Summary
The Cpx and σE regulons help maintain outer membrane integrity; the Cpx pathway monitors the biogenesis of cell surface structures, such as pili, while the σE pathway monitors the biogenesis of β-barrel outer membrane proteins (OMPs). In this study we revealed the importance of the Cpx regulon in the event of β-barrel OMP mis-assembly, by utilizing mutants expressing either a defective β-barrel OMP assembly machinery (Bam) or assembly defective β-barrel OMPs. Analysis of specific mRNAs showed that ΔcpxR bam double mutants failed to induce degP expression beyond the wild type level, despite activation of the σE pathway. The synthetic conditional lethal phenotype of ΔcpxR in mutant Bam or β-barrel OMP backgrounds was reversed by wild type DegP expressed from a heterologous plasmid promoter. Consistent with the involvement of the Cpx regulon in the event of aberrant β-barrel OMP assembly, the expression of cpxP, the archetypal member of the cpx regulon, was upregulated in defective Bam backgrounds or in cells expressing a single assembly-defective β-barrel OMP species. Together, these results showed that both the Cpx and σE regulons are required to reduce envelope stress caused by aberrant β-barrel OMP assembly, with the Cpx regulon principally contributing by controlling degP expression.
Introduction
In Escherichia coli, σE and CpxAR constitute the two major signal transduction pathways that help maintain envelope integrity. In this organism, the σE pathway is essential while the CpxAR pathway is dispensable. Activation of the σE pathway is triggered by the accumulation of misfolded β-barrel outer membrane proteins (OMPs) (for reviews, see Alba and Gross, 2004; Ades, 2008). RseA is an inner membrane anti-σE factor, which sequesters σE and prevents it from transcribing the downstream target genes (Rhodius et al., 2006). Under stress, the accumulation of misfolded envelope proteins triggers a two-step, DegS-and RseP-mediated degradation cascade of RseA (Ades et al., 1999; Alba et al., 2002; Inaba et al., 2008). This frees σE to transcribe genes whose products help restore envelope homeostasis by promoting protein folding/degradation or by inhibiting translation through activating the synthesis of small regulatory RNAs (Johansen et al., 2006; Papenfort et al., 2006).
The CpxAR gene products constitute a two-component signal transduction pathway in which CpxA functions as the inner membrane-bound sensor kinase while CpxR acts as a cytoplasmic response regulator (Raivio and Silhavy, 1997). Upon receiving the biochemical signals from CpxA, phosphorylated CpxR regulates the expression of a number of genes (De wulf et al., 2002; Yamamoto and Ishihama, 2006; Price and Raivio, 2009). The CpxAR pathway has been reported to be activated in response to diverse environmental signals and aberrant envelope or cell surface structures, including medium pH (Danese and Silhavy, 1998), medium osmolarity (Jubelin et al., 2005), overexpression of certain lipoproteins (Snyder et al., 1995; Miyadai et al., 2004), expression of misfolded P pilus subunits (Jones et al., 1997; Hung et al., 2001), defective protein secretion across the inner membrane (Shimohata et al., 2007), accumulation of enterobacterial common antigen (Danese et al., 1998) and defective membrane lipid composition (Mileykovskaya and Dowhan, 1997; Klein et al., 2009). Many, if not all, of the signals and defects listed above directly or indirectly influence envelope protein folding. Therefore, it is likely that the presence of misfolded envelope proteins activates the CpxAR signal transduction pathway. Indeed, the downstream targets of the CpxAR regulon, degP, dsbA and ppiA are involved in degrading or folding of envelope proteins, thus strongly suggesting a role for the CpxAR regulon in maintaining envelope integrity by sensing and appropriately responding to the folding status of envelope proteins.
Given that both the σE and CpxAR pathways respond to abnormal envelope biogenesis, it is not surprising that they share at least one very important downstream target gene, degP, whose product was originally thought to principally degrade misfolded envelope proteins (Strauch et al., 1989); however, subsequent studies revealed an additional chaperone role for DegP (Spiess et al., 1999; Krojer et al., 2008). It is well established that the σE pathway specifically responds to misfolded β-barrel OMPs because DegS, one of the two proteases that degrade RseA, recognizes the unique C-terminal sequence of misfolded β-barrel OMPs (Walsh et al., 2003). No systematic study has been conducted to determine whether the CpxAR pathway also plays a role when envelope stress is initiated due to misfolding of β-barrel OMPs. In the absence of such analysis, the defensive role of the CpxAR pathway appears to be reserved only for envelope stresses resulting from the aberrant assembly of cell surface organelles or other stresses not directly related to β-barrel OMPs (Raivio and Silhavy, 1999; Ruiz and Silhavy, 2005; Dorel et al., 2006).
In this study, we investigated the involvement of the CpxAR pathway in the biogenesis of β-barrel OMPs. More specifically, we examined the requirement of the CpxAR pathway in genetic backgrounds: (i) compromised in the proper assembly of β-barrel OMPs due to the presence of a defective β-barrel OMP assembly machine (Bam), (ii) lacking periplasmic chaperones/protease, SurA, Skp and DegP and (iii) expressing assembly defective β-barrel OMPs, OmpF and OmpC. The absence of CpxR in these genetic backgrounds, except Δskp, led to synthetic or conditional lethal phenotypes. In the absence of CpxR, cells failed to increase degP expression to a level needed to cope with the envelope stress resulting from the mis-assembly of β-barrel OMPs. Moreover, the conditional lethal phenotype of ΔcpxR in these genetic backgrounds was reversed when DegP was expressed from a heterologous plasmid promoter, indicating that the CpxAR pathway plays an important role in the event of aberrant β-barrel OMP assembly by contributing to the steady-state as well as regulated expression of degP. Together, our analysis shows that both the σE and CpxAR signal transduction pathways are required to fully cope with envelope stresses caused by misfolded β-barrel OMPs.
Results
Synthetic and conditional lethal phenotypes of ΔcpxR in genetic backgrounds defective in β-barrel OMP assembly
Assembly of β-barrel OMPs depends on two major envelope components: (i) the outer membrane-bound Bam complex comprised of the BamABCDE proteins and (ii) a periplasmic chaperone protein SurA. Another periplamic chaperone, Skp, plays a relatively minor role in β-barrel OMP assembly but its role becomes crucial in the absence of SurA (Rizzitello et al., 2001; Sklar et al., 2007). DegP is the major periplasmic protease (Strauch et al., 1989), which degrades misfolded OMPs (Misra et al., 1991; 2000; CastilloKeller and Misra, 2003). DegP has also been shown to act as a chaperone (Spiess et al., 1999); however, its role in actually promoting the assembly of E. coli β-barrel OMPs is not fully understood (Sklar et al., 2007; Krojer et al., 2008).
We asked whether the Cpx regulon plays a role when envelope stress is induced by mutations that directly affect β-barrel OMP assembly. For this, we introduced a ΔcpxR∷Cmr null allele in wild type, ΔbamB, bamA66, surA∷Kmr, Δskp and degP∷Tn10 backgrounds and measured cell growth at 30°C, 37°C and 40°C (Table 1; Fig. S1). In the wild type background, disabling of the Cpx regulon produced no growth defects at the three different incubation temperatures. Similarly, the absence of BamB, a non-essential lipoprotein component of the Bam complex (Wu et al., 2005; Vuong et al., 2008), by itself produced no growth defects but growth of the ΔbamB ΔcpxR∷Cmr mutant became increasingly worse with higher incubation temperatures. At 40°C, the mutant failed to form single colonies; instead, faster growing revertants arose frequently. The introduction of ΔcpxR in a bamA66 background, which expresses a mutant form of BamA in which a single amino acid residue was deleted from the POTRA domain (Bennion et al., unpubl. data), also resulted in a synthetic phenotype, although the growth defect at 37°C and 40°C was not as severe as observed in a ΔbamB ΔcpxR∷Cmr background (Table 1; Fig. S1). These results showed that some members of the Cpx regulon play an important role when envelope stress is triggered due to a defective Bam complex.
Table 1.
Synthetic phenotypes of ΔcpxR in backgrounds lacking or expressing defective component of β-barrel OMP assembly.
| Growtha temperature | |||
|---|---|---|---|
| Relevant genotype | 30°C | 37°C | 40°C |
| WT | ++ | +++ | +++ |
| ΔcpxR∷Cmr | ++ | +++ | +++ |
| ΔbamB | ++ | +++ | +++ |
| ΔbamB ΔcpxR∷Cmr | + | +/− | −/+ |
| ΔbamA66 | ++ | +++ | +++ |
| ΔbamA66 ΔcpxR∷Cmr | ++ | + | + |
| degP∷Tn10 | ++ | ++ | −/+ |
| degP∷Tn10 ΔcpxR∷Cmr | ++ | + | − |
| Δskp | ++ | +++ | +++ |
| Δskp ΔcpxR | ++ | +++ | +++ |
| surA∷Kmr | + | ++ | ++ |
| surA∷Kmr ΔcpxR∷Cmr | −/+ | +/− | +/− |
Wild type (WT) and various mutant strains were grown on LBA plates for 16 h at three temperatures shown. Growth resulting in homogeneous large-, medium- and small-sized colonies were scored as +++, ++ and + respectively. Occasionally, medium or small colonies lacked homogeneity, segregating into very small colonies. Due to this behaviour, such colonies were scored as +/− or −/+, reflecting increasing growth defects. Growth was scored as − when no isolated colonies were obtained.
Next, we investigated the significance of the Cpx regulon in genetic backgrounds lacking the periplasmic chaperones SurA, Skp or the protease/chaperone DegP (Table 1). Although the ΔcpxR∷Cmr allele could be transduced into a surA∷Kmr strain, purified transductants produced heterogeneous colonies at all growth temperatures. In contrast, the ΔcpxR∷Cmr allele could be readily transduced into a Δskp background and the resulting Δskp ΔcpxR∷Cmr double mutant grew just as well as the wild type strain regardless of the incubation temperature (Table 1). Thus, envelope stress generated by the loss of Skp was not sufficient to demand a requirement of the functional Cpx regulon. Lastly, we introduced ΔcpxR∷Cmr in a degP∷Tn10 background and observed a synthetic phenotype (Table 1; Fig. S1), indicating the involvement of some additional members of the Cpx regulon, other than DegP, in the absence of the major periplasmic protease/chaperone. Together, these results showed that the Cpx regulon is not only involved but plays an important role when envelopes are stressed due to a compromised β-barrel OMP assembly machinery or periplasmic folding/degradation environment.
Inability to fully activate degP expression causes synthetic phenotypes
Having shown that the Cpx regulon is needed when β-barrel OMP assembly is compromised, we set out to determine the status of four transcripts relevant to the β-barrel OMP-mediated envelope stress; namely degP, ompF, rseA and yfgC. The rseA and yfgC genes are positively regulated by σE (Rhodius et al., 2006), while rseA is partially repressed by the Cpx regulon (De wulf et al., 2002). degP is positively regulated by both the σE (Erickson and Gross, 1989) and Cpx (Danese et al., 1995) regulons, while ompF is negatively controlled by σE, which lowers ompF and ompC translation by increasing the synthesis of a small inhibitory RNA, rybB (Johansen et al., 2006; Papenfort et al., 2006). Transcription of ompF is also inhibited by an activated CpxAR system (Batchelor et al., 2005).
The transcriptional status of these genes was determined by real time, reverse transcription quantitative PCR (RT qPCR) (Fig. 1). For RNA isolation, cultures were gown overnight at 30°C, followed by growth at 37°C for about 2 h to a mid log phase. In a ΔcpxR∷Cmr background, rseA and yfgC transcript levels went up 1.8- and 1.3-fold, respectively, presumably as a result of the de-repression of the rpoE(σE)rseABC operon (De wulf et al., 2002). Interestingly, despite the slight activation of the σE regulon, degP transcript levels went down twofold, reaffirming a positive role for the Cpx regulon in controlling degP transcription independently of the σE regulon (Danese et al., 1995). No appreciable decrease in the ompF transcript level was noted in the absence of cpxR, indicating that the envelope was not significantly stressed. In degP∷Tn10 and degP∷Tn10 ΔcpxR∷Cmr backgrounds, the rseA and ompF transcript levels went up (rseA) and down (ompF) slightly relative to wild type, indicating the existence of mild envelope stress conditions.
Fig. 1.
Evaluation of degP, rseA, yfgC and ompF transcripts from wild type, ΔdegP, ΔbamB and bamA66 strains carrying a wild type cpxR or a ΔcpxR allele. Cultures were grown at 37°C to mid-log phase and RNA was extracted from approximately 5 × 108 cells. cDNA synthesized from RNA preparations was subjected to RT qPCR using gene-specific primers. Each PCR reaction was performed in triplicate and fold changes in transcript levels along with standard deviations were calculated from at least two experiments (n ≥ 2). Comparative critical threshold (ΔΔCt) values were obtained after normalizing data against a housekeeping gene, ftsL or purC, whose expression did not change in the presence of the various mutations used here.
In a ΔbamB background, the levels of both the rseA and yfgC transcripts increased by over two- and 1.5-fold, respectively, while the ompF transcript level went down almost fivefold, indicating that the cells are experiencing envelope stress (Fig. 1). Consistent with this assertion, degP transcript levels also went up over 2.4-fold (Fig. 1). In a ΔbamB ΔcpxR∷Cmr double mutant, the rseA and yfgC transcript levels rose slightly further over those present in a ΔbamB background, while ompF transcript levels remained low. Strikingly, despite the activation of the σE regulon, the degP transcript level remained threefold below that present in a ΔbamB background. It is important to note the degP transcript level did rise slightly in the ΔbamB ΔcpxR∷Cmr double mutant over that present in a ΔcpxR∷Cmr background (0.8 versus 0.5). Thus, while the activated σE regulon is capable of elevating degP expression under these conditions, the data suggest that without a functional Cpx regulon degP expression does not rise to a level needed to cope with envelope stress. We believe that this inability of the ΔbamB ΔcpxR∷Cmr mutant to increase degP expression is one of the reasons for the observed synthetic lethal phenotype at elevated growth temperatures (Table 1; Fig. S1). The effects of ΔcpxR∷Cmr in a bamA66 background were very similar to that observed in a ΔbamB background (Fig. 1), thus reaffirming that the inability of ΔcpxR cells to sufficiently elevate degP expression results in the synthetic phenotype.
The examination of β-barrel OMPs from membranes prepared from the same cultures used for RNA analysis showed that the levels of OmpF and LamB were significantly down in the ΔbamB and bamA66 strains compared with the wild type and degP∷Tn10 strains, with the absence of BamB exerting a slightly greater negative effect on OMP levels than the expression of a mutant BamA66 protein (Fig. 2). Consistent with the RT qPCR data, the absence of cpxR produced no further negative effect on OmpF and the three other OMPs examined (Fig. 2). Together, these RNA and protein data provided an explanation for the genetic data (Table 1), revealing that despite the fully operational σE regulon the growth defects are likely caused by the inability of a ΔcpxR strain to fully induce degP expression when the assembly of β-barrel OMPs is compromised.
Fig. 2.
Analysis of four β-barrel OMPs from wild type and various mutant backgrounds. Envelopes were prepared from the same cultures used for RNA analysis in Fig. 1. OMPs were detected by Western blot analysis using polyclonal antibodies that recognize LamB or OmpF, OmpC and OmpA. Plus and minus signs reflect presence (+) or absence (−) of cpxR.
Expression of DegP from a plasmid or the absence of rseA reverses the conditional lethal phenotype of ΔcpxR
To test whether the expression of DegP from a heterologous plasmid promoter can overcome the growth defects of the mutants listed in Table 1, we transformed selected strains that showed growth defects with an empty vector (pACYC184) and two derivatives expressing wild type or a protease-deficient DegPS210A variant and monitored growth at 30°C, 37°C and 40°C (Table 2). The expression of wild type DegP from a plasmid promoter fully reversed the growth defects in all cases, except in a surA∷Kmr ΔcpxR∷Cmr background where homogeneous growth was restored but the colony size was smaller compared with other strains. In contrast to wild type DegP, the expression of protease-deficient DegPS210A only partially reversed the growth defects, with no reversal observed in a surA∷Kmr ΔcpxR background. These results indicated that the protease activity of DegP is more crucial than its chaperone activity for lowering stress at elevated growth temperatures.
Table 2.
Expression of DegP from a plasmid reverses the conditional synthetic phenotype of degP and double mutants lacking cpxR.
| Plasmidsa and Growthb temperatures used for complementation | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| pACYC184 |
pACYC-degP (WT) |
pACYC-degP (S210A) |
|||||||
| Relevant mutant genotype | 30°C | 37°C | 40°C | 30°C | 37°C | 40°C | 30°C | 37°C | 40°C |
| degP∷Tn10 | ++ | ++ | +/− | +++ | +++ | +++ | +++ | +++ | ++ |
| degP∷Tn10 ΔcpxR∷Cmr | ++ | +/− | −/+ | ++ | +++ | +++ | ++ | ++ | +/− |
| bamA66 ΔcpxR∷Cmr | ++ | ++ | +/− | ++ | +++ | +++ | ++ | +++ | ++ |
| ΔbamB ΔcpxR∷Cmr | ++ | + | +/− | ++ | +++ | +++ | ++ | +++ | ++ |
| surA∷Kmr ΔcpxR∷Cmr | +/− | +/− | +/− | + | ++ | ++ | +/− | +/− | +/− |
Leaky expression of DegP or DegPS210A from plasmids was driven by a lac promoter in the absence of inducer.
Mutants were grown on LBA plates for 16 h. Growth resulting in homogeneous large-, medium- and small-sized colonies were scored as +++, ++ and + respectively. Small colonies occasionally produced mixed sized colonies and were scored as +/−, with −/+, reflecting a more debilitating growth defect.
In a ΔcpxR mutant, the σE pathway should be fully functional and slightly activated due to the absence of the repressive effect of CpxR, yet the activated σE pathway failed to alleviate growth defects when envelopes were stressed due to a defective Bam complex or periplasmic folding environment. It is possible that when the σE pathway is fully and constitutively activated due to the absence of its negative regulator, RseA, the collective actions of increased synthesis of factors that promote OMP assembly, degrade misfolded OMPs and inhibit OMP synthesis may be sufficient to overcome the envelope stress of a ΔcpxR mutant simultaneously defective in β-barrel OMP assembly. In agreement with our prediction, the introduction of ΔrseA restored almost normal growth of bam ΔcpxR and ΔdegP ΔcpxR mutants at 37°C and 40°C (Fig. S2). Thus, a fully activated σE pathway can substitute for the CpxAR pathway in cells experiencing envelope stress caused by mis-assembled β-barrel OMPs or the absence of degP.
Comparative analysis of the activation of Cpx and σE regulons in backgrounds defective in β-barrel OMP biogenesis
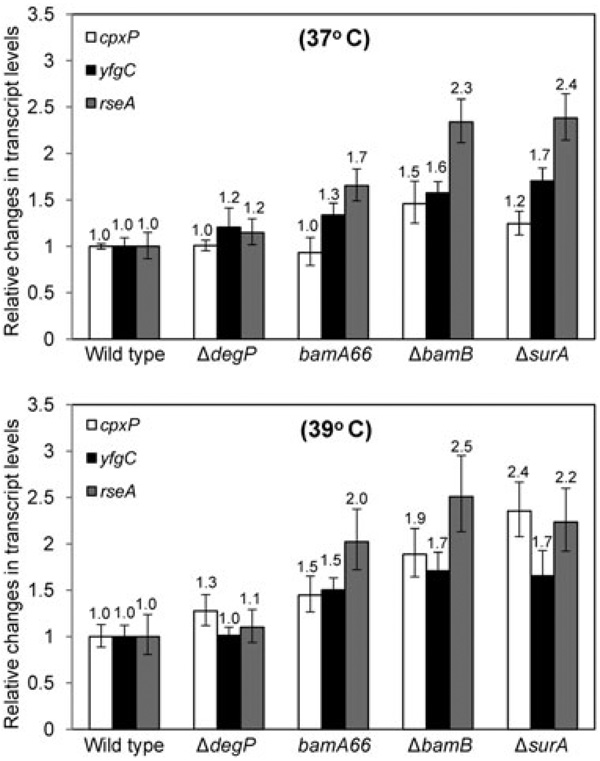
We carried out side-by-side assessments of the activation of Cpx and σE regulons in mutants expressing a defective Bam complex, lacking SurA, or lacking DegP. Wild type and mutant strains were grown overnight at 30°C, and next day after 1:100 dilution growth was resumed at 37°C or 39°C until cultures reached mid to late exponential phase (OD600 ≈ 0.6). RNA prepared from three independent cultures was converted to cDNA and subjected to RT qPCR analyses.
We focused on three key genes, cpxP, rseA and yfgC, which are regulated by Cpx (Danese and Silhavy, 1998), σE/Cpx (De wulf et al., 2002; Rhodius et al., 2006) and σE (Rhodius et al., 2006) respectively. We also included a ΔsurA mutant as no colony growth heterogeneity is observed in a ΔsurA cpxR+ background. At 37°C, expression of cpxP was mildly activated in ΔbamB and ΔsurA backgrounds, whereas no significant change in cpxP expression was noted in a bamA66 or ΔdegP background (Fig. 3, top panel). At 39°C, cpxP expression in all four mutant backgrounds increased compared with that at 37°C, indicating a greater demand for the Cpx regulon under conditions of increased β-barrel OMP assembly defects (Fig. 3, bottom panel). In general, fold increase in rseA and yfgC expression was greater than cpxP in all four mutant backgrounds at 37°C; however, this pattern was not obvious at 39°C. Together, these data showed that both the Cpx and σE regulons are activated in genetic backgrounds conducive for mis-assembly of β-barrel OMPs.
Fig. 3.
Evaluation of the status of the cpxAR and σE regulons in wild type and various mutant backgrounds. Relative RNA levels of cpxP yfgC and rseA, which are regulated by CpxAR, σE and CpxAR/σE, respectively, were determined by RT qPCR from RNA isolated from cultures grown at mid log phase at 37 and 39°C. RT qPCR analysis was performed as described Fig. 1 legend.
Lethal effects of ΔcpxR in genetic backgrounds expressing an assembly-defective β-barrel OMP
So far we have used Bam or chaperone/protease mutants to underscore the importance of a functional Cpx pathway in overcoming envelope stress. However, these mutants broadly affect OMP biogenesis and in some instances, e.g. bamA66 and ΔsurA, may also affect lipid biogenesis indirectly by interfering with the assembly of LptD (Imp), which is involved in the transport of LPS to the outer membrane (Bos et al., 2004). Indeed, defects in LPS biogenesis have been shown to activate the Cpx regulon (Klein et al., 2009). Therefore, to establish unequivocally that expression of misfolded β-barrel OMPs demands the intact Cpx regulon, we examined the phenotype of ΔcpxR in genetic backgrounds expressing individual mutant β-barrel OMPs with known assembly defects. The first group of mutants we used expressed OmpF proteins bearing a C-terminal residue other than the wild type phenylalanine (Misra et al., 2000). It is well established that the C-terminal phenylalanine, which is conserved in most β-barrel OMPs, plays a critical role in their folding and assembly (Struyvé et al., 1991; Misra et al., 2000). Peptides derived from the C-termini of β-barrel OMPs also bind to DegS and initiate a two-step proteolysis of RseA to induce σE (Walsh et al., 2003). The second group of mutants we used expressed OmpC proteins bearing one or two non-native cysteine residues (OmpC1Cys and OmpC2Cys; Misra, 1993; CastilloKeller and Misra, 2003). The formation of a disulfide bond in OmpC2Cys causes the protein to misfold (Misra, 1993; CastilloKeller and Misra, 2003). Expression of both the mutant OmpC and certain C-terminal mutant OmpF proteins demanded the presence of DegP (Misra et al., 2000; CastilloKeller and Misra, 2003).
We transduced the ΔcpxR mutation into the mutant OMP backgrounds at 30°C and tested whether the absence of the Cpx pathway interfered with bacterial growth at elevated incubation temperatures. Three of eight different OmpF mutants showed growth defects at 37°C but at 40°C six mutants grew poorly, with OmpFF340R displaying the most severe growth defects at 37°C and 40°C (Table 3; Fig. S3). Similarly, expression of OmpC2Cys in the absence of CpxR inhibited single colony formation at 37°C and 40°C (data not shown). These results showed that the expression of misfolded β-barrel OMPs, and not indirect effects, such as perturbed lipid biogenesis, demands the functional Cpx regulon. As with bam mutants, growth defects under these conditions are likely due to reduced synthesis of DegP, since expression of DegP from a plasmid restores normal growth (data not shown).
Table 3.
Effects of ΔcpxR on growth of strains expressing assembly-defective OmpF proteins.a
| 30°C |
37°C |
40°C |
||||
|---|---|---|---|---|---|---|
| OmpF Protein | cpxR+ | ΔcpxR | cpxR+ | ΔcpxR | cpxR+ | ΔcpxR |
| OmpFF340 (WT) | ++ | ++ | +++ | +++ | +++ | +++ |
| OmpF- | ++ | ++ | +++ | +++ | +++ | +++ |
| OmpFY340 | ++ | ++ | +++ | +++ | +++ | +++ |
| OmpFL340 | ++ | ++ | +++ | +++ | +++ | +++ |
| OmpFA340 | ++ | ++ | +++ | +++ | +++ | + |
| OmpFT340 | ++ | ++ | +++ | +++ | +++ | + |
| OmpFS340 | ++ | ++ | +++ | +++ | +++ | + |
| OmpFP340 | ++ | ++ | +++ | ++ | +++ | +/− |
| OmpFH340 | ++ | ++ | +++ | ++ | +++ | +/− |
| OmpFR340 | ++ | ++ | +++ | + | +++ | −/+ |
Strains were grown on LBA (plus 0.4 mM IPTG) plates for 16 h at temperatures shown. Growth resulting in large-, medium- and small-sized colonies were scored as +++, ++ and + respectively. Small or very small, but mixed-sized colonies were scored as +/− and −/+ respectively. OmpF in these strains was expressed from the lambda attachment site under the control of an IPTG-inducible promoter. No growth defects were observed in the absence of IPTG (see Fig. S2).
Expression of a single misfolded β-barrel OMP species induces the Cpx regulon
Next we asked whether the expression of an assembly-defective mutant OMP activates the Cpx regulon. For this, we introduced plasmids expressing the parental or an assembly defective OmpF under the control of an IPTG-inducible promoter into a strain carrying the cpxP∷lacZ fusion at the lambda attachment site. Cells were grown freshly at 37°C to an early log phase (OD600 ≈ 0.3), at which time cultures were split into three aliquots that received either none, 0.04 mM or 0.1 mM IPTG and growth was resumed for an hour so as to only transiently induce OmpF expression. β-galactosidase activities were measured from three independent cultures of each strain. The results showed that induction of the parental OmpF protein modestly elevated cpxP expression (about 1.8-fold; Fig. 4). In contrast, induction of an assembly-defective OmpFF340R protein increased cpxP∷lacZ activity fourfold over that of the control culture not induced with IPTG (Fig. 4), showing that the expression of a single assembly-defective β-barrel OMP species is sufficient to activate the Cpx regulon.
Fig. 4.
Activation of the CpxAR regulon by an assembly-defective OmpF protein. Status of the CpxAR regulon was examined through monitoring the activity of a cpxP∷lacZ fusion located at the lambda attachment site on the chromosome. See the Experimental procedures section for details concerning bacterial growth and β-galactosidase assay.
The absence of cpxP does not exacerbate envelope stress caused by aberrant β-barrel OMP assembly
Isaac et al. (2005) previously showed that in the absence of CpxP the toxic effects of overexpression of misfolded PapE and PapG were significantly exacerbated. Based on this and DegP-mediated protein degradation data they concluded that CpxP combats the toxic effects PapE and PapG. We asked whether CpxP similarly combats the toxic effects of misfolded β-barrel OMPs and if so, whether the absence of cpxP in defective β-barrel OMP assembly backgrounds would produce a synthetic phenotype. The introduction of a cpxP null allele in backgrounds expressing a defective Bam complex, misfolded OmpC or OmpF mutants did not produce a synthetic phenotype at 30°C, 37°C or 40°C (data not shown). These results suggest that CpxP, unlike CpxR or DegP, does not play a significant role in reducing the toxic effects of misfolded β-barrel OMPs. Because CpxP is shown to act as a negative regulator of CpxA (Raivio et al., 1999), it is possible that de-repression of the CpxAR regulon in a ΔcpxP background could potentially mask the negative effect of the loss of CpxP. In this regard, it is interesting to note that the absence of CpxP in a degP background allows the strain to form small but homogeneous single colonies at 40°C, thus reflecting a DegP-independent beneficiary effect resulting from the de-repression of the CpxAR regulon.
Discussion
Cpx regulon and β-barrel OMP assembly
Genetic and molecular analyses presented here showed the involvement and necessity of the Cpx regulon in the event of aberrant β-barrel OMP assembly. Several lines of evidence supported this conclusion: first, the absence of cpxR in mutant bam backgrounds, defective in the normal assembly of multiple β-barrel OMPs, caused a conditional synthetic lethal phenotype. Second, expression of a single assembly-defective species of β-barrel OMP in a ΔcpxR background caused a conditional synthetic lethal phenotype. Third, the Cpx regulon was activated in genetic backgrounds defective in correct assembly of β-barrel OMPs or expressing a single assembly-defective β-barrel OMP. All of these observations point to the involvement of the Cpx regulon, in addition to the σE regulon, in maintaining envelope integrity when perturbed due to the mis-assembly of β-barrel OMPs.
We noted that despite the activation of the σE regulon, the expression of degP in a bam ΔcpxR background was threefold lower compared with isogenic bam cpxR+ strains. This suggested that Cpx-mediated expression of degP is one of the reasons why the Cpx system is required under these conditions. Consistent with this notion, expression of a protease-active DegP protein from a plasmid reversed the synthetic lethal phenotype of bam ΔcpxR double mutants. Our results indicated that the Cpx regulon does not need to be activated beyond the wild type level for it to be relevant in reducing envelope stress. This was best exemplified by data obtained from the bamA66 mutant. When grown at 37°C, cpxP expression in the bamA66 mutant was very similar to that in the wild type strain, indicating no further activation of the Cpx regulon beyond what normally exists in a wild type background. Despite this, elimination of the Cpx regulon in a bamA66 ΔcpxR double mutant caused growth defects, owing to the inability of this mutant to fully induce degP expression. Therefore, the basal, unstimulated activity of the Cpx regulon can be sufficient to cope with envelope stress. Together, these results showed that the σE regulon alone is not sufficient to overcome envelope stress induced by mis-assembled β-barrel OMPs; rather, a concerted effort from both the Cpx and σE regulons is required (Fig. 5).
Fig. 5.
A cartoon showing selected Cpx- and σE-regulated genes whose products directly or indirectly help reduce envelope stress. Aberrant β-barrel OMP assembly resulting from a defective periplasmic folding environment or Bam complex or alterations in the primary sequence of OMPs stimulates both the Cpx and σE regulons. Elevated synthesis of proteins, such as the members of the Bam complex, LptD, SurA, PpiD and DsbA directly assists in OMP folding and assembly. No data exist showing a role for PpiA in OMP assembly (Kleerebezem et al., 2004). degP is the only gene in this list that is positively regulated by both the Cpx and σE regulons. The σE-induced synthesis of two small RNAs, rybB and micA, leads to translation inhibition of various OMPs, thus indirectly assisting in lowering envelope stress (Johansen et al., 2006; Papenfort et al., 2006). Activated CpxR can directly influence transcription of ompC and ompF (Batchelor et al., 2005), or via increasing expression of mzrA, whose inner membrane-localized product directly influences EnvZ’s kinase and/or phosphatase activities (Gerken et al., 2009). Besides affecting transcription of ompC and ompF, the activated EnvZ/OmpR two-component system influences transcription of a number of genes whose product help reduce stress (Gerken et al. 2009 and references therein).
Despite our demonstration here that the Cpx regulon is required for effectively combating envelope stress resulting from mis-assembled β-barrel OMPs, we believe that the σE pathway is the principal responder of stress signals generated under these conditions. One reason for this assertion is that a large number of σE-regulated gene products have been implicated either directly or indirectly in the assembly of β-barrel OMPs or maintaining envelope homeostasis by modulating OMP synthesis. (Fig. 5; Johansen et al., 2006; Papenfort et al., 2006; Rhodius et al., 2006). In contrast, expression of only a handful of genes, including dsbA, ppiA and ppiD, whose products could influence β-barrel OMP assembly, is uniquely controlled by the Cpx regulon (Danese and Silhavy, 1997; Pogliano et al., 1997; Dartigalongue and Raina, 1998; Fig. 5). However, none of these genes are essential for β-barrel OMP assembly, and no data exist for the involvement of PpiA in OMP assembly (Kleerebezem et al., 2004). Moreover, the two major β-barrel OMPs, OmpF and OmpC, lack any natural cysteine residues; therefore, rendering a direct involvement of DsbA in their assembly irrelevant. On the other hand, the further deterioration of growth in a degP mutant in the absence of cpxR suggests that at least one other cpx-regulated gene product, other than DegP, is needed for normal growth in the absence of the major periplasmic protease/chaperone activity of DegP. In this regard, it is interesting to note that the activated Cpx system has been recently shown to influence another two-component signal transduction comprised of the EnvZ/OmpR, via upregulating the synthesis of mzrA (Gerken et al., 2009). The inner membrane product of mzrA, whose expression is positively regulated by CpxAR, is shown to directly interact with EnvZ and influence EnvZ’s activity in a fashion similar to that of constitutively activated, pleiotropic envZ alleles (Gerken et al., 2009). Therefore, the activated Cpx regulon likely triggers a broad defensive response by influencing the expression of a number of genes, including those that are not under direct control of the Cpx regulon.
Connolly et al. (1997) first reported that a constitutively activated Cpx regulon can reduce the lethal effects of an rpoE null mutation. Here, we have shown that a constitutively activated σE regulon can overcome the lethal effects of ΔcpxR in backgrounds simultaneously defective in the assembly of β-barrel OMPs. This reciprocating behaviour in a large part appears to be due to the involvement of degP, which is common to both the regulons. Under normal growth conditions in rich medium and in the absence of a deliberately provoked envelope stress, degP expression appears to be evenly controlled by the σE and Cpx regulons (this study; Danese et al., 1995). We believe that because of this shared responsibility, the activities of both the regulons are needed to effectively combat envelope stress.
Signals that stimulate Cpx regulon
Signals originating from a wide range of factors, including aberrant synthesis or folding of envelope components, as well as medium osmolarity and pH are known to activate the Cpx regulon (summarized by Ruiz and Silhavy, 2005; Dorel et al., 2006; Price and Raivio, 2009). We have shown here that signals generated by misfolded β-barrel OMPs can also activate the Cpx regulon. However, unlike the σE regulon, the molecular nature of the signals that activate the Cpx regulon is poorly understood. In the case of σE, a two-step proteolysis of the anti-sigma factor, RseA, by DegS and RseP, leads to the release of σE from the membrane and subsequent activation. This event begins with the binding of C-terminal-derived β-barrel OMP peptides to the PDZ domain of DegS, activating its proteolytic domain, which then cleaves RseA (Walsh et al., 2003; Hasselblatt et al., 2007). A second proteolytic event is then carried out by RseP, which cleaves RseA near the cytoplasmic phase of the inner membrane (Grigorova et al., 2004).
The signalling pathway that activates CpxA, a membrane-bound receptor kinase, is poorly understood. Although CpxP is not involved in the signalling pathway, the available data suggest that it is a negative regulator of CpxA (Raivio et al., 1999; Buelow and Raivio, 2005). Isaac et al. (2005) showed that CpxP acts as an adapter protein, which upon binding to substrate proteins, including misfolded PapE and PapG, not only mediates their degradation by DegP, but also gets degraded in the process. The exacerbation of growth defects of a ΔcpxP mutant expressing misfolded PapE and PapG further signifies the importance of CpxP in reducing stress caused by certain misfolded envelope proteins. However, unlike ΔcpxR or ΔdegP, the introduction of ΔcpxP does not exacerbate the growth phenotype of the bam and OMP mutants used here. Therefore, DegP-mediated reduction in toxicity stemming from misfolded β-barrel OMPs appears to be the result of a direct action of DegP on misfolded β-barrel OMPs without involving CpxP.
The data presented here suggest that misfolded β-barrel OMPs activate the Cpx regulon independent of CpxP. Other envelope stresses resulting from alkaline pH or the production of misfolded PapE and PapG were also reported to activate the Cpx pathway independent of CpxP (DiGiuseppe and Silhavy, 2003). Mutational data have suggested that the periplasmic domain of CpxA is required for induction of the Cpx pathway by envelope stress (DiGiuseppe and Silhavy, 2003). This then raises the possibility that misfolded envelope proteins, including misfolded β-barrel OMPs, may directly interact with the periplasmic domain of the CpxA kinase to stimulate its activity. Consistent with this notion, a recent in vitro study showed a modest 1.6-fold increase in CpxA-mediated phosphorylation of CpxR when proteoliposomes were reconstituted with CpxA and MalE219, a mutant form of MalE known to misfold in vivo (Keller and Hunke, 2009). The implication of this finding is that misfolded MalE binds directly to CpxA and stimulates its phosphotransfer activity. In addition to stimulating the phosphotransfer activity of CpxA, it is possible that the binding of substrate proteins to CpxA in vivo may displace CpxP from CpxA and this could activate the autokinase activity of CpxA (Fleischer et al., 2007). Clearly, additional work is needed to better understand the molecular nature of stress signals that activate CpxA.
Experimental procedures
Bacterial strains and chemicals
All strains used here were derived from E. coli K-12 MC4100 and are listed in Table 4. Luria broth (LB) and agar (LBA) were prepared as described previously (Silhavy et al., 1984) and supplemented with ampicillin (50 µg ml−1), chloramphenicol (12.5 µg ml−1), kanamycin (25 µg ml−1), isopropyl β-D-1-thiogalactopyranoside (IPTG; 0.04, 0.1 or 0.4 mM) or arabinose (0.2%) as necessary. SuperSignal West Pico Chemiluminescent substrate was purchased from Thermo Scientific. All other chemicals were of analytical grade.
Table 4.
Bacterial strains and plasmids used in this study.
| Strain or plasmid | Characteristics | Reference or source |
|---|---|---|
| Strains | ||
| MC4100 | F- araD139 Δ(argF lac)U139 rspL150 relA1 fibB5301 ptsF25 deoC1 thi-1 rbsR | Casadaban (1976) |
| RAM1292 | MC4100 Δara714 | Werner and Misra (2005) |
| RAM1609 | RAM1292 ΔcpxR∷Cmr | This Study |
| RAM1315 | RAM1292ΔbamB∷scar | Charlson et al. (2006) |
| RAM1423 | RAM1292 bamA66-zad∷Tn10(Tcr) | This Study |
| RAM1316 | RAM1292 degP∷Tn10(Tcr) | Charlson et al. (2006) |
| RAM1446 | RAM1292 ΔsurA∷Kmr | This Study |
| RAM1610 | RAM1292 Δskp-Tn10(Tcr) | This Study |
| RAM1611 | RAM1315 ΔcpxR∷Cmr | This Study |
| RAM1612 | RAM1423 ΔcpxR∷Cmr | This Study |
| RAM1614 | RAM1316 ΔcpxR∷Cmr | This Study |
| RAM1615 | RAM1292 ΔcpxR∷scar | This Study |
| RAM1616 | RAM1315 ΔcpxR∷scar | This Study |
| RAM1617 | RAM1423 ΔcpxR∷scar | This Study |
| RAM1618 | RAM1316 ΔcpxR∷scar | This Study |
| RAM1619 | RAM1610 ΔcpxR∷scar | This Study |
| RAM1620 | RAM1609 ΔsurA∷Kmr | This Study |
| RAM1621 | RAM1615 pACYC184 | This Study |
| RAM1622 | RAM1615 pACYC184degP | This Study |
| RAM1623 | RAM1615 pACYC184degP[S210A] | This Study |
| RAM1624 | RAM1616 pACYC184 | This Study |
| RAM1625 | RAM1616 pACYC184degP | This Study |
| RAM1626 | RAM1616 pACYC184degP[S210A] | This Study |
| RAM1627 | RAM1617 pACYC184 | This Study |
| RAM1628 | RAM1617 pACYC184degP | This Study |
| RAM1629 | RAM1617 pACYC184degP[S210A] | This Study |
| RAM1630 | RAM1618 pACYC184 | This Study |
| RAM1631 | RAM1618 pACYC184degP | This Study |
| RAM1632 | RAM1618 pACYC184degP[S210A] | This Study |
| RAM1633 | RAM1615 pBAD24cpxR | This Study |
| RAM1634 | RAM1615 pBAD24cpxR ΔsurA∷Kmr | This Study |
| RAM1635–1637 | RAM1621–1623 ΔsurA∷Kmr | This Study |
| RAM1638–1641 | RAM1615–1618 ΔrseA∷Kmr | This Study |
| RAM1642 | RAM1292 ΔcpxP∷Kmr | This Study |
| RAM1643 | RAM1315 ΔcpxP∷Kmr | This Study |
| RAM1644 | RAM1423 ΔcpxP∷Kmr | This Study |
| RAM1645 | RAM1316 ΔcpxP∷Kmr | This Study |
| RAM1646 | RAM1292 ΔompF∷scar λInCh-pTrc99A cpxR+-zii∷Tn10 | This Study |
| RAM1647 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340] cpxR+zii∷Tn10 | This Study |
| RAM1648 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340Y] cpxR+zii∷Tn10 | This Study |
| RAM1649 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340L] cpxR+zii∷Tn10 | This Study |
| RAM1650 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340A] cpxR+zii∷Tn10 | This Study |
| RAM1651 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340T] cpxR+zii∷Tn10 | This Study |
| RAM1652 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340S] cpxR+zii∷Tn10 | This Study |
| RAM1653 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340P] cpxR+zii∷Tn10 | This Study |
| RAM1654 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340H] cpxR+zii∷Tn10 | This Study |
| RAM1655 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340R] cpxR+zii∷Tn10 | This Study |
| RAM1656 | RAM1292 ΔompF∷scar λInCh-pTrc99A ΔcpxR∷scar- zii∷Tn10 | This Study |
| RAM1657 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340] ΔcpxR∷scar-zii∷Tn10 | This Study |
| RAM1658 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340Y] ΔcpxR∷scar-zii∷Tn10 | This Study |
| RAM1659 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340L] ΔcpxR∷scar-zii∷Tn10 | This Study |
| RAM1660 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340A] ΔcpxR∷scar-zii∷Tn10 | This Study |
| RAM1661 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340T] ΔcpxR∷scar-zii∷Tn10 | This Study |
| RAM1662 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340S] ΔcpxR∷scar-zii∷Tn10 | This Study |
| RAM1663 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340P] ΔcpxR∷scar-zii∷Tn10 | This Study |
| RAM1664 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340H] ΔcpxR∷scar-zii∷Tn10 | This Study |
| RAM1665 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340R] ΔcpxR∷scar-zii∷Tn10 | This Study |
| RAM1666–1675 | RAM1646–1655 pACYC184 | This Study |
| RAM1676–1685 | RAM1646–1655 pACYC184degP | This Study |
| RAM1686–1695 | RAM1656–1665 pACYC184 | This Study |
| RAM1696–1705 | RAM1656–1665 pACYC184degP | This Study |
| RAM1706 | RAM1292 ompC+zei∷Tn10 pBAD24cpxR | This Study |
| RAM1707 | RAM1706 ΔcpxR∷Cmr | This Study |
| RAM1708 | RAM1292 ompC[1Cys]-zei∷Tn10 pBAD24cpxR | This Study |
| RAM1709 | RAM1708 ΔcpxR∷Cmr | This Study |
| RAM1710 | RAM1292 ompC[2Cys]-zei∷Tn10 pBAD24cpxR | This Study |
| RAM1711 | RAM1710 ΔcpxR∷Cmr | This Study |
| RAM1712 | RAM1292 ΔompF∷scar cpxP’:lacZ nadA∷Tn10 pTrc99A | This Study |
| RAM1713 | RAM1292 ΔompF∷scar cpxP’:lacZ nadA∷Tn10 pTrc99A–ompF[F340] | This Study |
| RAM1714 | RAM1292 ΔompF∷scar cpxP’:lacZ nadA∷Tn10 pTrc99A–ompF[F340R] | This Study |
| RAM1715 | RAM1292 ΔompF∷scar λInCh-pTrc99A ΔcpxP∷Kmr | This Study |
| RAM1716 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340] ΔcpxP∷Kmr | This Study |
| RAM1717 | RAM1292 ΔompF∷scar λInCh-pTrc99A–ompF[F340R] ΔcpxP∷Kmr | This Study |
| RAM1718 | RAM1292 ompC+zei∷Tn10 | This Study |
| RAM1719 | RAM1292 ompC[1Cys]-zei∷Tn10 | This Study |
| RAM1720 | RAM1292 ompC[2Cys]-zei∷Tn10 | This Study |
| RAM1721–1723 | RAM1718–1720 ΔcpxP∷Kmr | This Study |
| RAM1724–1726 | RAM1718–1720 pBAD24cpxR | This Study |
| RAM1727–1729 | RAM1718–1720 pBAD24cpxR ΔcpxR∷Cmr | This Study |
| Plasmids | ||
| pACYC184 | Tcr Cmr; cloning vector | Chang and Cohen (1978) |
| pACYC184degP | Cmr; Expresses degP under lac promoter control | This Study |
| pACYC184degP[S210A] | Cmr; Expresses degP[S210A] under lac promoter control | Spiess et al. (1999) |
| pBAD24 | Ampr; cloning and expression vector | Guzman et al. (1995) |
| pBAD24cpxR | Ampr; cloning and expression vector with cpxR | This Study |
| pKD3 | Cmr; Plasmid carrying Cmr gene cassette | Datsenko and Wanner (2000) |
| pKD4 | Kmr; Plasmid carrying Kmr gene cassette | Datsenko and Wanner (2000) |
| pKD46 | Ampr; Red recombinase expression plasmid | Datsenko and Wanner (2000) |
| pCP20 | Cmr and Ampr; FLP recombinase expression plasmid, temperature sensitive | Datsenko and Wanner (2000) |
DNA manipulations
Standard bacterial genetic methods were carried out as described previously (Silhavy et al., 1984). Primers used for cloning cpxR are listed in Table 5. A chromosomal fragment containing cpxR was amplified using gene-specific cloning primers. BspHI and HinDIII PCR-amplified fragments were cloned into NcoI and HinDIII digested pBAD24 (Guzman et al., 1995). The wild-type pACYCdegP clone was created by restoring the wild-type serine 210 codon in the pACY-CdegP (S210A) clone using the Quickchange Lightning Site-Directed Mutagenesis (SDM) Kit from Stratagene following the manufacturer’s protocol. Primers for the SDM are listed in Table 5.
Table 5.
Primers used for cloning, deletion and RT-qPCR.
| Purpose | Sequence (5′-3′)a | |
|---|---|---|
| For cloning | ||
| cpxR BspHI Fwd | GCGCATtcatgaATAAAATCCTGTTAGTT | |
| cpxR HinDIII Rev | CAGTaagcttAAGAAGCAGAAACCATCAG | |
| For SDM | ||
| degP [A210S] SDM Fwd | GCAGCGATCAACCGTGGTAACTCTGGTGGTGCGCTGGTTAACCTGAACGGCG | |
| degP [A210S] SDM Rev | CGCCGTTCAGGTTAACCAGCGCACCACCAGAGTTACCACGGTTGATCGCTGC | |
| For RT-qPCR | ||
| rseA Fwd RTPCR | CCGAGGTGCTCCATTTCG | |
| rseA Rev RTPCR | GTCGCCGGTTGACGTACTG | |
| yfgC Fwd RTPCR | ACTCTGCCCTGTTCCGTTATTC | |
| yfgC Rev RTPCR | TTCGTGCGCCATAACTGAAG | |
| degP Fwd RTPCR | AACTGAGCGATGGCCGTAAG | |
| degP Rev RTPCR | ATATCAGAGCGCGGATCTTTG | |
| cpxP Fwd RTPCR | ATTAAGCCACGCTGCTGAAGTC | |
| cpxP Rev RTPCR | TCACCCGGATGCCAGTTATC | |
| ompF Fwd RTPCR | CGATATGCTGCCAGAATTTGG | |
| ompF Rev RTPCR | CGAAGAAGTCATCGCTGTATGC | |
| ftsL Fwd RTPCR | TGCATTGCCTGGTGTTATCG | |
| ftsL Rev RTPCR | TGGCAGCTTCCCAAATCG | |
| purC Fwd RTPCR | TCGAATTCCGCAATGATACG | |
| purC Rev RTPCR | GCGATCAAACTGCTCAATGC | |
Restriction and mutagenesis sites are shown by lower case and underlined characters respectively.
RNA isolation and real-time PCR
Total RNA, from 5 ml of log phase cells (OD600 ~ 0.6–0.8) grown at 37 or 39°C, was extracted using TRIzol Max Bacterial RNA Isolation Kit (Invitrogen) and further purified with the RNeasy kit (Qiagen). cDNA was synthesized from 10 mg of RNA using 100 pM random hexamer primer (Integrated DNA Technologies) and M-MuLV Reverse Transcriptase (New England Biolabs). After reverse transcription, five units of RNaseH (New England Biolabs) were added and tubes were incubated for 20 min at 37°C followed by purification of cDNA with the QIAquick PCR purification kit (Qiagen). To measure RNA transcripts, 300 nM of each primer and 20 ng of cDNA was added to SYBR Green PCR Master Mix (Applied Biosystems) per each 20 µl of reaction. Gene-specific primers (Table 5) were designed following SYBR Green PCR Master Mix and RT-PCR Reagents manufacturer’s protocol. Critical threshold (Ct) values were determined using ABI Prism 7900HT Sequence Detection System (Applied Biosystems). Relative quantification of target transcripts were calculated according to the 2−ΔΔCt method (Livak and Schmittgen, 2001) using ftsL and purC endogenous control genes whose expression did not change by mutations used in this study. Each PCR reaction was performed in triplicate and fold changes in transcript levels along with standard deviations were calculated from at least two experiments (n ≥ 2).
Cell fractionation
Overnight cultures were diluted 1:100 and grown to log phase (OD600 ~ 0.8). Equivalent amounts of cells, based on OD600, were pelleted and resuspended in a lysis buffer (100 mM Tris pH 7.5, 10 mM MgCl2, 0.25 µg ml−1 DNase I and 2 mM PMSF) and lysed using a French press. Following a low speed spin to remove unlysed cells, cell lysates were centrifuged for 1 h at 105 000 g, 4°C to separate soluble (cytoplasm and periplasm) and insoluble (inner and outer membranes) fractions. Membrane fractions were then subjected to SDS(urea)-PAGE (as described below) followed by Western blot (described below).
Protein analyses
Membrane pellets were resuspended in 10 mM Tris-HCl pH 7.5 and diluted with SDS-PAGE sample buffer before heating at 95°C for 5 min and analysis by SDS-PAGE. 4 M urea was added to the SDS-polyacrylamide running gel in order to better resolve OmpC and OmpF. Following electrophoresis, proteins were transferred onto Immobilin-P (Millipore) using a mini transblot (Bio-Rad) and incubated in primary antibody for 1.5 h. Primary rabbit antibodies and dilutions used were OmpF/C/A (1:16 000) and LamB (1:10 000). Membranes were incubated with goat anti-rabbit HRP-conjugated immunoglobulin G secondary antibodies for 1 h and developed with SuperSignal West Pico Chemiluminescent substrate for 5 min. Protein bands were visualized with a Molecular-Imager-ChemiDoc-XRS System from Bio-Rad and quantified using Quantity One software (Bio-Rad).
Enzymatic assays
Overnight cultures grown at 30°C degrees with ampicillin were subcultured (1:100 dilution) without ampicillin and grown at 37°C to early log phase (OD600 ~ 0.2–0.3). Cells were split into two separate cultures, one of which ompF expression was induced with IPTG, while the other remained uninduced. Cells were grown for an additional hour and β-galactosidase activities determined as described previously (Michaelis et al., 1983; Miller, 1992), using a VersaMax microtiter plate reader (Molecular Dynamics). Assays were performed in quadruplicate and activity was calculated as the rate of o-nitrophenyl-β-galactoside (ONPG; Acros Organics) cleavage divided by the cell density in each well.
Supplementary Material
Acknowledgements
We would like to thank an anonymous reviewer of another manuscript for questioning the role of the CpxAR regulon in β-barrel OMP assembly and Leanne Misra for reading the manuscript. The work was supported by a grant R01-GM48167 from the National Institutes of Health. H. G. was partly supported by a supplemental fellowship from the ARCS foundation.
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Ades SE. Regulation by destruction: design of the σE envelope stress response. Curr Opin Microbiol. 2008;11:535–540. doi: 10.1016/j.mib.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Ades SE, Connolly LE, Alba BM, Gross CA. The Escherichia coli σE-dependent extracytoplasmic stress response is controlled by the regulated proteolysis of an anti-σ factor. Genes Dev. 1999;13:2449–2461. doi: 10.1101/gad.13.18.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba BM, Gross CA. Regulation of the Escherichia coli σE-dependent envelope stress response. Mol Microbiol. 2004;52:613–619. doi: 10.1111/j.1365-2958.2003.03982.x. [DOI] [PubMed] [Google Scholar]
- Alba BM, Leeds JA, Onufryk C, Lu CZ, Gross CA. DegS and YaeL participate sequentially in the cleavage of RseA to activate the σE-dependent extracytoplasmic stress response. Genes Dev. 2002;16:2156–2168. doi: 10.1101/gad.1008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor E, Walthers D, Kenney LJ, Goulian M. The Escherichia coli CpxA-CpxR envelope stress response system regulates expression of the porins OmpF and OmpC. J Bacteriol. 2005;187:5721–5731. doi: 10.1128/JB.187.16.5723-5731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MP, Tefsen B, Geurtsen J, Tommassen J. Identification of an outer membrane protein required for the transport of lipopolysaccharide to the bacterial cell surface. Proc Natl Acad Sci USA. 2004;101:9417–9422. doi: 10.1073/pnas.0402340101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelow DR, Raivio TL. Cpx signal transduction is influenced by a conserved N-terminal domain in the novel inhibitor CpxP and the periplasmic protease DegP. J Bacteriol. 2005;187:6622–6630. doi: 10.1128/JB.187.19.6622-6630.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban MJ. Transposition and fusion of the lac genes to select promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;141:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- CastilloKeller M, Misra R. Protease-deficient DegP suppresses lethal effects of a mutant OmpC protein by its capture. J Bacteriol. 2003;185:148–154. doi: 10.1128/JB.185.1.148-154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang ACY, Cohen SN. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ES, Werner JN, Misra R. Differential effects of yfgL mutation on the biogenesis of Escherichia coli outer membrane proteins and lipopolysaccharide. J Bacteriol. 2006;188:7186–7194. doi: 10.1128/JB.00571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly L, Peñas ADL, Alba BM, Gross CA. The response to extracytoplasmic stress in Escherichia coli is controlled by partially overlapping pathways. Genes Dev. 1997;11:2012–2021. doi: 10.1101/gad.11.15.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese PN, Silhavy TJ. The σE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 1997;11:1183–1193. doi: 10.1101/gad.11.9.1183. [DOI] [PubMed] [Google Scholar]
- Danese PN, Silhavy TJ. CpxP, a stress-combative member of the Cpx regulon. J Bacteriol. 1998;180:831–839. doi: 10.1128/jb.180.4.831-839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese PN, Snyder WB, Cosma CL, Davis LJB, Silhavy TJ. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 1995;9:387–398. doi: 10.1101/gad.9.4.387. [DOI] [PubMed] [Google Scholar]
- Danese PN, Oliver GR, Barr K, Bowman GD, Rick PD, Silhavy TJ. Accumulation of the entero-bacterial common antigen lipid II biosynthetic intermediate stimulates degP transcription in Escherichia coli. J Bacteriol. 1998;180:5875–5884. doi: 10.1128/jb.180.22.5875-5884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartigalongue C, Raina S. A new heat-shock gene, ppiD, encodes a peptidyl-prolyl isomerase required for folding of outer membrane proteins in Escherichia coli. EMBO J. 1998;17:3968–3980. doi: 10.1093/emboj/17.14.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wulf P, McGuire AM, Liu X, Lin ECC. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J Biol Chem. 2002;277:26652–26661. doi: 10.1074/jbc.M203487200. [DOI] [PubMed] [Google Scholar]
- DiGiuseppe PA, Silhavy TJ. Signal detection and target gene induction by the CpxRA two-component system. J Bacteriol. 2003;185:2432–2440. doi: 10.1128/JB.185.8.2432-2440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorel C, Lejeune P, Rodrigue A. The Cpx system of Escherichia coli, a strategic signaling pathway for confronting adverse conditions and for settling biofilm communities? Res Microbiol. 2006;157:306–314. doi: 10.1016/j.resmic.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Erickson J, Gross CA. Identification of the σE subunit of Escherichia coli RNA polymerase: a second alternate sigma factor involved in high-temperature gene expression. Genes Dev. 1989;3:1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- Fleischer R, Heermann R, Jung K, Hunke S. Purification, reconstitution, and characterization of the CpxRAP envelope stress system of Escherichia coli. J Biol Chem. 2007;282:8583–8593. doi: 10.1074/jbc.M605785200. [DOI] [PubMed] [Google Scholar]
- Gerken H, Charlson ES, Cicirelli EM, Kenney LJ, Misra R. MzrA, a novel modulator of the EnvZ/OmpR two-component regulon. Mol Microbiol. 2009;72:1408–1422. doi: 10.1111/j.1365-2958.2009.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorova IL, Chaba R, Zhong HJ, Alba BM, Rhodius V, Herman C, Gross CA. Fine-tuning of the Escherichia coli σE envelope stress response relies on multiple mechanisms to inhibit signal-independent proteolysis of the transmembrane anti-sigma factor, RseA. EMBO J. 2004;18:2686–2697. doi: 10.1101/gad.1238604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselblatt H, Kurzbauer R, Wilken C, Krojer T, Sawa J, Kurt J, et al. Regulation of the σE stress response by DegS: how the PDZ domain keeps the protease inactive in the resting state and allows integration of different OMP-derived stress signals upon folding stress. EMBO J. 2007;21:2659–2670. doi: 10.1101/gad.445307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung DL, Ravio TL, Jones CH, Silhavy TJ, Hultgren SJ. Cpx signaling pathway monitors biogenesis and affects assembly and expression of P pili. EMBO J. 2001;20:1508–1518. doi: 10.1093/emboj/20.7.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Suzuki M, Maegawa K-I, Akiyama S, Ito K, Akiyama Y. A pair of circularly permutated PDZ domains control RseP, the S2P family intramembrane protease of Escherichia coli. J Biol Chem. 2008;283 doi: 10.1074/jbc.M806603200. 35042-35042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac DD, Pinkner JS, Hultgren SJ, Silhavy TJ. The extracytoplasmic adaptor protein CpxP is degraded with substrate by DegP. Proc Natl Acd Sci USA. 2005;102:17775–17779. doi: 10.1073/pnas.0508936102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J, Rasmussen AA, Overgaard M, Valentin-Hansen P. Conserved small non-coding RNAs that belong to the σE regulon: role in down-regulation of outer membrane proteins. J Mol Biol. 2006;364:1–8. doi: 10.1016/j.jmb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Jones CH, Danese PN, Pinkner JS, Silhavy TJ, Hultgren SJ. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 1997;16:6394–6406. doi: 10.1093/emboj/16.21.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubelin G, Vianney A, Beloin C, Ghigo J-M, Lazzaroni J-C, Lejeune P, Dorel C. CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J Bactetiol. 2005;187:2038–2049. doi: 10.1128/JB.187.6.2038-2049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller RF, Hunke S. Misfolded maltose binding protein MalE219 induces the CpxRA envelope stress response by stimulating phosphoryl transfer from CpxA to CpxR. Res Microbiol. 2009;160:396–400. doi: 10.1016/j.resmic.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Kleerebezem M, Heutink M, Tommassen J. Characterization of an Escherichia coli rotA mutant, affected in periplasmic peptidyl-prolyl cis/trans isomerase. Mol Microbiol. 2004;18:313–320. doi: 10.1111/j.1365-2958.1995.mmi_18020313.x. [DOI] [PubMed] [Google Scholar]
- Klein G, Lindner B, Brabetz W, Brade H, Raina S. Escherichia coli K-12 suppressor-free mutants lacking early glycosyltransferases and late acyltransferases: minimal lipopolysaccharide structure and induction of envelope stress response. J Biol Chem. 2009;284:15369–15389. doi: 10.1074/jbc.M900490200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krojer T, Sawa J, Schäfer E, Saibil HR, Ehrmann M, Clausen T. Structural basis for the regulated protease and chaperone function of DegP. Nature. 2008;453:885–891. doi: 10.1038/nature07004. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Michaelis S, Inouye H, Oliver D, Beckwith J. Mutations that alter the signal sequence of alkaline phosphatase in Escherichia coli. J Bacteriol. 1983;154:366–374. doi: 10.1128/jb.154.1.366-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskaya E, Dowhan W. The Cpx two-component signal transduction pathway is activated in Escherichia coli mutant strains lacking phosphatidylethanolamine. J Bacteriol. 1997;179:1029–1034. doi: 10.1128/jb.179.4.1029-1034.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia Coli and Related Bacteria. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1992. pp. 71–74. [Google Scholar]
- Misra R. A novel ompC mutation of Escherichia coli K-12 that reduces OmpC and OmpF levels in the outer membrane. Mol Microbiol. 1993;10:1029–1035. doi: 10.1111/j.1365-2958.1993.tb00973.x. [DOI] [PubMed] [Google Scholar]
- Misra R, Peterson A, Ferenci T, Silhavy TJ. A genetic approach for analyzing the pathway of LamB assembly into the outer membrane of Escherichia coli. J Biol Chem. 1991;266:13592–13597. [PubMed] [Google Scholar]
- Misra R, CastilloKeller M, Deng M. Overexpression of protease deficient DegPS210A rescues the lethal phenotype of Escherichia coli OmpF assembly mutants in a degP background. J Bacteriol. 2000;182:4882–4888. doi: 10.1128/jb.182.17.4882-4888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyadai H, Tanaka-Masuda K, Matsuyama S, Tokuda H. Effects of lipoprotein overproduction on the induction of DegP (HtrA) involved in quality control in the Escherichia coli periplasm. J Biol Chem. 2004;279:39807–39813. doi: 10.1074/jbc.M406390200. [DOI] [PubMed] [Google Scholar]
- Papenfort K, Pfeiffer V, Mika F, Lucchini S, Hinton JCD, Vogel J. σE-dependent small RNAs of Salmonella respond to membrane stress by accelerating global omp mRNA decay. Mol Microbiol. 2006;62:1674–1688. doi: 10.1111/j.1365-2958.2006.05524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano J, Lynch AS, Belin D, Lin ECC, Beckwith J. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 1997;11:1169–1182. doi: 10.1101/gad.11.9.1169. [DOI] [PubMed] [Google Scholar]
- Price NL, Raivio TL. Characterization of the Cpx regulon in Escherichia coli strain MC4100. J Bacteriol. 2009;191:1798–1815. doi: 10.1128/JB.00798-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivio TL, Silhavy TJ. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J Bacteriol. 1997;179:7724–7733. doi: 10.1128/jb.179.24.7724-7733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivio TL, Silhavy TJ. The σE and Cpx regulatory pathways: overlapping but distinct envelope stress responses. Curr Opin Microbiol. 1999;2:159–165. doi: 10.1016/S1369-5274(99)80028-9. [DOI] [PubMed] [Google Scholar]
- Raivio TL, Popkin DL, Silhavy TJ. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J Bacteriol. 1999;181:5263–5272. doi: 10.1128/jb.181.17.5263-5272.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodius VA, Suh WC, Nonaka G, West J, Gross CA. Conserved and variable functions of the σE stress response in related genomes. Plos Biol. 2006;4:43–59. doi: 10.1371/journal.pbio.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzitello AE, Harper JR, Silhavy TJ. Genetic evidence for parallel pathways of chaperone activity in the periplasm of Escherichia coli. J Bacteriol. 2001;183:6794–6800. doi: 10.1128/JB.183.23.6794-6800.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz N, Silhavy TJ. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr Opin Microbiol. 2005;8:122–126. doi: 10.1016/j.mib.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Shimohata N, Nagamori S, Akiyama Y, Kaback HR, Ito K. SecY alterations that impair membrane protein folding and generate a membrane stress. J Cell Biol. 2007;176:307–317. doi: 10.1083/jcb.200611121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy TJ, Berman ML, Enquist LW. Experiments with Gene Fusions. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- Sklar JG, Wu T, Kahne D, Silhavy TJ. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes Dev. 2007;21:2473–2484. doi: 10.1101/gad.1581007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder WB, Davis LJB, Danese PN, Cosma CL, Silhavy TJ. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J Bacteriol. 1995;177:4216–4223. doi: 10.1128/jb.177.15.4216-4223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess C, Bell A, Ehrmann M. A temperature-dependent switch from chaperones to protease in a widely conserved heat shock protein. Cell. 1999;97:339–347. doi: 10.1016/s0092-8674(00)80743-6. [DOI] [PubMed] [Google Scholar]
- Strauch KL, Johnson K, Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989;171:2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyvé M, Moons M, Tommassen J. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J Mol Biol. 1991;218:141–148. doi: 10.1016/0022-2836(91)90880-f. [DOI] [PubMed] [Google Scholar]
- Vuong P, Bennion D, Mantei J, Frost D, Misra R. Analysis of YfgL and YaeT interactions through bioinformatics, mutagenesis, and biochemistry. J Bacteriol. 2008;190:1507–1517. doi: 10.1128/JB.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh NP, Alba BM, Bose B, Gross CA, Sauer RT. Peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell. 2003;113:61–71. doi: 10.1016/s0092-8674(03)00203-4. [DOI] [PubMed] [Google Scholar]
- Werner J, Misra R. YaeT (Omp85) affects the assembly of lipid-dependent and lipid-independent outer membrane proteins of Escherichia coli. Mol Microbiol. 2005;57:1450–1459. doi: 10.1111/j.1365-2958.2005.04775.x. [DOI] [PubMed] [Google Scholar]
- Wu T, Malinverni J, Ruiz N, Kim S, Silhavy TJ, Kahne D. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell. 2005;121:235–245. doi: 10.1016/j.cell.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ishihama A. Characterization of copper-inducible promoters regulated by CpxA/CpxR in Escherichia coli. Biosci Biotechnol Biochem. 2006;70:1688–1695. doi: 10.1271/bbb.60024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.