.
Fig. 2.
Cross sections of one-week-old mouse femoral diaphyses. A – C: Selected HR line. D – F: Control line. A, D. Gray scale µCT sections. B, E. Segmented bone. C, F. Lines delineating periosteal and endosteal perimeters. The scalebar in A is 100 µm.
Structural parameters were computed using a custom-written script routine in IPL (Image Processing Language), the internal imaging code provided by the µCT scanner manufacturer. Values were averaged over the 80 slices in each VOI. Calculation of average values was deemed appropriate to capture representative structure at the mid-diaphysis because considerable variation was present among the slices from individual bones. Parameters measured include the gross diaphyseal dimensions commonly interpreted by anthropologists, such as cortical area (Ct.Ar), periosteal and endosteal areas (Ps.Ar, Es.Ar), maximal and minimal second moments of area (Imax, Imin), and polar moment of area (J; abbreviations follow Parfitt et al., 1987). Area moments and Ct.Ar were quantified excluding all porosities (which is not common in anthropological analyses). In standard beam analysis, these parameters approximate diaphyseal strength if the material strength of the bone tissue is held constant: Ct.Ar approximates a cross section’s internal resistance to axial loads, Imax and Imin describe resistance to bending around principal axes, and J describes resistance to torsion. However, one-week-old mouse diaphyses consist largely of bone fragments that are irregularly connected via narrow struts (Fig. 2), violating one of the assumptions of the beam model (at least for bending and torsion) – that stress under loading will be distributed linearly across sections (Hibbeler, 1997). Therefore, in this case, moments of area should not be interpreted as indicators of strength, but as geometric parameters delineating bone shape (“apparent traits”, Price et al., 2005). Microstructural parameters measured include tissue mineral density (TMD) and intracortical porosity (Po) as a fraction of the area between the defined periosteal and endosteal surfaces. Tissue mineral density was quantified using calibration hydroxyapatite phantoms (Scanco Medical) for the conversion of linear attenuation of a given voxel to mgHA/cm3 (Miller et al., 2007). Visual inspection of the cross sections indicated that the distribution of the intracortical pores was similar in HR and C bones (Fig. 2). A previous study on ontogenetic series of inbred mouse strains also suggests that the distribution of porosities in developing mouse bones is similar in animals with different genetic make ups (Price et al., 2005). TMD and Po explain the overwhelming majority of variation across species and individuals in tissue strength as determined from material properties tests of bone specimens (Currey, 1988, 2002); tissue strength increases with decreasing Po and increasing TMD. Diaphyseal structural parameters of right and left femora were averaged for each individual to minimize measurement error.
Statistics
All statistical analyses were carried out using SPSS 11 (SPSS Inc., Chicago, IL, USA). Males and females were analyzed separately. Descriptive statistics for all parameters were calculated, and then data were converted to natural logarithms for subsequent analyses. While data were distributed approximately normally in both raw and ln space (insignificant Kolmogorov-Smirnoff Z values), logging them slightly improved r2 values and linearity in regressions (Durbin-Watson statistics closer to 2). Pearson correlations were calculated to test for associations between body mass and structural parameters. Areas and area moments were all found to be significantly correlated with body mass; however, TMD and Po were not. For areas and area moments, regression slopes were compared to confirm that associations between body mass and structural parameters were similar between the HR and C groups. All slope differences were found to be non-significant. One-way ANCOVAs were then used to test for differences in areas and area moments between the groups, with body mass as a covariate. For TMD and Po, single classification ANOVAs were used to test for differences between the groups. The Levene test statistic was calculated for each sample to determine if the assumption of homogeneous variance was violated. In one case (Es.Ar; males), the result of this test was significant and additional rank transformation of the data was necessary. This procedure permitted the analysis of variance without loss of power (Conover and Iman, 1982). Statistical significance was assessed using a 95% criterion (P < 0.05). All tests were two-tailed.
RESULTS
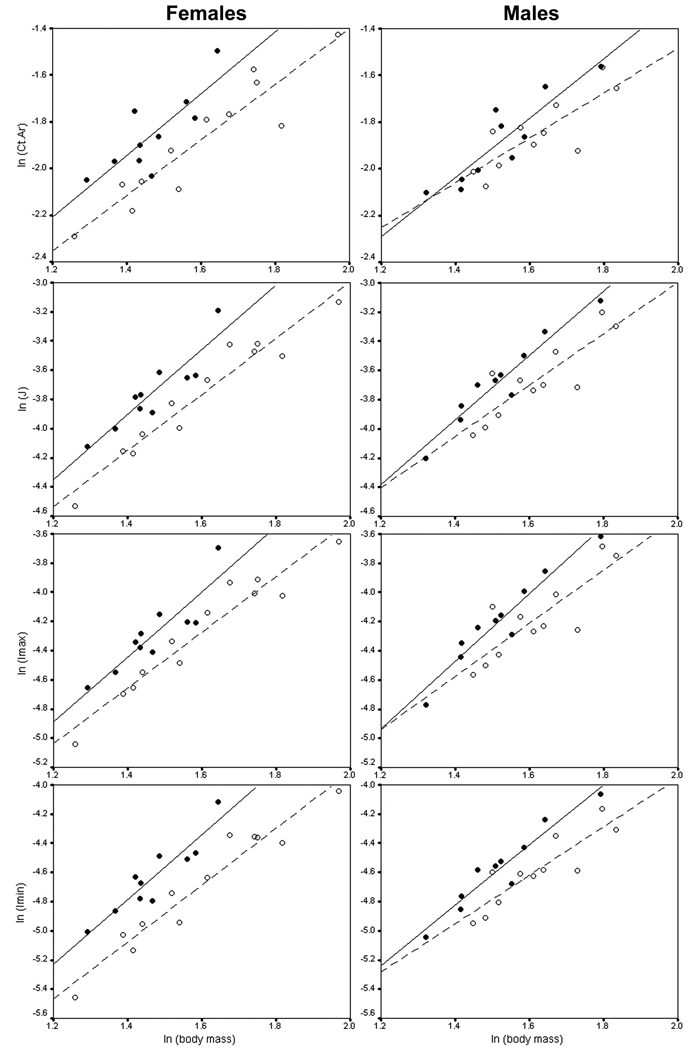
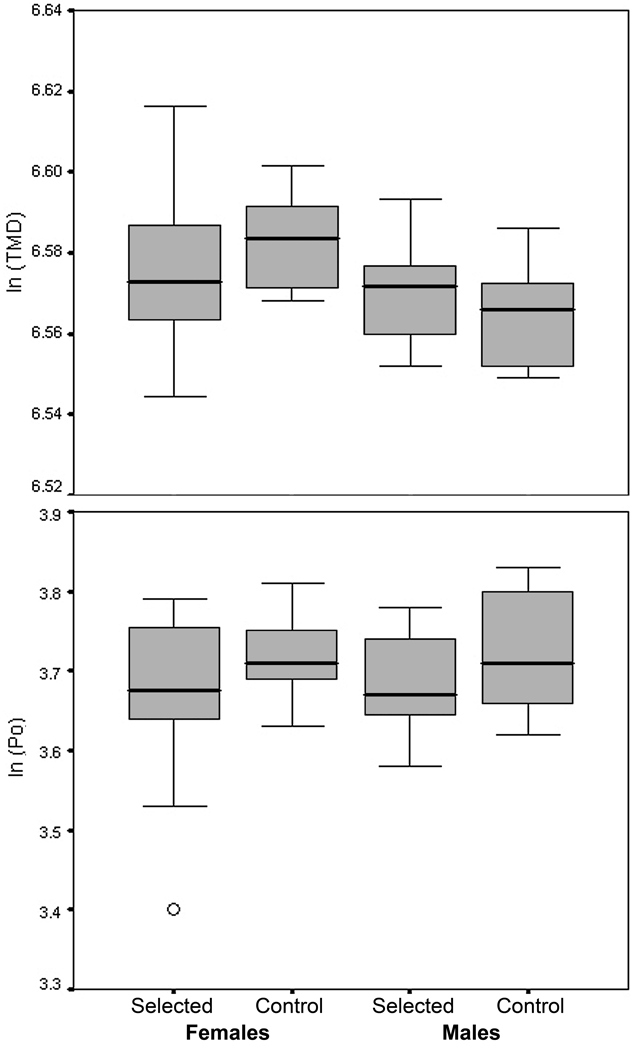
Descriptive statistics for body mass and femoral diaphyseal parameters, separated by sex and selection history, are presented in Table 1. Body mass-adjusted gross diaphyseal dimensions were significantly different between selected HR and non-selected C animals (Fig. 3; Table 2). Among both females and males, HR mice had larger mass-adjusted area moments compared to C mice, although the differences were generally greater in females. On average, selected females had 28% greater maximal second moments of area (P = 0.002), 37% greater minimal second moments of area (P < 0.0001), and 32% greater polar moments of area (P < 0.0001). Selected males had 21% greater maximal second moments of area (P = 0.0082), 22% greater minimal second moments of area (P = 0.0012), and 21% greater polar moments of area (P = 0.0036). Compared to controls, HR animals also had larger cortical areas (20% difference in female mass-adjusted means, and 8% difference in male mass-adjusted means); however, the difference was significant only for females (P = 0.0008; males: P = 0.10). Selected and unselected animals had similar relative endosteal areas (females: P = 0.29; males: P = 0.10), but selected mice had significantly larger mass-adjusted periosteal areas (females: 13% difference, P = 0.0005; males: 12% difference, P = 0.0017). Therefore, differences in cortical area were evidently driven primarily by periosteal expansion in the diaphyses of selected animals. Figure 4 graphically depicts data for tissue mineral density and porosity. No significant differences were found in either tissue mineral density (females: F1,20 = 1.04, P = 0.32; males: F1,19 = 0.85, P = 0.37) or porosity (females: F1,20 = 1.36, P = 0.26; males: F1,19 = 1.42, P = 0.25).
Table 1.
Descriptive statistics for body mass and femoral diaphyseal parameters of one-week-old mice. Values are means with standard deviations in parentheses.
| Females | Males | |||
|---|---|---|---|---|
| Trait (units) | Selected (n = 10) | Control (n = 12) | Selected (n = 10) | Control (n = 11) |
| Body Mass (g) | 4.37 (0.46) | 5.02 (1.05) | 4.61 (0.64) | 5.08 (0.67) |
| Ps.Ar (mm2) | 0.6068 (0.0692) | 0.6055 (0.1084) | 0.6611 (0.0959) | 0.6419 (0.0770) |
| Es.Ar (mm2) | 0.3378 (0.0486) | 0.3474 (0.0592) | 0.3964 (0.0648) | 0.3769 (0.0399) |
| Ct.Ar (mm2) | 0.1590 (0.0289) | 0.1568 (0.0418) | 0.1545 (0.0299) | 0.1590 (0.0253) |
| J (mm4) | 0.0242 (0.0069) | 0.0246 (0.0096) | 0.0266 (0.0084) | 0.0264 (0.0073) |
| Imax (mm4) | 0.0142 (0.0042) | 0.0148 (0.0057) | 0.0159 (0.0052) | 0.0159 (0.0048) |
| Imin (mm4) | 0.0100 (0.0027) | 0.0098 (0.0039) | 0.0107 (0.0032) | 0.0104 (0.0026) |
| TMD (mgHA/cm3) | 722 (8) | 717 (15) | 709 (9) | 713 (9) |
| Po (%) | 41.1 (2.4) | 39.4 (4.1) | 41.6 (3.3) | 39.9 (2.5) |
Ps.Ar = periosteal area, Es.Ar = endosteal area, Ct.Ar = cortical area, J = polar moment of area, Imax = maximal second moment of area, Imin = minimal second moment of area, TMD = tissue mineral density, Po = cortical porosity
Fig. 3.
Femoral gross diaphyseal dimensions in relation to body mass. Solid circles represent selected mice and open circles represent control mice. Lines are least-squares regressions through the selected HR (solid) and control (dashed) samples.
Table 2.
Statistical comparisons of femoral gross mid-diaphyseal dimensions of one-week-old mice selectively bred for high voluntary wheel running versus controls (with body mass as a covariate). Values are back-transformed adjusted (least squares) means with 95% confidence intervals in parentheses. Bold P-values indicate P < 0.05.
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Trait (units) |
Selected (n = 10) |
Control (n = 12) |
F1,19 | P | Selected (n = 10) |
Control (n = 11) |
F1,18 | P |
| Ps.Ar (mm2) |
0.6396 (0.6131–0.6673) |
0.5680 (0.5466–0.5902) |
17.69 | 0.0005 | 0.6852 (0.6550–0.7168) |
0.6119 (0.5862–0.6387) |
13.63 | 0.0017 |
| Es.Ar (mm2) |
0.3501 (0.3232–0.3793) |
0.3302 (0.3070–0.3550) |
1.21 | 0.2854 | 0.4070 (0.3797–0.4364) |
0.3619 (0.3387–0.3866) |
2.37a | 0.1411a |
| Ct.Ar (mm2) |
0.1704 (0.1590–0.1825) |
0.1418 (0.1332–0.1509) |
16.05 | 0.0008 | 0.1609 (0.1511–0.1713) |
0.1494 (0.1407–0.1586) |
3.04 | 0.0985 |
| J (mm4) |
0.0269 (0.0249–0.0289) |
0.0204 (0.0191–0.0219) |
30.24 | <0.0001 | 0.0282 (0.0259–0.0306) |
0.0233 (0.0215–0.0252) |
11.22 | 0.0036 |
| Imax (mm4) |
0.0157 (0.0145–0.0170) |
0.0123 (0.0115–0.0132) |
21.34 | 0.0002 | 0.0168 (0.0153–0.0185) |
0.0139 (0.0128–0.0152) |
8.80 | 0.0082 |
| Imin (mm4) |
0.0111 (0.0103–0.0120) |
0.0081 (0.0076–0.0087) |
42.45 | <0.0001 | 0.0113 (0.0105–0.0122) |
0.0093 (0.0087–0.0100) |
14.79 | 0.0012 |
Significance test performed on rank transformed data. See Table 1 legend for abbreviations.
DISCUSSION
In this study, we explored the degree to which limb bone diaphyseal structure reflects the historical activity levels of members of a lineage (population) using one-week-old mice selectively bred for high levels of voluntary wheel running. We sought to determine whether the femora of mice with an “evolutionary history” of high locomotor activity were structurally distinct from unselected mice prior to the ontogenetic onset of locomotion. As hypothesized, at one week postnatal, HR animals had larger body mass-adjusted gross diaphyseal dimensions, suggesting that selection for high activity has led to a concomitant evolutionary increase in diaphyseal dimensions. Interestingly, we found the difference between HR and C animals to be more pronounced in females than in males, and adult females also run more than males (Garland, 2003). Given that tissue mineral density and porosity did not significantly differ between HR and C animals, bone tissue strength was presumably similar across the groups. However, for reasons discussed previously (see Materials and Methods), it is unclear whether differences in diaphyseal dimension translate to differences in strength. This would need to be determined by whole-bone mechanical testing (e.g., Middleton et al., 2008a). In any case, results of the present study suggest that an evolutionary signal conditioned by ancestral activity patterns may be discerned in limb bone diaphyses.
An evolutionary signal is presumably most evident during perinatal development (or at the age selection has been acting if the genes act in an age-specific fashion), and becomes increasingly distorted throughout ontogeny as bones respond to changes in their loading environments. Furthermore, the degree to which adult morphology conveys an evolutionary signal may vary between individuals depending on their initial diaphyseal strength. One might predict that if a genome contains information that “instructs” cells to produce a stronger diaphysis, then that bone may resist load-induced strains without triggering an osteogenic response. In other words, the degree to which adult diaphyseal structure reflects individual behavior is likely to be a function of historical genetic changes to the entire genome, as well as the particular allelic complement of a given individual.
This scenario is consistent with the principle of initial value of exercise training, which states that individuals with low initial values of a physiologic variable will exhibit the greatest response to exercise (see Koch et al. [2005] and Middleton et al. [2008b] and references therein). On the other hand, adult structure might always primarily reflect individual behavior rather than evolutionary background if bone mass is regulated by strain control acting in a feedback loop (Rubin, 1984). This is because individuals born with stronger diaphyses would need to be highly active throughout life in order to maintain their elevated strength. Otherwise, they would lose bone mass because they would be “genetically overbuilt” for their customary mechanical environment. In this case adult diaphyseal structure would still reflect functional loading during life. Unfortunately, these alternative scenarios are difficult to evaluate given the paucity of research devoted to the interface between initial bone strength and mechanosensitivity (but see Akhter et al., 1998; Kodama et al., 2000; Robling and Turner, 2002; Robling et al., 2007). This is an issue that we plan to investigate in the future.
The mechanisms responsible for the evolutionary signal detected here remain elusive. In principle, however, the activity profiles of earlier generations could be incorporated into the genome of subsequent generations by the process of genetic assimilation (Price et al., 2003; Pigliucci et al., 2006). In this model, phenotypic traits individually acquired through a plastic response to environmental stimuli, such as some aspects of diaphyseal structure, are later, under the influence of selection, incorporated into an organism’s developmental repertoire if the environmental stimulus becomes constant. In the case of limb bones, if there is a shift in the loading environment that elicits an adaptive response in a given generation (e.g., increased loading associated with high activity levels causes individuals to grow thick diaphyses), and this new loading environment persists in subsequent generations (e.g., members of the lineage remain highly active), then the adaptive phenotype may become genetically assimilated and constitutively produced (see also Garland and Kelly [2006] on the possibility of self-induced adaptive plasticity). This scenario is consistent with the adaptationist perspective of Churchill (1999, see also Trinkaus et al., 1994), which predicts that selection favoring high activity should cause a concomitant evolutionary increase in diaphyseal strength. Thick diaphyses among fossil hominins, juvenile or adult, would thus be interpreted as an evolutionary (cross-generational) adaptation to high activity, rather than (only) a plastic response to individual activity level.
Alternatively, the evolutionary signal detected in this study need not be interpreted as adaptive. Indeed, from an energetic perspective, elevated bone mass might be considered maladaptive for highly active animals because it would increase the moment of inertia of their limbs and the metabolic cost of locomotion (Myers and Steudel, 1985; Steudel, 1990; McGillivray et al., 2009). Therefore, increased relative diaphyseal dimensions in HR mice may represent evolutionary “spandrels” (sensu Gould and Lewontin, 1979), genetically associated with physical activity through pleiotropic gene action (e.g., via molecules that regulate both activity and bone mass), rather than adaptations shaped by genetic assimilation. For example, as noted previously, HR mice have lower levels of plasma leptin (Girard et al., 2007), a key molecule in energy metabolism that also affects bone structure (Ducy et al., 2000; Elefteriou et al., 2004; Karsenty, 2006; Confavreux et al., 2009). Leptin deficiency has been demonstrated to cause substantial increases in bone mass (Ducy et al., 2000; Elefterion et al., 2004), suggesting that low leptin levels and high bone mass in HR mice might be causally related phenomena. Regardless of the specific genes and molecular pathways involved, if the observed differences in diaphyseal structure are non-adaptive and only indirectly related to activity, then the present results caution against using limb bone diaphyses to infer ancestral activity patterns. In other words, despite the presence of an evolutionary signal conditioned by ancestral behavior, inferring the latter from the former is not advisable unless they are related through causation and not simple correlation. Otherwise, if HR and C mice were also found to differ in, say, craniofacial morphology, then we would conclude that activity patterns of fossil taxa could also be reconstructed from these features. Indeed, several inbred strains of mice that differ significantly from one another in terms of activity levels (Lightfoot et al., 2004) also have distinct craniofacial morphologies (Cheverud et al., 1991; Vinyard and Payseur, 2008), but these differences are almost certainly not directly related. Therefore, the relevance of the results presented here for hominin functional morphology will depend on future investigations of the precise mechanisms driving the evolutionary signal (e.g., genetic assimilation, pleiotropy, or others).
We have assumed that diaphyseal structure in one-week-old mice is primarily determined by genetics rather than mechanical loads because mice do not actively locomote before this age. However, fetal and perinatal bones do not develop in an environment devoid of mechanical signals. In fact, loads applied through muscle contractions are critical to prenatal skeletal development (Hall and Herring, 1990). Therefore, the differences in diaphyseal structure observed between HR and C mice could be the result of differences in intrauterine and perinatal muscle activity. It is also possible that HR mothers are more active during pregnancy than C mothers and that this difference has a positive influence on fetal skeletal growth in HR mice. In this case, differences in perinatal diaphyseal structure would still reflect variation in ancestral activity patterns, but would not be the result of genetic inheritance (for further discussion of nongenetic hereditary mechanisms see Jablonka and Lamb [2007] and references therein). The activity patterns of pregnant mothers and newborn pups have not yet been examined rigorously, nor have fetal activity patterns. However, based on informal observations by Garland, Kelly, and Middleton, HR and C pups appear to be equally sedentary prior to one week of age, and this pattern presumably extends to prenatal development as well. Differences in activity between pregnant mothers are more likely, but there is currently no experimental evidence suggesting that maternal activity influences fetal bone growth directly (i.e., soma to soma). Note that both the HR and C dams in the present study did not have wheel access and that previous behavioral studies have shown that, after giving birth, dams from C and HR lines exhibit similar frequencies of maternal behavior and similar levels of locomotor activity. Litter size or litter mass at birth or at weaning also does not differ between lines (Girard et al., 2002). In sum, differences in maternal or pre- and perinatal activity are unlikely to have significantly influenced the results presented here. However, more detailed investigations are clearly needed.
It should be emphasized that the differences detected in this study were in mass-adjusted diaphyseal dimensions. Even though the infant mouse bones were not yet weight-bearing (i.e., transmitting body weight), we used body mass to control for variation in size because it could be more reliably determined than other size variables like body length, and some size correction seemed prudent as bone dimensions reflect size to a certain degree (e.g., Middleton et al., 2008a; 2010). Structural differences between lines may have been exaggerated by the fact that HR mice are less massive than C mice (Table 1), though not significantly so for our sample (females: P = 0.13; males: P = 0.35). Indeed, the mass of certain unadjusted parameters (females: J, Imax; males: Ct.Ar) are actually higher for C mice than HR mice (Table 1). That the differences in both body mass and mass-adjusted diaphyseal dimensions are generally more pronounced in females than males further suggests that the overall pattern in diaphyseal dimensions was driven, to some degree, by body mass. It remains true that HR mice have relatively larger diaphyses, but one is left to speculate that selection on activity influences only body mass and not bone mass directly, at least at the age of one week.
An important limitation of this study is that it lacked replication of experimental lines, so it is possible that differences between the selected and unselected animals are the result of founder effects and/or random genetic drift rather than the effects of selection (Henderson, 1989, 1997; Konarzewski et al., 2005; Garland and Rose, 2009). Therefore, the results will need to be confirmed by future analyses involving all eight lines. In presenting these preliminary results, we hope to stimulate discussion within the anthropological community about how genetics and phenotypic plasticity interact in the skeleton. In recent years, arguments about the role of genetics and functional loading in determining diaphyseal structure have been rather dichotomized (cf Ruff et al., 2006). Both factors are clearly important -- as are hormones, nutrition, and age -- and it is critical that functional morphologists begin to consider non-mechanical influences on diaphyseal structure as more than confounding variables, but as potential sources of scientific inquiry.
Fig. 4.
Box plots for tissue mineral density (TMD) and porosity (Po). Differences were not significant between selected and control animals for either females or males. Medians, middle 50% of cases, and expected ranges of scores are represented by lines, boxes, and whiskers, respectively. Circles represent outliers.
ACKNOWLEDGEMENTS
We thank William Jungers, Matthew O’Neill, Massimo Pigliucci, Jack Stern and Simon Wallace for their helpful discussions and Luci Betti-Nash for assisting with preparation of the figures. Christopher Ruff and two anonymous reviewers provided very helpful comments on the manuscript.
Grant sponsorship: Leakey Foundation (IJW), Stony Brook University (IJW), Turkana Basin Institute (IJW), NIAMS (KMM), NIAMS (SJ), NSF (TG)
Footnotes
The structural strength of a limb bone diaphysis (i.e., its resistance to failure) is the product of its gross size and shape, and the material properties of the bone tissue. Anthropologists commonly interpret only the gross structure of the diaphysis in cross section, expressed in terms of areas and area moments, and ignore the microstructural characteristics influencing tissue material properties. In the following, we will refer to gross size and shape as “gross dimensions” and use the term “structure” for a more inclusive reference to bone architecture that includes such microstructural characteristics as tissue mineral density and porosity.
LITERATURE CITED
- Akhter MP, Cullen DM, Pedersen EA, Kimmel DB, Recker RR. Bone response to in vivo mechanical loading in two breeds of mice. Calc Tissue Int. 1998;63:442–449. doi: 10.1007/s002239900554. [DOI] [PubMed] [Google Scholar]
- Akhter MP, Iwaniec UT, Covey MA, Cullen DM, Kimmel DB, Recker RR. Genetic variations in bone density, histomorphometry and strength in mice. Calcif Tissue Int. 2000;67:337–344. doi: 10.1007/s002230001144. [DOI] [PubMed] [Google Scholar]
- Arsuaga JL, Villaverde V, Quam R, Martínez I, Carretero JM, Lorenzo C, Gracia A. New neandertal remains from Cova Negra (Valencia, Spain) J Hum Evol. 2007;52:31–58. doi: 10.1016/j.jhevol.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Burnett CN, Johnson EW. Development of gait in childhood: part II. Develop Med Child Neurol. 1971;13:207–215. doi: 10.1111/j.1469-8749.1971.tb03246.x. [DOI] [PubMed] [Google Scholar]
- Cheverud JM, Hartman SE, Richtsmeier JT, Atchley WR. A quantitative genetic analysis of localized morphology in mandibles of inbred mice using finite element scaling analysis. J Craniofac Genet Dev Biol. 1991;11:122–137. [PubMed] [Google Scholar]
- Chiu C-H, Hamrick MW. Evolution and development of the primate limb skeleton. Evol Anthropol. 2002;11:94–107. [Google Scholar]
- Churchill SE. Cold adaptation, heterochrony, and neandertals. Evol Anthropol. 1999;7:46–60. [Google Scholar]
- Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolism. Mol Cell Endocrin. 2009;310:21–29. doi: 10.1016/j.mce.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover WJ, Iman RL. Analysis of covariance using the rank transformation. Biometrics. 1982;38:715–724. [PubMed] [Google Scholar]
- Cowgill LW. Ph.D. dissertation. St. Louis: Washington University; 2008. The ontogeny of recent and Late Pleistocene human postcranial robusticity. [Google Scholar]
- Cowgill LW. The ontogeny of Holocene and late Pleistocene human postcranial strength. Am J Phys Anthropol. 2009 doi: 10.1002/ajpa.21107. DOI 10.1002/ajpa.21107. [DOI] [PubMed] [Google Scholar]
- Cowgill LW, Hager LD. Variation in the development of postcranial robusticity: an example from Çatalhöyük, Turkey. Int J Osteoarchaeol. 2007;17:235–252. [Google Scholar]
- Currey JD. The effect of porosity and mineral content on the Young’s modulus of elasticity of compact bone. J Biomech. 1988;21:131–139. doi: 10.1016/0021-9290(88)90006-1. [DOI] [PubMed] [Google Scholar]
- Currey JD. Bones: structure and mechanics. Princeton: Princeton University Press; 2002. [Google Scholar]
- Demissie S, Dupuis J, Cupples LA, Beck TJ, Kiel DP, Krasik D. Proximal hip geometry is linked to several chromosomal regions: genome-wide linkage results from the Framingham Osteoporosis Study. Bone. 2006;40:743–750. doi: 10.1016/j.bone.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Dumke CL, Rhodes JS, Garland T, Jr, Maslowski E, Swallow JG, Wetter AC, Cartee GD. Genetic selection of mice for high voluntary wheel running: effect on skeletal muscle glucose uptake. J Appl Physiol. 2001;91:1289–1297. doi: 10.1152/jappl.2001.91.3.1289. [DOI] [PubMed] [Google Scholar]
- Eisman JA. Genetics of osteoporosis. Endocr Rev. 1999;20:788–804. doi: 10.1210/edrv.20.6.0384. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Ae Kim C, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson C, Nakao K, Capeau J, Karsenty G. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci USA. 2004;101:3258–3263. doi: 10.1073/pnas.0308744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T., Jr . Selection experiments: an under-utilized tool in biomechanics and organismal biology. In: Bels VL, Gasc J-P, Casinos A, editors. Vertebrate biomechanics and evolution. Oxford: BIOS; 2003. pp. 23–56. [Google Scholar]
- Garland T, Jr, Freeman PW. Selective breeding for high endurance running increases hindlimb symmetry. Evolution. 2005;59:1851–1854. [PubMed] [Google Scholar]
- Garland T, Jr, Kelly SA. Phenotypic plasticity and experimental evolution. J Exp Biol. 2006;209:2344–2361. doi: 10.1242/jeb.02244. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Rose MR. Experimental evolution: concepts, methods, and applications of selection experiments. Berkeley: University of California Press; 2009. [Google Scholar]
- Girard I, McAleer MW, Rhodes JS, Garland T., Jr Selection for high voluntary wheel-running increases speed and intermittency in house mice (Mus domesticus) J Exp Biol. 2001;204:4311–4320. doi: 10.1242/jeb.204.24.4311. [DOI] [PubMed] [Google Scholar]
- Girard I, Swallow JG, Carter PA, Koteja P, Rhodes JS, Garland T., Jr Maternal-care behavior and life-history traits in house mice (Mus domesticus) artificially selected for high voluntary wheel-running activity. Behav Processes. 2002;57:37–50. doi: 10.1016/s0376-6357(01)00206-6. [DOI] [PubMed] [Google Scholar]
- Girard I, Rezende EL, Garland T., Jr Leptin levels and body composition of mice selectively bred for high voluntary locomotor activity. Physiol Biochem Zool. 2007;80:568–579. doi: 10.1086/521086. [DOI] [PubMed] [Google Scholar]
- Goodship AE, Cunningham JL. Pathophysiology of functional adaptation of bone in remodeling and repair in vivo. In: Cowin S, editor. Bone mechanics handbook. Boca Raton: CRC Press; 2001. pp. 26-1–26-31. [Google Scholar]
- Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc R Soc Lond B. 1979;205:581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- Hall BK, Herring SW. Paralysis and growth of the musculoskeletal system in the embryonic chick. J Morphol. 1990;206:45–56. doi: 10.1002/jmor.1052060105. [DOI] [PubMed] [Google Scholar]
- Havill LM, Muhaney MC, Binkley TL, Specker BL. Effects of genes, sex, age, and activity on BMC, bone size, and areal and volumetric BMD. J Bone Miner Res. 2007;22:737–746. doi: 10.1359/jbmr.070213. [DOI] [PubMed] [Google Scholar]
- Henderson ND. Interpreting studies that compare high- and low-selected lines on new characters. Behav Genet. 1989;19:473–502. doi: 10.1007/BF01066250. [DOI] [PubMed] [Google Scholar]
- Henderson ND. Spurious associations in unreplicated selected lines. Behav Genet. 1997;27:145–154. doi: 10.1023/a:1025689425738. [DOI] [PubMed] [Google Scholar]
- Hesinger RN. Standards in pediatric orthopedics. New York: Raven Press; 1986. [Google Scholar]
- Hibbeler RC. Mechanics of materials. 3rd edition. Prentice Hall: Upper Saddle River; 1997. [Google Scholar]
- Jablonka E, Lamb MJ. The expanded evolutionary synthesis – a response to Godfrey-Smith, Haig, and West-Eberhard. Biol Philos. 2007;22:453–472. [Google Scholar]
- Jepsen KJ, Akkus O, Majeska RJ, Nadeau JH. Hierarchical relationship between bone traits and mechanical properties in inbred mice. Mamm Genome. 2003;14:97–104. doi: 10.1007/s00335-002-3045-y. [DOI] [PubMed] [Google Scholar]
- Jepsen KJ, Hu B, Tommasini SM, Courtland H-W, Price C, Cordova M, Nadeau JH. Phenotypic integration of skeletal traits during growth buffers genetic variants affecting the slenderness of femora in inbred mouse strains. Mamm Genome. 2009;20:21–33. doi: 10.1007/s00335-008-9158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judex S, Garman RA, Squire ME, Donahue LR, Rubin CT. Genetically based influences on the site-specific regulation of trabecular and cortical bone morphology. J Bone Miner Res. 2004;19:600–606. doi: 10.1359/JBMR.040101. [DOI] [PubMed] [Google Scholar]
- Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Kelly SA, Czech PP, Wight JT, Blank KM, Garland T., Jr Experimental evolution and phenotypic plasticity of hindlimb bones in high-activity house mice. J Morphol. 2006;267:360–374. doi: 10.1002/jmor.10407. [DOI] [PubMed] [Google Scholar]
- Koch LG, Green CL, Lee AD, Hornyak JE, Cicila GT, Britton SL. Test of the principle of initial value in rat genetic models of exercise capacity. Am J Regul Integr Comp Physiol. 2005;288:R466–R472. doi: 10.1152/ajpregu.00621.2004. [DOI] [PubMed] [Google Scholar]
- Kodama Y, Umemura Y, Nagasawa S, Beamer WG, Donahue LR, Rosen CR, Baylink DJ, Farley JR. Exercise and mechanical loading increase periosteal bone formation and whole bone strength in C57BL/6J mice but not in C3H/Hej mice. Calcif Tissue Int. 2000;66:298–306. doi: 10.1007/s002230010060. [DOI] [PubMed] [Google Scholar]
- Koller DL, Liu G, Econs MJ, Hui SL, Morin PA, Joslyn G, Rodriguez LA, Conneally PM, Christian JC, Johnston CC, Jr, Foroud T, Peacock M. Genome screen for quantitative trait loci underlying normal variation in femoral structure. J Bone Miner Res. 2001;16:985–991. doi: 10.1359/jbmr.2001.16.6.985. [DOI] [PubMed] [Google Scholar]
- Konarzewski M, Książek A, Łapo I. Artificial selection on metabolic rates and related traits in rodents. Integr Comp Biol. 2005;45:416–425. doi: 10.1093/icb/45.3.416. [DOI] [PubMed] [Google Scholar]
- Kondo O, Dodo Y. Postcranial bones of the Neanderthal child of the burial No. 1. In: Akazawa T, Muhesen S, editors. Neanderthal burials: excavations of the Dederiyeh Cave, Afrin, Syria. Kyoto: International Research Center for Japanese Studies; 2002. pp. 139–214. [Google Scholar]
- Larsen CS. Bioarchaeology: interpreting behavior from the human skeleton. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR. Genetic influence on daily wheel running activity level. Physiol Genomics. 2004;19:270–276. doi: 10.1152/physiolgenomics.00125.2004. [DOI] [PubMed] [Google Scholar]
- Lovejoy CO, McCollum MA, Reno PL, Rosenman BA. Developmental biology and human evolution. Annu Rev Anthropol. 2003;32:85–109. [Google Scholar]
- Lublinsky S, Ozcevici E, Judex S. An automated algorithm to detect the trabecularcortical bone interface in micro-computed tomographic images. Calcif Tissue Int. 2007;81:285–293. doi: 10.1007/s00223-007-9063-8. [DOI] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW, Gomes FR, Chappell MA, Garland T., Jr Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheelrunning behavior. Gen Comp Endocrinol. 2008;156:210–217. doi: 10.1016/j.ygcen.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW, Kolb EM, Wada H, Hannon RM, Chappell MA, Middleton KM, Garland T., Jr Behavioral despair and home-cage activity in mice with chronically elevated baseline corticosterone concentrations. Behav Genet. 2009;39:192–201. doi: 10.1007/s10519-008-9246-8. [DOI] [PubMed] [Google Scholar]
- McGillivray DG, Garland T, Jr, Dlugosz EM, Chappell MA, Syme DA. Changes in efficiency and myosin expression in the small-muscle phenotype of mice selectively bred for high voluntary running activity. J Exp Biol. 2009;212:977–985. doi: 10.1242/jeb.026625. [DOI] [PubMed] [Google Scholar]
- Meek TH, Lonquich BP, Hannon RM, Garland T., Jr Endurance capacity of mice selectively bred for high voluntary wheel running. J Exp Biol. 2009;212:2908–2917. doi: 10.1242/jeb.028886. [DOI] [PubMed] [Google Scholar]
- Middleton KM, Shubin CE, Moore DC, Carter PA, Garland T, Jr, Swartz SM. The relative importance of genetics and phenotypic plasticity in dictating bone morphology and mechanics in aged mice: evidence form an artificial selection experiment. Zoology. 2008a;111:135–147. doi: 10.1016/j.zool.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton KM, Kelly SA, Garland T., Jr Selective breeding as a tool to probe skeletal response to high voluntary locomotor activity in mice. Integr Comp Biol. 2008b;48:394–410. doi: 10.1093/icb/icn057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton KM, Goldstein BD, Guduru PR, Waters JF, Kelly SA, Swartz SM, Garland T., Jr Variation in within-bone stiffness measured by nanoindentation in mice bred for high levels of voluntary wheel running. J Anat. 2010;216:121–131. doi: 10.1111/j.1469-7580.2009.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LM, Little W, Schirmer A, Sheik F, Busa B, Judex S. Accretion of bone quantity and quality in the developing mouse skeleton. J Bone Miner Res. 2007;22:1037–1045. doi: 10.1359/jbmr.070402. [DOI] [PubMed] [Google Scholar]
- Myers MJ, Steudel K. Effect of limb mass and its distribution on the energetic cost of running. J Exp Biol. 1985;116:363–373. doi: 10.1242/jeb.116.1.363. [DOI] [PubMed] [Google Scholar]
- Nehrenberg DL, Hua K, Estrada-Smith D, Garland T, Jr, Pomp D. Voluntary exercise and its effects on body composition depend on genetic selection history. Obesity. 2009;17:1402–1409. doi: 10.1038/oby.2009.51. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Peacock M, Turner CH, Econs MJ, Foroud T. Genetics of osteoporosis. Endocr Rev. 2002;23:303–326. doi: 10.1210/edrv.23.3.0464. [DOI] [PubMed] [Google Scholar]
- Pearson OM, Lieberman DE. The aging of Wolff’s “law”: ontogeny and responses to mechanical loading in cortical bone. Yrbk Phys Anthropol. 2004;47:63–99. doi: 10.1002/ajpa.20155. [DOI] [PubMed] [Google Scholar]
- Pigliucci M, Murren CJ, Schlichting CD. Phenotypic plasticity and evolution by genetic assimilation. J Exp Biol. 2006;209:2362–2367. doi: 10.1242/jeb.02070. [DOI] [PubMed] [Google Scholar]
- Price C, Herman BC, Lufkin T, Goldman HM, Jepsen KJ. Genetic variation in bone growth patterns defines adult mouse bone fragility. J Bone Miner Res. 2005;20:1983–1991. doi: 10.1359/JBMR.050707. [DOI] [PubMed] [Google Scholar]
- Price TD, Quarnstrom A, Irwin DE. The role of phenotypic plasticity in driving genetic evolution. Proc R Soc Lond B. 2003;270:1433–1440. doi: 10.1098/rspb.2003.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende EL, Gomes FR, Malisch JL, Chappell MA, Garland T., Jr Maximal oxygen consumption in relation to subordinate traits in lines of house mice selectively bred for high voluntary wheel running. J Appl Physiol. 2006a;101:477–485. doi: 10.1152/japplphysiol.00042.2006. [DOI] [PubMed] [Google Scholar]
- Rezende EL, Garland T, Jr, Chappell MA, Malisch JL, Gomes FR. Maximum aerobic performance in lines of Mus selected for high wheel-running activity: effects of selection, oxygen availability and the mini-muscle phenotype. J Exp Biol. 2006b;209:115–127. doi: 10.1242/jeb.01883. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Gammie SC, Garland T., Jr Neurobiology of mice selected for high voluntary wheel-running activity. Integ Comp Biol. 2005;45:438–455. doi: 10.1093/icb/45.3.438. [DOI] [PubMed] [Google Scholar]
- Robbins G. Ph.D. dissertation. Eugene: University of Oregon; 2007. Population dynamics, growth and development in Chalcolithic sites of the Deccan Peninsula, India. [Google Scholar]
- Robling AG, Turner CH. Mechanotransduction in bone: genetic effects on mechanosensitivity in mice. Bone. 2002;31:562–569. doi: 10.1016/s8756-3282(02)00871-2. [DOI] [PubMed] [Google Scholar]
- Robling AG, Warden SJ, Shultz KL, Beamer WG, Turner CH. Genetic effects on bone mechanotransduction in congenic mice harboring bone size and strength quantitative trait loci. J Bone Min Res. 2007;22:984–991. doi: 10.1359/jbmr.070327. [DOI] [PubMed] [Google Scholar]
- Rubin CT. Skeletal strain and the functional significance of bone architecture. Calcif Tissue Int. 1984;36:S11–S18. doi: 10.1007/BF02406128. [DOI] [PubMed] [Google Scholar]
- Ruff CB. Biomechanical analysis of archaeological human skeletons. In: Katzenberg MA, Saunders SR, editors. Biological anthropology of the human skeleton. New York: Wiley-Liss; 2000. pp. 71–102. [Google Scholar]
- Ruff CB, Walker A, Trinkaus E. Postcranial robusticity in Homo. III: ontogeny. Am J Phys Anthropol. 1994;93:35–54. doi: 10.1002/ajpa.1330930103. [DOI] [PubMed] [Google Scholar]
- Ruff CB, Holt B, Trinkaus E. Who’s afraid of the big bad Wolff?: “Wolff’s law” and bone functional adaptation. Am J Phys Anthropol. 2006;129:484–498. doi: 10.1002/ajpa.20371. [DOI] [PubMed] [Google Scholar]
- Saless N, Litscher SJ, Lopez Franco GE, Houlihan MJ, Sudhkaran S, Abdul Raheem K, O’Neil TK, Vanderby R, Demant P, Blank RD. Quantitative trait loci for biomechanical performance and femoral geometry in an intercross of recombinant congenic mice: restriction of the Bmd7 candidate interval. FASEB J. 2009;23:2142–2154. doi: 10.1096/fj.08-118679. [DOI] [PubMed] [Google Scholar]
- Stanitski DF, Nietert PJ, Stanitski cL, Nadjarian RK, Barfield W. Relationship of factors affecting age of onset of independent ambulation. J Pediatr Orthop. 2000;20:686–688. doi: 10.1097/00004694-200009000-00026. [DOI] [PubMed] [Google Scholar]
- Steudel K. The work and energetic cost of locomotion. II. Partitioning the cost of internal and external work within a species. J Exp Biol. 1990;154:287–303. doi: 10.1242/jeb.154.1.287. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Carter PA, Garland T., Jr Artificial selection for increased wheel-running behavior in house mice. Behav Genet. 1998;28:227–237. doi: 10.1023/a:1021479331779. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Koteja P, Carter PA, Garland T., Jr Artificial selection for increased wheel-running activity in house mice results in decreased body mass at maturity. J Exp Biol. 1999;202:2513–2520. doi: 10.1242/jeb.202.18.2513. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Koteja P, Carter PA, Garland T., Jr Food consumption and body composition in mice selected for high wheel-running activity. J Comp Physiol B. 2001;171:651–659. doi: 10.1007/s003600100216. [DOI] [PubMed] [Google Scholar]
- Tracer DP. Infant carrying and prewalking locomotor development: proximate and evolutionary perspectives. Am J Phys Anthropol suppl. 2009;48:257. [Google Scholar]
- Trinkaus E, Churchill SE, Ruff CB. Postcranial robusticity in Homo. II: humeral bilateral asymmetry and bone plasticity. Am J Phys Anthropol. 1994;93:1–34. doi: 10.1002/ajpa.1330930102. [DOI] [PubMed] [Google Scholar]
- Trinkaus E, Ruff C. Early modern human remains from eastern Asia: the Yamashita-cho 1 immature postcrania. J Hum Evol. 1996;30:299–314. [Google Scholar]
- Trinkaus E, Ruff CB, Esteves F, Santos Coelho JM, Silva M, Mendonça M. The lower limb remains. In: Zilhão J, Trinkaus E, editors. Portrait of the artist as a young child. Lisbon: Instituto Português de Arqueología; 2002. pp. 435–465. [Google Scholar]
- Vaanholt LM, Meerlo P, Garland T, Jr, Visser GH, van Dijk G. Plasma adiponectin is increased in mice selectively bred for high wheel-running activity, but not by wheel running per se. Horm Metab Res. 2007;39:377–383. doi: 10.1055/s-2007-976542. [DOI] [PubMed] [Google Scholar]
- Vinyard CJ, Payseur BA. Of “mice” and mammals: utilizing classical inbred mice to study the genetic architecture of function and performance in mammals. Integr Comp Biol. 2008;48:324–337. doi: 10.1093/icb/icn063. [DOI] [PubMed] [Google Scholar]
- Wergedal JE, Sheng MHC, Ackert-Bicknell CL, Beamer WG, Baylink DJ. Genetic variation in femur extrinsic strength in 29 different inbred strains of mice is dependent on variations in femur cross-sectional geometry and bone density. Bone. 2005;36:111–122. doi: 10.1016/j.bone.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Williams E, Scott JP. The development of social behavior patterns in the mouse, in relation to natural periods. Behaviour. 1954;6:35–64. [Google Scholar]