Abstract
We tested the hypothesis that the protective effect of parity on fibroids is due to direct pregnancy-related effects by following women from early pregnancy to postpartum period with ultrasound. Of 171 women with one initial fibroid, 36% had no identifiable fibroid at the time of postpartum ultrasound and 79% of the remaining fibroids decreased in size.
Keywords: leiomyoma, postpartum, pregnancy, ultrasound, uterine remodeling
Uterine leiomyomata, or fibroids, are benign tumors with an estimated prevalence of at least 15% in white women and 40% in black women by age 35 (1,2). Higher parity is associated with reduced fibroid risk in most epidemiologic studies (3,4,5) and fibroid risk is lower among women with more recent pregnancies (4, 5) than with remote pregnancies. Whether the association is due to direct protective effects or to fibroid-related infertility is unclear. Baird and Dunson (6) argued that the parity association is likely due to a pregnancy-related protective effect citing experimental data from the Eker rat, an animal model for fibroids (7).
We evaluated pregnancy-related effects on fibroids within a large prospective pregnancy study using ultrasound to determine the proportion of tumors that are eliminated or changed in size after pregnancy. This analysis was conducted as part of an ongoing prospective study of pregnancy, Right from the Start. Methods have been described previously (2, 8).
The cohort study included an early pregnancy endovaginal ultrasound at radiologic referral facilities. All sonographers had training in pelvic sonography for a minimum of 3 years and annual study training. Probes for the study were 5-7.5 mHz. The ultrasound systematically screened for uterine fibroids of at least 0.5 cm in largest diameter. Of the 458 first-time study participants with fibroids, 282 participated in the postpartum endovaginal ultrasound, which was scheduled between three and six months after the birth. This analysis was limited to women who had a single fibroid at early pregnancy screening and who gave birth at ≥ 20 weeks gestation (n = 171).
Fibroid characteristics were recorded at each ultrasound. Size of fibroid was based on measuring the diameter in each of the three perpendicular planes. Triplicate measures, separated in time by intervening measurements of the gestational sac and fetus, were taken to reduce the chance of focal contractions being misclassified as fibroids. We averaged across planes to provide a single mean diameter per fibroid.
Using mutually exclusive categories, fibroid type was defined as: 1) submucous – any leiomyoma in contact with or distorting the uterine cavity; 2) subserous – distorting the external contour of the uterus; 3) intramural – within the myometrium, neither distorting contour nor cavity; and 4) pedunculated – attached to the uterus with an identifiable stalk. Location (fundus, corpus, lower uterine segment) was also mutually exclusive and position was marked as anterior, posterior or both. Fibroid images were saved initially as still photos and later as digital images on CD-ROM for review by study physicians (KH, SL).
We performed a validation study using magnetic resonance imaging (MRI) to evaluate the elimination of fibroids seen on postpartum ultrasounds. A subset of women who completed the postpartum ultrasound in the preceding 18 months was invited for pelvic MRI. Eleven women participated, four with ultrasound-documented postpartum fibroids (positive controls) and seven with no fibroid seen at postpartum ultrasound (validation sample). Participation rate was 26% (11/42 eligible). Of those who gave reasons for non-participation the major reasons were time restrictions and breastfeeding. MRI was performed using a 3.0T unit using a standard protocol. T2-weighted and contrast enhanced images were reviewed in the sagittal and axial planes. Radiologists had no prior knowledge of fibroid status.
Chi-squared and the Kolmogorov-Smirnov tests were used to compare fibroids that resolved to below detectable size with those that remained. We evaluated resolution by fibroid characteristics and by timing of the early pregnancy ultrasounds. The sign test was used to compare diameter change in a matched analysis of the persisting fibroids. We used linear regression to evaluate fibroid size change by fibroid characteristics using the difference between post-partum and early pregnancy fibroid diameter for remaining fibroids. Data were analyzed with STATA10.0 (Stata Corporation, College Station, TX).
Mean age of the women was 31.2 years (SD 4.8), 62% were white, and nearly half were nulliparous at enrollment. Median gestational age was 8 weeks (25th, 75th percentiles 7, 10) for early pregnancy ultrasound and 3.6 months (25th, 75th percentiles 3.2, 4.4) after delivery for postpartum ultrasounds. Most fibroids were either intramural or subserous and were located in the uterine corpus. Mean diameter of the fibroids ranged from 0.4 cm to 8.0 cm with a median of 1.9 cm (25th, 75th percentiles 1.1, 3.2).
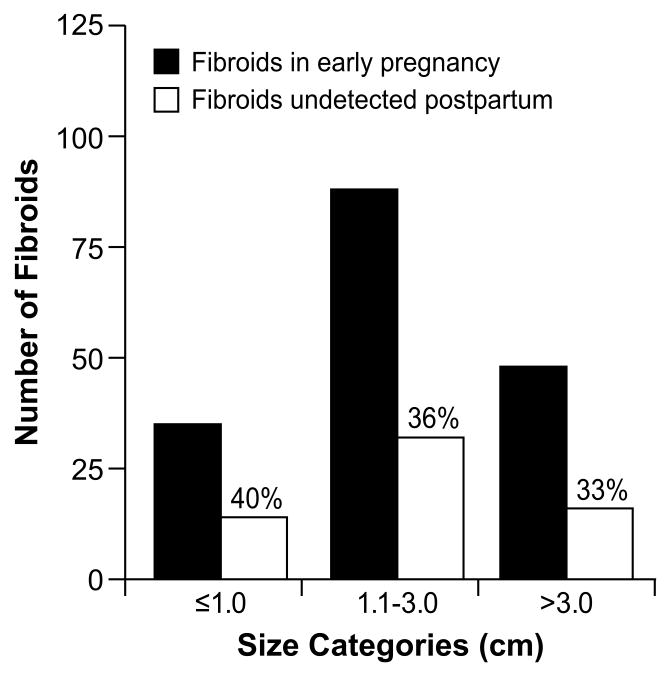
Of the 171 women, 54% (95% confidence interval (CI) 46, 61) had one fibroid at the time of postpartum ultrasound and 10% had more than one, leaving 36% of fibroids that were resolved beyond the ability to detect them (Figure 1). Fibroid type, location and position were not associated with fibroid resolution (p>0.2). Initial tumor size was also not related to fibroid resolution (p>0.4); one third of fibroids >3 cm in diameter resolved.
Figure 1.
Distribution of initial fibroid size for 171 solitary fibroids, and proportion of each group that were no longer detected on the postpartum ultrasound.
Fibroids seen at the postpartum ultrasound were generally smaller than at initial ultrasound. Among the 109 single fibroids detected at the postpartum ultrasound, 79% (95% CI 70, 86) were smaller than the initially-measured size. The median diameter on early pregnancy ultrasound was 1.9 cm (25th, 75th percentile 1.2, 3.3) and the postpartum diameter was significant smaller (p<0.001) with a median change in diameter of 0.5 cm (25th, 75th percentiles 0.1, 1.4). The reduction in diameter associated with submucous fibroids was greater (1.8 cm change) than the reduction associated with intramural (0.2 cm), subserous (0.6 cm) or pedunculated (0.5 cm) fibroids (p<0.002). Fibroids in the lower segment were associated with a greater change in fibroid diameter (1.4cm) when compared to fibroids in the corpus (0.5cm) or fundus (0.4cm, p=0.003). The reduction in fibroid size did not differ by position (left or right).
Our results were supported by the MRI. The four women who had fibroids on the postpartum ultrasound also had fibroids on MRI and measured fibroid sizes were consistent. Of the seven women with no fibroids on postpartum ultrasound, three had no fibroids on MRI and two had fibroids on MRI that were at or below the level of detection by ultrasound. The remaining two women had fibroids on MRI large enough that they could have been detected by ultrasound (mean diameters of 0.6 and 0.7 cm); however, these MRIs occurred more than 13 weeks after the ultrasound, so it is unclear whether the fibroids were missed or below the level of detection at the time of the ultrasound. Of the eight fibroids present on the MRI, one showed 100% non-enhancement and two showed central lack of enhancement after contrast indicating there may be central ischemia for some of the fibroids.
Our study was designed to test for a direct protective effect of pregnancy on fibroids. We found that 36% of fibroids were resolved below our ability to detect them. Fibroids that remained were reduced in diameter by a median of 0.5 cm. Previous studies that tracked fibroids during pregnancy were based on clinically identified fibroids rather than those detected by systematic ultrasound screening. Three studies included postpartum ultrasounds (9-11) and reported instances of fibroid elimination or shrinkage. The largest included 34 postpartum assessments and these were at four weeks postpartum when uterine involution may not be complete (12); they found up to an 18% reduction in fibroid size for small fibroids. A study of 262 tumors in non-pregnant premenopausal women showed that most fibroids grow over time (13). Though spontaneous regression occurred it was infrequent and shrinkage was limited; only 7% had a shrinkage rate of more than 20% per six months (13). This suggests that observed pregnancy-related regression is much more common than would be expected among non-pregnant women. Additionally, though breastfeeding creates a hypoestrogenic state that could confound our findings, prior studies have found no association of breastfeeding and fibroids (4, 6, 14, 15), especially after controlling for parity (14). A limitation of our study is that the fibroid screening was done in early pregnancy rather than before pregnancy. Fibroids may grow during the first trimester, as noted in 12 women from a prior study (11) that showed 8% increase at 10 weeks and 3% at 12 weeks over the pre-pregnancy size. Given the large size reduction in our study (median percent change in diameter of 30%), early first trimester growth is not likely to account for much of the volume loss. Furthermore, if early-pregnancy growth followed by later shrinkage back to prepregnancy size were an important contributor to our observed results, the fibroids measured toward the end of the first trimester would be expected to show the biggest change. However, we found no association of higher gestational age at first ultrasound and fibroid resolution (p=0.4). An additional limitation is that our sample included only eight tumors greater than 5 cm in diameter (5%), so we were unable to adequately evaluate pregnancy-related effects on very large tumors.
The mechanism by which fibroids resolve is of great interest. Ischemia of the myometrium and myomas at the time of delivery has been proposed as a mechanism (16). Studies on fibroid vasculature show abnormal vessels which are reduced in density compared to adjacent myometrium making fibroids more susceptible to ischemia (17, 18). Additionally, clot forms in both myometrial and fibroid tissue after delivery, but a decrease in fibrinolytic activity in fibroids compared to myometrium leads to more sustained ischemia in fibroids (16). Ischemia may continue during postpartum uterine involution which involves major remodeling of the vasculature (19). It is notable that MRI revealed decreased blood flow in three of eight postpartum fibroids. Further investigation of the natural mechanisms of pregnancy-related fibroid resolution may identify future directions for developing therapies that could limit the need for highly invasive treatments.
Acknowledgments
The authors would like to thank the staff and participants of the Right from the Start study for making our research possible. Special thanks to Allen Wilcox and Reem Hasan for reviewing earlier versions of this manuscript.
Financial support: The work was conducted as part of the Right from the Start study. The parent study received support from the American Water Works Association Research Foundation under Contract #2579 (Savitz); Pfizer Scholars Grants for Faculty Development in Clinical Epidemiology (Hartmann) and NICHD RO1 HD043883-04 and HD049675: “Consequences and Course of Uterine Fibroids in Pregnancy” (Hartmann). Laughlin was supported by the NIH Women's Health Fellowships in Intramural Women's Health Research. The postpartum data collection research was supported in part by the Intramural Research Program of the NIH and the National Institute of Environmental Health Sciences (P30ES10126).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–7. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 2.Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalence of uterine leiomyomas in the first trimester of pregnancy: an ultrasound-screening study. Obstet Gynecol. 2009;113:630–5. doi: 10.1097/AOG.0b013e318197bbaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:571–88. doi: 10.1016/j.bpobgyn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Wise LA, Palmer JR, Harlow BL, Spiegelman D, Stewart EA, Adams-Campbell LL, et al. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol. 2004;159:113–23. doi: 10.1093/aje/kwh016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall LM, Spiegelman D, Goldman MB, Manson JE, Colditz GA, Barbieri RL, et al. A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril. 1998;70:432–9. doi: 10.1016/s0015-0282(98)00208-8. [DOI] [PubMed] [Google Scholar]
- 6.Baird DD, Dunson DB. Why is parity protective for uterine fibroids? Epidemiology. 2003;14:247–50. doi: 10.1097/01.EDE.0000054360.61254.27. [DOI] [PubMed] [Google Scholar]
- 7.Walker CL, Cesen-Cummings K, Houle C, Baird D, Barrett JC, Davis B. Protective effect of pregnancy for development of uterine leiomyoma. Carcinogenesis. 2001;22:2049–52. doi: 10.1093/carcin/22.12.2049. [DOI] [PubMed] [Google Scholar]
- 8.Promislow JH, Makarushka CM, Gorman JR, Howards PP, Savitz DA, Hartmann KE. Recruitment for a community-based study of early pregnancy: the Right From The Start study. Paediatr Perinat Epidemiol. 2004;18:143–52. doi: 10.1111/j.1365-3016.2003.00546.x. [DOI] [PubMed] [Google Scholar]
- 9.Neiger R, Sonek JD, Croom CS, Ventolini G. Pregnancy-related changes in the size of uterine leiomyomas. J Reprod Med. 2006;51:671–4. [PubMed] [Google Scholar]
- 10.Aharoni A, Reiter A, Golan D, Paltiely Y, Sharf M. Patterns of growth of uterine leiomyomas during pregnancy. A prospective longitudinal study. Br J Obstet Gynaecol. 1988;95:510–3. doi: 10.1111/j.1471-0528.1988.tb12807.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosati P, Exacoustos C, Mancuso S. Longitudinal evaluation of uterine myoma growth during pregnancy. A sonographic study J Ultrasound Med. 1992;11:511–5. doi: 10.7863/jum.1992.11.10.511. [DOI] [PubMed] [Google Scholar]
- 12.Negishi H, Kishida T, Yamada H, Hirayama E, Mikuni M, Fujimoto S. Changes in uterine size after vaginal delivery and cesarean section determined by vaginal sonography in the puerperium. Arch Gynecol Obstet. 1999;263:13–6. doi: 10.1007/s004040050253. [DOI] [PubMed] [Google Scholar]
- 13.Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci U S A. 2008;105:19887–92. doi: 10.1073/pnas.0808188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumbiganon P, Rugpao S, Phandhu-fung S, Laopaiboon M, Vudhikamraksa N, Werawatakul Y. Protective effect of depot-medroxyprogesterone acetate on surgically treated uterine leiomyomas: a multicentre case--control study. Br J Obstet Gynaecol. 1996;103:909–14. doi: 10.1111/j.1471-0528.1996.tb09911.x. [DOI] [PubMed] [Google Scholar]
- 15.Samadi AR, Lee NC, Flanders WD, Boring JR, 3rd, Parris EB. Risk factors for self-reported uterine fibroids: a case-control study. Am J Public Health. 1996;86:858–62. doi: 10.2105/ajph.86.6.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burbank F. Childbirth and myoma treatment by uterine artery occlusion: do they share a common biology? J Am Assoc Gynecol Laparosc. 2004;11:138–52. doi: 10.1016/s1074-3804(05)60189-2. [DOI] [PubMed] [Google Scholar]
- 17.Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088–93. doi: 10.1093/humrep/deg213. [DOI] [PubMed] [Google Scholar]
- 18.Wei JJ, Zhang XM, Chiriboga L, Yee H, Perle MA, Mittal K. Spatial differences in biologic activity of large uterine leiomyomata. Fertil Steril. 2006;85:179–87. doi: 10.1016/j.fertnstert.2005.07.1294. [DOI] [PubMed] [Google Scholar]
- 19.Wakasa T, Wakasa K, Nakayama M, Kuwae Y, Matsuoka K, Takeuchi M, et al. Change in Morphology and Oxytocin Receptor Expression in the Uterine Blood Vessels during the Involution Process. Gynecol Obstet Invest. 2008;67:137–44. doi: 10.1159/000172805. [DOI] [PubMed] [Google Scholar]